Abstract
Aggregation of α‐synuclein is a hallmark of Parkinson's disease and dementia with Lewy bodies. We here investigate the relationship between cytosolic Ca2+ and α‐synuclein aggregation. Analyses of cell lines and primary culture models of α‐synuclein cytopathology reveal an early phase with reduced cytosolic Ca2+ levels followed by a later Ca2+ increase. Aggregated but not monomeric α‐synuclein binds to and activates SERCA in vitro, and proximity ligation assays confirm this interaction in cells. The SERCA inhibitor cyclopiazonic acid (CPA) normalises both the initial reduction and the later increase in cytosolic Ca2+. CPA protects the cells against α‐synuclein‐aggregate stress and improves viability in cell models and in Caenorhabditis elegans in vivo. Proximity ligation assays also reveal an increased interaction between α‐synuclein aggregates and SERCA in human brains affected by dementia with Lewy bodies. We conclude that α‐synuclein aggregates bind SERCA and stimulate its activity. Reducing SERCA activity is neuroprotective, indicating that SERCA and down‐stream processes may be therapeutic targets for treating α‐synucleinopathies.
Keywords: aggregation, alpha‐synuclein, calcium, endoplasmic reticulum, SERCA
Subject Categories: Membrane & Intracellular Transport, Molecular Biology of Disease, Neuroscience
Introduction
The small unfolded neuronal protein α‐synuclein (AS) is closely linked to Parkinson's disease (PD). This is evidenced by the findings that autosomal‐dominant familial PD can be caused by duplications and triplications of the normal SNCA gene encoding AS, by missense mutations causing exchange of single‐amino acid residues (A30P, E46K, H50Q, G51D and A53T, A53E), and by variations in the SNCA gene, which represents the greatest genetic risk factor for sporadic PD. Aggregated amyloid‐type fibrillar forms of AS accumulate in intra‐neuronal Lewy body inclusions, which are the pathological hallmark of PD. Similar AS‐containing intracellular inclusions also exist in other neurodegenerative diseases, so‐called α‐synucleinopathies, that besides PD are dominated by dementia with Lewy bodies (DLB) and multiple system atrophy (MSA). On the pathway from native protein to aggregated amyloid species, soluble oligomeric species are hypothesised to represent cytotoxic forms. The molecular mechanisms whereby AS aggregates contribute to the degeneration of neuronal populations are still unclear, but it has been proposed that they may be linked to perturbations of homeostatic mechanisms, for example proteostasis, mitochondrial functions, and direct toxic actions on membranes (reviewed in 1).
Disturbances in brain Ca2+ regulation have recently been linked to PD because treatment of hypertension with antagonists of L‐type Ca2+ CaV1 channels reduces the risk of PD 2, 3, 4 and the expression of CaV channels in the brain is changed in PD 4, 5. Average cytosolic Ca2+ concentrations are kept in the nM range, contrasting with the mM concentrations present outside cells and in the endoplasmic reticulum. The steep gradient makes Ca2+ an ideal signalling molecule because its cytosolic concentrations can be regulated precisely with spatio‐temporal precision by opening of Ca2+ channels. Once in the cytosol, Ca2+ has to be removed by active transporting pumps in ER, Golgi and the plasma membrane, by Na+/Ca2+ exchanger in the plasma membrane, and by mitochondrial buffering. The mechanism whereby Ca2+ channel antagonists modulate the disease course of PD is still unknown, but it is hypothesised that the mechanism reduces the oxidant stress of dopaminergic neurons of substantia nigra that display an energy‐consuming pacemaking firing pattern driven by Ca2+ influx via CaV1 channels 6. How this localised effect in dopaminergic neurons is related to the progressive nature of PD remains unclear, but recent data indicate a complex interplay between AS, cytosolic Ca2+ and CaV1 channels in dopaminergic substantia nigra neurons 7. Braak hypothesised that PD arises in the deep brainstem nuclei and spreads via the midbrain to neocortical regions 8. This spreading pattern can be clinically observed in some patients, where REM sleep behaviour disorder (RBD) represents a prodromal phase of PD, and most PD patients develop cognitive impairment and dementia in their later phases due to involvement of neocortex.
