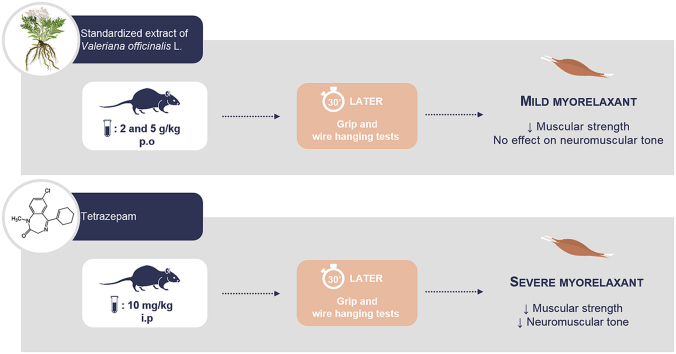
Abstract
Valeriana officinalis L. root extracts are traditionally taken for their sedative and anxiolytic properties and are also used for muscle relaxation. Relaxant effects were clearly observed on smooth muscle whereas data on effects on skeletal muscle are scarce and inconsistent. The aim of this study was to assess whether a standardized extract (SE) of V. officinalis had myorelaxant effects by decreasing skeletal muscle strength and/or neuromuscular tone in mice. Mice received an acute dose of V. officinalis SE (2 or 5 g/kg per os) or tetrazepam (10 mg/kg ip), a standard myorelaxant drug. Thirty minutes later, the maximal muscle strength was measured using a grip test, while global skeletal muscle function (endurance and neuromuscular tone) was assessed in a wire hanging test. Compared to tetrazepam, both doses of V. officinalis SE induced a pronounced decrease in skeletal muscle strength without any significant effects on endurance and neuromuscular tone. This study provides clear evidence that the extract of V. officinalis tested has a relaxant effect on skeletal muscle. By decreasing skeletal muscle strength without impacting endurance and neuromuscular tone, V. officinalis SE could induce less undesirable side effects than standard myorelaxant agents, and be particularly useful for avoiding falls in the elderly.
Keywords: Valeriana officinalis, Skeletal muscle relaxant, Strength, Hydroethanolic root extract, Acute treatment, Mouse
Graphical abstract
Abbreviations
- DMSO
dimethyl sulfoxide
- GABA
gamma–amino butyric acid
- HRE
hydroethanolic root extract
- HPLC
high performance liquid chromatography
- HPTLC
high performance thin layer chromatography
- UHPLC
ultra-high performance liquid chromatography
- SE
standardized extract
1. Introduction
Native from Europe and North Asia, Valeriana officinalis L. is a perennial plant with thick roots often used as an herbal supplement for its anxiolytic and sedative properties.1, 9, 25 The European Medicine Agency (EMA) deemed that dry ethanol root extracts of V. officinalis could be used for the relief of mild nervous tension and sleep disorders on the basis of their ‘well-established use’ (i.e. bibliographic data are available) and that other valerian extracts could be used for the relief of mild symptoms of mental stress and to aid sleep on the basis of their ‘traditional use’ (>30-year use).12 Besides these anxiolytic and sedative effects, V. officinalis was also shown to have myorelaxant properties. V. officinalis is used as a spasmolytic to treat gastrointestinal spasms and menstrual cramps.18, 19 Relaxant effects were observed on smooth muscle such as guinea pig ileum, vascular and bronchial muscle and isolated human uterine muscle.13, 6, 10, 19 As regards to the skeletal muscular system, in vivo studies provided inconsistent results concerning the capability of V. officinalis extracts to affect locomotor activity24, 16, 15, 14 and the only study we found that assessed relaxant properties on the skeletal muscle reported negative results.14 The objective of this study was to evaluate the relaxant effects of a standardized extract of V. officinalis on skeletal muscle after acute administration in mice, in comparison with tetrazepam, a benzodiazepine known to have robust myorelaxant properties.
2. Material and methods
2.1. Preparation of the liquid hydroethanolic root extract of V. officinalis
Roots of V. officinalis were harvested in north-western France in October 2013.
