Version Changes
Revised. Amendments from Version 1
We have revised the article in accordance with the suggestions made by the reviewers. We have phrased some of the conclusions (with regard to congruent risk factors and CMV vaccination) more cautiously, and added immunological detail on the role of Type I IFN and the effect of human CMV on NK cell function. See detailed responses to the reviewers' comments.
Abstract
Although several factors are known to increase the risk of tuberculosis, the occurrence of tuberculosis disease in an infected individual is difficult to predict. We hypothesize that active human cytomegalovirus infection due to recent infection, reinfection or reactivation plays an epidemiologically relevant role in the aetiology of tuberculosis by precipitating the progression from latent tuberculosis infection to disease. The most compelling support for this hypothesis comes from the striking similarity in age-sex distribution between the two infections, important because the age-sex pattern of tuberculosis disease progression has not been convincingly explained. Cytomegalovirus infection and tuberculosis have other overlapping risk factors, including poor socio-economic status, solid organ transplantation and, possibly, sexual contact and whole blood transfusion. Although each of these overlaps could be explained by shared underlying risk factors, none of the epidemiological observations refute the hypothesis. If this interaction would play an epidemiologically important role, important opportunities would arise for novel approaches to controlling tuberculosis.
Keywords: Tuberculosis, latent tuberculosis infection, human cytomegalovirus, epidemiology, age pattern, risk factor
Introduction
With 10.4 million new cases and 1.7 million deaths per year, tuberculosis (TB) remains a major global health problem 1. Only 5%–15% of individuals infected with Mycobacterium tuberculosis (Mtb) ever develop TB disease, and over 50% of these do so within two years after infection 2. Although risk factors for progression to TB disease have been identified 3, disease occurrence cannot be accurately predicted 4.
Recent data suggest that infection with human cytomegalovirus (HCMV) is a predictor of TB disease in infants. In a cohort study of South African infants, an HCMV-specific IFN-γ T-cell response was associated with a 2.2-fold increased risk of TB disease over a period of up to 3 years. A similar response to Epstein-Barr virus (EBV) showed no such associations 5. HCMV-positive and HCMV-negative infants had distinct immune pathways associated with TB disease. Although CD8+ T-cell activation was a distinguishing feature of HCMV-positive infants, the proposed immunological mechanism was impairment of the natural killer (NK) cell response. In African infants, HCMV infection induced profound CD8+ T-cell and NK cell differentiation and poor physical growth 5– 7.
The possibility that this association between HCMV and TB disease progression is causal, also holds in adults, and thus merits further study is dependent on its epidemiological plausibility. Only few published studies have investigated epidemiological associations between the two diseases 8– 10. Despite this paucity of direct evidence we argue that the epidemiology of TB and HCMV share important similarities that make HCMV infection a plausible candidate as a cause of TB disease progression.
Viral triggers of tuberculosis disease
Various etiological frameworks for TB disease progression have been developed. One proposed by Comstock considers TB disease the result of two hits or causes, one of which is Mtb infection, and the other (still) unknown 11. In this framework, factors that strongly increase the risk of TB disease such as HIV infection or anti-tumour necrosis alpha therapy may act as a second hit but would not account for all or most TB cases.
Several factors have been identified that increase the risk of disease progression, such as low body-mass index 12, diabetes 13, tobacco smoking 14, and alcohol abuse 15. As their effects are modest another framework has emerged that these are predisposing conditions for disease progression while other, yet unidentified precipitating events are needed to trigger progression to active disease 16. Among the precipitating events suggested are viral infections, possibly through induction of Type I interferons (IFN). Elevated Type I IFN signalling is a hallmark of viral control, however, Type I IFN is also associated with susceptibility to bacterial infections, including Mtb 17– 22. The Type I IFN response is tightly regulated by prostaglandins and the balance between prostoglandins PGE2 and LXA4 can be manipulated by Mtb to drive Type I IFN mediated necrosis and promote mybobacterial dissemination 19, 23. Type I IFN-associated impairment of the immunity against Mtb has been shown for influenza A 21. A role for influenza A infection has also been suggested by epidemiological data. Notification of TB tends to peak in the months after winter when most respiratory viruses circulate 24, and TB mortality has shown increases during influenza epidemics 25, 26. However, careful analysis of seasonality data suggests that it is TB transmission rather than disease progression that is increased in winter 27, and increased TB mortality during influenza epidemics may reflect increased case fatality among TB patients due to secondary influenza rather than increased TB incidence.
As with many viral infections, Type I IFN can control HCMV replication 28, 29. HCMV has been suggested in three studies from Nigeria, Russia and Uganda that all found higher prevalence or levels of IgG HCMV antibodies in diagnosed TB patients compared to healthy controls and patients diagnosed with other diseases 8– 10.
Human cytomegalovirus infection
HCMV, human herpesvirus 5, is a double-stranded DNA virus. After primary infection, usually through mucosal contact, HCMV remains dormant in the host’s myeloid tissues but can reactivate if immunity is compromised. Primary infection is often asymptomatic but can present as mononucleosis with fever, pharyngo-tonsillitis and lymphadenopathy. In congenitally infected infants HCMV may cause severe generalized infection with high case fatality and neurologic sequelae 30. Generalized infection also occurs in severely immunocompromised adults, usually through reactivation. During primary infection and reactivation virus is shed in the urine, saliva, breast milk, cervical fluid and semen 31. Common routes of transmission are from mother to child during delivery, between children and by sexual contact. Transmission through blood transfusion and solid organ transplantations also occurs.
HCMV viruses show genomic diversity, in particular in genes coding for envelope glycoproteins, and polymorphisms in these genes have been used to genotype strains 32, 33. Both immunocompromised and immunocompetent individuals can be re-infected and harbour multiple HCMV strains 34– 36.
Primary HCMV infection is characterized by profound expansion of antigen specific CD8+ and CD4+ T cells and NK cell populations with specificity for HCMV 37. HCMV expanded NK cells can display inappropriate homing to tissue infected with other pathogens and lower IFN-γ secretion in response to pathogens 38. HCMV infection drives the expansion of CD94/NKG2C NK cells and these cells are important for control of viral replication 39. In HCMV positive infants who progressed to TB disease in the South African cohort there was lower expression of CD94 and NKG2C (KLRD1 and KLRC3) transcripts and lower frequency of NK cells 5. The NKG2C receptor is encoded by the KLRC2 gene which is deleted in approximately 10% of individuals 40. The KLRC2 gene deletion is associated with lower numbers of mature NK cells and increased risk of HIV infection and disease progression 41, 42, as well as with susceptibility to autoimmune conditions and cancer 40. Susceptibility to TB in this infant population may be due to loss of control of CMV infection due to KLRC2 gene defects in some individuals.
HCMV has multiple immune evasion strategies 37, which may make the microenvironment around latently infected myeloid cells suppressive to T-cell function, potentially creating an environment permissive for mycobacterial growth 43, 44. This may be through effects of HCMV on the systemic immune response, but also through local effects. The lung is a reservoir of HCMV infection 45, 46 and frequently the site of viral reactivation 47, which drives inflammation and in mice may cause pulmonary fibrosis 48. It is therefore possible that active HCMV (re)infection or reactivation of latent HCMV could precipitate progression to TB disease.
