Abstract
Design
We have previously shown that IFN-α stimulation augments direct natural killer (NK) cell lysis of autologous CD4+ primary T cells infected with certain HIV-1 isolates based upon major histocompatibility complex class 1 (MHC-1) downregulation capacity. Here, we investigated if antibody-dependent cellular cytotoxicity (ADCC) could trigger lysis of HIV-1 isolates that were resistant to direct NK lysis and if IFN-α prestimulation of NK cells could further enhance ADCC.
Methods
Using broadly neutralizing monoclonal antibodies against gp120 (VRC01 or PGV04) or plasma from HIV-1-infected patients (ART-suppressed or elite controller) to trigger ADCC, we measured NK cell chromium release cytotoxicity against HIV-1-infected autologous CD4+ primary T cells and NK cell CD107a degranulation against gp120-coated CD4+ T cells. Total or NK-depleted peripheral blood mononuclear cells were used as effectors in the presence or absence of IFN-α prestimulation.
Results
Plasma from HIV-1-infected patients and monoclonal antibodies against gp120 could trigger NK-dependent ADCC lysis of viral isolates that were resistant to direct NK cell lysis following IFN-α stimulation. In contrast, viral isolates that exhibited potent MHC-I downregulation capacity could be lysed by NK cells through either IFN-α stimulated direct cytotoxicity or through ADCC. When utilized in combination, IFN-α prestimulation significantly augmented ADCC lysis of HIV-1-infected target cells and increased NK cell CD107a degranulation against gp120-coated ADCC targets (P <0.05, n =6).
Conclusion
HIV-1 isolates with lower MHC-I downregulation capacity are resistant to direct lysis following IFN-α stimulation but retain sensitivity to ADCC. IFN-α presti-mulation can significantly increase NK-mediated clearance of HIV-1-infected target cells by both ADCC and/or direct cytotoxicity depending on MHC downregulation status.
Keywords: antibody-dependent cellular cytotoxicity, cytotoxicity, HIV/AIDS, IFN-α, natural killer cells, VRC01
Introduction
The ability of natural killer (NK) cells to discriminate between normal and abnormal cells involves complex interactions between inhibitory and activating NK cell receptors [1–4]. Under physiological conditions, the binding of inhibitory NK cell receptors such as NKG2A, LIR/ILT2, and killer immunoglobulin-like receptors (KIRs) to autologous major histocompatibility complex class I (MHC-I) molecules induces negative regulatory signals that switch off NK cells [5–8]. Heterologous target cells expressing mismatched MHC-I ligands lead to reduced NK inhibitory receptor signaling that artificially increases their target cell sensitivity to NK cell lysis. In contrast, an autologous NK assay system represents the most physiologically relevant in-vitro model for measuring NK activity due to the complete match between MHC-I alleles on target cells and inhibitory receptors on NK effector cells [9–11]. However, previous research has shown that HIV-1-infected autologous CD4+ primary T cells remain largely resistant to direct NK lysis in vitro due to viral strategies of immune evasion such as selective MHC-I downregulation [9,12,13].
Following the reduction of inhibitory signals, NK cells also require the engagement of activating receptors to trigger the killing of susceptible target cells. Examples of activating NK cell receptors include the NKG2D receptor that recognizes stress-induced ligands, activating KIRs lacking inhibitory motifs, the natural cytotoxicity receptor family (NKp46, NKp30, and NKp44) which directly recognize viral or cellular antigens, and the Fc-γIII receptor (CD16) which mediates antibody-dependent cellular cytotoxicity (ADCC) [14–17]. Cytokines such as IL-2, IL-12, IL-15, IL-21, or IFN-α can also augment lysis of susceptible targets cells by preactivating NK cells [18–20].
We have previously shown that NK cytotoxicity against autologous HIV-1-infected CD4+ primary T cells can be triggered by IFN-α pretreatment [21] in a MHC-I downregulation-dependent process that requires the NK-activating receptors NKp46 and NKG2D [22]. Here, we identified that HIV-1 isolates with lower MHC-I downregulation capacity (IIIB and NL4-3) were resistant to direct lysis following IFN-α stimulation but retained sensitivity to ADCC. We also identified that IFN-α prestimulation could further increase NK-mediated ADCC lysis of autologous HIV-1-infected CD4+ primary T cells in the presence of antibodies against the HIV-1 gp120 viral envelope protein including the broadly neutralizing antibody VRC01 or plasma from HIV-1-infected patients.
Materials and methods
HIV-1 infection and gp120 coating
Peripheral blood mononuclear cells (PBMCs) were isolated from 20 healthy uninfected donors according to informed consent and Institutional Review Board approval from The Wistar Institute. PBMCs were stimulated for 3 days with 10 μg/ml PHA-p (Sigma Aldrich, St. Louis, Missouri, USA) and 100 IU/ml hIL-2 (PeproTech, Rocky Hill, New Jersey, USA). CD4+ primary T cells were isolated by positive selection using anti-CD4+ magnetic beads as described by the manufacturer (Miltenyi Corporation, San Diego, California, USA). For coating experiments, 1 ×106 uninfected CD4+ primary T cells were coated with 1 μg gp120 from the HIV-1 IIIB isolate (ProSpec Protein Specialists, New Jersey, USA) for 30 min. For HIV-1 infection, 5 ×106 activated CD4+ T cells were spinfected with 150 ng of p24 containing supernatant of the CXCR4-tropic HIV-1 isolates IIIB, NL4–3, or 96USH-IPS9 (SHIP) as previously described [21]. If infection levels were not above 50% at day 4 postinfection (as determined by intracellular p24 positivity through flow cytometry), we enriched HIV-1-infected cells that downregulated the CD4+ receptor during infection by removing uninfected CD4+ T cells using anti-CD4+ depletion magnetic beads (Miltenyi Corporation) as previously described [9].
