Abstract
This study was undertaken to examine the antinociceptive, antihyperglycemic, and membrane stabilizing activity with phytochemical screening of methanolic extract of Garcinia lanceifolia whole plant. The extracts were subjected to in-vivo antinociceptive, antihyperglycemic activity in laboratory animals and in-vitro membrane stabilizing activity. In peripheral antinociceptive activity, G. lanceifolia (400 and 200 mg/kg) exhibited significant (P < 0.001) inhibition of writhing with 59.15% and 49.30% respectively comparable to standard Diclofenac (54.92% inhibition). In central antinociceptive activity, the extract (400 and 200 mg/kg) exhibited significant analgesic activity having 78.31% (P < 0.05) and 89.95% (P < 0.01) elongation of reaction time respectively in 90 min after administration of sample comparable to the standard Morphine (708.99% elongation). In hypoglycemic activity, the extract (400 and 200 mg/kg) exhibit statistically significant (P < 0.001) antihyperglycemic activity compared to standard drug Glibenclamide (10 mg/kg) at different time interval. In membrane stabilizing activity assay, clearly evident that the methanolic extracts of G. lanceifolia were highly effective to prevent the lyses of erythrocytes induced by heat. The outcomes of the present study revealed that this plant possess noteworthy pharmacological activities that may be basis for further research to disclose feasible mode of action of the plant part.
Keywords: Garcinia lanceifolia, Clusiaceae, Phytochemicals, Antinociceptive, Antihyperglycemic, Membrane stabilizing
Graphical abstract
1. Introduction
Pain is an unpleasant sensation and emotional experience associated with either acute or chronic tissue damage.1 By acting in the central nervous system (CNS) or on the peripheral pain mechanism, analgesic compounds selectively relieves pain without substantial alteration of consciousness. Actually analgesics are applied when the noxious stimulus cannot be removed or as adjuvant to more etiological approach to pain.2 On the other diabetes is a pandemic disease in the whole world characterized by hyperglycemia (high blood glucose level). The approximate death rate in people with diabetes is about double than that of people without diabetes.3 To minimize the adverse drug reactions of existing hypoglycemic dugs, people are choosing natural products. It has been reviewed that above 80% world population trust on herbal medicine for their therapeutic benefits.4
Garcinia lanceifolia is an important unexplored endemic medicinal plant, frequently known as “Rupahi-thekera” (Assamese), “Pelh” (Mizo), “Rupohi tekera” (Mising), belonging to the family Clusiaceae, mainly found in Assam, Meghalaya and southern part of Bangladesh. Now-a-days, it is facing the risk of extinction in environment and is frequently cultured at homestead.5 This medicinal plant is a small, handsome, evergreen tree, growing up to 12 feet under the dense shade of other trees. The flowers mainly occur between February to March whereas the fruiting occurs between June to July.6 The plant parts of fruits and young leaves are eaten raw and cooked as vegetables or made into pickles by the local people of Northeast India.7
Different ethnic communities of Northeast India and the ethnic groups of Tinsukia district of upper Assam used traditionally this plant in various ailments5, 8 including as pain reliever9 and hypoglycemic agents.6 A report was validated on this plant, some recent phytochemical studies confirmed that the presence of bioactive compounds like tannins, saponins, flavonoids, terpenoids, alkaloids, reducing sugar, and cardiac glycosides on this plant parts of stem, leaf, fruits and this plant possess virtuous antioxidant activity with the potential source of high phenolic content.5, 10 A current report has validated on prominent anthelmintic and antibacterial actions in methanolic extract of this plant.11 Furthermore, the statement to investigate this plant was due to the genus Garcinia belongs to family Clusiaceae are identified to a rich source of bioactive mixtures such as benzophenones, xanthones, benzoquinones, biflavonoids, and triterpenes that have anti-inflammatory, antibacterial, antioxidant, and antifungal effects.5
Therefore, the present study was intended to evaluate the antinociceptive and antihyperglycemic activity in Swiss albino mice; in-vitro membrane stabilizing activity and to find the existence of phytochemicals in the methanolic crude extract of G. lanceifolia whole plant, which is an unfamiliar essential medicinal plant at yet. The experiments, which have been taken into consideration in this study, have not been accompanied previously on this plant in Bangladesh as well as other countries.
