Abstract
α-Lactalbumin is a whey protein that constitutes approximately 22% of the proteins in human milk and approximately 3.5% of those in bovine milk. Within the mammary gland, α-lactalbumin plays a central role in milk production as part of the lactose synthase complex required for lactose formation, which drives milk volume. It is an important source of bioactive peptides and essential amino acids, including tryptophan, lysine, branched-chain amino acids, and sulfur-containing amino acids, all of which are crucial for infant nutrition. α-Lactalbumin contributes to infant development, and the commercial availability of α-lactalbumin allows infant formulas to be reformulated to have a reduced protein content. Likewise, because of its physical characteristics, which include water solubility and heat stability, α-lactalbumin has the potential to be added to food products as a supplemental protein. It also has potential as a nutritional supplement to support neurological function and sleep in adults, owing to its unique tryptophan content. Other components of α-lactalbumin that may have usefulness in nutritional supplements include the branched-chain amino acid leucine, which promotes protein accretion in skeletal muscle, and bioactive peptides, which possess prebiotic and antibacterial properties. This review describes the characteristics of α-lactalbumin and examines the potential applications of α-lactalbumin for human health.
Keywords: infant formula, leucine, serotonin, sleep, tryptophan
INTRODUCTION
α-Lactalbumin constitutes approximately 22% of total protein and approximately 36% of the whey proteins in human milk and approximately 3.5% of total protein and approximately 17% of whey proteins in bovine milk (Figure 1)1,2. It has an amino acid composition that is high in essential amino acids and comparatively rich in tryptophan, lysine, cysteine, and the branched-chain amino acids (BCAAs) leucine, isoleucine, and valine.3 (Table 1)4. Because of its unique amino acid profile, α-lactalbumin has potential for multiple uses: (1) as a component of infant formulas, to make them more similar to breast milk; (2) as a supplement to promote gastrointestinal health or modulate neurological function, including sleep and depression; and (3) as a therapeutic agent with applications in conditions or diseases such as sarcopenia, mood disorders, seizures, and cancer.
Figure 1.
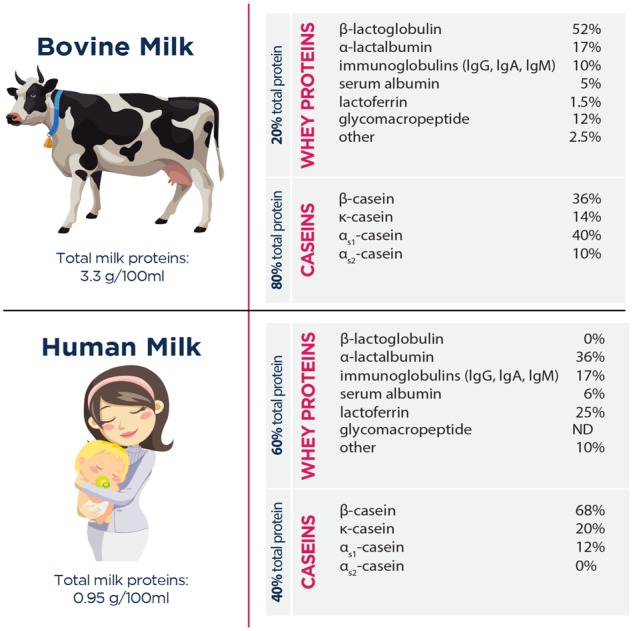
Protein concentrations of whey and casein fractions in bovine and human milk. 1 , 2 Abbreviations: Ig, immunoglobulin; ND, not detectable.
Table 1.
Amino acid composition of isolated proteins
| Amino acid | Milligrams of amino acid per gram of protein |
||||||
|---|---|---|---|---|---|---|---|
| α-LAa | Wheyb | Casein | Eggc | Beefd | Soy | Wheat | |
| Histidine | 30 | 22 | 25 | 26 | 34 | 19 | 20 |
| Isoleucine | 60 | 55 | 47 | 56 | 44 | 49 | 42 |
| Leucine | 108 | 122 | 89 | 94 | 82 | 78 | 68 |
| Lysine | 109 | 112 | 76 | 76 | 90 | 51 | 26 |
| Methionine | 10 | 23 | 26 | 39 | 29 | 13 | 17 |
| Cysteine | 48 | 30 | 3 | 26 | 11 | 12 | 22 |
| Phenylalanine | 41 | 36 | 51 | 66 | 39 | 43 | 58 |
| Threonine | 43 | 45 | 44 | 45 | 44 | 38 | 28 |
| Tryptophan | 48 | 27 | 12 | 17 | 12 | 13 | 9 |
| Valine | 43 | 52 | 59 | 73 | 46 | 47 | 43 |
CHARACTERISTICS OF α-LACTALBUMIN
The composition of milk differs between species. Human milk has a high proportion of whey proteins, with the whey and casein protein fractions constituting approximately 60% and 40% of the total protein, respectively, while bovine milk contains approximately 80% casein (Figure 1). Further, the concentration of whey proteins in human milk changes dramatically over the course of lactation, which does not occur in milk from other species. During the first few days after birth, whey proteins constitute over 90% of total protein, with casein being virtually undetectable. During the first month, the ratio of whey to casein declines to approximately 60:40, and by 100 days postpartum, the ratio approaches 50:50.5
The whey fraction of bovine milk is typically obtained by acid precipitation of casein during cheese production. The whey fraction contains more than 100 proteins, 5 of which make up over 85% of the total6 (Figure 1). In human milk, α-lactalbumin is the predominate protein in whey, followed by lactoferrin and immunoglobulins, whereas in bovine milk, β-lactoglobulin is the predominate protein in whey, followed by α-lactalbumin and immunoglobulins.1,2 Beta-lactoglobulin is absent from human milk.
α-Lactalbumin plays an important role during milk production. It is produced in the epithelial cells of the mammary gland and combines with the enzyme β-1,4-galactosyltransferase to form lactose synthase, which converts glucose and galactose into lactose. Synthesis of lactose is thought to be essential for milk production, creating an osmotic force to draw water into the mammary gland and driving the total volume of milk produced. Human and bovine α-lactalbumins share similar amino acid compositions, having 74% sequence homology and similar bioactivities.7
α-Lactalbumin is the second most abundant protein in bovine-derived whey protein concentrates and whey protein isolates, accounting for 15% to 20% of total protein. It is available commercially as α-lactalbumin–enriched whey protein concentrates obtained by filtration methods, with α-lactalbumin constituting approximately 45% of total protein. Highly purified α-lactalbumin, accounting for more than 93% of total protein, is obtained by ion exchange methods. α-Lactalbumin preparations have physical properties and food system behaviors similar to those of whey protein isolates, including high protein quality, clean flavor profile, high water solubility across a wide pH range (2.0 – 9.0), and heat stability, making them compatible for use in beverages. They are also useful in the development of emulsions, foams, and gels, providing flexibility in product formulation.8,9 These characteristics allow α-lactalbumin to be used in diverse food applications in which high-quality protein is important, including infant formulas, protein-fortified beverages, lactose-free and reduced-carbohydrate foods, and medical foods.
α-LACTALBUMIN AND INFANT NUTRITION
During the almost 100 years that infant formulas have been available, much research has been devoted to improving protein quality.10 The composition of formula has been modified frequently throughout the years in an effort to obtain a nutrient profile similar to that of breast milk. However, breast milk is a complex matrix of nutrients and bioactive compounds with unique digestibility and bioavailability. Breast milk is difficult to duplicate, and the optimum composition of infant formula remains unknown. A more realistic goal is to benchmark the growth and development of formula-fed infants against that of breastfed infants. Adding specific proteins such as α-lactalbumin, which contains essential amino acids, to infant formula may lead to improvements in intestinal health, immune responses, and growth as well as to increased absorption of essential trace elements like iron and zinc, all of which would provide formula-fed infants with benefits similar to those provided by breast milk.11
Growth and development
Human milk is the ideal food for infants because of its unique nutritional characteristics and its high-quality protein. However, because breast milk is not always available, infant formulas were developed as a substitute. Currently, there is no consensus about the optimum protein concentration of infant formulas. The US Food and Drug Administration12 and the Codex Alimentarius13 define the minimum protein-to-energy ratio of infant formulas, on the basis of cow’s milk and soy protein, as 1.8 g/100 kcal and 2.25 g/100 kcal, respectively, but do not address optimum intake. To meet the amino acid requirements of the neonate, the total protein content of most infant formulas (13 – 15 g/L for bovine milk–based formulas and ≈ 18 g/L for soy-based formulas) is higher than that of breast milk (9 – 11 g/L).14 The higher protein concentration of infant formulas compared with breast milk has been suggested to be a source of metabolic stress on tissues such as the liver and kidneys in the still-developing infant.15 It is also thought to be a contributing factor to growth differences observed between formula-fed and breastfed infants.16
Bovine milk has become the most common source of ingredients for infant formula. However, because human milk and bovine milk differ substantially in the ratio of whey to casein proteins, in the levels of specific proteins (Figure 1), and in their amino acid profiles,3 the growth and development of breastfed and formula-fed infants also differs. β-Lactoglobulin, for example, is a protein found in cow’s milk that is absent from human milk, and α-lactalbumin, the predominant protein in breast milk, is found in low concentrations in bovine milk. Furthermore, the amounts of the amino acids tryptophan and cysteine in bovine milk are approximately half those in human milk.3,10 As a result, in order to maintain plasma concentrations of essential amino acids similar to or higher than those in breastfed infants, the protein content of standard cow’s milk formula (13 – 15 g/L) is greater than that of breast milk.16 However, infants fed formulas with higher protein contents gain weight more quickly than breastfed infants. Excessive weight gain and metabolic programming during infancy are thought to make infants more susceptible to additional weight gain and obesity later in life.17
Tryptophan is one of the most limiting amino acids in food proteins, and plasma tryptophan is believed to be an important marker of protein adequacy of infant formulas.18 As the precursor to the neurotransmitter serotonin, oral tryptophan has repeatedly been linked to central nervous system functions such as appetite, sleep, memory and learning, temperature regulation, mood, behavior, and maturation of neurons and synaptic connections.19 Increasing the proportion of α-lactalbumin in infant formula while reducing total protein content is beneficial because α-lactalbumin has comparatively high tryptophan content (Table 1) and high protein quality. Clinical studies have shown that lowering the protein content of infant formula while increasing the proportion of α-lactalbumin can result in plasma concentrations of essential amino acids similar to those in breastfed infants.10
Since excessive protein in infant formulas is associated with potential risks, enriching formula with α-lactalbumin makes it possible to reduce the protein content of formula while also providing the required amino acids. Compared with whey-based, nonenriched formulas, α-lactalbumin–enriched formulas support normal growth and maintain adequate concentrations of plasma amino acids at lower protein intakes. In a study in which 134 infants aged 14 days or younger were fed either a control formula or a reduced-protein, α-lactalbumin–enriched formula for 12 weeks, growth and serum albumin (a clinical biomarker of protein status) were comparable in both groups, despite the lower protein content of the experimental formula.20
In a double-blind randomized controlled trial, Sandström et al21 fed infants standard formula or α-lactalbumin–enriched formula (25% of total protein vs 11% in the standard formula) from 6 weeks to 6 months of age and compared them with breastfed infants. The protein content of each formula was 13.1 g/L. Compared with infants fed the standard formula, infants fed the α-lactalbumin–enriched formula had a growth pattern more similar to that of breastfed infants and plasma amino acid concentrations similar to or higher than those of breastfed infants. In a similar study, formula-fed infants were fed either standard infant formula (protein, 15 g/L) or an experimental formula with added α-lactalbumin (2.2 g/L vs 1.3 g/L in standard formula) and lower protein content (14 g/L) for 120 days. Both groups showed age-appropriate growth, head circumference, and plasma essential amino acid concentrations. However, infants randomized to the α-lactalbumin–enriched formula had growth outcomes more similar to those observed in breastfed infants than in infants fed the standard formula.17 These studies demonstrate the benefits of using infant formulas enriched with α-lactalbumin.
Regulation of sleep/wake cycles
Sleep in the newborn is essential for proper development of the brain, and plasma tryptophan concentrations have been shown to influence sleep patterns.22 Research suggests that feeding infants formulas supplemented with tryptophan improves sleep in infants, which could influence neurobehavioral development.23 Dietary tryptophan influences the synthesis of both the neurotransmitter serotonin in the brain24 (Figure 2) and the hormone melatonin in the intestines,25 which are involved in regulating sleep.26 The mean concentration of tryptophan in breast milk is about 2.5% (2.5 g per 100 g of protein), whereas standard formulas contain only 1.0% to 1.5% (1.0–1.5 g per 100 g of protein).27 Furthermore, tryptophan levels in breast milk are maximal during the night.28 As expected, plasma tryptophan levels were found to be lower in formula-fed infants than in breastfed infants.23
Figure 2.
