Abstract
The maintenance of cellular identity requires continuous adaptation to environmental changes. This process is particularly critical for stem cells, which need to preserve their differentiation potential over time. Among the mechanisms responsible for regulating cellular homeostatic responses, mitochondria are emerging as key players. Given their dynamic and multifaceted role in energy metabolism, redox, and calcium balance, as well as cell death, mitochondria appear at the interface between environmental cues and the control of epigenetic identity. In this review, we describe how mitochondria have been implicated in the processes of acquisition and loss of stemness, with a specific focus on pluripotency. Dissecting the biological functions of mitochondria in stem cell homeostasis and differentiation will provide essential knowledge to understand the dynamics of cell fate modulation, and to establish improved stem cell‐based medical applications.
Keywords: differentiation, metabolism, mitochondria, pluripotency, PSCs
Subject Categories: Development & Differentiation, Metabolism, Stem Cells
Glossary
- ACL
ATP‐citrate lyase
- aKG
alpha‐ketoglutarate
- AMPK
AMP‐activated protein kinase
- BCL‐2
B cell lymphoma‐2
- DNMTs
DNA methyltransferases
- DRP1
dynamin‐related protein 1
- ESCs
embryonic stem cells
- GPX2
glutathione peroxidase‐2
- GSH
glutathione
- HDAC
histone deacetylases
- HIF1a
hypoxia inducible factor one alpha
- HMTs
histone methyltransferases
- HSCs
hematopoietic stem cells
- iPSCs
induced pluripotent stem cells
- JHDMs
Jumonji C domain demethylase
- LIF
leukemia inhibitory factor
- MFN
mitofusin
- MSCs
mesenchymal stem cells
- mtDNA
mitochondrial DNA
- NAD+
nicotinamide adenine dinucleotide (oxidized form)
- NADH
nicotinamide adenine dinucleotide (reduced form)
- NADPH
nicotinamide adenine dinucleotide phosphate (reduced form)
- NPCs
neural progenitor cells
- NRF2
nuclear respiratory factor 2
- NSCs
neural stem cells
- OPA1
optic atrophy 1
- OXPHOS
oxidative phoshorylation
- PDC
pyruvate dehydrogenase complex
- PDK
pyruvate dehydrogenase kinases
- POLGA
polymerase gamma A
- PPP
pentose phosphate pathway
- PSCs
pluripotent stem cells
- PTP
permeability transition pore
- RC
respiratory chain
- ROS
reactive oxygen species
- TCA
tricarboxylic
- TET
ten‐eleven translocation
- UCP2
uncoupling protein 2
Introduction
Living cells need to constantly respond to the environment. The cellular responses must be rapid and tightly regulated in order to allow the adaptation to environmental changes and the maintenance of cellular identity. These mechanisms are at the basis of cellular homeostasis and require epigenetic remodeling, that is, chromatin reorganization leading to a different gene expression program without changes in the DNA sequence 1. Environmental cues can also cause transcriptional responses that challenge the identity of the cell, resulting in survival or cell death depending on whether the cells are plastic enough to adapt.
Adaptation and plasticity are particularly relevant for stem cells, given their ability to generate different progenies. This feature is known as potency and varies among stem cells according to how many distinct “identities” they can give rise to. Pluripotent stem cells (PSCs)—including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs)—can differentiate into virtually any cell of the body (i.e., belonging to all three germ layers). Multipotent adult stem cells—including hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), and neural stem cells (NCSs)—can generate several cell types within a defined lineage. At the same time, all stem cells are capable to continuously proliferate while preserving their identity, a property known as self‐renewal. Cellular homeostasis is therefore critical for stem cells, given their need for constant preservation of both potency and self‐renewal.
Upon exposure to environmental stimuli, stem cells dynamically regulate the transcriptional machinery and constantly choose between the maintenance of stemness or the exit from stemness, which results in either differentiation or cell death. In the complexity of the cytoplasmic response of stem cells to the environment, mitochondria are poised to play an essential and unique role, given that they are at the center of numerous homeostatic processes 2, 3, 4.
In this review, we discuss how mitochondria may contribute to stem cell homeostasis. We focus particularly on pluripotency. In fact, since the discovery of iPSCs in 2006 5, pluripotent stem cells have acquired a remarkable importance in several biomedical applications, including regenerative medicine, disease modeling, and drug discovery, even in the context of diseases impairing mitochondrial function 6, 7. In order to dissect the role of mitochondria in stem cell homeostasis, we simplify the mitochondrial response to the environment and divide it into three branches: (i) mitochondrial bioenergetics and dynamics, (ii) mitochondrial regulation of redox and calcium balance, and (iii) crosstalk between mitochondrial metabolism and epigenetics (Fig 1).
Figure 1. Mitochondrial regulation of stem cell homeostasis.
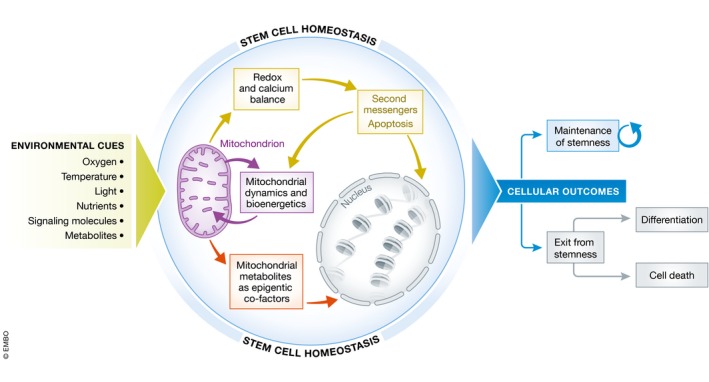
Summary of the three mechanisms that we describe in the text as being associated with the regulation of stem cell homeostasis. Upon exposure to environmental cues (left), mitochondria respond by modulating (i) their network morphology and bioenergetics (purple arrows), (ii) the redox and calcium balance (light green arrows), and (iii) the epigenetic landscape of the cells (orange arrows). Following these mitochondria‐based cellular responses, stem cells can either maintain their identity or exit stemness. This latter route can either lead to physiological differentiation or cell death (right).
The picture emerging from studies on PSCs and other stem cell types is that mitochondria can play a contributing role in the instruction of cell fate outcomes, given their dynamic ability to integrate environmental cues to modulate cellular homeostasis. Unraveling the plasticity of the mitochondrial response to the stem cell environment can provide critical insights into how cell fate decisions are established. Moreover, this knowledge may have implications for our understanding of disorders affecting mitochondrial function and could ultimately support the development of improved stem cell‐based clinical applications.
Mitochondrial metabolism and dynamics in stem cell homeostasis
The first and best known function of mitochondria is the production of energy in the form of ATP via oxidative phosphorylation (OXPHOS). This process takes place in the mitochondrial cristae through the action of respiratory chain (RC) complexes 8. A structural change to the morphology of the cristae or to the overall mitochondrial shape can have an impact on the cellular bioenergetic output. In fact, mitochondria do not operate as individual organelles but rather as an interconnected intracellular network 3. This mitochondrial network is constantly modulated by continuous cycles of mitochondrial fusion and fission, a process collectively known as mitochondrial dynamics 9. Given the lack of de novo mitochondrial biogenesis, the fusion and fission balance is essential for mitochondria to acquire the morphological structure needed to fulfill the specific cellular requirements. Hence, mitochondrial dynamics allow the cells to rapidly respond to environmental cues and adapt the bioenergetic needs. A fused interconnected mitochondrial architecture is generally present in cells that are metabolically active and rely on OXPHOS for energy production. Non‐fused spherical mitochondria are instead common in cells that are quiescent or that are using glycolytic metabolism 10. The state of the mitochondrial network is also changing in response to the nutrient availability, as nutrient‐rich environments associate with mitochondrial fragmentation and nutrient‐poor environments with mitochondrial elongation 11.
The first studies investigating the mitochondrial changes occurring during the induction of pluripotency observed that mitochondria in iPSCs acquire a non‐fused morphology with underdeveloped cristae 12, 13. At the same time, the metabolic profile of the reprogrammed cells shifts from OXPHOS to glycolysis 12, 14, 15, 16 (Fig 2). The activation of DRP1 (dynamin‐related protein 1), the protein regulating mitochondrial fission, is indeed critical for reprogramming to iPSCs 17, 18. During the differentiation of PSCs, oxidative metabolism is activated 12, 19. Consequently, the proteins that drive mitochondrial fusion, MFN (mitofusin) 1 and 2 and OPA1 (optic atrophy 1) are required for the differentiation of stem cells into cells that depend on OXPHOS metabolism, like cardiomyocytes and neurons 20, 21. Interestingly, reprogramming to iPSCs is significantly improved under high‐glucose conditions 22, which are supportive of non‐fused mitochondrial network 11. These findings underscore the importance of nutrient availability in the conversion to pluripotency and in the achievement of its correct mitochondrial and metabolic state 4, 23.