Ca2+ deregulation has also been hypothesised as playing a general role in several neurodegenerative diseases like Alzheimer's disease and Huntington's disease. In these diseases, increased cytosolic Ca2+ represents a common theme which suggests the presence of mechanisms that lead to the influx of Ca2+ from extracellular space, endoplasmic reticulum and mitochondria as targets for therapeutic intervention 9.
We have previously demonstrated that AS aggregate‐dependent degenerative processes are initiated at very early time points in cell models when no overt phenotypes are present 10. In the present study, we investigated the temporal development of changes in cytosolic Ca2+ in cellular and neuronal models of AS aggregate‐dependent degeneration. Surprisingly, we observed early reduction in cytosolic Ca2+ across models. This reduction was later followed by increased cytosolic Ca2+ when degeneration became apparent. Inhibitors of AS aggregation blocked both the early and late Ca2+ changes. This suggests activation of mechanisms whereby cytosolic Ca2+ is removed against large gradients. Co‐immunoprecipitation experiments demonstrated that soluble and insoluble AS aggregates bind to the Ca2+ pump SERCA in contrast to monomers. SERCA is located in the endoplasmic reticulum. Their interaction was validated in cells using proximity ligation assays (PLA) where the antibody pair against SERCA and AS only produced a signal when the aggregation was not prevented. In vitro biochemical experiments demonstrate that AS aggregates activate the transmembrane Ca2+ pumping and ATP hydrolysis by SERCA. Counteracting the activation of SERCA in cells with the SERCA inhibitor cyclopiazonic acid (CPA) abrogated both the early reduction and later increase in cytosolic Ca2+ and protected cells and neurons against AS aggregate‐dependent cell death. Treatment of AS‐transgenic Caenorhabditis elegans with CPA protected the dopaminergic neurons against AS‐dependent degeneration. Analyses of human brains by proximity ligation assay demonstrated the interaction between SERCA and aggregated AS in patients with DLB but not in controls. Moreover, SERCA was present in purified Lewy bodies from DLB patients and in glial cytoplasmic inclusions from MSA patients and SERCA copurified with insoluble AS in the detergent‐insoluble fraction of MSA brain.
We hypothesise that a slow build‐up of AS aggregates occurs within individual neurons during the process of disease spreading throughout the nervous system. We posit that the accumulating aggregates in the early stages activate SERCA and cause a reduction in cytosolic Ca2+, triggering decisive pathophysiological changes and leading to ensuing cell death (Fig 8). Counteracting this early phase or its down‐stream processes holds promise for increasing the vitality of the affected cells, thereby slowing and modifying the disease course.
Figure 8. AS aggregates stimulate SERCA activity leading to reduced cytosolic Ca2+ and neuron dysfunction and death.
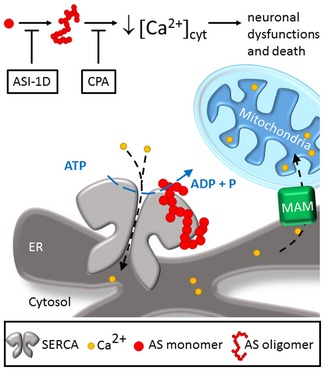
AS aggregates bind and stimulate the active SERCA‐mediated transport of cytosolic Ca2+ into the endoplasmic reticulum. During the building‐up process of cytosolic AS aggregates, the stimulation of SERCA causes an early reduction in cytosolic Ca2+, increased ER Ca2+ that could lead to increased mitochondrial Ca2+. The dysregulated Ca2+ perturbs Ca2+‐dependent processes, leading to progressive neuronal dysfunctions. Ultimately, compensatory mechanisms fail, whereafter cytosolic Ca2+ rise and the neurons die. Inhibition of AS aggregation by the inhibitor ASI‐1D and reducing the activity of SERCA by its inhibitor CPA counteracts the Ca2+ dysregulation and protect the neurons.