The hydroethanolic root extract (HRE) of V. officinalis was obtained according to the patented process WO2001056584A1. V. officinalis frozen fresh roots were crushed, then extracted with 20–70% (v/v) ethanolic leaching. The extract was concentrated under reduced pressure to evaporate ethanol. Final concentration is adjusted by adding glycerin to obtain a 0.01–0.02% (w/v) sesquiterpenic acid range. Batch used in this study (batch no. 111) contained glycerine (80%) with a dry drug: dry genuine extract ratio of 4:1. After the addition of glycerine, the HRE is a standardized extract (SE) of V. officinalis (EPS V. officinalis PiLeJe Laboratoire, France). To ensure a consistent quality and reproducible biological properties of our extracts, at each extraction, sesquiterpenes content is systematically determined using high performance liquid chromatography (HPLC) adapted from the European Pharmacopoeia 8.0 for valerian dry root monograph (07/2010:0453).
2.2. Chromatographic analyses of V. officinalis HRE for valerenic acid and derivatives
To further characterize the extract in terms of its content in valerenic acid and derivatives, the HRE of V. officinalis was analysed by high performance liquid chromatography (HPLC), mass spectrometry and high performance thin layer chromatography (HPTLC).
2.2.1. HPLC and mass spectrometry
The HPLC analyses were performed on an Agilent Technologies 1260 Infinity using a Nucleodur C18 ec column (100Å, 4 × 250 mm; 5 μm). The mobile phase was a mixture of 0.1% (v/v) formic acid in water (phase A) and 0.1% (v/v) formic acid in acetonitrile (phase B). The gradient of phase A was 100% (0–9 min), 100%–90% (10–15 min), 90%–0% (15–45 min) and then was held at 0% for 10 min. The flow rate was 0.8 mL/min, and the injection volume 5 μL.
Mass analyses were performed on a Ultimate 3000 RSLC ultra-high performance liquid chromatography (UHPLC) system (Thermo Fisher Scientific Inc., MA, USA) coupled to a quaternary rapid separation pump (Ultimate autosampler) and a rapid separation diode array detector. Compounds were separated on a Kinetex EVO C18 column (2.1 × 100 mm; 1.7μm; Phenomenex, CA, USA), which was controlled at 30 °C. The mobile phase was a mixture of 0.1% (v/v) formic acid in water (phase A) and 0.1% (v/v) formic acid in acetonitrile (phase B). The gradient of phase A was 95% (0 min), 95%–1% (0–7.5 min), 1% (7.5–8.5 min) 1%–95% (8.5–9 min) and then was held at 95% for 2 min. The flow rate was 0.45 mL/min, and the injection volume was 5 μL. The UHPLC system was connected to an Orbitrap (Thermo Fisher Scientific Inc., MA, USA) mass spectrometer, operated in negative electrospray ionization mode. Source operating conditions were: 3 kV spray voltage; 320 °C heated capillary temperature; 400 °C auxiliary gas temperature; sheath, sweep and auxiliary gas (nitrogen) flow rate 50, 10 and 2 arbitrary units, respectively; and collision cell voltage between 10 and 50 eV. Full scan data were obtained at a resolution of 70,000 whereas MS2 data were obtained at a resolution of 17,500. Data were processed using Xcalibur software (Thermo Fisher Scientific Inc., MA, USA).
The quantity of valerenic acid and its derivatives is known to be low in aqueous ethanolic extracts due to slight solubility.3 For that reason, to identify them, we had to re-extract the HRE (after elimination of the solvent) with an organic solvent (dichloromethane [DCM]) in which the compounds are more soluble.6 The resulting fraction was analysed by HPLC and mass spectrometry.
Compounds present in the HRE were characterized according to their retention times, mass spectral data and compared with authentic standards.
2.2.2. HPTLC
Analyses were performed using 0.20 mm silicagel 60 F254 (20 × 10 cm) glass HPTLC analytical plates (Merck, Darmstadt, Germany). Material included a Camag HPTLC system (Muttenz, Switzerland) equipped with an automatic TLC sampler (ATS 4), an automatic developing chamber ADC2 with humidity control, a TLC visualizer, WinCATS 1.4.9 software and for derivatization, a Chromatogram Immersion Device III and TLC Plate Heater III.
