Abstract
Translational repression of msl-2 mRNA in females of Drosophila melanogaster is an essential step in the regulation of X-chromosome dosage compensation. Repression is orchestrated by Sex-lethal (SXL), which binds to both untranslated regions (UTRs) of msl-2 and inhibits translation initiation by poorly understood mechanisms. Here we identify Hrp48 as a SXL co-factor. Hrp48 binds to the 3′ UTR of msl-2 and is required for optimal repression by SXL. Hrp48 interacts with eIF3d, a subunit of the eIF3 translation initiation complex. Reporter and RNA chromatography assays showed that eIF3d binds to msl-2 5′ UTR, and is required for efficient translation and translational repression of msl-2 mRNA. In line with these results, eIF3d depletion -but not depletion of other eIF3 subunits- de-represses msl-2 expression in female flies. These data are consistent with a model where Hrp48 inhibits msl-2 translation by targeting eIF3d. Our results uncover an important step in the mechanism of msl-2 translation regulation, and illustrate how general translation initiation factors can be co-opted by RNA binding proteins to achieve mRNA-specific control.
INTRODUCTION
Post-transcriptional regulation plays an important role in numerous biological situations. In particular, translational control by RNA binding proteins pervades embryonic development and adult cell homeostasis, yet few mechanisms of translational control have been described to date (1–4). Dosage compensation in Drosophila melanogaster, a mechanism that equalizes the expression of X-linked genes between males (XY) and females (XX), depends on translational regulation (5). In males, the chromatin remodeling dosage compensation complex (DCC) hyper-transcribes the single X-chromosome about 2-fold. In females, dosage compensation is repressed via a complex set of post-transcriptional events orchestrated by the female-specific RNA binding protein Sex-lethal (SXL) to inhibit the expression of the limiting DCC subunit MSL2 (6). SXL, a primarily nuclear protein, first inhibits the splicing of a facultative intron in the 5′ UTR of msl-2 pre-mRNA and promotes nuclear retention of msl-2 transcripts (7–9). SXL then inhibits translation in the cytoplasm by binding to both the 5′ and 3′ UTRs of msl-2 (10–12). SXL bound to the 3′ UTR recruits the co-repressor UNR to inhibit initial ribosome recruitment to the mRNA (13–16). SXL bound to the 5′ UTR inhibits ribosomal scanning by a mechanism that entails the recognition of an upstream AUG (17,18). The targets in the translation machinery for either 5′ or 3′- mediated regulation are unknown.
Here, we focus on 3′ UTR-mediated regulation of msl-2 mRNA. We found that the minimal region in the 3′ UTR required for translational repression contains binding sites for regulators distinct from SXL and UNR. Using a combination of GST pull-down and RNA affinity binding (GRAB) (9), we have identified Hrp48 as a novel msl-2 regulator binding to these sites. Hrp48 interacts with endogenous SXL, UNR and msl-2 mRNA. In vitro translation assays suggested that Hrp48 targets a factor downstream of cap structure recognition. A search for Hrp48 interactors identified eIF3d, a subunit of the translation initiation factor eIF3. eIF3d binds to msl-2 5′ UTR, is required for msl-2 translation, and is necessary for SXL-mediated translational repression. In addition, eIF3d depletion leads to a modest but consistent msl-2 de-repression in female flies. Our results identify two new factors in msl-2 regulation, Hrp48 and eIF3d, and suggest that eIF3d is a potential target within the translational machinery for 3′ UTR-mediated control. Further, the data illustrate how a general translation initiation factor can contribute to mRNA-specific regulation.
MATERIALS AND METHODS
Plasmids
Plasmids used for the synthesis of the WT and 5m msl-2 reporters were described previously (13). Plasmids WT and 5m have been renamed in this work for simplicity (named 3′EF and mut5 in 13, respectively). Plasmid (AB)m-5m was obtained by replacing the 5′ UTR of 5m with the full-length (626 nt) 5′ UTR of msl-2 containing mutated SXL binding sites. Plasmid (AB)m is as (AB)m-5m but contains wild type 3′ UTR sequences. Plasmid uORF-BL(EF)m has been described (18), and contains nucleotides 270–339 of msl-2 5′ UTR including AUG3. Plasmid uORF-BL(EF5)m is as uORF-BL(EF)m but contains a substitution of region 5 by (CT)8. Constructs BLEF and mLm have been previously described (13). UTR swap derivatives mLEF and BLm were obtained by exchanging the 3′ UTRs of mLm and BLEF using the EcoNI and BglII sites of these constructs. BruLEF was obtained by amplification of the 521 nt 5′ UTR of aret mRNA from embryo extracts and subsequent cloning into the SacI and BamHI sites of mLEF. To obtain construct 7, the fragment between the BlpI and NcoI sites of (AB)m was exchanged with the AflII/NcoI fragment of BruLEF. Constructs 8 and 9 were obtained by insertion of the corresponding msl-2 5′ UTR fragments into the 5′ UTR of BrunoLEF modified to contain BlpI and SacI sites (BruLEFmod). In the case of construct 8, the BlpI/SacI fragment of (AB)m was inserted in the same sites of BRuLEFmod. To obtain construct 9, we exchanged the BlpI/NcoI fragment of BruLEFmod with the fragment flanked by the same sites of (AB)m. Construct 10 was obtained by substituting the BlpI/AleI fragment of (AB)m by the AflII/DraI fragment of BrunoLEF using the Gibson cloning method (19).
Plasmids used for the generation of biotinylated probes were obtained by insertion of hybridized complementary oligonucleotides containing nucleotides 909–954 of the msl-2 3′ UTR or derivatives into pBluescript, as described (20).
The FC msl-2 reporter and pAc-Renilla constructs used for transfection were previously described (9,10). To obtain pAc-SXL, the SXL ORF was amplified by PCR and cloned into the EcoRI and XhoI sites of pAc5.1B-NheI plasmid.
Plasmids used for expression of UNR and recombinant SXL variants (pGEX-dRBD4 and pGEX-mRBD) have been described (14,20). The plasmid used for the expression of Hrp48 was obtained by cloning the Hrp48 ORF into the NdeI and XhoI sites of pET15b. The plasmid used for the expression of His-2HA-eIF3d was generated by insertion of the eIF3d ORF, obtained from the PGADT7-eIF3d plasmid (a generous gift of Jan Medenbach), into the pETM14 vector using the Gibson cloning method (19). Two HA tags were introduced at the N-terminus of the eIF3d sequence, separated by a linker (TTACGCTATGGCCATGTACCCAT).
Flies and crosses
UAS and GAL4 lines yw;actin5C-GAL4/TM6B, w;SgS3-GAL4, w;patched-GAL4 and yw;nubbin-GAL4;UAS-dicer-2 (the two latter kindly provided by Dr Jordi Bernués) were used in this study. Flies containing the eIF3d RNAi construct were obtained from National Institute of Genetics Fly Stock Center (NIG-FLY ID 10161R-3) and balanced with TM6B. A TM3 balanced eIF3e RNAi strain was obtained from VDRC (ID 27032) and re-balanced over TM6B. The eIF3h RNAi strain was also obtained from VDRC (ID 36086). For crosses with the eIF3d and eIF3e RNAi lines, control Tubby siblings within the same cross were scored. For the eIF3h RNAi line, parallel crosses with wild type w1118 flies were used as control. Flies were maintained in standard food at either 25°C or 29°C.
Recombinant proteins
SXL derivatives dRBD4 (amino acids 122–301 of Drosophila melanogaster SXL) and mRBD (amino acids 99–271 of Musca domestica SXL) were expressed in Escherichia coli as N-terminal GST-tagged fusions and purified as described (20). His-dRBD4 and His-FLAG-tagged, full length UNR were purified according to the pET systems user's manual, with a second purification step for UNR using FLAG columns (Novagen). His-2HA-eIF3d was expressed in E. Coli using autoinduction culture medium (Novagen, 71491-5). The protein was purified by two passages in Nickel affinity Trap Fast Flow columns followed by size exclusion chromatography using Superdex 200 columns (GE Healthcare). All proteins were dialyzed against buffer D (20 mM HEPES pH 8.0, 20% glycerol, 1 mM DTT, 0.01% NP-40, 0.2 mM EDTA). Hrp48 was expressed as a His-tagged fusion and used to generate antibodies in rabbits. Hrp48 was largely insoluble, and thus to increase solubility, Hrp48-transformed E. coli were induced with 1 mM IPTG for 3 h at 30°C, and purification was performed under 8 M urea as previously described (21). The portion of soluble recombinant Hrp48 after renaturation was used to perform the experiments.
RNA synthesis
Biotinylated RNAs were synthesized using the MEGAshort script kit (Ambion), adding bio-14-CTP (Invitrogen) at an equimolar ratio with CTP in the reaction, and were purified using G25 columns (GE Healthcare). Radiolabeled msl-2 probes used in gel-mobility shift assays were prepared by in vitro transcription from hybridized oligonucleotide templates containing the T7 promoter followed by the relevant msl-2 sequences. Biotinylated and radiolabeled probes contained an ApppG cap (KEDAR). Radiolabeled 7mGpppG-capped 5′ UTRs used in Figure 6C were generated by in vitro transcription from the corresponding plasmids digested with NcoI. dsRNA for depletion experiments was synthesized using the MEGAscript kit (Ambion). The synthesis of mRNAs used in in vitro translation reactions was performed as described (22). These mRNAs contained either a 7mGpppG cap or an ApppG cap, as indicated, and a poly(A) tail of 73 residues. All mRNAs used in the same experiment were synthesized and quantified in parallel, and the concentration and quality confirmed by separation in agarose gels.
Figure 6.