Results
Neuronal Ca2+ homeostasis has been linked to PD pathogenesis by virtue of a reduced prevalence of sporadic PD among patients treated for hypertension with Ca2+‐antagonists 2, 3 and genomewide association studies linking the protective effect of caffeine against PD to the GRIN2A gene, which encodes a subunit of the N‐methyl‐d‐aspartate receptor (NMDA)‐activated Ca2+ channel 11, 12. Using the Ca2+ indicator Fura‐2, we investigated the temporal changes in cytosolic Ca2+ concentration in three cell models of AS aggregation‐dependent cytotoxicity, the mitotic oligodendrocytic cell model OLN‐t40‐AS, the non‐mitotic retinoic acid‐differentiated neuroblastoma SH‐SY5Y model with inducible AS expression and primary hippocampal neuron cultures from mThy1‐αSyn (“line 61”) mice (ASO) overexpressing human AS (Fig 1) 13, 14, 15. The OLN‐t40‐AS model revealed a surprising reduction in cytosolic Ca2+ from approximately 195 to 180 nM when measured 8 h after inducing AS aggregation by co‐expression of the aggregation‐inducing protein p25α (Fig 1A). At this early time point, no overt morphological changes are observed, but early AS aggregation‐dependent gene expression responses have been detected like increases in the NF‐κB inhibitor, IκBiα 10. Not surprisingly, an increased cytosolic Ca2+ level was measured 24 h after induction of AS aggregation, where cells show visible degenerative changes such as perinuclear microtubule retraction and nuclear NF‐κB translocation (Fig EV4B) 10, 15. Both the early decrease and later increase in basal intracellular Ca2+ concentration were due to AS aggregation as they could be inhibited by the AS aggregation inhibitor ASI‐1D (Fig 1A) 16. Differentiated non‐mitotic SH‐SY5Y cells with inducible AS expression displayed a reduction of cytosolic Ca2+ from 170 to 150 nM after 5 days of AS expression and an increase after 10 days when compared to b‐gal‐expressing control cells. Aggregate inhibitor ASI‐1D rescued both the initial reduction and late increase (Fig 1B). The biphasic AS aggregation‐dependent cytosolic Ca2+ change also occurs in primary cultures of mouse hippocampal neurons expressing human AS that display a reduction in cytosolic Ca2+ from 150 to 125 nM after 5 days of culture compared to non‐transgenic controls and an increase from 150 to 175 nM after 14 days with both phases blocked by ASI‐1D (Fig 1C). Hence, using different cell models, we demonstrate a surprising link between early stages of intracellular AS aggregation and cytosolic Ca2+ reduction. In order to gain more insight to Ca2+ dynamics upon accumulation of AS, we measured Ca2+ over time in both the SH‐SY5Y model (Fig 1D) and primary hippocampal neurons (Fig 1E). We found that the early phase of decreased cytosolic Ca2+ in SH‐SY5Y cells is significant from day 4 until day 8, whereas the later Ca2+ increase is pronounced after day 10. This closely resembles what we see in primary hippocampal neurons where the early phase of reduced Ca2+ is significant between day 5 and 8 and the later phase, with increased Ca2+ occurring after day 12 and being significant at day 15 (Fig 1C and E). The OLN‐T40‐AS cells, the SH‐SY5Y AS cells and primary neurons isolated from AS‐transgenic mice are based on over‐expression of AS but their protein levels of AS are not many fold higher than in total brain lysate from C57BL/6J wt mice (Fig EV1). In the OLN model, the AS level is double as high as in brain homogenate and in the SH‐SY5Y model levels after 5 and 10 days of AS expression are approx. 75 and 150% of total brain homogenate, respectively. Primary neurons from wt mice have approx. 50% of the AS in adult mice brain whereas primary neurons from AS‐transgenic mice has approx. 80 and 95% at DIV 5 and DIV 14, respectively.
Figure 1. Cellular stress from AS aggregates causes early reduction in cytosolic calcium followed by later increase.