Test and reference solutions were applied at volumes of 7 μL and as 8 mm bands, 8 mm from the lower edge of the plate. The syringe was rinsed with absolute ethanol between samples. Mobile phase was a mixture of cyclohexane, ethyl acetate and acetic acid (60:38:2 by vol). Plates were developed over a distance of 70 mm from the lower edge using a twin trough glass chamber saturated for 20 min with mobile phase under controlled humidity. After development, plates were dried under a stream of cool air for 10 min in ADC2. Digital images were captured using 366-nm UV light or white light before derivatization.
The plate was first sprayed with HCl reagent (HCl/acetic acid: 8/2 by vol) and heated at 110 °C for 3 min with the TLC Plate Heater. An additional derivatization was required: the plate was immersed into para-anisaldehyde reagent (170 mL iced methanol, 20 mL acetic acid, 10 mL sulfuric acid and 1 mL para-anisaldehyde) using the Chromatogram Immersion Device (immersion time of 1 s and immersion speed of 5 cm/s) and heated at 100 °C for 3 min. Evaluation of the plates after each derivatization was carried out under visible and UV light (366 nm).
2.3. Evaluation of myorelaxant effects in mice
2.3.1. Animals
Experiments were performed in 131 male RjORL: Swiss mice (Janvier, Laval, France), aged 6 weeks and weighing 35.8 ± 0.2 g. Mice were housed 6 per cage and maintained on a 12/12 h light/dark schedule in a temperature controlled facility (22 ± 1 °C) with free access to food and water. Animals were kept undisturbed for 7 days before experiments. All procedures were conducted in conformity with European rules for animal experimentation (French Ethical Committee CEEA-PdL-2011-45/CEEA-PdL-01579.01).
2.3.2. Treatment
Animals were divided into 6 groups. Two groups of 24 mice received a single oral dose of the SE of V. officinalis at 2 or 5 g/kg. Two control groups of 18 and 17 mice received glycerine orally, the SE excipient (batch no. 3189711, 3i nature, France) at the corresponding doses of 1.6 or 4 g/kg. A group of 24 mice received tetrazepam (batch no. 0022P8, Sigma-Aldrich, Saint-Quentin Fallavier, France; 1 mL/kg, tetrazepam in 7.5% dimethyl sulfoxide [DMSO] and 3.75% Tween 20 in 0.9% NaCl solution) intraperitoneally at a dose of 10 mg/kg, and a control group of 24 mice was injected with the vehicle solution (both at a volume of 2.5 mL/kg).
2.3.3. Behavioural tests
The grip and the wire hanging tests were performed 30 min after treatment for all groups.
The grip test is a non-invasive method designed to evaluate muscle strength in vivo, by taking advantage of the mouse's tendency to grasp a grid while suspended by its tail. The grip test is then used to measure the maximal muscle strength of the four limbs. Mice were placed on a 10 × 10 cm grid and were gently pulled horizontally backward until they released their grip.8 A grip meter (Panlab, BIOSEB, France) attached to a force transducer measured the peak force generated by the four limbs together. Three measures were performed and averaged. The maximal strength developed by these four limbs was expressed in grams (g; absolute strength) and normalized to the body weight (g/g; relative strength).
The wire hanging test is used to assess global skeletal muscle function and endurance over time in mice. This test is also used in pharmacological studies for evaluating the neuromuscular tone, a continuous and passive partial skeletal muscle contraction.7, 23 The wire hanging apparatus is comprised of a stainless steel bar (49 cm length, 1.5 mm diameter), resting on two vertical supports and elevated 45 cm above a flat surface covered with litter in order to cushion falls.4, 23 Mice were hung onto the bar with the two forepaws midway between the supports. The time spent hanging was recorded until they fall, before being manually hung again. For each mouse, a score equalling to 10 was given at the beginning of the test. Each fall decreased this score by one unit. The test ended when the animal had felt 10 times or after 3 min. For each second of the test duration, a mean score was calculated for all animals from one group, and the mean score obtained at the “100 s” time point was used as an indicator of endurance. Moreover, the impulse parameter, which represents the ability to maintain a sufficient strength to stay on the bar, was calculated (g.s; body weight [g] multiplied by maximal duration between two falls [s]).5
2.3.4. Statistical analysis
The results are presented as means ± sem. Normal distribution of data was verified with a D'Agostino-Pearson test. When data were not normally distributed, the significance of differences between groups was evaluated using the Kruskal-Wallis ANOVA followed when significant, by a Mann-Whitney post hoc test between test groups and their respective controls. When data were normally distributed, the significance of differences between groups was evaluated using a one-way ANOVA followed when significant, by a Student t-test. Level of significance was set at p < 0.05. Statistical analyses were carried out with GraphPad Prism version 5.0.
