Abstract
Increasing evidence suggests that microRNAs (miRNAs) play a crucial role in the pathogenesis of tumor. In this study, miR-214 was found to be significantly down-regulated in lung tumor tissues and lung cell lines. From the gain-of-function experiment results, we found that ectopic expression of miR-214 in lung cancer cell lines significantly inhibited cell growth, as evidenced by cell viability and colony formation assays, and suppressed tumor growth in vivo. Besides, further investigations showed that miR-214 inhibited cell migration and invasion. The luciferase activity assay revealed that oncogene Janus kinase 1 (JAK1) was a direct target gene of miR-214, and its expression was inversely correlated with that of miR-214. Altogether, our findings demonstrated that miR-214 plays a pivotal role in lung cancer by inhibiting cell proliferation, invasion and migration by targeting oncogenic JAK1, and thus, miR-214 may provide a new potential therapeutic target in lung cancer.
Keywords: miR-214, lung cancer, JAK1, tumor suppressor
Introduction
Lung cancer is one of the highest morbidity and mortality diseases in China and worldwide, and approximately 80% of lung cancer is currently classified as non-small cell lung cancer (NSCLC) [1]. Even with current advanced treatment, 5-year survival rate of lung cancer is still less than 17%, and most patients eventually died in metastasis diseases [2,3]. Therefore, it is urgently needed to investigate the molecular mechanism of NSCLC for finding more effective therapeutic targets. Over the last decade, microRNAs (miRNAs) have emerged as key players in carcinogenesis [4]. The aberrant expression of miRNAs has been demonstrated to play a critical role in the initiation and progression of several human cancers through post-transcriptional regulation of gene expression [5].
miRNAs, typically a class of small noncoding RNAs in length of 18-22 nucleotides, could negatively regulate gene expression post-transcriptionally by pairing with the 3’-untranslated regions (UTRs) of target mRNAs leading to mRNA degradation and/or translational repression [6]. Accumulating evidences have proved that miRNAs play a critical role in the regulation and development of lung cancer [7,8]. miRNAs in lung cancer might serve as new therapeutic strategies for direct modulation of oncogenes or tumor suppressor genes. To date, miR-214 has been confirmed as a tumor suppressor in esophageal squamous cell carcinoma [9], lung cancer [10], cervical cancer [11], and ovarian cancer [12] by suppressing cellular proliferation, migration and invasion. However, the functional role and mechanistic action of miR-214 in lung cancer remain mostly unclear. In the present study, we aimed to determine its biological function, molecular basis and target genes in lung cancer.
Materials and methods
Clinical samples and cells
Specimens of primary lung cancer tissues and corresponding adjacent noncancerous tissues were collected and paired from 25 patients with lung cancer from October 2010 to May 2013 following surgical resection. All tissue specimens were immediately frozen in the liquid nitrogen and stored at -80°C refrigerator. Five human lung cancer cell lines (A549, H1299, H522, SPC-A1, PC-9) and the normal human bronchial epithelium cells BEAS-2B used as the control in the experiments were obtained from Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). All cells were cultured in DMEM medium (Life Technologies, Beijing, China) supplemented with 10% fetal bovine serum (HyClone, Logan, UT, USA) and 1% penicillin/streptomycin in a humidified incubator containing (5% CO2, 37°C).
RNA isolation and qRT-PCR assay
For confirming the mRNA expression of JAK1 and miR-214, RNAs were extracted using TRIzol reagent (Invitrogen Co., USA) following the instructions provided by manufacturers. Real-time quantitative PCR analysis was performed using a 7500 Real-Time PCR system (Thermo Fisher Scientific, Applied Biosystems). RT-primers of JAK1 and miR-214 mRNAs were designed and synthesized by Genepharma Company (Shanghai, China) as follows: JAK1, 5’-CTT TGC CCTG TAT GAC GAG AAC-3’ (forward) and 5’-ACC TCA TCC GGT AGT GGA GC-3’ (reverse); GAPDH, 5’-ACA ACT TTG GTA TCG TGG AAG G-3’ (forward) and 5’-GCC ATC ACG CCA CAG TTT C-3’ (reverse); miR-214, 5’-CTG GCT GGA CAG AGT TGT CAT-3’ (forward) and 5’-GCT GTA CAG GTG AGC GGA TG-3’ (reverse); U6, 5’-CTC GCT TCG GCA GCA CA-3’ (forward) and 5’-AAC GCT TCA CGA ATT TGC GT-3’ (reverse). The relative quantification of JAK1 mRNA was normalized using GAPDH. And, the relative quantification of miR-214 was normalized using U6.