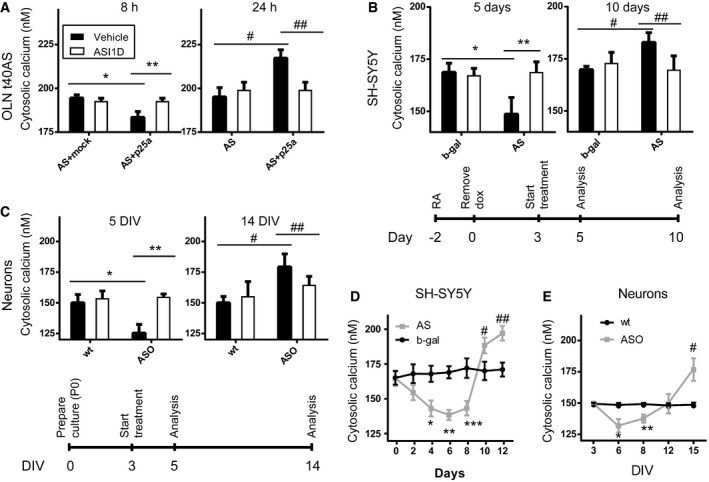
- Mitotic OLN‐t40‐AS cells were transfected with p25α and the fluorescent transfection marker tdTomato. OLN‐t40‐AS cells transfected with tdTomato and empty expression vectors served as negative controls. Bars display Ca2+ concentrations as mean ± SD, N = 3 (*P = 0.0001, **P = 0.0002, # P = 0.0011, ## P = 0.0033). The average Ca2+ level of individual experiments was calculated by measuring > 50 or more tdTomato expressing cells.
- Non‐mitotic SH‐SY5Y cells were generated by treatment with retinoic acid (RA; 10 μM) for 2 days, after which AS expression was induced by removal of doxycycline (dox) and cytosolic Ca2+ measured after 5 days and 10 days of AS expression. See timeline under bars. Cells induced to express β‐galactosidase (b‐gal) upon dox removal were used as negative controls. Bars display Ca2+ concentrations as mean ± SD, N = 4 (*P = 0.0005, **P = 0.0005, # P = 0.0062, ## P = 0.0055). The average Ca2+ level was calculated by measuring > 200 randomly selected cells in each experiment.
- Primary hippocampal neurons were isolated from new‐born (P0) mice expressing human AS under the mThy1 promoter and wild‐type (wt) littermates. Cytosolic Ca2+ was measured after 5 days in vitro (5 DIV) and 14 days in vitro culture (14 DIV). See timeline under graphs. Bars display Ca2+ concentrations as mean ± SD, N = 3 (*P = 0.002, **P = 0.0007, # P = 0.0071, ## P = 0.0455). The average cellular Ca2+ is based on > 500 neurons per experiment.
- Cytosolic Ca2+ in SHSY5Y cells as in (B) measured every second day. Points represent Ca2+ concentrations as mean ± SD, N = 3 (*P = 0.0377, **P = 0.0057, ***P = 0.03, # P = 0.045, ## P = 0.0229).
- Cytosolic Ca2+ in primary hippocampal neurons as in (C) measured every third day. Points represent Ca2+ concentrations as mean ± SD, N = 3 (*P = 0.01, **P = 0.0073, # P = 0.0058).
Figure EV4. Allosteric activation of SERCA mimics the Ca2+ dysregulation of aggregated AS. CPA reduces NF‐κB nuclear translocation in OLN cells and increases survival in neurons.