Figure 2. Mitochondrial plasticity during reprogramming and differentiation.
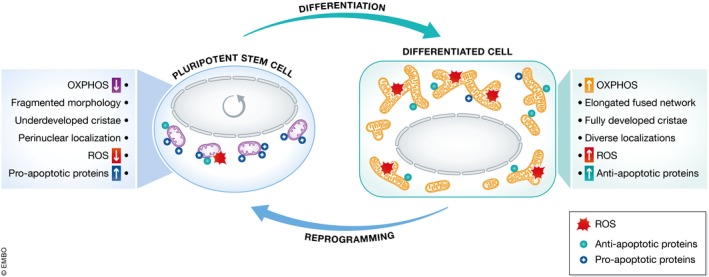
Mitochondria undergo several changes during the reprogramming of somatic cells into pluripotent stem cells (PSCs) and upon the differentiation of PSCs. These modifications impact the OXPHOS activity, the morphology and localization of the mitochondrial network, the appearance of the mitochondrial cristae, the production of reactive oxygen species (ROS), and the balance between pro‐apoptotic and anti‐apoptotic BCL‐2‐like proteins.
The metabolic switch from OXPHOS metabolism to glycolysis occurring during iPSC generation is reminiscent of the effect noticed by Otto Warburg in the context of cancer cells, which he described as being able to maintain high glycolytic rates even in the presence of oxygen, a phenomenon known as “aerobic glycolysis” or “Warburg effect” 24. The glycolytic state of both tumor cells and PSCs has been suggested to be related to their high proliferative rates that require biomass precursors derived from the higher branches of glycolysis and the pentose phosphate pathway (PPP) 25. In fact, non‐replicative cells, such as neurons and cardiomyocytes, typically rely on OXPHOS 26. However, adult stem cells, including HSCs and NSCs, also depend on glycolysis despite being lowly proliferative or even quiescent 27, 28, 29. This suggests that the preference of glycolysis over mitochondrial function may represent a feature of stemness irrespective of their proliferative features. One likely reason for the glycolytic state of stem cells may be that the reduction in mitochondrial metabolism allows the maintenance of low levels of harmful free radicals (see below).
Despite the importance of glycolysis, mitochondrial metabolism can also play a role in stemness. Even in the context of cancer, it is now evident that mitochondria are not simply defective, as initially postulated by Warburg, but are instead essential for tumor growth and progression and may even represent a therapeutic target 30. Accordingly, PSCs express high level of the mitochondrial protein uncoupling protein 2 (UCP2) 31, which is involved in the transport of metabolites out of the mitochondria, thereby regulating glucose oxidation 32. Although a glycolytic switch is required for the acquisition of pluripotency, the early phases of iPSC generation are characterized by an initial burst of OXPHOS activity and by the up‐regulation of RC complexes 33, 34, 35. Mitochondrial metabolism may also be important in the self‐renewal of human PSCs, as its activation is increased when the lipid presence in the media is reduced 36, further highlighting how nutrients in the environment can shape the metabolic and functional state of stem cells.
The relevance of mitochondrial metabolism for pluripotency is illustrated by recent studies aiming at dissecting the functional and molecular differences between two PSC states, namely naïve and primed. Naïve PSCs are believed to correspond to the pre‐implantation stage of embryo development, while primed PSCs should reflect the post‐implantation stage 37. Despite being potentially less developmentally mature, naïve PSCs exhibit higher OXPHOS activity than primed PSCs 38, 39, 40, 41. In accordance, the conversion from primed to naïve PSCs is facilitated by STAT3‐mediated activation of mitochondrial respiration 42 and by the induction of OXPHOS genes following the down‐regulation of LIN28 43, whose expression is low in naïve PSCs. However, the OXPHOS increase in naïve PSCs does not necessarily translate into reduced glycolysis. Naïve PSCs display a bivalent metabolism dependent on both glycolysis and OXPHOS 39 and also exhibit increased glycolytic metabolism 44, 45, 46. Therefore, the role of mitochondrial activity in pluripotency may be independent from glycolytic regulation.
Self‐renewing proliferative progenitors also show active mitochondrial metabolism. This is the case for both mouse embryonic NPCs and adult NPCs, which activate the OXPHOS program already during the initial stages of neurogenesis 47. NPCs derived from human PSCs also display a tubular mitochondrial network and OXPHOS‐dependent metabolism when they are generated and cultured using leukemia inhibitory factor (LIF) 48, itself capable of activating mitochondrial respiration 42.
The relationship between the architecture of the mitochondrial network and the cellular metabolism of stem cells is however more complex than previously thought 3. In fact, even if naïve PSCs show higher OXPHOS activity than primed PSCs, their mitochondrial morphology is less tubular and non‐fused compared to that of primed PSCs 4, 39, 40, 41. Moreover, glycolytic embryonic mouse NSCs have been found to contain a relatively connected mitochondrial network 49. The findings have been corroborated in adult mouse NSCs 47. Mesenchymal stem cells, although mainly glycolytic 50, also exhibit tubular mitochondria that can further elongate upon differentiation 51. Even HSCs, which are known to rely on glycolysis 27, show elongated mitochondria and require the expression of the fusion protein MFN2 52. Therefore, the general assumption that glycolytic cells, like stem cells, have fragmented non‐fused mitochondrial network is likely to be imprecise. In fact, by looking at different stem cell types and at cancer cells, it becomes clear that the association between proliferation, mitochondrial metabolism, and mitochondrial dynamics is rather complex and probably highly plastic and environment‐dependent (Table 1).
Table 1.
Mitochondrial properties in stem cells and cancer
| Stem cell type | Proliferation | Metabolism | Mitochondrial network | ROS | Sensitivity to cell death |
|---|---|---|---|---|---|
| Primed PSCs | High | Glycolysis | Non‐fused with underdeveloped cristae, but some tubular | Low | High |
| Naïve PSCs | High | Glycolysis but also OXPHOS | Non‐fused with underdeveloped cristae | Low | High |
| NSCs | Low | Glycolysis | Tubular | Low | Intermediate |
| NPCs | High | Glycolysis; OXPHOS (when grown with LIF) | Non‐fused; tubular (when grown with LIF) | Low | Intermediate |
| HSCs | Low | Glycolysis | Tubular | Low | Intermediate |
| MSCs | High | Glycolysis | Tubular | Low | Intermediate |
| Cancer cells | High | Glycolysis | Tubular with disorganized cristae | High | Low |
In Table 1, we report some of the mitochondrial features in distinct stem cell types and cancer cells. It is interesting to point out that OXPHOS metabolism can be associated also with stem/progenitor cells and that tubular mitochondrial network may also be present in stem/progenitor cells regardless of their metabolic state. The proliferative rate of the cells also does not seem to be uniquely correlated with a specific metabolism. By comparing the mitochondrial properties of stem/progenitor cells with that of cancer cells, it becomes clear that they exhibit key differences in the response to cell death.
Mitochondrial control of redox and calcium balance in stem cell homeostasis
Mitochondria play a critical role in the balance between cell survival and cell death. These mechanisms are at the bases of cellular homeostasis and regulate the potential outcomes in response to environmental cues. Mitochondria influence cell death pathways associated with necrosis, apoptosis, and autophagy 53 mainly through the modulation of redox and calcium balance 54.
The constant and tight regulation of cell death is essential for stem cells in order to preserve genome integrity, which ensures the faithful derivation of functional progeny 55. The main insult causing loss of genome integrity is evoked by oxidative stress that occurs when the amount of free radicals is not properly counterbalanced by the cellular antioxidant defenses. Reactive oxygen species (ROS), generated upon OXPHOS metabolism during the transport of electrons in the RC 56, can have detrimental consequences on DNA, proteins, or lipids. Oxidative damage of DNA is particularly harmful for stem cells as it can cause unwanted mutations 57. ROS may also damage mitochondrial DNA (mtDNA), which is particularly susceptible to oxidative stress, given the proximity to free radicals and the lack of histones 58, 59. Importantly, PSCs carrying high mtDNA mutation load have been shown to generate defective differentiated cells 60, 61, 62, 63. Furthermore, mtDNA mutagenesis can impair the derivation of iPSCs 64. Increased ROS levels and mtDNA mutagenesis can also lead to defective NSCs and HSCs 65.