miRNA transfection and lentivirus transfection
Human miR-214 precursor (miR-214, SC400277) and the miRNA mimic negative control (Ctrl) were purchased from OriGene Technologies, Inc. The cells were seeded in 24-well plate at 60% confluence and transfected with 15 pmole miRNA per well using Lipofectamine 2000 (Invitrogen). Lentiviruses overexpressing miR-214 and negative control were purchased from GenePharma (Shanghai, China).
Cell viability assay and colony formation assay
A549 and H522 were transfected with miR-214 mimics and negative control by reverse transfection according to the manufacturer’s instructions and plated in 96-well plates (4 × 103 cells per well) for cell viability assay using MTT (MTT, Sigma, St Louis, MO, USA) assay. For each treatment group, triplicate wells were analyzed for cell viability.
A549 and H522 cells (5 × 104/well) were plated in a 24-well plate and transfected with miR-214, control 214. After 24 h, the cells collected and seeded (1000-1500/well) in a fresh 6-well plate for 12 days. Surviving colonies containing at least 50 cells were record after fixed with methanol/acetone (1:1) and stained with 5% crystal violet. The number of colonies was counted under a light microscope. The experiment was carried out in triplicate wells for three times.
Cell invasion assays and wound healing assay
To measure cell invasion, a transwell invasion chamber coated with matrigel (Corning Glass Works, Corning, New York, USA) was used to determine the cell invasion ability. After fixed with 95% absolute alcohol, the cells invaded in the membrane then stained and counted. The number of cells that had invaded through the matrigel was counted in five fields for triplicate membranes at × 200 magnification using an inverted microscope (Olympus).
To further investigate the role of miR-214 in cell migration, we used a wound-healing assay to detect this ability. When the cells were cultured to approximately 90% confluence, wounds were scratched with pipette tips. Then, cells were rinsed three times with phosphate buffered saline (PBS) and fresh culture medium was added. The residual gap widths were evaluated from photomicrographs after 96 h of wound establishment. All the experiments were performed in triplicate wells for three times.
Western blotting and Immunohistochemistry
To determine the expression of protein, approximately 30 mg/lane of protein was loaded and then divided by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Separated protein bands were transferred into polyvinylidene fluoride (PVDF) membranes (Millipore Corporation, MA, USA). After blocked with 5% nonfat dry milk-TBS-0.1% Tween 20 for 2 h, the membranes were incubated with the specific primary antibodies against JAK1 (1:1,000 dilution; polyclonal antibody, ab47435, abcam, USA) and β-actin (1:3,000 dilution; monoclonal antibody, Cell Signaling Technology Inc, USA, #4970S) overnight at 4°C followed by incubation with a horseradish peroxidase (HRP)-conjugated second antibodies (1:5,000 dilution; Santa Cruz). The abundance of target protein bands was densitometrically determined using Bandscan 5.0 software (Glyko Inc, Novato, CA) and quantifying was presented as fold changes after normalization to according invariant control.
Tissues of formalin-fixed paraffin-embedded surgical specimens were constructed. Detection of Ki67 and JAK1 was performed on paraffin sections (4 μm) with the anti-Ki67 antibody (1:200) and anti-JAK1 antibody (1:200). The analysis was carried out by Image-Pro Plus 6.0 (Media Cybernetics Company, America).
Luciferase reporter assay
The fragments including the 3’-UTR-WT or 3’-UTR-MUT regions of JAK1 and a mutant reporter (Luc-JAK1-mu), in which the predicted miR-214 binding site on JAK1 was mutated, was cloned into luciferase reporters and co-transfected with either a miR-214 mimic or control. After 48 h transfection, the firefly and renilla luciferase activities were measured by GloMax20/20 Luminometer (Promega) using the Dual-Luciferase Reporter Assay System (Promega) followed the manufacturer’s suggestions.
