Abstract
In addition to a role in the processing of nascent RNA polymerase III transcripts, La proteins are also associated with promoting cap-independent translation from the internal ribosome entry sites of numerous cellular and viral coding RNAs. La binding to RNA polymerase III transcripts via their common UUU-3’OH motif is well characterized, but the mechanism of La binding to coding RNAs is poorly understood. Using electromobility shift assays and cross-linking immunoprecipitation, we show that in addition to a sequence specific UUU-3’OH binding mode, human La exhibits a sequence specific and length dependent poly(A) binding mode. We demonstrate that this poly(A) binding mode uses the canonical nucleic acid interaction winged helix face of the eponymous La motif, previously shown to be vacant during uridylate binding. We also show that cytoplasmic, but not nuclear La, engages poly(A) RNA in human cells, that La entry into polysomes utilizes the poly(A) binding mode, and that La promotion of translation from the cyclin D1 internal ribosome entry site occurs in competition with cytoplasmic poly(A) binding protein (PABP). Our data are consistent with human La functioning in translation through contacts to the poly(A) tail.
INTRODUCTION
La proteins have been characterized in nearly all eukaryotes examined and have conserved functions in the processing of RNA polymerase III transcripts (1). First identified as an autoantigen in patients suffering from systemic lupus erythematosus and Sjögren's syndrome (2,3), immunoprecipitations using anti-La antibodies revealed that human La (hLa) associates with precursor forms of RNA polymerase III transcripts (4–6) as well as the uridylate-tailed adenoviral VA RNA and the Epstein-Barr virus encoded EBER RNAs (7,8). The importance of the uridylate tail was subsequently validated by experiments showing that the number of uridylates directly influenced the efficiency of La binding to a pre-tRNA or VA RNA substrate, with high-affinity binding generally requiring at least three terminal uridylates (9,10). Subsequent structural and biochemical work deciphered the specific mechanism of UUU-3’OH recognition, in which the UUU-3’OH motif is sandwiched between the N-terminal La motif (LAM) and RNA recognition motif (RRM1; together the so-called ‘La module’). Furthermore it was demonstrated that uridylate specific contacts are mediated largely by conserved amino acids on the La motif (11,12). Surprisingly, these structures indicated that neither of the expected nucleic acid binding surfaces of the La module (the winged helix interaction surface of the LAM nor the β-sheet of RRM1) contribute to UUU-3’OH recognition (12,13), leaving the function of these canonical interaction surfaces unclear.
In addition to a sequence-specific UUU-3’OH-dependent binding mode, other work using a variety of substrates has demonstrated that the canonical RNA binding surface of RRM1, RRM2 and the disordered CTD also contribute to La RNA binding in a relatively non-specific manner (14–17). The canonical RNA binding surface of RRM1 enhances human La binding to the main body of pre-tRNAs, which in combination with the UUU-3’OH dependent binding modes, assists La in the discrimination of pre-tRNA processing intermediates (14,18). The RRM1, RRM2 and disordered C-terminal regions of La have also been implicated in RNA binding via modes that lack sequence specificity but can nevertheless rely on the presence of RNA secondary structure (15,16,19). For example, the La motif, RRM1 and RRM2 all contribute to the binding of a small hairpin with a short, single-stranded 3’ tail derived from the Hepatitis C IRES, and while the presence of the hairpin and single stranded extension were both shown to be critical for maximal binding, the actual sequence of these was much less important (15). Often, these UUU-3’OH independent binding modes have been implicated in La function as an RNA chaperone. RNA chaperone activity in human La has been mapped to the RRM1 as well as the disordered CTD (17,20), and it has been proposed that one of the functions of the UUU-3’OH depending binding mode is to recruit non-specific La-associated RNA chaperone activity to UUU-3’OH containing substrates (21). It is thus hypothesized that binding of La to RNA targets in vivo occurs through the co-operation of a number of RNA binding modes in combination (15,22), and that the specificity determinants for some of these binding modes are still nebulous.
Consistent with the presence of UUU-3’OH independent La-RNA binding modes, La proteins also immunoprecipitate coding RNAs lacking this motif (23,24). Human La was identified as the first internal ribosome entry site (IRES) trans-acting factor (ITAF) due to its ability to enhance translation of polioviral RNA (25), and has since been shown to likewise enhance cap-independent translation of other (+) stranded viral RNAs associated with challenges to human health. IRESs are also found in some cellular mRNAs, and it has been hypothesized that these can be more efficiently translated under conditions of cellular stress (reviewed in (26)). Consistent with a role in the translation of RNA polymerase II transcripts, La immunoprecipitates mRNAs in a variety of experimental systems, including yeast, Xenopus oocytes, and human tissue culture cells (23,24,27). La promotes IRES based translation from several cellular mRNAs, including those encoding BiP, cyclin D, NRF2 and laminin B1, as well as the upstream open reading frame (uORF) containing mRNA for the oncogene MDM2 (28–32). The effect of La on classical cap-dependent translation is less well understood, with various studies indicating that La can activate or inhibit cap-dependent protein synthesis, suggesting that La influence in this process may be context specific (reviewed in (33)). While the mechanism by which La proteins recognize coding RNAs is still poorly understood, it has been hypothesized to rely on electrostatic, RNA structure dependent contacts mapping largely to the RRM1 and RRM2 domains (in those La proteins that harbour a second RRM) (15,33). Importantly, a sequence specific RNA binding mode for La targets that lack UUU-3’OH has yet to be characterized.
Recently, the study of human La has substantially expanded into the study of the human La-related proteins (hLARPs), which similar to hLa share a La-motif and can harbor RNA chaperone activity but are hypothesized to bind RNA targets distinct from those of La (1,33,34). Like La, the hLARPs 1, 4 and 4b have also been implicated in the control of protein translation. Notably, these La-related factors are hypothesized to associate with the poly(A) tail directly and/or through interactions with poly(A) binding protein (35–39). However, a mechanism by which the LARPs may engage poly(A) sequences is still lacking. Given the established role of La and the La-related proteins in the translation of cellular mRNAs, we decided to test whether La might contact mRNAs, at least in part, through the poly(A) tail. In this work, we demonstrate that human La specifically binds poly(A) RNA in vitro with significantly higher affinity than other non-uridylate homopolymers, provided the RNA sequence is extended (i.e. 20 nucleotides), as is common in the poly(A) tails of mRNAs. We also show the canonical winged-helix face of the La motif plays a role in poly(A) but not UUU-3’OH binding, as mutagenesis of this region impairs (a) poly(A) binding in vitro, (b) entry of cytoplasmic La into polysomes (c) crosslinking immunoprecipitation (CLIP) of poly(A) tails in human cells and (d) translation from a bicistronic cyclin D1 IRES containing reporter construct. Together, our data are consistent with a model in which two specific binding modes (UUU-3’OH and poly(A)) direct La RNA chaperone activity to its two classes of RNA substrate in their respective cellular compartments. The importance of the La motif in poly(A) binding may also have relevance for the mechanism of how the cytoplasmic La-related proteins associated in translation perform related functions.
MATERIALS AND METHODS
Electromobility shift assays (EMSAs)
All radiolabeled RNAs were PAGE purified after 5′ end labeling. Recombinant His-tagged human La (40) or hLa point mutants (generated by QuikChange; Agilent) were purified from Escherichia coli first over a Ni2+ column (His-Trap, GE-Amersham) then a heparin column (Hi-Trap, GE-Amersham). Proteins were then concentrated and quantitated via Bradford and SDS-PAGE, and A260/A280 ratios were taken to confirm purified proteins were free from contaminating RNAs that might have co-purified from E. coli. EMSAs were performed as described (14). Briefly, 3000 cpm (∼0.1 nM) of various RNA substrates (IDT) were incubated with various concentrations of recombinant human La or human La mutants in a 20 μl reaction containing 1X EMSA buffer (20 mM Tris pH 7.6, 100 mM KCl, 1 mM EDTA and 5 mM β-mercaptoethanol) and 50 ng HepC (5′ rCrGrU rGrCrA rCrCrA rUrGrA rGrCrA rCrGrA rArUrC rCrA 3’; (15)) or 10 ng C10 as cold RNA competitor. RNAs were initially slow-cooled from 95°C to room temperature and then incubated with protein at 37°C for 20 min. Complexes were resolved on 10% polyacrylamide nondenaturing gels at 4°C at 100 V. Supershifts were treated as supplementary binding events to the primary binding event, and binding curves were fit using a non-linear specific binding curve fitting program (GraphPad, Prism). Kd values were approximated as the concentration of protein at which half of the RNA substrate was bound.
For competition experiments, radiolabeled RNAs were mixed with indicated amounts of cold RNA in 1× EMSA buffer, after which 2 uM recombinant hLa was added and incubated for 20 min at 37°C, prior to separation on native PAGE as described above.