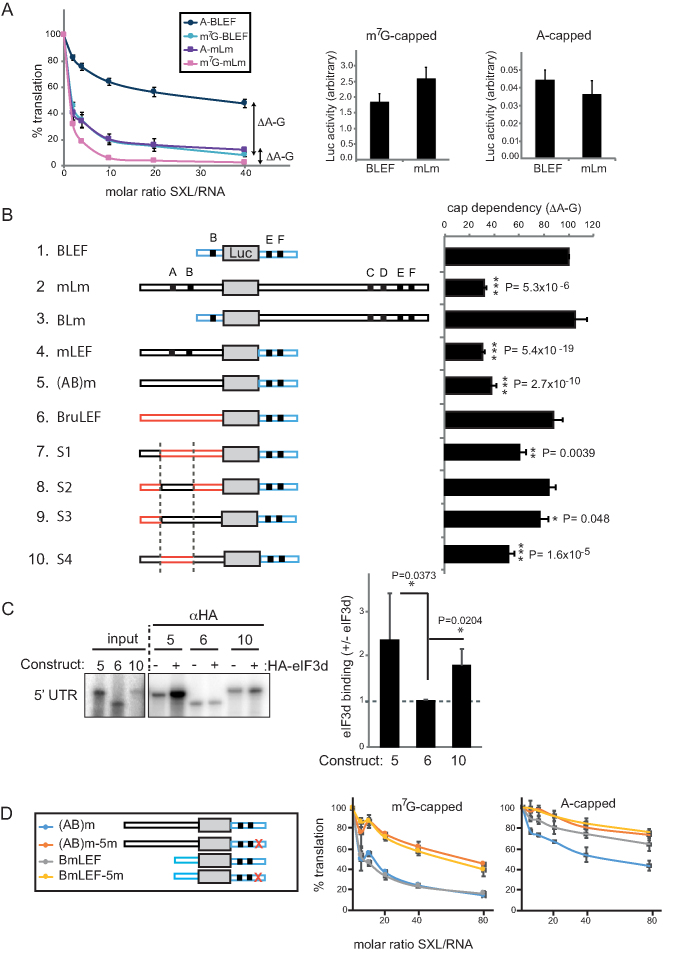
eIF3d binding to the 5′ UTR of msl-2 correlates with cap-independent repression. (A) Cap-dependency of repression of full length (mLm) and minimal (BLEF) msl-2 reporters. BLEF contains nucleotides 270–339 and 909–954 of the msl-2 5′ and 3′ UTRs, respectively (see also Figure 1A). Left, in vitro translation reactions were performed as described in the legend of Figure 1B using A-capped or m7G-capped mRNAs. The distance between the A and m7G repression lines (ΔA-G) was defined as the cap-dependency of the construct. Error bars represent the standard error of three independent experiments. Basal translation among m7G-capped (middle) or A-capped (right) construct was comparable. Notice the difference in absolute translation levels (ordinates) of m7G-capped versus A-capped constructs. (B) Analysis of cap-dependency of repression of msl-2 derivatives. Only the data obtained at SXL/RNA ratio = 40 are shown for simplicity. Error bars represent the standard error of at least three independent replicates. (C) eIF3d binds to msl-2 5′ UTR. Left, radiolabeled 5′ UTRs of constructs 5, 6 and 10 were incubated with Drosophila embryo extract in the presence (+) or absence (–) of HA-eIF3d and pulled-down with αHA beads. Immunoprecipitated material was resolved by SDS-PAGE. Right, quantification of four independent experiments. Error bars represent the standard deviation. (D) Effect of region 5 mutation on translational repression of constructs containing long versus short 5′ UTRs. Where it applies in this figure, significance was determined by unpaired Student's t-test (*P< 0.05, **P < 0.01, ***P < 0.001).
Gel mobility shift assays
Radiolabelled msl-2 probes were incubated with increasing amounts of recombinant dRBD4 and UNR as described previously (14). RNA–protein complexes were resolved in non-denaturing 4% polyacrylamide gels.
Antibodies and immunoprecipitation
Antibodies against full-length Hrp48 were generated in rabbits and characterized by Western blot and immunoprecipitation of Hrp48 from Drosophila embryo extracts. Anti-Hrp48 antibodies used in initial experiments were kindly provided by Anne Ephrussi (23) and Marco Blanchette (24). Anti-MSL2 antibodies were generated in rabbits and characterized by Western blot, immunoprecipitation and immunostaining. Anti-UNR, anti-SXL and anti-HOW antibodies were previously described (9,14). Anti-tubulin (Sigma, T6199) and anti-HA (Abcam) antibodies, and anti-HA magnetic beads (Pierce, 88837) were provided commercially.
Immunoprecipitations shown in Figure 4B were performed using purified IgGs covalently cross-linked to protein A Dynabeads (Invitrogen) which were pre-blocked with Drosophila embryo extract. Thirty microliters of the corresponding bead slurry were incubated with 1.2 mg of either Drosophila embryo, Kc or SL2 cell extract, complete protease inhibitor cocktail (Roche), and 0.1 M sodium phosphate pH 8.0 to a final volume of 360 μl. After 2 h of incubation at 4°C, five washes were performed with 10× bead volumes of cold 1× NET buffer (50 mM Tris–HCl pH7.5, 150 mM NaCl, 0.1% NP40 and 1 mM EDTA). Half of the beads were then treated with a mix of 5 μg RNase A and 2.5 units RNase ONE (Promega) in RNase ONE buffer, and the other half with buffer alone in a total volume of 30 μl, for 30 min at 37°C. The supernatant was removed and the beads were washed five additional times with 10 volumes of cold 1× NET buffer. Proteins were recovered with 1× Laemmli buffer and resolved by SDS-PAGE.
Figure 4.
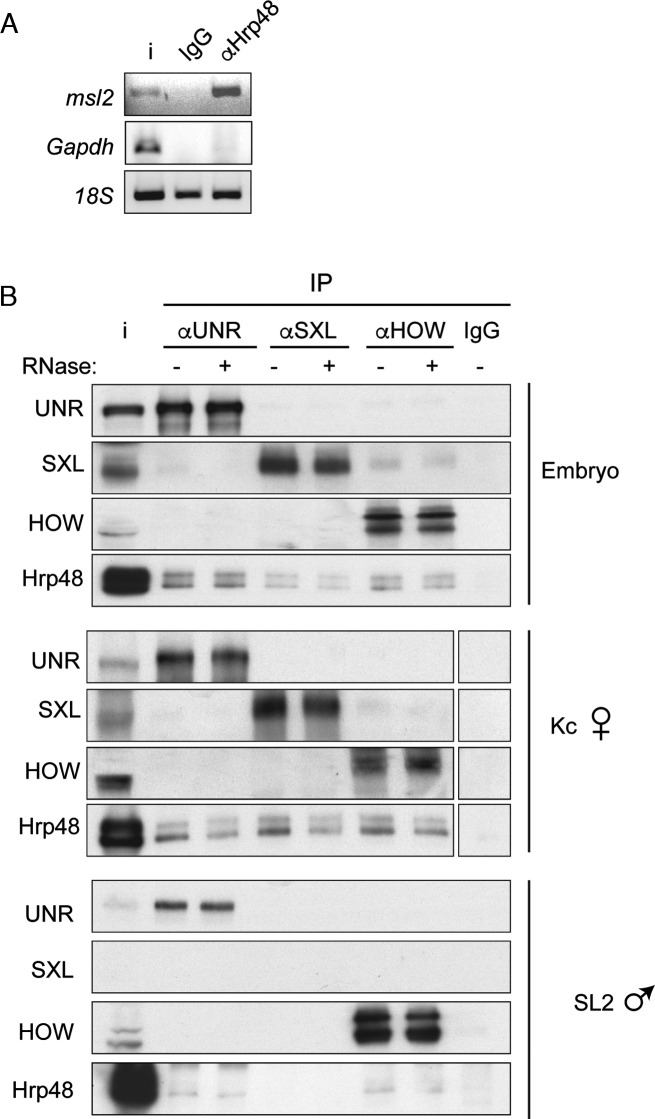
Hrp48 interacts with components of the msl-2 repressor complex. (A) Hrp48 interacts with endogenous msl-2 mRNA. Hrp48 was immunoprecipitated from Drosophila embryo extracts, and the presence of msl-2 and Gapdh mRNAs in the pellet was tested by semi-quantitative PCR. Immunoprecipitation with non-specific IgG was carried as negative control. 18S RNA is shown as a measure of background. (B) Hrp48 interacts with msl-2 repressors. SXL, UNR and HOW were immunoprecipitated from Drosophila embryo, Kc and SL2 cell extracts, and the presence of Hrp48 in the pellet was tested by Western blot. Samples were treated with RNase (+) or buffer (–). Non-specific IgG was carried as negative control. i, input.
For the immunoprecipitations shown in Figure 5C, 0.7 μg of recombinant HA-eIF3d were added to ∼1 mg of Drosophila embryo extract, incubated for 30 min at room temperature in a rotating wheel, and pulled-down with 100 μl of anti-HA magnetic bead slurry previously washed with 1× NET (50 mM Tris–HCl pH 7.5, 150 mM NaCl, 0.1% NP40, 1 mM EDTA). After 1 h incubation at 4°C, beads were washed three times with 10 volumes of 1× NET. Proteins were recovered with 2× Laemmli buffer and resolved by SDS-PAGE. For the immunoprecipitation of recombinant Hrp48 and eIF3d, 150 ng of His-Hrp48 were incubated in the presence or absence of 1 μg of HA-eIF3d in buffer X (50 mM Tris–HCl pH 7.5, 150 mM KCl, 0.05% NP40, 1 mM EDTA, 5% glycerol, 0.6 mM DTT) for 30 min at 4°C. Where indicated, 100 units of RNAse ONE (Promega) were added. One hundred microliter of anti-HA magnetic beads equilibrated in buffer X were then added, and incubated for 2 h at 4°C on a rotating wheel. Beads were then washed three times in 10 volumes of buffer X and proteins resolved by SDS-PAGE.
Figure 5.