Tumorigenicity assay in nude mice
A549 cells (1 × 106, infected with inducible miR-214 and NC miRNA) were injected subcutaneously into the left flank of 4-week-old female BALB/c nude mice. Tumor size was measured with every three day, and the tumor volume (V) was calculated as (l × w × w)/2, with l indicating length and w indicating width. All mice were killed after 28 days inoculation. The tumor tissues were isolated and weighted, and parts of the tumor tissues were fixed in 4% formaldehyde or snap frozen in liquid nitrogen and stored at -80°C for further molecular detection.
Statistical analysis
All data were expressed as mean values ± standard deviation (SD). All statistical analyses were performed using the SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). Differences between groups was analyzed by one-way ANOVA with the Tukey multiple comparison test using GraphPad Prism 5.0. P-values less than 0.05 could be considered as statistically significant changes.
Results
miR-214 is down-regulated in tissues and cell lines of lung cancer
We firstly analyzed the expression of miR-214, which was determined by qRT-PCR in 25 lung cancerous and corresponding noncancerous tissues (Figure 1A). The results showed that the average expression level of miR-214 was down-regulated in lung cancer tissues in comparison with that in the paired normal lung tissue (P = 0.0055). Moreover, we also found that the expression of miR-214 in lung cancer cell lines was considerably lower than that in the normal human bronchial epithelium cells BEAS-2B (Figure 1B). Therefore, these outcomes suggested that depleted miR-214 expression may be related with lung carcinogenesis.
Figure 1.
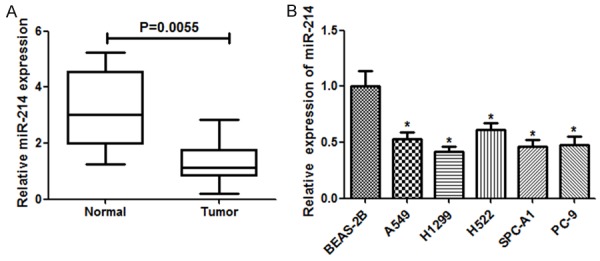
The expression of miR-214 was down-regulated in lung cancer. A. Relative miR-214 expression in 25 surgical specimens of lung carcinoma tissues and their matched non-tumor tissues was detected by qRT-PCR. U6 was used as a control. miR-214 expression was significantly down-regulated in lung cancer patient tissues compared to the adjacent normal tissues. B. miR-214 expression in lung carcinoma cell lines was reduced compared with that in BEAS-2B cells. Each bar represents the mean ± SD. Each experiment was performed in triplicate. *P < 0.05, student t-test.
miR-214 suppresses lung cancer cell growth in vitro
To investigate the potential functional roles of miR-214 in lung cancer, the lentiviral vector containing the miR-214 was transfected into lung cancer cell lines (A549 and H522). From the MTT assays, ectopic expression of miR-214 in A549 and H522 cells caused a significant decrease in cell viability (P < 0.05 in both cell lines) (Figure 2A).
Figure 2.

miR-214 inhibited lung cancer cell growth in vitro. A. Cell viability was validated by MTT assay in lung carcinoma A549 and H522 cells transfected with miR-214 mimics (miR-214) or controls (Ctrl). B. Representative images (left) and quantification (right) of the colony formation assay of the indicated cells. Each bar represents the mean ± SD. *P < 0.05, **P < 0.01, student t-test.
Furthermore, the colony formation assay was used to further assess the inhibitory effect of miR-214 on lung cancer cell growth. The colony formation assay consistently revealed that enforced expression of miR-214 dramatically reduced the number of colonies in the two lung cancer cell lines (P < 0.01 in both cell lines) (Figure 2B). These results were further proved that miR-214 plays a tumor suppressive role in lung cancer.
miR-214 suppresses lung cancer cell migration and invasion of lung cancer cell lines
To investigate the impact of miR-214 on cell invasion and migration, we analyzed the outcomes of transwell matrigel assays (Figure 3A) and wound healing assays (Figure 3B), showing A549 and H522 cells transfected with miR-214 significantly decreased the invasive cells in A549 and H522 compared to control cells. Consolidated with all results, we elucidated that miR-214 overexpression suppressed cell invasion and migration capability of A549 cells and H522 considerably.
Figure 3.