2. Materials and methods
2.1. Drugs and chemicals
Acetic acid, glucose, tween 80 (Sigma chemicals, USA), glibenclamide, morphine (Square Pharmaceuticals Ltd., Bangladesh), aceclofenace, diclofenac sodium (ACI Pharmaceuticals Ltd., Bangladesh), normal saline (Opsonin Pharmaceuticals Ltd., Bangladesh) were procured from the mentioned sources.
2.2. Experimental animals
An average weight of 20–25 g Swiss-albino mice (aged 4–5 weeks) were used in this study and obtained from International Centre for Diarrheal Disease Research, Bangladesh (ICDDR,B). They were kept under controlled room temperature (24 ± 2 °C; relative humidity 60–70%) in a 12 h light-dark cycle and fed ICDDR; B formulated rodent food and water (ad-libitum). As these animals are very subtle to environmental changes, they are kept before the test for at least 3–4 days in the environment where the test will take place. The Institutional Animal Ethical Committee of Noakhali Science and Technology University, Bangladesh has approved the study protocol.
2.3. Collection of plant and identification
The whole plant of G. lanceifolia Roxb. was collected from Moheshkhali, Bangladesh in April, 2014. This plant was identified and authenticated by proficient botanist of Bangladesh National Herbarium (DACB), Mirpur, Dhaka and the given authentication number is 38329, where the plant was placed for further reference.
2.4. Preparation of methanol extract
The powdered plant material of about 238 g was taken in a fresh, flat-bottomed glass container and soaked in 900 ml of 80% methanol (Merck, Germany). The container with its contents was closed and retained for a period of 15 days accompanying infrequent shaking and stirring. Initially the entire mixture was filtered by a piece of clean and white cotton material and finally using Whatman No. 42 filter paper. The obtaining clear filtrates were kept at room temperature for the purposes of evaporation. It condensed a sticky concentrate of slight greenish color and stored at 4 °C until analysis.
2.5. Phytochemical screening
The presence of different constituents like flavonoids, saponins, glycosides, reducing sugars, diterpenes, alkaloids, phenols, tannins, proteins and amino acids, phytosterol, terpenoids etc in the freshly prepared crude methanolic extract were analyzed by using standard phytochemical procedures previously described.12, 13
2.6. Peripheral antinociceptive activity
The peripheral antinociceptive activity of the crude methanolic extract was evaluated by acetic acid induced writhing method in Swiss albino mice.14, 15 Laboratory animals were divided into four groups of five mice in each group. Each group received particular treatment like, Group-I served as control group treated with 1% Tween-80 in normal saline, Group-II received standard drug Diclofenac sodium (50 mg/kg body weight), Group-III and IV received extract at doses of 200 and 400 mg per kg body weight, respectively. All substances were administered orally before intra-peritoneal administration of 0.7% acetic acid. The writhing (constriction of abdomen, turning of trunk and extension of hind legs) was observed on each mice for 5 min after 15 min of time interval of acetic acid administration. Analgesic activity was expressed as writhing inhibition (%) and was calculated by using the following formula16:
| Writhing inhibition (%) = ([Wc − Ws]/Wc) × 100 |
where, Wc is the mean number of writhing of control and Ws is the mean number of writhing of the test sample.
