Abstract
The present study aimed to investigate the antitumor efficacy of di-2-pyridylketone-4-cyclohexyl-4-methyl-3-thiosemicarbazone (DpC) and di-2-pyridylketone-4,4,-dimethyl-3-thiosemicarbazone (Dp44mT) on head and neck squamous cell carcinoma (HNSCC) cells. The proliferation and apoptosis of HNSCC cells treated with the iron chelators DpC and Dp44mT were detected. The mechanism of DpC-induced apoptosis on HNSCC cells was investigated. The human HNSCC cell lines FaDu, Cal-27 and SCC-9 were cultured in vitro and exposed to gradient concentrations of DpC and Dp44mT. A Cell Counting Kit-8 assay was used to detect the viability of FaDu, Cal-27, SCC-9 cells. Double staining with annexin V and propidium iodide was performed for the detection of the proportion of apoptotic FaDu, Cal-27 and SCC-9 cells following treatment. The nuclear damage to Cal-27 cells that were treated with DpC was detected by Hoechst staining. Finally, western blot analysis was used to detect the expression of proteins associated with the DNA damage pathway in Cal-27 cells that were treated with DpC. The CCK-8 assay showed that treatment with DpC and Dp44mT was able to markedly inhibit the viability of FaDu, Cal-27 and SCC-9 cells in a concentration-dependent manner. In comparison to Dp44mT, treatment with DpC exhibited a more effective inhibitory effect on the viability of HNSCC cells. The proportion of apoptotic cells detected by flow cytometry increased in a dose-dependent manner in all cell lines following DpC and Dp44mT treatment, with the proportion of apoptotic HNSCC cells induced by DpC treatment being significantly higher compared with Dp44mT (P<0.05). The results of Hoechst staining revealed that the nuclei of Cal-27 cells exhibited morphological changes in response to DpC treatment, including karyopyknosis and nuclear fragmentation. The expression of DNA damage-associated proteins, including phosphorylated (p)-serine-protein kinase ATM, p-serine/threonine-protein kinase Chk1 (p-Chk-1), p-serine/threonine-protein kinase ATR (p-ATR), p-Chk-2, poly (ADP-ribose) polymerase, p-histone H2AX, breast cancer type 1 susceptibility protein, p-tumor protein P53, increased with increasing concentration of DpC in Cal-27 cells. Treatment with DpC and Dp44mT markedly inhibited cell viability and increased the apoptotic rates in human HNSCC cells in a concentration-dependent manner. DpC exhibited a stronger antitumor effect compared with Dp44mT, potentially inducing the apoptosis of HNSCC cells via the upregulation of DNA damage repair-associated proteins.
Keywords: di-2-pyridylketone-4-cyclohexyl-4-methyl-3-thiosemicarbazone; di-2-pyridylketone-4,4,-dimethyl-3-thiosemicarbazone; proliferation; apoptosis; DNA damage; head and neck squamous cell carcinoma
Introduction
Head and neck squamous cell carcinoma (HNSCC) is one of the most common malignant tumor in the world, which occurs in upper respiratory and upper digestive tract. HNSCC includes cancer of the lip, oral cavity, pharynx, larynx, hypopharynx, salivary gland, nose, head and facial soft tissue, and thyroid (1). In recent years, the incidence of HNSCC has been increasing (2). Management strategies for HNSCC are varied, including surgery, radiotherapy, chemotherapy, biological therapy and traditional Chinese medicine (3). Despite the integrated application of these therapies, the 5-year survival rate of HNSCC has not increased substantially in recent years (4). However, the combination of surgery, radiotherapy and chemotherapy can severely reduce the quality of life of patients with the disease (5).
Compared with normal cells, cancer cells with rapid growth exhibit a higher demand for iron (6). Generally, the expression of transferrin receptor 1 (TfR1) is higher in cancer cells compared with normal cells, which allows cancer cells to absorb iron from transferrin at a higher rate (7), and this results in selectively increased iron chelation (8). Previous studies have shown that iron chelators confer anti-tumor activity as they can chelate metal ions (2,9).
Di-2-pyridylketone thiosemicarbazone (DpT) is an iron chelator with good anti-tumor activity and selectivity (10–12), which was first reported by Yuan et al (13). Di-2-pyridylketone-4,4,-dimethyl-3-thiosemicarbazone (Dp44mT), one of the most effective chelators of the DpT family, has been shown to exhibit a substantial inhibitory effect in transplanted tumors in mice (14) Di-2-pyridylketone-4-cyclohexyl-4-methyl-3-thiosemicarbazone (DpC) is a second-generation iron chelator. DpC exhibits a synergistic effect with chemotherapy on mouse lung tumors and a potential independent antitumor activity (8). In comparison to the first-generation iron chelator Dp44mT, DpC exhibits advantages in the treatment of tumors (3). At present, there are a limited number of studies on the anti-tumor activity of DpC and Dp44mT in HNSCC. The present study aims to clarify the anti-tumor effect of DpC and Dp44mT on several HNSCC cell lines in vitro, and investigate the mechanisms involved.