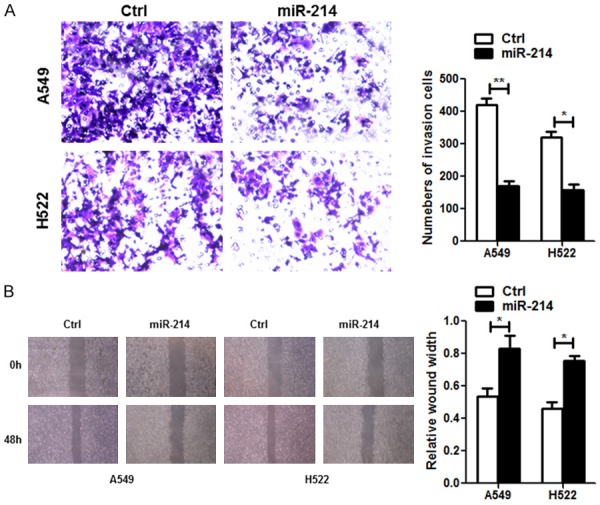
miR-214 inhibited lung carcinoma cell growth in vitro. Transwell Matrigel assay was used to determine cell invasive capability (A). Wound-healing assay was used to determine cell migratory capability (B). Each bar represents the mean ± SD. *P < 0.05, **P < 0.01, student t-test.
Effects of miR-214 overexpression on the tumor growth in nudemurine xenografts
To validate the observations in vitro that overexpression of miR-214 may have tumor-inhibitory effects, an in vivo xenograft tumor model of lung cancer was established. Stably expressing A549 cells were prepared by infection with lentivirus carrying the miR-214 gene or control, and their tumorigenic effects were examined in nude mice. Consistent with the in vitro results, miR-214 overexpression inhibited tumor growth in vivo, and the tumor size and volume derived from miR-214 overexpressing cells were dramatically smaller than those of the control group (Figure 4A and 4B). The average tumor weight in the control group was significantly higher than that in the miR-214 group (Figure 4C). qRT-PCR analysis of tumor tissues confirmed that miR-214 expression levels were increased in miR-214 overexpressing tumors (Figure 4D). Analysis of lung cancer proliferation activity showed that miR-214 over-expression decreased the levels of the cell proliferation marker Ki67 in tumor cells compared with those in the control group (Figure 4E). These data supported an essential role for miR-214 in the suppression of lung cancer growth in vivo.
Figure 4.

miR-214 overexpression suppressed lung carcinoma xenograft tumor growth in vivo. Lung carcinoma A549 cells were transduced with lentivirus (miR-214 or Ctrl) and then subcutaneously injected into nude mice. A. Tumor growth curve in the nude mice was measured on the indicated days. B. Representative pictures of subcutaneous tumors are shown. C. Mean tumor weights. D. Relative expression of miR-214 in the tumors. E. Ki67 levels in tumors measured by IHC staining. Each bar represents the mean ± SD. *P < 0.05, **P < 0.01, student t-test.
miR-214 targets JAK1 via binding to its 3’UTR
In order to detect the possible target for miR-214, online informatic tools such as TargetScan, MiRanda were used. As illustrated in Figure 5A, JAK1 possesses a putative binding site of miR-214. Luciferase reporter assay was executed in A549 cells and H522, suggesting that miR-214 mimics scaled down the luciferase activity of the construct containing the wild-type JAK1 3’UTR reporter considerably, but this diminished activity was not detected in the JAK1 3’UTR reporter with mutant miR-214 binding seed sites (Figure 5B). Our data thus demonstrated that JAK1 was a direct target of miR-214.
Figure 5.