3. Results
3.1. Content in valerenic acid and derivatives
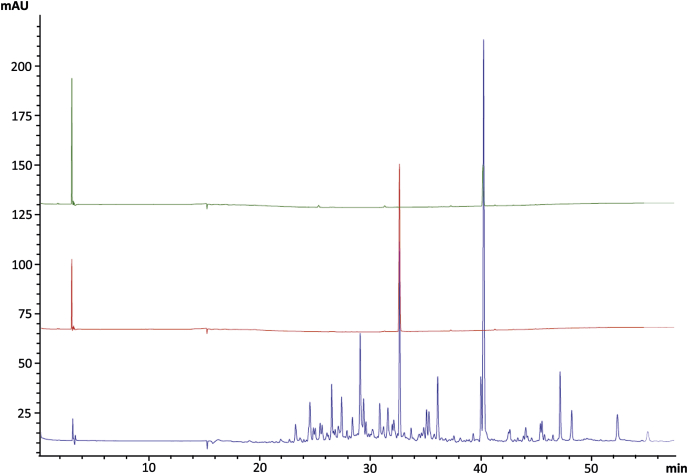
Valerenic acid and its derivative, hydroxyvalerenic acid, were clearly identified by HPLC in comparison to standards. The HPLC analysis of the DCM fraction and standards in the same conditions confirmed the presence of valerenic and hydroxyvalerenic acids in that fraction and so, in the V. officinalis HRE (Fig. 1). Under the extraction conditions used to obtain the DCM fraction, acetoxyvalerenic acid underwent degradation and was thus not detected.
Fig. 1.
Superposition of the V. officinalis HRE DCM fraction (blue) with hydroxyvalerenic acid standard (red) and valerenic acid standard (green).
The mass spectra showed that the major compounds of the DCM fraction were valerenic acid derivatives; the other compounds could not be identified as known compounds in V. officinalis extracts. The presence of valerenic and hydroxyvalarenic acids was confirmed by mass spectrometry.
HPTLC analysis confirmed the presence of the two sesquiterpenes, valerenic and hydroxyvalerenic acids and showed the presence of acetoxyvalerenic acid. White light observation of the plate after derivatization with para-anisaldehyde allowed the detection of hydroxyvalerenic, acetoxyvalerenic and valerenic acids at retention factor (Rf) 0.25, 0.43 and 0.61, respectively (Fig. 2). These sesquiterpenes were clearly identified in the V. officinalis HRE fingerprint in comparison with standards; other weak zones correspond to nonidentified minor substances.
Fig. 2.
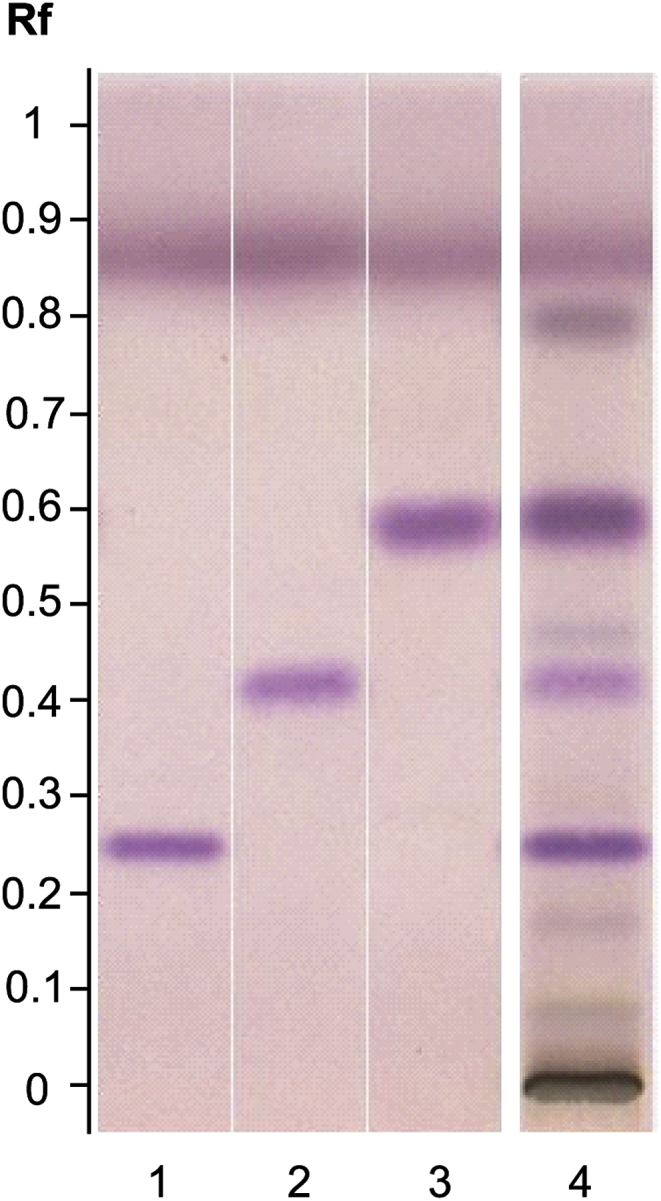