Cross-linking immunoprecipitation (CLIP)
CLIP was performed as described (41) with the following modifications. Briefly, two 15 cm dishes of HEK293T cells per CLIP were transfected with pEGFPC1-hLa (42), or indicated GFP-hLa variants or myc-tagged pcDNA-PABPC1 (43) (PolyJet, SignaGen). Twenty four hours post-transfection, cells were UV-crosslinked (Stratalinker at 254 nm, 1000 mJ) and cells were lysed in RIPA buffer (10 mM Tris–HCl pH8.0, 1 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, 140 mM NaCl, 1 mM PMSF). Lysates were then treated with 3 μl of 1:100 RNAse I (100 U/μl AM2294 Invitrogen) or 40 μl of a 1:100 dilution of RNase T1 (1 U/μl AM2283 Ambion) and 2 μl DNase I (2 U/μl AM2238 Ambion) at 37°C for 3 min. Antibodies used were anti-cmyc (Abcam ab21060) or anti-GFP (Abcam ab1218). coIPs were performed using Protein G magnetic Dynabeads (Invitrogen). Antibodies were incubated with Dynabeads in 150 μl RIPA buffer for 1 h at room temperature, then antibodies/Dynabeads were incubated with lysates for 2 h at 4°C. Complexes were washed 2× with RIPA buffer and 3× with proteinase K buffer prior to digestion with 10 μl proteinase K (buffer and enzyme supplied by Invitrogen, #100005393 20 mg/ml). Eluted RNA was probed with 32P radiolabeled dT (40), stripped and reprobed for pre-tRNA Met-e (probe sequence AAA TTA TTG TGC CCC G) via northern blot.
qPCR of hLa precipitated mRNAs
Crosslinking of RNA protein complexes was performed as described (44). Briefly, one 15 cm plate of HEK293T cells was transfected with pEGFPC1-hLa 1–375, pEGFPC1 hLa 1–375 K86A/T87A/K88A or the pEGFPC1 vector control and crosslinked with 1% formaldehyde at room temperature for 10 min. The reaction was stopped with 0.25 M glycine pH 7.0 for 5 min at room temperature. Cells were washed twice in 1× PBS followed by lysis in 1 ml RIPA buffer with DNase treatment (4 U, 5 min at 37°C). Immunoprecipitations were performed using anti-GFP and Protein G beads with three 500 ul washes in RIPA buffer then three 500 ul washes in PNK buffer (50 mM Tris–HCl pH 7.0, 5 mM EDTA, 10 mM DTT, 1% SDS) followed by reversal of crosslink by incubation at 70°C for 45 min. RNA was Trizol extracted followed by isopropanol precipitation. Input and eluted RNAs were converted to cDNA using BioRad iScript cDNA synthesis kit and assessed by quantitative real-time PCR using SensiFAST SYBR No-ROX Kit (Bioline) using the following settings: Hot start @ 95°C for 5 min (denaturation for 5 s @ 95°C, annealing/extension @ 60°C for 10 s) × 40 cycles. Relative abundance was calculated as enrichment over empty vector normalized to input using the ΔΔCt method. Primers used: BiP Forward: GAAAGAAGGTTACCCATGCAGT, BiP Reverse: CAGGCCATAAGCAATAGCAGC; CCND1 Forward: CTCTCCAGAGTGATCAAGTGTGACCC, CCND1 Reverse: TGTGCAAGCCAGGTCCACC; 5.8S rRNA Forward: TCTTAGCGGTGGATCACTCGGC, 5.8S rRNA Reverse: GCTCAGACAGGCGTAGCCC. Values presented represent mean enrichments over a minimum of three biological replicates.
Polysome analysis
Polysome analysis was performed as described (45). Briefly, two 15 cm dishes of transfected HEK293T cells were lysed 24 h post transfection in hypotonic lysis buffer [5 mM Tris–HCl (pH 7.5), 2.5 mM MgCl2, 1.5 mM KCl, 100 μg/ml cycloheximide, 2 mM DTT, 0.5% Triton X-100 and 0.5% sodium deoxycholate] and cell debris was removed by centrifugation. Twenty A260 nm of cell lysate was loaded over a seven step 20–50% sucrose gradient prepared according to the method of Luthe (46) in 20 mM HEPES–KOH (pH 7.6), 100 mM KCl, 5 mM MgCl2 followed by centrifugation at 30 000 rpm for 3 h in a Beckman SW41 rotor. Gradients were fractionated into 500 μl fractions of which 20 μl was separated by SDS-PAGE and blotted using the relevant antibodies. Anti-Rpl9 antibody was from Abcam (ab182556). For puromycin treatment, cell lysates were treated with 25 μM puromycin for 15 min and cyclohexamide was omitted (47).
CCND1 IRES luciferase reporter assay
The CCND1 IRES was cloned as a cDNA from HeLa poly(A) purified RNA into the SalI-BamHI site of the pDL-N dual luciferase construct (48) and confirmed by sequencing. This construct was then used as a template for a PCR reaction using primers that added an SP6 promoter upstream of the renilla 5′UTR and a 20A or 40A sequence after the firefly 3’UTR. The PCR product was used as a template for a SP6 RNA polymerase mediated in vitro transcription of capped mRNA using the SP6 mMESSAGE mMACHINE kit from Ambion as per manufacturer's protocol. HEK 293T cells were plated in six well plates 18 h prior to transfection in antibiotic free media and transfected with 4 ug plasmid DNA (pEGFPC1-hLa (42) and PABPC1-cmyc (43) or vector controls) and Lipofectamine 2000 as per the manufacturer's instructions. Twenty four hours post-transfection, cells were re-plated into 96-well plates and transfected with 150 ng of mRNA. Twenty four hours post-transfection, lysates were measured using Dual-Luciferase Reporter Assay (Promega). To test for reporter mRNA levels, qPCR of the firefly cistron was performed using the Qiagen one-step RT-PCR kit (Qiagen #210210) and TaqMan based primers, normalized to the U5 snRNA as measured by SYBR qPCR as described above. Primers: U5 snRNA Forward: TGG TTT CTC TTC AGA TCG CAT AAA, U5 snRNA Reverse: CCA AGG CAA GGC TCA AAA AAT; Firefly Forward: GAC GAT GAC GCC GGT G, Firefly Reverse: GAC TGG CGA CGT AAT CCA, Firefly TaqMan Probe: /56-FAM/CC GCC GTT G/ZEN/T TGT TTT GGA GCA C/3IABkFQ/. TaqMan probe was from IDT.
RESULTS
La binds to poly(A) in a sequence specific and length dependent manner
While La preference for terminal uridylates has previously been established, La binding to extended adenylate sequences has not been tested, despite the established association of La with cellular mRNAs in both yeast and humans cells (23,28–31). To test La binding to polyadenylate sequences, we compared hLa binding to various RNA homopolymers using electromobility shift assay (EMSA; Figure 1, Supplementary Figure S1 and Supplementary Table 1). To better assess the nature of specificity of RNA binding in our analysis, we included one of two competitors in our EMSA analysis. The first was a small hairpin with a single stranded 3’ tail derived from a La target, the Hepatitis C IRES, as La has previously been demonstrated to bind to this RNA in a manner that relies on its secondary structure in the absence of sequence specificity (15). The alternate competitor was a C10 homopolymer, which is not expected to form secondary structure.
Figure 1.
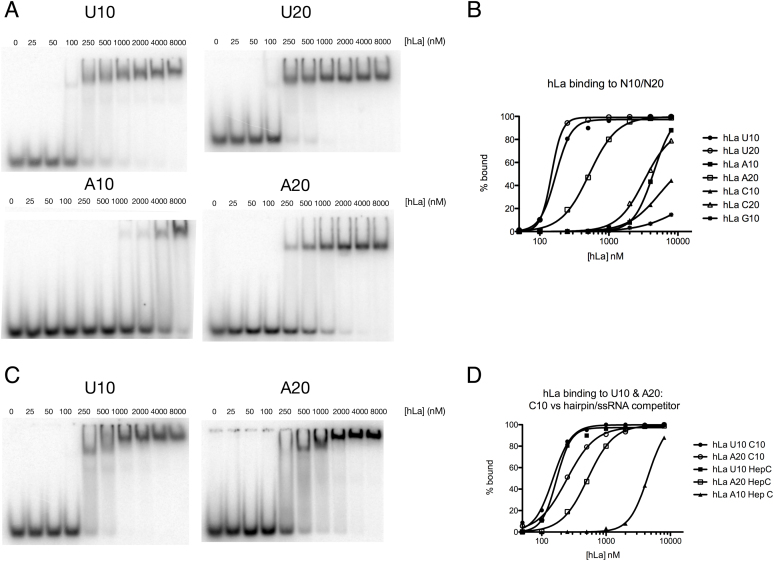
Human La displays length dependent affinity for poly(A). (A) Binding of recombinant human La for U10, U20, A10 and A20 was tested by electromobility shift assay (EMSA) using HepC hairpin RNA as competitor; gels for C10, C20, G10 and G20 provided in Supplementary Figure S1. (B) Graphical representation of EMSA results. (C) EMSA of human La for U10 and A20 in the presence of C10 as competitor. (D) Graphical representation of binding curves comparing La affinity for U10 and A20 with C10 versus HepC hairpin competitors.
