Abstract
The aim of this study was to evaluate the protective effects of dietary flavonoid quercetin against Alzheimer’s disease (AD) and detect the explicit administration of quercetin in early-middle or middle-late stage of AD pathology could play the effect as well as its mechanism of action. In this study, APP/PS1 mice were used to investigate cognitive impairment and related pathologies. The results showed that quercetin enrich diet could play an ameliorated pathology development of AD in APP/PS1 mice. And then we next determined administration of quercetin in early-middle and middle-late stage of AD pathology, which exerted that only quercetin enrich diet during the early-middle stage of AD pathological development period ameliorates cognitive dysfunction and the protection effect was mainly related to increased Aβ clearance and reduced astrogliosis. These findings suggest a possible new protective role for dietary flavonoids on AD. This new role might expand the preventive and/or therapeutic use of AD in conditions.
Keywords: Quercetin, alzheimer’s disease, flavonoid
Introduction
Alzheimer’s disease (AD), a degenerative neurological disease, is clinically characterized by progressive cognitive dysfunction [1]. It is most common cause of dementia in elderly patients, which typically begins with deterioration of memory. It is estimated that the global incidence of dementia is as high as 36 million and will reach 66 million by 2030 and 115 million by 2050, with approximately two-thirds of those patients living in developing countries [2].
At present, although important progress has been made in understanding the pathogenesis of AD, the etiology and course of AD is not clear enough. The treatments for AD currently used have had little effect on symptoms and no effective treatment has been found to delay or prevent the disease [3]. The United States FDA has approved very little medication for AD; and these drugs improve symptoms but do not alter the course of disease progression and have even shown some adverse effects [4]. In this case, the use of natural substances to treat neurodegenerative diseases such as AD is increasing.
Recent epidemiological and experimental studies have determined that fruit and vegetable juices containing various flavonoid compounds provides a reduced risk of AD a slower cognitive decline [5]. Quercetin (a bioflavonoid), frequently found in many plants, leaves, fruits, mostly in the form of glycosides, has many beneficial effects on human health, mainly including anti-cancer [6], anti-inflammation [7], anti-oxidative stress effects [8]. It has also been reported that quercetin may have neuro-protective effects and slow down the progression of degenerative diseases [9]. Meanwhile, quercetin has been shown to attenuate behavioural alterations and cognitive impairment in Parkinson’s disease models and chronic cerebral ischemia models [10]. And quercetin has also been indicated to penetrate in and rigidify the bilayer of membranes and reduce the interaction of the membrane with the Aβ peptide, which is a core marker in the AD pathological process [11]. It also has been reported that quercetin have some effect on the treatment of AD but there is some studies to show no effect on the AD for quercetin. Due to the controversy of protection role of quercetin on AD, we firstly confirmed that quercetin enrich diet an ameliorated pathology development of AD in APP/PS1 mice. Then we speculated that the different outcomes of quercetin administration may possibly because the different time of therapy they chosen, therefore we next determined administration of quercetin in early-middle and middle-late stage of AD pathology, which exerted that only quercetin enrich diet during the early-middle stage of AD pathological development period ameliorates cognitive dysfunction and the protection effect was mainly related to increased Aβ clearance and reduced astrogliosis.
Materials and methods
Animals
All mice used in this study, APP/PS1 mice were purchased from Model Animal Research Center of Nanjing University (Nanjing, China) and group-housed in a temperature-controlled room with a 12 h: 12 h light-dark cycle. All animals used in this study were performed humanely and following the institutional and national guidelines for ethical animal research. The experimental procedures were approved by the Administration Committee of Experimental Animals. The APP/PS1 mice were divided into group mice received normal diet without any supplementation, and another group mice received diet supplemented with quercetin (2 mg/g diet).
Behavioral test
The Morris water navigation task, also known as the Morris water maze (MWM), is a behavioral procedure mostly used with rodents. It is widely used to analyze the spatial memory and to evaluate both the working and reference memory functions in the animals. The circular pool (diameter of 120 cm) filled with water (20 ± 0.5°C) with a bath heater. Briefly, the whole test lasted 8 days and was divided into three sections, including visible platform training, hidden platform training, and preference quadrant test without a platform. On days 1-2, visible platform training took place, during which a cylindrical white-colored platform (7 cm in diameter) was placed 0.5 cm above the water. On the other hand, hidden platform training (1 cm below the water’s surface) was performed on days 3-7, and then moved to the opposite quadrant from the visible platform training. Eventually, the platform was removed, while the preference quadrant test was conducted on the 8th day. The swimming paths were recorded and average swimming speed (cm/s) and latency (s) to reach the visible platform or the hidden-platform were analyzed during the training and testing. The percentage of time spent in each quadrant was assessed using the preference quadrant test performed on day 8. In visible platform training and hidden platform training section, mice were placed to the opposite quadrant and let them to find the platform with maximum time of 60 s. In preference quadrant test, mice were placed to the opposite quadrant and let them swim in the pool for 60 s.