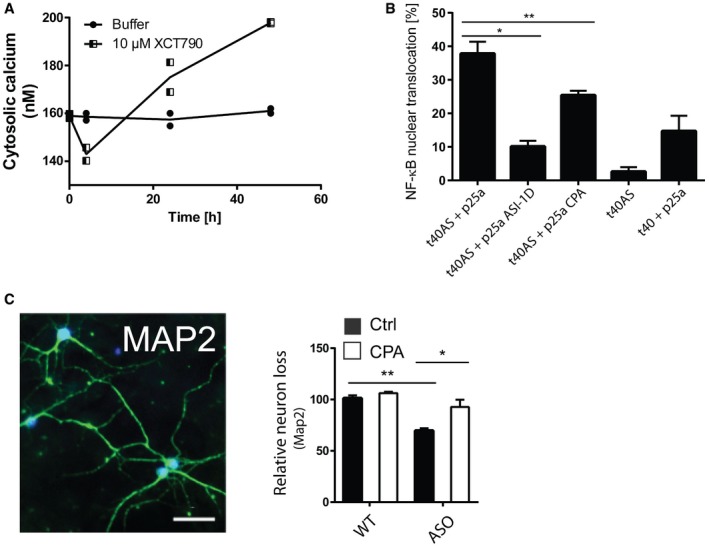
- Allosteric activation of SERCA by XCT790 mimics the early decrease and later increase in Ca2+ observed upon intracellular AS aggregation. Cytosolic Ca2+ levels were quantified by the Ca2+ sensor Fura‐2 and converted to absolute values using the Fura‐2 Calcium Imaging Calibration Kit. Non‐mitotic SH‐SY5Y wt cells were generated by treatment with 10 μM retinoic acid for 2 days before treatment with SERCA activator, 10 μM XCT790 (Sigma‐Aldrich). The cytosolic Ca2+ in SH‐SY5Y cells was measured after 4, 24 and 48 h. Points represent Ca2+ concentrations as mean ± SD, N = 2.
- CPA reduces NF‐κB nuclear translocation in OLN cells. Aggregation of AS in OLN cells by co‐expression of AS and p25a generates a cell stress that increases nuclear translocation of NF‐κB (p65), which can be rescued by ASI‐1D treatment 10. Bars represent the average percentage of transfected cells with NF‐κB translocated to the nucleus ± SD in > 100 transfected cells in each experiment (one‐way ANOVA multiple comparisons with Sidak post hoc test, *P = 0.0001 and **P = 0.0006). N = 3.
- Survival of neurons from day 6 to day 14 was quantified by counting MAP2‐positive cells. Representative MAP2 staining pattern (green) of primary hippocampal neurons with NeuN marking neuronal nuclei. Scale bar is 50 μm. Bars represent remaining MAP‐2‐positive neurons at day 14 normalised to the number present at day 6, presented as means of three individual experiments ± SD each of > 300 neurons (one‐way ANOVA multiple comparisons with Sidak post hoc test, *P = 0.0003, **P = 0.0001).
Figure EV1. Evaluation of the AS level in cell models used for study.
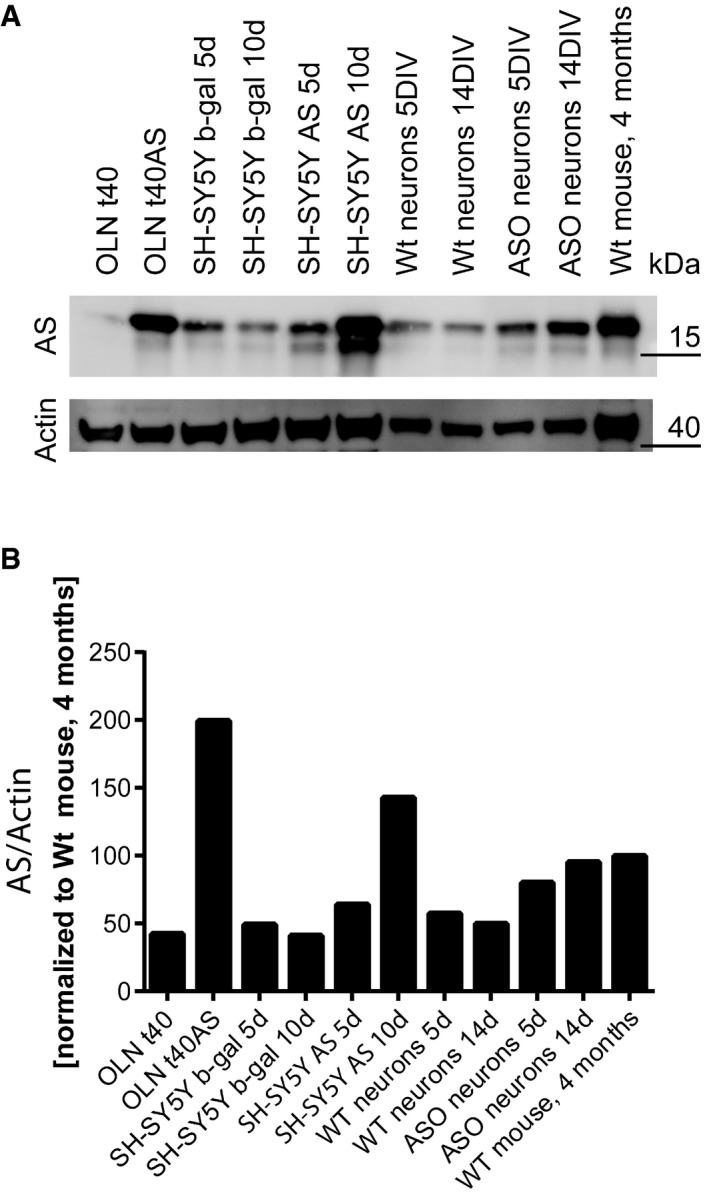
- About 10 μg total lysate from the different cell models, primary mouse hippocampal neurons from wild‐type and AS‐transgenic mice (ASO), and total brain homogenate from a wild‐type C57BL/6J mouse were resolved by 10–16% SDS–PAGE probed for α‐syn (ASY‐1), and actin.
- The level of AS was quantified and normalised to the actin level. The data are presented with the level in total brain homogenate from 4‐month‐old C57BL/6J mouse arbitrarily numbered as 100.