Materials and methods
Chemical regents
DpC, Dp44mT and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). DpC and Dp44mT were dissolved in DMSO (final concentration, 20 mM) and stored at −20°C. Cell counting kit-8 was purchased from Dojindo Molecular Technologies, Inc. (Kumamoto, Japan). The Annexin V-Propidium Iodide (PI) Double Staining assay kit was used for apoptosis detection by flow cytometry (Hangzhou Lianke Biotechnology Co., Ltd., Zhejiang, China). Antibodies against GAPDH, phosphorylated (p)-serine-protein kinase ATM (ATM), p-serine/threonine-protein kinase Chk1 (Chk-1), p-serine/threonine-protein kinase ATR (ATR), p-Chk-2, poly (ADP-ribose) polymerase, p-histone H2AX, breast cancer type 1 susceptibility protein (BRCA1), p-tumor protein P53 (P53) were all obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Cell culture
The HNSCC Cal-27, SCC-9 and FaDu cell lines were cultured in Dulbecco's modified Eagle's Medium (DMEM; HyClone;) with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in 37°C at 5% CO2. The cells in the logarithmic phase of growth were used in the experiment.
Cytotoxicity test
FaDu, Cal-27 and SCC-9 cells (1×103) in the logarithmic growth phase were seeded into wells in a 96-well plate. A range of concentrations of DpC (0, 1, 5, 10 and 50 µM) and Dp44mT (0, 1, 5, 10 and 50 µM) were added to the cells for treatment for 24 h. Next, 10 µl/well CCK-8 reagent was added to 96-well plates, which were incubated at 37°C for 1 h, then the optical density (OD) values were detected using a microplate reader at a wavelength of 450 nm. The experiment was repeated three times independently. The effect of DpC and Dp44mT on cell activity was measured by normalizing the OD value of the experimental group to the OD value of the control group (0 nM), and the concentration required to inhibit viability by 50% (IC50) was calculated.
Flow cytometry analysis
FaDu, Cal-27 and SCC-9 HNSCC cells in the logarithmic growth phase were treated with a range of concentrations of DpC (0, 2.5, 5 and 7.5 µM) and Dp44mT (0, 2.5, 5 and 7.5 µM) for 24 h and the cells were collected. The adherent cells and supernatant were washed with precooled PBS twice, and then the cells were resuspended with 500 µl 1X Binding buffer. Next 5 µl Annexin V-fluorescein isothiocyanate (Annexin V-FITC) and 10 µl propidium iodide were added for staining at room temperature for 15 min. All the regents used in this experiment were contained in the Annexin V/PI kit. A flow cytometer was used to detect the levels of cell apoptosis. The software CellQuest Pro (BD Biosciences, San Jose, CA, USA) was used for the analysis.
Hoechst staining
The Cal-27 cells in the logarithmic growth phase were collected and seeded into 6-well plates, and the cells were treated with a range of concentrations of DpC (0, 2.5, 5 and 7.5 µM) at 37°C for 24 h. The cells were then washed once with PBS, and 1 ml Hoechst staining solution was added per well (Beyotime Institute of Biotechnology, Jiangsu, China). The cells were incubated in the dark for 30 min and then washed twice with PBS. The cells were then observed with a fluorescent microscope (×40). The staining results were quantified using Image Studio v3.1 software (LI-COR Biosciences, Lincoln, NE, USA)
Western blot analysis
Cal-27 HNSCC cells in the logarithmic growth phase were treated with DpC (0, 2.5, 5 and 7.5 µM) for 24 h. Next, the cells were collected, and the total protein was extracted. Cells were harvested and lysed in the protein lysate buffer (Beyotime Institute of Biotechnology). A bicinchoninic acid assay (Pierce™ BCA Protein Assay kit; cat. no. 23225; Thermo Fisher Scientific, Inc.) was used to determine the protein concentration. The protein sample used per lane was 25 µg. Lysates were resolved by SDS-PAGE on a 10% gel. The proteins were transferred onto the PVDF membrane by electrophoresis and electrotransfer. Following incubation with the blocking solution (5% non-fat milk powder) at room temperature for 2 h, the primary antibodies against GAPDH, p-ATM, p-Chk-1, p-ATR p-Chk-2, PARP, p-histone H2A.X, BRCA1 and p-P53 were added, and the membrane was incubated at 4°C overnight. The samples were washed with TBST three times, and the secondary antibody [IRDye® 800CW Donkey anti-rabbit IgG (cat. no. 925-32213; LI-COR Biosciences; 1:10,000)] was added for incubation for 1 h. The membranes were visualized using the Odyssey CLx Infrared Imaging system (LI-COR Biosciences, Lincoln, NE, USA). And the western blotting bands were quantified using Image Studio v3.1 software.
Statistical analysis
SPSS v17.0 software (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. Data between two groups were compared using unpaired t-test, and multiple groups were compared using one way-analysis of variance, post-hoc analysis was performed with the Tukey test. P<0.05 was considered to indicate a statistically significant difference.