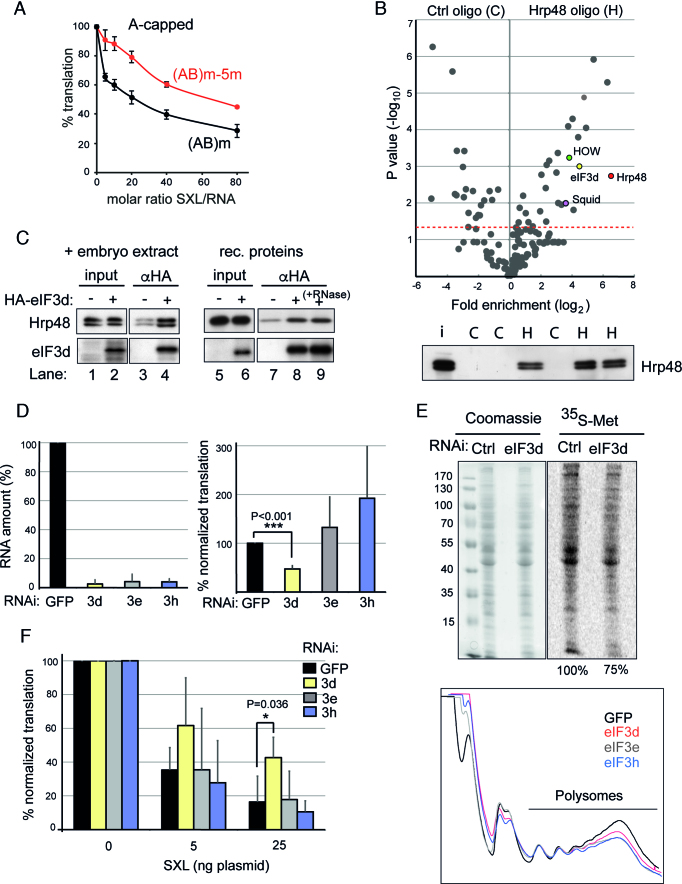
Hrp48 binds to eIF3d, an initiation factor required for msl-2 mRNA regulation. (A) Region 5 is required for repression of A-capped mRNAs. In vitro translation reactions were performed as described in the legend of Figure 1B using A-capped (AB)m and (AB)m-5m mRNAs to measure 3′-mediated regulation. Error bars represent the standard error of three independent replicates. (B) Top, Volcano plot showing the mass spectrometry analysis of triplicate pull-downs of Drosophila embryo extracts with an oligomer containing Hrp48 binding sites. An unrelated oligomer (polyC) was used as control. Relevant proteins are marked with color. The red line indicates the significance threshold (Pval = 0.05). Bottom, Western blot of Hrp48 in the eluates to test the efficiency and specificity of pull-down. H, Hrp48 oligo; C, control oligo. (C) Hrp48 co-immunoprecipitates with eIF3d. Left, recombinant HA-tagged eIF3d was incubated with Drosophila embryo extract, captured with αHA beads, and the presence of Hrp48 in the pellet was tested by Western blot. Right, purified recombinant Hrp48 and HA-eIF3d were mixed in the absence (lanes 5–8) or presence of 100 units of RNase ONE (lane 9), captured with αHA beads, and the presence of Hrp48 in the pellet tested by Western blot. (D) eIF3d is necessary for msl-2 mRNA translation. eIF3d was depleted from SL2 cells and the efficiency of translation of the FC msl-2 reporter was measured. Depletion of two additional eIF3 subunits (eIF3e, eIF3h) and RNAi against GFP were carried as controls. The depletion efficiency was measured by RT-qPCR, and plotted relative to the amount of the corresponding eIF3 subunit in GFP RNAi cells (Left panel). To obtain the efficiency of FC translation, βGal activity was normalized for co-transfected Renilla expression and corrected for the levels of the reporter RNA. The data were plotted relative to the βGal activity in GFP RNAi cells (Right panel). Error bars represent the standard deviation from five experiments. (E) Depletion of eIF3d causes a mild defect in global translation. (Top) De novo protein synthesis was assessed by metabolic labeling with 35S-methionine. A Coomassie stained gel is shown as loading reference. Numbers represent quantification of the 35S signal corrected for loading. (Bottom) Polysome analysis of cells depleted of eIF3d, eIF3e or eIF3h. RNAi against GFP was carried as negative control. (F) eIF3d is required for efficient SXL-mediated repression. The ability of SXL to repress translation of the FC msl-2 reporter was measured in eIF3d depleted cells. A Renilla luciferase encoding plasmid was co-transfected as an internal control. RNAi against eIF3e, eIF3h and GFP were carried as controls. The data were processed as described for Figure 3B.
RNAi, transfections, reporter activity assays and 35S-methionine labeling
RNA interference and transfections were performed as described previously (9). The βgal FC reporter construct was co-transfected with pAc-Renilla control and increasing amounts of pAc-SXL. Renilla luciferase and βgalactosidase activities were measured with luciferase (Promega) and Galacto-Star (Tropix) kits, respectively. βgal activity was corrected for co-transfected Renilla, and normalized for reporter RNA levels.
For 35S-Methionine labeling, cells were seeded in six-well plates and depletion of eIF3 subunits was performed for 3 days. Cells were then washed with fresh Schneider's medium, and incubated for 2 h at 25°C with 2 μl 35S-meth 10 mCi/ml in the medium. After incubation, cells were washed with cold PBS and lysed with passive lysis buffer (Promega). Samples were quantified, resolved on SDS-PAGE, and visualized by Coomassie staining and exposure to Phosphorimager.
RNA extraction and quantification
Total RNA was extracted from SL2 cells or salivary glands using Trizol (Invitrogen) following the manufacturer's instructions. RNA was treated with Turbo DNase (Ambion) and reverse-transcribed using random primers and SuperScript II (Invitrogen). cDNA was amplified by qPCR with SYBR Green (Applied Biosystems). Reporter βgal RNA levels were normalized for co-transfected Renilla luciferase RNA levels. Endogenous eIF3d mRNA levels were corrected by actin.
GRAB and RNA affinity chromatography
GRAB was performed as described previously (9). For direct RNA affinity chromatography, 30 μl of streptavidin Dynabead slurry (Invitrogen) were pre-blocked for 10 min with 100 ng/μl tRNA in binding buffer (5 mM Tris pH 7.4, 0.5 mM EDTA, 1 M NaCl). Beads were washed with binding buffer and subsequently incubated with 100 pmol of biotinylated RNA for 30 min at room temperature. Beads were then washed with 20 bead volumes of ice-cold TCB (17 mM creatine phosphate, 80 ng/μl creatine kinase, 25 mM HEPES pH 8.0, 0.6 mM Mg(OAc)2, 80 mM KOAc). Washed beads were mixed with 5 mg of Drosophila embryo extract and 20 pmol of GST-dRBD4 or GST-mRBD, in TCB supplemented with 100 U RNAsin (Promega) and 1× Complete protease inhibitor cocktail (Roche). The mix was incubated for 1 h at 4°C. Beads were subsequently washed with 15 bead volumes of cold TCB supplemented with 10% glycerol and 0.01% Triton X-100. RNA bound proteins were recovered by elution with 20 μl RNase mix (10–20U RNase ONE and 40 μg RNase A in RNase ONE buffer) after incubation for 30 min at 37°C. Eluted proteins were resolved by SDS-PAGE and analyzed by immunoblotting.
Extract depletion
Two hundred microliters of streptavidin Dynabeads were pre-blocked with Drosophila embryo extract for 1 h at 4°C, washed with binding buffer (see above) and further incubated with a 5′ biotinylated RNA oligomer containing two Hrp48 binding sites (bio-ACCACCUAGGAUUAAGACCUAGGAUUAAG) or with bio-polyC as control. After incubation for 30 min at room temperature, beads were washed with 15 volumes of 20 mM HEPES pH 7.4, and divided in 15 aliquots. Embryo extract (1 mg diluted with 60 mM HEPES pH 7.4 in a ratio of 2:1) was passed sequentially from one aliquot to the other, after incubation with each aliquot for 10 min at 4°C (i.e. a total of 15 rounds of depletion). The efficiency of depletion was tested by western blot.
In vitro translation assays
In vitro translation assays were performed as described (22). Briefly, 12 pmol of msl-2 Firefly reporter mRNA (20–40 ng depending on the RNA) were mixed with 10 ng Renilla luciferase mRNA and incubated with increasing amounts of His-dRBD4 in a final volume of 12.5 μl containing 40% Drosophila embryo extract, 60 μM amino acids, 16.8 mM creatine phosphate, 80 ng/μl creatine kinase, 24 mM HEPES pH 7.5, 0.6 mM Mg(OAc)2 and 80 mM KOAc. The reaction was incubated at 25°C for 90 min, and the Firefly and Renilla activities measured using the Dual Luciferase kit (Promega).
Oligonucleotide pull-down
Groups of 15 bead aliquots from independent depletion experiments (see above for depletion protocol) were pooled and washed four times with 1 ml of ice-cold 20 mM HEPES pH 7.4. Beads were then resuspended in 100 μl RNase mix (10–20 U RNase ONE and 40 μg RNase A in RNase ONE buffer) and incubated at 37°C for 30 min. The supernatant was recovered and analyzed by quantitative mass spectrometry.
Polysome profiles
Cell extracts were prepared in lysis buffer (15 mM Tris–HCl pH 7.4, 15 mM MgCl2, 300 mM NaCl, 1% Triton X-100, 40 U/ml RNasin, 100 μg/ml cycloheximide, 1× Promega protease inhibitor cocktail) and 10 A260 units were loaded on a 10–50% sucrose gradient also containing 100 μg/ml cycloheximide. Polysomes were separated by centrifugation at 35 000 rpm for 3 h using a Beckmann SW41 rotor. Fifteen fractions of 800 μl were collected while polysomes were monitored by following the absorbance at 254 nm.