In order to limit the occurrence of DNA lesions, stem cells must be equipped with a specific strategy. To this aim, the glycolytic metabolism of stem cells may represent a protective mechanism, as it avoids excessive ROS production by lowering OXPHOS activity. In accordance, PSCs have been found to exhibit reduced ROS and reduced ROS‐mediated damage to lipids, proteins, and DNA 12, 66. Pluripotent stem cells are also highly proficient in DNA repair capacity and antioxidant defenses, including glutathione (GSH) and glutathione peroxidase‐2 (GPX2), which undergo down‐regulation upon differentiation 67, 68, 69. Furthermore, the high energy flux of glycolysis and PPP support stem cell antioxidant defenses by providing NADPH that maintains GSH in its reduced form 70. HSCs also display enhanced self‐renewal potential under conditions of low ROS content 71. NPCs exhibit decreased amount of free radical production in comparison with neuronal and astrocytic counterparts 72. MSCs show low levels of ROS and high level of GSH 73.
As mentioned above, aerobic glycolysis can be favored by stem cells and proliferative cells even in the presence of oxygen. The simple absence of oxygen also causes increased glycolysis, a phenomenon known as “anaerobic glycolysis”. The underlying mechanisms might be similar and may involve the induction of a gene expression program activated by hypoxia inducible factors such as the hypoxia inducible factor one alpha (HIF1a). Accordingly, low oxygen conditions increase the glycolytic flux of ESCs 74, 75 and improve iPSC derivation 76. At the same time, glycolysis and HIF1a are activated during reprogramming to pluripotency even under normoxic culture 77, 78. Low oxygen in in vitro cultures is more reminiscent of the actual in vivo situation in the inner cell mass, where the physiological oxygen concentration is lower than 5% and thus far less of the 20% of atmospheric oxygen 2. Physiological oxygen levels are also present in the in vivo niches of adult stem cells, including HSCs and MSCs 79, 80, and NSCs 81, 82. Given the direct effect of environmental oxygen on transcriptional regulation, it may be possible that differences in the oxygen exposure could cause slightly altered gene expression programs in distinct cells within the in vivo niche. This effect may contribute to the functional heterogeneity of stem cells in vivo 83.
Despite the decreased OXPHOS‐mediated ROS generation, oxidative damage may still occur in stem cells. Therefore, there should be mechanisms in place to ensure the efficient elimination of damaged cells. Consequently, PSCs are highly sensitive to agents causing DNA lesions, including pro‐apoptotic chemicals and gamma irradiation 84, 85. This hyper‐sensitivity of PSCs has been dubbed “mitochondrial priming”, as the cell death pathways of PSCs appear specific for mitochondria‐triggered apoptosis and not receptor‐mediated apoptosis 68, 86, 87. Pluripotent stem cells exhibit a specific state of BCL‐2 (B cell lymphoma‐2) family proteins, with high amount of pro‐apoptotic BCL‐2‐like proteins (like BAX, BAK, and BOK/MTD) and low levels of anti‐apoptotic BCL‐2‐like proteins (like BCL‐2, BCL‐XL, BCL‐W, and MCL‐1) 84, 88. This mitochondrial priming allows PSCs to rapidly undergo cell death when DNA damage has occurred. The mitochondrial apoptotic pathway is also crucial during the early exit from pluripotency, where it can cause the elimination of those cells that are not efficiently undergoing differentiation. Interestingly, cellular apoptosis typically involves mitochondrial fission. Accordingly, BCL‐2 family members can contribute to the regulation of mitochondrial dynamics 89. This suggests that the processes underlying the changes mentioned above in mitochondrial morphology and metabolism occurring during the acquisition and loss of pluripotency may also be important for the configuration of a PSC‐like apoptotic sensitivity (Fig 2).
Adult stem cells are considered to be more resistant to cell death than PSCs 90. Nonetheless, adult stem cells may still retain the ability to undergo cell death upon DNA damage 91, 92, 93. On the other hand, the sensitivity to cell death is radically different in cancer cells, which are capable of surviving in the presence of high levels of ROS and DNA damage (Table 1).
In addition to their role in redox biology, free radicals can act as second messengers to modulate cellular processes. The biological role of mitochondrial ROS may be particularly relevant for stem cell homeostasis 94. An increase in ROS, independently from OXPHOS, can promote the differentiation of PSCs 95 and adult stem cells, including MSCs 73, 96 HSCs 97 and NSCs 98. Modulation of ROS may also be important in the regulation of stem cell self‐renewal 99. Physiological free radicals can trigger a nuclear response that includes the expression of nuclear respiratory factor (NRF) 2, which in turn leads to stem cell differentiation 49. ROS‐mediated NRF2 induction occurs also during the initiation of iPSC reprogramming, where it causes an initial burst of OXPHOS followed by the activation of glycolytic metabolism 35.
Reactive oxygen species are also important for the stem cell response to low oxygen 100. In order for the physiological ROS production to successfully induce a HIF1a response, the mitochondrial network acquires a perinuclear clustering. The typical perinuclear localization of mitochondria that has been detected in PSCs 12, 101, 102 has been therefore suggested to play a role in the oxygen‐dependent regulation of cell fate in PSCs 2.
In accordance with the physiological importance of ROS in stem cell homeostasis, the exogenous administration of antioxidants may not always be beneficial. Although vitamin C was initially found to improve the derivation of iPSCs 103, the use of various antioxidants failed to increase the efficiency of iPSC reprogramming and did not ameliorate the growth defects of iPSCs carrying mtDNA mutations 64. Even in the context of tumor cells, antioxidants may promote cancer proliferation by down‐regulating the endogenous protective mechanisms 104.
Intracellular calcium is another mechanism that is regulated by mitochondria and is implicated in both cell death activation and physiological signaling pathways 105, 106. Mitochondria regulate calcium balance in the cells by acting as high capacity buffers 53. Intra‐mitochondrial calcium positively affects energy metabolism through the stimulation of ATP production by OXPHOS. At the same time, excessive accumulation of calcium into the mitochondria can lead to apoptosis through the opening of the permeability transition pore (PTP) and the consequent release of cytochrome c in the cytoplasm 107.
The regulation of intracellular calcium might be crucial for the physiological differentiation of PSCs 21 and adult stem cells, including MSCs 108 and NSCs 109. Nonetheless, the specific importance of mitochondrial calcium regulation for stem cell homeostasis remains overall under‐investigated 110, 111.
Mitochondrial metabolism–epigenetics crosstalk in stem cell homeostasis
A growing body of evidence suggests that the metabolic profile of the cells can influence the cytoplasmic signaling, connecting environmental inputs with transcriptional programming 1, 112. Metabolism‐driven chromatin regulation is crucial for cellular plasticity for dictating the changes required to modulate cellular identity, a key process during cancer transformation and reprogramming to iPSCs 4.
Mechanistically, the importance of mitochondrial metabolism for stem cell fate regulation may be due to the action of metabolites generated in the tricarboxylic (TCA) cycle that can function as co‐factors or substrates for chromatin modifying enzymes. The mechanisms through which mitochondrial metabolites influence stem cell epigenetics is actively investigated in the context of PSCs. However, the metabolism–epigenetic crosstalk could be important for stemness in general 113. A schematic representation of the interplay between mitochondrial metabolism and epigenetics in stem cells is shown in Fig 3A and B.
Figure 3. Mitochondria metabolism and epigenetic modulation.
