Abstract
Primary amoebic meningoencephalitis (PAM) is a rare, fulminating, hemorrhagic infection of the brain caused by Naegleria fowleri, a thermophilic, free-living amoeba. A 74-year male presented with sudden severe global headache and fever with features of anomic aphasia. Magnetic resonance imaging (MRI) suggested herpes encephalitis and acyclovir (IV) was started but the patient developed altered sensorium, agitation and progressive weakness of lower limbs with gradual truncal weakness. Repeat MRI showed increase in lesion size and edema with confluent blood areas. Dexamethasone showed significant improvement. Ten days after completion of acyclovir, there was recurrence of altered sensorium with seizures. Repeat MRI showed new lesions appearing. Excisional biopsy of brain confirmed N. fowleri. Amphotericin B and miltefosin was started but patient succumbed to his illness after 10 days. This is a first case of PAM in Nepal, involving elderly immune-competent male without environmental exposure to freshwater, mimicking as herpes encephalitis.
INTRODUCTION
Amoebic encephalitis is a rare disease that can be rapidly fatal if not diagnosed early. Around 300 cases are reported world wide [1]. The main organisms responsible are free-living amoeba of three species: Acanthamoeba, Balamuthia and Naegleria. The first two are known to present as fatal granulomatous amebic encephalitis (GAE) whereas Naegleria mainly presents as primary amoebic meningoencephalitis (PAM) [2]. Naegleria fowleri is naturally found in warm freshwater environments such as lakes, hot springs, sewage, poorly maintained swimming pools, hydrotherapy and remedial pools and soil [3]. The diagnosis is usually missed because of rarity of disease, low suspicion and lack of diagnostic tests [3]. Many cases reported are diagnosed on autopsy and even when diagnosed ante mortem, the reported survival is very low [4] with only few survivors world-wide. We report first case of amoebic encephalitis presenting as herpes encephalitis in a 74-year-old immunocompetent individual with no risk factors from Nepal.
CASE REPORT
A 74-year-old gentleman, otherwise healthy, presented with 4 days history of low grade fever followed by 2 days history of severe global headache. He had features of anomic aphasia but there were no symptoms of vomiting, seizure or other neurological deficit at presentation. Additional medical history was positive for hypertension only. He was not on any immunosuppressant; there was no history of swimming in lifetime and no recent history of travel.
An empirical treatment with intravenous ceftriaxone and dexamethasone was started. A magnetic resonance imaging (MRI) brain was obtain on 4th day of presentation, which showed edema and temporal lobe lesion (T1 hypo and T2 hyper intense) with few areas of micro-hemorrhages suggestive of cerebritis (likely Herpes) (Fig. 1). Intravenous acyclovir was started at 10 mg/kg three times a day on the same day along with phenytoin. Cerebrospinal fluid (CSF) findings are summarized in Table 1. Dexamethasone was stopped with provisional diagnosis of herpes encephalitis and rest continued. Patient developed altered sensorium and agitation on the same day. After 5 days, he started developing gradually progressive weakness of bilateral lower limbs with gradual truncal weakness. A repeat MRI was done which showed increase in size of lesion with confluent blood areas and increase in edema (Fig. 2). Dexamethasone was restarted for weakness and showed rapid improvement (from 1 to 4-power in lower limbs) in weakness and hence was continued. However, his sensorium continued to fluctuate over next 1 week and a repeat CSF was done. In view of non-improvement, anti-tubercular treatment and meropenem was started empirically. He gradually improved over next 1 week and was discharged after completion of 14 days of meropenem and 21 days of acyclovir (Table 2).