2.7. Central antinociceptive activity
Central antinociceptive activity of the crude methanolic extract was evaluated by tail immersion. In tail immersion method, the test animals were divided into four groups of three mice each. Group 1 served as control treated with 1% Tween-80 in normal saline, Group 2 received standard drug Morphine, Group 3 and 4 received extract at doses of 200 and 400 mg per kg body weight, respectively. All substances were administered orally. At zero hour, 1–2 cm of the tail of mice was immersed in warm water kept constant at 55 °C. The reaction time is the time required by the mice to deflect their tails. The first reading is discarded and the reaction time is recorded as a mean of the next three reading. A latency period of 20 s was defined as complete analgesia and the measurement was stopped to avoid injury to mice. The latent period of tail-flick response was determined before and 0, 30, 60 and 90 min after the administration of drugs. A 30 min interval was given to ensure proper absorption of the administered substances. Then morphine solution was administered subcutaneously to the mice. After 30 min, 60 min and 90 min, the tail immersion time was measured.
2.8. Antihyperglycemic activity
Antihyperglycemic activity of extract was determined by the most acceptable method Glucose Tolerance Test (GTT), which is formerly described by Joy and Kuttan17 with slight modifications. Briefly, at first mice were randomly selected and grouped into four groups of three mice each. Each grouped received a particular treatment like Group 1 received vehicle (1% Tween 80 in saline, 0.1 ml/10 g of body weight) and served as control, Group 2 received standard drug (Glibenclamide, 10 mg/kg body weight), Group 3 and 4 received extract at doses of 200 and 400 mg per kg body weight, respectively. All substances were administered orally. After a period of 1 h, all mice were treated with 10% glucose solution orally (2 g glucose/kg body weight). Then blood samples were collected from each mice tail vein at 30, 60, 120 and 180 min after the glucose administration and blood glucose level was measured by using glucometer.
2.9. Membrane stabilizing activity
This activity was measured by using hypnotic solution and heat induced hemolysis of human RBC by the method developed by Omale and Okafor as previously described.18
2.10. Statistical analysis
The statistical analysis was performed in this study by using SPSS software package (version 19.0). Calculated values are expressed as mean ± SEM. Data analysis among the groups was compared using one-way ANOVA followed by Dunnett's post Hoc test. P value < 0.05 in all cases was measured as statistical significant.
3. Results
3.1. Phytochemical screening
The initiatory phytochemical screening revealed that crude extract of G. lanceifolia confirmed the presence of alkaloid, saponines, flavonoids, cardiac glycoside, terpenoids, phytosterol, and tannins (Table 1). Furthermore, it is noted that the presence of alkaloid, saponines and flavonoids extensively.
Table 1.
Phytochemical screening of the methanolic extract of G. lanceifolia.
| Name of phytochemical | Observation |
|---|---|
| Alkaloid | + + |
| Cardiac glycoside | + |
| Carbohydrate | − |
| Saponines | + + |
| Triterpene | + |
| Phytosterol | + |
| Flavonoids | + + |
| Protein and amino acids | − |
| Tannins | + |
Here, (+) = presence of constituents; (−) = absence of constituents; (++) = presence of constituents extensively.
3.2. Peripheral antinociceptive activity
The effects of methanolic extract of G. lanceifolia to subside the pain caused by acetic acid were observed as follows to evaluate antinociceptive activity by taking samples at doses of 200 mg/kg and 400 mg/kg body weight. This extract of G. lanceifolia at both doses (200 mg/kg and 400 mg/kg b.w.) showed highly statistically significant (P < 0.001) antinociceptive activity having 49.30% and 59.15% of writhing inhibition, respectively compared to standard diclofenac 59.92% (Table 2).
Table 2.
Acetic acid induced peripheral antinociceptive activity of G. lanceifolia.
| Group | Dose (mg/kg) | Number of writhing (mean ± SEM) | % of inhibition of writhing |
|---|---|---|---|
| Control | – | 14.20 ± 0.86 | – |
| Standard | 125 | 6.40 ± 0.60 | 54.92 |
| ME 200 | 200 | 7.20 ± 0.37c | 49.30c |
| ME 400 | 400 | 5.80 ± 0.37c | 59.15c |
Each value represents the mean ± SEM (n = 5). cP < 0.001 compared with standard. (One way ANOVA followed by Dunnett's ‘t’-test). ME 200, ME 400 = Methanolic extract of G. lanceifolia at 200 and 400 mg/kg body weight respectively.