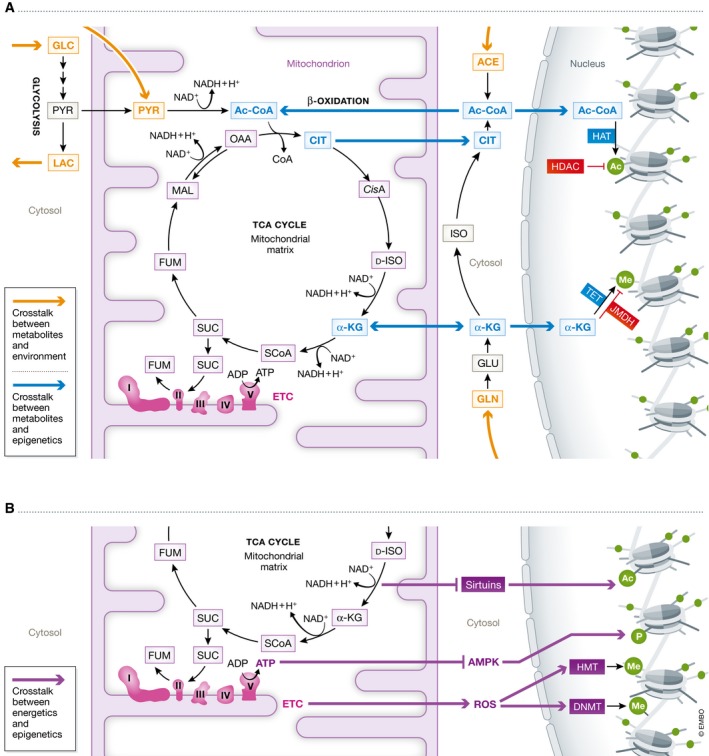
Schematic representation of the interaction between mitochondrial metabolism and chromatin regulation mechanisms. (A) Interplay between mitochondrial metabolites and epigenetics. Orange arrows indicate the connection with the extracellular environment. Blue arrows refer to the mitochondrial metabolites (like citrate, acetyl‐CoA, and aKG). (B) Interplay between mitochondrial bioenergetics and epigenetics. Bordeaux arrows indicate pathways related to mitochondrial bioenergetics (like AMPK, sirtuins, and ROS).
An important TCA metabolite associated with epigenetic modulation is citrate. Citrate can be exported outside the mitochondria, where it is converted into acetyl‐CoA by the enzyme ATP‐citrate lyase (ACL) 114. Cytosolic acetyl‐CoA is the primary donor for the acetylation of histones, an epigenetic modification associated with transcriptional activation. High levels of acetyl‐CoA induce the expression of proliferation‐related genes in yeast 115, a response that is highly relevant for cancer transformation 116. Acetyl‐CoA‐dependent histone acetylation plays a role in the maintenance of primed PSCs 117.
Mitochondrial citrate is primarily obtained from mitochondrial acetyl‐CoA. Mitochondrial acetyl‐CoA can be imported from the cytoplasm under conditions promoting lipid oxidation. Alternatively, mitochondrial acetyl‐CoA can be generated from glycolysis‐derived pyruvate through the action of pyruvate dehydrogenase complex (PDC). This latter route is the preferred one of primed PSCs 117. At the same time, pyruvate dehydrogenase kinases (PDK) 1 and 3, which inhibit the PDC and redirect pyruvate away from the mitochondria into lactate, have been reported to be over‐expressed in cancer cells and primed PSCs 16, 78, 118, 119. This apparent controversy may perhaps be explained by the fact that PDC has been suggested to translocate into the nuclear compartment in response to mitochondrial inhibition 120. Therefore, we can speculate that pyruvate (glucose‐derived and/or exogenously provided) might have two fates in PSCs. On the one hand, pyruvate could be shunt away from mitochondria to generate lactate following PDC inhibition within the mitochondria. On the other hand, pyruvate might be converted into acetyl‐CoA by PDC localized in the nucleus in order to enable rapid histone acetylation. Further studies are needed to fully address these mechanisms (Fig 4).
Figure 4. Energy flux and pyruvate fate in PSCs.
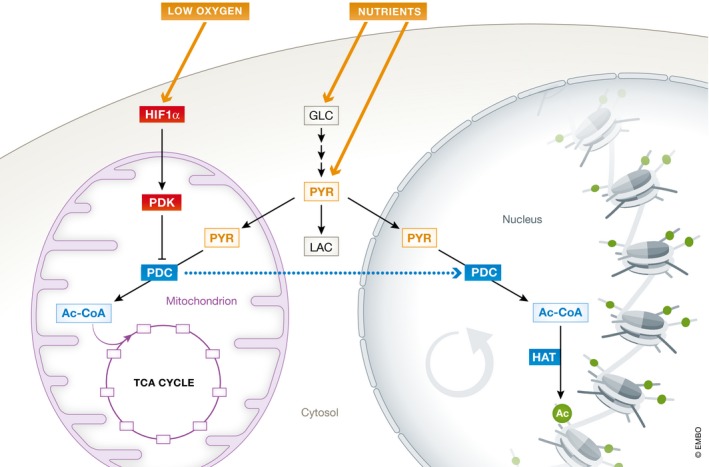
Intracellular pyruvate can be obtained from glucose or directly taken up from the environment. The fate of pyruvate depends on the activity of pyruvate dehydrogenase complex (PDC), which converts pyruvate into acetyl‐CoA that is in turn used in the TCA cycle in the mitochondria. In PSCs, hypoxia inducible factors (HIF) are activated and lead to increased expression of pyruvate dehydrogenase kinase (PDK)1‐3 that inhibit mitochondrial PDC. The inhibition of mitochondrial PDC re‐routes pyruvate outside the mitochondria to be used for lactate generation. However, this mechanism is in apparent contrast with the importance of pyruvate‐derived acetyl‐CoA for histone acetylation. Given that it has been reported that PDC can translocate outside the mitochondria and into the nucleus under conditions of mitochondrial inhibition, we speculate that a PDC translocation may also occur in PSCs. This might help explaining the double importance of pyruvate in PSCs for glycolytic metabolism and epigenetic regulation.
Another important TCA metabolite is alpha‐ketoglutarate (aKG). aKG is transported into the cytosol via the aKG‐malate shuttle that also facilitates the NAD+/NADH mitochondria–cytosol transfer 121. alpha‐ketoglutarate can enter the nucleus where it is used as a substrate of ten‐eleven translocation (TET) proteins for DNA methylation (associated with transcriptional repression) and of Jumonji C domain demethylase (JHDMs) for histone demethylation (linked to context‐dependent gene silencing or activation) 116. Global DNA hypomethylation is a known feature of PSCs that must be obtained during cellular reprogramming for the faithful establishment of pluripotency 122. The level of genomic methylation can also have an impact on the mtDNA copy number. PSCs and cancer cells may in fact maintain low level of mtDNA replication by reducing the activity of the nuclear‐encoded mitochondrial polymerase gamma A (POLGA) through the hypermethylation at its exon 2 123. Finally, aKG‐dependent DNA and histone methylation may be important for promoting naïve pluripotency 124, 125. In primed PSCs, aKG has been found to both accelerate differentiation 126 and support the undifferentiated state 127. Therefore, aKG might exert opposite effects in cell fate regulation depending on the specific cellular context.
Tricarboxylic intermediates can be derived not only from glucose but also from glutamine, which is converted into glutamate and afterward into aKG in the cytoplasm. aKG can then function in the cytoplasm or be transferred into the mitochondria. The glutamine dependence of the TCA cycle is known as reductive carboxylation, and it is believed to be important for cancer metabolism 128. Glutamine appears also indispensable for the survival of primed PSCs 129. This supports the notion that mitochondrial metabolism and active TCA cycle are maintained during pluripotency and can be fueled by different sources.
In addition to TCA metabolites, the bioenergetic state of the cells can influence the epigenetic landscape. The energy sensor AMP‐activated protein kinase (AMPK), which is activated when intracellular ATP levels lower, can phosphorylate histones and histone acetylases. By doing so, AMPK induces the expression of genes involved in cellular metabolic stress and inhibits cellular growth 130. AMPK activation may function as a barrier for reprogramming to pluripotency 131. Another example of bioenergetics‐related regulation of epigenetics is represented by sirtuins. Sirtuins are activated following increased NAD+/NADH ratio that occurs for example upon energy starvation due to glucose depletion. Sirtuins can act as histone deacetylases (HDAC) to achieve transcriptional silencing 1, 116, 132. The chromatin regulation of sirtuins may contribute to cell fate transition. Indeed, sirtuins have been implicated in enhancing the generation of iPSCs 133, 134.
Finally, the epigenetic landscape might also be affected by the redox state of the cells. In addition to their described importance in signaling, free radicals can alter chromatin remodeling through the oxidation of DNA methyltransferases (DNMTs), histone methyltransferases (HMTs), or through the direct oxidation of nucleotide bases 135, 136. The role of vitamin C in the reprogramming to pluripotency may in fact be linked to both its antioxidant features and its action as cofactor for epigenetic enzymes 103.
Concluding remarks
The ability of mitochondria to integrate environmental cues to influence cellular homeostatic responses is emerging as a key aspect of stem cell biology. A growing body of work suggests that mitochondria play an active role in shaping the cellular fate through the modulation of bioenergetics, redox and calcium balance, and epigenetics. Moreover, mitochondria regulate cell death pathways, which are crucial to allow stem cells to preserve their genome integrity and the functionality of the differentiated progeny.