Flow cytometry
All antibodies were obtained from BD Biosciences (San Jose, California, USA) unless otherwise noted and used at the recommended dilution of 0.25 μg antibody/million cells. Cell surface staining for the CD69 activation marker and the CD16 FcγIII receptor was carried on CD56+/ CD3− gated NK cells with gates set upon isotype control. Cell surface staining for gp120 on uninfected, HIV-1 infected, or gp120-coated CD4+ T cells was carried out using a 1 : 1000 dilution or plasma from a representative HIV-1-infected elite controller patient (viral load <50 copies/ml, ART-naïve) followed by detection with a phycoerythrin (PE)-conjugated goat secondary antibody to the Fc portion of human IgG. For intracellular staining of the HIV-1 p24 gag protein, CD4+ primary T cells were permeabilized with the Cytofix/Cytoperm kit (BD Biosciences) and stained with the antip24 KC57 FITC antibody (Beckman Coulter, Brea, California, USA) as described by the manufacturer. Samples were collected on a LSRII Cytometer and were analyzed with FlowJo software (Tree Star Inc., Ashland, Oregon, USA).
Natural killer chromium release cytotoxicity assay
HIV-1-infected and uninfected CD4+ primary T cells were generated over a 7-day period as described above. On the day of chromium lysis assay, HIV-1-infected and uninfected CD4+ primary T cells were labeled with 100 μCu Na251CrO4 for 3 h. For experiments in which ADCC was compared with CpG oligodeoxynucleotides (CpG)-induced IFN-α stimulation (Fig. 1), PBMCs were stimulated for 18 h in the presence or absence of 10 μg/ml CpG-ODN 2216 to induce endogenous IFN-α production, washed, and then incubated with chromium labeled autologous HIV-1-infected or uninfected CD4+ primary T cells at a 100 : 1 effector/target cell ratio for 4 h. Plasma from a representative HIV-1-infected ART-suppressed patient (used at a 1 : 1000 final concentration) was added to target cells 30 min prior to and during 4 h cytotoxicity assay to induce ADCC. For additive experiments in which IFN-α was added to ADCC (Fig. 2), PBMCs were stimulated for 18 h in the presence or absence of 5000 U/ml IFN-α, washed, and then incubated with chromium labeled autologous HIV-1-infected and uninfected CD4+ primary T cells at a 25 : 1 effector/target cell ratio for 4 h. VRC01 and PGV04 monoclonal antibodies to the CD4+-binding site of gp120 (used at a 10 μg/ml final concentration) or Plasma from a representative HIV-1-infected elite controller patient (used at a 1 : 1000 final concentration) was added to target cells 30 min prior to and during 4 h cytotoxicity assay to induce ADCC. For NK depletion experiments, more than 99.5% of NK cells were depleted from PBMCs using anti-CD56 magnetic depletion beads as described by the manufacturer (Miltenyi Corporation). Percentage cytotoxicity was calculated as previously described [21] and HIV-specific cytotoxicity was calculated as percentage cytotoxicity of HIV-1 infected minus uninfected CD4+ primary T cells.
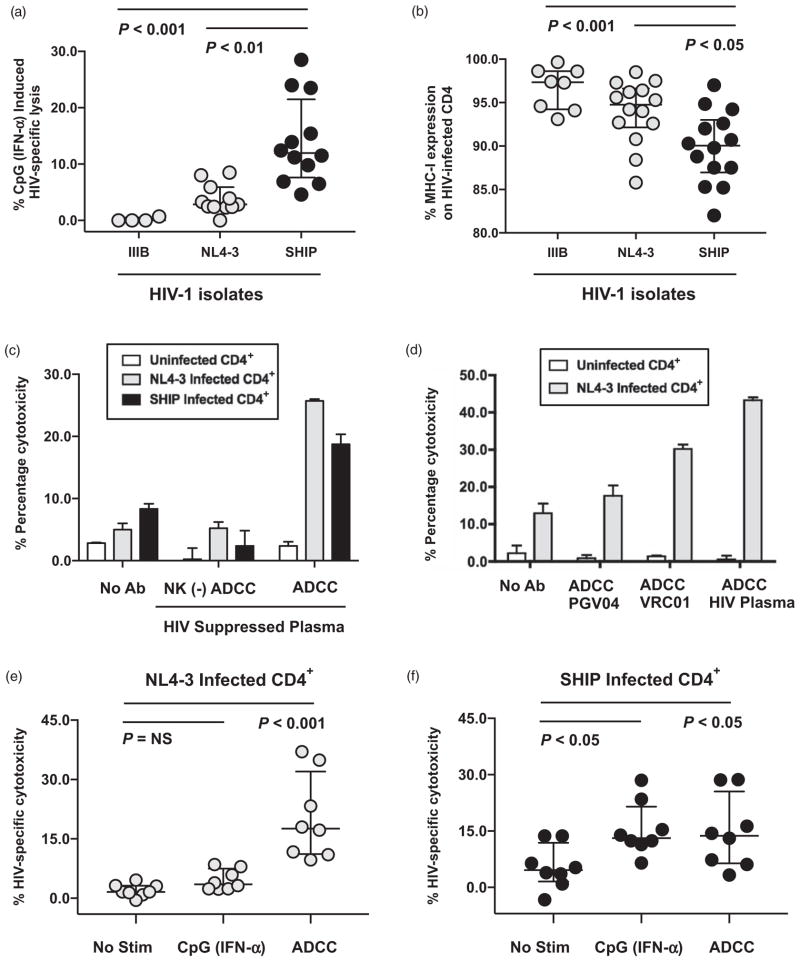
Fig. 1. Antibodies against HIV gp120 can trigger natural killer-mediated antibody-dependent cellular cytotoxicity lysis of HIV-1-infected autologous CD4+ T cells independently of MHC-I downregulation.