Toxic AS oligomers associate with ER of presymptomatic AS‐transgenic mice and in brain fractions from PD brain tissue enriched in ER 17. The free Ca2+ concentration in the ER is approximately 0.5 mM, compared to a concentration of 100 nM in cytosol 18. This steep gradient is maintained by the high‐capacity transmembrane P‐type Ca2+ ATPase SERCA and is critical for cellular signalling, for example by inositol‐1,4,5 tris‐phosphate‐activated ER channels. Lowering cytosolic Ca2+ requires an active process whereby Ca2+ is removed from the cytosol against steep gradients. We hypothesised that the decreased cytosolic Ca2+ was caused by AS oligomers activating SERCA. To explore this hypothesis, we first conducted a co‐immunoprecipitation experiment to test whether toxic AS oligomers could bind SERCA. The AS oligomers used as bait were purified by gel filtration 19, 20 and share aggregate‐specific epitopes with AS species in pathological human and mouse brain tissue and insoluble AS filaments 16, 17, 21. Freshly eluted from the gel filtration column, the oligomers reveal heterogeneous morphologies in negative stain transmission electron microscopy. Here, an underlying structure of ribbons with a width of 13 nm form spirals with a diameter of around 20 nm which further displayed a tendency to form closed and open circular structures with a diameter of around 40 nm (Fig 2A). As sources for SERCA we used detergent extracts of (i) SERCA2b‐rich membrane fractions from mice brains of C57BL/6 OlaHsd (ASdel) mice, which do not express AS, to avoid interference from endogenous mouse AS (Fig 2B), and (ii) SERCA1a‐rich rabbit muscle microsomes (Fig EV2A). The extracts were supplemented with purified recombinant human monomeric and oligomeric AS (or PBS as negative control) followed by co‐immunoprecipitation using anti‐AS IgG conjugated to Sepharose beads. SERCA displayed preferential binding to oligomeric AS with negligible binding to monomeric AS irrespective of its source being muscle or brain (Figs 2B and EV2A). During the pumping cycle, SERCA exhibits two grossly different conformations, namely a Ca2+‐bound E1 state and a low Ca2+‐affinity E2 state. Using 5 μM Ca2+ or 1 μM of the SERCA inhibitor, thapsigargin, it was possible to capture SERCA in the E1 and E2 conformations, respectively 22. Co‐immunoprecipitation of the conformation‐trapped SERCA, and AS revealed that AS oligomers bind preferentially to the Ca2+‐bound E1 conformation, although binding to E2 is not completely abolished (Fig 2C and D). The higher binding to the Ca2+‐bound E1 state is unlikely to be due to Ca2+ interactions with AS because this binding is of low affinity with a Kd around 0.5 mM 23. This indicates that AS oligomer interaction with SERCA displays a high degree of structural specificity as physiological changes in the SERCA structure are able to modulate the binding strength.