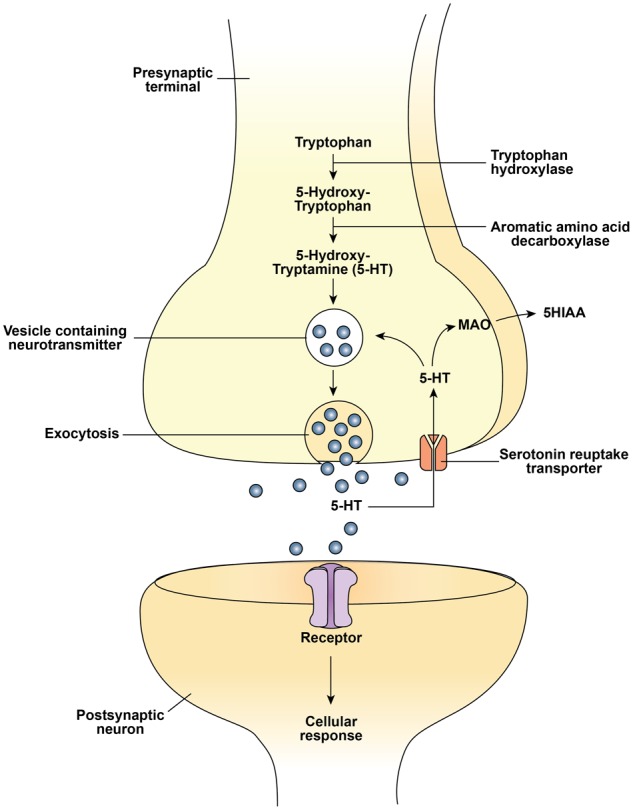
Serotonin synthesis and function in the neuron. Abbreviations: 5-HT, 5-hydroxytryptamine (serotonin); MAO, monoamino oxidase; 5HIAA, 5-hydroxyindole acetic acid.
Serotonin does not cross the blood–brain barrier, so it must be synthesized within the brain, and synthesis is in large part dependent on the availability of tryptophan.24 Tryptophan is transported into the brain by the large neutral amino acid (LNAA) carrier, which also transports the BCAAs (leucine, isoleucine, and valine), phenylalanine, and tyrosine. Transport is competitive, and the LNAA carrier is almost fully saturated at normal blood levels of the LNAAs.29,30 Hence, tryptophan uptake into the brain depends not only on tryptophan concentrations in blood but also on the concentrations of tryptophan’s LNAA transport competitors (ie, the ratio of tryptophan to other LNAAs). Brain uptake of tryptophan (and serotonin synthesis) can therefore be modified by altering either the blood tryptophan levels or the levels of other LNAAs in blood.29 The importance of this relationship was demonstrated in an early study. Twenty healthy newborns (aged 2–3 days) were randomly assigned to receive either formula or a glucose solution containing tryptophan or valine. The infants fed the tryptophan solution entered active sleep sooner than the formula-fed infants, while infants fed the valine solution entered sleep much later. The results demonstrate that the amino acid composition of the formula can influence sleep behavior in newborns.31
Oral administration of tryptophan to infants has been shown to increase urinary levels of serotonin and melatonin,32 and, similar to findings in rats, administration of tryptophan at night is known to increase circulating concentrations of both serotonin and melatonin.33 Tryptophan is necessary for synchronization of the wake/sleep cycle in infants, and feeding a tryptophan-supplemented formula at night was shown to significantly improve the development of the wake/sleep cycle.32 In the few studies conducted in infants, who have somewhat different sleep characteristics than adults, tryptophan administration was reported to reduce sleep latency (the time required to fall asleep),23 lengthen both rapid eye movement (REM) sleep (when most dreaming occurs) and slow-wave sleep (ie, deep sleep) periods,31,32,34 and improve sleep efficiency (total length of time asleep).32 Studies in infants fed a α-lactalbumin–enriched formula demonstrate plasma profiles of essential amino acids similar to those observed in breastfed infants10,17; however, the effect of α-lactalbumin–enriched formulas on infant sleep patterns has not been examined.
Gut development and immunity
The human neonatal gut is immature in both cellular structure and immune function. Breast milk provides essential nutrients and bioactive compounds to facilitate gut development.35,36 Protein is a major component of these functional nutrients, and breast milk contains over 400 different proteins.36,37 Whey proteins represent the largest fraction of breast milk proteins (Figure 1), with the predominate proteins being α-lactalbumin, lactoferrin, immunoglobulins, and lysozyme. These proteins, along with the associated amino acids and peptides released during digestion, exert diverse physiological effects on gastrointestinal function, including motility and antimicrobial activity.
The specific effect of α-lactalbumin on gut development has not been fully elucidated. Evidence suggests that benefits likely derive, at least in part, from bioactive peptides, from the unique content of tryptophan and cysteine,3 and, potentially, from the activity of post-translational modifications, including disulfide bridges38 or glycosylation.39 Tryptophan, the direct precursor to serotonin, has regulatory roles in the gut,40 and cysteine is the direct precursor to the anti-inflammatory, antioxidant glutathione.41 Furthermore, tryptophan is degraded through the kynurenine pathway, producing glutamate and quinolinic acid, both reported to influence intestinal motility and immune competence.42 Bioactive peptides can be released during normal digestion in the relatively immature digestive tract of the infant or produced by commercial processing of whey proteins using techniques such as enzymatic hydrolysis or by microbial fermentation. The physiological significance of these peptides remains largely unknown.43
Beyond its role in central nervous system function, serotonin has diverse roles in regulating food intake and energy balance, in gastrointestinal and endocrine function, and in cardiovascular and pulmonary physiology.40 Approximately 1% to 2% of daily tryptophan intake is converted into serotonin, with 95% of serotonin synthesized outside the central nervous system, mostly within the enterochromaffin cells of the gastric endothelium.40 These cells release serotonin upon exposure to food, stimulating gastrointestinal motility and secretions. Oral administration of free tryptophan stimulates gut synthesis of serotonin.44 Approximately 95% of daily tryptophan intake is degraded through the kynurenine pathway, located predominately in the liver and small intestine.42 Recent evidence suggests the involvement of kynurenine metabolites in intestinal motility, although the mechanism behind this is unknown. In addition, quinolinic acid is recognized to be immunoprotective in macrophages.42 At present, experimental evaluation of the effects of α-lactalbumin on gut development has been largely focused on changes in the gut microbiome and on antibacterial actions.18,45
Dupont et al46 compared α-lactalbumin–enriched formula with standard formula in 66 infants with colic aged 3 weeks to 3 months in a double-blind randomized controlled trial. The standard formula contained protein at 15 g/L and the α-lactalbumin–enriched formula provided 14 g/L. Growth was similar in the 2 groups, but feeding-related gastrointestinal adverse events were significantly lower in the group fed the α-lactalbumin–enriched formula. However, the experimental formula was also supplemented with probiotics, and thus it is not possible to determine which component produced the beneficial effects.
Ushida et al47 reported α-lactalbumin to have protective effects on gastric tissue in young rats. α-Lactalbumin administered by oral intubation increased prostaglandin production in gastric tissue, increasing both mucin production and bicarbonate secretion into the protective mucin layer.
α-Lactalbumin is digested in the small intestine, where amino acids are absorbed both individually and as small peptides. No intact α-lactalbumin is found in the stool of breastfed infants. Several peptides released from α-lactalbumin during digestion have been proposed to elicit biological effects.48 An immunostimulatory tripeptide (Gly-Leu-Phe) was originally isolated following in vitro digestion of milk proteins by trypsin.49 This peptide, present in both human and bovine α-lactalbumin, has been shown to stimulate phagocytic activity of macrophages in vitro and to exert a protective effect against Klebsiella pneumoniae infection in mice.43 It also stimulates phagocytosis by human macrophages.50 Other peptides have been shown to have prebiotic activity by stimulating the growth of bifidobacteria.51 The microbiome of breastfed infants is dominated by Lactobacillus and, in particular, a diverse group of Bifidobacterium species,52 while the microbiome of formula-fed infants has a composition more similar to that of adults.53 Bifidobacteria are thought to protect infants from certain gastrointestinal diseases caused by a variety of intestinal pathogens.54
Antibacterial peptides active against Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus, Staphylococcus epidermis, streptococci, and Candida albicans may also be formed during digestion of α-lactalbumin.38 Peptide fractions from α-lactalbumin have been shown to prevent enteropathogenic Escherichia coli, Salmonella typhimurium, and Shigella flexneri from adhering to intestinal cells and thus may prevent infection. These small peptides likely resist further digestion and exert their antibacterial activity in the colon. This may explain the inhibitory effect of α-lactalbumin–supplemented infant formula on enteropathogenic E. coli–induced diarrhea in infant rhesus monkeys.55 The antimicrobial activity of α-lactalbumin peptides may be associated, at least in part, with the 4 disulfide bridges within α-lactalbumin.38 Trypsin digestion of α-lactalbumin yielded 2 pentapeptides linked by a disulfide bridge, which exhibited antimicrobial properties, while the individual peptides showed no antimicrobial activity.38 Dairy proteins, in general, and α-lactalbumin and its peptides, specifically, have been shown to affect the gut microbiome, which in turn could potentially affect immune responses. Elucidating the effect of dietary proteins and infant formulas on the gut microbiome and innate immunity is a critical area for future research.
Mineral absorption
A sufficient supply of trace minerals, such as iron and zinc, is essential during the rapid growth of infants. Infant formulas often contain trace mineral concentrations higher than those in human milk to compensate for lower bioavailability.56 α-Lactalbumin has 2 binding sites for calcium, 1 of which may be occupied by iron or zinc. Studies in infant rhesus monkeys and in human infants report that α-lactalbumin may increase absorption of zinc, but not iron.21,57,58 However, it has also been reported that peptides released during digestion of α-lactalbumin have a high affinity for iron59 and that peptide–iron complexes formed in vitro can promote iron uptake by human intestinal Caco-2 cells.60 A short-term (14 days) absorption study in human infants found no such enhancing effect. It is possible that an enhancing effect of α-lactalbumin was too small to detect during the short period studied and that an improvement in iron status would be noted during a longer period of consumption.21,61 Furthermore, α-lactalbumin stimulates the growth of beneficial microorganisms like bifidobacteria, which reduce pH in the intestinal tract and thereby may enhance mineral absorption.18 Clinical studies of α-lactalbumin–enriched infant formulas have largely evaluated infant growth, acceptability by infants, and levels of plasma amino acids following ingestion. Future studies need to assess specific effects of α-lactalbumin on immune function, gut microflora, and trace element status.
α-LACTALBUMIN AND ADULT NUTRITION
Whey protein isolates, a rich source of essential amino acids, have been used extensively in adult supplements because of their palatability, water solubility, and heat stability as well as their nutritional properties. Perhaps most notable, whey proteins are recognized as an excellent source of the BCAA leucine, which has a unique role in postmeal stimulation of muscle protein synthesis via the mechanistic target of rapamycin complex 1 (mTORC1) anabolic signal cascade. In addition to their direct effect in activating mTORC1, whey protein isolates contain high levels of essential amino acids, which support optimal muscle protein anabolism with lower protein intake and reduced total calories. Because of these qualities, whey proteins are used as supplements for athletes and for individuals susceptible to declining skeletal muscle mass during weight loss, bed rest, and aging.62–64
While whey protein isolate is widely used in adult nutrition, specific whey proteins, such as α-lactalbumin, have received relatively little attention for use in adult nutrition, in part because it is difficult to obtain purified proteins with the processes used for isolation. However, recent advances in large-scale ion exchange and filtration methods have led to highly purified α-lactalbumin (> 93%) that is free of other proteins, lipids, and lactose. High-purity α-lactalbumin allows researchers to further evaluate the nutritional value of α-lactalbumin as well as its unique amino acid profile (Table 1) and bioactive peptides. Currently, most research on α-lactalbumin for adult nutrition has focused on whether the high tryptophan content of α-lactalbumin can be leveraged to modify neurological or behavioral outcomes such as sleep and mood.