Epidemiological convergence
Both Mtb and HCMV infections are ubiquitous 1, 49, and during millions of years of co-evolution have become highly human host-specific 50– 52. An animal reservoir has been described for neither Mtb nor HCMV (several monkey and rodent species have their distinct CMV species), implying that their epidemiological patterns are entirely determined by transmission between, and carriage by, humans.
We hypothesize that immunologically active HCMV infection, whether primary, reactivation or re-infection, acts as (depending on one’s preferred framework) second-hit or precipitating factor for progression of latent TB infection to TB disease at an epidemiologically relevant scale. We base this on two arguments: their striking similarity in age distribution, and the existence of congruent risk factors [ Box 1].
Box 1. Approach to evidence gathering.
We systematically searched PubMed for the following combinations of keywords: tuberculosis and cytomegalovirus; cytomegalovirus and prevalence or seroprevalence; cytomegalovirus and age; cytomegalovirus and reinfection; cytomegalovirus and sexual; tuberculosis and sexual; tuberculosis and sexual transmitted infections or Chlamydia or gonorrhoea or human papillomavirus; tuberculosis and blood transfusion; cytomegalovirus and blood transfusion; tuberculosis and gastrectomy; cytomegalovirus and renal dialysis; tuberculosis and renal dialysis; tuberculosis and organ transplantation; cytomegalovirus and organ transplantation.
We in addition made use of an extensive review of the literature on age-sex distribution of tuberculosis incidence published by Nagelkerke (2012) 53.
Age distribution
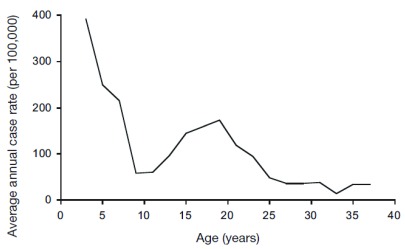
The probability of progressing from TB infection to disease has a highly typical age distribution. The classical description of this age pattern is by Comstock et al, who followed 82,269 Puerto Rican children reacting to tuberculin enrolled in 1949–1951 for 8 to 20 years [ Figure 1] 54. This pattern, confirmed in a systematic review of studies from the pre-chemotherapy era 55, is defined by a peak in the first 1–4 years of life, followed by a trough until early puberty, rising to a second peak around the age of 20 years. Analyses of notification and prevalence data from high-incidence countries show that incidence starts to rise again from the sixth decade 56, 57. Although several explanations for this age pattern have been suggested, none has been proven.
Figure 1. Age-specific incidence of tuberculosis disease among tuberculin-reactive children.
Average annual rate of tuberculosis disease in a cohort of 82,269 Puerto Rican children with a positive tuberculin skin test, by age of disease occurrence. Children were enrolled in the period 1949–1951, and followed for 8 to 20 years. Figure reproduced with permission from Comstock et al. (1974) 54.
Infants. Studies from the pre-chemotherapy era showed that, while the risk of infection with Mtb in the first year of life was over 10-fold lower than later in childhood, the risk of progression to disease once infected was much higher with up to 50% of infected infants developing disease 55. These high progression rates have been attributed to age-specific maturation of immune responses 58, although the mechanisms responsible for this vulnerability have not been elucidated 59.
HCMV infection in infants is common 60. Depending on the country and socio-economic status of the mother, between 10 and 60% of children are HCMV IgG seropositive (reflecting current or past active infection) by the age of 12–36 months 61– 68. Important causes are congenital infection and transmission through breastfeeding; >85% of HCMV seropositive women excrete virus in the breastmilk 60, 69– 72. Infants infected through breastfeeding do not develop disease, probably due to protection by maternal antibodies, but do shed virus in saliva and urine intermittently for months, by which they may transmit HCMV to other children and caregivers 31, 60, 64, 73. Shedding of HCMV shows a steep decline by the age of 5 years 31, coinciding with the age at which TB incidences drop 54, 55.
Adolescents. The rate of progression to TB disease then remains low until puberty. Several studies have observed an increase in TB incidence from this age onward among children who were exposed to infectious TB patients or had a positive tuberculin response, leading to a peak in incidence in the first half of the third decade 54, 74– 79. This phenomenon has been attributed to hormonal changes, but again without a putative mechanistic pathway 80.
Most population-based studies of HCMV seroprevalence show exactly this age pattern: a slow increase in HCMV IgG seroprevalence up to the age of 10–15 years, followed by an acceleration during adolescence 61, 63– 66, 68, 81– 88. One explanation for this increase in seroprevalence is sexual transmission. Various studies found that HCMV conversion among women was associated with sexual activity 89– 93. However, as several studies of adolescents found no association of HCMV seroprevalence with sexual exposure 83, 94, 95, other transmission routes such as mouth-to-mouth kissing may also be important.
Another indication that HCMV infection may be implicated is the sex difference in TB disease progression in the second decade. For girls the increase in TB incidence starts 2–4 years earlier than for boys, and progression rates tend to remain higher in women than in men for the subsequent two decades, a pattern that was observed before the HIV era in various populations 54, 75, 76, 78, 79, 96– 99. This pattern is again reflected in that of HCMV infection. The acceleration of HCMV seroprevalence during puberty and adolescence is steeper in girls than in boys and is higher in women of childbearing age than in men in populations with relatively low HCMV seroprevalence 49, 64, 65, 84, 100– 102. Age-adjusted HCMV seroprevalence does not differ between men and women in populations with high seroprevalence 49, 103. This may be because IgG seroprevalence measures cumulative infection experience and thus ignores reinfection. HCMV reinfection, identified by DNA typing or strain-specific antibody responses, is a common occurrence in sexually exposed women 104– 106.
Elderly. Although there is little data on TB progression rates in the elderly, age patterns of TB notifications suggest increased progression rates from the sixth decade onward 1, 56. In populations with declining incidence rates over the past decades this is partially a cohort effect, whereby younger generations have lower prevalence of latent infection 107, 108. However in high-incidence countries with little change in TB incidence, notification rates clearly increase at older age 1. This is also observed for TB prevalence in population surveys, suggesting that this is not explained by better access to diagnosis 1. HCMV infection has been implicated as a cause of age-related decrease in naïve T cells and increase in memory T cells known as immunosenescence 109. However, reactivation of HCMV infection is also common at old age, probably reflecting weakening immune control 37. Detection of viral DNA increases after the age of 60–70 years 110, 111, and viral DNA is frequently detected in urine and plasma of elderly people 112, 113.
Congruent risk factors
Our hypothesis predicts that factors that drive CMV (re-)infection are also risk factors for TB. We highlight the four most important: socio-economic status, sexual contact, blood transfusion, and solid organ transplantation.
Socio-economic status. Incidence and prevalence of CMV infection are associated with poor socio-economic status (SES), between countries as well as within countries and communities 49, 64, 65, 100, 114– 116. This includes association with crowding, in particular the number of young children in household 117– 119. Several studies found ethnicity or migrant status to be independently associated with age-adjusted CMV prevalence 65, 84, 120, which may partly reflect higher background infection rates in the country of origin. In a US study the association with ethnicity was explained by differences in exposure to infants and sexual risk 93.
Also the incidence of TB, often regarded as the archetypal poverty disease, shows a remarkable inverse gradient with SES at the household, regional and country level 121– 123. This association has been explained mainly by crowding in ill-ventilated spaces conducive to Mtb transmission 124, poor nutritional status 12, 125, alcohol abuse 15 and, possibly, indoor air pollution 126. Similarly, in low-incidence countries, TB incidences are higher in particular ethnic groups and immigrants 127, 128, which also may reflect socio-economic disparities and differences in background infection rates 129. Very few studies have attempted to investigate whether these and other known risk factors explain all of the observed variation in SES-related TB incidence 130.