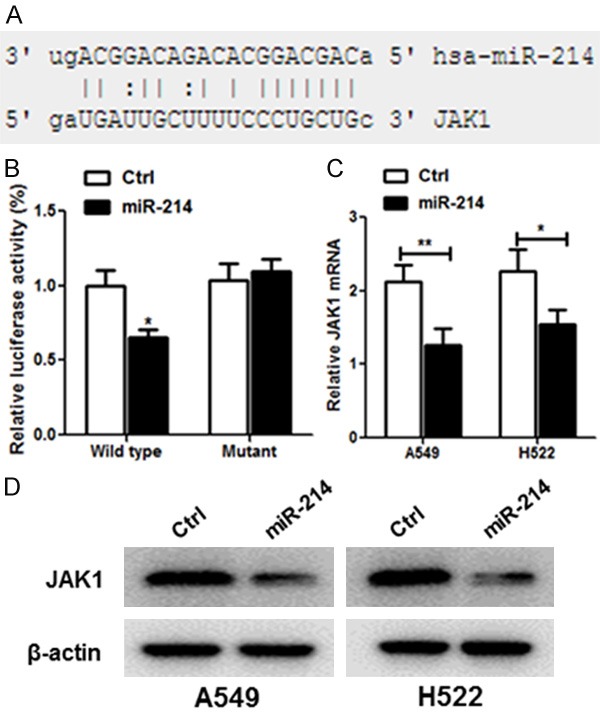
JAK1 is a direct target of miR-214 in lung cancer. A. The human JAK1 3’UTR binding site for miR-214. B. miR-214 targeted the wild-type but not the mutant 3’UTR of JAK1. C. Ectopic expression of miR-214 down-regulated JAK1 mRNA expression in A549 and H522 cells as determined by qRT-PCR. D. miR-214 decreased JAK1 protein level in A549 and H522 by western blot. Each bar represents the mean ± SD. *P < 0.05, student t-test.
To further confirm that miR-214 targets JAK1, miR-214 or control mimic was transfected into A549 and H522 cells. Transfection of miR-214 resulted in significant reduction of JAK1 mRNA and protein expression by qRT-PCR (Figure 5C) and western blot (Figure 5D), respectively.
JAK1 is up-regulated in primary lung cancer tumors and inverse correlated with miR-214 expression
To assess the importance of JAK1 in primary lung cancer, we compared the level of JAK1 in 25 paired tumor and adjacent normal tissues. The expression of the JAK1 mRNA was significantly increased in lung cancer tumors compared to the adjacent normal tissues (P < 0.01; Figure 6A). JAK1 was overexpressed in 72% (18/25) of tumors compared with their normal counterparts.
Figure 6.
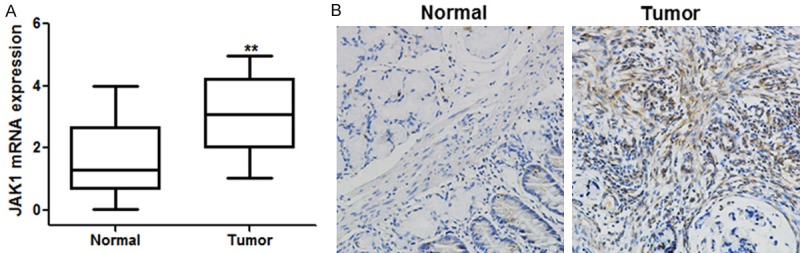
A. Expression of JAK1 is significantly up-regulated in primary lung tumors compared with their adjacent normal tissues (n = 25, left panel). B. Representative immunohistochemical staining of JAK1 in lung cancer tissue and the corresponding adjacent non-tumor tissue (right panel). Each bar represents the mean ± SD. **P < 0.01, student t-test.
To further study the relationship between miR-214 and JAK1 in human lung cancer, we measured the protein expressions in 25 paired lung cancer and adjacent normal tissues using immunohistochemistry. From the results, lung cancer tissues exhibited significantly higher JAK1 expression compared to the adjacent normal tissues.
Discussion
Lung cancer is still a major health problem due to its high incidence and mortality rates [13]. Tumor-related molecular abnormalities such as oxidative stress and inflammation [14-16] play a key role in lung cancer progression and therapy. And NSCLC accounts for about 80% of all lung cancer and the most common cause of tumor death [17]. In the past few decades, with the improvement of surgical techniques and the rapid development of chemical drugs and the treatment of targeted systemic therapy, the prognosis of patients with lung cancer has been improved effectively [18]. But the overall prognosis of patients with NSCLC is still unsatisfactory: the 5-year survival rate is only about 15% for patients with stage III of NSCLC, even if combined with radiotherapy and chemotherapy [19]. In the United States alone, it is reckoned that over 200,000 new cases of lung cancer are reported each year, and 150,000 people die from lung cancer every year [20]. Therefore, early diagnosis of NSCLC and further development of treatment for the improvement of lung cancer patients with the prognosis is of particular importance.