RESULTS
Identification of Hrp48 as a candidate factor for msl-2 translational regulation
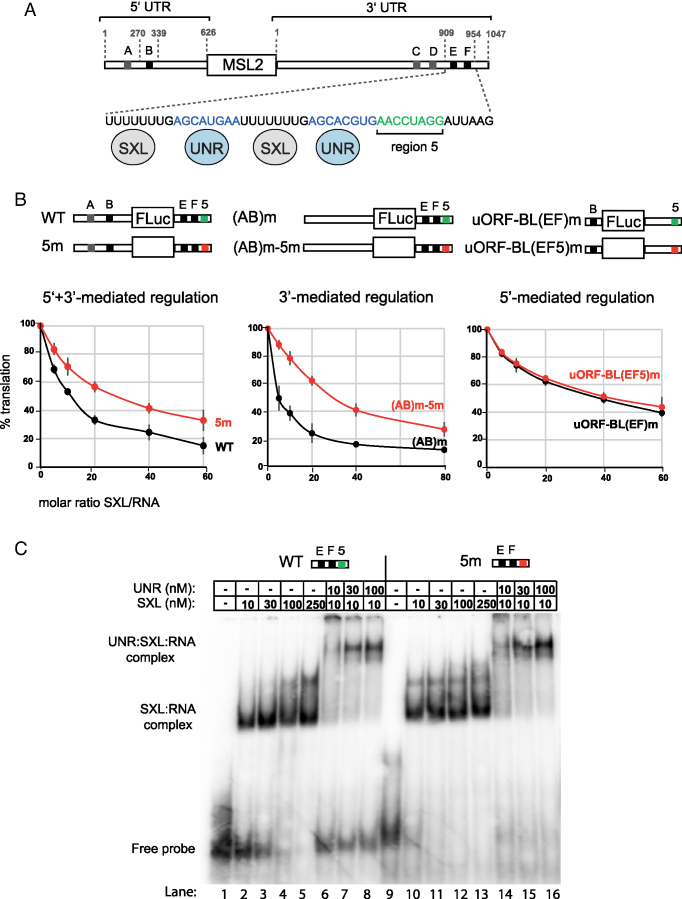
The msl-2 transcript contains long 5′ and 3′ UTRs with multiple uridine stretches that serve as SXL-binding sites (sites A–F, Figure 1A). Sites B, E and F (black boxes) are required for translational repression, while sites A, C and D (gray boxes) are dispensable. The minimal region of the 3′ UTR required for regulation consists of 46 nucleotides containing sites E and F, each followed by a UNR-binding site (blue). Sequences downstream of these sites are also important for translational repression (13). We performed in vitro translation assays in Drosophila embryo extracts to thoroughly evaluate the relevance of these sequences. Translation of msl-2 reporters was assessed in extracts supplemented with increasing amounts of a recombinant SXL derivative that is fully competent for translational repression (dRBD4) (20). Substitution of the 8-nucleotide segment immediately downstream of the SXL and UNR binding sites (Figure 1A, green, hereafter termed ‘region 5’) by (CU)4 de-repressed msl-2 translation (Figure 1B, left panel). To evaluate the contribution of region 5 to regulation mediated by each msl-2 UTR, we tested the substitution mutant (5m) in the framework of transcripts that lacked SXL-binding sites in either UTR. While mutation of region 5 was irrelevant for 5′ UTR-mediated regulation (Figure 1B, right panel), 3′ UTR-driven repression was less efficient in 5m mutants (middle panel), indicating that region 5 plays an important role in msl-2 silencing mediated by the 3′ UTR.
Figure 1.
‘Region 5’ is important for translational repression of msl-2 mRNA independent of SXL and UNR binding. (A) Schematic representation of msl-2 mRNA. SXL binding sites are depicted with gray and black boxes (A–F); sites A, C, D (grey) are dispensable for translational repression while sites B, E and F (black) are required. Numbers indicate the length of the 5′ and 3′ UTRs (626 and 1047 nucleotides, respectively) and the position of the minimal sequences required for translational repression (nt 270–339 in the 5′ UTR and nt 909–954 in the 3′ UTR). A detail of the minimal functional 3′ UTR is shown, with the UNR binding sites in blue and the region 5 in green. SXL and UNR proteins are shown binding to their corresponding sites. (B) Region 5 is important for 3′-mediated regulation. In vitro translation assays were performed with a series of indicator constructs that support either 5′, 3′ or 5′+3′-mediated regulation, schematically represented above each graph. Constructs WT and 5m contain a 5′ UTR of 354 nt including sites A and B. Constructs (AB)m and (AB)m-5m contain a 5′ UTR of 626 nt lacking sites A and B. Constructs uORF-BL(EF)m and uORF-BL(EF5)m contain the minimal functional 5′ UTR. All constructs contain minimal 3′ UTR derivatives, as indicated. Region 5 was mutated to (CU)8 and is highlighted in red. In vitro translation assays were performed with increasing amounts of recombinant His-dRBD4. Renilla luciferase mRNA was co-translated as an internal control. Firefly luciferase was corrected for Renilla expression, and the data were plotted relative to the percentage of translation in the absence of SXL. Error bars represent the standard deviation of three experiments. (C) Gel mobility shift assays using the wild type minimal msl-2 3′ UTR (WT), or a derivative lacking region 5 (5m). Increasing amounts of GST-dRBD4 or UNR were added to the reaction, as indicated. The positions of the protein-RNA complexes and the free probe are indicated.
One possibility to explain these effects is that region 5 contributes to SXL and/or UNR binding to the 3′ UTR. To assess this possibility, we used gel mobility shift assays to test the binding of recombinant UNR and dRBD4 to 3′ UTR fragments containing or lacking region 5 (Figure 1C). dRBD4 binds with high affinity to a wild type 3′ UTR fragment (lanes 1–5). As previously reported, UNR also binds with high affinity after addition of dRBD4 (lanes 6–8) (14,16). Binding of both proteins was not decreased by mutation of region 5 (lanes 9–16). These results indicate that region 5 is not required for binding of SXL and/or UNR and, thus, may contribute to msl-2 repression by binding to a novel regulator.
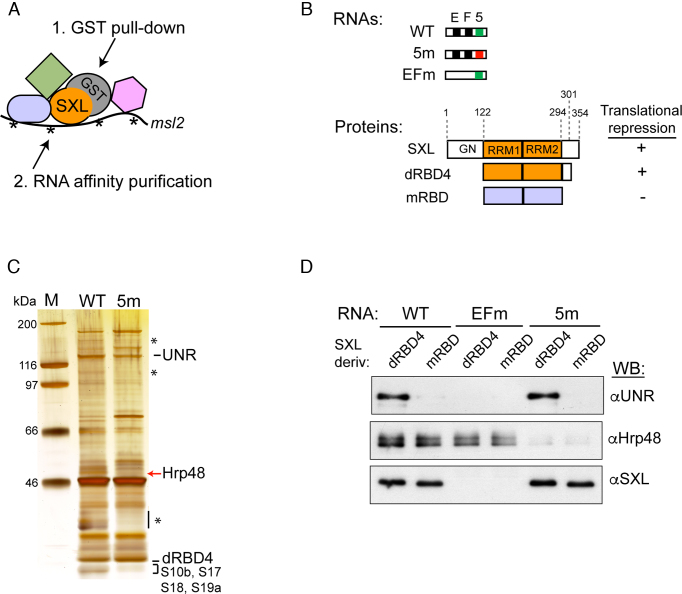
To identify factors binding to region 5 that could potentially contribute to translational repression we used a technology previously optimized in our laboratory termed GRAB (9). GRAB allows for the enrichment of SXL RNPs from a mix containing recombinant GST-SXL, biotinylated msl-2 mRNA and Drosophila embryo extracts following two affinity purification steps: i) GST-pull down and elution with TEV protease that separates the GST moiety, and ii) RNA affinity purification using streptavidin beads (Figure 2A). We compared the GRAB profiles obtained with wild type (WT) or 5m 3′ UTR fragments together with GST-dRBD4 (see Figure 2B for the collection of RNA fragments and proteins used in GRAB and RNA affinity chromatography). A protein of 48 kDa was present in the WT but absent in the 5m eluates (Figure 2C). Mass spectrometry analysis identified this protein as the Heterogeneous Ribonucleoprotein 48 (Hrp48, also known as Hrb27C). A group of ribosomal proteins (S10b, S17, S18, S19a) was also reproducibly absent in the 5m profile while, as expected, UNR and dRBD4 bound equally to both WT and 5m RNAs. We focused on Hrp48, as this protein has been previously shown to participate in translational regulation of oskar and other Drosophila mRNAs by mechanisms that are poorly understood (23,25,26).
Figure 2.
Hrp48 binds to region 5. (A) Schematic representation of the GRAB purification protocol. Recombinant GST-dRBD4 was incubated with biotinylated msl-2 RNA probes and Drosophila embryo extract in translation reaction conditions. A first purification step includes GST pull-down and elution with TEV protease, which separates the GST moiety. In the second purification step, the biotinylated RNA is pulled-down with streptavidin beads, and complexes are eluted with SDS buffer. (B) Schematic representation of the msl-2 RNA probes and the SXL derivatives used in this study. WT and 5m RNAs are as described in the legend of Figure 1C. EFm RNA lacks sites E and F, which have been substituted by CU repeats. D. melanogaster SXL is a 354 amino acid protein containing two RRM-type RNA binding domains and a glycine/ asparagine (GN)-rich amino-terminal region. The deletion derivative dRBD4 is fully competent for translational repression. mRBD contains the RNA-binding domains of the SXL homolog from Musca domestica, sharing 95% identity with Drosophila SXL but inactive in translational repression. (C) GRAB eluates obtained with WT and 5m RNAs were analyzed by PAGE and silver stained. Selected bands were cut and sent for identification by mass spectrometry. Asterisks denote bands that were not reproducibly absent in the 5m eluate. (D) Hrp48 binds to region 5 independently of SXL and UNR. RNA affinity chromatography was performed with WT, EFm and 5m RNAs, using the SXL derivatives described in part B. WB, Western blot.
To test whether binding of Hrp48 to msl-2 3′ UTR depends on prior SXL or UNR binding, we performed direct RNA chromatography using SXL derivatives that either support (dRBD4) or not (mRBD) binding of UNR (20), or an msl-2 construct lacking sites E and F (EFm) which binds neither SXL nor UNR (13) (see legend of Figure 2B for details). As control, we used 5m RNA which should not support Hrp48 binding. The results indicated that Hrp48 bound efficiently to WT and EFm RNAs—but not to 5m RNA- independently on whether dRBD4 or mRBD were used (Figure 2D). This contrasts with UNR, which is strictly dependent on SXL for msl-2 RNA recognition. Therefore, Hrp48 binds to region 5 independently of known msl-2 translational regulators.