Using the hairpin-containing competitor (Figure 1A), we found that hLa bound U10 with substantially higher apparent affinity than C10, G10 or A10, consistent with previous results demonstrating high affinity binding of La to terminal uridylates. However, when the length of the homopolymers was increased to twenty nucleotides, the apparent affinity of hLa for A20 increased substantially. This increase in affinity was specific for adenylates, as C20 bound hLa with low affinity, similar to C10 (Figure 1A, B, Supplementary Figure S1). We noted that G20 tended to dimerize, with the free RNA running as two bands (Supplementary Figure S1). Interestingly, hLa shifted the G20 dimer with reasonably high affinity, while the single stranded G20 band had similarly poor affinity as the G10 homopolymer, reminiscent of previous work indicating binding of hLa to RNAs with secondary structure in the absence of sequence specificity (14,15).
We then compared hLa binding to U10 and A20 in the presence of C10 as a competitor to our previous result using the hairpin-containing competitor (Figure 1C, D). We found that the apparent affinity for the U10 substrate using C10 as a competitor was nearly identical to that when using the hairpin-containing competitor. However, the apparent affinity for A20 increased when substituting C10 as a competitor, to the extent that the apparent Kd of hLa for A20 was only slightly lower than that of U10. These data suggest that a) hLa binds A20 with high affinity in a manner that is length dependent and that discriminates adenylates from C or (single-stranded) G, and b) structured RNAs compete slightly better for A20 binding than single-stranded RNA, suggesting that some A20 contacts may partially overlap with contacts associated with structure dependent, UUU-3’OH independent binding (15).
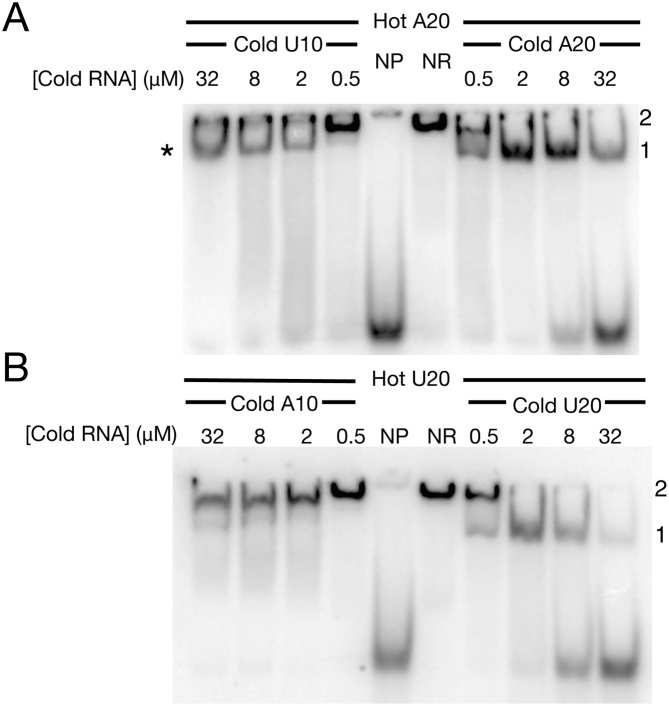
In order to better understand the potential overlap between the UUU-3’OH dependent binding mode and those contacts important for A20 binding, we performed direct competition experiments between uridylate and adenylate containing RNAs (Figure 2). We determined an amount of recombinant human La (2 μM) sufficient to achieve >95% binding of radiolabelled A20 (NR lane, Figure 2A) or U20 (NR lane, Figure 2B) in the absence of competitor, then added this amount of protein to pre-mixed radiolabelled and cold RNAs containing increasing concentrations of cold competing RNA substrates to determine their propensities to compete our radiolabelled RNAs from hLa. In order to demonstrate a successful competition reaction, we performed competitions with the identical cold U20 or A20 RNAs (Figure 2A and B, right hand sides). These experiments revealed that at this concentration, La-RNA binding was occurring in two stages: in the absence of competitor (NR), La protein at 2 μM supershifts U20 and A20 (i.e. greater than one La per RNA, as has been described previously at higher concentrations of La (reviewed in (22)). This supershift would then shift down to a 1:1 La-RNA RNP with increasing like competitor, as the cold RNAs titrated hLa causing a supershift away from the radiolabelled substrates. (Figure 2A and B, right hand sides; 1:1 La-RNA indicated as ‘1’, La-RNA supershifts as ‘2’). We found that addition of cold competing U10 to radiolabelled A20 titrated La resulting in a supershift away from A20, causing a clear mobility shift to the 1:1 La-A20 species at lower concentration of competitor (Figure 2A, left hand side, marked with ‘*’). However, increasing addition of U10 did not result in a release of A20 from the hLa-A20 RNP even at the highest concentration of U10 competitor tested (32 μM). These data indicate that despite U10 being active in our assay (addition of U10 titrated La that caused a supershift away from A20) it did not result in release of A20 from the A20:hLa RNP, suggesting that the U10 competes poorly for A20 and that their binding modes are at least partially distinct.
Figure 2.
Uridylate containing RNAs compete poorly for poly(A) binding to hLa. Sufficient recombinant human La was added (2 μM) to radiolabelled A20 to achieve >95% binding (NR: no cold RNA added) and increasing concentrations of various cold RNAs were added to assess their ability to displace A20 from hLa. (A) Comparison of U10 (left-hand series) and A20 (right-hand series) for radiolabelled A20 binding. (B) Comparison of A10 (left-hand series) and U20 (right-hand series) for radiolabelled U20 binding. NP: no recombinant hLa protein added (i.e. free radiolabeled A20 or U20). ‘1’: indicative of 1:1 La–RNA complex; ‘2’: indicative of multimeric La–RNA complex. * (asterisk): new RNP formed between La and A20 with addition of U10.
We considered that the La RNP in the presence of both A20 and U10 could be a double stranded RNA duplex, and that the La RNP observed with increasing competitor could be the result of La binding to double stranded RNA. To test this, we added increasing concentration of A10 to the hLa:U20 RNP (Figure 2B, left panel), as U20:A10 should have a similar propensity to form a duplex as A20:U10. Unlike the A20:U10 competition, however, A10 had no effect on the hLa:U20 RNP, as measured by the persistence of supershift with increasing concentration of A10, consistent with our previous data (Figure 1) showing low affinity for A10. In sum, these data are consistent with a relatively poor ability of adenylates to compete with uridylates on hLa and at least partially distinct poly(A) and UUU-3’OH binding modes.
The winged-helix face of the La motif functions in adenylate binding
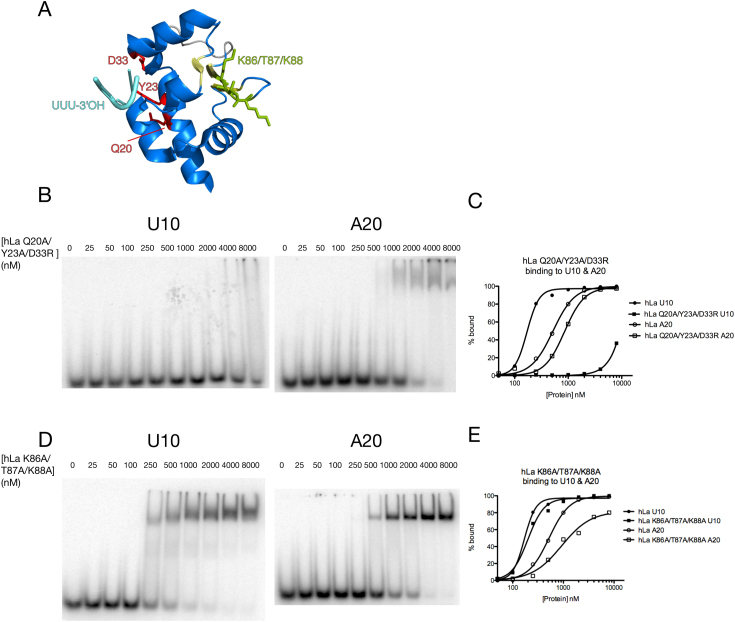
High resolution La module/UUU-3’OH co-crystal structures have previously identified the regions of La required for uridylate recognition (11,12), and these surprisingly revealed that the expected nucleic acid binding surface of the LAM (the winged helix face) does not make any contacts to the UUU-3’OH motif (Figure 3A). To further investigate potential differences between the UUU-3’OH and A20 binding modes, we compared U10 versus A20 binding across some La mutants (Figure 3). We tested a mutant we have previously demonstrated as having significantly compromised affinity for terminal uridylates (49) by virtue of mutations to three amino acids critical for UUU-3’OH binding (hLa Q20A/Y23A/D33R) for its differential ability to bind U10 and A20. Consistent with our competition data arguing for at least partially distinct U10 and A20 binding modes, this mutant had nearly negligible U10 binding yet still bound A20. We observed a slight decrease in A20 affinity relative to wild-type, (Figure 3B, C), consistent with either a slight folding defect for this variant, as has been noted previously (14), or the possibility that amino acids critical for UUU-3’OH binding may also play a role in adenylate binding. Nevertheless, the near complete loss of binding of this mutant to U10 relative to the continued binding to A20 are consistent with the uridylate and adenylate RNA binding modes still being substantially distinct.