Immunofluorescence
The brain tissue was fixed with 4% paraformaldehyde overnight at 4°C. After dehydrating in sucrose buffer, the brain tissue was sectioned coronally using a freezing microtome (CM1950, Leica, Wetzlar, Germany) at 10 μm. The slides were permeabilized using 0.1% Triton X-100 for 15 min and blocked with 10% normal goat serum for 1 h at room temperature. After incubated with related primary antibodies (anti-GFAP (rabbit monoclonal primary antibody, 1:300; Abcam, Cambridge, UK) or anti-6E10 (mice monoclonal primary antibody, Covance, Princeton, NJ, USA)) at 4°C overnight, brain tissue then incubated with Alexa Fluo-488 conjugated goat anti-rabbit IgG antibody (1:500, Invitrogen) or Alexa Fluo-555 conjugated goat anti-mice IgG antibody (1:500, Invitrogen)), and counterstained with DAPI in a cassette for 5 min. Fluorescence was detected and imaged under a confocal laser scanning microscope.
β-secretase and γ-secretase concentration measurement
The levels of β-Secretase and γ-Secretase concentration in the brain tissue were measured using enzyme-linked immunosorbent assay (ELISA) kit (Invitrogen) following the manufacturer’s instructions.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
The total RNA was isolated using TRIzol reagent (Invitrogen, CA, USA) from the brain tissues. RT-qPCR was performed using SYBR Green PCR Kit (Takara) according to the manufacturer’s instructions. Real-time quantitative PCR analysis was performed using a 7500 Real-Time PCR system (Thermo Fisher Scientific, Applied Biosystems). The housekeeping gene GAPDH was used as endogenous controls for the mRNA experiments.
Western blotting
Total protein was extracted from the separated brain as previously described and analyzed by western blot with specific antibodies as described. Briefly, total protein was extracted by collecting the pellet after centrifugation at 12,000 rpm at 4°C for 10 min. and the supernatant was collected. The protein concentration was determined by BSA assay. Equal amounts of protein were separated by 10% SDS-PAGE and electrobloted onto PVDF membranes. After blocking with 5% non-fat milk for 1.5 h at 37°C, and then the membranes were incubated with the corresponding primary antibody, including anti-APP (1:2000; Abcam, Cambridge, UK), anti-GFAP (1:1000; Abcam), anti-BACE1 (1:1000; Abcam), anti-PS1 loop (1:2000; Millipore), pSmad2 (1:1000; Abcam), Smad2 (1:1000; Abcam), p-STAT3 (1:1000; Abcam), STAT3 (1:1000; Abcam), Hevin (1:1000; Abcam), SPARC (1:1000; Abcam), CTFβ (82E1, 1:500; IBL international, Humburg, Germany), and anti-β-actin (1:500; Santa Cruz, Dallas, TX, USA) overnight at 4°C, then rinsed with TBST three times, followed by horseradish peroxidase-conjugated secondary antibody (anti-rabbit IgG or anti-mouse IgG, 1:1000; Cell Signaling Technology, Inc) for 1 h at room temperature. After three washes in TBST, immunoreactive protein bands were visualized with Tanon 5200 Chemiluminescence imaging system and analyzed the intensity with Quantity One soft-ware (Bio-Rad, Hercules, CA, USA).
Statistical analysis
All data were analyzed using SPSS software (version 16.0, SPSS Inc., Chicago, IL, USA). Differences among means were assessed for significance using either Student’s t-test or one-factor analysis of variance (ANOVA) followed by post hoc Tukey’s test. Differences at P < 0.05 were considered statistically significant. All results were represented as means ± standard error of the mean (SEM) using GraphPad Prism 5 (La Jolla, CA, USA).