HPTLC fingerprint (sesquiterpenes) of V. officinalis HRE. Track assignment: 1 hydroxyvalerenic acid standard; 2 acetoxyvalerenic acid standard; 3 valerenic acid standard; 4 V. officinalis HRE.
3.2. Myorelaxant effect in mice
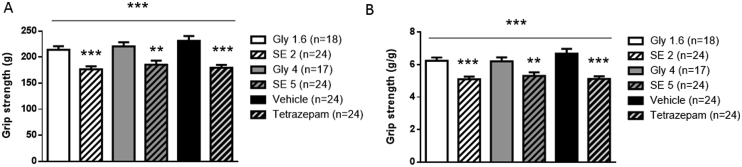
In the grip test, treatment with the SE of V. officinalis at the two doses tested significantly reduced the maximal skeletal muscle strength when compared to the respective control groups (Fig. 3). Specifically, the 2 g/kg dose induced a significant decrease in maximal strength of 17.6% for the absolute (p < 0.001, Fig. 3A) and 18.2% for the relative strength in comparison with the control groups (p < 0.001, Fig. 3B). The higher dose of 5 g/kg was also effective: the absolute and relative strengths were significantly reduced by 15.9% (p < 0.01, Fig. 3A) and 14.5% (p < 0.01, Fig. 3B), respectively. As usually observed, maximal skeletal muscle strength was also significantly decreased with tetrazepam; we measured a decrease of 22.4% for the absolute and 23.3% for the relative strength in comparison with the vehicle (p < 0.001, Fig. 3). At the two doses tested, the SE of V. officinalis induced a significant decrease in the maximal skeletal muscle strength which was similar to that observed with tetrazepam.
Fig. 3.
Mean (±sem) grip strength developed by the four paws 30 min after acute treatment with V. officinalis SE (2 g/kg, SE 2 and 5 g/kg, SE 5), glycerine (1.6 g/kg, Gly 1.6 and 4 g/kg, Gly 4), tetrazepam (10 mg/kg) or vehicle, in grams (g) (A, absolute values) and grams per gram of body weight (g/g) (B, relative values). **p < 0.01, ***p < 0.001, one-way ANOVA (overall symbol) followed by post-hoc Student t-test (test group versus corresponding control group).