Sexual contact. The risk of CMV (re-)infection in adults is correlated with measures of sexual activity such as age at first intercourse, recent and lifetime number of sexual partners and condom use, as well as with prevalence of other sexually transmitted infections 89, 90, 92, 131– 133. Historically, TB has also been associated with sexual promiscuity in medical and popular literature (reviewed in 53) but no systematic epidemiological data exist. Investigation of associations between TB disease and sexually transmitted infections has been strongly dominated by HIV infection, which may obviously be a major confounder. There have been few studies from low HIV prevalence populations. One from China found an association between history of TB and human papilloma virus infection 134.
Interestingly, the declining TB mortality rates in The Netherlands and England and Wales in the 20 th century showed no surge during the Great Depression 121, 135, when SES status deteriorated thereby affecting several of these known risk factors, in particular nutritional status. They did however surge during and shortly after the Second World War 121, 135. In England and Wales this was not paralleled by major deterioration in nutritional status; in The Netherlands famine only started in the winter of 1944–45 while the increase in TB mortality started already from 1942 53. In both countries during this period major increases were seen in sexually transmitted infections, mainly related to presence of large numbers of Allied and Axis troops 53.
Blood transfusion. Transfusion-associated CMV infection occurs in particular following multiple transfusions of whole blood or granulocytes, and can be prevented by removal of white blood cells 136– 138. Increased incidences of TB have indeed been described in two categories of patients who in the past often received multiple whole blood transfusions: patients who underwent (partial) gastrectomy, mainly for bleeding gastric ulcers 139, 140, and patients with end-stage renal disease on haemodialysis 141. For both these categories alternative explanations for increased TB incidences are possible: low body mass index for gastrectomy 139, 140, and impaired cellular immunity due to uraemia for haemodialysis 142. Nonetheless, several studies among haemodialysis patients have suggested increased rates of CMV (re)infection, either or not associated with transfusion of blood or blood products 143– 147, as well as increased rates of CMV reactivation 143.
Solid organ transplantation. The incidence of symptomatic CMV infection is strongly increased in solid organ transplant patients, mainly due to infection from a CMV IgG positive donor 148. Solid organ transplantation also increases the risk of TB disease 141, 149– 151. TB incidence is highest in lung transplant patients and associated with presence of latent TB infection, clinical condition and intensity of the immunosuppressive therapy; the latter has been brought forward as the sole explanation for the increased TB risk 152. Interestingly, a study among Korean solid organ transplant patients found that the risk of developing TB was associated with CMV infection within the prior 3 months 153.
Potential impact on tuberculosis control and elimination
If indeed CMV (re-)infection or reactivation commonly precipitates progression from latent infection to active TB, this will suggest novel approaches to TB control. Combining a test for Mtb infection with one for ongoing or recent active CMV infection may strongly increase our ability to predict the development of TB disease and allow the targeting of preventive treatment to those most at risk 4. Vaccination against CMV might prevent TB in those with TB infection. A wide range of CMV vaccines are currently in clinical development including plasmid-based vaccines, viral vector vaccines, attenuated HCMV strains, and recombinant protein and peptide vaccines 154. Recently, a genetically modified CMV vector expressing antigens from Mtb (RhCMV/TB) has shown some protection against Mtb in a non-human primate study 155. If the human version of this vaccine was able to afford (partial) protection against CMV, it could also significantly impact the TB epidemic. However, there is currently no widely available HCMV vaccine and it is unclear if vaccines based on HCMV in humans will offer similar protection to those based on RhCMV in non-human primates. Since CMV infection may also affect TB treatment response, another potential application therefore could be the provision of CMV antiviral treatment as an adjunct to TB treatment, for example of patients with multidrug resistance.
Future research
There is an urgent need for elucidating the role of CMV infection in TB disease progression. Further serological and cellular studies should be done to confirm the association between TB disease and CMV infection. However, in settings with high CMV seroprevalence (also those with highest TB incidence) it will be important to identify recent reinfection for which various diagnostic approaches exist 30. Their relative merits are beyond the scope of this article, but some may potentially signal reactivation due to Mtb replication, i.e. consequence rather than cause. Therefore, ultimately longitudinal studies are needed in which the incidence of TB disease among those with latent TB infection is measured over time comparing those with CMV (re-)infection or reactivation to those without. These studies should be supplemented with immunological studies to define the mechanisms through which CMV precipitates progression to active TB disease. Finally, it will be important to study the role of CMV reactivation during TB disease and its effect on the response to TB treatment.
Data availability
No data is associated with this article.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 2; referees: 2 approved]
References
- 1. WHO: Global tuberculosis report 2017.WHO: Geneva,2017. Reference Source [Google Scholar]
- 2. Ferebee SH: Controlled chemoprophylaxis trials in tuberculosis. A general review. Bibl Tuberc. 1970;26:28–106. [PubMed] [Google Scholar]
- 3. Dheda K, Barry CE, 3rd, Maartens G: Tuberculosis. Lancet. 2016;387(10024):1211–26. 10.1016/S0140-6736(15)00151-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cobelens F, Kik S, Esmail H, et al. : From latent to patent: rethinking prediction of tuberculosis. Lancet Respir Med. 2017;5(4):243–4. 10.1016/S2213-2600(16)30419-2 [DOI] [PubMed] [Google Scholar]