3.3. Central antinociceptive activity
The central antinociceptive activity of the crude methanolic extract by tail immersion method is expressed in Table 3. In this method, the crude extract (200 and 400 mg/kg b.w) showed significant analgesic activity having 89.95% (P < 0.01) and 78.31% (P < 0.05) elongation of reaction time respectively in 90 min after administration of sample compared to control, whereas the standard drug morphine having 708.99% elongation (P < 0.001).
Table 3.
Central antinociceptive activity of methanolic crude extract of G. lanceifolia by tail immersion method.
| Group | Dose (mg/kg) | Reaction time (s) |
||
|---|---|---|---|---|
| 30 min (% elongation) | 60 min (% elongation) | 90 min (% elongation) | ||
| CTL | – | 4.61 ± 0.62 | 3.10 ± 0.78 | 1.89 ± 0.06 |
| STD | 2 | 19.45 ± 0.55c (321.91) | 19.12 ± 0.88c (516.67) | 15.29 ± 0.39c (708.99) |
| ME 1 | 200 | 8.00 ± 2.31 (73.53) | 7.40 ± 2.94 (138.71) | 3.59 ± 0.37b (89.95) |
| ME 2 | 400 | 6.67 ± 1.20 (44.68) | 6.01 ± 1.12 (93.87) | 3.37 ± 0.23a (78.31) |
Each value represents the mean ± SEM (n = 3). cP < 0.001, bP < 0.01, aP<0.05 compared with control. (One way ANOVA followed by Dunnett's ‘t’-test). CTL: Control; STD: Standard; ME: Methanolic extract.
3.4. Antihyperglycemic activity
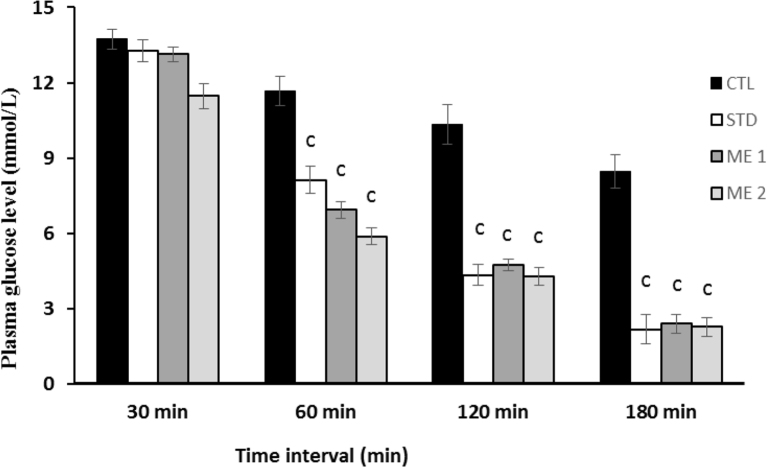
The results obtained from the present study of the effect of methanolic crude extract on lowering blood glucose level are presented in Fig. 1. At both doses of 200 and 400 mg per kg body weight, the lowering of blood glucose levels were significant (P < 0.001) in experimental animals compared to blood glucose level in control group at different time intervals of 60, 120 and 180 min.
Fig. 1.
Hypoglycemic activity of methanolic crude extract of G. lanceifolia. Here, values are presented as mean ± SEM (n = 3); CTL: Control (10 ml/kg); STD: Standard (10 mg/kg); ME 1: Methanolic extract (200 mg/kg); ME 2: Methanolic extract (400 mg/kg). cP < 0.001 compared with control (One way ANOVA followed by Dunnett's ‘t’-test).