Dissecting the importance of mitochondria for stemness (see also Box 1) will help designing improved stem cell‐based medical applications. Furthermore, it may lead us to broaden our understanding of the pathogenetic mechanisms of diseases causing mitochondrial dysfunction. If mitochondrial impairment can impact stem cell function, then mitochondrial disorders may also affect the stem cell compartment and not only fully differentiated cells. This might be the case of aging and neurological diseases, where defects in neural stem cells and neurogenesis are starting to be identified 47, 137, 138. Finally, uncovering the mitochondrial control of stem cell homeostasis will shed light on the cellular strategies underlying the establishment of cell fate identity and how to modulate its plasticity.
Conflict of interest
The authors declare that they have no conflict of interest.
Box 1: In need of answers.
Does mitochondrial impairment affect stem cell function in vivo? Can stem cells be a therapeutic target for diseases causing mitochondrial dysfunction?
Is it possible to modulate cell fate identity only through metabolic‐related changes? If so, are these mechanisms also relevant in vivo, suggesting for example that our environment can shape the function of our somatic stem cells?
Can we untangle the relationship between energy metabolism, cellular proliferation, and mitochondrial dynamics?
What is the importance of calcium balance for cell fate plasticity?
How can we effectively target redox homeostasis to enhance stem cell function and improve efficient differentiation?
Acknowledgements
We apologize to the authors whose important works we could not cite due to space limitations. We acknowledge financial support from the German Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF) (e:Bio Young Investigator Grant #AZ.031A318 to A.P.), and the National Science Center, Poland (Grant No. 2016/22/M/NZ2/00548 to P.L).
EMBO Reports (2018) 19: e45432
See the Glossary for abbreviations used in this article.
References
- 1. Gut P, Verdin E (2013) The nexus of chromatin regulation and intermediary metabolism. Nature 502: 489–498 [DOI] [PubMed] [Google Scholar]
- 2. Lees JG, Gardner DK, Harvey AJ (2017) Pluripotent stem cell metabolism and mitochondria: beyond ATP. Stem Cells Int 2017: 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen H, Chan DC (2017) Mitochondrial dynamics in regulating the unique phenotypes of cancer and stem cells. Cell Metab 26: 39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mathieu J, Ruohola‐Baker H (2017) Metabolic remodeling during the loss and acquisition of pluripotency. Development 144: 541–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676 [DOI] [PubMed] [Google Scholar]
- 6. Inak G, Lorenz C, Lisowski P, Zink A, Mlody B, Prigione A (2017) Concise review: induced pluripotent stem cell‐based drug discovery for mitochondrial disease. Stem Cells 35: 1655–1662 [DOI] [PubMed] [Google Scholar]
- 7. Wu J, Ocampo A, Belmonte JCI (2016) Cellular metabolism and induced pluripotency. Cell 166: 1371–1385 [DOI] [PubMed] [Google Scholar]
- 8. Dyall SD, Brown MT, Johnson PJ (2004) Ancient invasions: from endosymbionts to organelles. Science 304: 253–257 [DOI] [PubMed] [Google Scholar]
- 9. Campello S, Scorrano L (2010) Mitochondrial shape changes: orchestrating cell pathophysiology. EMBO Rep 11: 678–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Collins TJ, Berridge MJ, Lipp P, Bootman MD (2002) Mitochondria are morphologically and functionally heterogeneous within cells. EMBO J 21: 1616–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liesa M, Shirihai OS (2013) Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab 17: 491–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prigione A, Fauler B, Lurz R, Lehrach H, Adjaye J (2010) The senescence‐related mitochondrial/Oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells 28: 721–733 [DOI] [PubMed] [Google Scholar]
- 13. Suhr ST, Chang EA, Tjong J, Alcasid N, Perkins GA, Goissis MD, Ellisman MH, Perez GI, Cibelli JB (2010) Mitochondrial rejuvenation after induced pluripotency. PLoS One 5: e14095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu S, Li W, Zhou H, Wei W, Ambasudhan R, Lin T, Kim J, Zhang K, Ding S (2010) Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell 7: 651–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Folmes CD, Nelson TJ, Martinez‐Fernandez A, Arrell DK, Lindor JZ, Dzeja PP, Ikeda Y, Perez‐Terzic C, Terzic A (2011) Somatic oxidative bioenergetics transitions into pluripotency‐dependent glycolysis to facilitate nuclear reprogramming. Cell Metab 14: 264–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Varum S, Rodrigues AS, Moura MB, Momcilovic O, Easley CA, Ramalho‐Santos J, Van Houten B, Schatten G (2011) Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS One 6: e20914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prieto J, León M, Ponsoda X, Sendra R, Bort R, Ferrer‐Lorente R, Raya A, López‐García C, Torres J (2016) Early ERK1/2 activation promotes DRP1‐dependent mitochondrial fission necessary for cell reprogramming. Nat Commun 7: 11124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vazquez‐Martin A, Cufí S, Corominas‐Faja B, Oliveras‐Ferraros C, Vellon L, Menendez JA (2012) Mitochondrial fusion by pharmacological manipulation impedes somatic cell reprogramming to pluripotency: new insight into the role of mitophagy in cell stemness. Aging (Albany NY) 4: 393–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yanes O, Clark J, Wong DM, Patti GJ, Sánchez‐Ruiz A, Benton HP, Trauger SA, Desponts C, Ding S, Siuzdak G (2010) Metabolic oxidation regulates embryonic stem cell differentiation. Nat Chem Biol 6: 411–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fang D, Yan S, Yu Q, Chen D, Yan SS (2016) Mfn2 is required for mitochondrial development and synapse formation in human induced pluripotent stem cells/hiPSC derived cortical neurons. Sci Rep 6: 31462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kasahara A, Cipolat S, Chen Y, Dorn GW, Scorrano L (2013) Mitochondrial fusion directs cardiomyocyte differentiation via calcineurin and notch signaling. Science 342: 734–737 [DOI] [PubMed] [Google Scholar]
- 22. Jang H, Kim TW, Yoon S, Choi S‐Y, Kang T‐W, Kim S‐Y, Kwon Y‐W, Cho E‐J, Youn H‐D (2012) O‐GlcNAc regulates pluripotency and reprogramming by directly acting on core components of the pluripotency network. Cell Stem Cell 11: 62–74 [DOI] [PubMed] [Google Scholar]
- 23. Prieto J, Torres J (2017) Mitochondrial dynamics: in cell reprogramming as it is in cancer. Stem Cells Int 2017: 8073721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Warburg O (1956) On the origin of cancer cells. Science 123: 309–314 [DOI] [PubMed] [Google Scholar]
- 25. Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the warburg effect: the metabolic requirements of cell proliferation. Science 324: 1029–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hall CN, Klein‐Flugge MC, Howarth C, Attwell D (2012) Oxidative phosphorylation, not glycolysis, powers presynaptic and postsynaptic mechanisms underlying brain information processing. J Neurosci 32: 8940–8951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simsek T, Kocabas F, Zheng J, DeBerardinis RJ, Mahmoud AI, Olson EN, Schneider JW, Zhang CC, Sadek HA (2010) The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell 7: 380–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takubo K, Nagamatsu G, Kobayashi CI, Nakamura‐Ishizu A, Kobayashi H, Ikeda E, Goda N, Rahimi Y, Johnson RS, Soga T et al (2013) Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell 12: 49–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Candelario KM, Shuttleworth CW, Cunningham LA (2013) Neural stem/progenitor cells display a low requirement for oxidative metabolism independent of hypoxia inducible factor‐1alpha expression. J Neurochem 125: 420–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zong W‐X, Rabinowitz JD, White E (2016) Mitochondria and cancer. Mol Cell 61: 667–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang J, Khvorostov I, Hong JS, Oktay Y, Vergnes L, Nuebel E, Wahjudi PN, Setoguchi K, Wang G, Do A et al (2011) UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J 