(a) Composite data from multiple donors measuring HIV-specific lysis of IIIB, NL4-3, or 96USHIPS9 (SHIP) infected autologous CD4+ primary T cells by peripheral blood mononuclear cells (PBMCs) following CpG-ODN 2216 stimulation at a 100 : 1 effector-to-target cell ratio in a standard 4-h chromium lysis assay. A total 10 μg/ml CpG-2216 was added to PBMCs 18 h prior to chromium lysis assay to stimulate plasmacytoid dendrtitic cells to secrete endogenous IFN-α that activates natural killer cells and triggers cytotoxicity [21]. CpG (IFN-α) induced lysis was calculated after subtracting lysis by unstimulated PBMCs from the same donor tested in parallel. HIV-specific cytotoxicity was calculated as lysis of HIV-1-infected CD4+ primary T cells minus background lysis of uninfected CD4+ primary T cells. Statistical analysis of three groups was carried out with a Kruskal–Wallis unpaired, nonparametric analysis of variance (ANOVA) with a Dunn posttest and a two-tailed P value. (b) Composite analysis of HIV-specific MHC-I downregulation as determined by measuring the percentage of HLA-A, B, and C expression on HIV-1 infected (p24 positive cells) in each experiment relative to mock uninfected cells (set at 100%). PHA/IL-2 stimulated CD4+ primary T cells were infected with either laboratory adapted (IIIB or NL4-3) or primary (SHIP) HIV-1 isolates for 4 days, stained with antibodies to MHC class I (pan W6/32), and permeabilized for intracellular p24 expression by flow cytometry. Statistical analysis of three groups was carried out with a Kruskal–Wallis unpaired, nonparametric ANOVA with a Dunn posttest and a two-tailed P value. (c) 4-h standard chromium release assay measuring the ability of total or natural killer–depleted (−) PBMCs to mediate antibody-dependent cellular cytotoxicity (ADCC) lysis against uninfected (white), HIV-1 NL4-3 (light gray), or HIV-1 SHIP infected (black) autologous CD4+ primary T cells at a 100 : 1 effector-to-target cell ratio in the presence or absence of a 1/1000 dilution of plasma from a representative HIV-1-infected ART-suppressed patient. (d) ADCC chromium release assay measuring the percentage cytotoxicity of uninfected or NL4-3-infected autologous CD4+ primary T cells by PBMCs at a 100 : 1 effector/target ratio in the presence or absence of 10 μg/ml PGV04 or 10 μg/ml VRC01 monoclonal antibodies to gp120 or a 1/1000 dilution of plasma from a representative HIV-1-infected ART suppressed patient (HIV plasma) to trigger ADCC. (e and f) Composite data from multiple donors measuring HIV-specific natural killer lysis of NL4-3 (e) or SHIP (f) infected autologous CD4+ primary T cells in the presence of different stimuli. Direct lysis in the absence of stimulation (no stim) was compared with 10 μg/ml CpG-2216 (IFN-α) induced direct lysis and to ADCC lysis (triggered with a 1/1000 dilution of plasma from a representative HIV-1-infected ART suppressed patient). HIV-specific cytotoxicity was calculated as lysis of HIV-1 infected CD4+ primary T cells minus background lysis of uninfected CD4+ primary T cells. Statistical analysis of three groups was carried out with a Friedman paired, nonparametric ANOVA with a Dunn posttest and a two-tailed P value.
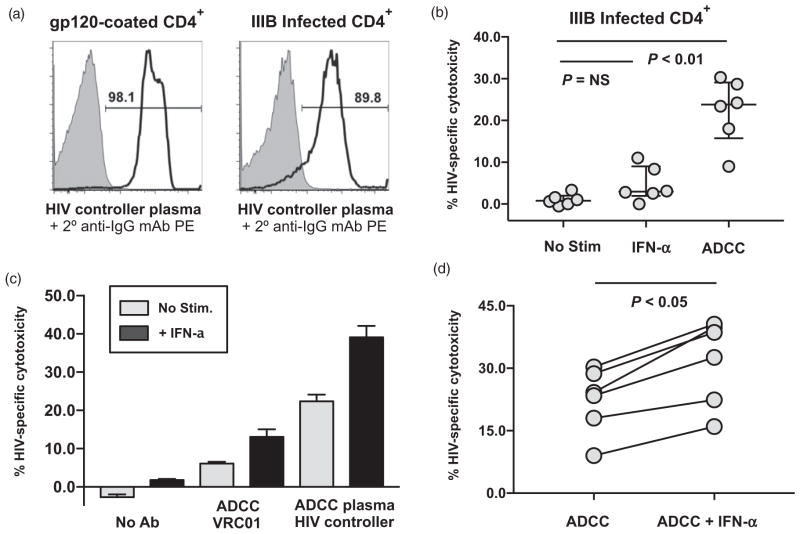
Fig. 2. IFN-α stimulation Augments antibody-dependent cellular cytotoxicity lysis of HIV-1 infected autologous CD4+ T cells.
(a) Flow cytometric staining of gp120 on uncoated (gray shaded histogram) and gp120-coated (black histogram) CD4+ primary T cells or uninfected (gray shaded histogram) and HIV-1 IIIB infected (black histogram) CD4+ primary T cells after staining with a 1/1000 dilution of plasma from a representative HIV-1-infected elite controller patient. A phycoerythrin (PE)-conjugated secondary antibody to the Fc portion of human IgG was used at a 1/100 dilution for detection. (b) Composite data from multiple donors measuring HIV-specific natural killer lysis of IIIB infected autologous CD4+ primary T cells in the presence of different stimuli in a standard 4-h chromium lysis assay at a 25 : 1 effector/target ratio. Peripheral blood mononuclear cell (PBMCs) lysis in the absence of stimulation (direct lysis) was compared with PBMCs prestimulated with 5000 U/ml interferon-α (IFN-α) or antibody-dependent cellular cytotoxicity (ADCC) lysis in the presence of a 1/1000 dilution of plasma from a representative HIV-1-infected elite controller patient (ADCC). Statistical analysis of three groups was carried out with a Friedman paired, nonparametric ANOVA with a Dunn posttest and a two-tailed P value. (c) Chromium release assay measuring HIV-specific cytotoxicity of IIIB infected autologous CD4+ primary T cells by unstimulated (light gray bars) or IFN-α stimulated (dark black bars) PBMCs at a 25 : 1 effector/ target ratio in the presence or absence of 10 μg/ml VRC01 mAb to gp120 or a 1/1000 dilution of plasma from a representative HIV-1-infected elite controller patient to trigger ADCC. (d) Composite data from six donors measuring HIV-specific ADCC lysis (triggered with a 1/1000 dilution of plasma from a representative HIV-1-infected elite controller patient) of IIIB infected autologous CD4+primary T cells at a 25 : 1 effector-to-target cell ratio by unstimulated PBMCs or PBMCs prestimulated with 5000 U/ml IFN-α. Statistical analysis of two groups was carried out with a Wilcoxon matched pairs, nonparametric t test with a two-tailed P value.