- 5. Muller J, Matsumiya M, Snowden MA, et al. : Cytomegalovirus infection is a risk factor for TB disease in Infants. bioRxiv. 2017;222646 10.1101/222646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garcia-Knight MA, Nduati E, Hassan AS, et al. : Cytomegalovirus viraemia is associated with poor growth and T-cell activation with an increased burden in HIV-exposed uninfected infants. AIDS. 2017;31(13):1809–18. 10.1097/QAD.0000000000001568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miles DJ, van der Sande M, Jeffries D, et al. : Cytomegalovirus infection in Gambian infants leads to profound CD8 T-cell differentiation. J Virol. 2007;81(11):5766–76. 10.1128/JVI.00052-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Olaleye OD, Omilabu SA, Baba SS: Cytomegalovirus infection among tuberculosis patients in a chest hospital in Nigeria. Comp Immunol Microbiol Infect Dis. 1990;13(2):101–6. 10.1016/0147-9571(90)90522-U [DOI] [PubMed] [Google Scholar]
- 9. Sirenko IA, Shmat’ko SA, Smelianskaia MV, et al. : [Impact of cytomegalovirus infection on the course of tuberculosis in children and adolescents]. Probl Tuberk Bolezn Legk. 2003; (8):7–9. [PubMed] [Google Scholar]
- 10. Stockdale L, Nash S, Nalwoga A, et al. : Human cytomegalovirus epidemiology and relationship to tuberculosis and cardiovascular disease risk factors in a rural Ugandan cohort. PLoS One. 2018;13(2):e0192086. 10.1371/journal.pone.0192086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Comstock GW: Tuberculosis--a bridge to chronic disease epidemiology. Am J Epidemiol. 1986;124(1):1–16. 10.1093/oxfordjournals.aje.a114352 [DOI] [PubMed] [Google Scholar]
- 12. Lönnroth K, Williams BG, Cegielski P, et al. : A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol. 2010;39(1):149–55. 10.1093/ije/dyp308 [DOI] [PubMed] [Google Scholar]
- 13. Jeon CY, Murray MB: Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5(7):e152. 10.1371/journal.pmed.0050152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin HH, Ezzati M, Murray M: Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. PLoS Med. 2007;4(1):e20. 10.1371/journal.pmed.0040020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lönnroth K, Williams BG, Stadlin S, et al. : Alcohol use as a risk factor for tuberculosis - a systematic review. BMC Public Health. 2008;8:289. 10.1186/1471-2458-8-289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Esmail H, Barry CE, Jr, Young DB, et al. : The ongoing challenge of latent tuberculosis. Philos Trans R Soc Lond B Biol Sci. 2014;369(1645):20130437. 10.1098/rstb.2013.0437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Freudenberg MA, Merlin T, Kalis C, et al. : Cutting edge: a murine, IL-12-independent pathway of IFN-gamma induction by gram-negative bacteria based on STAT4 activation by Type I IFN and IL-18 signaling. J Immunol. 2002;169(4):1665–8. 10.4049/jimmunol.169.4.1665 [DOI] [PubMed] [Google Scholar]
- 18. Fehr T, Schoedon G, Odermatt B, et al. : Crucial role of interferon consensus sequence binding protein, but neither of interferon regulatory factor 1 nor of nitric oxide synthesis for protection against murine listeriosis. J Exp Med. 1997;185(5):921–31. 10.1084/jem.185.5.921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mayer-Barber KD, Andrade BB, Oland SD, et al. : Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature. 2014;511(7507):99–103. 10.1038/nature13489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Manca C, Tsenova L, Freeman S, et al. : Hypervirulent M. tuberculosis W/Beijing strains upregulate type I IFNs and increase expression of negative regulators of the Jak-Stat pathway. J Interferon Cytokine Res. 2005;25(11):694–701. 10.1089/jir.2005.25.694 [DOI] [PubMed] [Google Scholar]
- 21. Redford PS, Mayer-Barber KD, McNab FW, et al. : Influenza A virus impairs control of Mycobacterium tuberculosis coinfection through a type I interferon receptor-dependent pathway. J Infect Dis. 2014;209(2):270–4. 10.1093/infdis/jit424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zak DE, Penn-Nicholson A, Scriba TJ, et al. : A blood RNA signature for tuberculosis disease risk: a prospective cohort study. Lancet. 2016;387(10035):2312–2322. 10.1016/S0140-6736(15)01316-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Divangahi M, King IL, Pernet E: Alveolar macrophages and type I IFN in airway homeostasis and immunity. Trends Immunol. 2015;36(5):307–14. 10.1016/j.it.2015.03.005 [DOI] [PubMed] [Google Scholar]
- 24. Fares A: Seasonality of Tuberculosis. J Glob Infect Dis. 2011;3(1):46–55. 10.4103/0974-777X.77296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walaza S, Cohen C, Nanoo A, et al. : Excess Mortality Associated with Influenza among Tuberculosis Deaths in South Africa, 1999–2009. PLoS One. 2015;10(6):e0129173. 10.1371/journal.pone.0129173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zürcher K, Zwahlen M, Ballif M, et al. : Influenza Pandemics and Tuberculosis Mortality in 1889 and 1918: Analysis of Historical Data from Switzerland. PLoS One. 2016;11(10):e0162575. 10.1371/journal.pone.0162575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tedijanto C, Hermans S, Cobelens F, et al. : Drivers of seasonal variation in tuberculosis incidence: insights from a systematic review and mathematical model. Epidemiology.in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McSharry BP, Forbes SK, Avdic S, et al. : Abrogation of the interferon response promotes more efficient human cytomegalovirus replication. J Virol. 2015;89(2):1479–83. 10.1128/JVI.02988-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fielding CA, Aicheler R, Stanton RJ, et al. : Two novel human cytomegalovirus NK cell evasion functions target MICA for lysosomal degradation. PLoS Pathog. 2014;10(5):e1004058. 10.1371/journal.ppat.1004058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dioverti MV, Razonable RR: Cytomegalovirus. Microbiol Spectr. 2016;4(4). 10.1128/microbiolspec.DMIH2-0022-2015 [DOI] [PubMed] [Google Scholar]
- 31. Cannon MJ, Hyde TB, Schmid DS: Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev Med Virol. 2011;21(4):240–55. 10.1002/rmv.695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chou S: Comparative analysis of sequence variation in gp116 and gp55 components of glycoprotein B of human cytomegalovirus. Virology. 1992;188(1):388–90. 10.1016/0042-6822(92)90771-G [DOI] [PubMed] [Google Scholar]
- 33. Pignatelli S, Dal Monte P, Landini MP: gpUL73 (gN) genomic variants of human cytomegalovirus isolates are clustered into four distinct genotypes. J Gen Virol. 2001;82(Pt 11):2777–84. 10.1099/0022-1317-82-11-2777 [DOI] [PubMed] [Google Scholar]
- 34. Leach CT, Detels R, Hennessey K, et al. : A longitudinal study of cytomegalovirus infection in human immunodeficiency virus type 1-seropositive homosexual men: molecular epidemiology and association with disease progression. J Infect Dis. 1994;170(2):293–8. 10.1093/infdis/170.2.293 [DOI] [PubMed] [Google Scholar]
- 35. Novak Z, Ross SA, Patro RK, et al. : Cytomegalovirus strain diversity in seropositive women. J Clin Microbiol. 2008;46(3):882–6. 10.1128/JCM.01079-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bale JF, Jr, Petheram SJ, Souza IE, et al. : Cytomegalovirus reinfection in young children. J Pediatr. 1996;128(3):347–52. 10.1016/S0022-3476(96)70279-2 [DOI] [PubMed] [Google Scholar]