3.5. Membrane stabilizing activity
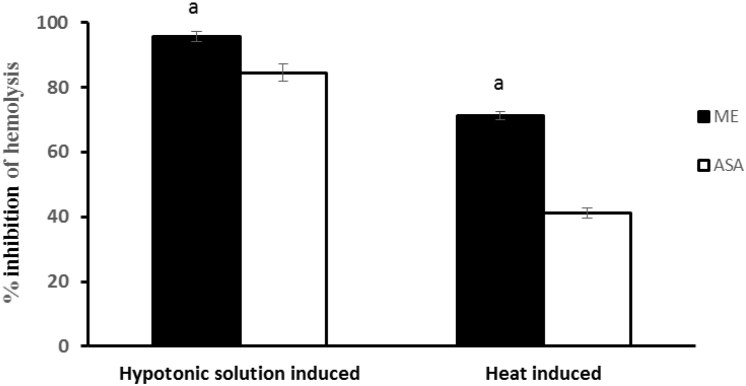
The methanolic extracts of G. lanceifolia at concentration 2.0 mg/ml significantly protected the lysis of human erythrocyte membrane induced by hypnotic solution and heat as compared to the standard acetyl salicylic acid (ASA) and the obtained results were presented in Fig. 2. In a conditions of hypnotic solution and heat induced, the methanolic extract was found to inhibit 95.59% and 71.35% hemolysis of erythrocyte membrane respectively, whereas in the same situations, ASA exhibited 84.44% and 41.12% inhibition of hemolysis of erythrocyte.
Fig. 2.
Effect of extraction of G. lanceifolia on hypnotic solution and heat induced hemolysis of erythrocyte membrane. Here, ME = Methanolic extract (2 mg/ml); ASA = Acetyl salicylic acid (0.10 mg/ml). aP<0.05 compared with standard (One way ANOVA followed by Dunnett's ‘t’-test).
4. Discussion
This study confirmed the presence of numerous phytochemical constituents, namely alkaloid, saponines, flavonoids, cardiac glycoside, terpenoids, phytosterol, and tannins as a natural product in the title plant extract. Furthermore, it can be mentioned that the content of alkaloid, saponines and flavonoids was found to be very high. This finding was in accordance with other reported works.10, 11
It was established that acetic acid induced pain triggering by localized inflammatory response generated by discharge of free arachidonic acid from tissue phospholipid via cyclooxygenase (COX), and prostaglandin biosynthesis and correlated with increased level of PGE2 and PGF2α in peritoneal fluids.19 This method is considered to be useful for determining peripherally active analgesics. The number of writhing mitigated by this agent will render analgesic outcome preferably by prohibition of prostaglandin synthesis, a peripheral mechanism of pain inhibition.20 So, the significant pain reduction of the plant extracts might be due to the prostaglandin pathways. It was reported that the existence of phytochemicals like saponin, terpenoids, flavonoids, diterpene and steroids in the plant extract are the reasonable cause for demonstrating the antinociceptive activity20, 21 by inhibiting prostaglandin synthesis.22 Therefore, it can be anticipated that the presence of saponins, terpenoids, flavonoids, and phytosterol are responsible for observed antinociceptive activity in accordance with above mentioned studies.