Figure 3.
The winged-helix face of the La motif is involved in adenylate binding. (A) Structure of La motif (11) with uridylate binding amino acids labeled in red, UUU-3’OH RNA in cyan, winged helix face of La motif in yellow and mutated amino acids of winged-helix face in green. (B) EMSAs of hLa Q20A/Y23A/D33R binding to U10 and A20. (C) Graphical representation of hLa and hLa Q20A/Y23A/D33R bound to U10 and A20. (D) EMSAs of hLa K86A/T87A/K88A binding to U10 and A20. (E) Graphical representation of hLa and hLa K86A/T87A/K88A bound to U10 and A20.
We considered the possibility that the expected winged helix-fold nucleic acid interaction surface of the La motif might comprise one of the regions important for A20 recognition, as this region has not yet been demonstrated to engage an RNA ligand. We therefore tested several sets of point mutants around the La motif for their differential ability to bind U10 versus A20. The hLa mutant R32A/K34A/K37A, whose mutated amino acids map to neither the winged-helix face nor the UUU-3’OH recognition site, had no defect in binding either substrate, while the hLa F65A/N66A/E70A and E70A/K74A/K76A mutants, whose mutations map to the canonical winged-helix recognition helix of the La motif, showed slightly impaired binding to both U10 and A20, suggesting they may be misfolded (data not shown). The hLa mutant K86A/T87A/K88A comprises mutations to the first ‘wing’ of the La motif's winged helix fold; while this mutant bound U10 nearly indiscernibly from wild-type, it had a more substantial drop in affinity for the A20 substrate, as well as a more gradual binding curve (Figure 3E), suggesting the possible loss of one of several cooperative binding sites. These data suggest these amino acids contribute to the affinity of La for poly(A) but not UUU-3’OH, even if this mutant still contains other surfaces that contribute to poly(A) binding, consistent with our observed length dependence of the adenylate binding mode.
To further test these ideas, we compared U10 and A20 binding across a series of hLa deletion mutants (Supplementary Figure S2). Consistent with previous work indicating that the LAM and RRM1 form a single binding pocket for UUU-3’OH, deletion of the LAM (hLa dLAM) or RRM1 (hLa dRRM1) resulted in near complete loss of U10 binding. However, each of these mutants were still able to completely shift the A20 substrate, albeit at significantly higher concentrations than the wild-type protein. Deletion of RRM2 and amino acids C-terminal to this (1–235) had no effect on U10 binding, as expected, while A20 binding was slightly enhanced, suggesting that contacts that contribute to A20 binding are contained within the La module and the unstructured region between RRM1 and RRM2. In sum, our results suggest that while the binding of La to UUU-3’OH is an event that relies integrally on the presence of both the LAM and RRM1 forming a single binding site, the adenylate binding mode uses sites on the LAM and RRM1 that function more additively and that cooperate for high-affinity binding.
Cytoplasmic La binds directly to poly(A) in human cells
Human La is largely nuclear where it engages RNA polymerase III transcripts, but also shuttles to the cytoplasm where it can accumulate during conditions of cellular stress or viral infection (32,50–52). To test whether hLa can directly engage poly(A) tails in human cells, we performed cross-linking immunoprecipitation (CLIP), which included a limited RNase I digestion step in order to degrade RNA not in direct contact to hLa (Figure 4A). To differentiate hLa RNA binding profiles in the nucleus versus the cytoplasm, we transfected GFP-tagged wild-type and nuclear localization signal deleted (ΔNLS (53,54); hLa 1–375; localization of constructs in Supplementary Figure S3) hLa constructs into HEK293T cells. We also tested our point mutant with compromised A20 binding (K86A/T87A/K88A) in both the full length (nuclear) and 1–375 (cytoplasmic) contexts, as well as a myc-tagged cytoplasmic PABP construct as a poly(A) binding positive control.
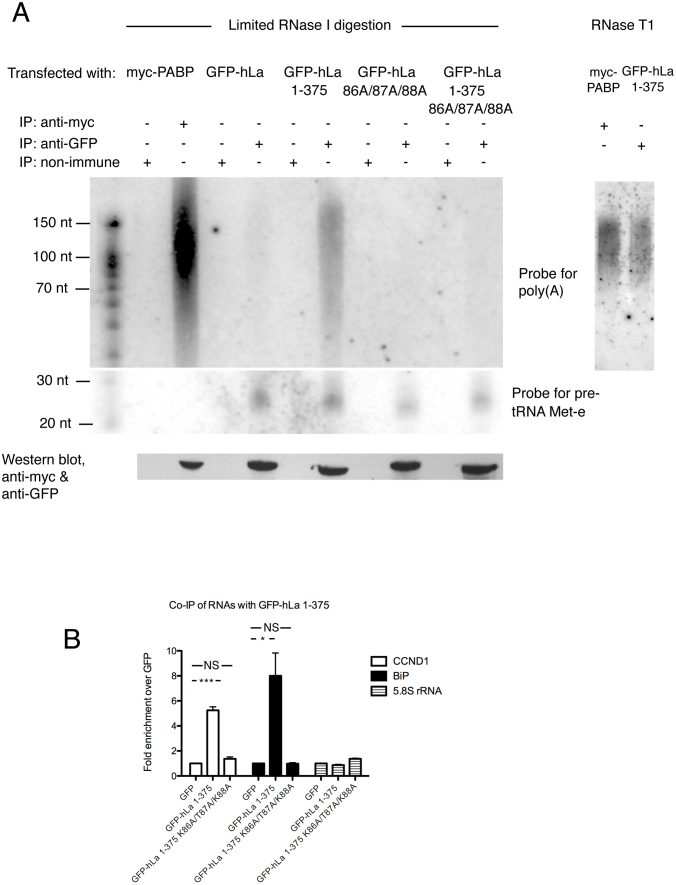
Figure 4.
Human La binds to poly(A) in human cells. (A) Crosslinking immunoprecipitation of transfected myc-PABP and GFP-hLa or GFP-hLa mutants in HEK293T cells. Left: limiting digestion with RNase I; right: Digestion with RNase T1. IP: immunoprecipitation. Presence of poly(A) or pre-tRNA Met-e in immunoprecipitated RNPs was assessed by Northern blot. Bottom: Western blot confirming transfection of myc-PABP or GFP-hLa. (B) Co-immunoprecipitation of cyclin D (CCND1) mRNA, BiP mRNA or 5.8S rRNA with GFP-hLa 1–375 versus GFP-hLa 1-375 K86A/T87A/K88A relative to GFP vector only control as measured by qPCR. Error bars correspond to standard error of the mean, asterisks highlight statistically significant changes (*P-value < 0.05; ***P-value < 0.001 two-tailed Student's t-test).
We observed that PABP was highly effective in pulling down a range of poly(A) RNA lengths relative to the non-immune sera control, consistent with the heterogeneous length of poly(A) tails observed in the cytoplasm. However, PABP was incapable of pulling down a La RNA polymerase III substrate previously demonstrated to be an unusually stable and a very highly abundant hLa target, pre-tRNA Met-e (55,56), as expected. We found that the cytoplasmic GFP-hLa 1–375 immunoprecipitated poly(A) RNA of similar size range as PABP, and to a substantially greater extent than our nuclear GFP-hLa construct, relative to the poly(A) independent hLa target pre-tRNA Met-e. However, the GFP-hLa 1–375 K86A/T87A/K88A mutant was substantially impaired in poly(A) immunoprecipitation compared to GFP-hLa 1–375, despite still pulling down pre-tRNA Met-e. These data are consistent with hLa binding to poly(A) directly in the cytoplasm and further support the importance of the LAM winged helix in binding poly(A) over UUU-3’OH containing substrates. To test the nature of this interaction further, we repeated the CLIP using excess RNase T1, which cuts specifically at ‘G’ residues, as this should separate RNA upstream of the poly(A) tail but leave the poly(A) tail intact. We found that GFP-hLa 1–375 still immunoprecipitated poly(A) signal similar to PABP (Figure 4A, right-hand side), also consistent with hLa 1–375 making contact to the poly(A) tail directly.
To further assess the importance of the winged helix face of the La motif in the binding of previously characterized La mRNA targets, we immunoprecipitated GFP-hLa 1–375 and GFP-hLa 1–375 K86A/T87A/K88A associated transcripts in the context of reversible formaldehyde crosslink and determined their relative abundance using quantitative RT-PCR (Figure 4B). We observed a significant enrichment of GFP-hLa 1–375 with the cyclin D1 and BiP mRNA transcripts relative to the GFP alone control, as expected. However, the ability of GFP-hLa 1–375 to immunoprecipitate these mRNAs was substantially impaired in the context of the K86A/T87A/K88A mutations, and was more similar to the vector control, in agreement with our UV-CLIP data (Figure 4A). As a negative control, we observed no enrichment for the 5.8S rRNA (which lacks a poly(A) tail) relative to the vector control. Thus our immunoprecipitation data suggest that cytoplasmic La binds mRNAs in cells at least in part via the poly(A) tail, and that the winged-helix face of the La motif contributes to this binding, similar to our data in vitro.