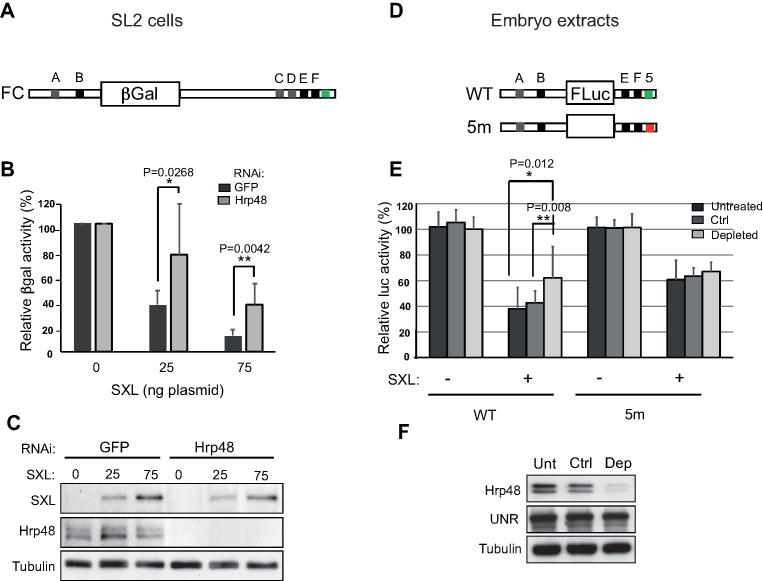
Hrp48 is required for msl-2 translational repression
We next performed functional assays to determine whether Hrp48 is involved in msl-2 translational regulation. We used male SL2 cells, which lack endogenous SXL. Transfection of a SXL-encoding plasmid allows for tight control of SXL levels in these cells. We depleted Hrp48 from SL2 cells and tested the ability of exogenous SXL to inhibit the translation of a reporter containing full length msl-2 5′ and 3′ UTRs (Figure 3A). Depletion of Hrp48 indeed reduced the capacity of SXL to inhibit translation of the reporter (Figure 3B). Depletion efficiency and SXL expression were monitored by western blot (Figure 3C). Similar results were obtained in vitro, after depletion of Hrp48 from Drosophila embryo extracts using an oligonucleotide containing two Hrp48 binding sites. As Hrp48 is very abundant, a total of 15 rounds of depletion were required to observe reduction of Hrp48 protein levels (Figure 3F). During these experiments, we carried a poly(C) oligonucleotide as control. WT and 5m reporters were compared (Figure 3D). Repression of the WT RNA reporter was less efficient in the Hrp48-depleted extract compared to control or untreated extracts (Figure 3E, WT RNA). As expected, 5m RNA was less efficiently repressed in untreated or control extracts compared to WT RNA (Figure 3E, 5m). Importantly, depletion of Hrp48 had no effect on 5m RNA, as 5m RNA was repressed equally less efficiently in all extracts. Altogether, these results indicate that Hrp48 is required for optimal translational repression of msl-2 by SXL, and that the effect of Hrp48 is mediated through region 5.
Figure 3.
Hrp48 contributes to msl-2 mRNA translational repression. (A) Schematic representation of the βGal reporter containing the full length 5′ and 3′ UTRs of msl-2 (FC) used in transfection assays. (B) Depletion of Hrp48 impairs SXL-mediated repression. Hrp48 was depleted from male SL2 cells, which were then transfected with FC, a control Renilla luciferase plasmid, and increasing amounts of a SXL-encoding plasmid. GFP RNAi was carried as negative control. βGal activity was normalized for Renilla expression and corrected for the levels of the reporter RNA. The data were plotted relative to the βGal activity in the absence of SXL. Error bars represent the standard deviation from seven independent experiments (Unpaired Student's t-test *P< 0.05, **P < 0.01). (C) The levels of Hrp48 and SXL were monitored by Western blot. Tubulin was used as loading control. (D) Schematic representation of msl-2 Luciferase reporters containing (WT) or lacking (5m) region 5. (E) Hrp48 functions through region 5. Hrp48 was depleted from embryo extracts after 15 rounds of incubation with an RNA oligomer containing two Hrp48 binding sites. Depletion with poly(C) was carried as control (Ctrl). The repression of the msl-2 Luciferase reporters was tested upon addition of 10 ng GST-dRBD4. Renilla luciferase was co-translated as internal control. Firefly luciferase activity was corrected for Renilla expression and plotted relative to the activity in the absence of SXL. Error bars represent the standard deviation from at least 11 replicates in 4 independent experiments. (F) The efficiency of depletion was assessed by western blot.
Hrp48 interacts with components of the repressed msl-2 mRNP
To determine whether Hrp48 interacts with components of the repressed msl-2 mRNP, we first tested binding of Hrp48 to endogenous msl-2 by immunoprecipitation followed by semi-quantitative PCR. The results showed that Hrp48 specifically interacts with msl-2 mRNA, as msl-2 but not Gapdh transcripts were pulled-down (Figure 4A). We then used co-immunoprecipitation assays to test if Hrp48 interacts with the msl-2 mRNP components UNR, SXL and HOW. As mentioned above, UNR and SXL are involved in msl-2 translational regulation, while HOW binds to the msl-2 5′ UTR intron and promotes nuclear pre-mRNA retention (9). To evaluate a potential sex-specificity of interactions, we used embryo (mixed of males and females), Kc (female) and SL2 (male) cell extracts. We found that Hrp48 interacts with all components in an RNA-independent fashion (Figure 4B). Except for SXL, which is expressed only in females, Hrp48 interactions were non sex-specific. These results suggest that Hrp48 is part of the repressed msl-2 mRNP.
Hrp48 may interfere with eIF3d to repress translation
To gain insight into the molecular mechanism used by Hrp48 to repress msl-2 translation, we first tested whether a cap structure was required. Transcripts containing or lacking region 5 were synthesized in the presence of a non-functional ApppG cap analog (hereafter termed A-cap) and tested for repression by SXL in Drosophila embryo extracts. The results showed that mutation of region 5 de-repressed translation of A-capped transcripts and, therefore, Hrp48 must target a translation initiation factor acting downstream of cap recognition (Figure 5A). To identify this factor, we searched for novel Hrp48 interactors in Drosophila embryo extracts using oligonucleotide pull-downs (Figure 5B). The same Hrp48-binding oligonucleotide used for depletions was used in three independent pull-down replicates (H), carrying in parallel poly(C) as control (C). After extensive washing, associated proteins were eluted by RNase digestion. Efficient and specific Hrp48 pull-down was assessed by Western blot (Figure 5B, bottom panel). Mass-spectrometry analysis revealed a number of proteins significantly enriched in the Hrp48 oligo eluate (Figure 5B and Supplementary Table S1). The presence of HOW and Squid in the group of co-purified proteins validate the use of oligo pull-down to reveal bona fide interactors of Hrp48. Indeed, Squid is a partner of Hrp48 in the regulation of gurken mRNA expression and HOW is a component of the msl-2 mRNP that co-immunoprecipitates with Hrp48 (Figure 4B) (9,27,28). Interestingly, the subunit d of eIF3 -but no other eIF3 subunit- was also enriched in the Hrp48 oligo eluate. Interaction between eIF3d and Hrp48 was confirmed by independent co-immunoprecipitation analysis using HA-tagged eIF3d (Figure 5C). Recombinant HA-eIF3d interacts with endogenous (lanes 1–4) and purified recombinant (lanes 5–8) Hrp48 in an RNA-independent fashion (lane 9). Thus, Hrp48 interacts directly with eIF3d.
eIF3d is a component of the multi-subunit translation initiation factor eIF3, a factor that coordinates several steps of translation initiation (29,30). We hypothesized that Hrp48 might interfere with translation initiation by targeting eIF3d. To test this hypothesis, we first examined whether eIF3d was required for msl-2 translation. Depletion of eIF3d from SL2 cells reduced translation of an msl-2 reporter, while depletion of other subunits (eIF3e, eIF3h) had no effect (Figure 5D). Importantly, eIF3d depletion did not cause major defects in cellular translation (Figure 5E) nor affected the viability of cultured cells (data not shown). These results indicate that eIF3d promotes the translation of specific transcripts like msl-2 mRNA.
To test whether eIF3d could be a target for msl-2 regulation, we measured the ability of SXL to repress the translation of the full-length msl-2 reporter in SL2 cells after depletion of eIF3d. Depletion of eIF3d, but not eIF3e or eIF3h, reduced the inhibitory effect of SXL, suggesting that eIF3d is a potential target of the repressor complex within the translational machinery (Figure 5F).
eIF3d binds to the 5′ UTR of msl-2 mRNA
We next wondered why translation of msl-2 was sensitive to eIF3d depletion. To address this question, we took advantage of the observation that msl-2 can be translated –albeit at a lesser efficiency- in the absence of a cap structure and this translation is sensitive to repression by SXL (13) (see also Figure 6A). Because Hrp48 contributes to cap-independent repression (Figure 5A), we hypothesized that the Hrp48 target, eIF3d, can be recruited to msl-2 mRNA in a cap-independent fashion, and that this property distinguishes msl-2 from other messages. We, thus, set to identify features of msl-2 mRNA necessary for cap-independent repression, and wondered if these features were related to eIF3d recruitment.