Historically over 400 plant species have been recognized in the literature with significant hypoglycemic activity.23 It was observed that, the plant extract of G. lanceifolia exhibited dose-dependent significant hypoglycemic activity at different time interval in comparison to that of the standard of glibenclamide, evaluated by the most acceptable method of GTT. Earlier study data reported that the genus of Garcinia belongs to family Clusiaceae contain rich sources of bioactive compounds, those have scientifically proven antidiabetic activity with the mechanism of action of α-glucosidase inhibition.5, 6
It is evidence that methanolic extract protected RBC membrane against lysis induced by hypotonic solution and heat induced. In inflammation, lysosomal enzymes and hydrolytic components are exempt from the phagocytes to the extracellular space, which are responsible for the damages of the enclosure organelles and tissues and also supports a diversity of ailments.21 This study was undertaken for measuring the mechanism of anti-inflammatory activity of the plant extract, because human RBC membranes are calculated alike to lysosomal membrane constituents.24 One can say that the potential mode of action of the extracts and typical anti-inflammatory drugs may be linked with binding to the erythrocyte membranes with compliant deviation of surface charges of cells. It is predict that, this could have clogged physical interaction with agents of aggregation or promote diffraction by mutual expulsion of the charges as being involved in the hemolysis of RBCs. In-vivo studies in experimental animals showed that the flavonoids exert stabilizing effects largely on lysosomes25 as tannin and saponins are capable of binding cations and other biomolecules, and are capable of stabilizing the erythrocyte membrane. In harmony with these studies, presence of tannin, saponins, and flavonoids in the plant extract and findings revealed that the plant extract exposed powerful RBC membrane stabilizing action in case of hypotonic solution and heat-induced lysis.
5. Conclusion
In light of the outcomes of the present study led us to the interpretation that the plant extract have noteworthy antinociceptive, hypoglycemic and membrane stabilizing activity with potential sources of bioactive compounds. Since this plant is used in traditional medicine the extracts should be advance discovered for its phytochemical profile to identify active component responsible for those activity scientifically.
Conflict if interest
Authors declared that they have no conflict of interest.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Loeser J.D., Treede R.D. The Kyoto protocol of IASP basic pain terminology. Pain. 2008;137:473–477. doi: 10.1016/j.pain.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 2.Sarker M.A.A., Banik S., Hussain M.S., Ghosh A., Hossain M.S. In-vitro and in-vivo pharmacological activities with phytochemical evaluation of methanolic extract of Microcos paniculata stem barks. Curr Drug Ther. 2016;11:142–149. [Google Scholar]
- 3.Paul S.R., Hakim M.L. In vivo hypoglycemic study of Manilkara zapota leaf and seed. Bangladesh J Pharmacol. 2015;10:246–250. [Google Scholar]
- 4.Tiwari S. Plant: a rich source of herbal medicine. J Nat Prod. 2008;1:27–35. [Google Scholar]
- 5.Chowdhury T., Handique P.J. Evaluation of antibacterial activity and phytochemical activity of Garcinia lanceifolia ROXB. IJPSR. 2012;3(6):1663–1667. [Google Scholar]
- 6.Baruah A. Less known local fruits of NE India: a digest with emphasis to ethno-medico-botany and chemical constituents. In: Trivedi P.C., editor. Ethnomedicinal Plants of India. 1st edition. Aavishkar; 2007. pp. 122–135. [Google Scholar]
- 7.Bora N.S., Kakoti B.B., Gogoi B. Antibacterial Activity of the methanolic extract of the bark of Garcinia lanceifolia Roxb. J Pharma Sci Tech. 2015;4(2):34–35. [Google Scholar]