Results
Proliferation of HNSCC cells is efficiently inhibited by DpC and Dp44mT
To evaluate the effect of DpC and Dp44mT on the proliferation of HNSCC cell lines in vitro, a cell viability test was performed using the CCK-8 method (Fig. 1). Gradient concentrations of DpC (0, 1, 5, 10 and 50 µM) and Dp44mT (0, 1, 5, 10 and 50 µM) were used to treat the FaDu, Cal-27, SCC-9 HNSCC cell lines for 24 h. The results demonstrated that DpC and Dp44mT had an anti-proliferative effect on HNSCC cells, and the effect was concentration-dependent. The IC50 values at 24 h were assessed, and the values are as follows: FaDu Dp44mT, 24.37 µM and DpC, 3.93 µM; Cal-27 Dp44mT, 15.15 µM and DpC, 2.79 µM; and SCC-9 Dp44mT, 95.36 µM and DpC, 15.61 µM. The results of the present study indicated the anti-proliferative effect of DpC was stronger compared with Dp44mT in HNSCC cells, and that the IC50 of Cal-27 cells was the lowest for treatment with DpC and Dp44mT, indicating that Cal-27 cells were the most sensitive to DpC and Dp44mT.
Figure 1.
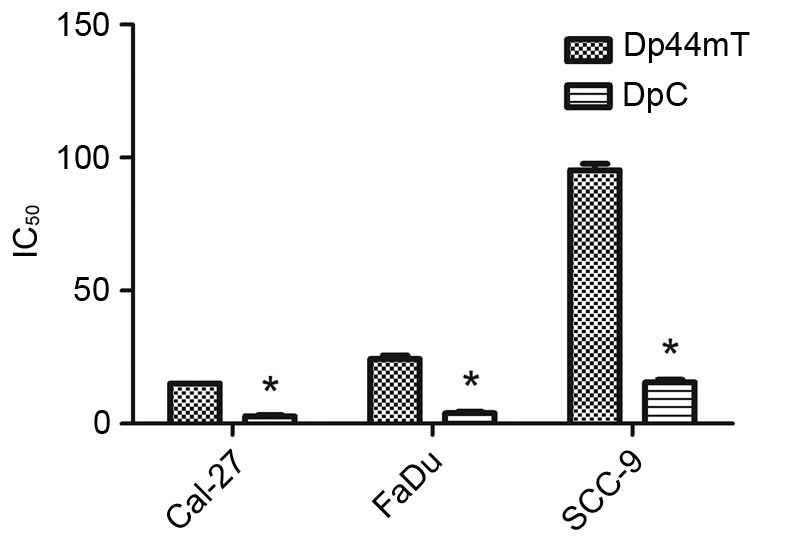
Dp44mT and DpC inhibit the viability of Cal-27, FaDu and SCC-9 cells. The Cal-27, SCC-9 and FaDu HNSCC cell lines were treated with a range of concentrations of DpC (0, 1, 5, 10 and 50 µM) and Dp44mT (0, 1, 5, 10 and 50 µM) for 24 h, and the viability of these cell lines were subsequently assessed. The IC50 values at 24 h were assessed, and it was indicated the anti-proliferative effect of DpC was stronger compared with Dp44mT in HNSCC cells. *P<0.001 vs. Dp44mT. Dp44mT, di-2-pyridylketone-4,4,-dimethyl-3-thiosemicarbazone; DpC, di-2-pyridylketone-4-cyclohexyl-4-methyl-3-thiosemicarbazone; HNSCC, head and neck squamous cell carcinoma; IC50, half-maximal inhibitory concentration.
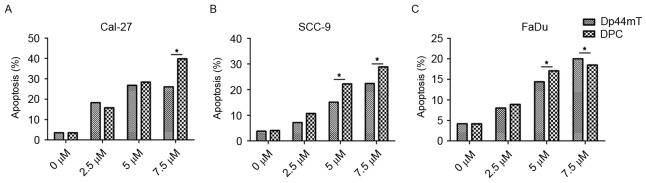
Apoptosis of HNSCC cells is efficiently induced by DpC and Dp44mT
To determine the effect of DpC and Dp44mT on the apoptosis of HNSCC cells in vitro, flow cytometry was performed to detect the proportion of cells in early apoptosis and late apoptosis were included (Fig. 2). Gradient concentration of DpC (0, 1, 5, 10 and 50 µM) and Dp44mT (0, 1, 5, 10 and 50 µM) were used to treat HNSCC Cal-27, SCC-9 and FaDu cell lines for 24 h. The results revealed that the percentage of apoptotic cells increased with the increase of the concentration of DpC and Dp44mT, and the effect was concentration-dependent (Fig. 2). The percentages of apoptotic cells for 0, 1, 5, 10, 50 µM Dp44mT treatment were as follows: Cal-27, 3.5, 18.3, 26.8 and 26.1% (Fig. 2A); SCC-9, 3.8, 7.2, 15.1 and 22.4% (Fig. 2B); FaDu, 4.2, 8.0, 14.4 and 20.0%, respectively (Fig. 2C). The percentages of apoptotic cells for 0, 1, 5, 10, 50 µM DpC treatment were as follows: Cal-27, 3.5, 15.8, 28.4, 39.8% (Fig. 2A); SCC-9, 4.1, 10.7, 22.3 and 28.9% (Fig. 2B); FaDu, 4.2, 8.9, 17.1 and 18.5%, respectively (Fig. 2C). These findings indicated DpC and Dp44mT were able to promote the apoptosis of HNSCC cells. The effect of DpC on the apoptosis of HNSCC cells was greater compared with Dp44mT, and Cal-27 was the most sensitive cell line to DpC and Dp44mT treatment.