The poly(A) binding mode contributes to La entry into polysomes
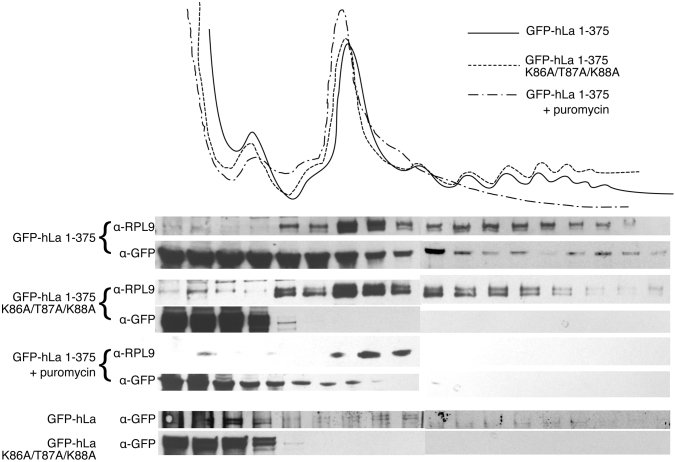
La proteins have been implicated in the translation of several mRNAs and have been previously demonstrated to enter into polysomes (57). We therefore hypothesized that the adenylate binding mode could play an important role in the capacity of La to enter polysomes via contacts to the poly(A) tail. We transfected GFP-hLa and the cytoplasmic GFP-hLa 1–375 into HEK293T cells and performed polysome fractionation followed by Western blot against GFP (Figure 5). We indeed observed entry of GFP-hLa 1–375, and to a lesser extent GFP-hLa, into polysomal fractions, confirming that hLa (and predominantly cytoplasmic hLa) associates with translating polysomes, consistent with previous work (57). To test the importance of the hypothesized poly(A) binding mode, we compared these results to the entry of GFP-hLa K86A/T87A/K88A and GFP-hLa 1–375 K86A/T87A/K88A into polysomal fractions. We observed a substantial decrease in the ability of these mutants to enter into polysomes, and instead observed them in abundance exclusively at the top of the polysome gradient. Addition of puromycin to GFP-hLa 1–375 transfected samples resulted in a collapse of polysomes and a concomitant loss of GFP-hLa 1–375 in the higher molecular weight fractions, confirming that GFP-hLa 1–375 is indeed associating with polysomes. These data are consistent with the winged-helix face of the La motif previously identified as a component of an hLa poly(A) binding mode playing an important role in La engagement of translating mRNAs.
Figure 5.
The poly(A) binding mode promotes hLa entry into polysomes. Top: Trace of ribosomes/polysomes fractionated from HEK293T cells transfected with indicated constructs or treated with puromycin. Bottom: Western blots versus Rpl9 and GFP-hLa, GFP hLa 1–375 and indicated mutants, as well as a puromycin treated control for GFP hLa 1–375.
La binding to poly(A) in cap-dependent and cap-independent translation
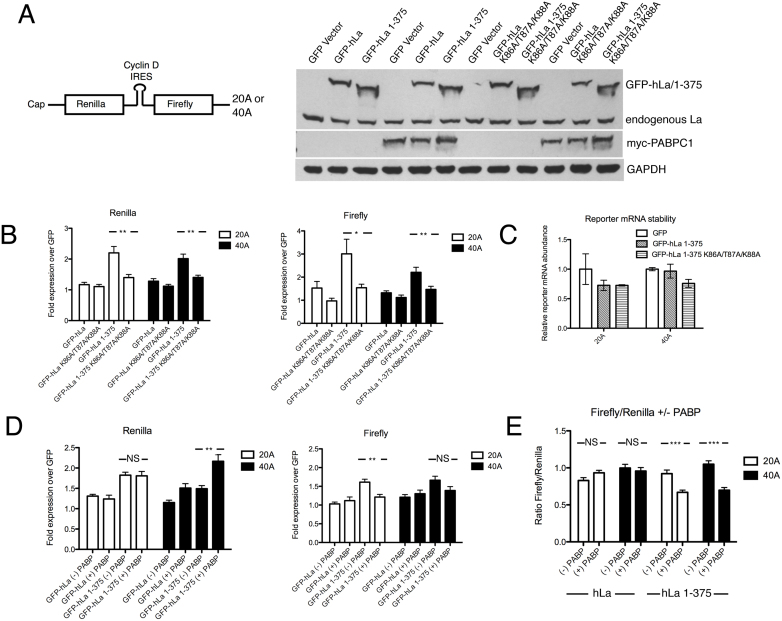
La function has been associated in the enhancement of cap-independent translation, and has been associated with either the enhancement or inhibition of cap-dependent translation depending on the identity of the transcript. To further test the importance of the poly(A) dependent binding mode in translation, we cloned the La-associated IRES from the human cyclin D (CCND1) gene into a dual-luciferase reporter construct, similar to what has been described previously (30), and used this to generate SP6 transcription templates from which we made capped mRNAs for direct transfection into HEK293T cells (Figure 6A). This experimental design allowed us to control the poly(A) tail length of our transfected messages, which we set at twenty or forty adenosines (20A or 40A). These reporter mRNAs were transfected into cells that had been transfected twenty-four hours prior with plasmids encoding GFP-hLa (nuclear), GFP-hLa 1–375 (cytoplasmic) or the poly(A) binding impaired versions of these (GFP-hLa K86A/T87A/K88A or GFP-hLa 1–375 K86A/T87A/K88A), or the GFP vector control, and the effects on cap-dependent and cap-independent translation were assessed. We observed that overexpression of GFP-hLa 1–375 had a substantially greater positive effect on both cap-dependent (renilla) and cap-independent translation (firefly) than GFP-hLa, consistent with cytoplasmic hLa promoting the translation and/or stability of our transfected bicistronic reporter mRNAs (Figure 6B). Notably, GFP-hLa 1–375 K86A/T87A/K88A had a significantly lesser positive effect on translation of both the renilla and firefly cistrons than GFP-hLa 1–375 despite equal levels of GFP-hLa 1–375 K86A/T87A/K88A expression (Figure 6A), consistent with the poly(A) associated binding mode functioning in La enhancement of expression from the reporter. qPCR of the reporter mRNAs eight hours post-transfection revealed that increased expression from the reporter mRNAs was not due to enhanced mRNA stability in the context of GFP-hLa 1–375 transfected relative to GFP or GFP 1–375 K86A/T87A/K88A transfected cells (Figure 6C).
Figure 6.
The human La poly(A) binding mode contributes to La function in translation. (A) Left: Schematic of bicistronic reporter construct used for direct transfection into HEK293T cells. Right: Western blots of transfected myc-PABP, GFP-hLa, GFP-hLa 1–375 or K86A/T87A/K88A mutants of these. GAPDH shown as loading control. (B) Relative expression of cap-dependent (left; renilla) and cap-independent (right; firefly) reporter genes in the presence of overexpressed GFP-hLa, GFP-hLa 1–375 or the K86A/T87A/K88A mutants on the 20A or 40A tailed mRNA reporter constructs normalized to level expression in GFP vector control. (C) Enhanced expression from bicistronic reporter mRNAs upon co-expression of GFP-hLa 1–375 is not due to enhanced reporter mRNA stability. Total RNA was isolated and levels of transfected 20A or 40A bicistronic reporters were assessed by qPCR 8 h post-transfection in cells that had been previously transfected by indicated GFP or GFP-hLa 1–375 constructs. Reporter mRNA levels are provided relative to amounts in the GFP-vector transfected cells after normalization for total RNA abundance via qPCR for the U5 snRNA. (D) Effect of overexpression of myc-PABP on the GFP-hLa and GFP-hLa 1–375 associated expression of cap-dependent (left, renilla) and cap-independent (right, firefly), normalized to the expression levels in the context of the GFP vector control ± overexpression of myc-PABP. (E) Ratios of renilla/luciferase expression in the context of GFP-hLa or GFP-hLa 1–375 expression ± the expression of myc-PABP. (*P-value < 0.05; ** P-value <0.01; ***P-value < 0.001). Error bars: SEM.