Natural killer CD107a degranulation assay
PBMCs were stimulated for 18 h in the presence or absence of 5000 U/ml IFN-α, washed, and then incubated with uncoated or gp120-coated autologous CD4+ primary T cells a 10 : 1 effector to target cell ratio in a standard 4-h degranulation assay in the presence of anti-CD107a PE-conjugated mAb and 5 μg/ml mon-ensin. Plasma from a representative HIV-1-infected elite controller patient (used at a 1 : 1000 final concentration) was added to target cells 30 min prior to and during 4-h degranulation assay to induce ADCC. Degranulation was measured on CD56+/CD3−-gated NK cells and the percentage of CD107a positive NK cells following incubation with gp120-coated or uncoated CD4+ primary T cells was calculated after subtraction of background degranulation from the No Target Control (which was incubated for 4 h with anti-CD107a PE-conjugated mAb, 5 μg/ml monensin, and a 1/1000 dilution of plasma from a representative HIV-1-infected elite controller patient but without target cells).
Statistical analysis
All graphic presentations and statistical analysis were performed with Prism software (GraphPad Software, La Jolla, California, USA). In individual representative experiments, error bars depict the SD. In composite graphs of multiple experiments, cross bars represent the median and interquartile range. Statistical analysis of two groups was carried out with a Wilcoxon matched pair, nonparametric t test. Statistical analysis of three groups was carried out with a Kruskal–Wallis unpaired, nonparametric ANOVA or a Friedman matched pair, nonparametric ANOVA with a Dunn Posttest. All P values were two-sided with significance of P value less than 0.05.
Results
Antibodies against HIV gp120 can trigger natural killer-mediated antibody-dependent cellular cytotoxicity lysis of HIV-1-infected autologous CD4+ T cells independently of MHC-I downregulation
Previously, we observed that pretreatment of PBMC with the Toll-like receptor 9 ligand CpG-2216, which induces endogenous IFN-α secretion by plasmacytoid dendritic cells (pDCs) [21], could activate NK cells and trigger lysis of HIV-1-infected autologous CD4+ primary T cells. However, CD4+ primary T cells infected with certain HIV-1 isolates like IIIB and NL4-3 remained resistant to CpG-induced IFN-α-stimulated NK lysis (Fig. 1a) and this was directly related to their limited ability to downregulate MHC-I proteins as compared with primary HIV-1 isolates such as 96USHIPS9 (SHIP) (Fig. 1b). Here, we investigated if HIV-specific antibodies against the gp120 (including naturally occurring antibodies from HIV-1-infected plasma or broadly neutralizing monoclonal antibodies) could trigger NK-mediated lysis of HIV-1-infected targets through ADCC regardless of MHC-1 downregulation capacity. We observed that lysis of SHIP or NL4-3 HIV-1-infected CD4+ primary T cells by unstimulated PBMCs was minimal, but could be increased substantially if we added plasma from a representative HIV-1-infected ART-suppressed patient containing naturally occurring antibodies against HIV-1 (Fig. 1c). NK depletion from PBMCs prior to interaction with target cells showed that HIV-specific ADCC was NK-mediated (Fig. 1c). Importantly, plasma from a representative HIV-1-infected ART-suppressed patient induced NK-mediated ADCC against autologous CD4+ primary T cells infected with either the NL4-3 lab adapted or SHIP primary isolates despite dramatic differences in their ability to downregulate MHC-I (Fig. 1c).
We next tested if broadly neutralizing antibodies to HIV-1 could also facilitate NK-mediated ADCC against HIV-1-infected targets. As shown in Fig. 1d, the VRC01 mAb against the CD4+-binding site of gp120 could mediate ADCC against NL4–3-infected autologous CD4+ primary T cells, although to a lesser extent than HIV-1-infected patient plasma. In contrast, another broadly neutralizing antibody also directed to the CD4+-binding site of gp120, PGV04 (used at the same concentration of 10 μg/ml), did not show equal ADCC activity as observed with VRC01 (Fig. 1d). Together, these results show that HIV-1 isolates with lower MHC-I down-regulation capacity such as NL4-3 are resistant to direct lysis following IFN-α prestimulation but can be significantly lysed by ADCC (P <0.01, n =8), whereas HIV-1 primary isolates like SHIP with strong MHC-I downregulation capacity can be lysed through either IFN-α stimulated direct cytotoxicity or ADCC (P <0.05, n =8) (Fig. 1e and f).
IFN-α stimulation augments antibody-dependent cellular cytotoxicity lysis of HIV-1-infected autologous CD4+ T cells
We next measured if IFN-α prestimulation could show an additive effect with ADCC in increasing NK lysis of HIV-1-infected autologous CD4+ primary T cells. We utilized plasma from a representative elite controller patient and confirmed its HIV-1 specificity by Flow Cytometric staining showing the naturally occurring antibodies in the plasma could only stain HIV-1 infected or gp-120 coated CD4+ primary T cells (black histogram) but not uninfected or uncoated CD4+ primary T cells (gray, shaded histograms) (Fig. 2a). We chose the IIIB viral isolate as it was resistant to IFN-α induced direct NK cytotoxicity but was susceptible to ADCC (Fig. 2b), thereby allowing us to separate any potential stimulatory effect of IFN-α on ADCC independent on its known effect on direct cytotoxicity. As shown in Fig. 2c, we observed that ADCC lysis of IIIB infected autologous CD4+ primary T cells increased following IFN-α prestimulation regardless of the antibody source utilized to trigger ADCC including the VRC01 broadly neutralizing mAb or plasma from an HIV-1-infected elite controller patient (Fig. 2c). The increase in ADCC lysis mediated by HIV-plasma following IFN-α prestimulation was significant (Fig. 2d, P <0.05, n =6) and indicated that IFN-α can indeed increase ADCC activity by NK cells independent of its ability to increase direct NK cytotoxicity.