As expected, translational repression of a full-length msl-2 reporter (mLm) was largely cap-independent (Figure 6A, left panel, compare purple and pink lines). However, repression of a reporter containing the minimal sites was not (BLEF, compare blue and black lines). The different behaviors of the mLm and BLEF constructs were not due to differences in basal translation (middle and right panels). These results suggest that cap-independency is conferred by an RNA feature present in mLm but lacking in BLEF. To identify this feature, we first swapped the UTRs of the mLm and BLEF constructs. To simplify our analysis, we defined ‘cap-dependency’ as the distance between the repression lines of A-capped and m7G-capped mRNAs (ΔA-G). This distance was constant at all SXL/RNA ratios for any given construct. We, thus, represented the cap dependency of each construct as the ΔA-G value obtained at a SXL/RNA molar ratio of 40, normalized to the ΔA-G value obtained for BLEF (Figure 6B). The results of the UTR swap mutants showed that cap-independency in repression is conferred by the msl-2 5′ UTR (compare constructs 1–4). Importantly, this cap-independency was maintained in the absence of SXL binding sites in the 5′ UTR (construct 5), indicating that the 3′ UTR complex represses translation efficiently in the absence of a m7G cap.
To address whether the feature that confers cap-independent repression is simply the length of the 5′ UTR, we substituted the 5′ UTR by one of similar length from an unrelated transcript (the 521 nt 5′UTR of aret mRNA, encoding the morphogen Bruno) (construct 6). The results indicated that the length did not explain cap-independent repression. Point mutations in sequence elements previously shown to affect msl-2 regulation (uAUGs, splice sites) did not alter cap-independent repression (data not shown). Analysis of constructs containing hybrid msl-2:aret 5′ UTRs (constructs 7–10) indicated that the first and the last thirds of msl-2 5′ UTR contribute to cap-independent repression. In summary, a sequence or structural feature conformed by the distal regions of the long msl-2 5′ UTR contribute to cap-independent repression, although maximal cap-independency requires the full 5′ UTR.
A scenario to explain cap-independent repression is that, in the absence of the cap structure, eIF3d is recruited to msl-2 5′ UTR and is targeted by the repressor complex. To test whether eIF3d binds to msl-2 5′ UTR and whether this binding correlates with cap-independent repression, we measured the association of radioactively labeled 5′ UTRs to HA-eIF3d in translation conditions, in the presence of embryo extract. The results revealed association of eIF3d with the 5′ UTRs of msl-2 constructs 5 and 10, but not with the aret construct 6 (Figure 6C, see quantification of several experiments in the right panel). Thus, eIF3d binds to the 5′ UTR of msl-2 in a manner that correlates with cap-independent repression.
We next reasoned that, if eIF3d binds to the long msl-2 5′ UTR and is targeted by Hrp48, then lack of Hrp48 binding should mimic lack of eIF3d binding and result in decreased cap-independent repression. To test this hypothesis, we analyzed the effect of mutating region 5 in the repression of constructs containing long and short 5′ UTRs. We found that mutation of region 5 decreased repression of m7G-capped transcripts whether or not the 5′ UTR is long or short (Figure 6D, left panel). However, for A-capped transcripts, where the long 5′ UTR supports eIF3d binding, only repression of this transcript is efficient, and this repression is lost when region 5 is mutated (Figure 6D, right panel). Repression of the short A-capped transcript is weak and barely affected by mutation of region 5. These results agree with our hypothesis that the long 5′ UTR supports eIF3d binding and translational repression in the absence of a cap structure. As expected, the results are similar for a construct containing SXL-binding sites in the 5′ UTR (data not shown). We propose that eIF3d can be recruited to msl-2 mRNA in two ways, either through the cap structure and/or via binding to the 5′ UTR, and both types of recruitment are targeted by the repressor complex assembled at the 3′ UTR (see model in Figure 8).
Figure 8.
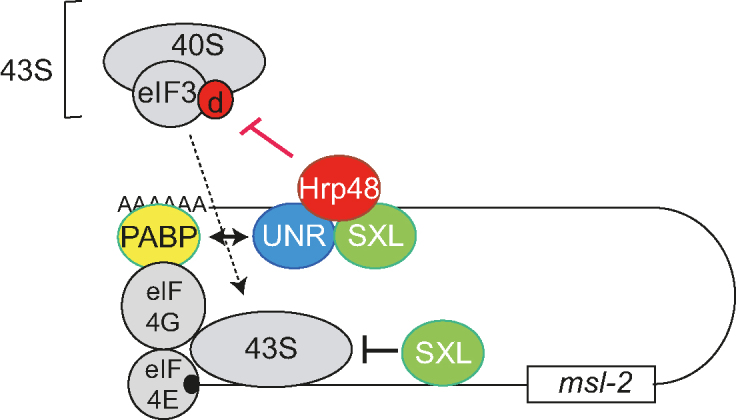
Model for translational repression of msl-2 mRNA. In this model, the findings of this manuscript are integrated with previous knowledge. SXL orchestrates a fail-safe mechanism of translational repression by binding to both the 5′ and 3′ UTRs of msl-2 mRNA. SXL bound to the 3′ UTR recruits UNR and interacts with Hrp48. Contacts of these factors with PABP and eIF3d contribute to inhibit 43S ribosomal complex recruitment. SXL bound to the 5′ UTR inhibits the scanning of those 43S complexes that presumably have escaped the 3′ UTR-mediated control. Findings in this work are highlighted in red.
eIF3d depletion de-represses msl-2 translation in female flies
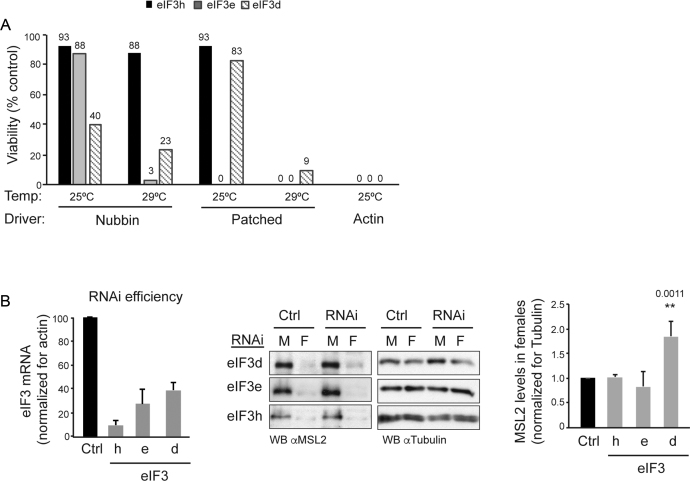
To address the role of eIF3d in vivo, we performed depletion by RNA interference in flies. We also depleted eIF3e and h as controls. Depletion of these subunits had consequences on viability. Using a systemic actin 5C-GAL4 driver resulted in full lethality at an early developmental stage for all subunits, as no third instar larvae were recovered (Figure 7A). Using tissue-restricted drivers (nubbin-GAL4 or patched-GAL4), the extent of viability increased depending on the driver and the eIF3 subunit that was depleted (Figure 7A). These results suggest non-overlapping roles of eIF3d, e and h during development.
Figure 7.
eIF3d is necessary for development and contributes to repression of msl-2 in female flies. (A) The eIF3 subunits d, e and h are required for development and display non-overlapping functions. The eIF3 subunits were depleted by expression of RNAi constructs under the nubbin, patched and actin-5c drivers, at 25 and 29°C. The percentage of embryos reaching pupae with respect to control was measured (exact percentage indicated on the top of each bar). Controls for eIF3d and e were Tubby siblings within the same cross. The control for eIF3h was a parallel w1118x RNAi cross. None of the pupae survived to adulthood, except for patched at 25°C. (B) eIF3d depletion de-represses msl-2 in vivo. eIF3d was depleted in salivary glands using the SgS3-GAL4 driver at 29°C. eIF3e and eIF3h depletions were carried as controls. The efficiency of depletion was assessed by RT-qPCR (left). MSL2 levels were monitored by Western blot (middle) and quantified (right). Error bars represent the standard deviation of three independent experiments. M, male; F, female; Ctrl, control crosses as in (A).
As dosage compensation is usually assessed in salivary glands, we next depleted eIF3d from salivary glands using the SgS3-GAL4 driver and tested for MSL2 levels in females. We expected a mild de-repression of msl-2 upon eIF3d depletion, because endogenous msl-2 is repressed by multiple mechanisms, including 5′UTR-mediated repression by SXL and additional 3′ UTR-mediated mechanisms which are presumably independent of eIF3d and should remain intact after eIF3d depletion. Indeed, a mild but consistent de-repression of msl-2 was observed upon eIF3d depletion, but not upon depletion of eIF3e or eIF3h (Figure 7B, see quantification of several experiments in the right panel, and efficiency of depletion in the left panel). These results indicate that eIF3d contributes to msl-2 repression in female flies.
DISCUSSION
The regulation of msl-2 expression is a prime example of translational control where binding of RNA binding proteins to both UTRs of the transcript coordinate protein output. Despite efforts to unravel the msl-2 regulatory mechanism, the full set of regulators and how they interact with the translational machinery have remained elusive. Here, we identify Hrp48 as a novel msl-2 regulator, and eIF3d as a potential target in the protein synthesis machinery.
Hrp48 is an hnRNP A/B family member involved in post-transcriptional regulation at multiple levels, including splicing, mRNA localization and translation (23,31,32). Using in vitro and ex vivo assays, we show here that Hrp48 inhibits msl-2 translation by interacting with the 3′ UTR of the transcript in complex with SXL and UNR (Figures 2–4). A standing question is why Hrp48 needs to be in the SXL:UNR complex to repress translation, given that Hrp48 binds to the mRNA and interacts with eIF3d in the absence of these components. Indeed, in embryo extracts, the basal translation of constructs containing or lacking the Hrp48 binding sites is similar, and differences are only observed upon addition of SXL (see Supplementary Figure S1). A possible explanation is that binding of Hrp48 to eIF3d in the competitive conditions of the extract is more efficient in the presence of SXL and UNR. Alternatively, binding to eIF3d might be necessary but not sufficient for repression, and further events operated by SXL or UNR downstream of eIF3d binding might be required.