We then attempted to further design an experiment that might uncouple the potential positive effects of La on translation from those on mRNA stability. While testing for effects on the expression of mRNAs ± a poly(A) tail in cells is challenging due to the anticipated degradation of messages lacking a poly(A) tail, we hypothesized that if La promotes translation at least in part through engagement of the poly(A) tail, then this might occur in competition with cytoplasmic PABP. We therefore repeated our GFP-hLa and GFP-hLa 1–375 overexpression experiments in the presence and absence of overexpressed myc-PABPC1, bearing in mind that both hLa and PABPC1 have an apparent minimum poly(A) tail binding length: ∼20A for hLa (this work) and 12–25 nt for PABPC1 (58). Co-transfection of myc-PABPC1 resulted in further enhanced expression from the renilla reporter relative to hLa 1–375 for the 40A tailed construct but not the 20A tailed construct (Figure 6D), consistent with both PABPC1 and hLa 1–375 promoting cap-dependent translation on this reporter but with the 20A construct possibly being too short for these factors to act additively. Most importantly for this work, co-transfection of myc-PABPC1 had the opposite effect on cap-independent translation, significantly mitigating GFP-hLa 1–375 associated enhancement of firefly expression for the 20A construct (Figure 6D), as well as the firefly/renilla ratio for both the 20A and 40A constructs (Figure 6E). Since lowering the ratio of cap-independent translation to cap-dependent translation in the presence of excess PABP (Figure 6E) is not expected to result from altered stability of a bicistronic mRNA, this result is consistent with La-dependent enhancement of expression from the IRES reporter being due, at least partially, to La promoting translation at the IRES through contacts to the poly(A) tail. While the specific nature by which PABP and hLa functionally interact in this system remains to be determined, these data further corroborate the link between La-associated function in translation and the poly(A) tail.
DISCUSSION
Human La binds to poly(A) sequences in vitro and in human cells
In this work, we propose that human La binds adenylate sequences with high affinity, and that this binding mode represents at least one important way that La contacts coding RNAs in the cytoplasm. La proteins have been associated extensively with the engagement of mRNAs with consequent effects on their translation, but until this work, it was previously hypothesized that La engages such coding transcripts exclusively via non-specific recognition of structured RNA elements. In this work, we demonstrate that human La has higher affinity for adenylate containing sequences compared to poly(C) or single stranded poly(G) in a length dependent manner. We show that point-mutation of the winged-helix face of the La motif results in decreased binding to A20 but not a UUU-3’OH containing RNA, consistent with the adenylate and UUU-3’OH binding modes being at least partially distinct. Using UV-CLIP, we demonstrate that cytoplasmic La contacts poly(A) tails directly in a manner analogous to PABP in tissue culture cells, then demonstrate that the adenylate binding mode contributes to immunoprecipitation of previously identified La-mRNA targets, La entry in to polysomes, and La function in translation using a bicistronic cap-dependent/IRES reporter containing defined adenylate lengths. While the complete mechanism by which La binds to poly(A) tail and its consequent functional overlap with the role of PABP have not yet been fully explored, our data are consistent with La binding to poly(A) acting as an important determinant for La function in mRNA expression in the cytoplasm.
La function in cap-independent and cap-dependent translation
In the nucleus, La proteins are recruited to nascent RNA polymerase III transcripts, such as pre-tRNAs, via sequence specific recognition of UUU-3’OH (9,10). La then assists these targets attain their native fold via RNA chaperone activity prior to UUU-3’OH processing and removal (14,49,59). In the cytoplasm, La was identified as the first cellular factor important for the enhancement of IRES dependent, cap-independent translation, a theme that has since recurred for a number of viral and cellular IRES containing transcripts, leading to hypotheses that La may also function as an RNA chaperone for these (60). For IRES containing cellular mRNAs that contain a poly(A) tail, our data are consistent with a model in which La recognition of poly(A) may direct La-associated RNA chaperone activity to its mRNA targets in an analogous manner to how UUU-3’OH does the same for nascent RNA polymerase III transcripts. In such a scenario, hLa might remain bound at the poly(A) tail and function as an RNA chaperone by looping the RNA between the poly(A) tail and the 5′UTR/IRES, or hLa could first be recruited to the mRNA via the poly(A) tail and then translocate to these sites. Previous work noting a number of potential RNA binding sites on La support the idea that La may be able to engage the poly(A) tail as well as other regions of an mRNA simultaneously (14–17, 61), but this has yet to be demonstrated formally.
La function in cap-dependent translation appears to be more complex, as various studies have proposed either an activating or inhibitory role for La at the 5′ cap. It has been previously noted that mRNAs whose cap-dependent expression is inhibited by La tend to have short 5′UTRs, such as 5′TOPs, while those that tend to be enhanced often have complex 5′UTRs that may rely on La-dependent RNA remodeling for optimal translation, similar to the role proposed for La at IRESs (62). One factor whose role in the complex interplay coordinating expression at the cap is becoming increasingly appreciated is the La-related protein LARP1. Similar to La, human LARP1 (hLARP1) has also been hypothesized to bind to poly(A) directly (35), as well as via contacts with PABP (36), and reminiscent of La function on coding transcripts, has been associated with both the enhancement and inhibition of cap-dependent translation (38,63). Very recently, a structural domain unique to LARP1 family members and lacking in genuine La proteins, the DM15 region, has been demonstrated to bind the 7-methylguanosine cap in the context of a cytosine at +1, as is commonly observed in 5′TOP sequences (64,65). These insights on LARP1 function in the expression from the cap have recently been expanded by work showing that LARP1 can be phosphorylated directly by mTORC1 and Akt to switch from an inhibitory to an activating role (66), as well other work demonstrating a role for hLARP1 in controlling the stability of 5′TOP mRNAs on 40S ribosomes independent of translation (67), which is reminiscent of other work linking LARP1 family members and mRNA transcript stability (35,68,69). Future work will be required to deconvolute the complex interplay of LARP1, PABP and La at the poly(A) tail and the various effects of these on mRNA translation and degradation.
Poly(A) binding in the LAM superfamily
While La proteins have documented functions for both RNA polymerase III and coding transcripts, each of the various LARP families target only one of these classes: the LARP7 family members hLARP7 and p65 engage RNA polymerase III transcripts, also using a UUU-3’OH dependent RNA binding mode, and the LARP1, LARP4 and LARP6 families are associated with mRNA translation (reviewed in (33,70)). While members from the LARP1, LARP4 and LARP6 families have been documented to interact with PABP (37,39,71,72), several LARPs have also been hypothesized to engage poly(A) directly, with human LARP4 and LARP1 demonstrating affinity for adenylates in a length dependent manner, similar to La (35,39). Consistent with our findings, one study that screened for factors that could be affinity purified in a poly(A) dependent manner identified not only hLARP1, but also human La (35). Future structural work will be very helpful in determining the precise contacts between La and poly(A) and whether LARPs that engage poly(A) use the same LAM surface as in hLa, given the high conservation of the La motif between the La and La-related proteins.
Our data suggest that the hLa LAM and RRM domains are important for both the UUU-3’OH and poly(A) binding modes, but that the respective RNA binding surfaces on these domains do not entirely overlap. In La proteins, the linker region bridging the LAM and RRM domains is flexible in the RNA-free form, but for those LARPs that have been tested, this appears to not be the case (11). Notably, the observation that this linker region is much shorter in La modules of the LARP4 family and in hLARP6 (1,73), as well as the observation of LAM-RRM interdomain contacts in the hLARP7 protein (74), has led to the hypothesis that unlike genuine La, the orientation of the linker region in the LARPs is fixed and may constitute an important RNA binding determinant (73). It will thus be fascinating to investigate whether the flexibility observed between the LAM and RRM of hLa may relate to its capacity to accommodate both the poly(A) and UUU-3’OH binding modes, while the fixation of the orientation between the LAM and RRM in the LARPs is an outcome of their specialization for a single class of target.
Supplementary Material
ACKNOWLEDGEMENTS
We thank O. Rissland for assistance in the CLIP methodology and A. Ashok & R. Maraia for helpful discussions.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Canadian Institutes of Health Research's Institute of Genetics (to M.A.B.). Funding for open access charge: CIHR.
Conflict of interest statement. None declared.