IFN-α stimulation can augment natural killer degranulation against gp120-coated autologous CD4+ T cells and increase the frequency of NK cells mediating antibody-dependent cellular cytotoxicity
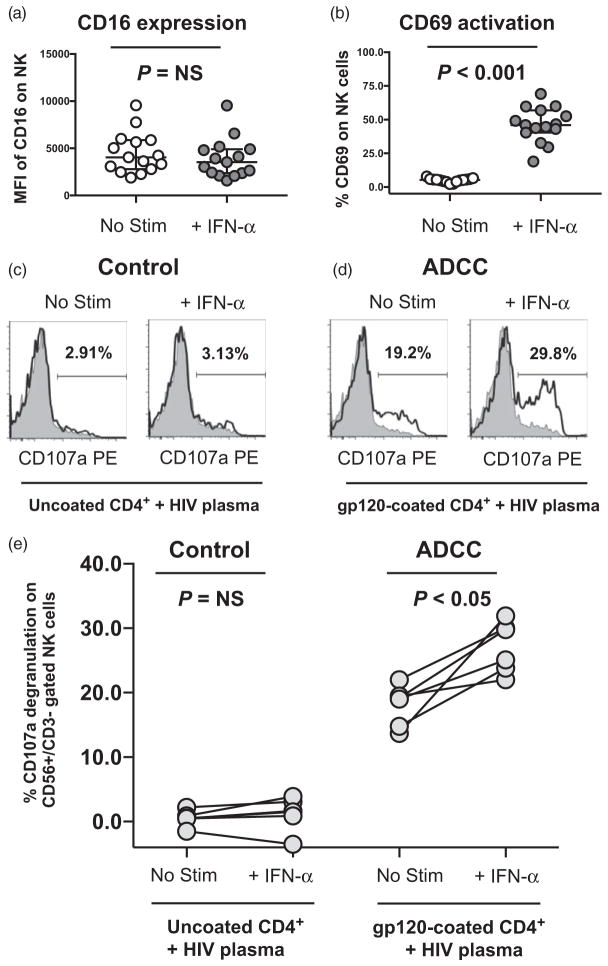
To investigate how IFN-α could augment ADCC lysis, we first measured the surface expression of the CD16 FcGamma receptor on NK cells and observed that CD16 levels were unchanged on CD56+/CD3− gated NK cells following IFN-α stimulation of PBMCs (Fig. 3a). In contrast, the CD69 activation marker was significantly upregulated (P <0.001, n =15) on CD56+/CD3− gated NK cells following IFN-α stimulation (Fig. 3b). This data suggested that IFN-α did not increase ADCC by altering the CD16 receptor that triggers ADCC but rather increased the number of activated NK cells that could mediate ADCC. To investigate this possibility further, we utilized a flow cytometry–based CD107a degranulation assay to quantify the percentage of CD56+/CD3− gated NK cells mediating ADCC from PBMCs incubated with or without IFN-α. As target cells, autologous CD4+ primary T cells were coated with exogenous HIV-1 envelope gp120 protein and ADCC was triggered with plasma from the same representative HIV-1-infected elite controller patient utilized earlier. After subtraction of No Target Control background (gray, shaded histogram), CD56+/CD3− gated NK cells exhibited strong ADCC-induced CD107a degranulation (19.2%) against gp120 coated autologous CD4+ primary T cells in the presence of HIV-plasma, whereas uncoated CD4+ primary T cells incubated with HIV-plasma led to minimal CD107a degranulation (2.91%) over the No Target Control background (Fig. 3c and d). Following IFN-α stimulation, the percentage of CD56+/CD3− gated NK cells exhibiting ADCC-induced CD107a degranulation against gp120-coated autologous CD4+ primary T cells increased over the IFN-α stimulated No Target Control background (29.8%), whereas the no such increase was observed with uncoated autologous CD4+ primary T cells over the IFN-α stimulated No Target Control background (3.13%) (Fig. 3c and d). The increase in ADCC-triggered CD107a degranulation (with gp120-coated CD4+ primary T cells) following IFN-α stimulation was significant (P <0.05, n =6) and not observed with uncoated CD4+ primary T cells (Fig. 3e). Together, these data show that the ADCC enhancement effect of IFN-α is mediated through an increase in the percentage of NK cells engaged in ADCC target cell recognition and degranulation.
Fig. 3. IFN-α stimulation can augment natural killer degranulation against gp120-coated autologous CD4+ T cells and increase the frequency of natural killer cells mediating antibody-dependent cellular cytotoxicity.