Hrp48 inhibits SXL expression in monomorphic tissues of female flies (26). We could confirm these results in female Kc cells, where depletion of Hrp48 augmented SXL expression (Supplementary Figure S2). These results suggest cross-regulation between factors belonging to the same complex, and compromise the assessment of the role of Hrp48 on msl-2 repression in vivo, as depletion of one inhibitor (Hrp48) would result in increased levels of another inhibitor (SXL). Nevertheless, it has been reported that Hrp48 loss causes sex-specific defects: hypomorph females display strong lethality while males are less affected (33). Our results suggest that regulation of msl-2 expression by Hrp48 could contribute to the observed female-specific lethality.
Hrp48 participates in the localization and translational regulation of oskar and gurken mRNAs during Drosophila early embryogenesis (23,27,28,32). In the case of oskar, Hrp48 binds to both UTRs of the transcript and represses oskar translation during transport (23). In the case of msl-2, Hrp48 binds to the 3′ UTR, although indirect contacts with the 5′ UTR via interactions with HOW—a 5′ UTR binding factor (9)—could be evoked (Figure 4). Hrp48 and its mammalian ortholog DAZAP1 have also been shown to stimulate translation of certain transcripts (25,34). DAZAP1 modulates translation initiation downstream of the recognition of the 5′ cap structure by initiation factors (34). As Hrp48 interacts with a subunit of eIF3 (Figure 5), our data raise the possibility that DAZAP1 exploits eIF3 interactions to stimulate translation.
eIF3 is involved in practically all steps of translation initiation, as it controls the formation of the 43S pre-initiation complex (PIC), the binding of this complex to the mRNA and the stringency of start codon selection (29,30). The composition of eIF3 varies across species (30). In metazoa, eIF3 consists of 12 subunits and one associated factor, named eIF3a to eIF3m, and the specific contributions of these to the variety of eIF3 functions has only started to be elucidated. Although subunit eIF3d is not conserved in budding yeast and is not part of the structural eIF3 octameric core, it is essential in some organisms (30,35–37). Our data indicate that eIF3d, as well as the non-conserved eIF3e and h subunits, is also essential in Drosophila (Figure 7). Intriguingly, the three subunits have different phenotypes regarding viability, suggesting non-overlapping functions during development. These functions may reflect regulation of distinct sets of mRNAs. As precedent, eIF3d was found in a screen for factors required to cope with stress in S. pombe (38), contributes to message-specific translation in this system (39), and has recently been shown to regulate mRNA-specific translation in human cells (40,41). In line with a message-specific role, we show here that Drosophila eIF3d is required for msl-2 regulation (Figures 5 and 7).
Previous reports have shown that eIF3d and eIF3e form a module that is incorporated to the eIF3 complex in the late steps of assembly (42), and that depletion of eIF3e leads to co-depletion of eIF3d in Hela cells (43). In Drosophila SL2 cells, we do not find co-depletion at the mRNA level (Supplementary Figure S3). Although assessment of protein levels would be required, the distinct effects of eIF3d and 3e depletion in translational regulation of msl-2 strongly suggest that there is no co-depletion of eIF3d after knock-down of eIF3e. We suspect that co-depletion effects might be species- or even cell type-specific, as for example eIF3h depletion in Hela cells leads to co-depletion of eIF3l (43) while this is not observed after CRISPR knock-out of eIF3h in HEK293T cells (42).
It has been shown that mammalian eIF3d binds directly to the 5′ UTR of specific mRNAs during cap-dependent translation (40) and that it can also directly recognize the cap structure (41). Our results show that eIF3d binds to the 5′ UTR of msl-2 mRNA (Figure 6). At present, our attempts to assess whether this binding is direct have failed. However, binding does not require the cap structure, because it occurs also on ApppG-capped RNAs (data not shown). Determinants of eIF3d binding are specific mRNA features contributed by two distal portions of the 5′ UTR which we have been unable to further delineate. Interestingly, Meyer et al. recently showed that eIF3 binds to methylated adenosines (m6A) in the 5′ UTR of transcripts, promoting cap-independent translation (44). It will be interesting to decipher whether m6A also contributes to binding of eIF3d to the 5′ UTR of msl-2.
How do Hrp48 and eIF3d contribute to msl-2 translational regulation? Several scenarios can be envisaged:
eIF3d may function as a co-factor for repression independently of any role as an eIF3 component. There is precedent for this type of situation with ribosomal protein L13a (reviewed in 45). During resolution of inflammation, L13a is phosphorylated and detaches from the ribosome to become part of the GAIT complex, an assembly that represses the translation of several mRNAs encoding inflammatory factors. Within the GAIT complex, L13a binds eIF4G to block ribosome recruitment. We think this scenario is unlikely for eIF3d because this factor is required for efficient translation of msl-2 reporters (Figure 5D) and, thus, it functions as a translation factor for msl-2 mRNA.
Hrp48 may interfere with eIF3d association to the rest of the eIF3 complex. In support of this hypothesis, we detect no other eIF3 subunit in oligonucleotide-mediated Hrp48 pull-downs (Figure 5B and Supplementary Table S1). However, it has been shown that both eIF3h and eIF3e are required for incorporation of eIF3d into the complex (42), and the fact that depletion of these subunits does not affect repression of msl-2 by SXL argues against a role of Hrp48 in detaching eIF3d from the rest of eIF3.
Hrp48 may interfere with interactions of eIF3d required for efficient 43S PIC recruitment to the mRNA. This is our favored scenario. Reports have found that eIF3d is required for efficient binding of eIF3 to eIF4G, an initiation factor that is part of the mRNA cap-binding complex and participates in ribosome recruitment to the mRNA (37,46). Recent structural studies have placed eIF3d near the mRNA exit channel, consistent with cross-linking of this factor to mRNA at several positions upstream of the start codon in reconstituted mammalian pre-initiation complexes (47,48). This is a suitable location for eIF3d to mediate interactions with eIF4G during recruitment of the ribosome to the mRNA, because eIF4G is also located in the vicinity of the mRNA exit channel (reviewed in 30,49). Targeting of eIF3d, therefore, fits with the proposed role of the msl-2 3′UTR complex in inhibiting ribosome recruitment (13). To lead to a net reduction in 43S PIC recruitment to the mRNA, the interactions between Hrp48 and eIF3d, or between Hrp48 and the repressor complex in the 3′ UTR of msl-2 should be dynamic, such that the 43S PIC is not ‘frozen’ on the mRNA. Further work is required to test this scenario.
In summary, our results support a model where a repressor complex composed of SXL, UNR and Hrp48 assembles at the 3′ UTR of msl-2 mRNA; Hrp48 then contributes to inhibit ribosome recruitment by targeting eIF3d. This mechanism may act in concert with other inhibitory mechanisms contributed by the 5′ or 3′ UTRs of msl-2 (see integrated model in Figure 8). For example, PABP has been shown to interact with UNR for optimal msl-2 repression (50), and SXL represses translation via the 5′ UTR in a manner independent of UNR (15) and likely also Hrp48 (this report). Further studies are required to analyze the interplay between PABP, eIF3d and other factors in the translational repression of msl-2 mRNA.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Jan Medenbach for the eIF3d plasmid and for useful discussions. We are grateful to Marco Blanchette and Anne Ephrussi for kindly providing anti-Hrp48 antibodies, to Jordi Bernués for fly stocks and to Marco Blanchette for advice on Hrp48 purification. We thank Marija Mihailovich for cloning of the UTR swap constructs. We also thank the CRG Protein Service and the CRG-UPF Proteomics Facility for protein production and identification, and Juan Valcárcel for critically reading this manuscript.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Spanish Ministry of Economy and Competitiveness MINECO and the European Regional Development Fund (ERDF) [BFU2012-37135, BFU2015-68741, Consolider CSD2009-00080]; Spanish Ministry of Economy and Competitiveness, ‘Centro de Excelencia Severo Ochoa 2013–2017’ [SEV-2012-0208]; La Caixa Foundation (to M.G.-B.); Fondation pour la Recherche Médicale (FRM) (to A.G.). Funding for open access charge: Spanish Ministry of Economy and Competitiveness (MINECO) [BFU2015-68741].
Conflict of interest statement. None declared.