Results
Quercetin enrich diet prevented cognitive dysfunction through increasing Aβ clearance and astrocyte function
In the first, we designed the scheme to investigate the protection effect of quercetin on AD (Figure 1A). Our data showed that quercetin enrich diet (1 month-13 month) significantly decreased the latency in APP/PS1 mice compared with the normal diet APP/PS1 mice, but not affected the swim speed (Figure 1B and 1C). And we withdrew the platform on the last day and recorded the swim path (Figure 1D and 1E). The results showed that normal diet mice spend less time in the target quadrant compared with quercetin enrich diet (1 month-13 month) mice. Meanwhile, we assessed amyloid plaques and astrogliosis in cortex and hippocampus of APP/PS1 mice by immunostaining (Figure 1F, 1G, 1I and 1J). The pictures showed that the number and area of amyloid plaques and astrogliosis were significantly decreased in cortex and hippocampus of quercetin enrich diet (1 month-13 month) mice compared with normal diet mice. We also identified the protein level of APP, CTFβ and GFAP to assess amyloid plaques and astrogliosis in cortex and hippocampus of quercetin enrich diet mice (1 month-13 month) compared with normal diet mice, and the results showed that CTFβ and GFAP protein were decreased but APP protein was not changed in quercetin enrich diet (1 month-13 month) mice compared with normal diet mice, which indicated quercetin enrich diet prevents AD pathological development through decreasing amyloid deposition and astrogliosis (Figure 1H and 1K).
Figure 1.
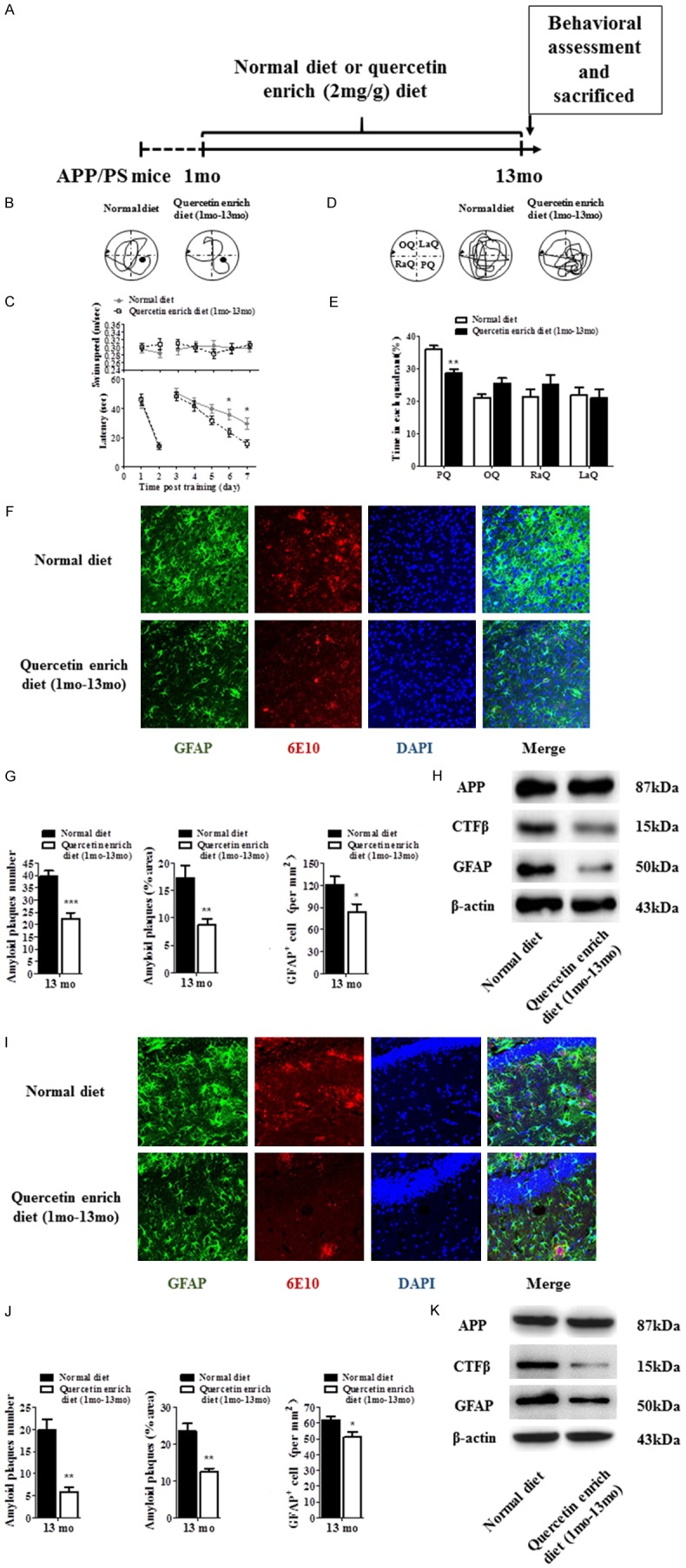
Quercetin enrich diet (1 month-13 month) prevented cognitive dysfunction and decreased amyloid plaques and astrogliosis. Schematic for administration of normal or quercetin enrich (2 mg/g) diet and all of experimental examination (A). Spatial learning and memory functions were improved at 13 month in quercetin enrich diet (1 mo-13 mo) mice compared with normal diet mice identified by Morris water maze (B-E). Immunostaining was performed to identify GFAP, 6E10 and DAPI in cortex and hippocampus of quercetin enrich diet (1 month-13 month) mice and normal diet mice (F and I). The number and area of amyloid plaques and GFAP+ cells were decreased in cortex and hippocampus of quercetin enrich diet (1 month-13 month) mice compared with normal diet mice (G and J). The expression of CTFβ and GFAP were decreased but the expression of APP was not changed in cortex and hippocampus of quercetin enrich diet (1 month-13 month) mice compared with normal diet mice (H and K). All data are presented as means ± standard error of the mean, n = 12, *P < 0.05, **P < 0.01, ***P < 0.001 compared with normal diet mice, mo represented as months.