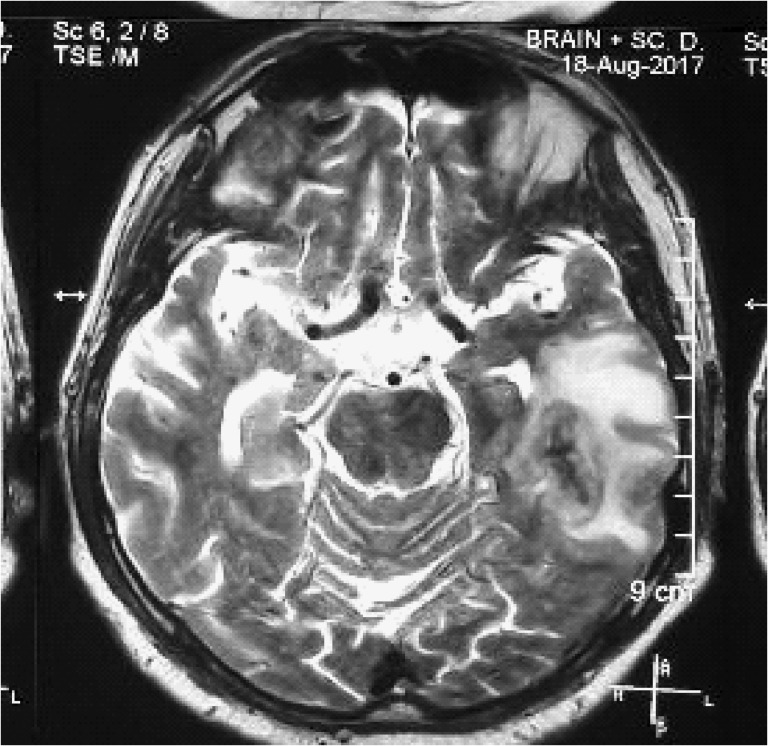
Figure 1:
MRI at presentation: T2 hyper intense lesion at left temporal lobe with minimal enhancement and small bleeding areas consistent with hemorrhagic encephalitis.
Table 1:
CSF findings.
| Parameters | First 14.08.2017 | Second 21.08.2017 |
|---|---|---|
| Total count | 4 | 95 |
| Differential | 85% Lymphocytes | 86% Lymphocytes |
| Protein | 80 mg/dl | 100 mg/dl |
| Sugar | 120 mg/dl | 132 mg/dl |
| Gm stain | Negative | Negative |
| AFB stain | Negative | Negative |
| ADA | 2 | 4 |
| TB PCR | Negative | Negative |
| HSV PCR | Negative | Negative |
| KOH | Negative | |
| ACE level | Negative |
Comparative table of CSF findings at admission and after 1 week of treatment.
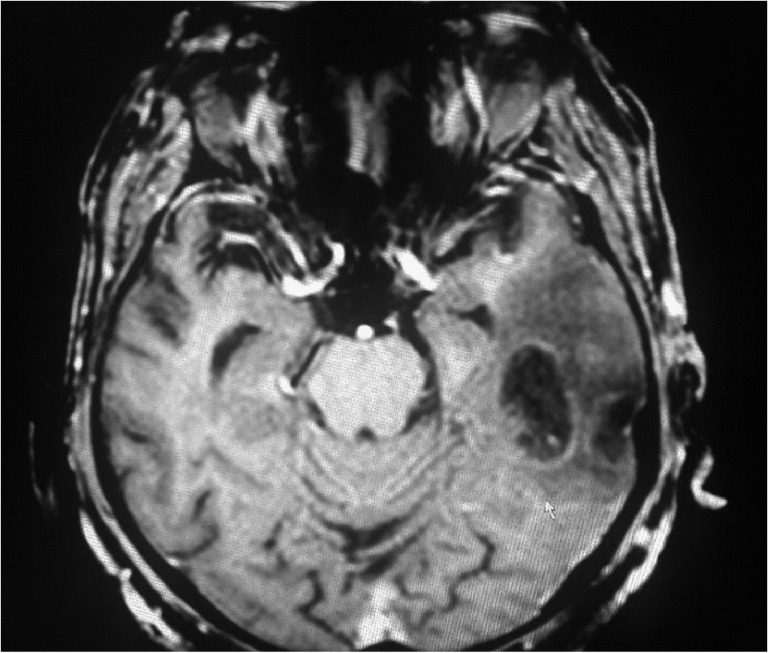
Figure 2:
MRI at 5 days: T1 hypointense lesion increased upto the posterior limb of internal capsule with confluence and enlargement of hemorrhagic areas indicating interval increase in lesion.