Tryptophan and brain responses
Serotonin (5-hydroxytryptamine) is a monoamine found in both the central nervous system and diverse peripheral tissues, including the lung, kidney, and gastrointestinal tract.42 It is synthesized from tryptophan in a 2-step process involving hydroxylation followed by decarboxylation (Figure 2). The initial enzyme in the pathway, tryptophan hydroxylase, catalyzes the conversion of tryptophan to serotonin. This step is rate limiting in the pathway, and tryptophan hydroxylase is only partly saturated with substrate at normal levels of tryptophan in brain. Hence, raising brain tryptophan levels increases the saturation of hydroxylase and accelerates the synthesis of serotonin.24
The regulation of brain tryptophan levels and the competitive transport of tryptophan across the blood–brain barrier were originally observed in a study to investigate why blood and brain tryptophan levels in rats rose shortly after intake of a meal containing carbohydrates,65 yet brain levels did not increase in animals that ingested a meal that contained both carbohydrate and protein (casein), despite larger increases in blood tryptophan concentrations.66 The protein effect was shown to be due to large increases in the blood levels of the other LNAAs, relative to the rise in blood tryptophan, which competitively limited tryptophan access to the LNAA transporters at the blood–brain barrier.66 The ratio of the plasma concentration of tryptophan to the summed concentrations of the other LNAAs has proven surprisingly accurate in predicting changes in brain tryptophan levels.67
α-Lactalbumin and brain tryptophan
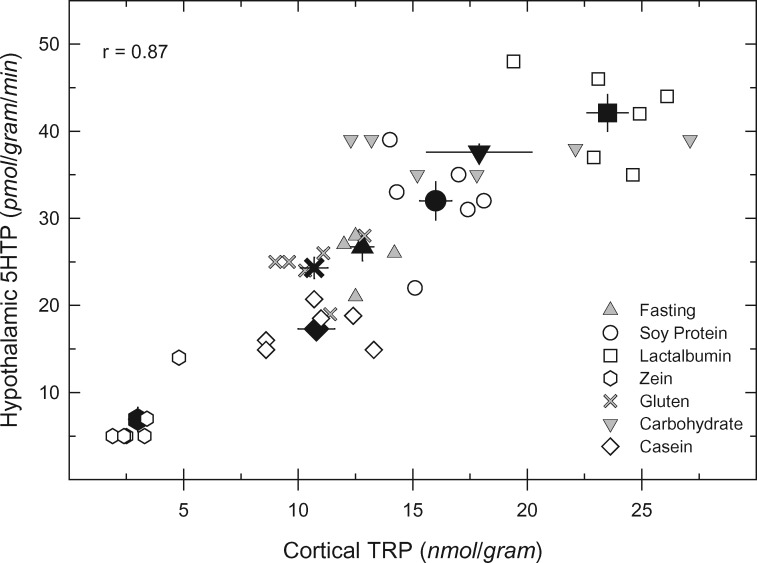
With regard to the effects of dietary protein on brain uptake of tryptophan, it was assumed for many years that the ingestion of any protein would produce no rise in brain tryptophan levels, despite the increases in blood tryptophan levels, since the sum of the other LNAAs was always larger and thus the ratio of tryptophan to LNAAs would not increase in blood.24 This assumption was dispelled by the report of Markus et al in 2000,68 who noted a rise in the ratio of tryptophan to LNAAs in serum from human study participants who ingested a meal containing α-lactalbumin. Shortly thereafter, Feurte et al69 observed that a diet containing α-lactalbumin increased the ratio of tryptophan to LNAAs in plasma from rats significantly more than a casein-containing diet. Orosco et al70 then showed that serotonin released directly from brain neurons was higher in rats fed α-lactalbumin than in those fed a casein diet. Subsequently, Choi et al71 examined the responses of rats to different dietary proteins and found that brain tryptophan levels and rates of serotonin synthesis were tightly correlated with the serum ratio of tryptophan to LNAAs. Of particular interest, a meal containing “lactalbumin” (a partially purified extract of whey protein) elicited substantially higher increases in the serum ratio of tryptophan to LNAAs, in brain tryptophan concentrations, and in the rate of serotonin synthesis compared with meals containing other proteins (Figure 3).71
Figure 3.
Correlation between cortical tryptophan concentration and rate of hypothalamic 5HTP synthesis (an in vivo estimate of serotonin synthesis) in rats ingesting meals containing different proteins. White (open) symbols are data from individual rats in each treatment group (symbols defined in figure); black symbols are mean values for each group, and bars are standard errors of the mean. The value of the linear correlation coefficient (r) is indicated. In this experiment, groups of 6 male rats were fasted during the light period, and, at onset of dark, given free access to a meal containing 1 of the proteins (17% by weight), a meal lacking protein (“carbohydrates”), or nothing (“fasting”). Two hours into the dark period, all rats received an injection of m-hydroxybenzylhydrazine (100 mg/kg IP, to allow measurement of 5HTP synthesis rate) and were killed 30 min later. From Choi et al71 (with permission). Abbreviations: TRP, tryptophan; 5HTP, 5-hydroxytryptophan.
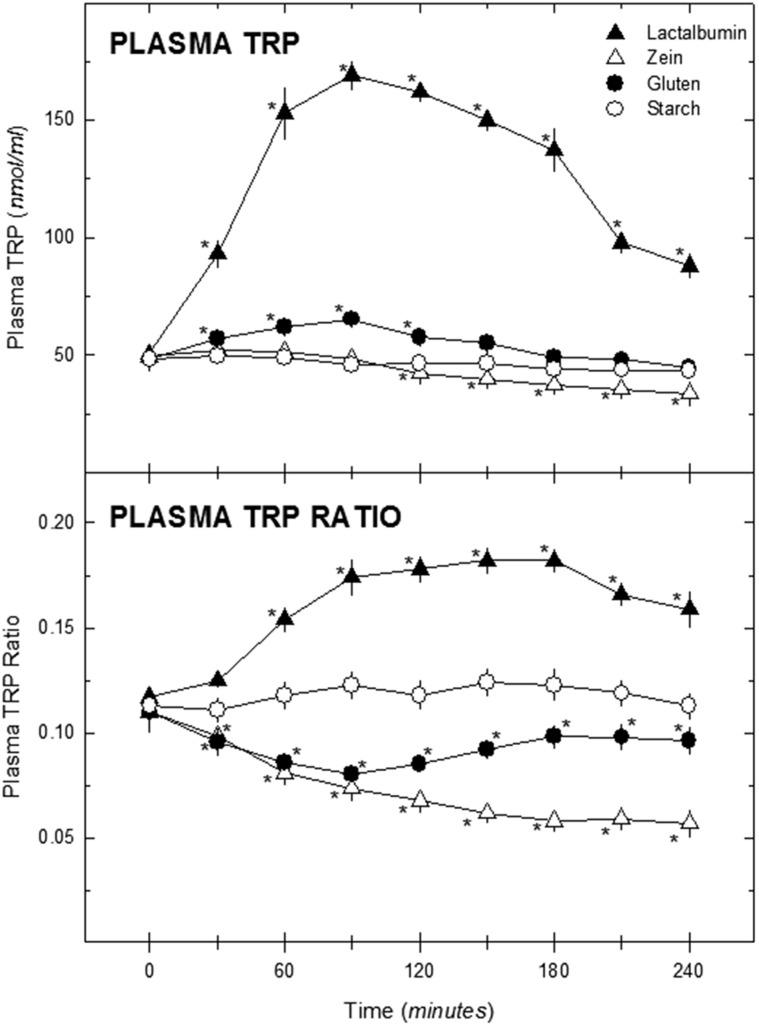
These findings led Fernstrom et al30 to conduct a small trial in healthy human study participants (n = 6) fed a protein-containing beverage after an overnight fast to determine if similar changes in the plasma ratio of tryptophan to LNAAs would occur in humans. Three proteins, α-lactalbumin, gluten, and zein, were selected on the basis of previous findings in animal models showing these proteins to increase, to produce no change, or to decrease the ratio of tryptophan to LNAAs, respectively.71 The beverage base was V-8 Vegetable Juice (500 mL, 102 kcal), to which 40 g of protein or cornstarch (total energy content, 262 kcal) was added. The results for each protein paralleled the findings in animals.71 First, the ingestion of the α-lactalbumin elicited a marked increase in plasma tryptophan concentrations, with values increasing more than 3-fold and remaining elevated for approximately 3 hours (Figure 4).30 The ratio of plasma tryptophan to LNAAs rose about 50% above baseline and remained elevated for at least 4 hours. Ingestion of the cornstarch beverage caused no change in plasma tryptophan or the ratio of tryptophan to LNAAs (rats differ from humans in this regard72), while ingestion of the gluten- or zein-containing drinks elicited a small, transient decline and a larger, prolonged reduction in the ratio of tryptophan to LNAAs, respectively. This study provided proof of concept that ingestion of different dietary proteins can produce markedly different effects on the plasma ratio of tryptophan to LNAAs in humans and, presumably, changes in brain tryptophan concentrations and serotonin synthesis.73
Figure 4.
Effect of protein ingestion on plasma tryptophan concentrations and the plasma ratio of tryptophan to large neutral amino acid in human study participants. Fasting male participants (n = 6) ingested a 16-oz drink containing 40 g of the indicated protein on 4 separate occasions. Blood samples were collected at the indicated times thereafter (the first sample was taken before ingesting the drink). Data are presented as means ± standard errors of the means. For plasma tryptophan, the effects of protein (F = 80.36, df = 3) and time (F = 30.49, df = 8) were significant (P < 0.001), and the interaction (F = 26.21, df = 24) was also significant (P < 0.001). For the plasma tryptophan ratio, the effects of protein (F = 81.95, df = 3; P < 0.001) and time (F = 2.48, df = 8, P < 0.05) were significant, and the interaction (F = 46.17, df = 24) was also significant (P < 0.01). *P < 0.05 vs value at time 0. From Fernstrom et al30 (with permission). Abbreviation: TRP, tryptophan.
If ingestion of moderate amounts of α-lactalbumin leads to increases in serotonin synthesis and release from brain neurons, a reasonable extension of these findings is to consider beneficial or therapeutic applications to influence serotonin-linked brain functions. While specific research of α-lactalbumin and brain function is limited, some insight into potential benefits can be gained from previous studies of the effect of tryptophan on blood pressure, pituitary hormone secretion, sleep, and mood.24,74,75 The best-characterized therapeutic uses of tryptophan are for sleep and mood, with some evidence suggesting a role for tryptophan in epilepsy control.
Tryptophan and sleep
Sleep deprivation is a stress that affects physiological and cognitive functions ranging from increased blood pressure, weakened immune response, and weight gain to cognitive dysfunction, memory problems, and depression. An estimated 50 to 70 million US adults have a sleep disorder.76 Interest in the link between tryptophan, serotonin, and sleep began in the 1960s following observations of sleepiness in normal study participants who ingested tryptophan77 and in patients given mood-altering drugs that modified neuronal serotonin release.78 Later, animal studies examined the effect on sleep of a variety of treatments that modified brain serotonin levels.79 In general, treatments that reduced serotonin tended to disrupt features of sleep, while those that raised serotonin enhanced sleep.79 Similar observations were made in humans, though there were some differences.80,81 With these preliminary findings, researchers began to study the effects of tryptophan on sleep and the production of serotonin in brain.82–84
In adults, tryptophan supplements have been reported to improve sleep, increasing total sleep time and reducing waking time in individuals with and without insomnia.84–86 Tryptophan supplements were also found to modify specific sleep stages (eg, to increase slow-wave sleep, or to increase REM sleep).83–85 Single doses of tryptophan (taken at bedtime) ranged from small (1 g) to very large (15 g) and were administered acutely or chronically for periods of up to 2 years. Tryptophan generally appears to improve sleep, but the specific improvements are not uniform across studies. Consistent with these findings, depletion of serotonin in the brain, studied using the “acute tryptophan depletion paradigm,” a method for rapidly depleting the brain of tryptophan and serotonin,73 predictably produces sleep effects opposite of those elicited by tryptophan administration, including reduced REM latency, increased REM sleep time, reduced total sleep time, and increased sleep latency.87
Ingestion of α-lactalbumin, which has a high proportion of tryptophan to LNAAs, might be expected to produce effects like those seen following the ingestion of tryptophan. In support of this hypothesis, Markus et al88 examined 14 healthy adults with mild sleep complaints and 14 adults without sleep complaints. Each participant was given 2 nighttime protein shakes (at 18:30 and 19:30), each containing either 20 g of α-lactalbumin (4.8 g of tryptophan per 100 g) or 20 g of casein (1.4 g of tryptophan per 100 g), in a crossover design. At 2 hours after consumption of the second shake, the α-lactalbumin shakes produced higher plasma ratios of tryptophan to LNAAs than did the casein shakes, suggesting that serotonin levels were probably higher after consumption of the α-lactalbumin shakes. There was no detectable difference in sleep quality or duration between trials, but individuals receiving the shake with higher tryptophan content reported less morning sleepiness, and those with prior sleep complaints demonstrated improved daytime alertness and attention. The investigators suggested that the reduced sleepiness and improved alertness after α-lactalbumin ingestion most likely indicated that study participants had slept better because of presumed increases in serotonin.
Finally, the effects of tryptophan on sleep may also involve the hormone melatonin. Melatonin is synthesized in a variety of cell types in the body, including the enteroendocrine cells of the gastrointestinal tract.89 Tryptophan is the precursor of melatonin, and the pathway follows the normal route to serotonin, which is then N-acetylated and O-methylated to melatonin. Melatonin synthesis and release by gut enteroendocrine cells is stimulated by oral intake of tryptophan, leading to elevated blood melatonin levels.25 As a fat-soluble hormone, melatonin is readily transported from blood to brain. Since the ingestion of melatonin improves sleep90 and since tryptophan ingestion stimulates production of melatonin in the gut, tryptophan ingestion may contribute to improvements in sleep. In general, tryptophan ingestion appears to have beneficial effects on sleep, but the potential of dietary proteins to improve sleep remains speculative. α-Lactalbumin produces changes in both the plasma tryptophan concentration and the ratio of tryptophan to LNAAs, consistent with changes following tryptophan intake. Future studies should evaluate the potential of habitual dietary intake of α-lactalbumin to improve sleep conditions, characterizing both the protein dose and the timing of the meal.