Quercetin enrich diet during early-middle AD pathological progression was sufficient to prevent pathological development
To determine the effect of quercetin enrich diet in early-middle (1 month-9 month) AD pathological progression, we designed the experimental scheme (Figure 2A). Cognitive function was detected by Morris Water Maze (Figure 2B), and the latency was decreased and swim speed was not affected in quercetin enrich diet (1 month-9 months) mice compared with normal diet mice (Figure 2B and 2C). And the swim path was recorded on the last day after withdrawing the platform and time spent in each quadrant were analyzed (Figure 2D and 2E). The results showed that quercetin enrich diet (1 month-9 months) increased the time spent in target quadrant compared with normal diet. To identify the mechanism of cognitive dysfunction prevention, firstly, we examined the mRNA and protein level of APP, CTFβ, BACE1 and PS1 loop in brain of APP/PS1 mice to assess the amyloid plaques formation and clearance (Figure 2F and 2G). The results showed that the protein level of CTFβ and BACE1 was decreased but the mRNA and protein level of APP and PS1 loop were not changed in quercetin enrich diet (1 month-9 months) mice compared with normal diet mice. Additionally, the results of β- and γ-secretase activity assay exerted that β-secretase activity was not altered but γ-secretase activity was significantly upregulated (Figure 2H), suggesting quercetin enrich diet cover the early-middle stage of AD pathological development depress Aβ producing. Secondly, we examined the mRNA and protein level of Smad2 and STAT3 and the protein level of p-Smad2 and p-STAT3 which play critical role in astrogliosis (Figure 2I and 2J). The mRNA and protein level of Smad2 and STAT3 were not affected but the protein level of p-Smad2 and p-STAT3 were decreased in quercetin enrich diet (1 month - 9 months) mice compared with normal diet mice indicated the reduction of astrogliosis. Finally, we identified the mRNA and protein level of Hevin and SPARC which are important in protein secretion (Figure 2K and 2L). The results showed that quercetin enrich diet (1 month-9 months) significantly reduced the mRNA and protein level of Hevin and SPARC compared with normal diet.
Figure 2.