Table 2:
Other investigations.
| Investigation | Reports | Remarks |
|---|---|---|
| ANA by (IFA) | Negative | |
| dsDNA (ELISA) | Negative | |
| Anti-Smith Antibody (ELISA) | Negative | |
| Toxoplasma serology | Negative | Both IgG/IgM |
| HIV serology | Negative | Both I and II (repeated) |
| CD4 count/ percentage | 320/45% | Normal |
| Brain tissue TB PCR | Negative | |
| Paragonimia ELISA | Negative | |
| Brain tissue for toxoplasma antibody stain | Negative | |
| Amoebic serology (Entamoeba) | Positive | Common in our population |
| HSV I serology IgG | Positive | Common in our population |
| HSV I serology IgM | Negative |
Next 10 days, he was doing well except for episodes of accelerated hypertension and headache which was managed with antihypertensive. There was no motor deficit except for anomic aphasia. After 10 days, he developed vomiting with slight hematemesis and was rushed back to hospital. Hematemesis did not recur but he again started to develop agitation and altered sensorium. A repeat MRI again showed an increase in lesion with new lesions appearing in right high-parietal area (Fig. 3). Patient’s sensorium deteriorated to E1M5V1 and he was electively intubated and an excisional brain biopsy was done. Histopathology revealed areas of necrosis with some evidence of thrombosed arteries but without any vessel wall involvement or any mitotic pathology. Finally, few slides showed trophozoite from of parasite engulfing red blood cells and it was later confirmed as N. Fowleri by three independent pathologists (Figs 4 and 5). A combination of amphotericin B lipid complex, flucytosine, voriconazole, azithromycin, rifampicin and miltefosine were started. However, by the time diagnosis was established (7 weeks from disease onset), the patient had dilated and unresponsive pupils. After 10 days of treatment, he started having raised bilirubin, deranged renal function and oligura. He developed metabolic acidosis and finally succumbed to his illness.
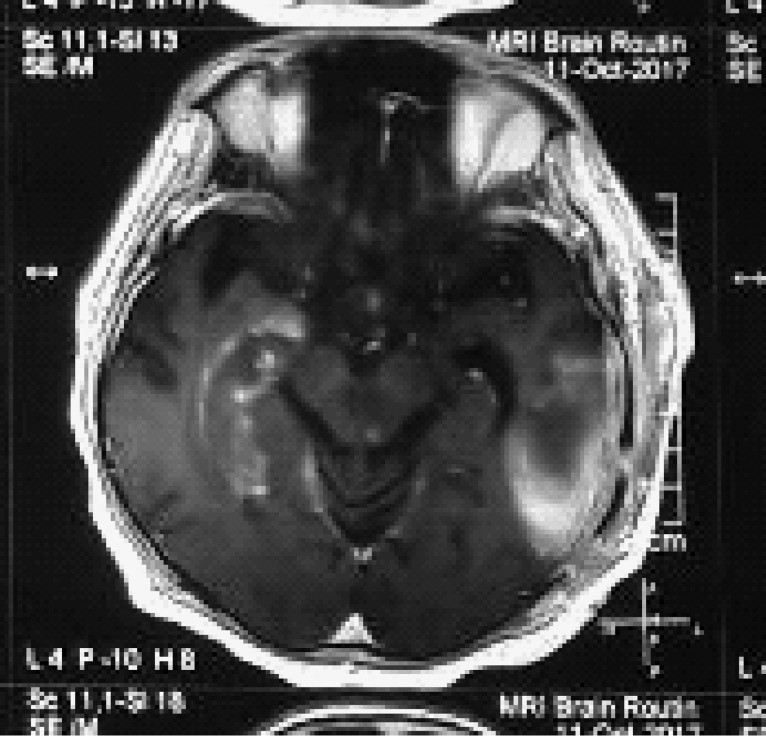
Figure 3:
MRI at 6weeks: persistent lesion in left temporal lobe with appearance of new lesion in right hippocampal and mid brain area.