REFERENCES
- 1. Bousquet-Antonelli C., Deragon J.-M.. A comprehensive analysis of the La-motif protein superfamily. RNA. 2009; 15:750–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alspaugh M.A., Tan E.M.. Antibodies to cellular antigens in Sjögren's syndrome. J. Clin. Invest. 1975; 55:1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mattioli M., Reichlin M.. Heterogeneity of RNA protein antigens reactive with sera of patients with systemic lupus erythematosus. Description of a cytoplasmic nonribosomal antigen. Arthritis Rheum. 1974; 17:421–429. [DOI] [PubMed] [Google Scholar]
- 4. Hendrick J.P., Wolin S.L., Rinke J., Lerner M.R., Steitz J.A.. Ro small cytoplasmic ribonucleoproteins are a subclass of La ribonucleoproteins: further characterization of the Ro and La small ribonucleoproteins from uninfected mammalian cells. Mol. Cell. Biol. 1981; 1:1138–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lerner M.R., Boyle J.A., Hardin J.A., Steitz J.A.. Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science. 1981; 211:400–402. [DOI] [PubMed] [Google Scholar]
- 6. Rinke J., Steitz J.A.. Precursor molecules of both human 5S ribosomal RNA and transfer RNAs are bound by a cellular protein reactive with anti-La lupus antibodies. Cell. 1982; 29:149–159. [DOI] [PubMed] [Google Scholar]
- 7. Francoeur A.M., Mathews M.B.. Interaction between VA RNA and the lupus antigen La: formation of a ribonucleoprotein particle in vitro. Proc. Natl. Acad. Sci. U.S.A. 1982; 79:6772–6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lerner M.R., Andrews N.C., Miller G., Steitz J.A.. Two small RNAs encoded by Epstein-Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus. Proc. Natl. Acad. Sci. U.S.A. 1981; 78:805–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stefano J.E. Purified lupus antigen La recognizes an oligouridylate stretch common to the 3′ termini of RNA polymerase III transcripts. Cell. 1984; 36:145–154. [DOI] [PubMed] [Google Scholar]
- 10. Mathews M.B., Francoeur A.M.. La antigen recognizes and binds to the 3′-oligouridylate tail of a small RNA. Mol. Cell. Biol. 1984; 4:1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kotik-Kogan O., Valentine E.R., Sanfelice D., Conte M.R., Curry S.. Structural analysis reveals conformational plasticity in the recognition of RNA 3′ ends by the human La protein. Structure. 2008; 16:852–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Teplova M., Yuan Y.-R., Phan A.T., Malinina L., Ilin S., Teplov A., Patel D.J.. Structural basis for recognition and sequestration of UUU(OH) 3′ temini of nascent RNA polymerase III transcripts by La, a rheumatic disease autoantigen. Mol. Cell. 2006; 21:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maraia R.J., Bayfield M.A.. The La protein-RNA complex surfaces. Mol. Cell. 2006; 21:149–152. [DOI] [PubMed] [Google Scholar]
- 14. Bayfield M.A., Maraia R.J.. Precursor-product discrimination by La protein during tRNA metabolism. Nat. Struct. Mol. Biol. 2009; 16:430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martino L., Pennell S., Kelly G., Bui T.T.T., Kotik-Kogan O., Smerdon S.J., Drake A.F., Curry S., Conte M.R.. Analysis of the interaction with the hepatitis C virus mRNA reveals an alternative mode of RNA recognition by the human La protein. Nucleic Acids Res. 2012; 40:1381–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brown K.A., Sharifi S., Hussain R., Donaldson L., Bayfield M.A., Wilson D.J.. Distinct dynamic modes enable the engagement of dissimilar ligands in a promiscuous atypical RNA recognition motif. Biochemistry. 2016; 55:7141–7150. [DOI] [PubMed] [Google Scholar]
- 17. Kuehnert J., Sommer G., Zierk A.W., Fedarovich A., Brock A., Fedarovich D., Heise T.. Novel RNA chaperone domain of RNA-binding protein La is regulated by AKT phosphorylation. Nucleic Acids Res. 2015; 43:581–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gogakos T., Brown M., Garzia A., Meyer C., Hafner M., Tuschl T.. Characterizing expression and processing of precursor and mature human tRNAs by hydro-tRNAseq and PAR-CLIP. Cell Rep. 2017; 20:1463–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Horke S., Reumann K., Rang A., Heise T.. Molecular characterization of the human La protein.hepatitis B virus RNA.B interaction in vitro. J. Biol. Chem. 2002; 277:34949–34958. [DOI] [PubMed] [Google Scholar]
- 20. Naeeni A.R., Conte M.R., Bayfield M.A.. RNA chaperone activity of human La protein is mediated by variant RNA recognition motif. J. Biol. Chem. 2012; 287:5472–5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vakiloroayaei A., Shah N.S., Oeffinger M., Bayfield M.A.. The RNA chaperone La promotes pre-tRNA maturation via indiscriminate binding of both native and misfolded targets. Nucleic Acids Res. 2017; 45:11341–11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wolin S.L., Cedervall T.. The La protein. Annu. Rev. Biochem. 2002; 71:375–403. [DOI] [PubMed] [Google Scholar]
- 23. Inada M., Guthrie C.. Identification of Lhp1p-associated RNAs by microarray analysis in Saccharomyces cerevisiae reveals association with coding and noncoding RNAs. Proc. Natl. Acad. Sci. U.S.A. 2004; 101:434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Intine R.V., Tenenbaum S.A., Sakulich A.L., Keene J.D., Maraia R.J.. Differential phosphorylation and subcellular localization of La RNPs associated with precursor tRNAs and translation-related mRNAs. Mol. Cell. 2003; 12:1301–1307. [DOI] [PubMed] [Google Scholar]
- 25. Meerovitch K., Pelletier J., Sonenberg N.. A cellular protein that binds to the 5′-noncoding region of poliovirus RNA: implications for internal translation initiation. Genes Dev. 1989; 3:1026–1034. [DOI] [PubMed] [Google Scholar]
- 26. Spriggs K.A., Stoneley M., Bushell M., Willis A.E.. Re-programming of translation following cell stress allows IRES-mediated translation to predominate. Biol. Cell. 2008; 100:27–38. [DOI] [PubMed] [Google Scholar]
- 27. Pellizzoni L., Cardinali B., Lin-Marq N., Mercanti D., Pierandrei-Amaldi P.. A Xenopus laevis homologue of the La autoantigen binds the pyrimidine tract of the 5′ UTR of ribosomal protein mRNAs in vitro: implication of a protein factor in complex formation. J. Mol. Biol. 1996; 259:904–915. [DOI] [PubMed] [Google Scholar]
- 28. Kim Y.K., Back S.H., Rho J., Lee S.H., Jang S.K.. La autoantigen enhances translation of BiP mRNA. Nucleic Acids Res. 2001; 29:5009–5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petz M., Them N., Huber H., Beug H., Mikulits W.. La enhances IRES-mediated translation of laminin B1 during malignant epithelial to mesenchymal transition. Nucleic Acids Res. 2012; 40:290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sommer G., Dittmann J., Kuehnert J., Reumann K., Schwartz P.E., Will H., Coulter B.L., Smith M.T., Heise T.. The RNA-binding protein La contributes to cell proliferation and CCND1 expression. Oncogene. 2011; 30:434–444. [DOI] [PubMed] [Google Scholar]
- 31. Trotta R., Vignudelli T., Candini O., Intine R.V., Pecorari L., Guerzoni C., Santilli G., Byrom M.W., Goldoni S., Ford L.P. et al. BCR/ABL activates mdm2 mRNA translation via the La antigen. Cancer Cell. 2003; 3:145–160. [DOI] [PubMed] [Google Scholar]
- 32. Zhang J., Dinh T.N., Kappeler K., Tsaprailis G., Chen Q.M.. La autoantigen mediates oxidant induced de novo Nrf2 protein translation. Mol. Cell Proteomics. 2012; 11, doi:10.1074/mcp.M111.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bayfield M.A., Yang R., Maraia R.J.. Conserved and divergent features of the structure and function of La and La-related proteins (LARPs). Biochim. Biophys. Acta. 2010; 1799:365–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hussain R.H., Zawawi M., Bayfield M.A.. Conservation of RNA chaperone activity of the human La-related proteins 4, 6 and 7. Nucleic Acids Res. 2013; 41:8715–8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aoki K., Adachi S., Homoto M., Kusano H., Koike K., Natsume T.. LARP1 specifically recognizes the 3′ terminus of poly(A) mRNA. FEBS Lett. 2013; 587:2173–2178. [DOI] [PubMed] [Google Scholar]
- 36. Burrows C., Abd Latip N., Lam S.-J., Carpenter L., Sawicka K., Tzolovsky G., Gabra H., Bushell M., Glover D.M., Willis A.E. et al. The RNA binding protein Larp1 regulates cell division, apoptosis and cell migration. Nucleic Acids Res. 2010; 38:5542–5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schäffler K., Schulz K., Hirmer A., Wiesner J., Grimm M., Sickmann A., Fischer U.. A stimulatory role for the La-related protein 4B in translation. RNA. 2010; 16:1488–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tcherkezian J., Cargnello M., Romeo Y., Huttlin E.L., Lavoie G., Gygi S.P., Roux P.P.. Proteomic analysis of cap-dependent translation identifies LARP1 as a key regulator of 5′TOP mRNA translation. Genes Dev. 2014; 28:357–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang R., Gaidamakov S.A., Xie J., Lee J., Martino L., Kozlov G., Crawford A.K., Russo A.N., Conte M.R., Gehring K. et al. La-related protein 4 binds poly(A), interacts with the poly(A)-binding protein MLLE domain via a variant PAM2w motif, and can promote mRNA stability. Mol. Cell. Biol. 2011; 31:542–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goodier J.L., Fan H., Maraia R.J.. A carboxy-terminal basic region controls RNA polymerase III transcription factor activity of human La protein. Mol. Cell. Biol. 1997; 17:5823–5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. König J., Zarnack K., Rot G., Curk T., Kayikci M., Zupan B., Turner D.J., Luscombe N.M., Ule J.. iCLIP reveals the function of hnRNP particles in splicing at individual nucleotide resolution. Nat. Struct. Mol. Biol. 2010; 17:909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Intine R.V., Dundr M., Misteli T., Maraia R.J.. Aberrant nuclear trafficking of La protein leads to disordered processing of associated precursor tRNAs. Mol. Cell. 2002; 9:1113–1123. [DOI] [PubMed] [Google Scholar]