Many miRNAs were identified as metastasis promoter or suppressor and thus provided us a new perspective on the metastatic process [21]. Therefore, the purpose of this study is to identify miRNAs’ role in NSCLC metastasis by comparing the miRNA profiles. In the present study, miR-214 was proved to a decreased expression in the lung cancer tissues and lung cell lines. Moreover, we found that miR-214 overexpression inhibited cell proliferation and colony formation, and also suppressed the cell migration and invasion, indicating that miR-214 might act as a tumor suppressor in NSCLC. Having shown the crucial role of miR-214 in suppressing NSCLC development, we sought for the possible gene effectors participating in its function. Among the miRNAs predicted to target genes, we found that JAK1 acts as a critical effector of miR-214. JAK1, a member of the JAK family, is widely expressed in mammalian cells [22]. There has reported that JAK1 stimulates the phosphorylation of STAT3 and promotes cell proliferation, migration, invasion, and angiogenesis [23]. In this study, we determined that miR-214 was able to significantly repress the luciferase activity of Luc-JAK1-3’UTR by targeting the 3’UTR of JAK1 gene’s mRNA. In NSCLC patients, the mRNA and protein expression of JAK1 gene were increased, suggesting miR-214 may play the potential therapeutic effects on lung cancer via inhibition of JAK1 expression.
In conclusion, our data provided new evidence to support the tumor suppressor function of miR-214 in NSCLC. Meanwhile, we have determined that JAK1 is a novel target of miR-214 in NSCLC. Future study will focus on miR-214’s role as an innovative diagnostic biomarker and therapeutic target in the treatment of NSCLC.
Acknowledgements
This work is supported by Natural Science Foundation of China (No. 81501966) and 2014 Shanghai Pujiang Talent 14PJ009.
Disclosure of conflict of interest
None.
References
- 1.Kang JU, Koo SH, Kwon KC, Park JW, Kim JM. Identification of novel candidate target genes, including EPHB3, MASP1 and SST at 3q26.2-q29 in squamous cell carcinoma of the lung. BMC Cancer. 2009;9:237. doi: 10.1186/1471-2407-9-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao F, Liu D, Guo Y, Shi B, Song Z, Tian Y, Zhang Z, Liang C. Survival rate and prognostic factors of surgically resected clinically synchronous multiple primary non-small cell lung cancer and further differentiation from intrapulmonary metastasis. J Thorac Dis. 2017;9:990–1001. doi: 10.21037/jtd.2017.03.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenmotsu H, Ohde Y, Wakuda K, Nakashima K, Omori S, Ono A, Naito T, Murakami H, Kojima H, Takahashi S. Survival data for postoperative adjuvant chemotherapy comprising cisplatin plus vinorelbine after complete resection of non-small cell lung cancer. Cancer Chemother Pharmacol. 2017:1–6. doi: 10.1007/s00280-017-3400-z. [DOI] [PubMed] [Google Scholar]
- 4.Li Q, Li S, Wu Y, Gao F. miRNA-708 functions as a tumour suppressor in hepatocellular carcinoma by targeting SMAD3. Oncol Lett. 2017;14:2552. doi: 10.3892/ol.2017.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Liu H, Shao J, Xing G. miR-320a serves as a negative regulator in the progression of gastric cancer by targeting RAB14. Mol Med Rep. 2017;16:2652–2658. doi: 10.3892/mmr.2017.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu L, Sturgis EM, Lu Z, Zhang H, Wei P, Wei Q, Li G. Association between miRNA-binding site polymorphisms in double-strand break repair genes and risk of recurrence in patients with squamous cell carcinomas of the nonoropharynx. Carcinogenesis. 2017:38. doi: 10.1093/carcin/bgx019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Zhang H, Dong Y, Fan Y, Li Y, Zhao C, Wang C, Liu J, Li X, Dong M. MiR-146b-5p functions as a suppressor miRNA and prognosis predictor in non-small cell lung cancer. J Cancer. 2017;8:1704–1716. doi: 10.7150/jca.16961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dejima H, Iinuma H, Kanaoka R, Matsutani N, Kawamura M. Exosomal microRNA in plasma as a non-invasive biomarker for the recurrence of non-small cell lung cancer. Oncol Lett. 2017;13:1256. doi: 10.3892/ol.2017.5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M, Wang L, Zhang M, Li X, Zhu Z, Wang H. MiR-214 inhibits the proliferation and invasion of esophageal squamous cell carcinoma cells by targeting CDC25B. Biomed Pharmacother. 2017;95:1678–1683. doi: 10.1016/j.biopha.2017.09.048. [DOI] [PubMed] [Google Scholar]