30: 4860–4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vozza A, Parisi G, De Leonardis F, Lasorsa FM, Castegna A, Amorese D, Marmo R, Calcagnile VM, Palmieri L, Ricquier D et al (2014) UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc Natl Acad Sci USA 111: 960–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hansson J, Rafiee MR, Reiland S, Polo JM, Gehring J, Okawa S, Huber W, Hochedlinger K, Krijgsveld J (2012) Highly coordinated proteome dynamics during reprogramming of somatic cells to pluripotency. Cell Rep 2: 1579–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kida YS, Kawamura T, Wei Z, Sogo T, Jacinto S, Shigeno A, Kushige H, Yoshihara E, Liddle C, Ecker JR et al (2015) ERRs mediate a metabolic switch required for somatic cell reprogramming to pluripotency. Cell Stem Cell 16: 547–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hawkins KE, Joy S, Delhove JMKM, Kotiadis VN, Fernandez E, Fitzpatrick LM, Whiteford JR, King PJ, Bolanos JP, Duchen MR et al (2016) NRF2 orchestrates the metabolic shift during induced pluripotent stem cell reprogramming. Cell Rep 14: 1883–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang H, Badur MG, Divakaruni AS, Parker SJ, Jäger C, Hiller K, Murphy AN, Metallo CM (2016) Distinct metabolic states can support self‐renewal and lipogenesis in human pluripotent stem cells under different culture conditions. Cell Rep 16: 1536–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Theunissen TW, Friedli M, He Y, Planet E, O'Neil RC, Markoulaki S, Pontis J, Wang H, Iouranova A, Imbeault M et al (2016) Molecular criteria for defining the naive human pluripotent state. Cell Stem Cell 19: 502–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Takashima Y, Guo G, Loos R, Nichols J, Ficz G, Krueger F, Oxley D, Santos F, Clarke J, Mansfield W et al (2014) Resetting transcription factor control circuitry toward ground‐state pluripotency in human. Cell 158: 1254–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhou W, Choi M, Margineantu D, Margaretha L, Hesson J, Cavanaugh C, Blau CA, Horwitz MS, Hockenbery D, Ware C et al (2012) HIF1α induced switch from bivalent to exclusively glycolytic metabolism during ESC‐to‐EpiSC/hESC transition. EMBO J 31: 2103–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sperber H, Mathieu J, Wang Y, Ferreccio A, Hesson J, Xu Z, Fischer KA, Devi A, Detraux D, Gu H et al (2015) The metabolome regulates the epigenetic landscape during naive‐to‐primed human embryonic stem cell transition. Nat Cell Biol 17: 1523–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ware CB, Nelson AM, Mecham B, Hesson J, Zhou W, Jonlin EC, Jimenez‐Caliani AJ, Deng X, Cavanaugh C, Cook S et al (2014) Derivation of naive human embryonic stem cells. Proc Natl Acad Sci USA 111: 4484–4489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carbognin E, Betto RM, Soriano ME, Smith AG, Martello G (2016) Stat3 promotes mitochondrial transcription and oxidative respiration during maintenance and induction of naive pluripotency. EMBO J 35: 618–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang J, Ratanasirintrawoot S, Chandrasekaran S, Wu Z, Ficarro SB, Yu C, Ross CA, Cacchiarelli D, Xia Q, Seligson M et al (2016) LIN28 regulates stem cell metabolism and conversion to primed pluripotency. Cell Stem Cell 19: 66–80 [DOI] [PubMed] [Google Scholar]
- 44. Gu W, Gaeta X, Sahakyan A, Chan AB, Hong CS, Kim R, Braas D, Plath K, Lowry WE, Christofk HR (2016) Glycolytic metabolism plays a functional role in regulating human pluripotent stem cell state. Cell Stem Cell 19: 476–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mlody B, Prigione A (2016) A glycolytic solution for pluripotent stem cells. Cell Stem Cell 19: 419–420 [DOI] [PubMed] [Google Scholar]
- 46. Sone M, Morone N, Nakamura T, Tanaka A, Okita K, Woltjen K, Nakagawa M, Heuser JE, Yamada Y, Yamanaka S et al (2017) Hybrid cellular metabolism coordinated by Zic3 and esrrb synergistically enhances induction of naive pluripotency. Cell Metab 25: 1103–1117.e6 [DOI] [PubMed] [Google Scholar]
- 47. Beckervordersandforth R, Ebert B, Schäffner I, Moss J, Fiebig C, Shin J, Moore DL, Ghosh L, Trinchero MF, Stockburger C et al (2017) Role of mitochondrial metabolism in the control of early lineage progression and aging phenotypes in adult hippocampal neurogenesis. Neuron 93: 560–573.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lorenz C, Lesimple P, Bukowiecki R, Zink A, Inak G, Mlody B, Singh M, Semtner M, Mah N, Auré K et al (2017) Human iPSC‐derived neural progenitors are an effective drug discovery model for neurological mtDNA disorders. Cell Stem Cell 20: 659–674.e9 [DOI] [PubMed] [Google Scholar]
- 49. Khacho M, Clark A, Svoboda DS, Azzi J, MacLaurin JG, Meghaizel C, Sesaki H, Lagace DC, Germain M, Harper M‐E et al (2016) Mitochondrial dynamics impacts stem cell identity and fate decisions by regulating a nuclear transcriptional program. Cell Stem Cell 19: 232–247 [DOI] [PubMed] [Google Scholar]
- 50. Chen C‐T, Shih Y‐RV, Kuo TK, Lee OK, Wei Y‐H (2008) Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells 26: 960–968 [DOI] [PubMed] [Google Scholar]
- 51. Forni MF, Peloggia J, Trudeau K, Shirihai O, Kowaltowski AJ (2016) Murine mesenchymal stem cell commitment to differentiation is regulated by mitochondrial dynamics. Stem Cells 34: 743–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Luchsinger LL, de Almeida MJ, Corrigan DJ, Mumau M, Snoeck H‐W (2016) Mitofusin 2 maintains haematopoietic stem cells with extensive lymphoid potential. Nature 529: 528–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rizzuto R, De Stefani D, Raffaello A, Mammucari C (2012) Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 13: 566–578 [DOI] [PubMed] [Google Scholar]
- 54. Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu S‐S (2004) Calcium, ATP, and ROS: a mitochondrial love‐hate triangle. Am J Physiol Cell Physiol 287: C817–C833 [DOI] [PubMed] [Google Scholar]
- 55. Weissbein U, Benvenisty N, Ben‐David U (2014) Genome maintenance in pluripotent stem cells. J Cell Biol 204: 153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24: R453–R462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cervantes RB, Stringer JR, Shao C, Tischfield JA, Stambrook PJ (2002) Embryonic stem cells and somatic cells differ in mutation frequency and type. Proc Natl Acad Sci 99: 3586–3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Alexeyev M, Shokolenko I, Wilson G, LeDoux S (2013) The maintenance of mitochondrial DNA integrity–critical analysis and update. Cold Spring Harb Perspect Biol 5: a012641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wallace DC (1994) Mitochondrial DNA sequence variation in human evolution and disease. Proc Natl Acad Sci USA 91: 8739–8746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wahlestedt M, Ameur A, Moraghebi R, Norddahl GL, Sten G, Woods N‐B, Bryder D (2014) Somatic cells with a heavy mitochondrial DNA mutational load render induced pluripotent stem cells with distinct differentiation defects. Stem Cells 32: 1173–1182 [DOI] [PubMed] [Google Scholar]
- 61. Ma H, Folmes CDL, Wu J, Morey R, Mora‐Castilla S, Ocampo A, Ma L, Poulton J, Wang X, Ahmed R et al (2015) Metabolic rescue in pluripotent cells from patients with mtDNA disease. Nature 524: 234–238 [DOI] [PubMed] [Google Scholar]
- 62. Kirby DM, Rennie KJ, Smulders‐Srinivasan TK, Acin‐Perez R, Whittington M, Enriquez J‐A, Trevelyan AJ, Turnbull DM, Lightowlers RN (2009) Transmitochondrial embryonic stem cells containing pathogenic mtDNA mutations are compromised in neuronal differentiation. Cell Prolif 42: 413–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yokota M, Hatakeyama H, Ono Y, Kanazawa M, Goto Y (2017) Mitochondrial respiratory dysfunction disturbs neuronal and cardiac lineage commitment of human iPSCs. Cell Death Dis 8: e2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hämäläinen RHH, Ahlqvist KJJ, Ellonen P, Lepistö M, Logan A, Otonkoski T, Murphy MPP, Suomalainen A (2015) mtDNA mutagenesis disrupts pluripotent stem cell function by altering redox signaling. Cell Rep 11: 1614–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ahlqvist KJ, Hämäläinen RH, Yatsuga S, Uutela M, Terzioglu M, Götz A, Forsström S, Salven P, Angers‐Loustau A, Kopra OH et al (2012) Somatic progenitor cell vulnerability to mitochondrial DNA mutagenesis underlies progeroid phenotypes in polg mutator mice. Cell Metab 15: 100–109 [DOI] [PubMed] [Google Scholar]