- 43. Walters R.W., Bradrick S.S., Gromeier M.. Poly(A)-binding protein modulates mRNA susceptibility to cap-dependent miRNA-mediated repression. RNA. 2010; 16:239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Niranjanakumari S., Lasda E., Brazas R., Garcia-Blanco M.A.. Reversible cross-linking combined with immunoprecipitation to study RNA-protein interactions in vivo. Methods. 2002; 26:182–190. [DOI] [PubMed] [Google Scholar]
- 45. Dowling R.J.O., Zakikhani M., Fantus I.G., Pollak M., Sonenberg N.. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007; 67:10804–10812. [DOI] [PubMed] [Google Scholar]
- 46. Luthe D.S. A simple technique for the preparation and storage of sucrose gradients. Anal. Biochem. 1983; 135:230–232. [DOI] [PubMed] [Google Scholar]
- 47. Gao X., Wan J., Liu B., Ma M., Shen B., Qian S.-B.. Quantitative profiling of initiating ribosomes in vivo. Nat. Methods. 2015; 12:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Venkatesan A., Sharma R., Dasgupta A.. Cell cycle regulation of hepatitis C and encephalomyocarditis virus internal ribosome entry site-mediated translation in human embryonic kidney 293 cells. Virus Res. 2003; 94:85–95. [DOI] [PubMed] [Google Scholar]
- 49. Huang Y., Bayfield M.A., Intine R.V., Maraia R.J.. Separate RNA-binding surfaces on the multifunctional La protein mediate distinguishable activities in tRNA maturation. Nat. Struct. Mol. Biol. 2006; 13:611–618. [DOI] [PubMed] [Google Scholar]
- 50. Meerovitch K., Svitkin Y.V., Lee H.S., Lejbkowicz F., Kenan D.J., Chan E.K., Agol V.I., Keene J.D., Sonenberg N.. La autoantigen enhances and corrects aberrant translation of poliovirus RNA in reticulocyte lysate. J. Virol. 1993; 67:3798–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Romero V., Fellows E., Jenne D.E., Andrade F.. Cleavage of La protein by granzyme H induces cytoplasmic translocation and interferes with La-mediated HCV-IRES translational activity. Cell Death Differ. 2009; 16:340–348. [DOI] [PubMed] [Google Scholar]
- 52. Fok V., Friend K., Steitz J.A.. Epstein-Barr virus noncoding RNAs are confined to the nucleus, whereas their partner, the human La protein, undergoes nucleocytoplasmic shuttling. J. Cell Biol. 2006; 173:319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shiroki K., Isoyama T., Kuge S., Ishii T., Ohmi S., Hata S., Suzuki K., Takasaki Y., Nomoto A.. Intracellular redistribution of truncated La protein produced by poliovirus 3Cpro-mediated cleavage. J. Virol. 1999; 73:2193–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Simons F.H., Broers F.J., Van Venrooij W.J., Pruijn G.J.. Characterization of cis-acting signals for nuclear import and retention of the La (SS-B) autoantigen. Exp. Cell Res. 1996; 224:224–236. [DOI] [PubMed] [Google Scholar]
- 55. Harada F., Matsubara M., Kato N.. Stable tRNA precursors in HeLa cells. Nucleic Acids Res. 1984; 12:9263–9269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ali N., Pruijn G.J., Kenan D.J., Keene J.D., Siddiqui A.. Human La antigen is required for the hepatitis C virus internal ribosome entry site-mediated translation. J. Biol. Chem. 2000; 275:27531–27540. [DOI] [PubMed] [Google Scholar]
- 57. Cardinali B., Carissimi C., Gravina P., Pierandrei-Amaldi P.. La protein is associated with terminal oligopyrimidine mRNAs in actively translating polysomes. J. Biol. Chem. 2003; 278:35145–35151. [DOI] [PubMed] [Google Scholar]
- 58. Sachs A.B., Davis R.W., Kornberg R.D.. A single domain of yeast poly(A)-binding protein is necessary and sufficient for RNA binding and cell viability. Mol. Cell. Biol. 1987; 7:3268–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chakshusmathi G., Kim S.D., Rubinson D.A., Wolin S.L.. A La protein requirement for efficient pre-tRNA folding. EMBO J. 2003; 22:6562–6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Holcik M., Sonenberg N.. Translational control in stress and apoptosis. Nat. Rev. Mol. Cell Biol. 2005; 6:318–327. [DOI] [PubMed] [Google Scholar]
- 61. Ehlers I., Horke S., Reumann K., Rang A., Grosse F., Will H., Heise T.. Functional characterization of the interaction between human La and hepatitis B virus RNA. J. Biol. Chem. 2004; 279:43437–43447. [DOI] [PubMed] [Google Scholar]
- 62. Schwartz E.I., Intine R.V., Maraia R.J.. CK2 is responsible for phosphorylation of human La protein serine-366 and can modulate rpL37 5′-terminal oligopyrimidine mRNA metabolism. Mol. Cell. Biol. 2004; 24:9580–9591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fonseca B.D., Zakaria C., Jia J.-J., Graber T.E., Svitkin Y., Tahmasebi S., Healy D., Hoang H.-D., Jensen J.M., Diao I.T. et al. La-related protein 1 (LARP1) represses terminal oligopyrimidine (TOP) mRNA translation downstream of mTOR complex 1 (mTORC1). J. Biol. Chem. 2015; 290:15996–16020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lahr R.M., Fonseca B.D., Ciotti G.E., Al-Ashtal H.A., Jia J.-J., Niklaus M.R., Blagden S.P., Alain T., Berman A.J.. La-related protein 1 (LARP1) binds the mRNA cap, blocking eIF4F assembly on TOP mRNAs. Elife. 2017; 6:e24146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lahr R.M., Mack S.M., Héroux A., Blagden S.P., Bousquet-Antonelli C., Deragon J.-M., Berman A.J.. The La-related protein 1-specific domain repurposes HEAT-like repeats to directly bind a 5′TOP sequence. Nucleic Acids Res. 2015; 43:8077–8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hong S., Freeberg M.A., Han T., Kamath A., Yao Y., Fukuda T., Suzuki T., Kim J.K., Inoki K.. LARP1 functions as a molecular switch for mTORC1-mediated translation of an essential class of mRNAs. Elife. 2017; 6:e25237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gentilella A., Morón-Duran F.D., Fuentes P., Zweig-Rocha G., Riaño-Canalias F., Pelletier J., Ruiz M., Turón G., Castaño J., Tauler A. et al. Autogenous control of 5′TOP mRNA stability by 40S ribosomes. Mol. Cell. 2017; 67:55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Merret R., Descombin J., Juan Y., Favory J.-J., Carpentier M.-C., Chaparro C., Charng Y., Deragon J.-M., Bousquet-Antonelli C.. XRN4 and LARP1 are required for a heat-triggered mRNA decay pathway involved in plant acclimation and survival during thermal stress. Cell Rep. 2013; 5:1279–1293. [DOI] [PubMed] [Google Scholar]
- 69. Hopkins T.G., Mura M., Al-Ashtal H.A., Lahr R.M., Abd-Latip N., Sweeney K., Lu H., Weir J., El-Bahrawy M., Steel J.H. et al. The RNA-binding protein LARP1 is a post-transcriptional regulator of survival and tumorigenesis in ovarian cancer. Nucleic Acids Res. 2016; 44:1227–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Maraia R.J., Mattijssen S., Cruz-Gallardo I., Conte M.R.. The La and related RNA-binding proteins (LARPs): structures, functions, and evolving perspectives. Wiley Interdiscip. Rev. RNA. 2017; 8, doi:10.1002/wrna.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Blagden S.P., Gatt M.K., Archambault V., Lada K., Ichihara K., Lilley K.S., Inoue Y.H., Glover D.M.. Drosophila Larp associates with poly(A)-binding protein and is required for male fertility and syncytial embryo development. Dev. Biol. 2009; 334:186–197. [DOI] [PubMed] [Google Scholar]
- 72. Merret R., Martino L., Bousquet-Antonelli C., Fneich S., Descombin J., Billey E., Conte M.R., Deragon J.-M.. The association of a La module with the PABP-interacting motif PAM2 is a recurrent evolutionary process that led to the neofunctionalization of La-related proteins. RNA. 2013; 19:36–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Martino L., Pennell S., Kelly G., Busi B., Brown P., Atkinson R.A., Salisbury N.J.H., Ooi Z.-H., See K.-W., Smerdon S.J. et al. Synergic interplay of the La motif, RRM1 and the interdomain linker of LARP6 in the recognition of collagen mRNA expands the RNA binding repertoire of the La module. Nucleic Acids Res. 2015; 43:645–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Uchikawa E., Natchiar K.S., Han X., Proux F., Roblin P., Zhang E., Durand A., Klaholz B.P., Dock-Bregeon A.-C.. Structural insight into the mechanism of stabilization of the 7SK small nuclear RNA by LARP7. Nucleic Acids Res. 2015; 43:3373–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.