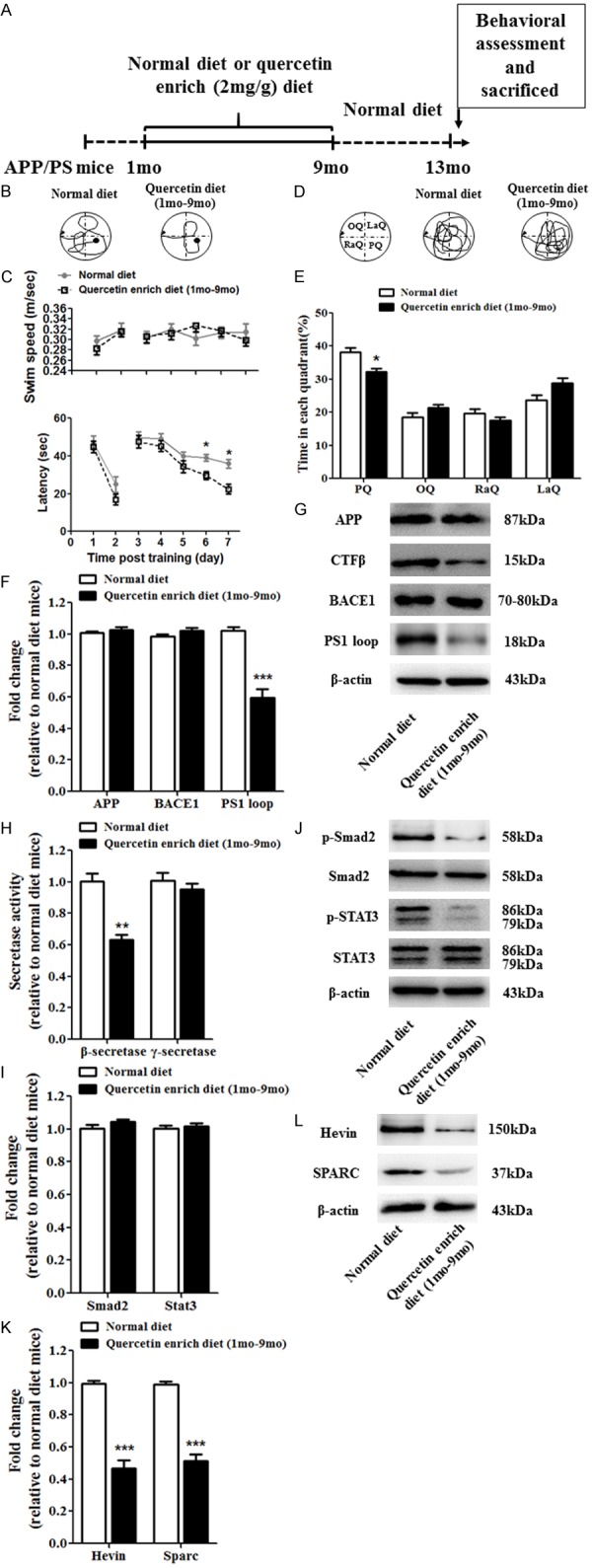
Quercetin enrich diet during early-middle AD pathological progression prevented cognitive dysfunction and decreased amyloid plaques and astrogliosis. Schematic for administration of normal or quercetin enrich (2 mg/g) diet and all of experimental examination (A). Spatial learning and memory functions were improved at 13 months in quercetin enrich diet (1 month-9 months) mice compared with normal diet mice identified by Morris water maze (B-E). The mRNA (F) and protein (G) level of APP, BACE1 and PS1 loop were not affected but the protein level of CTFβ was decreased in quercetin enrich diet (1 month-9 months) mice compared with normal diet mice. The concentration of γ-Secretase was not affected but the level of β-Secretase concentration was decreased in quercetin enrich diet (1 month-9 months) mice compared with normal diet mice (H). The mRNA (I) and protein (J) level of Smad2 and STAT3 were not affected but the protein level of p-Smad2 and p-STAT3 were decreased in quercetin enrich diet (1 month-9 months) mice compared with normal diet mice. The mRNA (K) and protein (L) level of Hevin and SPARC were decreased in quercetin enrich diet (1 month-9 months) mice compared with normal diet mice. All data are presented as means ± standard error of the mean, n = 12, *P < 0.05, **P < 0.01, ***P < 0.001 compared with normal diet mice, mo represented as months.