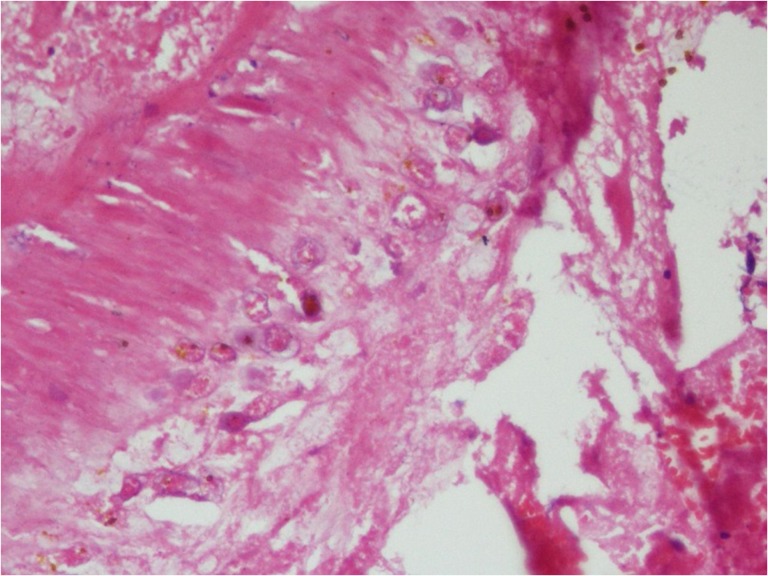
Figure 4:
H&E stain of section showing trophozoites of N.fowleri with engulfed erythrocytes.
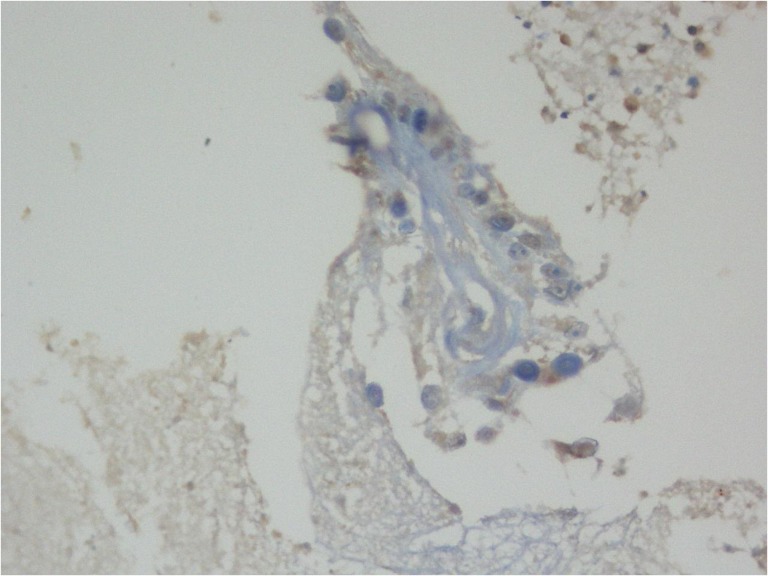
Figure 5:
Toulidine blue stain showing trophozoite with multiple nuclei.
DISCUSSION
Naegleria is a free water living, thermophilic amoeba that can be pathogenic to immunocompetent individuals. Over 30 species have been isolated from soil and water but only N. fowleri has been associated with human disease and the usual port of entry is via olfactory route to the brain through the cribiform plate [2]. PAM is the usual presentation and has been reported to be highly virulent with death within 1 week of onset [4]. Out of 143 PAM infections from 1962 through 2016, there were only four survivors in the USA with 97% mortality [5].