Figure 2.
Dp44mT and DpC promote the apoptosis of Cal-27, FaDu and SCC-9 cells. DpC (0, 1, 5, 10 and 50 µM) and Dp44mT (0, 1, 5, 10 and 50 µM) were used to treat Cal-27, SCC-9 and FaDu HNSCC cells for 24 h. (A) The apoptotic rate of the Cal-27 cells when treated with DpC (0, 1, 5, 10 and 50 µM) and Dp44mT (0, 1, 5, 10 and 50 µM). (B) The apoptotic rate of the SCC-9 cells when treated with DpC (0, 1, 5, 10 and 50 µM) and Dp44mT (0, 1, 5, 10 and 50 µM). (C) The apoptotic rate of the FaDu cells when treated with DpC (0, 1, 5, 10 and 50 µM) and Dp44mT (0, 1, 5, 10 and 50 µM). The apoptotic rate of the cells increased with the increase of the concentration of DpC and Dp44mT, indicating that DpC and Dp44mT are able to promote the apoptosis of HNSCC cells. The effect of DpC on the apoptosis of HNSCC cells was stronger compared with Dp44mT. *P<0.001. Dp44mT, di-2-pyridylketone-4,4,-dimethyl-3-thiosemicarbazone; DpC, di-2-pyridylketone-4-cyclohexyl-4-methyl-3-thiosemicarbazone; HNSCC, head and neck squamous cell carcinoma.
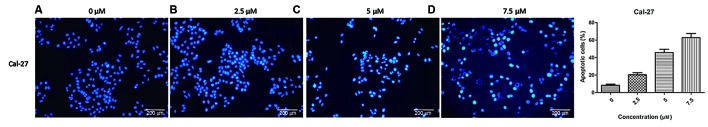
Nuclear damage in Cal-2 cells7 increased with the increase of DpC drug concentration
Flow cytometric analysis revealed a significant increase in apoptosis upon treatment with increasing drug concentrations. Compared with the first-generation iron-chelating agent Dp44mT, DpC had a more marked effect on the regulation of proliferation and apoptosis of HNSCC cell lines, particularly in Cal-27 cells. In order to confirm this result, the changes in nuclear morphology in Cal-27 cell line mediated by DpC treatment was examined by Hoechst staining (Fig. 3). The Cal-27 cell line was observed to be the more sensitive cell line to DpC. Hoechst is a specific fluorescent DNA probe, which can be combined with DNA in living cells. The nucleus exhibits a bright blue fluorescence when excited by ultraviolet light and viewed with a fluorescence microscope. This experiment revealed that with increasing concentrations of DpC (0, 2.5, 5 and 7.5 µM) treatment, the proportion of nuclei with bright blue fluorescence decreased significantly.
Figure 3.
DpC induces nuclear damage of Cal-27. The change in nuclear morphology by DpC on the Cal-27 cell line was detected by Hoechst staining. The experimental results showed that upon treatment with a range of concentrations of DpC (0, 2.5, 5 and 7.5 µM). (A) The nuclear staining of the Cal-27 cells when treated with 0 µM DpC. (B) The nuclear staining of the Cal-27 cells when treated with 2.5 µM DpC. (C) The nuclear staining of the Cal-27 cells when treated with 5 µM DpC. (D) The nuclear staining of the Cal-27 cells when treated with 7.5 µM DpC; the proportion of nuclei with bright blue fluorescence decreased significantly. DpC, di-2-pyridylketone-4-cyclohexyl-4-methyl-3-thiosemicarbazone (magnification, ×40).
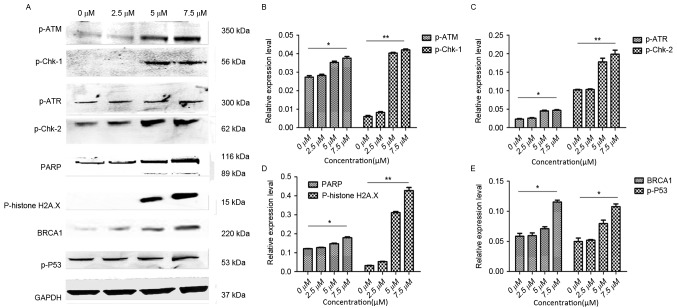
Expression of proteins associated with DNA damage was upregulated in Cal-27 following treatment with DpC
As aforementioned, DpC has an important role in regulating proliferation and apoptosis of HNSCC cells. Therefore, the expression of several important proteins in the DNA damage pathway associated with proliferation and apoptosis may also change accordingly. Western blot analysis was performed to detect the expression of proteins that are associated with DNA damage (Fig. 4). GAPDH was used as a loading control. Cal-27 cells were treated with an increasing concentration of DpC (0, 2.5, 5 and 7.5 µM). It as observed that PARP, a protein that in involved in the monitoring of DNA damage, was upregulated as the concentration of DpC increased. Furthermore, the levels of p-ATM and p-ATR proteins were upregulated, and it was hypothesized that ATM and ATR carried out DNA damage repair in two ways after DNA damage has occurred. Which resulted in increased expression of p-histone H2AX and BRCA1 (15). In addition, the expression of p-Chk-1, p-Chk-2 and p-P53 were also upregulated.