Quercetin enrich diet during middle-late AD pathological progression failed to prevent pathological development
Next, we also investigated the effect of quercetin enrich diet in middle-late stage of AD pathological development and designed the experimental scheme (Figure 3A). Morris Water Maze was performed to evaluate the spatial learning and memory in quercetin enrich diet (6 months-13 months) mice and normal diet mice. Swim speed and the latency were analyzed and the results showed that no significant difference was obtained between quercetin enrich diet (6 months-13 months) mice and normal diet mice (Figure 3B and 3C). Time in each quadrant during the preference quadrant test was also recorded and the results also showed no significant difference in quercetin enrich diet (6 months-13 months) mice compared with normal diet mice (Figure 3D and 3E). Furthermore, the mRNA and protein expression of APP, CTFβ, BACE1 and PS1 loop were examined to assess the amyloid plaques formation and clearance, and all of them showed no difference in quercetin enrich diet (6 months-13 months) mice compared with normal diet mice (Figure 3F and 3G). Moreover, the activity of β- and γ-secretase activity were also exerted no significant difference (Figure 3H). Then the mRNA and protein level of Smad2 and STAT3 and the protein level of p-Smad2 and p-STAT3 were examined to evaluate the astrogliosis (Figure 3I and 3J) and all of them were not affected in quercetin enrich diet (6 months-13 months) mice compared with normal diet mice. Eventually, the mRNA and protein level of Hevin and SPARC which play important role in protein secretion were identified in quercetin enrich diet (6 months-13 months) mice and normal diet mice (Figure 3K and 3L). The results showed that quercetin enrich diet (6 months-13 months) unaffected the mRNA and protein level of Hevin and SPARC compared with normal diet.
Figure 3.
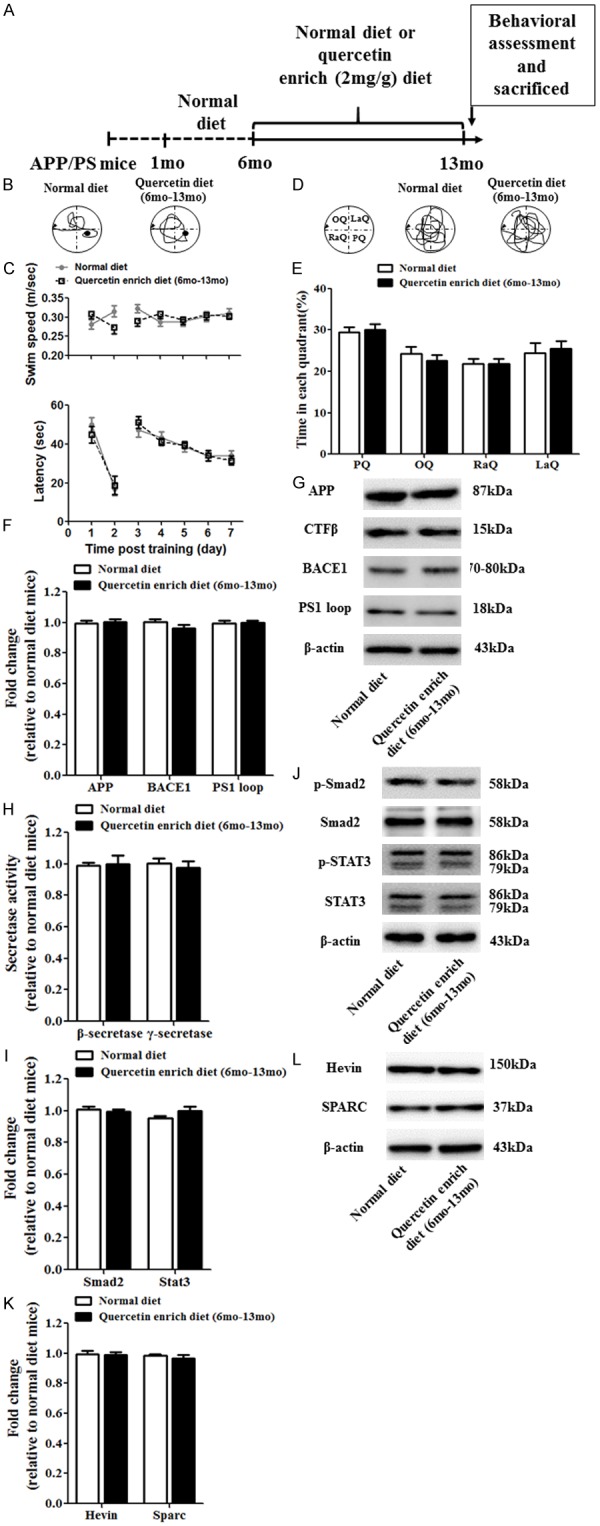
Quercetin enrich diet during middle-late AD pathological progression unaffected cognitive dysfunction, amyloid plaques and astrogliosis. Schematic for administration of normal or quercetin enrich (2 mg/g) diet and all of experimental examination (A). Spatial learning and memory functions were unaffected at 13 months in quercetin enrich diet (1 month-9 months) mice compared with normal diet mice identified by Morris water maze (B-E). The mRNA (F) and protein (G) level of APP, CTFβ, BACE1 and PS1 loop were not affected in quercetin enrich diet (6 months-13 months) mice compared with normal diet mice. The concentration of β-Secretase and γ-Secretase was not affected in quercetin enrich diet (1 month-9 months) mice compared with normal diet mice (H). The mRNA (I) and protein (J) level of Smad2 and STAT3 and the protein level of p-Smad2 and p-STAT3 were not changed in quercetin enrich diet (6 mo-13 mo) mice compared with normal diet mice. The mRNA (K) and protein (L) level of Hevin and SPARC were similar in quercetin enrich diet (6 mo-13 mo) mice compared with normal diet mice. All data are presented as means ± standard error of the mean, n = 12, mo represented as months.