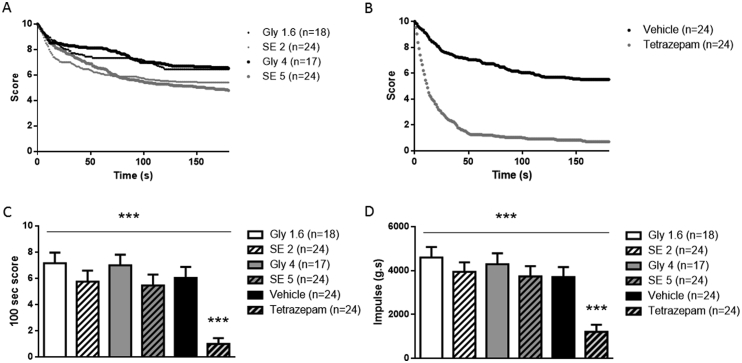
In the wire hanging test, mice treated with the SE of V. officinalis exhibited a reduced latency to fall (Fig. 4A). However, although the “100 s” scores were decreased by approximately 20% at the two doses, the decrease was not significantly different from those observed in the control groups. In good agreement, the impulse parameter was also decreased by approximately 14% in the two SE groups but the difference with controls was not statistically significant (Fig. 4D). By contrast, the latency to fall was significantly reduced in mice treated with tetrazepam (Fig. 4B). The “100 s” score was strongly and significantly decreased by 83.3% (p < 0.001, Fig. 4C) and impulse parameter by 67.3% (p < 0.001, Fig. 4D).
Fig. 4.
Score evolution on the wire hanging test 30 min after acute treatment with (A) V. officinalis SE (2 g/kg: SE 2 and 5 g/kg: SE 5) or glycerine (1.6 g/kg, Gly 1.6 and 4 g/kg, Gly 4) or (B) tetrazepam (10 mg/kg) or vehicle. C: Mean score at 100 s (±sem). D: mean impulse (±sem) in grams second (g.s). ***p < 0.001, Kruskal-Wallis ANOVA (overall symbol) followed by Mann-Whitney post hoc test (test group versus corresponding control group).
4. Discussion
Our results provide clear evidence that the SE of V. officinalis tested has a relaxant effect on skeletal muscle. This effect of V. officinalis had never been clearly shown previously. These results suggest that V. officinalis could not only be used for its anxiolytic and sedative properties but also for its relaxant effects on the skeletal muscle. We observed that the SE of V. officinalis reduces skeletal muscle strength: the SE of V. officinalis led to a marked impairment of the performance of mice in the grip test at an extent similar to that of tetrazepam. By contrast, the impact of the SE of V. officinalis on global skeletal muscle function (endurance and neuromuscular tone) measured in the wire hanging test was much less severe than that induced by tetrazepam. This selective effect of V. officinalis SE on the skeletal muscle is interesting because tetrazepam (withdrawn from the market because of an unpredictable risk of serious skin reactions)11 and in a general way benzodiazepines are commonly used in the elderly to treat sleep and anxiety problems but were reported to increase the risk of falls by approximately 50% in that population.17, 21 By decreasing skeletal muscle strength without impacting endurance and neuromuscular tone, V. officinalis SE could induce less undesirable side effects than benzodiazepines and therefore, be a useful alternative myorelaxant, particularly for avoiding falls in the elderly.
In the study by Hattesohl and colleagues,14 several V. officinalis extracts were tested and reported to have anxiolytic and antidepressant properties but no myorelaxant properties. It should be noted that although the term “strength” was used throughout the article, the design was more for measurement of tone than strength. The effect of V. officinalis was indeed evaluated in a test similar to the wire hanging test used in our study: the authors assessed the percentage of mice that had lost the ability to grasp a wire with at least one paw, when tested consecutively every 10 min over a period of 1 h. These results are therefore in agreement with ours showing that the SE of V. officinalis had no effect on neuromuscular tone. These results are also in good agreement with an in vivo study on V. officinalis extracts that demonstrated no effect on mice locomotor activity.15
The phytochemical analysis of our extract revealed the presence of the main family of compounds known to be present in V. officinalis. The three main sesquiterpenes corresponding to the reference substances, hydroxyvalerenic acid, acetoxyvalerenic acid and valerenic acid, were found. The mechanisms involved in the myorelaxant effects of V. officinalis SE remain to be elucidated. However, available data suggest that V. officinalis SE and notably the sesquiterpenes identified in the extract could be responsible for the effects we observed via an action on the central nervous system and the gamma–amino butyric acid (GABA) system. An aqueous extract was shown to increase the release and inhibit the recapture of GABA.3 V. officinalis was shown to increase GABA levels in rat brain homogenate and hippocampal neuronal cultures.2, 20 Valerenic acid was found to have an inhibitory effect on muscimol-sensitive neurons in vitro via GABAA-receptors26; to be a subunit-specific allosteric modulator of GABAA receptors; and extract fractions with high content of valerenic acid exhibited strong receptor activation.22
We report here clear evidence for the relaxant effect on skeletal muscle of a standardized extract of V. officinalis, the effect being mild compared to that of tetrazepam. By decreasing skeletal muscle strength without impacting endurance and tone, the extract could induce less undesirable effects than standard myorelaxants. Current knowledge suggests an implication of valerenic acid and the GABA system. However, other compounds and mechanisms could be involved such as effects on blood pressure. Next step would be to study more closely the compounds and mechanisms at the origin of the myorelaxant effect observed.