(a and b) Flow cytometric analysis of the (a) geometric mean fluorescence intensity of the CD16 FcGamma receptor, and (b) the percentage CD69 activation marker expression on CD56+/CD3− gated natural killer cells from peripheral blood mononuclear cells (PBMCs) incubated in the absence (no stim) or presence of 5000 U/ml IFN-α (IFN-α) for 18 h. Statistical analysis of two groups was carried out with a Wilcoxon matched pair, nonparametric t test with a two-tailed P value. (c and d) CD107a degranulation was measured on CD56+/CD3− gated natural killer cells from unstimulated or 5000 U/ml IFN-α prestimulated PBMCs incubated with uncoated (c) or gp120-coated (d) CD4+ primary T cells (empty, black histogram) for 4 h at a 10 : 1 effector-to-target cell ratio in the presence of anti-CD107a phycoerythin (PE)-conjugated mAb, 5 μg/ml monensin, and a 1/1000 dilution of plasma from a representative HIV-1-infected elite controller patient. The percentage of CD107a positive natural killer cells following incubation with gp120-coated or uncoated CD4+ primary T cells was calculated after subtraction of background degranulation of No Target Control (gray, shaded histogram) which was incubated for 4 h with anti-CD107a PE-conjugated mAb, 5 μg/ml monensin, and a 1/1000 dilution of plasma from a representative HIV-1-infected elite controller patient but without target cells. (e) Composite graph of six individual experiments where CD107a degranulation was measured on CD56+/CD3− gated natural killer cells from unstimulated or IFN-α stimulated PBMC incubated with autologous uncoated control or gp120-coated antibody-dependent cellular cytotoxicity (ADCC) target cells and a 1/1000 dilution of plasma from a representative HIV-1-infected elite controller as described above. Statistical analysis of two groups was carried out with a Wilcoxon matched pairs, nonparametric t test with a two-tailed P value.
Discussion
We have previously shown that IFN-α prestimulation can augment direct NK cell lysis of HIV-1-infected autologous CD4+ primary T cells in a MHC-I down-regulation-dependent process [21] through the NK activating receptors NKp46 and NKG2D [22]. Here, we now show that IFN-α prestimulation can also independently increase the NK-mediated ADCC activity (induced by broadly neutralizing monoclonal antibodies to gp120 or endogenously produced antibodies from HIV-1-infected plasma) against HIV-1-infected autologous CD4+ primary T cells. This finding was corroborated by our flow cytometry–based NK cell CD107a degranulation results showing that the percentage of NK cells mediating HIV-specific ADCC degranulation against gp120-coated CD4+ target cells was significantly increased following IFN-α prestimulation. Overall, our findings suggest that in addition to increasing direct NK cytotoxicity of autologous HIV-1-infected CD4+ primary T cells, IFN-α can also enhance clearance of HIV-1-infected target cells by NK-mediated ADCC in the presence of naturally occurring HIV-specific antibodies or broadly neutralizing monoclonal antibodies to gp120.
Another important finding of this article is our data showing that the addition of antibodies against gp120 can trigger NK-mediated ADCC against HIV-1 viral isolates that exhibited low (IIIB and NL4-3) or high (SHIP) sensitivity to IFN-α stimulated direct NK lysis based upon MHC-I downregulation capacity. Previously, we have shown that circulating viral isolates have differing abilities to downregulate MHC-I proteins and these differences impact their ability to be recognized by NK cells [21]. Our results here now show that IFN-α stimulation in conjunction with antibodies to mediate ADCC can help NK cells eliminate infected cells by a wide variety of circulating viral isolates independently of differences in their ability to downregulate MHC-I proteins.
Of note, plasma from HIV-1-infected patients triggered a better NK-mediated ADCC response against HIV-1-infected autologous CD4+ primary T cells compared with the broadly neutralizing mAb, VRC01 (Fig. 1d and Fig. 2c). This is not unexpected due the polyclonal nature of envelope-specific antibodies in plasma as opposed to a mAb targeting only one epitope of gp120. Nevertheless, VRC01 did trigger levels of ADCC activity that was superior to another neutralizing antibody targeted to the CD4+-binding site of gp120, PGV04, which was largely ineffective (Fig. 1d). Importantly, the increase in ADCC responses following IFN-α prestimulation was observed regardless if ADCC was triggered by HIV-1-infected patient plasma or VRC01. These data suggest that broadly neutralizing monoclonal antibodies have different abilities to induce ADCC and it will remain to be determined how these differences may impact their activity when used as prevention or immunotherapy [23]. Although both IFN-α2 immunotherapy [24,25] and broadly neutralizing antibodies [26] have been used individually in HIV-1-infected persons interrupting ART, their combination has not yet been tested. Indeed, suppressing viral replication without inhibiting HIV-1 expression from infected reservoir cells may increase IFN-α antiviral effects on escape mutants via ADCC while also enhancing NK-mediated direct clearance of infected cells that express stress molecules. It also remains to be determined to what extent IFN-α versus IL-15 prestimulated NK cells provide a more effective effector response against autologous infected targets as IL-15 has been reported to increase both NK detection of cancer lines and ADCC responses against gp-120-coated targets [27].
In vivo, the correlation between NK responses, ADCC, IFN-α stimulation, and HIV-1 control is supported by several important observations; HIV-1-infected controllers have retained pDC and NK function along with enhanced HIV-specific ADCC antibodies that are associated with viral control [28–33], IFN-α immunotherapy results in suppression of viral replication and leads to decreased cell-associated HIV-1 DNA integration in antiretroviral therapy suppressed patients [24], genetic data have shown a consistent association between certain NK cell Killer Inhibitory Receptor alleles of the KIR3DL1 locus with lower viral loads and/or delayed progression to AIDS [34–36], and viremia in HIV-1-infected noncontrollers is associated with defective NK, pDC, and ADCC responses [37–45]. Taken together, our results show preclinical data that in addition to increasing direct NK cytotoxicity, IFN-α can potentiate humoral pressure on HIV-1-infected target lysis via ADCC. The cooperative role between NK cells, humoral ADCC responses, and IFN-α stimulation in the elimination of HIV-1-infected cells supports the evaluation of NK-ADCC activity as a mechanism that may contribute to HIV-1 control in absence of therapy, and evaluation of novel immunotherapy strategies combining broadly neutralizing HIV-specific monoclonal antibodies together with IFN-α therapy to maximize immune control over HIV-1.
Acknowledgments
All HIV-1 viral isolates were expanded and titered at the University of Pennsylvania Centers for AIDS Research Viral Core Facility. The following reagents were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: Anti-HIV-1 gp120 Monoclonal antibodies PGV04 and VRC01 (from Dr John Mascola).