Tryptophan and mood
Experiments in both animals and humans provide evidence that brain serotonin can modulate a wide array of cognitive functions, including mood, alertness, memory, attention, executive functions, and, potentially, depression.91 The association between tryptophan and mood was made over 60 years ago, when investigators examined potential links between serotonin and depression.92 At that time, the principal antidepressant drugs were monoamine oxidase inhibitors and tricyclic antidepressants.93
In the earliest studies examining tryptophan and mood, tryptophan was administered to patients with depression for several days or weeks, alone and in combination with an antidepressant drug. Tryptophan was found to have an antidepressant action by itself94 and to enhance the antidepressant efficacy of monoamine oxidase inhibitors95 and chlorimipramine, a moderately serotonin-selective tricyclic antidepressant.96 In 1 study, a subeffective dose of a monoamine oxidase inhibitor combined with tryptophan produced an effective antidepressant action.97 The hypothesis behind the combination studies was that serotonin function would be amplified by stimulating serotonin production with tryptophan supplementation and by inhibiting serotonin removal and reuptake with a monoamine oxidase inhibitor or chlorimipramine. Each combination would result in more serotonin being present in the synapse to stimulate serotonin receptors (and, thus, improve mood). Such findings led investigators to suggest that some forms of depression may result from endogenous, suboptimal levels of serotonin in the brain.98
These early studies involved administration of large daily doses of tryptophan (2.5 – 6 g) given in divided doses throughout the day, often in combination with an antidepressant drug for an extended period of time (3 days to 3 weeks).94–97,99,100 In contrast, recent studies that examined dietary proteins or protein hydrolysates for their effects on mood have not included these experimental design elements. Instead, in the majority, tryptophan-equivalent doses of less than 1 g of tryptophan were used as a constituent of a small amount of dietary protein and, typically, normal individuals were studied over the course of a single day.68,101,102 For example, 1 experiment compared the acute mood effects of ingesting pure tryptophan (0.8 g), α-lactalbumin (15 g containing 0.8 g of tryptophan and 9.4 g of LNAAs), casein (20 g containing 0.4 g of tryptophan and 10 g of LNAAs), a hydrolyzed animal protein (containing 0.8 g of tryptophan and 4 g of LNAAs), or a tryptophan-containing dipeptide (synthetic dipeptide/serine-tryptophan, containing 0.8 g of tryptophan).101 Casein served as the control treatment, as it does not raise the plasma ratio of tryptophan to LNAAs. Mood was evaluated regularly during the experimental period (3.5 hours). All study participants were tested on separate days with each treatment. All treatments except casein (the control) elevated the plasma ratio of tryptophan to LNAAs over the pretreatment ratio (≈ 0.11), with hydrolyzed animal protein and synthetic dipeptide eliciting the largest increases (peak ratios ≈ 0.40 at 60 – 90 min), followed by tryptophan (peak ratio ≈ 0.30 at 90 – 120 min). α-Lactalbumin produced the smallest increase (peak ratio ≈ 0.20 at 120 min). Somewhat consistent with changes in the ratio of tryptophan to LNAAs, mood was significantly improved following treatment with hydrolyzed animal protein and tryptophan, modestly but not significantly improved following synthetic dipeptide treatment, and not improved following α-lactalbumin treatment.
Another study compared a casein hydrolysate (12 g of hydrolysate containing 0.12 g of tryptophan; ratio of tryptophan to LNAAs, 0.02) with a hydrolysate of egg white (12 g of hydrolysate containing 0.66 g of tryptophan; ratio of tryptophan to LNAAs, 0.19).102 In this study, the egg white protein increased the plasma ratio of tryptophan to LNAAs to about 0.23, produced a positive effect on mood (compared with the casein hydrolysate), and activated brain areas associated with mood (as demonstrated by magnetic resonance imaging).
In a study with larger doses of protein in healthy females, Scrutton et al103 compared the effects of ingestion of 40 g of α-lactalbumin or casein (control) after an overnight fast. The ratio of tryptophan to LNAAs rose from 0.098 to 0.138 (fasting to 150 min) after α-lactalbumin, and fell from 0.099 to 0.078 after casein. No effects on measures of emotional processing were observed. However, the rise in the plasma ratio of tryptophan to LNAAs (Figure 4) was somewhat larger when examined in healthy males who ingested the same amount of α-lactalbumin from the same manufacturer, administered under similar conditions.30
Merens et al104 studied protein intake in individuals without depression and in individuals with depression in remission. Following an overnight fast, study participants ingested 20 g of either α-lactalbumin or casein in 2 doses separated by 2 hours. The total protein dose of 40 g provided 493 mg of tryptophan and 5680 mg of LNAAs from α-lactalbumin and 380 mg of tryptophan and 8000 mg of LNAAs from casein. The plasma ratio of tryptophan to LNAAs rose from the baseline fasting level of 0.11 to 0.13 at 90 minutes after lunch for α-lactalbumin (though plasma tryptophan did increase) and declined from 0.11 to 0.08 for casein (the changes in ratio did not differ between individuals without depression and individuals with depression in remission for either protein). There was a significant difference in the plasma ratio of tryptophan to LNAAs between proteins at 90 minutes after the second protein dose, but no effect of treatment on mood was detected in either study group with either protein.
Finally, in the only longer study published thus far, Mohajeri et al105 examined women who consumed a casein hydrolysate or an egg white protein hydrolysate supplement twice daily for 19 days.105 The protein dose was small, 0.5 g of hydrolysate, taken twice daily, and provided approximately 10 mg of tryptophan per day from the casein hydrolysate (ratio of tryptophan to LNAAs, 0.02) and approximately 70 mg of tryptophan per day from the egg white hydrolysate (ratio of tryptophan to LNAAs, 0.19). At the end of the study, no difference was evident between treatment groups in the ratio of tryptophan to LNAAs in fasting plasma samples, but there was a significant difference in the ratio 80 minutes after ingestion of the last dose of the hydrolysates. The investigators observed improvements in cognitive and emotional processing consistent with the higher ratio of tryptophan to LNAAs.
Summarizing these studies, it is clear that chronic administration (> 2 weeks) of large doses of tryptophan (> 3 g) can alter brain serotonin levels and enhance mood. However, the influence of dietary proteins on mood and cognitive function remains speculative, perhaps because of weak study design. Additional research is needed to examine dose response and duration of treatment with dietary proteins in relation to plasma changes in the ratio of tryptophan to LNAAs and, ultimately, in relation to behavior and mood outcomes.
Tryptophan and epilepsy
Epilepsy is a condition characterized by repeated spontaneous seizures. Approximately 1 of every 10 people is likely to have at least 1 epileptic seizure in their lifetime, and approximately one-third who experience an epileptic seizure experience a recurrence.106 A link between brain serotonin levels and epilepsy has been explored for over 50 years, yielding the broad view that treatments that enhance serotonin may inhibit the generation of seizures.19,107,108
A few relevant studies in animals have been published. Of particular interest, Meldrum et al109 and Wada et al110 (from the same laboratory) examined the effect of tryptophan on photically induced epilepsy in baboons., Large single doses of tryptophan (≤600 mg/kg, IP) did not reduce the severity of photically induced epilepsy, however injection of 5-hydroxytryptophan, the immediate precursor of serotonin, attenuated the seizures. Likewise, 5-hydroxytryptophan administration was reported to enhance the antiepileptic action of fluoxetine, a serotonin reuptake blocker, in genetically epilepsy-prone rats.111 Together, these findings suggest that, if tryptophan does have an antiseizure effect, the supplemental dose required would be sizeable.
Initial clinical trials have largely failed to support the usefulness of tryptophan therapy to increase serotonin in patients with epilepsy. For example, adults with epilepsy under poor control with standard medications participated in a multiple-arm crossover trial that included each of the following treatment arms added to their current treatment protocol: (1) placebo; (2) tryptophan (8 g/d or 100 mg/kg/d for an 80-kg person); and tryptophan (6–8 g/d) in combination with a monoamine oxidase inhibitor. Each treatment arm lasted 3 months. No effects of any treatment on seizure frequency or severity were noted.112 In another study, children (7 – 14 years) received tryptophan (40 mg/kg/d) or placebo in a double-blind crossover trial, with each arm lasting 5 weeks. The authors reported that the amino acid was well tolerated, but seizure frequency was unaffected.113 Like the adults in the study described above,112 the children were not removed from their current antiepileptic medications during the trial, for obvious reasons, and this may have obscured the effects of tryptophan. However, in support of a tryptophan effect, an interesting study was conducted in adults who were not epileptic but were undergoing electroconvulsive therapy for depression. Individuals received electroconvulsive therapy twice, separated by 2 to 3 days. On the first test day, electroconvulsive therapy was given alone, and on the second test day, electroconvulsive therapy was preceded by oral tryptophan (6 g given the day before electroconvulsive therapy, and 3 g given 4 h before electroconvulsive therapy). The duration of the induced seizure, recorded by electroencephalography, was significantly reduced by tryptophan administration.114
Against this background, Citraro et al115 and Russo et al116 recently reported that chronic oral administration of α-lactalbumin reduced epileptogenesis in animal models of epilepsy. These investigators proposed that the effect might be caused by an increase in brain tryptophan concentrations, secondary to ingestion of the protein, and that the action may result from increased production of serotonin115 and/or kynurenic acid.116 Kynurenic acid, a product of tryptophan metabolism via the kynurenine pathway, is formed in brain as well as in the gut and liver.117 The potential link between kynurenic acid and epilepsy derives from (1) kynurenic acid’s action as an antagonist to a specific glutamate receptor, the N-methyl-d-aspartate receptor118 and (2) the ability of N-methyl-d-aspartate to induce seizures in animals.119 Administration of tryptophan stimulates kynurenic acid synthesis in animals,120 and, thus, if α-lactalbumin ingestion also has this effect, the result might be to protect against seizures. It will be important to determine if α-lactalbumin ingestion can increase kynurenic acid levels in brain. In addition, the concentration of another metabolite of the kynurenine pathway, quinolinic acid, is increased following tryptophan loading121 and stimulates the N-methyl-d-aspartate receptor.122 The brain level of quinolinic acid should also be measured to determine its change following α-lactalbumin ingestion and to establish whether the net effect of α-lactalbumin ingestion is ultimately to stimulate or to inhibit the action of N-methyl-d-aspartate receptors. These findings with α-lactalbumin warrant further study in additional trials.
CYSTEINE, OXIDATIVE STRESS, AND IMMUNE FUNCTION
Whey proteins, because of their unique amino acid composition, are recognized for their ability to optimize aspects of immune function, with perhaps their best-characterized action being stimulation of glutathione and glutamine production.123 Glutathione is synthesized from cysteine, glutamate, and glycine. It plays a major role in protection of cells against oxidative stress. Glutamine is produced in skeletal muscle from catabolism of BCAAs and is an essential fuel for the immune system.
Glutathione plays a central role in the body’s ability to scavenge reactive oxygen species and also has diverse roles in signal transduction, gene expression, apoptosis, and nitric oxide metabolism.124 Depletion of tissue glutathione is observed in a wide range of physical stress conditions, ranging from intense exercise and aging to pathologies such as cancer, neurodegenerative disorders, cystic fibrosis, and HIV infection.124,125 Diets supplemented with dairy (ie, milk or yogurt)126 or whey protein127,128 have been shown to increase tissue levels of glutathione.