Figure 4.
Changes in the expression of proteins in the DNA-damage-associated pathway following DpC treatment in Cal-27 cells. (A) Western blot analysis was performed to detect the expression of the indicated proteins, and GAPDH was used as a loading control. Cal-27 cells treated with a range of concentrations of DpC (0, 2.5, 5 and 7.5 µM). (B) Statistical view of western blot analysis for p-ATM and p-Chk-1. (C) Statistical views of western blot analysis for p-ATR and p-Chk-2. (D) Statistical views of western blot analysis for PARP and p-histone H2A.X. (E) Statistical views of western blot analysis for BRCA1 and p-P53. As the concentration of DpC increased, the expression of (B) p-ATM, (C) p-ATR, (D) PARP, (E) BRCA1 and p-P53 also increased. *P<0.05; **P<0.01. DpC, di-2-pyridylketone-4-cyclohexyl-4-methyl-3-thiosemicarbazone; p-, phosphorylated; PARP, poly (ADP-ribose) polymerase; BRCA1, breast cancer type 1 susceptibility protein; P53, tumor protein P53.
Discussion
Thiosemicarbazone compounds with antitumor activity were initially identified by Hamre et al (16) in 1950. The DpT group of compounds, which includes Dp44mT and DpC, were derived from an analysis of structural activity associations over a period of 20 years and were derived from assessment of the pyridoxal isonicotinoyl hydrazone analogue group of agents (17–20). Since the derivation of DpT compounds, numerous types of biological activity, including antiviral, antibacterial, antitumor, anti-leprosy, anti-tubercular and anti-malarial activities, were reported in multiple studies (21–25). The N1NH(CS)N4H structure is the key active structure, and is necessary for the biological activity of the compound. Due to the presence of N, S and other elements, and the C=N group, thiosemicarbazones readily form stable complexes with a variety of metal ions. The biological activity of thiosemicarbazones was markedly enhanced following the formation of complexes, particularly in terms of anticancer and anti-HIV activity (26). Compared with 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP), the double-ketone thiosemicarbazone compounds, DpTs, are better ferrous ion chelating agents, which are able to inhibit tumor occurrence and development by chelating iron required for tumor growth (10). Compared with the commonly used iron chelator 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP), DpTs possess significantly higher antitumor activity and selectivity. As DpTs are more selective compounds, they also lack the side effects that are caused by 3-AP, which include methemoglobinemia and hypoxia (27). As a first-generation DpT drug, Dp44mT was reported to effectively inhibit breast and lung cancer cells by Lovejoy et al (28). Kovacevic et al (29) demonstrated that the second-generation DpT drug DpC exhibited a superior antitumor activity in pancreatic cancer cells compared with Dp44mT.
In the present study, DpC and Dp44mT efficiently inhibited the proliferation of HNSCC cell lines in vitro and induced apoptosis in these cells. DpC was able to effectively inhibit proliferation and induce the apoptosis of HNSCC cells, and the effects were greater compared with the first-generation DpT, Dp44mT. These antitumor functions may be mediated via the regulation of the expression of DNA damage signaling pathway-associated proteins p-ATM, Chk-1, p-ATR, p-Chk-2, PARP, p-histone, H2A.X, BRCA1 and p-P53.
The DNA-damage signaling pathway is closely associated with the occurrence and development of HNSCC. DNA damage is caused by DNA modifications that can initiate apoptosis, induce DNA double-strand breaks (DSBs) and block DNA replication, which can cause DSBs (30). It has been widely reported that ATM, ATR, nibrin, meiotic recombination 11 homolog 1, Rad50, PARP, histone H2AX, BRCA1, topoisomerase IIβ-binding protein 1, p-Chk-1, p-Chk-2, and p-P53 are involved in the repair of DNA damage (31). ATM is primarily activated by DSBs, whereas ATR is primarily activated by blocks in DNA replication (15).
Chen et al (32) reported that iron mediates the production of reactive oxygen species, which leads to oxidative damage of DNA (32). Iron chelators are able to repair oxidative damage to DNA in cells by chelating excessive iron normal cells (33). However, iron is also an essential element required for cellular metabolism. When iron is required for the growth of tumor cells, it may also initiate DNA oxidative damage to the tumor cells (34). Therefore, it can be inferred that DpC is able to inhibit the proliferation of HNSCC cells and induce apoptosis by regulating the DNA-damage-signaling pathway, which is closely associated with the occurrence and development of HNSCC.