- 37. Jackson SE, Redeker A, Arens R, et al. : CMV immune evasion and manipulation of the immune system with aging. GeroScience. 2017;39(3):273–91. 10.1007/s11357-017-9986-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goodier MR, Jonjić S, Riley EM, et al. : CMV and natural killer cells: shaping the response to vaccination. Eur J Immunol. 2018;48(1):50–65. 10.1002/eji.201646762 [DOI] [PubMed] [Google Scholar]
- 39. Gumá M, Cabrera C, Erkizia I, et al. : Human cytomegalovirus infection is associated with increased proportions of NK cells that express the CD94/NKG2C receptor in aviremic HIV-1-positive patients. J Infect Dis. 2006;194(1):38–41. 10.1086/504719 [DOI] [PubMed] [Google Scholar]
- 40. Goncalves A, Makalo P, Joof H, et al. : Differential frequency of NKG2C/ KLRC2 deletion in distinct African populations and susceptibility to Trachoma: a new method for imputation of KLRC2 genotypes from SNP genotyping data. Hum Genet. 2016;135(8):939–51. 10.1007/s00439-016-1694-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thomas R, Low HZ, Kniesch K, et al. : NKG2C deletion is a risk factor of HIV infection. AIDS Res Hum Retroviruses. 2012;28(8):844–51. 10.1089/AID.2011.0253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goodier MR, White MJ, Darboe A, et al. : Rapid NK cell differentiation in a population with near-universal human cytomegalovirus infection is attenuated by NKG2C deletions. Blood. 2014;124(14):2213–22. 10.1182/blood-2014-05-576124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brinkmann MM, Dağ F, Hengel H, et al. : Cytomegalovirus immune evasion of myeloid lineage cells. Med Microbiol Immunol. 2015;204(3):367–82. 10.1007/s00430-015-0403-4 [DOI] [PubMed] [Google Scholar]
- 44. Fielding CA, Weekes MP, Nobre LV, et al. : Control of immune ligands by members of a cytomegalovirus gene expansion suppresses natural killer cell activation. eLife. 2017;6: pii: e22206. 10.7554/eLife.22206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Poole E, Juss JK, Krishna B, et al. : Alveolar Macrophages Isolated Directly From Human Cytomegalovirus (HCMV)-Seropositive Individuals Are Sites of HCMV Reactivation In Vivo. J Infect Dis. 2015;211(12):1936–42. 10.1093/infdis/jiu837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gordon CL, Miron M, Thome JJ, et al. : Tissue reservoirs of antiviral T cell immunity in persistent human CMV infection. J Exp Med. 2017;214(3):651–67. 10.1084/jem.20160758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Papazian L, Hraiech S, Lehingue S, et al. : Cytomegalovirus reactivation in ICU patients. Intensive Care Med. 2016;42(1):28–37. 10.1007/s00134-015-4066-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Balthesen M, Messerle M, Reddehase MJ: Lungs are a major organ site of cytomegalovirus latency and recurrence. J Virol. 1993;67(9):5360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cannon MJ, Schmid DS, Hyde TB: Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20(4):202–13. 10.1002/rmv.655 [DOI] [PubMed] [Google Scholar]
- 50. Brosch R, Gordon SV, Marmiesse M, et al. : A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc Natl Acad Sci U S A. 2002;99(6):3684–9. 10.1073/pnas.052548299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gutierrez MC, Brisse S, Brosch R, et al. : Ancient origin and gene mosaicism of the progenitor of Mycobacterium tuberculosis. PLoS Pathog. 2005;1(1):e5. 10.1371/journal.ppat.0010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Reddehase MJ: The immunogenicity of human and murine cytomegaloviruses. Curr Opin Immunol. 2000;12(4):390–6. 10.1016/S0952-7915(00)00106-0 [DOI] [PubMed] [Google Scholar]
- 53. Nagelkerke NJ: Courtesans and consumption. How sexually transmitted infections drive tuberculosis epidemics.Delft: Eburon,2012. Reference Source [Google Scholar]
- 54. Comstock GW, Livesay VT, Woolpert SF: The prognosis of a positive tuberculin reaction in childhood and adolescence. Am J Epidemiol. 1974;99(2):131–8. 10.1093/oxfordjournals.aje.a121593 [DOI] [PubMed] [Google Scholar]
- 55. Marais BJ, Gie RP, Schaaf HS, et al. : The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. 2004;8(4):392–402. [PubMed] [Google Scholar]
- 56. Stead WW, Lofgren JP: Does the risk of tuberculosis increase in old age? J Infect Dis. 1983;147(5):951–5. [DOI] [PubMed] [Google Scholar]
- 57. Onozaki I, Law I, Sismanidis C, et al. : National tuberculosis prevalence surveys in Asia, 1990–2012: an overview of results and lessons learned. Trop Med Int Health. 2015;20(9):1128–45. 10.1111/tmi.12534 [DOI] [PubMed] [Google Scholar]
- 58. Schaaf HS, Collins A, Bekker A, et al. : Tuberculosis at extremes of age. Respirology. 2010;15(5):747–63. 10.1111/j.1440-1843.2010.01784.x [DOI] [PubMed] [Google Scholar]
- 59. Vanden Driessche K, Persson A, Marais BJ, et al. : Immune vulnerability of infants to tuberculosis. Clin Dev Immunol. 2013;2013: 781320. 10.1155/2013/781320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pass RF, Anderson B: Mother-to-Child Transmission of Cytomegalovirus and Prevention of Congenital Infection. J Pediatr Infect Dis Soc. 2014;3 Suppl 1:S2–6. 10.1093/jpids/piu069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liu Z, Wang E, Taylor W, et al. : Prevalence survey of cytomegalovirus infection in children in Chengdu. Am J Epidemiol. 1990;131(1):143–50. 10.1093/oxfordjournals.aje.a115467 [DOI] [PubMed] [Google Scholar]
- 62. Natali A, Valcavi P, Medici MC, et al. : Cytomegalovirus infection in an Italian population: antibody prevalence, virus excretion and maternal transmission. New Microbiol. 1997;20(2):123–33. [PubMed] [Google Scholar]
- 63. Lanzieri TM, Kruszon-Moran D, Amin MM, et al. : Seroprevalence of cytomegalovirus among children 1 to 5 years of age in the United States from the National Health and Nutrition Examination Survey of 2011 to 2012. Clin Vaccine Immunol. 2015;22(2):245–7. 10.1128/CVI.00697-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Voigt S, Schaffrath Rosario A, Mankertz A: Cytomegalovirus Seroprevalence Among Children and Adolescents in Germany: Data From the German Health Interview and Examination Survey for Children and Adolescents (KiGGS), 2003–2006. Open Forum Infect Dis. 2016;3(1):ofv193. 10.1093/ofid/ofv193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Korndewal MJ, Mollema L, Tcherniaeva I, et al. : Cytomegalovirus infection in the Netherlands: seroprevalence, risk factors, and implications. J Clin Virol. 2015;63:53–8. 10.1016/j.jcv.2014.11.033 [DOI] [PubMed] [Google Scholar]
- 66. Fang FQ, Fan QS, Yang ZJ, et al. : Incidence of cytomegalovirus infection in Shanghai, China. Clin Vaccine Immunol. 2009;16(11):1700–3. 10.1128/CVI.00385-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. O’Brien TP, Thompson JM, Black PN, et al. : Prevalence and determinants of cytomegalovirus infection in pre-school children. J Paediatr Child Health. 2009;45(5):291–6. 10.1111/j.1440-1754.2009.01495.x [DOI] [PubMed] [Google Scholar]