The bioavailability of cysteine is rate limiting for glutathione synthesis.124 Cysteine is generally considered a nonessential or dispensable amino acid in adults because it can be synthesized from the essential amino acid methionine. While nutritional requirements for these 2 sulfur-containing amino acids are often listed together as a sum, methionine and cysteine participate in different metabolic processes. Methionine is central to 1-carbon metabolism, which requires folate, vitamin B12, and choline and is essential for synthesis of the purines, pyrimidines, and polyamines required for nuclear development and function, while cysteine is essential for synthesis of taurine and glutathione, which are involved in immune and antioxidant responses.129
The first step in cysteine synthesis is transmethylation of methionine to homocysteine, which is then combined with serine to form cystathionine. Cystathionine is subsequently converted to cysteine. Methionine can be resynthesized from homocysteine by remethylation, but conversion of cystathionine to cysteine is irreversible.129 Hence, while the sulfur amino acids are linked, they are not entirely interchangeable, and some investigators suggest that diet is limiting in cysteine availability.124,130 Furthermore, elevated plasma concentrations of homocysteine are associated with increased risk of cardiovascular disease, while increased levels of glutathione appear to reduce the risk of cardiovascular disease.129,131 Dietary supplements of cysteine increase glutathione levels without increasing homocysteine.124,129,131 Whey proteins also provide a rich source of cysteine with relatively lower levels of methionine (Table 1). Most proteins have a ratio of cysteine to methionine that is higher in methionine, but whey protein isolate has a cysteine-to-methionine ratio of approximately 1.3, and α-lactalbumin has a cysteine-to-methionine ratio of approximately 4.8 (Table 1). The cysteine present in α-lactalbumin occurs in the form of 4 disulfide bridges that can be released by digestion and appear in the blood as either the disulfide cystine or free cysteine.38,129,130
In animal studies examining humoral immune response to infection, whey proteins, when compared with other dietary proteins (ie, soy, casein, or wheat), have been shown to enhance antibody production.132,133 Furthermore, animal studies demonstrate that consumption of α-lactalbumin protein can increase liver glutathione in a dose-dependent response134 and can increase total white cell counts (CD4+) and lymphocyte counts in mice with parasitic infections.135 Studies have also shown that glutathione can modulate glucose tolerance and improve insulin sensitivity,136 presumably through a mechanism of nitric oxide signal transmission.124 A study in rats given either an α-lactalbumin–rich whey concentrate or total milk proteins during a high-sucrose meal, with and without cysteine supplementation, demonstrated that both α-lactalbumin and cysteine supplementation increased glutathione concentrations and improved glucose homeostasis.136
Likewise, in humans, increasing the supply of cysteine or cystine can increase glutathione synthesis and prevent glutathione deficiency under nutritional or pathological stress (ie, protein malnutrition, respiratory distress, or HIV infection).125 Several studies found that supplementing with cysteine-rich whey proteins increased blood levels of glutathione and improved quality of life among HIV-positive individuals.137–139
BRANCHED-CHAIN AMINO ACIDS AND SKELETAL MUSCLE METABOLISM
α-Lactalbumin, like other whey proteins, has a high concentration of the BCAAs leucine, isoleucine, and valine and a greater leucine content than egg, soy, beef, or wheat proteins (Table 1). Leucine is a key dietary regulator of skeletal muscle anabolism via leucine-induced activation of the mTORC1 signal complex, which plays a central role in the activation of translation factors that stimulate postmeal synthesis of skeletal muscle protein.62 The importance of the leucine threshold for maintenance of skeletal muscle mass and function was recognized by the PROT-AGE Study Group,140 which recommended a minimum of 2.5 g of leucine at each meal for muscle health in older adults. Metabolism of the BCAAs, which occurs largely in skeletal muscle, not only provides fuel for muscle contraction but also provides the amine substrate (−NH2) for de novo synthesis of glutamine and alanine. Glutamine is a primary fuel for lymphocytes, macrophages, and intestinal epithelial cells, and alanine is a primary substrate for gluconeogenesis and homeostatic regulation of blood glucose.141,142
The importance of leucine in the regulation of muscle protein synthesis has been shown in catabolic conditions such as after overnight fasting, during prolonged bed rest, and post exercise. Leucine is required to activate the mTORC1 signaling pathway to initiate anabolic recovery. Furthermore, research has shown that free leucine is not an effective supplement; instead, leucine must be provided as part of a meal that contains proteins with complete essential amino acid profiles.143 In this context, whey proteins are most effective in producing maximal anabolic stimulation with the least amount of total protein.144 The efficiency of the response is particularly important for persons who often consume small meals, eg, the elderly, or when small meals are desired, eg, post surgery, post exercise, or at breakfast.62
Recent research also suggests that nighttime intake of protein may have the potential to protect and enhance muscle mass and strength.145 A Dutch group has shown that consumption of 28 g of casein (a milk protein providing 2.5 g of leucine) prior to sleep increased skeletal muscle protein synthesis in young adults (≈ 22 years).146 When combined with resistance exercise, the nighttime protein increased muscle mass and strength after 12 weeks.147 The investigators suggest nighttime protein intake provides an important source of precursor amino acids to sustain muscle protein accretion during sleep. These investigators found similar increases in muscle protein synthesis and positive balance in whole-body protein turnover in older adults (≈ 74 years) provided with 40 g of casein.145 Timing the intake coincident with sleep is, at least in part, consistent with circadian cycles of growth factors, specifically with the daily peak of growth hormone that occurs during the early period of slow-wave sleep.148 α-Lactalbumin, as a rich source of both tryptophan and leucine, may be an ideal protein to optimize both sleep and muscle protein synthesis.
Glutamine is utilized as a primary fuel source by cells of the immune system in support of both optimal proliferation of lymphocytes and production of cytokines by lymphocytes and macrophages.141 In humans, plasma and muscle glutamine levels are depleted during stress conditions such as surgery, sepsis, burn injury, and endurance exercise, and it has been suggested that lower glutamine concentrations contribute to stress-induced immunosuppression. This view is supported by studies showing that maintenance of plasma glutamine enhances immune function in patients.142 Skeletal muscle is the principal site of glutamine production. Muscle maintains a high concentration of glutamine, which can be released into circulation during stress conditions.149 Muscle glutamine is derived from BCAA degradation, and studies have shown that supplementation with BCAAs helps maintain immune function in long-distance athletes.150
While BCAAs exert anabolic effects on muscle protein synthesis and enhance immune function, the potential for diets high in BCAAs or total protein to produce abnormal metabolism has raised concerns. Elevated blood levels of BCAAs have been correlated with insulin resistance, obesity, and type 2 diabetes in both humans and rodents.151–153 However, contrary to these correlations, controlled studies have demonstrated that leucine supplementation or leucine-rich diets improve insulin sensitivity.154,155 Diets designed around the threshold of 2.5 grams of leucine per meal trigger the postmeal anabolic response and protect metabolically active tissue during weight loss while increasing the loss of body fat.156 Positive associations have also been demonstrated between a diet rich in BCAAs, body weight, and glucose homeostasis.157–160 It is unclear whether leucine and other BCAAs are direct contributors to the development of insulin resistance or whether they are secondary biomarkers of impaired insulin action.158
APPLICATION OF α-LACTALBUMIN IN CANCER TREATMENTS
The ability of whey proteins to increase glutathione concentrations and enhance immunity, along with the discovery of the unique antitumor complex called HAMLET (Human Alpha-lactalbumin Made Lethal to Tumor cells), led scientists to investigate the potential use of whey proteins in cancer therapy. In an early study with mice, Bounous et al161 reported that a diet formulated with whey protein concentrate, when compared with a standard casein-based diet, reduced the incidence and size of chemically induced colon tumors. Subsequently, a different group replicated this finding, showing that whey protein had twice the protective effect of soy protein.162 The anticarcinogenic properties of whey protein have also been demonstrated with dimethylbenz(a)anthracene-induced mammary tumors in rats.163 Hakkak et al163 found a lower incidence of tumors in animals consuming whey protein than in animals fed casein or soy diets. The authors speculated that the beneficial effects of whey protein could be associated with enhanced tissue glutathione levels or active peptides. In support of the glutathione mechanism, Kent et al164 used a human prostate cell line and showed that treatment of the cells with hydrolyzed whey protein isolate or a cysteine-donating compound (N-acetylcysteine) increased cellular glutathione concentrations and protected cells against reactive oxygen species and cell death in prostate epithelial cells.
The HAMLET complex was discovered by researchers examining the potential of dairy protein extracts to reduce the adhesion of pneumonia-causing bacteria to respiratory epithelial cells.165 When these investigators serendipitously switched their cell line to lung carcinoma cells, they found that the dairy protein product killed both the bacteria and the cancer cells. Investigating this apoptotic response further, they fractionated the dairy product and isolated the active compound, identifying it as a protein-lipid complex of α-lactalbumin and oleic acid, which they named HAMLET.
HAMLET is a potent anticancer agent, killing tumor cells of approximately 50 cancer cell types with high selectivity165,166 while leaving healthy cells largely unaffected both in vitro and in vivo.167 The structure of HAMLET compounds consists of an aggregation of partially unfolded proteins with fatty acid molecules bound in the hydrophobic core.168 Similarly, BAMLET (Bovine Alpha-Lactalbumin Made Lethal to Tumor), a complex of bovine α-lactalbumin and oleic acid, also kills tumor cells, and both HAMLET and BAMLET appear to penetrate the cell membrane and trigger a pathway for lysosomal cell death. α-Lactalbumin–oleic acid complexes such as HAMLET and BAMLET appear to have the ability to kill tumors cells that are otherwise highly resistant to apoptosis.165,167
HAMLET (or BAMLET) is not a normal component of milk but is formed during low-pH precipitation of the casein fraction, which allows for partial unfolding of the α-lactalbumin structure and binding with the fatty acid.169 Mechanisms of action for these protein–lipid complexes are not well understood. There is debate over whether the key cytotoxic component of HAMLET/BAMLET complexes is α-lactalbumin or oleic acid. Some studies suggest the oleic acid component of these complexes accounts for the cytotoxicity, with α-lactalbumin acting only as a solubilizing agent that enhances membrane permeability for the fatty acid.168,170 Although a binding site for oleic acid was identified in the α-lactalbumin molecule, several other proteins, such as milk lysozyme, apomyoglobin, and α-2-microglobulin, also form HAMLET-like complexes with oleic acid and show antitumor activity.171 Delgado et al170 concluded that the protein component is not the cytotoxic component of the HAMLET protein–oleic acid complex but instead is involved in the delivery of cytotoxic oleic acid across the tumor cell membrane.
It is clear that the protein–lipid complex must come in direct contact with the tumor cell surface, which creates obvious delivery problems in pharmacological applications. Bruck et al172 showed that in vitro digestion by pepsin and trypsin abolished the apoptotic effect of α-lactalbumin. Sullivan et al173 gave human adults α-lactalbumin with or without oleic acid and monitored digestion via nasogastric tube and capsule endoscopy. α-Lactalbumin was rapidly digested, and no cytotoxic complex of α-lactalbumin and oleic acid was detected.
HAMLET has shown therapeutic efficacy when administered directly in 4 in vivo models. In humans with therapy-resistant skin papillomas, topical application of HAMLET significantly reduced lesion size and eliminated all lesions in over 80% of patients within 2 years.174 In patients diagnosed with superficial bladder cancer, local injections of HAMLET into the bladder reduced tumor cell size and increased tumor cell apoptosis.175 Similar outcomes have been demonstrated in animal studies. In mice with bladder cancer, localized administration of HAMLET reduced tumor size and delayed tumor development.176 In rats transplanted with human glioblastoma cells, local infusion of HAMLET delayed tumor development, and the HAMLET complex appeared to penetrate the tumor, triggering apoptosis.177 Finally, in an intriguing study using a mouse model of colon cancer, investigators found that oral delivery of HAMLET produced a significant reduction in tumor size and polyp number.178 In summary, the potential use of α-lactalbumin in cancer treatment remains experimental but merits continued research.
CONCLUSION
α-Lactalbumin is a milk protein that constitutes over one-third of the total protein in human milk. Through recent advances in food processing, pure α-lactalbumin has become commercially available, generating new opportunities to research the use of α-lactalbumin in nutritional and medical applications and in creating functional food products. α-Lactalbumin is an attractive protein, both because of its physical characteristics, which include a clean flavor profile, high water solubility, and heat stability (which combined allow for diverse food applications), and because of its biological properties derived from its protein quality, which create the potential for diverse nutrition applications. The most frequently studied uses for α-lactalbumin are as a component of infant formulas designed to be more similar to breast milk, and as supplement to enhance sleep or improve mood in adults. However, potential new applications in cancer treatment or to enhance immune response have also garnered interest. To date, most uses of α-lactalbumin derive from its unique amino acid composition, with most attention focused on the essential amino acid tryptophan, a precursor to the neurotransmitter serotonin. However, α-lactalbumin is also a rich source of the sulfur amino acids, with a highly unusual 5:1 ratio of cysteine to methionine. Moreover, it contains BCAAs, lysine, and potentially bioactive peptides. Because of its amino acid composition and associated biopeptides, α-lactalbumin has potential as a protein supplement to support infant development, promote gastrointestinal function, and protect muscle mass in aging adults. Moreover, it may have clinical applications in cancer therapy or to enhance immune function.
Acknowledgments
The authors would like to thank Densie Webb, PhD, RD, for editorial support.
Author contributions. D.K.L., B.L., and J.D.F. shared equally in development, writing, and review of this manuscript.
Funding/support. The authors thank Agropur for financial support in preparing this manuscript.
Declaration of interest. D.K.L. is a consultant to Agropur.
References
- 1. Heine WE, Klein PD, Reeds PJ.. The importance of α-lactalbumin in infant nutrition. J Nutr. 1991;121:277–283. [DOI] [PubMed] [Google Scholar]
- 2. Kunz C, Lönnerdal B.. Re-evaluation of the whey protein/casein ratio of human milk. Acta Paediatr. 1992;81:107–112. [DOI] [PubMed] [Google Scholar]
- 3. Krissansen GW. Emerging health properties of whey proteins and their clinical implications. J Am Coll Nutr. 2007;26:713S–723S. [DOI] [PubMed] [Google Scholar]
- 4.US Department of Agriculture, Agriculture Research Service. USDA Food Composition Database. Beltsville, MD: Nutrient Data Laboratory; 2015. https://ndb.nal.usda.gov/ndb/. Slightly revised May 2016. Accessed February 28, 2018.