Conflict of interest
Isabelle Guinobert is a scientific project manager at PiLeJe. Valérie Bardot, César Cotte, Isabelle Ripoche and Pierre Chalard were involved in the chromatographic analyses. Dorian Caudal, Aude Lafoux and Corinne Huchet performed the animal experiments for PiLeJe.
Funding
The research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sector.
Acknowledgements
The authors acknowledge writing and editorial assistance provided by Claude Blondeau (PiLeJe Laboratoire). They also thank Martin Leremboure (Institut de Chimie de Clermont-Ferrand) for his involvement in mass analyses.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jtcme.2017.06.011.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Andreatini R., Sartori V.A., Seabra M.L. Effect of valepotriates (valerian extract) in generalized anxiety disorder: a randomized placebo-controlled pilot study. Phytother Res. 2002;16:650–654. doi: 10.1002/ptr.1027. [DOI] [PubMed] [Google Scholar]
- 2.Awad R., Levac D., Cybulska P. Effects of traditionally used anxiolytic botanicals on enzymes of the gamma-aminobutyric acid (GABA) system. Can J Physiol Pharmacol. 2007;85:933–942. doi: 10.1139/Y07-083. [DOI] [PubMed] [Google Scholar]
- 3.Bruneton J. 5th ed. Lavoisier; Paris, France: 2016. Pharmacognosie: phytochimie, plantes médicinales; pp. 920–928. [Google Scholar]
- 4.Carre-Pierrat M., Lafoux A., Tanniou G. Pre-clinical study of 21 approved drugs in the mdx mouse. Neuromuscul Disord. 2011;21:313–327. doi: 10.1016/j.nmd.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Carlson C.G., Rutter J., Bledsoe C. A simple protocol for assessing inter-trial and inter-examiner reliability for two noninvasive measures of limb muscle strength. J Neurosci Methods. 2010;186:226–230. doi: 10.1016/j.jneumeth.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Circosta C., De Pasquale R., Samperi S. Biological and analytical characterization of two extracts from Valeriana officinalis. J Ethnopharmacol. 2007;112:361–367. doi: 10.1016/j.jep.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Crestani F., Löw K., Keist R. Molecular targets for the myorelaxant action of diazepam. Mol Pharmacol. 2001;59:442–445. doi: 10.1124/mol.59.3.442. [DOI] [PubMed] [Google Scholar]
- 8.De Luca A. 2008. Use of Grip Strength Meter to Assess the Limb Strength of Mdx Mice.http://www.treat-nmd.eu/downloads/file/sops/dmd/MDX/DMD_M.2.2.001.pdf SOP DMD_M.2.2.001. Version 2.0. Accessed 15 February 2017. [Google Scholar]
- 9.Dimpfel W., Suter A. Sleep improving effects of a single dose administration of a valerian/hops fluid extract – a double blind, randomized, placebo-controlled sleep-EEG study in a parallel design using electrohypnograms. Eur J Med Res. 2008;13:200–204. [PubMed] [Google Scholar]
- 10.Emami-Abarghouei M., Vafaei A., Akhavan M. The effect of hydro-alcoholic extract of Valeriana officinalis on the ileum movement of Guinea pig. J Gorgan Uni Med Sci. 2009;11:1–5. [Google Scholar]
- 11.European Medicines Agency (EMA) April 29, 2013. Co-ordination Group for Mutual Recognition and Decentralised Procedures – Human (CMDh). Recommendation to Suspend Tetrazepam-containing Medicines Endorsed by CMDh.http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2013/04/WC500142552.pdf EMA/256383/2013 dated. Accessed 15 February 2017. [Google Scholar]