- 68. Seale H, MacIntyre CR, Gidding HF, et al. : National serosurvey of cytomegalovirus in Australia. Clin Vaccine Immunol. 2006;13(11):1181–4. 10.1128/CVI.00203-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stagno S, Reynolds DW, Pass RF, et al. : Breast milk and the risk of cytomegalovirus infection. N Engl J Med. 1980;302(19):1073–6. 10.1056/NEJM198005083021908 [DOI] [PubMed] [Google Scholar]
- 70. Hotsubo T, Nagata N, Shimada M, et al. : Detection of human cytomegalovirus DNA in breast milk by means of polymerase chain reaction. Microbiol Immunol. 1994;38(10):809–11. 10.1111/j.1348-0421.1994.tb01862.x [DOI] [PubMed] [Google Scholar]
- 71. Vochem M, Hamprecht K, Jahn G, et al. : Transmission of cytomegalovirus to preterm infants through breast milk. Pediatr Infect Dis J. 1998;17(1):53–8. [DOI] [PubMed] [Google Scholar]
- 72. Hamprecht K, Maschmann J, Vochem M, et al. : Epidemiology of transmission of cytomegalovirus from mother to preterm infant by breastfeeding. Lancet. 2001;357(9255):513–8. 10.1016/S0140-6736(00)04043-5 [DOI] [PubMed] [Google Scholar]
- 73. Cannon MJ, Stowell JD, Clark R, et al. : Repeated measures study of weekly and daily cytomegalovirus shedding patterns in saliva and urine of healthy cytomegalovirus-seropositive children. BMC Infect Dis. 2014;14:569. 10.1186/s12879-014-0569-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pope AS, Sartwell PE, Zacks D: Development of Tuberculosis in Infected Children. Am J Public Health Nations Health. 1939;29(12):1318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zeidberg LD, Dillon A: Risk of developing tuberculosis among children of tuberculous parents. Am Rev Tuberc. 1954;70(6):1009–19. [DOI] [PubMed] [Google Scholar]
- 76. Zeidberg LD, Gass RS, Dillon A, et al. : The Williamson County Tuberculosis Study. A twenty-four-year epidemiologic study. Am Rev Respir Dis. 1963;87((3) Pt 2):1–88. [PubMed] [Google Scholar]
- 77. BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescence and early adult life. Bull World Health Organ. 1972;46(3):371–85. [PMC free article] [PubMed] [Google Scholar]
- 78. Tuberculosis in a rural population of South India: a five-year epidemiological study. Bull World Health Organ. 1974;51(5):473–88. [PMC free article] [PubMed] [Google Scholar]
- 79. Comstock GW, Ferebee SH, Hammes LM: A controlled trial of community-wide isoniazid prophylaxis in Alaska. Am Rev Respir Dis. 1967;95(6):935–43. [DOI] [PubMed] [Google Scholar]
- 80. Horton KC, MacPherson P, Houben RM, et al. : Sex Differences in Tuberculosis Burden and Notifications in Low- and Middle-Income Countries: A Systematic Review and Meta-analysis. PLoS Med. 2016;13(9):e1002119. 10.1371/journal.pmed.1002119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Vyse AJ, Hesketh LM, Pebody RG: The burden of infection with cytomegalovirus in England and Wales: how many women are infected in pregnancy? Epidemiol Infect. 2009;137(4):526–33. 10.1017/S0950268808001258 [DOI] [PubMed] [Google Scholar]
- 82. Lopo S, Vinagre E, Palminha P, et al. : Seroprevalence to cytomegalovirus in the Portuguese population, 2002–2003. Euro Surveill. 2011;16(25): pii: 19896. [PubMed] [Google Scholar]
- 83. Stadler LP, Bernstein DI, Callahan ST, et al. : Seroprevalence of cytomegalovirus (CMV) and risk factors for infection in adolescent males. Clin Infect Dis. 2010;51(10):e76–81. 10.1086/656918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bate SL, Dollard SC, Cannon MJ: Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988–2004. Clin Infect Dis. 2010;50(11):1439–47. 10.1086/652438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dowd JB, Aiello AE, Alley DE: Socioeconomic disparities in the seroprevalence of cytomegalovirus infection in the US population: NHANES III. Epidemiol Infect. 2009;137(1):58–65. 10.1017/S0950268808000551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Staras SA, Dollard SC, Radford KW, et al. : Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin Infect Dis. 2006;43(9):1143–51. 10.1086/508173 [DOI] [PubMed] [Google Scholar]
- 87. Aarnisalo J, Ilonen J, Vainionpää R, et al. : Development of antibodies against cytomegalovirus, varicella-zoster virus and herpes simplex virus in Finland during the first eight years of life: a prospective study. Scand J Infect Dis. 2003;35(10):750–3. 10.1080/00365540310015881 [DOI] [PubMed] [Google Scholar]
- 88. Almeida LN, Azevedo RS, Amaku M, et al. : Cytomegalovirus seroepidemiology in an urban community of São Paulo, Brazil. Rev Saude Publica. 2001;35(2):124–9. 10.1590/S0034-89102001000200004 [DOI] [PubMed] [Google Scholar]
- 89. Coonrod D, Collier AC, Ashley R, et al. : Association between cytomegalovirus seroconversion and upper genital tract infection among women attending a sexually transmitted disease clinic: a prospective study. J Infect Dis. 1998;177(5):1188–93. 10.1086/515292 [DOI] [PubMed] [Google Scholar]
- 90. Collier AC, Handsfield HH, Roberts PL, et al. : Cytomegalovirus infection in women attending a sexually transmitted disease clinic. J Infect Dis. 1990;162(1):46–51. 10.1093/infdis/162.1.46 [DOI] [PubMed] [Google Scholar]
- 91. Francisse S, Revelard P, De Maertelaer V, et al. : Human cytomegalovirus seroprevalence and risk of seroconversion in a fertility clinic population. Obstet Gynecol. 2009;114(2 Pt 1):285–91. 10.1097/AOG.0b013e3181af3d6f [DOI] [PubMed] [Google Scholar]
- 92. Staras SA, Flanders WD, Dollard SC, et al. : Influence of sexual activity on cytomegalovirus seroprevalence in the United States, 1988–1994. Sex Transm Dis. 2008;35(5):472–9. 10.1097/OLQ.0b013e3181644b70 [DOI] [PubMed] [Google Scholar]
- 93. Lanzieri TM, Kruszon-Moran D, Gambhir M, et al. : Influence of parity and sexual history on cytomegalovirus seroprevalence among women aged 20–49 years in the USA. Int J Gynaecol Obstet. 2016;135(1):82–5. 10.1016/j.ijgo.2016.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Patrick EJ, Higgins CD, Crawford DH, et al. : A cohort study in university students: investigation of risk factors for cytomegalovirus infection. Epidemiol Infect. 2014;142(9):1990–5. 10.1017/S0950268813002720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Foxworth MK, 2nd, Wilms IR, Brookman RR, et al. : Prevalence of CMV infection among sexually active adolescents: a matched case-control study. Adolesc Health Med Ther. 2014;5:73–8. 10.2147/AHMT.S60103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Frost WH: The age selection of mortality from tuberculosis in successive decades. 1939. Am J Epidemiol. 1995;141(1):4–9; discussion 3. 10.1093/oxfordjournals.aje.a117343 [DOI] [PubMed] [Google Scholar]