- 5. Atkinson SA, Lönnerdal B.. Nitrogenous components of milk In: Handbook of Milk Composition. In: Jensen RG, ed. San Diego, CA: Academic Press; 1995:351–387. [Google Scholar]
- 6. Swaisgood HE. Protein and amino acid composition of bovine milk In: Jensen RG, ed. Handbook of Milk Composition. San Diego, CA: Academic Press; 1995:464–467. [Google Scholar]
- 7. Kamau SM, Cheison SC, Chen W et al. , Alpha-lactalbumin: its production technologies and bioactive peptides. Comp Rev Food Sci. 2010;9:197–212. [Google Scholar]
- 8. Morr CV, Ha EY.. Whey protein concentrates and isolates: processing and functional properties. Crit Rev Food Sci Nutr. 1993;33:431–476. [DOI] [PubMed] [Google Scholar]
- 9. Permyakov EA, Berliner LJ.. α-Lactalbumin: structure and function. FEBS Lett. 2000;473:269–274. [DOI] [PubMed] [Google Scholar]
- 10. Lien EL. Infant formulas with increased concentrations of alpha-lactalbumin. Am J Clin Nutr. 2003;77:1555S–1558S. [DOI] [PubMed] [Google Scholar]
- 11. Lönnerdal B. Infant formula and infant nutrition: bioactive proteins of human milk and implications for composition of infant formulas. Am J Clin Nutr. 2014;99:712S–717S. [DOI] [PubMed] [Google Scholar]
- 12. US Food and Drug Administration. Infant formula. 2017. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=107.100. Accessed February 28, 2018.
- 13. Food and Agriculture Organization of the United Nations. Revised standard for infant formula and formulas for special medical purposes intended for infants. Codex Stan 72–1981. In: Codex Alimentarius Rome, Italy: Food and Agriculture Organization of the United Nations; Revision 2007.
- 14. Rudloff S, Kunz C.. Protein and nonprotein nitrogen components in human milk, bovine milk, and infant formula: quantitative and qualitative aspects in infant nutrition. J Pediatr Gastroenterol Nutr. 1997;24:328–344. [DOI] [PubMed] [Google Scholar]
- 15. Räihä NC, Axelsson IE.. Protein nutrition during infancy. An update. Pediatr Clin North Am. 1995;42:745–764. [DOI] [PubMed] [Google Scholar]
- 16. Koletzko B, von Kries R, Closa R et al. , Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. Am J Clin Nutr. 2009;89:1836–1845. [DOI] [PubMed] [Google Scholar]
- 17. Trabulsi J, Capeding R, Lebumfacil J et al. , Effect of an α-lactalbumin-enriched infant formula with lower protein on growth. Eur J Clin Nutr. 2011;65:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lönnerdal B, Lien EL.. Nutritional and physiologic significance of alpha-lactalbumin in infants. Nutr Rev. 2003;61:295–305. [DOI] [PubMed] [Google Scholar]
- 19. Bagdy G, Kecskemeti V, Riba P et al. , Serotonin and epilepsy. J Neurochem. 2007;100:857–873. [DOI] [PubMed] [Google Scholar]
- 20. Lien EL, Davis AM, Euler AR et al. , Growth and safety in term infants fed reduced-protein formula with added bovine alpha-lactalbumin. J Pediatr Gastroenterol Nutr. 2004;38:170–176. [DOI] [PubMed] [Google Scholar]
- 21. Sandström O, Lönnerdal B, Graverholt G et al. , Effects of α-lactalbumin-enriched formula containing different concentrations of glycomacropeptide on infant nutrition. Am J Clin Nutr. 2008;87:921–928. [DOI] [PubMed] [Google Scholar]
- 22. Mirmiran M, Van Someren E.. Symposium: normal and abnormal REM sleep regulation: the importance of REM sleep for brain maturation. J Sleep Res. 1993;2:188–192. [DOI] [PubMed] [Google Scholar]
- 23. Steinberg LA, O'Connell NC, Hatch TF et al. , Tryptophan intake influences infants' sleep latency. J Nutr. 1992;122:1781–1791. [DOI] [PubMed] [Google Scholar]
- 24. Fernstrom JD. Role of precursor availability in control of monoamine biosynthesis in brain. Physiol Rev. 1983;63:484–546. [DOI] [PubMed] [Google Scholar]
- 25. Huether G, Poeggeler B, Reimer A et al. , Effect of tryptophan administration on circulating melatonin levels in chicks and rats: evidence for stimulation of melatonin synthesis and release in the gastrointestinal tract. Life Sci. 1992;51:945–953. [DOI] [PubMed] [Google Scholar]
- 26. Vanecek J. Cellular mechanisms of melatonin action. Physiol Rev. 1998;78:687–721. [DOI] [PubMed] [Google Scholar]
- 27. Heine WE. The significance of tryptophan in infant nutrition. Adv Exp Med Biol. 1999;467:705–710. [DOI] [PubMed] [Google Scholar]
- 28. Cubero J, Valero V, Sanchez J et al. , The circadian rhythm of tryptophan in breast milk affects the rhythms of 6-sulfatoxymelatonin and sleep in newborn. Neuro Endocrinol Lett. 2005;26:657–661. [PubMed] [Google Scholar]
- 29. Pardridge WM. The role of blood-brain barrier transport of tryptophan and other neutral amino acids in the regulation of substrate-limited pathways of brain amino acid metabolism. J Neural Transm Suppl. 1979;15:43–54. [DOI] [PubMed] [Google Scholar]
- 30. Fernstrom JD, Langham KA, Marcelino LM et al. , The ingestion of different dietary proteins by humans induces large changes in the plasma tryptophan ratio, a predictor of brain tryptophan uptake and serotonin synthesis. Clin Nutr. 2013;32:1073–1076. [DOI] [PubMed] [Google Scholar]
- 31. Yogman MW, Zeisel SH.. Diet and sleep patterns in newborn infants. N Engl J Med. 1983;309:1147–1149. [DOI] [PubMed] [Google Scholar]
- 32. Aparicio S, Garau C, Esteban S et al. , Chrononutrition: use of dissociated day/night infant milk formulas to improve the development of the wake–sleep rhythms. Effects of tryptophan. Nutr Neurosci. 2007;10:137–143. [DOI] [PubMed] [Google Scholar]
- 33. Esteban S, Nicolaus C, Garmundi A et al. , Effect of orally administered L-tryptophan on serotonin, melatonin, and the innate immune response in the rat. Mol Cell Biochem. 2004;267:39–46. [DOI] [PubMed] [Google Scholar]
- 34. Yogman MW, Zeisel SH, Roberts C.. Assessing effects of serotonin precursors on newborn behavior. J Psychiatr Res. 1982;17:123–133. [DOI] [PubMed] [Google Scholar]
- 35. Andreas NJ, Kampmann B, Mehring Le-Doare K.. Human breast milk: a review on its composition and bioactivity. Early Human Dev. 2015;91:629–635. [DOI] [PubMed] [Google Scholar]
- 36. Newburg DS, Walker WA.. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res. 2007;61:2–8. [DOI] [PubMed] [Google Scholar]
- 37. Lönnerdal B. Nutritional and physiologic significance of human milk proteins. Am J Clin Nutr. 2003;77:1537S–1543S. [DOI] [PubMed] [Google Scholar]
- 38. Pellegrini A, Thomas U, Bramaz N et al. , Isolation and identification of three bactericidal domains in the bovine α-lactalbumin molecule. Biochim Biophys Acta. 1999;1426:439–448. [DOI] [PubMed] [Google Scholar]
- 39. Giuffrida MG, Cavaletto M, Ciunta C et al. , The unusual amino acid triplet Asn-Ile-Cys is a glycosylation consensus site in human α-lactalbumin. J Protein Chem. 1997;16:747–753. [DOI] [PubMed] [Google Scholar]
- 40. Berger M, Gray JA, Roth BL.. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu G. Synthesis of amino acids In: Amino Acids: Biochemistry and Nutrition. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2013:63–96. [Google Scholar]
- 42. Keszthelyi D, Troost FJ, Masclee AAM.. Understanding the role of tryptophan and serotonin metabolism in gastrointestinal function. Neurogastroenterol Motil. 2009;21:1239–1249. [DOI] [PubMed] [Google Scholar]
- 43. Madureira AR, Tavares T, Gomes AM et al. , Invited review: physiological properties of bioactive peptides obtained from whey proteins. J Dairy Sci. 2010;93:437–455. [DOI] [PubMed] [Google Scholar]
- 44. Yuwiler A, Brammer GL, Morley JE et al. , Short-term and repetitive administration of oral tryptophan in normal men: effects on blood tryptophan, serotonin, and kynurenine concentrations. Arch Gen Psychiatry. 1981;38:619–626. [DOI] [PubMed] [Google Scholar]
- 45. Migliore-Samour D, Roch-Arveiller M, Tissot M et al. , Effects of tripeptides derived from milk proteins on polymorphonuclear oxidative and phosphoinositide metabolisms. Biochem Pharmacol. 1992;44:673–680. [DOI] [PubMed] [Google Scholar]
- 46. Dupont C, Rivero M, Grillon C et al. , α-Lactalbumin-enriched and probiotic-supplemented infant formula in infants with colic: growth and gastrointestinal tolerance. Eur J Clin Nutr. 2010;64:765–767. [DOI] [PubMed] [Google Scholar]
- 47. Ushida Y, Shimokawa Y, Matsumoto H et al. , Effects of bovine α-lactalbumin on gastric defense mechanisms in naïve rats. Biosci Biotechnol Biochem. 2003;67:577–583. [DOI] [PubMed] [Google Scholar]
- 48. Kamau SM, Cheison SC, Chen W et al. , Alpha-lactalbumin: its production technologies and bioactive peptides. Comp Rev Food Sci Food Safey. 2010;9:197–212. [Google Scholar]
- 49. Berthou J, Migliore-Samour D, Lifchitz A et al. , Immunostimulating properties and three-dimensional structure of two tripeptides from human and cow caseins. FEBS Lett. 1987;218:55–58. [DOI] [PubMed] [Google Scholar]
- 50. Jaziri M, Migliore-Samour D, Casabianca-Pignede MR et al. , Specific binding sites on human phagocytic blood cells for Gly-Leu-Phe and Val-Glu-Pro-Ile-Pro-Tyr, immunostimulating peptides from human milk proteins. Biochim Biophys Acta. 1992;1160:251–261. [DOI] [PubMed] [Google Scholar]
- 51. Kee HJ, Hong YH, Kim ER et al. , Effect of enzymatically hydrolyzed alpha-LA fractions with pepsin on growth-promoting of Bifidobacgterium longum ATCC 15707. Korean J Dairy Sci. 1998;20:61–68. [Google Scholar]
- 52. Roger LC, Costabile A, Holland DT et al. , Examination of faecal Bifidobacterium populations in breast- and formula-fed infants during the first 18 months of life. Microbiology. 2010;156(pt 11):3329–3341. [DOI] [PubMed] [Google Scholar]
- 53. Harmsen HJ, Wildeboer-Veloo AC, Raangs GC et al. , Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr. 2000;30:61–67. [DOI] [PubMed] [Google Scholar]
- 54. Le Huerou-Luron I, Blat S, Boudry G.. Breast- v. formula-feeding: impacts on the digestive tract and immediate and long-term health effects. Nutr Res Rev. 2010;23:23–36. [DOI] [PubMed] [Google Scholar]
- 55. Bruck WM, Kelleher SL, Gibson GR et al. , rRNA probes used to quantify the effects of glycomacropeptide and alpha-lactalbumin supplementation on the predominant groups of intestinal bacteria of infant rhesus monkeys challenged with enteropathogenic Escherichia coli. J Pediatr Gastroenterol Nutr. 2003;37:273–280. [DOI] [PubMed] [Google Scholar]
- 56. Lönnerdal B, Keen CL, Ohtake M et al. , Iron, zinc, copper, and manganese in infant formulas. Am J Dis Child. 1983;137:433–437. [DOI] [PubMed] [Google Scholar]
- 57. Kelleher SL, Chatterton D, Nielsen K et al. , Glycomacropeptide and α-lactalbumin supplementation of infant formula affects growth and nutritional status in infant rhesus monkeys. Am J Clin Nutr. 2003;77:1261–1268. [DOI] [PubMed] [Google Scholar]
- 58. Szymlek-Gay EA, Lönnerdal B, Abrams SA et al. , α-Lactalbumin and casein-glycomacropeptide do not affect iron absorption from formula in healthy term infants. J Nutr. 2012;142:1226–1231. [DOI] [PubMed] [Google Scholar]
- 59. Baumy JJ., Brule G.. Effect of pH and iconic strength on the binding of bivalent cations to β-casein. Le Lait Dairy Sci Technol. 1988;68:409–417. [Google Scholar]
- 60. Wang X, Ai T, Meng XL et al. , In vitro iron absorption of α-lactalbumin hydrolysate-iron and β-lactoglobulin hydrolysate-iron complexes. J Dairy Sci. 2014;97:2559–2566. [DOI] [PubMed] [Google Scholar]
- 61. Szymlek-Gay EA, Domellof M, Hernell O et al. , Mode of oral iron administration and the amount of iron habitually consumed do not affect iron absorption, systemic iron utilisation or zinc absorption in iron-sufficient infants: a randomised trial. Br J Nutr. 2016;116:1046–1060. [DOI] [PubMed] [Google Scholar]