- 12.European Medicines Agency (EMA) February 02, 2016. Committee on Herbal Medicinal Products (HMPC). European Union Herbal Monograph on Valeriana Officinalis L., Radix.http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_Herbal_monograph/2016/04/WC500205376.pdf EMA/HMPC/150848/2015 dated. Accessed 15 February 2017. [Google Scholar]
- 13.Hazelhoff B., Malingré T.M., Meijer D.K. Antispasmodic effects of valeriana compounds: an in-vivo and in-vitro study on the guinea-pig ileum. Arch Int Pharmacodyn Ther. 1982;257:274–287. [PubMed] [Google Scholar]
- 14.Hattesohl M., Feistel B., Sievers H. Extracts of Valeriana officinalis L. s.l. show anxiolytic and antidepressant effects but neither sedative nor myorelaxant properties. Phytomedicine. 2008;15:2–15. doi: 10.1016/j.phymed.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 15.Hiller K.O., Zetler G. Neuropharmacological studies on ethanol extracts of Valeriana officinalis L.: behavioural and anticonvulsant properties. Phytother Res. 1996;10:145–151. [Google Scholar]
- 16.Leuschner J., Müller J., Rudmann M. Characterisation of the central nervous depressant activity of a commercially available valerian root extract. Arzneimittelforschung. 1993;43:638–641. [PubMed] [Google Scholar]
- 17.Maxwell C.J., Neutel C.I., Hirdes J.P. A prospective study of falls after benzodiazepine use: a comparison of new and repeat use. Pharmacoepidemiol Drug Saf. 1997;6:27–35. doi: 10.1002/(SICI)1099-1557(199701)6:1<27::AID-PDS240>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 18.Monograph Valeriana officinalis. Altern Med Rev. 2004;9:438–441. [PubMed] [Google Scholar]
- 19.Occhiuto F., Pino A., Palumbo D.R. Relaxing effects of Valeriana officinalis extracts on isolated human non-pregnant uterine muscle. J Pharm Pharmacol. 2009;61:251–256. doi: 10.1211/jpp/61.02.0016. [DOI] [PubMed] [Google Scholar]
- 20.Ortiz J.G., Nieves-Natal J., Chavez P. Effects of Valeriana officinalis extracts on [3H]flunitrazepam binding, synaptosomal [3H]GABA uptake, and hippocampal [3H]GABA release. Neurochem Res. 1999;24:1373–1378. doi: 10.1023/a:1022576405534. [DOI] [PubMed] [Google Scholar]
- 21.Pariente A., Fourrier-Réglat A., Letenneur L. Benzodiazepine use and occurrence of severe falls in community dwelling elderly: data from the PAQUID study. Pharmacoepidemiol Drug Saf. 2005;14:S193–S194. [Google Scholar]
- 22.Trauner G., Khom S., Baburin I. Modulation of GABAA receptors by valerian extracts is related to the content of valerenic acid. Planta Med. 2008;74(1):19–24. doi: 10.1055/s-2007-993761. [DOI] [PubMed] [Google Scholar]
- 23.van Putten M. 2011. The Use of Hanging Wire Tests to Monitor Muscle Strength and Condition over Time.http://www.treat-nmd.eu/downloads/file/sops/dmd/MDX/DMD_M.2.1.004.pdf SOP DMD_M.2.1.004 Version 4.0. Accessed 15 February 2017. [Google Scholar]
- 24.Wagner H., Jurcic K., Schaette R. Comparative studies on the sedative action of Valeriana extracts, valepotriates and their degradation products. Planta Med. 1980;39:358–365. doi: 10.1055/s-2008-1074930. [DOI] [PubMed] [Google Scholar]
- 25.Wheatley D. Medicinal plants for insomnia: a review of their pharmacology, efficacy and tolerability. J Psychopharmacol. 2005;19:414–421. doi: 10.1177/0269881105053309. [DOI] [PubMed] [Google Scholar]
- 26.Yuan C.S., Mehendale S., Xiao Y. The gamma-aminobutyric acidergic effects of valerian and valerenic acid on rat brainstem neuronal activity. Anesth Analg. 2004;98:353–358. doi: 10.1213/01.ANE.0000096189.70405.A5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.