- 97. Grzybowski S, Allen EA: The Challenge Of Tuberculosis In Decline. A Study Based On The Epidemiology Of Tuberculosis In Ontario, Canada. Am Rev Respir Dis. 1964;90:707–20. [DOI] [PubMed] [Google Scholar]
- 98. Groth-Petersen E, Knudsen J, Wilbek E: Epidemiological basis of tuberculosis eradication in an advanced country. Bull World Health Organ. 1959;21:5–49. [PMC free article] [PubMed] [Google Scholar]
- 99. Comstock GW, Edwards PQ: The competing risks of tuberculosis and hepatitis for adult tuberculin reactors. Am Rev Respir Dis. 1975;111(5):573–7. [DOI] [PubMed] [Google Scholar]
- 100. Ibrahim S, Siddiqui AA, Siddiqui AR, et al. : Sociodemographic factors associated with IgG and IgM seroprevalence for human cytomegalovirus infection in adult populations of Pakistan: a seroprevalence survey. BMC Public Health. 2016;16(1):1112. 10.1186/s12889-016-3772-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Antona D, Lepoutre A, Fonteneau L, et al. : Seroprevalence of cytomegalovirus infection in France in 2010. Epidemiol Infect. 2017;145(7):1471–8. 10.1017/S0950268817000103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Badami KG, McQuilkan-Bickerstaffe S, Wells JE, et al. : Cytomegalovirus seroprevalence and ‘cytomegalovirus-safe’ seropositive blood donors. Epidemiol Infect. 2009;137(12):1776–80. 10.1017/S0950268809990094 [DOI] [PubMed] [Google Scholar]
- 103. Conde-Glez C, Lazcano-Ponce E, Rojas R, et al. : Seroprevalences of varicella-zoster virus, herpes simplex virus and cytomegalovirus in a cross-sectional study in Mexico. Vaccine. 2013;31(44):5067–74. 10.1016/j.vaccine.2013.08.077 [DOI] [PubMed] [Google Scholar]
- 104. Chandler SH, Handsfield HH, McDougall JK: Isolation of multiple strains of cytomegalovirus from women attending a clinic for sexually transmitted disease. J Infect Dis. 1987;155(4):655–60. 10.1093/infdis/155.4.655 [DOI] [PubMed] [Google Scholar]
- 105. Boppana SB, Rivera LB, Fowler KB, et al. : Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N Engl J Med. 2001;344(18):1366–71. 10.1056/NEJM200105033441804 [DOI] [PubMed] [Google Scholar]
- 106. Yamamoto AY, Mussi-Pinhata MM, Boppana SB, et al. : Human cytomegalovirus reinfection is associated with intrauterine transmission in a highly cytomegalovirus-immune maternal population. Am J Obstet Gynecol. 2010;202(3):297.e1–8. 10.1016/j.ajog.2009.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hinman AR, Judd JM, Kolnik JP, et al. : Changing risks in tuberculosis. Am J Epidemiol. 1976;103(5):486–97. 10.1093/oxfordjournals.aje.a112250 [DOI] [PubMed] [Google Scholar]
- 108. Raviglione MC, Sudre P, Rieder HL, et al. : Secular trends of tuberculosis in western Europe. Bull World Health Organ. 1993;71(3–4):297–306. [PMC free article] [PubMed] [Google Scholar]
- 109. Weltevrede M, Eilers R, de Melker HE, et al. : Cytomegalovirus persistence and T-cell immunosenescence in people aged fifty and older: A systematic review. Exp Gerontol. 2016;77:87–95. 10.1016/j.exger.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 110. Parry HM, Zuo J, Frumento G, et al. : Cytomegalovirus viral load within blood increases markedly in healthy people over the age of 70 years. Immun Ageing. 2016;13:1. 10.1186/s12979-015-0056-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Furui Y, Satake M, Hoshi Y, et al. : Cytomegalovirus (CMV) seroprevalence in Japanese blood donors and high detection frequency of CMV DNA in elderly donors. Transfusion. 2013;53(10):2190–7. 10.1111/trf.12390 [DOI] [PubMed] [Google Scholar]
- 112. Stowe RP, Kozlova EV, Yetman DL, et al. : Chronic herpesvirus reactivation occurs in aging. Exp Gerontol. 2007;42(6):563–70. 10.1016/j.exger.2007.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Thomasini RL, Pereira DS, Pereira FSM, et al. : Aged-associated cytomegalovirus and Epstein-Barr virus reactivation and cytomegalovirus relationship with the frailty syndrome in older women. PLoS One. 2017;12(7):e0180841. 10.1371/journal.pone.0180841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lantos PM, Hoffman K, Permar SR, et al. : Neighborhood Disadvantage is Associated with High Cytomegalovirus Seroprevalence in Pregnancy. J Racial Ethn Health Disparities. 2017;1–5. 10.1007/s40615-017-0423-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Enders G, Daiminger A, Lindemann L, et al. : Cytomegalovirus (CMV) seroprevalence in pregnant women, bone marrow donors and adolescents in Germany, 1996–2010. Med Microbiol Immunol. 2012;201(3):303–9. 10.1007/s00430-012-0232-7 [DOI] [PubMed] [Google Scholar]
- 116. Wujcicka W, Gaj Z, Wilczyński J, et al. : Impact of socioeconomic risk factors on the seroprevalence of cytomegalovirus infections in a cohort of pregnant Polish women between 2010 and 2011. Eur J Clin Microbiol Infect Dis. 2014;33(11):1951–8. 10.1007/s10096-014-2170-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Stadler LP, Bernstein DI, Callahan ST, et al. : Seroprevalence and Risk Factors for Cytomegalovirus Infections in Adolescent Females. J Pediatr Infect Dis Soc. 2013;2(1):7–14. 10.1093/jpids/pis076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Schmink S, Kruszon-Moran D, Dollard SC, et al. : Effect of Breastfeeding and Additional Household Children on Cytomegalovirus Seroprevalence among U.S. Children 1 to 5 Years of Age. Clin Vaccine Immunol. 2017;24(11): pii: e00243-17. 10.1128/CVI.00243-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Hannachi N, Marzouk M, Harrabi I, et al. : [Seroprevalence of rubella virus, varicella zoster virus, cytomegalovirus and parvovirus B19 among pregnant women in the Sousse region, Tunisia]. Bull Soc Pathol Exot. 2011;104(1):62–7. 10.1007/s13149-010-0119-z [DOI] [PubMed] [Google Scholar]
- 120. Jansen MA, van den Heuvel D, Bouthoorn SH, et al. : Determinants of Ethnic Differences in Cytomegalovirus, Epstein-Barr Virus, and Herpes Simplex Virus Type 1 Seroprevalence in Childhood. J Pediatr. 2016;170:126–134.e1–6. 10.1016/j.jpeds.2015.11.014 [DOI] [PubMed] [Google Scholar]
- 121. Lönnroth K, Jaramillo E, Williams BG, et al. : Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med. 2009;68(12):2240–6. 10.1016/j.socscimed.2009.03.041 [DOI] [PubMed] [Google Scholar]
- 122. Hoa NB, Tiemersma EW, Sy DN, et al. : Household expenditure and tuberculosis prevalence in VietNam: prediction by a set of household indicators. Int J Tuberc Lung Dis. 2011;15(1):32–7. [PubMed] [Google Scholar]
- 123. Janssens JP, Rieder HL: An ecological analysis of incidence of tuberculosis and per capita gross domestic product. Eur Respir J. 2008;32(5):1415–6. 10.1183/09031936.00078708 [DOI] [PubMed] [Google Scholar]
- 124. Chapman JS, Dyerly MD: Social and other factors in intrafamilial transmission of tuberculosis. Am Rev Respir Dis. 1964;90:48–60. [DOI] [PubMed] [Google Scholar]
- 125. Cegielski JP, McMurray DN: The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis. 2004;8(3):286–98. [PubMed] [Google Scholar]