- 8.Buragohain J. Ethnomedicinal plants used by the ethnic communities of Tinsukia district of Assam, India. Rec Res Sci Tech. 2011;3(9):31–42. [Google Scholar]
- 9.Bora N.S., Kakoti B.B., Bairy P.S., Gogoi B. Garcinia lanceifolia Roxb; an endemic medicinal plant of Assam relieves pain and delays nociceptive response: an assay for its analgesic and anti-inflammatory activity. Int J Pharm Sci Drug Res. 2014;6(3):216–219. [Google Scholar]
- 10.Policegoudra R.S., Saikia S., Das J., Chattopadhyay P., Singh L., Veer V. Phenolic content, antioxidant activity, antibacterial activity and phytochemical composition of Garcinia lanceifolia. Ind J Pharm Sci. 2012;74(3):268–271. doi: 10.4103/0250-474X.106075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bora N.S., Kakoti B.B., Gogoi B. Study on antibacterial activity of the bark of Garcinia lanceifolia Roxb. Int Sch Res Not. 2014 doi: 10.1155/2014/784579. Article ID 784579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosh A., Banik S., Islam M.A. In vitro thrombolytic, anthelmintic, antioxidant and cytotoxic activity with phytochemical screening of methanolic extract of Xanthium indicum leaves. Bangladesh J Pharmacol. 2015;10:854–859. [Google Scholar]
- 13.Nandagoapalan V., Doss A., Marimuthu C. Phytochemical analysis of some traditional medicinal plants. Biosci Discov. 2016;7(1):17–20. [Google Scholar]
- 14.Koster R., Anderson M., de Beer E.J. Acetic acid for analgesic screening. Fed Proc. 1959;18:412. [Google Scholar]
- 15.Vogel H.G., Vogel W.H. Springer-Verlag; New York: 1997. Drug Discovery and Evaluation-Pharmacological Assays. 370-1, 402. [Google Scholar]
- 16.Apu A.S., Hossain F., Rizwan F., Bhuyan S.H., Matin M., Jamaluddin A. Study of pharmacological activities of methanol extract of Jatropha gossypifolia fruits. J Basic Clin Pharma. 2013;4:20–24. doi: 10.4103/0976-0105.109404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joy K.L., Kuttan R.J. Anti-diabetic activity of Picrorrhiza kurroa extract. J Ethnopharmacol. 1999;67:143–148. doi: 10.1016/s0378-8741(98)00243-8. [DOI] [PubMed] [Google Scholar]
- 18.Omale J., Okafor P.N. Compartive antioxidant capacity, membrane stabilization, polyphenol composition and cytotoxicity of the leaf and stem of Cissus multistriata. Afr J Biotechnol. 2008;7:3129–3133. [Google Scholar]
- 19.Ahmed F., Hossain M.H., Rahman A.A., Shahid I.Z. Antinociceptive and sedative effects of the bark of Cerbera odollam Gaertn. Orient Pharm Exp Med. 2006;6:344–348. [Google Scholar]
- 20.Oweyele V.B., Oloriegbe Y.Y., Balogun E.A., Soladoye A.O. Analgesic and anti-inflammatory properties of Nelsonia canescens leaf extract. J Ethnopharmacol. 2005;99:153–156. doi: 10.1016/j.jep.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Adeolu A.A., Margaret O.S., Viola M., Moyo B., Masika P.J., Afolayan A.J. Anti-inflammatory and analgesic activities of the aqueous extract of Cussonia paniculata stem bark. Rec Nat Prod. 2008;2:46–53. [Google Scholar]
- 22.Gupta A.K., Parasar D., Sagar A. Analgesic and anti-inflammatory properties of gelsolin in acetic acid induced writhing, tail immersion and Carrageenan induced paw edema in mice. PLoS One. 2015;10(8):e0135558. doi: 10.1371/journal.pone.0135558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belhekar S.N., Chaudhari P.D., Saryawanshi J.S., Mail K.K., Pandhare R.B. Antidiabetic and antihyperlipidemic effects of Thespesia populnea fruit pulp extracts on alloxan-induced diabetic rats. Indian J Pharm Sci. 2013;75:217–221. [PMC free article] [PubMed] [Google Scholar]
- 24.Feirrali M., Signormi C., Ciccolili L., Comporti M. Iron release and membrane damage in erythrocytes exposed to oxidizing agents, phenyl hydrazine, devicene and iso-uranil. Biochem J. 1992;285:295–301. doi: 10.1042/bj2850295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vinod R.K., Chandrasekhar J., Sudhakar K., Rajeswar T., Sandhya S.K., Venkatramana K.R. Membrane stabilizing potency of two Tephrosia species. J Phytol. 2010;2:42–46. [Google Scholar]