To elucidate the association between DNA damage signaling pathways and antitumor effects of DpC in HNSCC, the HNSCC cell line Cal-27 was incubated with a range of concentrations of DpC, and the changes in protein expression levels of the DNA damage signaling pathway were analyzed. It was also observed that the expression of PARP, a protein involved in the monitoring of DNA damage, was upregulated as the concentration of DpC increased. In addition, the levels of p-ATM and p-ATR proteins were also upregulated as the concentration of DpC increased, and the expression of ATM and ATR was hypothesized to be induced once DNA damage occurred. Furthermore, p-Chk-1 and p-Chk-2 were also upregulated as the concentration of DpC increased, leading to increased expression of p-histone H2AX and BRCA1, and the cascade of pro-apoptotic genes was activated (35). In addition, the expression of P53 was also upregulated, although the role of P53 in the process requires further investigation. The results of the present study revealed that the DNA-damage signaling pathway is closely associated with the occurrence and development of HNSCC, and DpC is able to cause DSBs and induce arrest of DNA replication in tumor cells.
Studies have reported that iron chelators are able to upregulate the expression of N-Myc downstream regulated 1, which is considered to be an iron-regulated metastasis suppressor (36,37). Dp44mT and DpC were revealed to selectively activate the lysosomal apoptotic pathway in cancer cells by sequestration of redox-active copper, indicating that treatment with these compounds may represent a novel generalized strategy for chemotherapeutic intervention against cancer (28,38).
Conventional radiotherapy and chemotherapy cause damage to normal tissues and organs. Despite the development of novel chemotherapeutic agents and technologies for radiotherapy, the side effects of these treatments remain (39). The present study confirmed that DpC is able to effectively inhibit the proliferation and induce the apoptosis of HNSCC cells, indicating that DpC may be an effective drug for the treatment of HNSCC, and that in the future, DpC may be used for the clinical treatment of HNSCC patients.
Acknowledgements
The present study was supported by a grant from the National Natural Science Foundation of China (grant no. 81372880).
Competing interests
The authors declare that they have no competing interests.
References
- 1.D'Cruz A, Lin T, Anand AK, Atmakusuma D, Calaguas MJ, Chitapanarux I, Cho BC, Goh BC, Guo Y, Hsieh WS, et al. Consensus recommendations for management of head and neck cancer in Asian countries: A review of international guidelines. Oral Oncol. 2013;49:872–877. doi: 10.1016/j.oraloncology.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Quach P, Gutierrez E, Basha MT, Kalinowski DS, Sharpe PC, Lovejoy DB, Bernhardt PV, Jansson PJ, Richardson DR. Methemoglobin formation by triapine, di-2-pyridylketone-4,4-dimethyl-3-thiosemicarbazone (Dp44mT), and other anticancer thiosemicarbazones: Identification of novel thiosemicarbazones and therapeutics that prevent this effect. Mol Pharmacol. 2012;82:105–114. doi: 10.1124/mol.112.078964. [DOI] [PubMed] [Google Scholar]
- 4.Molinolo AA, Amornphimoltham P, Squarize CH, Castilho RM, Patel V, Gutkind JS. Dysregulated molecular networks in head and neck carcinogenesis. Oral Oncol. 2009;45:324–334. doi: 10.1016/j.oraloncology.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bian X, Xu ZG, Lu CM, Tang PZ, Luo J. Cancer and surgica l treatment impact the qua lity of life in patients w ith head and neck cancer. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2005;40:606–610. (In Chinese) [PubMed] [Google Scholar]
- 6.Padmanabhan H, Brookes MJ, Iqbal T. Iron and colorectal cancer: Evidence from in vitro and animal studies. Nutr Rev. 2015;73:308–317. doi: 10.1093/nutrit/nuu015. [DOI] [PubMed] [Google Scholar]