Figure 2. The endoplasmic reticulum calcium ATPase, SERCA, is an AS oligomer‐interacting protein.
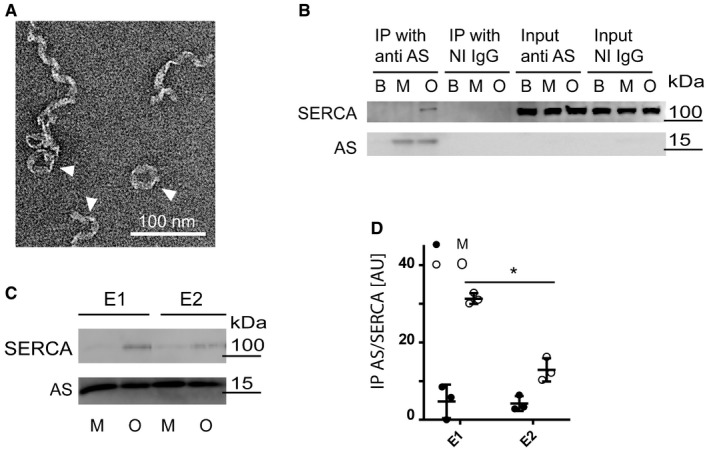
- Transmission electron microscopy of freshly isolated oligomers. Arrowheads appoint heterogeneous population of twisted ribbons with a maximum width of 15 nm. 100 nm scale bar is presented.
- Purified AS oligomers (O) and monomers (M) were incubated with detergent extract of ASdel mouse brain before being subjected to co‐immunoprecipitation (IP) using ASY‐1 (anti‐AS) and non‐immune rabbit IgG (NI IgG) coupled to sepharose. PBS was used as additional negative control. 2% input of each sample was used as input control. The co‐IP samples were analysed by immunoblotting anti‐SERCA (J15.5) and rabbit polyclonal anti‐AS (ASY‐1). One representative immunoblot of three independent experiments is shown.
- The Ca2+‐bound E1‐state of SERCA was stabilised by 5 μM free Ca2+, and the Ca2+‐free E2‐state was stabilised by 1 μM thapsigargin. A representative blot of three replicates is shown.
- Quantification of immunoprecipitation experiments in (C). Bars represent geometric mean ± 95% CI of SERCA signal relative to the AS signal. N = 3 (Wilcoxon signed rank test, *P = 0.0382).
Figure EV2. Aggregated AS interacts with SERCA.
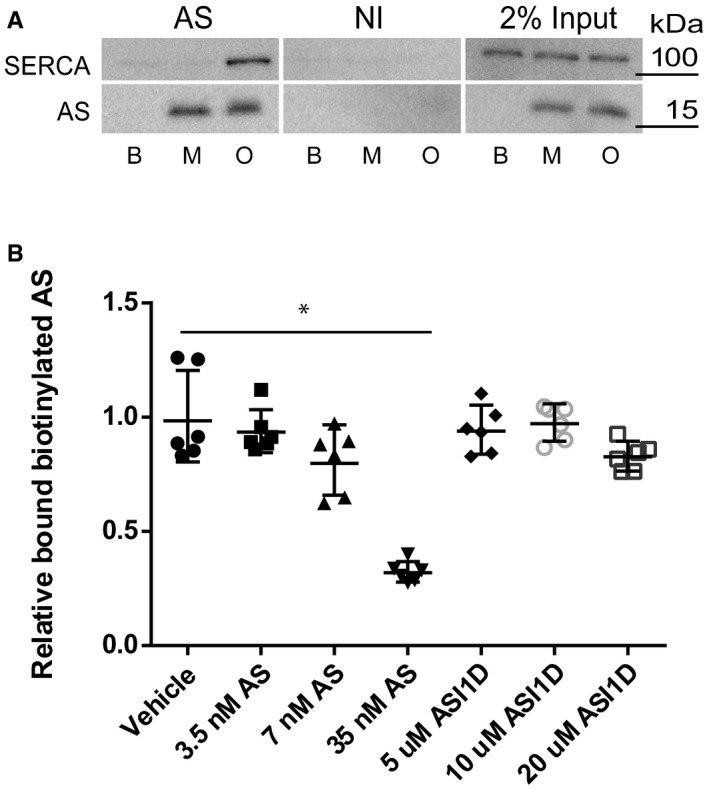
- Aggregated AS interacts with SERCA 1A. Purified AS oligomers (O) and monomers (M) were incubated with detergent extract of SERCA1a from sarcoplasmic reticuli isolated from rabbit muscle before being subjected to co‐immunoprecipitation (co‐IP) using anti‐AS (AS) and non‐immune rabbit IgG (NI) coupled to sepharose. PBS was used as additional negative control. 2% input of each sample was used as input control. The co‐IP samples were analysed by immunoblotting using rabbit polyclonal anti‐SERCA (J15.5) and rabbit polyclonal anti‐AS (ASY‐1). A representative blot of three replicates is shown.
- ELISA shows that the aggregation inhibitor ASI‐1D does not bind the primary anti‐AS antibody and thereby quench the proximity ligation assay. The aggregation inhibitor ASI‐1D was able to completely abolish the signal from the proximity ligation assay (Fig 3B); therefore, we tested if the binding of the Syn211 antibody to AS can be inhibited by the aggregate inhibitor ASI‐1D. An ELISA assay with 0.7 