- 62. Layman DK, Anthony TG, Rasmussen BB et al. , Defining meal requirements for protein to optimize metabolic roles of amino acids. Am J Clin Nutr. 2015;101:1330S–1338S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. English KL, Mettler JA, Ellison JB et al. , Leucine partially protects muscle mass and function during bed rest in middle-aged adults. Am J Clin Nutr. 2016;103:465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Murphy CH, Saddler NI, Devries MC et al. , Leucine supplementation enhances integrative myofibrillar protein synthesis in free-living older men consuming lower- and higher-protein diets: a parallel-group crossover study. Am J Clin Nutr. 2016;104:1594–1606. [DOI] [PubMed] [Google Scholar]
- 65. Fernstrom JD, Wurtman RJ.. Brain serotonin content: increase following ingestion of carbohydrate diet. Science. 1971;174:1023–1025. [DOI] [PubMed] [Google Scholar]
- 66. Fernstrom JD, Wurtman RJ.. Brain serotonin content: physiological regulation by plasma neutral amino acids. Science. 1972;178:414–416. [DOI] [PubMed] [Google Scholar]
- 67. Fernstrom JD, Faller DV.. Neutral amino acids in the brain: changes in response to food ingestion. J Neurochem. 1978;30:1531–1538. [DOI] [PubMed] [Google Scholar]
- 68. Markus CR, Olivier B, Panhuysen GE et al. , The bovine protein α-lactalbumin increases the plasma ratio of tryptophan to the other large neutral amino acids, and in vulnerable subjects raises brain serotonin activity, reduces cortisol concentration, and improves mood under stress. Am J Clin Nutr. 2000;71:1536–1544. [DOI] [PubMed] [Google Scholar]
- 69. Feurte S, Gerozissis K, Regnault A et al. , Plasma Trp/LNAA ratio increases during chronic ingestion of an alpha-lactalbumin diet in rats. Nutr Neurosci. 2001;4:413–418. [DOI] [PubMed] [Google Scholar]
- 70. Orosco M, Rouch C, Beslot F et al. , Alpha-lactalbumin-enriched diets enhance serotonin release and induce anxiolytic and rewarding effects in the rat. Behav Brain Res. 2004;148:1–10. [DOI] [PubMed] [Google Scholar]
- 71. Choi S, Disilvio B, Fernstrom MH et al. , Meal ingestion, amino acids and brain neurotransmitters: effects of dietary protein source on serotonin and catecholamine synthesis rates. Physiol Behav. 2009;98:156–162. [DOI] [PubMed] [Google Scholar]
- 72. Fernstrom MH, Fernstrom JD.. Brain tryptophan concentrations and serotonin synthesis remain responsive to food consumption after the ingestion of sequential meals. Am J Clin Nutr. 1995;61:312–319. [DOI] [PubMed] [Google Scholar]
- 73. Williams WA, Shoaf SE, Hommer D et al. , Effects of acute tryptophan depletion on plasma and cerebrospinal fluid tryptophan and 5-hydroxyindoleacetic acid in normal volunteers. J Neurochem. 1999;72:1641–1647. [DOI] [PubMed] [Google Scholar]
- 74. Fernstrom JD. Effects and side effects associated with the non-nutritional use of tryptophan by humans. J Nutr. 2012;142:2236S–2244S. [DOI] [PubMed] [Google Scholar]
- 75. Lieberman HR, Agarwal S, Fulgoni VL III. Tryptophan intake in the US adult population is not related to liver or kidney function but is associated with depression and sleep outcomes. J Nutr. 2016;146:2609S–2615S. [DOI] [PubMed] [Google Scholar]
- 76.Institute of Medicine. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem Washington, DC: National Academies Press; 2006:2–5. [PubMed]
- 77. Smith B, Prockop DJ.. Central-nervous-system effects of ingestion of L-tryptophan by normal subjects. N Engl J Med. 1962;267:1338–1341. [DOI] [PubMed] [Google Scholar]
- 78. Oswald I. Drugs and sleep. Pharmacol Rev. 1968;20:273–303. [PubMed] [Google Scholar]
- 79. Jouvet M. Biogenic amines and the states of sleep. Science. 1969;163:32–41. [DOI] [PubMed] [Google Scholar]
- 80. Wyatt RJ, Engelman K, Kupfer DJ et al. , Effects of para-chlorophenylalanine on sleep in man. Electroencephalogr Clin Neurophysiol. 1969;27:529–532. [DOI] [PubMed] [Google Scholar]
- 81. Wyatt RJ, Zarcone V, Engelman K et al. , Effects of 5-hydroxytryptophan on the sleep of normal human subjects. Electroencephalogr Clin Neurophysiol. 1971;30:505–509. [DOI] [PubMed] [Google Scholar]
- 82. Eccleston D, Ashcroft GW, Crawford TB.. Effect of tryptophan administration on 5HIAA in cerebrospinal fluid in man. J Neurol Neurosurg Psychiatry. 1970;33:269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Oswald I, Ashcroft GW, Berger RJ et al. , Some experiments in the chemistry of normal sleep. Br J Psychiatry. 1966;112:391–399. [DOI] [PubMed] [Google Scholar]
- 84. Griffiths WJ, Lester BK, Coulter JD et al. , Tryptophan and sleep in young adults. Psychophysiology. 1972;9:345–356. [DOI] [PubMed] [Google Scholar]
- 85. Schneider-Helmert D. Interval therapy with L-tryptophan in severe chronic insomniacs. A predictive laboratory study. Int Pharmacopsychiatry. 1981;16:162–173. [DOI] [PubMed] [Google Scholar]
- 86. Wyatt RJ, Engelman K, Kupfer DJ et al. , Effects of L-tryptophan (a natural sedative) on human sleep. Lancet. 1970;296:842–846. [DOI] [PubMed] [Google Scholar]
- 87. Bhatti T, Gillin JC, Seifritz E et al. , Effects of a tryptophan-free amino acid drink challenge on normal human sleep electroencephalogram and mood. Biol Psychiatry. 1998;43:52–59. [DOI] [PubMed] [Google Scholar]
- 88. Markus CR, Jonkman LM, Lammers JH et al. , Evening intake of alpha-lactalbumin increases plasma tryptophan availability and improves morning alertness and brain measures of attention. Am J Clin Nutr. 2005;81:1026–1033. [DOI] [PubMed] [Google Scholar]
- 89. Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci. 202;47:2336–2348. [DOI] [PubMed] [Google Scholar]
- 90. Srinivasan V, Pandi-Perumal SR, Trahkt I et al. , Melatonin and melatonergic drugs on sleep: possible mechanisms of action. Int J Neurosci. 2009;119:821–846. [DOI] [PubMed] [Google Scholar]
- 91. Silber BY, Schmitt JA.. Effects of tryptophan loading on human cognition, mood, and sleep. Neurosci Biobehav Rev. 2010;34:387–407. [DOI] [PubMed] [Google Scholar]
- 92. Hess SM, Doepfner W.. Behavioral effects and brain amine content in rats. Arch Int Pharmacodyn Ther. 1961;134:89–99. [PubMed] [Google Scholar]
- 93. Schildkraut JJ. The catecholamine hypothesis of affective disorders. Am J Psychiat. 1965;122:509–522. [DOI] [PubMed] [Google Scholar]
- 94. Jensen K, Fruensgaard K, Ahlfors UG et al. , Letter: tryptophan/imipramine in depression. Lancet. 1975;306:920.. [DOI] [PubMed] [Google Scholar]
- 95. Coppen A, Shaw DM, Farrell JP.. Potentiation of the antidepressive effect of a monoamine-oxidase inhibitor by tryptophan. Lancet. 1963;281:79–81. [DOI] [PubMed] [Google Scholar]
- 96. Walinder J, Skott A, Carlsson A et al. , Potentiation of the antidepressant action of clomipramine by tryptophan. Arch Gen Psychiatry. 1976;33:1384–1389. [DOI] [PubMed] [Google Scholar]
- 97. Pare CM. Potentiation of monoamine-oxidase inhibitors by tryptophan. Lancet. 1963;282:527–528. [DOI] [PubMed] [Google Scholar]
- 98. Cowen PJ. Serotonin and depression: pathophysiological mechanism or marketing myth? Trends Pharmacol Sci. 2008;29:433–436. [DOI] [PubMed] [Google Scholar]
- 99. Glassman AH, Platman SR.. Potentiation of a monoamine oxidase inhibitor by tryptophan. J Psychiatr Res. 1969;7:83–88. [DOI] [PubMed] [Google Scholar]
- 100. Rao B, Broadhurst AD.. Letter: tryptophan and depression. Br Med J. 1976;1:460.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Markus CR, Firk C, Gerhardt C et al. , Effect of different tryptophan sources on amino acids availability to the brain and mood in healthy volunteers. Psychopharmacology (Berl). 2008;201:107–114. [DOI] [PubMed] [Google Scholar]
- 102. Kroes MC, van Wingen GA, Wittwer J et al. , Food can lift mood by affecting mood-regulating neurocircuits via a serotonergic mechanism. Neuroimage. 2014;84:825–832. [DOI] [PubMed] [Google Scholar]
- 103. Scrutton H, Carbonnier A, Cowen PJ et al. , Effects of alpha-lactalbumin on emotional processing in healthy women. J Psychopharmacol. 2007;21:519–524. [DOI] [PubMed] [Google Scholar]
- 104. Merens W, Booij L, Markus R et al. , The effects of a diet enriched with α-lactalbumin on mood and cortisol response in unmedicated recovered depressed subjects and controls. Br J Nutr. 2005;94:415–422. [DOI] [PubMed] [Google Scholar]
- 105. Mohajeri MH, Wittwer J, Vargas K et al. , Chronic treatment with a tryptophan-rich protein hydrolysate improves emotional processing, mental energy levels and reaction time in middle-aged women. Br J Nutr. 2015;113:350–365. [DOI] [PubMed] [Google Scholar]
- 106. Engel J. Epilepsy in the world today: medical point of view. Epilepsia. 2002;43(suppl 6):12–13. [DOI] [PubMed] [Google Scholar]
- 107. Bonnycastle DD, Giarman NJ, Paasonen MK.. Anticonvulsant compounds and 5-hydroxytryptamine in rat brain. Br J Pharmacol Chemother. 1957;12:228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Mainardi P, Leonardi A, Albano C.. Potentiation of brain serotonin activity may inhibit seizures, especially in drug-resistant epilepsy. Med Hypotheses. 2008;70:876–879. [DOI] [PubMed] [Google Scholar]
- 109. Meldrum BS, Balzamo E, Wada JA et al. , Effects of L-tryptophan, L-3, 4, dihydroxyphenylalanine and tranylcypromine on the electroencephalogram and on photically induced epilepsy in the baboon, Papio papio. Physiol Behav. 1972;9:615–621. [DOI] [PubMed] [Google Scholar]
- 110. Wada JA, Balzamo E, Meldrum BS et al. , Behavioural and electrographic effects of L-5-hydroxytryptophan and D, L-parachlorophenyl-alanine on epileptic Senegalese baboon (Papio papio). Electroencephalogr Clin Neurophysiol. 1972;33:520–526. [DOI] [PubMed] [Google Scholar]
- 111. Yan QS, Jobe PC, Dailey JW.. Evidence that a serotonergic mechanism is involved in the anticonvulsant effect of fluoxetine in genetically epilepsy-prone rats. Eur J Pharmacol. 1994;252:105–112. [DOI] [PubMed] [Google Scholar]
- 112. Chadwick D, Trimble M, Jenner P et al. , Manipulation of cerebral monoamines in the treatment of human epilepsy: a pilot study. Epilepsia. 1978;19:3–10. [DOI] [PubMed] [Google Scholar]
- 113. Ghose KL. Tryptophan in hyperactive child syndrome associated with epilepsy: a controlled study. Neuropsychobiology. 1983;10:111–114. [DOI] [PubMed] [Google Scholar]
- 114. Raotma H. Has tryptophan any anticonvulsive effect? Acta Psychiatr Scand. 1978;57:253–258. [DOI] [PubMed] [Google Scholar]
- 115. Citraro R, Scicchitano F, De Fazio S et al. , Preclinical activity profile of α-lactoalbumin, a whey protein rich in tryptophan, in rodent models of seizures and epilepsy. Epilepsy Res. 2011;95:60–69. [DOI] [PubMed] [Google Scholar]
- 116. Russo E, Scicchitano F, Citraro R et al. , Protective activity of α-lactoalbumin (ALAC), a whey protein rich in tryptophan, in rodent models of epileptogenesis. Neuroscience. 2012;226:282–288. [DOI] [PubMed] [Google Scholar]
- 117. Fernstrom JD. A perspective on the safety of supplemental tryptophan based on its metabolic fates. J Nutr. 2016;146:2601S–2608S. [DOI] [PubMed] [Google Scholar]
- 118. Stone TW, Stoy N, Darlington LG.. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol Sci. 2013;34:136–143. [DOI] [PubMed] [Google Scholar]
- 119. Toscano CD, Kingsley PJ, Marnett LJ et al. , NMDA-induced seizure intensity is enhanced in COX-2 deficient mice. Neurotoxicology. 2008;29:1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bender DA. Biochemistry of tryptophan in health and disease. Mol Aspects Med. 1983;6:101–197. [DOI] [PubMed] [Google Scholar]