- 7.Miljuš G, Malenković V, Đukanovic B, Kolundžić N, Nedić O. IGFBP-3/transferrin/transferrin receptor 1 complexes as principal mediators of IGFBP-3 delivery to colon cells in non-cancer and cancer tissues. Exp Mol Pathol. 2015;98:431–438. doi: 10.1016/j.yexmp.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 8.Dragset MS, Poce G, Alfonso S, Padilla-Benavides T, Ioerger TR, Kaneko T, Sacchettini JC, Biava M, Parish T, Argüello JM, et al. A novel antimycobacterial compound acts as an intracellular iron chelator. Antimicrob Agents Chemother. 2015;59:2256–2264. doi: 10.1128/AAC.05114-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang BA, Kovačević Ž, Park KC, Kalinowski DS, Jansson PJ, Lane DJ, Sahni S, Richardson DR. Molecular functions of the iron-regulated metastasis suppressor, NDRG1, and its potential as a molecular target for cancer therapy. Biochim Biophys Acta. 2014;1845:1–19. doi: 10.1016/j.bbcan.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Richardson DR, Sharpe PC, Lovejoy DB, Senaratne D, Kalinowski DS, Islam M, Bernhardt PV. Dipyridyl thiosemicarbazone chelators with potent and selective antitumor activity form iron complexes with redox activity. J Med Chem. 2006;49:6510–6521. doi: 10.1021/jm0606342. [DOI] [PubMed] [Google Scholar]
- 11.Jansson PJ, Kalinowski DS, Lane DJ, Kovacevic Z, Seebacher NA, Fouani L, Sahni S, Merlot AM, Richardson DR. The renaissance of polypharmacology in the development of anti-cancer therapeutics: Inhibition of the ‘Triad of Death’ in cancer by Di-2-pyridylketone thiosemicarbazones. Pharmacol Res. 2015;100:255–260. doi: 10.1016/j.phrs.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Yu Y, Suryo Rahmanto Y, Richardson DR. Bp44mT: An orally active iron chelator of the thiosemicarbazone class with potent anti-tumour efficacy. Br J Pharmacol. 2012;165:148–166. doi: 10.1111/j.1476-5381.2011.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan J, Lovejoy DB, Richardson DR. Novel di-2-pyridyl-derived iron chelators with marked and selective antitumor activity: In vitro and in vivo assessment. Blood. 2004;104:1450–1458. doi: 10.1182/blood-2004-03-0868. [DOI] [PubMed] [Google Scholar]
- 14.Whitnall M, Howard J, Ponka P, Richardson DR. A class of iron chelators with a wide spectrum of potent antitumor activity that overcomes resistance to chemotherapeutics. Proc Natl Acad Sci USA. 2006;103:14901–14906. doi: 10.1073/pnas.0604979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka T, Huang X, Jorgensen E, Traganos F, Darzynkiewicz Z, Albino AP. ATM activation accompanies histone H2AX phosphorylation in A549 cells upon exposure to tobacco smoke. BMC Cell Biol. 2007;8:26. doi: 10.1186/1471-2121-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamre D, Bernstein J, Donovick R. The chemotherapy of experimental tuberculosis. II. Thiosemicarbazones and analogues in experimental tuberculosis in the mouse. J Bacteriol. 1950;59:675–860. doi: 10.1128/jb.59.5.675-680.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson DR, Tran EH, Ponka P. The potential of iron chelators of the pyridoxal isonicotinoyl hydrazone class as effective antiproliferative agents. Blood. 1995;86:4295–4306. [PubMed] [Google Scholar]
- 18.Richardson DR, Milnes K. The potential of iron chelators of the pyridoxal isonicotinoyl hydrazone class as effective antiproliferative agents II: The mechanism of action of ligands derived from salicylaldehyde benzoyl hydrazone and 2-hydroxy-1-naphthylaldehyde benzoyl hydrazone. Blood. 1997;89:3025–3038. [PubMed] [Google Scholar]
- 19.Darnell G, Richardson DR. The potential of iron chelators of the pyridoxal isonicotinoyl hydrazone class as effective antiproliferative agents III: The effect of the ligands on molecular targets involved in proliferation. Blood. 1999;94:781–792. [PubMed] [Google Scholar]
- 20.Gao J, Richardson DR. The potential of iron chelators of the pyridoxal isonicotinoyl hydrazone class as effective antiproliferative agents, IV: The mechanisms involved in inhibiting cell-cycle progression. Blood. 2001;98:842–850. doi: 10.1182/blood.V98.3.842. [DOI] [PubMed] [Google Scholar]
- 21.Pahontu E, Julea F, Rosu T, Purcarea V, Chumakov Y, Petrenco P, Gulea A. Antibacterial, antifungal and in vitro antileukaemia activity of metal complexes with thiosemicarbazones. J Cell Mol Med. 2015;19:865–878. doi: 10.1111/jcmm.12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu TH, Cao SW, Yu YY. Synthesis, characterization and biological evaluation of paeonol thiosemicarbazone analogues as mushroom tyrosinase inhibitors. Int J Biol Macromol. 2013;62:589–595. doi: 10.1016/j.ijbiomac.2013.09.056. [DOI] [PubMed] [Google Scholar]
- 23.Gan C, Cui J, Su S, Lin Q, Jia L, Fan L, Huang Y. Synthesis and antiproliferative activity of some steroidal thiosemicarbazones, semicarbazones and hydrozones. Steroids. 2014;87:99–107. doi: 10.1016/j.steroids.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Xie W, Xie S, Zhou Y, Tang X, Liu J, Yang W, Qiu M. Design and synthesis of novel 5,6-disubstituted pyridine-2,3-dione-3-thiosemicarbazone derivatives as potential anticancer agents. Eur J Med Chem. 2014;81:22–27. doi: 10.1016/j.ejmech.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Akgemci EG, Saf AO, Tasdemir HU, Türkkan E, Bingol H, Turan SO, Akkiprik M. Spectrophotometric, voltammetric and cytotoxicity studies of 2-hydroxy-5-methoxyacetophenone thiosemicarbazone and its N(4)-substituted derivatives: A combined experimental-computational study. Spectrochim Acta A Mol Biomol Spectrosc. 2015;136:719–725. doi: 10.1016/j.saa.2014.09.087. [DOI] [PubMed] [Google Scholar]