- 126. Lin HH, Suk CW, Lo HL, et al. : Indoor air pollution from solid fuel and tuberculosis: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2014;18(5):613–21. 10.5588/ijtld.13.0765 [DOI] [PubMed] [Google Scholar]
- 127. Scott C, Kirking HL, Jeffries C, et al. : Tuberculosis trends--United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(10):265–9. [PMC free article] [PubMed] [Google Scholar]
- 128. Hollo V, Beauté J, Ködmön C, et al. : Tuberculosis notification rate decreases faster in residents of native origin than in residents of foreign origin in the EU/EEA, 2010 to 2015. Euro Surveill. 2017;22(12): pii: 30486. 10.2807/1560-7917.ES.2017.22.12.30486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Cantwell MF, McKenna MT, McCray E, et al. : Tuberculosis and race/ethnicity in the United States: impact of socioeconomic status. Am J Respir Crit Care Med. 1998;157(4 Pt 1):1016–20. 10.1164/ajrccm.157.4.9704036 [DOI] [PubMed] [Google Scholar]
- 130. Pedrazzoli D, Boccia D, Dodd PJ, et al. : Modelling the social and structural determinants of tuberculosis: opportunities and challenges. Int J Tuberc Lung Dis. 2017;21(9):957–64. 10.5588/ijtld.16.0906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Pereira LH, Embil JA, Haase DA, et al. : Cytomegalovirus infection among women attending a sexually transmitted disease clinic: association with clinical symptoms and other sexually transmitted diseases. Am J Epidemiol. 1990;131(4):683–92. 10.1093/oxfordjournals.aje.a115552 [DOI] [PubMed] [Google Scholar]
- 132. Fowler KB, Pass RF: Risk factors for congenital cytomegalovirus infection in the offspring of young women: exposure to young children and recent onset of sexual activity Pediatrics. 2006;118(2):e286–292. 10.1542/peds.2005-1142 [DOI] [PubMed] [Google Scholar]
- 133. Fowler KB, Ma Y, Moscicki B, et al. : Seroprevalence and risk factors of hepatitis B, hepatitis C, and human cytomegalovirus among HIV-infected and high-risk uninfected adolescents: findings of the REACH Study. Adolescent Medicine HIV/AIDS Research Network. Sex Transm Dis. 2000;27(5):296–303. 10.1097/00007435-200005000-00012 [DOI] [PubMed] [Google Scholar]
- 134. Zhao FH, Forman MR, Belinson J, et al. : Risk factors for HPV infection and cervical cancer among unscreened women in a high-risk rural area of China. Int J Cancer. 2006;118(2):442–8. 10.1002/ijc.21327 [DOI] [PubMed] [Google Scholar]
- 135. KNCV Tuberculosefonds: Tuberculose in Nederland.The Hague,2008. Reference Source [Google Scholar]
- 136. Adler SP: Transfusion-associated cytomegalovirus infections. Rev Infect Dis. 1983;5(6):977–93. 10.1093/clinids/5.6.977 [DOI] [PubMed] [Google Scholar]
- 137. Forbes BA: Acquisition of cytomegalovirus infection: an update. Clin Microbiol Rev. 1989;2(2):204–16. 10.1128/CMR.2.2.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Pamphilon DH, Rider JR, Barbara JA, et al. : Prevention of transfusion-transmitted cytomegalovirus infection. Transfus Med. 1999;9(2):115–23. 10.1046/j.1365-3148.1999.00193.x [DOI] [PubMed] [Google Scholar]
- 139. THORN PA, BROOKES VS, WATERHOUSE JA: Peptic ulcer, partial gastrectomy, and pulmonary tuberculosis. Br Med J. 1956;1(4967):603–8. 10.1136/bmj.1.4967.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Snider DE, Jr: Tuberculosis and gastrectomy. Chest. 1985;87(4):414–5. 10.1378/chest.87.4.414 [DOI] [PubMed] [Google Scholar]
- 141. Al-Efraij K, Mota L, Lunny C, et al. : Risk of active tuberculosis in chronic kidney disease: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2015;19(12):1493–9. 10.5588/ijtld.15.0081 [DOI] [PubMed] [Google Scholar]
- 142. Chou KJ, Fang HC, Bai KJ, et al. : Tuberculosis in maintenance dialysis patients. Nephron. 2001;88(2):138–43. 10.1159/000045974 [DOI] [PubMed] [Google Scholar]
- 143. Hardiman AE, Butter KC, Roe CJ, et al. : Cytomegalovirus infection in dialysis patients. Clin Nephrol. 1985;23(1):12–7. [PubMed] [Google Scholar]
- 144. Tolkoff-Rubin NA, Rubin RH, Keller EE, et al. : Cytomegalovirus infection in dialysis patients and personnel. Ann Intern Med. 1978;89(5 Pt 1):625–8. 10.7326/0003-4819-89-5-625 [DOI] [PubMed] [Google Scholar]
- 145. Vilibic-Cavlek T, Kolaric B, Beader N, et al. : Seroepidemiology of cytomegalovirus infections in Croatia. Wien Klin Wochenschr. 2017;129(3–4):129–35. 10.1007/s00508-016-1069-7 [DOI] [PubMed] [Google Scholar]
- 146. Vilibic-Cavlek T, Kolaric B, Ljubin-Sternak S, et al. : Prevalence and dynamics of cytomegalovirus infection among patients undergoing chronic hemodialysis. Indian J Nephrol. 2015;25(2):95–8. 10.4103/0971-4065.139488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Cantisán S, Rodelo-Haad C, Páez-Vega A, et al. : Factors related to the development of CMV-specific CD8+ T cell response in CMV-seropositive solid organ transplant candidates. Am J Transplant. 2015;15(3):715–22. 10.1111/ajt.13012 [DOI] [PubMed] [Google Scholar]
- 148. Lumbreras C, Manuel O, Len O, et al. : Cytomegalovirus infection in solid organ transplant recipients. Clin Microbiol Infect. 2014;20 Suppl 7:19–26. 10.1111/1469-0691.12594 [DOI] [PubMed] [Google Scholar]
- 149. Ergun I, Ekmekci Y, Sengul S, et al. : Mycobacterium tuberculosis infection in renal transplant recipients. Transplant Proc. 2006;38(5):1344–5. 10.1016/j.transproceed.2006.03.029 [DOI] [PubMed] [Google Scholar]
- 150. Torre-Cisneros J, Doblas A, Aguado JM, et al. : Tuberculosis after solid-organ transplant: incidence, risk factors, and clinical characteristics in the RESITRA (Spanish Network of Infection in Transplantation) cohort. Clin Infect Dis. 2009;48(12):1657–65. 10.1086/599035 [DOI] [PubMed] [Google Scholar]
- 151. Canet E, Dantal J, Blancho G, et al. : Tuberculosis following kidney transplantation: clinical features and outcome. A French multicentre experience in the last 20 years. Nephrol Dial Transplant. 2011;26(11):3773–8. 10.1093/ndt/gfr156 [DOI] [PubMed] [Google Scholar]
- 152. Aguado JM, Torre-Cisneros J, Fortún J, et al. : Tuberculosis in solid-organ transplant recipients: consensus statement of the group for the study of infection in transplant recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology. Clin Infect Dis. 2009;48(9):1276–84. 10.1086/597590 [DOI] [PubMed] [Google Scholar]
- 153. Ha YE, Joo EJ, Park SY, et al. : Tacrolimus as a risk factor for tuberculosis and outcome of treatment with rifampicin in solid organ transplant recipients. Transpl Infect Dis. 2012;14(6):626–34. 10.1111/j.1399-3062.2012.00721.x [DOI] [PubMed] [Google Scholar]
- 154. Anderholm KM, Bierle CJ, Schleiss MR: Cytomegalovirus Vaccines: Current Status and Future Prospects. Drugs. 2016;76(17):1625–45. 10.1007/s40265-016-0653-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Hansen SG, Zak DE, Xu G, et al. : Prevention of tuberculosis in rhesus macaques by a cytomegalovirus-based vaccine. Nat Med. 2018;24(2):130–43. 10.1038/nm.4473 [DOI] [PMC free article] [PubMed] [Google Scholar]