Discussion
Neurodegenerative disorders including AD and PD represent a major burden on our society, whether it is from normal aging or as a consequence of a neurodegenerative disorder [12]. As an insidious progression of progressive neurodegenerative diseases, AD is clinically characterized by comprehensive dementia such as memory impairment, aphasia, dementia, dementia, impairment of visual skills, executive dysfunction, and personality and behavioral changes [13]. But at present, the pathogenesis of AD is not clear. So far, there are no effective drugs, and developing an effective drug of AD is becoming an increasingly important subject.
As traditional Chinese medicine has a long history and rich experience in the treatment of AD disease, in recent years, many scholars worldwide have researched the traditional Chinese medicine and its active ingredients, for the treatment of AD natural medicine extensive research [14-16]. It has the unique advantage of screening and developing the single herb medicine with effective curative effect, safe and non-toxic, and the active ingredients of traditional Chinese medicine and compound prescriptions from natural products [17]. However, it also brings the opportunity for the study of the role of Chinese medicine in AD. The traditional Chinese medicine has a moderate and long lasting effect with little side effects. There is a growing interest in the development of dietary flavonoids compounds for preventing and/or treating AD, as well as other neurodegenerative conditions [18]. Flavonoids, a large group of natural compounds commonly included in food additives and heath food supplements, have been considered as substitutes for estrogen [19]. In addition to the classical estrogenic effect of flavonoids, these compounds have been proven to possess neuroprotective effects [20]. To search for potential therapeutic agents against AD, quercetin, as a well-known research compound, has been demonstrated that it could inhibit the aggregation of Aβ in vitro, and pre-treatment of quercetin reduced cytotoxicity of Aβ in cultured neurons. Lastly, administration of quercetin reduced the severity of scopolamine-induced amnesia in rats, suggesting its potential role in developing a drug for the treatment of AD patients.
In the present study, we first detected feeding mice a quercetin-enriched diet resulted in a significant improvement in the APP/PS1 mice. Further research revealed that treatment of quercetin during the whole AD pathological development significantly prevented spatial cognitive dysfunction. Meanwhile, we found quercetin enrich diet only covered the early-middle stage (1 month-9 months) of AD pathology could reduce the Aβ accumulation by inhibiting of the amyloidogenic processing of APP according to the results from immunofluorescence and western blot, but not the middle-late stage of AD pathology. Collectively, outcomes from our studies provided, we determined only the pre-treatment of quercetin could improve the AD progress not in the inprocess AD. This important information provides the logical basis for developing novel therapeutic strategies incorporating dietary polyphenols, or novel pharmacological approaches using brain-targeted flavonoid components, either individually or in combination, to modulate the onset and progress of AD, as well as other neurodegenerative disorders.
Disclosure of conflict of interest
None.