- 26.Zhang N, Tai Y, Li M, Ma P, Zhao J, Niu J. Main group bismuth(III), gallium(III) and diorganotin(IV) complexes derived from bis(2-acetylpyrazine)thiocarbonohydrazone: Synthesis, crystal structures and biological evaluation. Dalton Trans. 2014;43:5182–5189. doi: 10.1039/c4dt00077c. [DOI] [PubMed] [Google Scholar]
- 27.Ma B, Goh BC, Tan EH, Lam KC, Soo R, Leong SS, Wang LZ, Mo F, Chan AT, Zee B, Mok T. A multicenter phase II trial of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, Triapine) and gemcitabine in advanced non-small-cell lung cancer with pharmacokinetic evaluation using peripheral blood mononuclear cells. Invest New Drugs. 2008;26:169–173. doi: 10.1007/s10637-007-9085-0. [DOI] [PubMed] [Google Scholar]
- 28.Lovejoy DB, Jansson PJ, Brunk UT, Wong J, Ponka P, Richardson DR. Antitumor activity of metal-chelating compound Dp44mT is mediated by formation of a redox-active copper complex that accumulates in lysosomes. Cancer Res. 2011;71:5871–5880. doi: 10.1158/0008-5472.CAN-11-1218. [DOI] [PubMed] [Google Scholar]
- 29.Kovacevic Z, Chikhani S, Lovejoy DB, Richardson DR. Novel thiosemicarbazone iron chelators induce up-regulation and phosphorylation of the metastasis suppressor N-myc down-stream regulated gene 1: A new strategy for the treatment of pancreatic cancer. Mol Pharmacol. 2011;80:598–609. doi: 10.1124/mol.111.073627. [DOI] [PubMed] [Google Scholar]
- 30.Merolla F, Mascolo M, Ilardi G, Siano M, Russo D, Graziano V, Celetti A, Staibano S. Nucleotide excision repair and head and neck cancers. Front Biosci (Landmark Ed) 2016;21:55–69. doi: 10.2741/4376. [DOI] [PubMed] [Google Scholar]
- 31.Han Yue CD, Guo Hongliang. Advances in DNA damage response. Chin J Cancer Prev Treat. 2013;20:1775–78. [Google Scholar]
- 32.Chen Z, Zhou Q, Zou D, Tian Y, Liu B, Zhang Y, Wu Z. Chloro-benzoquinones cause oxidative DNA damage through iron-mediated ROS production in Escherichia coli. Chemosphere. 2015;135:379–386. doi: 10.1016/j.chemosphere.2015.04.076. [DOI] [PubMed] [Google Scholar]
- 33.Melis JP, van Steeg H, Luijten M. Oxidative DNA damage and nucleotide excision repair. Antioxid Redox Signal. 2013;18:2409–2419. doi: 10.1089/ars.2012.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park JS, Na HJ, Pyo JH, Jeon HJ, Kim YS, Yoo MA. Requirement of ATR for maintenance of intestinal stem cells in aging Drosophila. Aging (Albany NY) 2015;7:307–318. doi: 10.18632/aging.100743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang S, Hou Y, Zhang H, Tu G, Yang L, Sun Y, Lang L, Tang X, Du YE, Zhou M, et al. Oxidized ATM promotes abnormal proliferation of breast CAFs through maintaining intracellular redox homeostasis and activating the PI3K-AKT, MEK-ERK, and Wnt-β-catenin signaling pathways. Cell Cycle. 2015;14:1908–1924. doi: 10.1080/15384101.2015.1041685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le NT, Richardson DR. Iron chelators with high antiproliferative activity up-regulate the expression of a growth inhibitory and metastasis suppressor gene: A link between iron metabolism and proliferation. Blood. 2004;104:2967–2975. doi: 10.1182/blood-2004-05-1866. [DOI] [PubMed] [Google Scholar]
- 37.Sun J, Zhang D, Zheng Y, Zhao Q, Zheng M, Kovacevic Z, Richardson DR. Targeting the metastasis suppressor, NDRG1, using novel iron chelators: Regulation of stress fiber-mediated tumor cell migration via modulation of the ROCK1/pMLC2 signaling pathway. Mol Pharmacol. 2013;83:454–469. doi: 10.1124/mol.112.083097. [DOI] [PubMed] [Google Scholar]
- 38.Seebacher NA, Lane DJ, Jansson PJ, Richardson DR. Glucose modulation induces lysosome formation and increases lysosomotropic drug sequestration via the P-glycoprotein drug transporter. J Biol Chem. 2016;291:3796–3820. doi: 10.1074/jbc.M115.682450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong FC, Ng AW, Lee VH, Lui CM, Yuen KK, Sze WK, Leung TW, Tung SY. Whole-field simultaneous integrated-boost intensity-modulated radiotherapy for patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2010;76:138–145. doi: 10.1016/j.ijrobp.2009.01.084. [DOI] [PubMed] [Google Scholar]