- 66. Armstrong L, Tilgner K, Saretzki G, Atkinson SP, Stojkovic M, Moreno R, Przyborski S, Lako M (2010) Human induced pluripotent stem cell lines show stress defense mechanisms and mitochondrial regulation similar to those of human embryonic stem cells. Stem Cells 28: 661–673 [DOI] [PubMed] [Google Scholar]
- 67. Maynard S, Swistowska AM, Lee JW, Liu Y, Liu S‐T, Da Cruz AB, Rao M, de Souza‐Pinto NC, Zeng X, Bohr VA (2008) Human embryonic stem cells have enhanced repair of multiple forms of DNA damage. Stem Cells 26: 2266–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dannenmann B, Lehle S, Hildebrand DG, Kübler A, Grondona P, Schmid V, Holzer K, Fröschl M, Essmann F, Rothfuss O et al (2015) High glutathione and glutathione peroxidase‐2 levels mediate cell‐type‐specific DNA damage protection in human induced pluripotent stem cells. Stem Cell Reports 4: 886–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Saretzki G, Walter T, Atkinson S, Passos JF, Bareth B, Keith WN, Stewart R, Hoare S, Stojkovic M, Armstrong L et al (2008) Downregulation of multiple stress defense mechanisms during differentiation of human embryonic stem cells. Stem Cells 26: 455–464 [DOI] [PubMed] [Google Scholar]
- 70. Stincone A, Prigione A, Cramer T, Wamelink MMC, Campbell K, Cheung E, Olin‐Sandoval V, Grüning N‐M, Krüger A, Tauqeer Alam M et al (2015) The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol Rev 90: 927–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jang Y‐Y, Sharkis SJ (2007) A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low‐oxygenic niche. Blood 110: 3056–3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tsatmali M, Walcott EC, Crossin KL (2005) Newborn neurons acquire high levels of reactive oxygen species and increased mitochondrial proteins upon differentiation from progenitors. Brain Res 1040: 137–150 [DOI] [PubMed] [Google Scholar]
- 73. Atashi F, Modarressi A, Pepper MS (2015) The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: a Review. Stem Cells Dev 24: 1150–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lees JG, Rathjen J, Sheedy JR, Gardner DK, Harvey AJ (2015) Distinct profiles of human embryonic stem cell metabolism and mitochondria identified by oxygen. Reproduction 150: 367–382 [DOI] [PubMed] [Google Scholar]
- 75. Forristal CE, Christensen DR, Chinnery FE, Petruzzelli R, Parry KL, Sanchez‐Elsner T, Houghton FD (2013) Environmental oxygen tension regulates the energy metabolism and self‐renewal of human embryonic stem cells. PLoS One 8: e62507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S (2009) Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell 5: 237–241 [DOI] [PubMed] [Google Scholar]
- 77. Mathieu J, Zhou W, Xing Y, Sperber H, Ferreccio A, Agoston Z, Kuppusamy KT, Moon RT, Ruohola‐Baker H (2014) Hypoxia‐inducible factors have distinct and stage‐specific roles during reprogramming of human cells to pluripotency. Cell Stem Cell 14: 592–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Prigione A, Rohwer N, Hoffmann S, Mlody B, Drews K, Bukowiecki R, Blümlein K, Wanker EE, Ralser M, Cramer T et al (2014) HIF1α modulates cell fate reprogramming through early glycolytic shift and upregulation of PDK1‐3 and PKM2. Stem Cells 32: 364–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, Zaher W, Mortensen LJ, Alt C, Turcotte R et al (2014) Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 508: 269–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Suda T, Takubo K, Semenza GL (2011) Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell 9: 298–310 [DOI] [PubMed] [Google Scholar]
- 81. Clarke L, van der Kooy D (2009) Low oxygen enhances primitive and definitive neural stem cell colony formation by inhibiting distinct cell death pathways. Stem Cells 27: 1879–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mohyeldin A, Garzón‐Muvdi T, Quiñones‐Hinojosa A (2010) Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 7: 150–161 [DOI] [PubMed] [Google Scholar]
- 83. Crisan M, Dzierzak E (2016) The many faces of hematopoietic stem cell heterogeneity. Development 143: 4571–4581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Momcilovic O, Knobloch L, Fornsaglio J, Varum S, Easley C, Schatten G (2010) DNA damage responses in human induced pluripotent stem cells and embryonic stem cells. PLoS One 5: e13410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Prigione A, Hossini AM, Lichtner B, Serin A, Fauler B, Megges M, Lurz R, Lehrach H, Makrantonaki E, Zouboulis CC et al (2011) Mitochondrial‐associated cell death mechanisms are reset to an embryonic‐like state in aged donor‐derived iPS cells harboring chromosomal aberrations. PLoS One 6: e27352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Liu JC, Guan X, Ryan JA, Rivera AG, Mock C, Agarwal V, Letai A, Lerou PH, Lahav G, Lahav G (2013) High mitochondrial priming sensitizes hESCs to DNA‐damage‐induced apoptosis. Cell Stem Cell 13: 483–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dumitru R, Gama V, Fagan BM, Bower JJ, Swahari V, Pevny LH, Deshmukh M (2012) Human embryonic stem cells have constitutively active Bax at the golgi and are primed to undergo rapid apoptosis. Mol Cell 46: 573–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. TeSlaa T, Setoguchi K, Teitell MA (2016) Mitochondria in human pluripotent stem cell apoptosis. Semin Cell Dev Biol 52: 76–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Martinou J‐C, Youle RJ (2011) Mitochondria in apoptosis: Bcl‐2 family members and mitochondrial dynamics. Dev Cell 21: 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mohrin M, Bourke E, Alexander D, Warr MR, Barry‐Holson K, Le Beau MM, Morrison CG, Passegué E (2010) Hematopoietic stem cell quiescence promotes error‐prone DNA repair and mutagenesis. Cell Stem Cell 7: 174–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Milyavsky M, Gan OI, Trottier M, Komosa M, Tabach O, Notta F, Lechman E, Hermans KG, Eppert K, Konovalova Z et al (2010) A distinctive DNA damage response in human hematopoietic stem cells reveals an apoptosis‐independent role for p53 in self‐renewal. Cell Stem Cell 7: 186–197 [DOI] [PubMed] [Google Scholar]
- 92. Lane AA, Scadden DT (2010) Stem cells and DNA damage: persist or perish? Cell 142: 360–362 [DOI] [PubMed] [Google Scholar]
- 93. Barazzuol L, Jeggo PA (2016) In vivo sensitivity of the embryonic and adult neural stem cell compartments to low‐dose radiation. J Radiat Res 57(Suppl. 1): i2–i10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Maryanovich M, Gross A (2013) A ROS rheostat for cell fate regulation. Trends Cell Biol 23: 129–134 [DOI] [PubMed] [Google Scholar]
- 95. Schmelter M, Ateghang B, Helmig S, Wartenberg M, Sauer H (2006) Embryonic stem cells utilize reactive oxygen species as transducers of mechanical strain‐induced cardiovascular differentiation. FASEB J 20: 1182–1184 [DOI] [PubMed] [Google Scholar]
- 96. Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B, Chandel NS (2011) Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab 14: 537–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Owusu‐Ansah E, Banerjee U (2009) Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature 461: 537–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hou Y, Ouyang X, Wan R, Cheng H, Mattson MP, Cheng A (2012) Mitochondrial superoxide production negatively regulates neural progenitor proliferation and cerebral cortical development. Stem Cells 30: 2535–2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, Wu H, Kornblum HI (2011) Proliferative neural stem cells have high endogenous ROS levels that regulate self‐renewal and neurogenesis in a PI3K/Akt‐dependant manner. Cell Stem Cell 8: 59–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS (2005) Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab 1: 409–414 [DOI] [PubMed] [Google Scholar]
- 101. Facucho‐Oliveira JM, Alderson J, Spikings EC, Egginton S, St. John JC (2007) Mitochondrial DNA replication during differentiation of murine embryonic stem cells. J Cell Sci 120: 4025–4034 [DOI] [PubMed] [Google Scholar]