REFERENCES
- 1. Sonenberg N., Hinnebusch A.G.. Regulation of translation initiation in Eukaryotes: Mechanisms and biological targets. Cell. 2009; 136:731–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kong J., Lasko P.. Translational control in cellular and developmental processes. Nat. Rev. Genet. 2012; 13:383–394. [DOI] [PubMed] [Google Scholar]
- 3. Szostak E., Gebauer F.. Translational control by 3′UTR binding proteins. Brief. Funct. Genomics. 2013; 12:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blackinton J.G., Keene J.D.. Post-transcriptional RNA regulons affecting cell cycle and proliferation. Semin. Cell Dev. Biol. 2014; 34:44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Graindorge A., Militti C., Gebauer F.. Post-transcriptional control of X-chromosome dosage compensation. Wiley Interdiscip. Rev. RNA. 2011; 2:534–545. [DOI] [PubMed] [Google Scholar]
- 6. Moschall R., Gaik M., Medenbach J.. Promiscuity in post-transcriptional control of gene expression: Drosophila sex-lethal and its regulatory partnerships. FEBS Lett. 2017; 591:1471–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Merendino L., Guth S., Bilbao D., Martinez C., Valcarcel J.. Inhibition of msl-2 splicing by Sex-lethal reveals interaction between U2AF35 and the 3′ splice site AG. Nature. 1999; 402:838–841. [DOI] [PubMed] [Google Scholar]
- 8. Forch P., Merendino L., Martinez C., Valcarcel J.. Modulation of msl-2 5′ splice site recognition by sex-lethal. RNA. 2001; 7:1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Graindorge A., Carre C., Gebauer F.. SXL promotes nuclear retention of msl2 mRNA via interactions with the STAR protein HOW. Genes Dev. 2013; 27:1421–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bashaw G.J., Baker B.S.. The regulation of the Drosophila msl-2 gene reveals a function for Sex-lethal in translational control. Cell. 1997; 89:789–798. [DOI] [PubMed] [Google Scholar]
- 11. Kelley R.L., Wang J., Bell L., Kuroda M.I.. Sex lethal controls dosage compensation in Drosophila by a non-splicing mechanism. Nature. 1997; 387:195–199. [DOI] [PubMed] [Google Scholar]
- 12. Gebauer F., Merendino L., Hentze M.W., Valcarcel J.. The Drosophila splicing regulator Sex-lethal directly inhibits translation of male-specific-lethal-2 mRNA. RNA. 1998; 4:142–150. [PMC free article] [PubMed] [Google Scholar]
- 13. Gebauer F., Grskovic M., Hentze M.W.. Drosophila sex-lethal inhibits the stable association of the 40S ribosomal subunit with msl-2 mRNA. Mol. Cell. 2003; 11:1397–1404. [DOI] [PubMed] [Google Scholar]
- 14. Abaza I., Coll O., Patalano S., Gebauer F.. Drosophila UNR is required for translational repression of male-specific lethal 2 mRNA during regulation of X- chromosome dosage compensation. Genes Dev. 2006; 20:380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duncan K., Grskovic M., Strein C., Beckmann K., Niggeweg R., Abaza I., Gebauer F., Wilm M., Hentze M.W.. Sex-lethal imparts a sex-specific function to UNR by recruiting it to the msl-2 mRNA 3′UTR: translational repression for dosage compensation. Genes Dev. 2006; 20:368–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hennig J., Militti C., Popowicz G.M., Wang I., Sonntag M., Geerlof A., Gabel F., Gebauer F., Sattler M.. Structural basis for the assembly of the Sxl-Unr translation regulatory complex. Nature. 2014; 515:287–290. [DOI] [PubMed] [Google Scholar]
- 17. Beckmann K., Grskovic M., Gebauer F., Hentze M.W.. A dual inhibitory mechanism restricts msl-2 mRNA translation for dosage compensation in Drosophila. Cell. 2005; 122:529–540. [DOI] [PubMed] [Google Scholar]
- 18. Medenbach J., Seiler M., Hentze M.W.. Translational control via Protein-Regulated upstream open reading frames. Cell. 2010; 145:902–913. [DOI] [PubMed] [Google Scholar]
- 19. Gibson D.G., Young L., Chuang R.Y., Venter J.C., Hutchison C.A. 3rd, Smith H.O.. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods. 2009; 6:343–345. [DOI] [PubMed] [Google Scholar]
- 20. Grskovic M., Hentze M.W., Gebauer F.. A co-repressor assembly nucleated by Sex-lethal in the 3′UTR mediates translational control of Drosophila msl-2 mRNA. EMBO J. 2003; 22:5571–5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Siebel C.W., Kanaar R., Rio D.C.. Regulation of tissue-specific P-element pre-mRNA splicing requires the RNA-binding protein PSI. Genes Dev. 1994; 8:1713–1725. [DOI] [PubMed] [Google Scholar]
- 22. Gebauer F., Corona D.F., Preiss T., Becker P.B., Hentze M.W.. Translational control of dosage compensation in Drosophila by Sex-lethal: cooperative silencing via the 5′ and 3′ UTRs of msl-2 mRNA is independent of the poly(A) tail. EMBO J. 1999; 18:6146–6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yano T., López de Quinto S., Matsui Y., Shevchenko A., Shevchenko A., Ephrussi A.. Hrp48, a Drosophila hnRNPA/B homolog, binds and regulates translation of oskar mRNA. Dev. Cell. 2004; 6:637–648. [DOI] [PubMed] [Google Scholar]
- 24. Blanchette M., Green R.E., Brenner S.E., Rio D.C.. Global analysis of positive and negative pre-mRNA splicing regulators in Drosophila. Genes Dev. 2005; 19:1306–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nelson M.R., Luo H., Vari H.K., Cox B.J., Simmonds A.J., Krause H.M., Lipshitz H.D., Smibert C.A.. A multiprotein complex that mediates translational enhancement in Drosophila. J. Biol. Chem. 2007; 282:34031–34038. [DOI] [PubMed] [Google Scholar]
- 26. Suissa Y., Kalifa Y., Dinur T., Graham P., Deshpande G., Schedl P., Gerlitz O.. Hrp48 attenuates Sxl expression to allow for proper notch expression and signaling in wing development. Proc. Natl. Acad. Sci. U.S.A. 2010; 107:6930–6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goodrich J.S., Clouse K.N., Schüpbach T.. Hrb27C, Sqd and Otu cooperatively regulate gurken RNA localization and mediate nurse cell chromosome dispersion in Drosophila oogenesis. Development. 2004; 131:1949–1958. [DOI] [PubMed] [Google Scholar]
- 28. Geng C., Macdonald P.M.. Imp associates with squid and Hrp48 and contributes to localized expression of gurken in the oocyte. Mol. Cell. Biol. 2006; 26:9508–9516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hinnebusch A.G. The scanning mechanism of eukaryotic translation initiation. Annu. Rev. Biochem. 2014; 83:779–812. [DOI] [PubMed] [Google Scholar]
- 30. Valášek L.S., Zeman J., Wagner S., Beznosková P., Pavlíková Z., Mohammad M.P., Hronová V., Herrmannová A., Hashem Y., Gunišová S1.. Embraced by eIF3: structural and functional insights into the roles of eIF3 across the translation cycle. Nucleic Acids Res. 2017; 45:10948–10968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blanchette M., Green R.E., MacArthur S., Brooks A.N., Brenner S.E., Eisen M.B., Rio D.C.. Genome-wide analysis of alternative pre-mRNA splicing and RNA-binding specificities of the Drosophila hnRNP A/B family members. Mol. Cell. 2009; 33:438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huynh J.R., Munro T.P., Smith-Litière L., Lepesant J.A.. The Drosophila hnRNPA/B homolog, Hrp48, is specifically required for a distinct step in osk mRNA localization. Dev. Cell. 2004; 6:625–635. [DOI] [PubMed] [Google Scholar]
- 33. Hammond L.E., Rudner D.Z., Kanaar R., Rio D.C.. Mutations in the hrp48 gene, which encodes a Drosophila heterogeneous nuclear ribonucleoprotein particle protein, cause lethality and developmental defects and affect P-element third-intron splicing in vivo. Mol. Cell. Biol. 1997; 17:7260–7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith R.W., Anderson R.C., Smith J.W., Brook M., Richardson W.A., Gray N.K.. DAZAP1, an RNA-binding protein required for development and spermatogenesis, can regulate mRNA translation. RNA. 2011; 17:1282–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smith M.D., Gu Y., Querol-Audí J., Vogan J.M., Nitido A., Cate J.H.. Human-like eukaryotic translation initiation factor 3 from Neurospora crassa. PLoS One. 2013; 8:e78715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Masutani M., Sonenberg N., Yokoyama S., Imataka H.. Reconstitution reveals the functional core of mammalian eIF3. EMBO J. 2007; 26:3373–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sun C., Todorovic A., Querol-Audí J., Bai Y., Villa N., Snyder M., Ashchyan J., Lewis C.S., Hartland A., Gradia S., Fraser C.S. et al. . Functional reconstitution of human eukaryotic translation initiation factor 3 (eIF3). Proc. Natl. Acad. Sci. U.S.A. 2011; 108:20473–20478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Calvo I.A., Gabrielli N., Iglesias-Baena I., García-Santamarina S., Hoe K.L., Kim D.U., Sansó M., Zuin A., Pérez P., Ayté J. et al. . Genome-wide screen of genes required for caffeine tolerance in fission yeast. PLoS One. 2009; 4:e6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shah M., Su D., Scheliga J.S., Pluskal T., Boronat S., Motamedchaboki K., Campos A.R., Qi F., Hidalgo E., Yanagida M. et al. . A transcript-specific eIF3 complex mediates global translational control of energy metabolism. Cell Rep. 2016; 16:1891–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee A.S., Kranzusch P.J., Cate J.H.. eIF3 targets cell-proliferation messenger RNAs for translational activation or repression. Nature. 2015; 522:111–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee A.S., Kranzusch P.J., Doudna J.A., Cate J.H.. eIF3d is an mRNA cap-binding protein that is required for specialized translation initiation. Nature. 2016; 536:96–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smith M.D., Arake-Tacca L., Nitido A., Montabana E., Park A., Cate J.H.. Assembly of eIF3 mediated by mutually dependent subunit insertion. Structure. 2016; 24:886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wagner S., Herrmannová A., Šikrová D., Valášek L.S.. Human eIF3b and eIF3a serve as the nucleation core for the assembly of eIF3 into two interconnected modules: the yeast-like core and the octamer. Nucleic Acids Res. 2016; 44:10772–10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meyer K.D., Patil D.P., Zhou J., Zinoviev A., Skabkin M.A., Elemento O., Pestova T.V., Qian S.B., Jaffrey S.R.. 5′ UTR m(6)A promotes Cap-Independent translation. Cell. 2015; 163:999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Arif A., Yao P., Terenzi F., Jia J., Ray P.S., Fox P.L.. The GAIT translational control system. WIREs RNA. 2018; 9:e1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Villa N., Do A., Hershey J.W., Fraser C.S.. Human eukaryotic initiation factor 4G (eIF4G) protein binds to eIF3c, -d, and -e to promote mRNA recruitment to the ribosome. J. Biol. Chem. 2013; 288:32932–32940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Des Georges A., Dhote V., Kuhn L., Hellen C.U., Pestova T.V., Frank J., Hashem Y.. Structure of mammalian eIF3 in the context of the 43S preinitiation complex. Nature. 2015; 525:491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pisarev A.V., Kolupaeva V.G., Yusupov M.M., Hellen C.U., Pestova T.V.. Ribosomal position and contacts of mRNA in eukaryotic translation initiation complexes. EMBO J. 2008; 27:1609–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hinnebusch A. Structural insights into the mechanisms of scanning and start codon recognition in eukaryotic translation initiation. Trends Biochem. Sci. 2017; 42:589–611. [DOI] [PubMed] [Google Scholar]
- 50. Duncan K.E., Strein C., Hentze M.W.. The SXL-UNR corepressor complex uses a PABP-mediated mechanism to inhibit ribosome recruitment to msl-2 mRNA. Mol. Cell. 2009; 36:571–582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.