- 10.Zhao X, Lu C, Chu W, Zhang Y, Zhang B, Zeng Q, Wang R, Li Z, Lv B, Liu J. microRNA-214 governs lung cancer growth and metastasis by targeting carboxypeptidase-D. DNA Cell Biol. 2016;35:715. doi: 10.1089/dna.2016.3398. [DOI] [PubMed] [Google Scholar]
- 11.Wang JM, Ju BH, Pan CJ, Gu Y, Li MQ, Sun L, Xu YY, Yin LR. MiR-214 inhibits cell migration, invasion and promotes the drug sensitivity in human cervical cancer by targeting FOXM1. Am J Transl Res. 2017;9:3541–3557. [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Q, Zhang S. miR-214 promotes radioresistance in human ovarian cancer cells by targeting PETN. Biosci Rep. 2017:37. doi: 10.1042/BSR20170327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onat S, Ates G, Avcı A, Yıldız T, Birak A, Ozmen CA, Ulku R. The role of mediastinoscopy in the diagnosis of non-lung cancer diseases. Ther Clin Risk Manag. 2017;13:939. doi: 10.2147/TCRM.S144393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao R, Saracini C, Ginsbach JW, Kieberemmons MT, Siegler MA, Solomon EI, Fukuzumi S, Karlin KD. Peroxo and superoxo moieties bound to copper ion: electron-transfer equilibrium with a small reorganization energy. J Am Chem Soc. 2016;138:7055–66. doi: 10.1021/jacs.6b02404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rui C, Elrod LT, Lehane RL, Kim E, Karlin KD. A peroxynitrite dicopper complex: formation via Cu-NO and Cu-O2 intermediates and reactivity via O-O cleavage chemistry. J Am Chem Soc. 2016;138:16148–16158. doi: 10.1021/jacs.6b10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan XY, Yi W, Hong L. Overexpression of PTEN suppresses non-small-cell lung carcinoma metastasis through inhibition of integrin αVβ6 signaling. Am J Transl Res. 2017;9:3304–3314. [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HY, Mohammed KA, Kaye F, Moudgil BM, Nasreen N. EphA2 targeted intratumoral therapy for non-small cell lung cancer using albumin mesospheres. Am J Transl Res. 2017;9:3293. [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S, Zhang B, Li C, Cui C, Yue D, Shi B, Zhang Q, Zhang Z, Zhang X, Wang C. Prognostic value of number of negative lymph node in patients with stage II and IIIa non-small cell lung cancer. Oncotarget. 2017;8:79387–79396. doi: 10.18632/oncotarget.18154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mino-Kenudson M, Chirieac LR, Law K, Hornick JL, Lindeman N, Mark EJ, Cohen DW, Johnson BE, Jänne PA, Iafrate AJ. A novel, highly sensitive antibody allows for the routine detection of ALK-rearranged lung adenocarcinomas by standard immunohistochemistry. Clin Cancer Res. 2010;16:1561. doi: 10.1158/1078-0432.CCR-09-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mjd E, Farhangi B, Montazeri M, Monfared H, Sistani RN, Sadeghizadeh M. Up-regulation of miR-21 decreases chemotherapeutic effect of dendrosomal curcumin in breast cancer cells. Iran J Basic Med Sci. 2017;20:350. doi: 10.22038/IJBMS.2017.8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu YJ, Sun WY, Zhang S, Li XR, Wei W. Targeted blockade of JAK/STAT3 signaling inhibits proliferation, migration and collagen production as well as inducing the apoptosis of hepatic stellate cells. Int J Mol Med. 2016;38:903. doi: 10.3892/ijmm.2016.2692. [DOI] [PubMed] [Google Scholar]
- 23.Sun L, Ma K, Wang H, Xiao F, Gao Y, Zhang W, Wang K, Gao X, Ip N, Wu Z. JAK1-STAT1-STAT3, a key pathway promoting proliferation and preventing premature differentiation of myoblasts. J Cell Biol. 2007;179:129. doi: 10.1083/jcb.200703184. [DOI] [PMC free article] [PubMed] [Google Scholar]