This work was supported by UM1AI126620 (cofunded by NIAID, NIMH, NINDS, and NIDA) as well as other grants from the National Institutes of Health (AI51225, AI47760, U01AI065279, AI07632, and AI068405) and foundation support from the Philadelphia Foundation and the CLAWS Foundation. Support for Shared Resources utilized in this study was provided by Cancer Center Support Grant (CCSG) P30CA010815 to The Wistar Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest
The article is not currently submitted or accepted elsewhere. All authors have approved the content of this manuscript and fulfilled the criteria for authorship. The authors have no conflicts of interest and no writing assistance was utilized in the preparation of this manuscript.
References
- 1.Blery M, Olcese L, Vivier E. Early signaling via inhibitory and activating NK receptors. Hum Immunol. 2000;61:51–64. doi: 10.1016/s0198-8859(99)00157-3. [DOI] [PubMed] [Google Scholar]
- 2.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 3.Middleton D, Curran M, Maxwell L. Natural killer cells and their receptors. Transpl Immunol. 2002;10:147–164. doi: 10.1016/s0966-3274(02)00062-x. [DOI] [PubMed] [Google Scholar]
- 4.Tomasello E, Blery M, Vely F, Vivier E. Signaling pathways engaged by NK cell receptors: double concerto for activating receptors, inhibitory receptors and NK cells. Semin Immunol. 2000;12:139–147. doi: 10.1006/smim.2000.0216. [DOI] [PubMed] [Google Scholar]
- 5.Biassoni R, Ugolotti E, De Maria A. NK cell receptors and their interactions with MHC. Curr Pharm Des. 2009;15:3301–3310. doi: 10.2174/138161209789105225. [DOI] [PubMed] [Google Scholar]
- 6.Long EO, Burshtyn DN, Clark WP, Peruzzi M, Rajagopalan S, Rojo S, et al. Killer cell inhibitory receptors: diversity, specificity, and function. Immunol Rev. 1997;155:135–144. doi: 10.1111/j.1600-065x.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 7.Vivier E, Romagne F. Good news, bad news for missing-self recognition by NK cells: autoimmune control but viral evasion. Immunity. 2007;26:549–551. doi: 10.1016/j.immuni.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Yokoyama WM, Kim S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol Rev. 2006;214:143–154. doi: 10.1111/j.1600-065X.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 9.Bonaparte MI, Barker E. Killing of human immunodeficiency virus-infected primary T-cell blasts by autologous natural killer cells is dependent on the ability of the virus to alter the expression of major histocompatibility complex class I molecules. Blood. 2004;104:2087–2094. doi: 10.1182/blood-2004-02-0696. [DOI] [PubMed] [Google Scholar]
- 10.Fogli M, Mavilio D, Brunetta E, Varchetta S, Ata K, Roby G, et al. Lysis of endogenously infected CD4+ T cell blasts by rIL-2 activated autologous natural killer cells from HIV-infected viremic individuals. PLoS Pathog. 2008;4:e1000101. doi: 10.1371/journal.ppat.1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward J, Bonaparte M, Sacks J, Guterman J, Fogli M, Mavilio D, et al. HIV modulates the expression of ligands important in triggering natural killer cell cytotoxic responses on infected primary T-cell blasts. Blood. 2007;110:1207–1214. doi: 10.1182/blood-2006-06-028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonaparte MI, Barker E. Inability of natural killer cells to destroy autologous HIV-infected T lymphocytes. AIDS. 2003;17:487–494. doi: 10.1097/00002030-200303070-00003. [DOI] [PubMed] [Google Scholar]
- 13.Ward JP, Bonaparte MI, Barker E. HLA-C and HLA-E reduce antibody-dependent natural killer cell-mediated cytotoxicity of HIV-infected primary T cell blasts. AIDS. 2004;18:1769–1779. doi: 10.1097/00002030-200409030-00005. [DOI] [PubMed] [Google Scholar]
- 14.Andre P, Castriconi R, Espeli M, Anfossi N, Juarez T, Hue S, et al. Comparative analysis of human NK cell activation induced by NKG2D and natural cytotoxicity receptors. Eur J Immunol. 2004;34:961–971. doi: 10.1002/eji.200324705. [DOI] [PubMed] [Google Scholar]
- 15.Biassoni R, Cantoni C, Pende D, Sivori S, Parolini S, Vitale M, et al. Human natural killer cell receptors and co-receptors. Immunol Rev. 2001;181:203–214. doi: 10.1034/j.1600-065x.2001.1810117.x. [DOI] [PubMed] [Google Scholar]
- 16.Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16:359–393. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 17.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 18.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 19.Robertson MJ. Role of chemokines in the biology of natural killer cells. J Leukoc Biol. 2002;71:173–183. [PubMed] [Google Scholar]
- 20.Varchetta S, Oliviero B, Mavilio D, Mondelli MU. Different combinations of cytokines and activating receptor stimuli are required for human natural killer cell functional diversity. Cytokine. 2013;62:58–63. doi: 10.1016/j.cyto.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 21.Tomescu C, Chehimi J, Maino VC, Montaner LJ. NK cell lysis of HIV-1-infected autologous CD4 primary T cells: requirement for IFN-mediated NK activation by plasmacytoid dendritic cells. J Immunol. 2007;179:2097–2104. doi: 10.4049/jimmunol.179.4.2097. [DOI] [PubMed] [Google Scholar]
- 22.Tomescu C, Mavilio D, Montaner LJ. Lysis of HIV-1-infected autologous CD4+primary T cells by interferon-alpha-activated NK cells requires NKp46 and NKG2D. AIDS. 2015;29:1767–1773. doi: 10.1097/QAD.0000000000000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruel T, Guivel-Benhassine F, Amraoui S, Malbec M, Richard L, Bourdic K, et al. Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nat Commun. 2016;7:10844. doi: 10.1038/ncomms10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azzoni L, Foulkes AS, Papasavvas E, Mexas AM, Lynn KM, Mounzer K, et al. Pegylated Interferon alfa-2a monotherapy results in suppression of HIV type 1 replication and decreased cell-associated HIV DNA integration. J Infect Dis. 2013;207:213–222. doi: 10.1093/infdis/jis663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatzakis A, Gargalianos P, Kiosses V, Lazanas M, Sypsa V, Anastassopoulou C, et al. Low-dose IFN-alpha monotherapy in treatment-naive individuals with HIV-1 infection: evidence of potent suppression of viral replication. J Interferon Cytokine Res. 2001;21:861–869. doi: 10.1089/107999001753238114. [DOI] [PubMed] [Google Scholar]