- 121. During MJ, Freese A, Heyes MP et al. , Neuroactive metabolites of L-tryptophan, serotonin and quinolinic acid, in striatal extracellular fluid. Effect of tryptophan loading. FEBS Lett. 1989;247:438–444. [DOI] [PubMed] [Google Scholar]
- 122. Guillemin GJ. Quinolinic acid, the inescapable neurotoxin. FEBS J. 2012;279:1356–1365. [DOI] [PubMed] [Google Scholar]
- 123. Cross ML, Gill HS.. Immunomodulatory properties of milk. Br J Nutr. 2000;84:S81–S89. [DOI] [PubMed] [Google Scholar]
- 124. Wu G, Fang YZ, Yang S et al. , Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–492. [DOI] [PubMed] [Google Scholar]
- 125. Townsend DM, Tew KD, Tapiero H.. The importance of glutathione in human disease. Biomed Pharmacother. 2003;57:145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Choi IY, Lee P, Denney DR et al. , Dairy intake is associated with brain glutathione concentration in older adults. Am J Clin Nutr. 2015;101:287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bounous G, Gervais F, Amer V et al. , The influence of dietary whey protein on tissue glutathione and the diseases of aging. Clin Invest Med. 1989;12:343–349. [PubMed] [Google Scholar]
- 128. Grey V, Mohammed SR, Smountas AA et al. , Improved glutathione status in young adult patients with cystic fibrosis supplemented with whey protein. J Cyst Fibros. 2003;2:195–198. [DOI] [PubMed] [Google Scholar]
- 129. Stipanuk MH, Watford M.. Amino acid metabolism In: Biochemical and Physiological Aspects of Human Nutrition. Stipanuk MH, ed. Philadelphia, PA: WB Saunders; 2000: 233–286. [Google Scholar]
- 130. Baker DH. Comparative nutrition and metabolism: explication of open questions with emphasis on protein and amino acids. Proc Nat Acad Sci U S A. 2005;102:17897–17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. McCarty MF, DiNicolantonio JJ.. An increased need for dietary cysteine in support of glutathione synthesis may underlie the increased risk for mortality associated with low protein intake in the elderly. Age (Dordr). 2015;37:96. doi:10.1007/s11357-015-9823-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Bounous G, Kongshavn PAL.. Influence of dietary proteins on the immune system of mice. J Nutr. 1982;112:1747–1755. [DOI] [PubMed] [Google Scholar]
- 133. Bounous G, Letourneau L, Kongshavn PAL.. Influence of dietary protein type on the immune system of mice. J Nutr. 1983;113:1415–1421. [DOI] [PubMed] [Google Scholar]
- 134. Mariotti F, Simbelie KL, Makarios-Lahham K et al. , Acute ingestion of dietary proteins improves post-exercise liver glutathione in rats in a dose-dependent relationship with their cysteine content. J Nutr. 2004;134:128–131. [DOI] [PubMed] [Google Scholar]
- 135. Ford JT, Wong CW, Colditz IG.. Effects of dietary protein types on immune respnses and levels of infecion with Eimeria vermiformis in mice. Immunol Cell Biol. 2001;79:23–28. [DOI] [PubMed] [Google Scholar]
- 136. Blouet C, Mariotti F, Mikogami T et al. , Meal cysteine improves postprandial glucose control in rats fed a high-sucrose meal. J Nutr Biochem. 2007;18:519–524. [DOI] [PubMed] [Google Scholar]
- 137. Micke P, Beeh KM, Buhl R.. Effects of long-term supplementation with whey proteins on plasma glutathione levels of HIV-infected patients. Eur J Nutr. 2002;41:12–18. [DOI] [PubMed] [Google Scholar]
- 138. Micke P, Beeh KM, Schlaak JF et al. , Oral supplementation with whey proteins increases plasma glutathione levels of HIV-infected patients. Eur J Clin Invest. 2001;31:171–178. [DOI] [PubMed] [Google Scholar]
- 139. Agin D, Kotler DP, Papandreou D et al. , Effects of whey protein and resistance exercise on body composition and muscle strength in women with HIV infection. Ann N Y Acad Sci. 2000;904:607–609. [DOI] [PubMed] [Google Scholar]
- 140. Bauer J, Biolo G, Cederholm T et al. , Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14:542–559. [DOI] [PubMed] [Google Scholar]
- 141. Calder PC, Yaqoob P.. Glutamine and the immune system. Amino Acids. 1999;17:227–241. [DOI] [PubMed] [Google Scholar]
- 142. Calder PC. Branched-chain amino acids and immunity. J Nutr. 2006;136:288S–293S. [DOI] [PubMed] [Google Scholar]
- 143. Wolfe RR. Branched-chain amino acids and muscle protein synthesis in humans: myth or reality? J Inter Soc Sports Nutr. 2017;17:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Tang JE, Phillips SM.. Maximizing muscle protein anabolism: the role of protein quality. Curr Opin Clin Nutr Metab Care. 2009;12:66–71. [DOI] [PubMed] [Google Scholar]
- 145. Groen BB, Res PT, Pennings B et al. , Intragastric protein administration stimulates overnight muscle protein synthesis in elderly men. Am J Physiol Endocrinol Metab. 2012;302:E52–E60. [DOI] [PubMed] [Google Scholar]
- 146. Res PT, Groen B, Pennings B et al. , Protein ingestion before sleep improves postexercise overnight recovery. Med Sci Sports Exerc. 2012;44:1560–1569. [DOI] [PubMed] [Google Scholar]
- 147. Snijders T, Res PT, Smeets JS et al. , Protein ingestion before sleep increases muscle mass and strength gains during prolonged resistance-type exercise training in healthy young men. J Nutr. 2015;145:1178–1184. [DOI] [PubMed] [Google Scholar]
- 148. Van Cauter E, Plat L, Copinschi G.. Interrelations between sleep and the somatotropic axis. Sleep. 1998;21:553–566. [PubMed] [Google Scholar]
- 149. Marliss EB, Aoki TT, Pozefsky T et al. , Muscle and splanchnic glutamine and glutamate metabolism in postabsorptive and starved man. J Clin Invest. 1971;50:814–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Bassit RA, Sawada LA, Bacurau RFP et al. , Branched-chain amino acid supplementation and the immune response of long-distance athletes. Nutrition. 2002;18:376–379. [DOI] [PubMed] [Google Scholar]
- 151. Newgard CB, An J, Bain JR et al. , A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Giesbertz P, Daniel H.. Branched-chain amino acids as biomarkers in diabetes. Curr Opin Clin Nutr Metab Care. 2016;19:48–54. [DOI] [PubMed] [Google Scholar]
- 153. Knebel B, Strassburger K, Szendroedi J et al. , Specific metabolic profiles and their relationship to insulin resistance in recent-onset type 1 and type 2 diabetes. J Clin Endocrinol Metab. 2016;101:2130–2140. [DOI] [PubMed] [Google Scholar]
- 154. Macotela Y, Emanuelli B, Bang AM et al. , Dietary leucine—an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS One. 2011;6:e21187. doi:10.1371/journal.pone.0021187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Lackey DE, Lynch CJ, Olson KC et al. , Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am J Physiol Endocrinol Metab. 2013;304:E1175–E1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Devkota S, Layman DK.. Protein metabolic roles in treatment of obesity. Curr Opin Clin Nutr Metab Care. 2010;13:403–407. [DOI] [PubMed] [Google Scholar]
- 157. Layman DK, Walker DA.. Potential importance of leucine in treatment of obesity and the metabolic syndrome. J Nutr. 2006;136(1 suppl):319S–323S. [DOI] [PubMed] [Google Scholar]
- 158. Lynch CJ, Adams SH.. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10:723–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Wang TJ, Larson MG, Vasan RS et al. , Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Pedroso JA, Zampieri TT, Donato J Jr.. Reviewing the effects of L-leucine supplementation in the regulation of food intake, energy balance, and glucose homeostasis. Nutrients. 2015;7:3914–3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Bounous G, Shenouda N, Kongshavn PAL et al. , Mechanism of altered B-cell response induced by changes in dietary protein type in mice. J Nutr. 1985;115:1409–1417. [DOI] [PubMed] [Google Scholar]
- 162. Mcintoch GH, Regester GO, Le Leu RK et al. , Dairy proteins protect against dimethylhydrazine-induced intestinal cancers in rats. J Nutr. 1995;125:809–816. [DOI] [PubMed] [Google Scholar]
- 163. Hakkak R, Korourian S, Shelnutt SR et al. , Diets containing whey proteins or soy protein isolate protect against 7,12-dimethylbenz(a)anthracene-induced mammary tumors in female rats. Cancer Epidemiol Biomarkers Prev. 2000;9:113–117. [PubMed] [Google Scholar]
- 164. Kent KD, Harper WJ, Bomser JA.. Effect of whey proein isolate on intracellular glutathione and oxidant-induced cell death in human prostate epithelial cells. Toxicol in Vitro. 2003;17:27–33. [DOI] [PubMed] [Google Scholar]
- 165. Ho JCS, Nadeem A, Svanborg C.. HAMLET—a protein-lipid complex with broad tumoricidal activity. Biochem Biophys Res Comm. 2017;482:454–458. [DOI] [PubMed] [Google Scholar]
- 166. Rath EM, Duff AP, Hakansson AP et al. , Structure and potential cellular targets of HAMLET-like anti-cancer compounds made from milk components. J Pharm Pharm Sci. 2015;18:773–824. [DOI] [PubMed] [Google Scholar]
- 167. Rammer P, Groth-Pedersen L, Kirkegaard T et al. , BAMLET activates a lysosomal cell death program in cancer cells. Mol Cancer Ther. 2010;9:24–32. [DOI] [PubMed] [Google Scholar]
- 168. Fontana A, Spolaore B, Polverino de Laureto P.. The biological activities of protein/oleic acid complexes reside in the fatty acid. Biochim Biophys Acta. 2013;1834:1125–1143. [DOI] [PubMed] [Google Scholar]
- 169. Pettersson J, Mossberg AK, Svanborg C.. α-Lactalbumin species variation, HAMLET formation, and tumor cell death. Biochem Biophys Res Comm. 2006;345:260–270. [DOI] [PubMed] [Google Scholar]
- 170. Delgado Y, Morales-Cruz M, Figueroa CM et al. , The cytotoxicity of BAMLET complexes is due to oleic acid and independent of the α-lactalbumin component. FEBS Open Bio. 2015;5:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Nakamura T, Aizawa T, Kariya R et al. , Molecular mechanisms of the cytotoxicity of human α-lactalbumin made lethal to tumor cells (HAMLET) and other protein-oleic acid complexes. J Biol Chem. 2013;288:14408–14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Bruck WM, Gibson GR, Bruck TB.. The effect of proteolysis on the induction of cell death by monomeric alpha-lactalbumin. Biochimie. 2014;97:138–143. [DOI] [PubMed] [Google Scholar]
- 173. Sullivan LM, Kehoe JJ, Barry L et al. , Gastric digestion of α-lactalbumin in adult human subjects using capsule endoscopy and nasogastric tube sampling. Br J Nutr. 2014;112:638–646. [DOI] [PubMed] [Google Scholar]
- 174. Gustafsson L, Leijonhufvud I, Aronsson A et al. , Treatment of skin papillomas with topical α-lacalbumin–oleic acid. N Engl J Med. 2004;350:2663–2672. [DOI] [PubMed] [Google Scholar]
- 175. Mossberg AK, Wullt B, Gustafsson L et al. , Bladder cancers respond to intravesical instillation of HAMLET (human α-lactalbumin made lethal to tumor cells). Int J Cancer. 2007;121:1352–1359. [DOI] [PubMed] [Google Scholar]
- 176. Mossberg AK, Hou Y, Svensson M et al. , HAMLET treatment delays bladder cancer development. J Urol. 2010;183:1590–1597. [DOI] [PubMed] [Google Scholar]
- 177. Fischer W, Gustafsson L, Mossberg AK et al. , Human α-lactalbumin made lethal to tumor cells (HAMLET) kills human glioblastoma cells in brian xenografts by an apoptosis-like mechanism and prolongs survival. Cancer Res. 2004;64:2105–2112. [DOI] [PubMed] [Google Scholar]
- 178. Puthia M, Storm P, Nadeem A et al. , Prevention and treatment of colon cancer by peroral administratioin of HAMLET (human α-lactalbumin made lethal to tumour cells). Gut. 2013;63:131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]