There are no early characteristic clinical or radiological findings that can help in diagnosis of amoebic encephalitis and the mortality is very high unless aggressive treatment is started early in the course of disease. Moreover, our case presented with many atypical features: most of the reported cases affected children and young adults [6] but our patient was an elderly male; there was no history of any exposure to free water; the disease followed a relatively indolent and waxing-waning course and the meningeal signs were absent. Detection of amoeba on wet mount preparation of CSF is considered important aid to the diagnosis [4, 7]. However, though the CSF was done twice, amoeba was not detected due to lack of expertize and low index of clinical suspicion. None of the features typically described in cases of PAM were present in our patient in early phase of disease. The presence of low fever, headache, isolated hemorrhagic temporal lobe lesion and lymphocytic pleocytosis all suggested herpes encephalitis. The waxing and waning course also mimicked natural history of herpes encephalitis adding to the diagnostic confusion.
There is no proven effective treatment protocol for amoebic encephalitis. Amphotericin B is the agent with best effectiveness against Naegleria sp [8]. Though a combination of treatment including amphotericin B and miltefosine was initiated after confirmation of treatment, it was already 7 weeks after diagnosis and the results were unrewarding.
Making diagnostic tests more accessible and epidemiological screening for the presence of pathogenic organism in the water source are important to estimate the risks for the general population. High index of clinical suspicion is very important, especially if the initial diagnostic tests are negative. The disease can even have an indolent and waxing-waning course without definite signs of meningitis.
CONCLUSION
Amoebic encephalitis is a life-threatening condition with rapid course and poor outcome. It should be suspected in early phases of encephalitis if diagnostic tests for other etiologies are negative.
ACKNOWLEDGEMENTS
We are thankful to the clinicians and nurses who managed this patient. The relative to the deceased is also acknowledged for consenting to have this case published.
CONFLICT OF INTEREST STATEMENT
None declared.
ETHICAL APPROVAL
Ethical approval was given by Ethical Review Committee of National Center for Rheumatic Disease via letter number CR-07/17.
FUNDING
No funding was received to publish this case report.
REFERENCES
- 1. De Jonckheere JF. What do we know by now about the genus Naegleria? Exp Parasitol 2014;145:S2–9. [DOI] [PubMed] [Google Scholar]
- 2. Marciano-Cabral F, Cabral G. The immune response to Naegleria fowleri amebae and pathogenesis of infection. FEMS Immunol Med Microbiol 2007;51:243–59. [DOI] [PubMed] [Google Scholar]
- 3. Visvesvara GS. Free-living amebae as opportunistic agents of human disease. J Neuroparasitol 2010;1:6–8. [Google Scholar]
- 4. Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free-living amoebae: Acanthamoebaspp., Balamuthia mandrillaris, Naegleria fowleri and Sappinia diploidea. FEMS Immunol Med Microbiol 2007;50:1–26. [DOI] [PubMed] [Google Scholar]
- 5.CDC: Parasites—Naegleria fowleri—Primary Amebic Meningoencephalitis (PAM)—Amebic Encephalitis. Available from: https://www.cdc.gov/parasites/naegleria/index.html (7 December 2017, date last accessed).
- 6. Yoder JS, Eddy BA, Visvesvara GS, Capewell L, Beach MJ. The epidemiology of primary amoebic meningoencephalitis in the USA, 1962–2008. Epidemiol Infect 2010;138:968–75. [DOI] [PubMed] [Google Scholar]
- 7. Martinez AJ. Free-Living Ameba’s: Natural History, Prevention, Diagnosis, Pathology and Treatment of Disease. Boca Raton, FL: CRC Press Inc., 1985. [Google Scholar]
- 8. Seidel JS, Harmatz P, Visvesvara GS, Cohen A, Edwards J, Turner J. Successful treatment of primary amebic meningoencephalitis. New Engl J Med 1982;306:346–8. [DOI] [PubMed] [Google Scholar]