- 26.Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP, Jr, Buckley N, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522:487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loubeau M, Ahmad A, Toma E, Menezes J. Enhancement of natural killer and antibody-dependent cytolytic activities of the peripheral blood mononuclear cells of HIV-infected patients by recombinant IL-15. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:137–145. doi: 10.1097/00042560-199711010-00001. [DOI] [PubMed] [Google Scholar]
- 28.Ackerman ME, Mikhailova A, Brown EP, Dowell KG, Walker BD, Bailey-Kellogg C, et al. Polyfunctional HIV-specific antibody responses are associated with spontaneous HIV control. PLoS Pathog. 2016;12:e1005315. doi: 10.1371/journal.ppat.1005315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lambotte O, Ferrari G, Moog C, Yates NL, Liao HX, Parks RJ, et al. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS. 2009;23:897–906. doi: 10.1097/QAD.0b013e328329f97d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Machmach K, Leal M, Gras C, Viciana P, Genebat M, Franco E, et al. Plasmacytoid dendritic cells reduce HIV production in elite controllers. J Virol. 2012;86:4345–4352. doi: 10.1128/JVI.07114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomescu C, Duh FM, Hoh R, Viviani A, Harvill K, Martin MP, et al. Impact of protective KIR/HLA genotypes on NK cell and T cell function in HIV-1 infected controllers. AIDS. 2012;26:1869–1878. doi: 10.1097/QAD.0b013e32835861b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomescu C, Liu Q, Ross BN, Yin X, Lynn K, Mounzer KC, et al. A correlate of HIV-1 control consisting of both innate and adaptive immune parameters best predicts viral load by multivariable analysis in HIV-1 infected viremic controllers and chronically-infected noncontrollers. PLoS One. 2014;9:e103209. doi: 10.1371/journal.pone.0103209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vieillard V, Fausther-Bovendo H, Samri A, Debre P. Specific phenotypic and functional features of natural killer cells from HIV-infected long-term nonprogressors and HIV controllers. J Acquir Immune Defic Syndr. 2010;53:564–573. doi: 10.1097/QAI.0b013e3181d0c5b4. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Vazquez A, Mina-Blanco A, Martinez-Borra J, Njobvu PD, Suarez-Alvarez B, Blanco-Gelaz MA, et al. Interaction between KIR3DL1 and HLA-B*57 supertype alleles influences the progression of HIV-1 infection in a Zambian population. Hum Immunol. 2005;66:285–289. doi: 10.1016/j.humimm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Martin MP, Gao X, Lee JH, Nelson GW, Detels R, Goedert JJ, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31:429–434. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 36.Martin MP, Qi Y, Gao X, Yamada E, Martin JN, Pereyra F, et al. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat Genet. 2007;39:733–740. doi: 10.1038/ng2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Almeida M, Cordero M, Almeida J, Orfao A. Different subsets of peripheral blood dendritic cells show distinct phenotypic and functional abnormalities in HIV-1 infection. AIDS. 2005;19:261–271. [PubMed] [Google Scholar]
- 38.Averill L, Lee WM, Karandikar NJ. Differential dysfunction in dendritic cell subsets during chronic HCV infection. Clin Immunol. 2007;123:40–49. doi: 10.1016/j.clim.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chehimi J, Campbell DE, Azzoni L, Bacheller D, Papasavvas E, Jerandi G, et al. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J Immunol. 2002;168:4796–4801. doi: 10.4049/jimmunol.168.9.4796. [DOI] [PubMed] [Google Scholar]
- 40.De Maria A, Fogli M, Costa P, Murdaca G, Puppo F, Mavilio D, et al. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44) Eur J Immunol. 2003;33:2410–2418. doi: 10.1002/eji.200324141. [DOI] [PubMed] [Google Scholar]
- 41.Feldman S, Stein D, Amrute S, Denny T, Garcia Z, Kloser P, et al. Decreased interferon-alpha production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin Immunol. 2001;101:201–210. doi: 10.1006/clim.2001.5111. [DOI] [PubMed] [Google Scholar]
- 42.Hu PF, Hultin LE, Hultin P, Hausner MA, Hirji K, Jewett A, et al. Natural killer cell immunodeficiency in HIV disease is manifest by profoundly decreased numbers of CD16+CD56+cells and expansion of a population of CD16dimCD56-cells with low lytic activity. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:331–340. [PubMed] [Google Scholar]
- 43.Jia M, Li D, He X, Zhao Y, Peng H, Ma P, et al. Impaired natural killer cell-induced antibody-dependent cell-mediated cytotoxicity is associated with human immunodeficiency virus-1 disease progression. Clin Exp Immunol. 2013;171:107–116. doi: 10.1111/j.1365-2249.2012.04672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lucia B, Jennings C, Cauda R, Ortona L, Landay AL. Evidence of a selective depletion of a CD16+ CD56+ CD8+ natural killer cell subset during HIV infection. Cytometry. 1995;22:10–15. doi: 10.1002/cyto.990220103. [DOI] [PubMed] [Google Scholar]
- 45.Mavilio D, Benjamin J, Daucher M, Lombardo G, Kottilil S, Planta MA, et al. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc Natl Acad Sci U S A. 2003;100:15011–15016. doi: 10.1073/pnas.2336091100. [DOI] [PMC free article] [PubMed] [Google Scholar]