- 102. Mandal S, Lindgren AG, Srivastava AS, Clark AT, Banerjee U (2011) Mitochondrial function controls proliferation and early differentiation potential of embryonic stem cells. Stem Cells 29: 486–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Esteban MA, Wang T, Qin B, Yang J, Qin D, Cai J, Li W, Weng Z, Chen J, Ni S et al (2010) Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell 6: 71–79 [DOI] [PubMed] [Google Scholar]
- 104. Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO (2014) Antioxidants accelerate lung cancer progression in mice. Sci Transl Med 6: 221ra15. [DOI] [PubMed] [Google Scholar]
- 105. Giacomello M, Drago I, Pizzo P, Pozzan T (2007) Mitochondrial Ca2+ as a key regulator of cell life and death. Cell Death Differ 14: 1267–1274 [DOI] [PubMed] [Google Scholar]
- 106. Tait SWG, Green DR (2010) Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol 11: 621–632 [DOI] [PubMed] [Google Scholar]
- 107. Rasola A, Bernardi P (2011) Mitochondrial permeability transition in Ca2+‐dependent apoptosis and necrosis. Cell Calcium 50: 222–233 [DOI] [PubMed] [Google Scholar]
- 108. Sun S, Liu Y, Lipsky S, Cho M (2007) Physical manipulation of calcium oscillations facilitates osteodifferentiation of human mesenchymal stem cells. FASEB J 21: 1472–1480 [DOI] [PubMed] [Google Scholar]
- 109. Gu X, Spitzer NC (1995) Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature 375: 784–787 [DOI] [PubMed] [Google Scholar]
- 110. Tonelli FMP, Santos AK, Gomes DA, da Silva SL, Gomes KN, Ladeira LO, Resende RR (2012) Stem cells and calcium signaling. Adv Exp Med Biol 740: 891–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Apáti Á, Berecz T, Sarkadi B (2016) Calcium signaling in human pluripotent stem cells. Cell Calcium 59: 117–123 [DOI] [PubMed] [Google Scholar]
- 112. Lu C, Thompson CB (2012) Metabolic regulation of epigenetics. Cell Metab 16: 9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ryall JG, Cliff T, Dalton S, Sartorelli V (2015) Metabolic reprogramming of stem cell epigenetics. Cell Stem Cell 17: 651–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB (2009) ATP‐citrate lyase links cellular metabolism to histone acetylation. Science 324: 1076–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Cai L, Sutter BM, Li B, Tu BP (2011) Acetyl‐CoA induces cell growth and proliferation by promoting the acetylation of histones at growth genes. Mol Cell 42: 426–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Yun J, Johnson JL, Hanigan CL, Locasale JW (2012) Interactions between epigenetics and metabolism in cancers. Front Oncol 2: 163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Moussaieff A, Rouleau M, Kitsberg D, Cohen M, Levy G, Barasch D, Nemirovski A, Shen‐Orr S, Laevsky I, Amit M et al (2015) Glycolysis‐mediated changes in acetyl‐CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell Metab 21: 392–402 [DOI] [PubMed] [Google Scholar]
- 118. Kim J, Tchernyshyov I, Semenza GL, Dang CV (2006) HIF‐1‐mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3: 177–185 [DOI] [PubMed] [Google Scholar]
- 119. Prigione A, Lichtner B, Kuhl H, Struys EA, Wamelink M, Lehrach H, Ralser M, Timmermann B, Adjaye J (2011) Human iPSCs harbor homoplasmic and heteroplasmic mitochondrial DNA mutations while maintaining hESC‐like metabolic reprogramming. Stem Cells 29: 1338–1348 [DOI] [PubMed] [Google Scholar]
- 120. Sutendra G, Kinnaird A, Dromparis P, Paulin R, Stenson TH, Haromy A, Hashimoto K, Zhang N, Flaim E, Michelakis ED (2014) A nuclear pyruvate dehydrogenase complex is important for the generation of acetyl‐CoA and histone acetylation. Cell 158: 84–97 [DOI] [PubMed] [Google Scholar]
- 121. Locasale JW, Cantley LC (2011) Metabolic flux and the regulation of mammalian cell growth. Cell Metab 14: 443–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lister R, Pelizzola M, Kida YS, Hawkins RD, Nery JR, Hon G, Antosiewicz‐Bourget J, O'Malley R, Castanon R, Klugman S et al (2011) Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature 471: 68–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lee WTY, St. John JC (2016) Mitochondrial DNA as an initiator of tumorigenesis. Cell Death Dis 7: e2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Carey BW, Finley LWS, Cross JR, Allis CD, Thompson CB (2014) Intracellular α‐ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 518: 413–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hwang I‐Y, Kwak S, Lee S, Kim H, Lee SE, Kim J‐H, Kim YA, Jeon YK, Chung DH, Jin X et al (2016) Psat1‐dependent fluctuations in α‐Ketoglutarate affect the timing of ESC differentiation. Cell Metab 24: 494–501 [DOI] [PubMed] [Google Scholar]
- 126. TeSlaa T, Chaikovsky AC, Lipchina I, Escobar SL, Hochedlinger K, Huang J, Graeber TG, Braas D, Teitell MA (2016) α‐Ketoglutarate accelerates the initial differentiation of primed human pluripotent stem cells. Cell Metab 24: 485–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Zhu C, Gao Y, Guo H, Xia B, Song J, Wu X, Zeng H, Kee K, Tang F, Yi C (2017) Single‐cell 5‐formylcytosine landscapes of mammalian early embryos and ESCs at single‐base resolution. Cell Stem Cell 20: 720–731.e5 [DOI] [PubMed] [Google Scholar]
- 128. Mullen AR, Wheaton WW, Jin ES, Chen P‐H, Sullivan LB, Cheng T, Yang Y, Linehan WM, Chandel NS, DeBerardinis RJ (2011) Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 481: 385–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Tohyama S, Fujita J, Hishiki T, Matsuura T, Hattori F, Ohno R, Kanazawa H, Seki T, Nakajima K, Kishino Y et al (2016) Glutamine oxidation is indispensable for survival of human pluripotent stem cells. Cell Metab 23: 663–674 [DOI] [PubMed] [Google Scholar]
- 130. Bungard D, Fuerth BJ, Zeng P‐Y, Faubert B, Maas NL, Viollet B, Carling D, Thompson CB, Jones RG, Berger SL (2010) Signaling kinase AMPK activates stress‐promoted transcription via histone H2B phosphorylation. Science 329: 1201–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Vazquez‐Martin A, Vellon L, Quirós PM, Cufí S, Ruiz de Galarreta E, Oliveras‐Ferraros C, Martin AG, Martin‐Castillo B, López‐Otín C, Menendez JA (2012) Activation of AMP‐activated protein kinase (AMPK) provides a metabolic barrier to reprogramming somatic cells into stem cells. Cell Cycle 11: 974–989 [DOI] [PubMed] [Google Scholar]
- 132. Tang S, Fang Y, Huang G, Xu X, Padilla‐Banks E, Fan W, Xu Q, Sanderson SM, Foley JF, Dowdy S et al (2017) Methionine metabolism is essential for SIRT1‐regulated mouse embryonic stem cell maintenance and embryonic development. EMBO J 36: 3175–3193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lee YL, Peng Q, Fong SW, Chen ACH, Lee KF, Ng EHY, Nagy A, Yeung WSB (2012) Sirtuin 1 facilitates generation of induced pluripotent stem cells from mouse embryonic fibroblasts through the miR‐34a and p53 pathways. PLoS One 7: e45633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Sharma A, Diecke S, Zhang WY, Lan F, He C, Mordwinkin NM, Chua KF, Wu JC (2013) The role of SIRT6 protein in aging and reprogramming of human induced pluripotent stem cells. J Biol Chem 288: 18439–18447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Grzybek M, Golonko A, Walczak M, Lisowski P (2017) Epigenetics of cell fate reprogramming and its implications for neurological disorders modelling. Neurobiol Dis 99: 84–120 [DOI] [PubMed] [Google Scholar]
- 136. Cyr AR, Hitchler MJ, Domann FE (2013) Regulation of SOD2 in cancer by histone modifications and CpG methylation: closing the loop between redox biology and epigenetics. Antioxid Redox Signal 18: 1946–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Agnihotri SK, Shen R, Li J, Gao X, Büeler H (2017) Loss of PINK1 leads to metabolic deficits in adult neural stem cells and impedes differentiation of newborn neurons in the mouse hippocampus. FASEB J 31: 2839–2853 [DOI] [PubMed] [Google Scholar]
- 138. Voloboueva LA, Sun X, Xu L, Ouyang Y‐B, Giffard RG (2017) Distinct effects of miR‐210 reduction on neurogenesis: increased neuronal survival of inflammation but reduced proliferation associated with mitochondrial enhancement. J Neurosci 37: 3072–3084 [DOI] [PMC free article] [PubMed] [Google Scholar]