References
- 1.Brambilla D. Drug discovery, development and delivery in Alzheimer’s disease. Pharm Res. 2017;35:3. doi: 10.1007/s11095-017-2329-6. [DOI] [PubMed] [Google Scholar]
- 2.Oustric S, Rouge-Bugat ME, Vellas B. Primary care practitioners on the front line of Alzheimer’s disease care. J Am Med Dir Assoc. 2011;12:545. doi: 10.1016/j.jamda.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Yiannopoulou KG, Papageorgiou SG. Current and future treatments for Alzheimer’s disease. The Adv Neurol Disord. 2013;6:19–33. doi: 10.1177/1756285612461679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nd TT, Filley CM, Mitchell WD, Culig KM, Loverde M, Byyny RL. Lack of efficacy of hydergine in patients with Alzheimer’s disease. N Eng J Med. 1990;323:445–8. doi: 10.1056/NEJM199008163230704. [DOI] [PubMed] [Google Scholar]
- 5.Borenstein AR, Dai Q, Wu Y, Jackson JC, Larson EB. Consumption of fruit and vegetable juices predicts a reduced risk of Alzheimer’s disease: the kame project. Alzheimers & Dementia. 2005;1:S60–S61. [Google Scholar]
- 6.Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008;269:315–25. doi: 10.1016/j.canlet.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 7.Xue F, Nie X, Shi J, Liu Q, Wang Z, Li X, Zhou J, Su J, Xue M, Chen WD. Quercetin inhibits lps-induced inflammation and ox-ldl-induced lipid deposition. Front Pharmacol. 2017;8:40. doi: 10.3389/fphar.2017.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuentes J, Atala E, Pastene E, Carrasco-Pozo C, Speisky H. Quercetin oxidation paradoxically enhances its antioxidant and cytoprotective properties. J Agric Food Chem. 2017;65:11002–11010. doi: 10.1021/acs.jafc.7b05214. [DOI] [PubMed] [Google Scholar]
- 9.Denny Joseph KM Muralidhara. Enhanced neuroprotective effect of fish oil in combination with quercetin against 3-nitropropionic acid induced oxidative stress in rat brain. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40:83–92. doi: 10.1016/j.pnpbp.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Mehdizadeh M, Joghataei MT, Nobakht M, Aryanpour R. Neuroprotective effect of quercetin in a model of Parkinson’s disease in rat: a histochemical analysis. Basic Clin Neuroscience. 2009:3–6. [Google Scholar]
- 11.Ansari MA, Abdul HM, Joshi G, Opii WO, Butterfield DA. Protective effect of quercetin in primary neurons against Aβ(1-42): relevance to Alzheimer’s disease. J Nut Biochem. 2009;20:269–275. doi: 10.1016/j.jnutbio.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10(Suppl):S10–7. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 13.Grober E, Hall CB, Lipton RB, Zonderman AB, Resnick SM, Kawas C. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer’s disease. J Int Neuropsychol Soc. 2008;14:266–78. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung TS, Song TH, Ng TB, Wu FH, Lao LX, Tang SC, Ho JC, Zhang KY, Sze SC. Therapeutic effects of herbal chemicals in traditional Chinese medicine on Alzheimer’s disease. Curr Med Chem. 2015;22:2392–403. doi: 10.2174/0929867322666150520095509. [DOI] [PubMed] [Google Scholar]
- 15.Li L, Zhang L, Yang CC. Multi-target strategy and experimental studies of traditional Chinese medicine for Alzheimer’s disease therapy. Curr Top Med Chem. 2016;16:537–48. doi: 10.2174/1568026615666150813144003. [DOI] [PubMed] [Google Scholar]
- 16.Wei S. Potential therapeutic action of natural products from traditional Chinese medicine on Alzheimer’s disease animal models targeting neurotrophic factors. Fundam Clin Pharmacol. 2016;30:490–501. doi: 10.1111/fcp.12222. [DOI] [PubMed] [Google Scholar]
- 17.Sun ZK, Yang HQ, Chen SD. Traditional Chinese medicine: a promising candidate for the treatment of Alzheimer’s disease. Transl Neurodegener. 2013;2:6. doi: 10.1186/2047-9158-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baptista FI, Henriques AG, Silva AM, Wiltfang J, da Cruz e Silva OA. Flavonoids as therapeutic compounds targeting key proteins involved in Alzheimer’s disease. Acs Chem Neurosci. 2014;5:83–92. doi: 10.1021/cn400213r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker ME, Medlock KL, Sheehan DM. Flavonoids inhibit estrogen binding to rat alpha-fetoprotein. Proc Soc Exp Bio Med. 1998;217:317–321. doi: 10.3181/00379727-217-44238. [DOI] [PubMed] [Google Scholar]
- 20.Zhu JT, Choi RC, Chu GK, Cheung AW, Gao QT, Li J, Jiang ZY, dong TT, Tsim KW. Flavonoids possess neuroprotective effects on cultured pheochromocytoma pc12 cells: a comparison of different flavonoids in activating estrogenic effect and in preventing β-amyloid-induced cell death. J Agric Food Chem. 2007;55:2438–45. doi: 10.1021/jf063299z. [DOI] [PubMed] [Google Scholar]