nM biotinylated AS was developed to test whether increasing amounts of ASI‐1D can compete with biotinylated AS bound to Syn211. Increasing amounts of ASI‐1D cannot compete against the binding of biotinylated AS to Syn211 even at a concentration of 20 μM. As control for the competition, increasing amounts of non‐biotinylated AS were tested, and a concentration of 7 nM AS resulted in ˜20% less bound biotinylated AS, and 35 nM AS reduced the biotinylated AS to ˜30%. This shows that the effect of the aggregate inhibitor in our PLA assay is not caused by inhibition of the binding between Syn211/AS by ASI‐1D. Bars represent mean absorbance ± SD (Kruskal–Wallis one‐way rank test with Dunn's post hoc test, *P = 0.001). N = 3.
To test whether SERCA and AS aggregates also interact in a cell model of AS aggregation‐dependent degeneration, we investigated the SH‐SY5Y model with proximity ligation assay using an antibody pair of rabbit anti‐SERCA and mouse anti‐AS (Syn211). The cells were analysed after 5 days of AS expression where they exhibit reduced cytosolic Ca2+ (Fig 1B). Proximity ligation assay (PLA) takes advantage of specific secondary antibodies tagged with short, unique DNA strands, termed PLA probes. When complementary PLA probes bind to primary antibodies that are in close proximity (< 40 nm), the DNA strands anneal, allowing enzymatic ligation and amplification of the signal. Fluorescent complementary oligonucleotide probes then label the amplified DNA sequences. AS‐SERCA PLA of SH‐SY5Y cells that have expressed AS for 5 days reveals that AS is in close proximity with SERCA, as shown by the amplified red punctate signals (Fig 3A). Inhibiting the formation of AS aggregates by ASI‐1D treatment completely abolishes the interaction of AS and SERCA (Fig 3B and D), which corroborates the immunoprecipitation data in Fig 2B of only aggregated AS interacting with SERCA. We used a competitive ELISA to validate that ASI‐1D does not bind the primary anti‐AS antibody Syn211 and thereby artefactually quenches the PLA signal (Fig EV2B). To further validate the specificity of the PLA signal, we suppressed AS expression by doxycycline treatment of transgenic SH‐SY5Y cells, and this abolished any PLA signal (Fig 3C and D).
Figure 3. AS aggregates interact with SERCA in SH‐SY5Y cells.
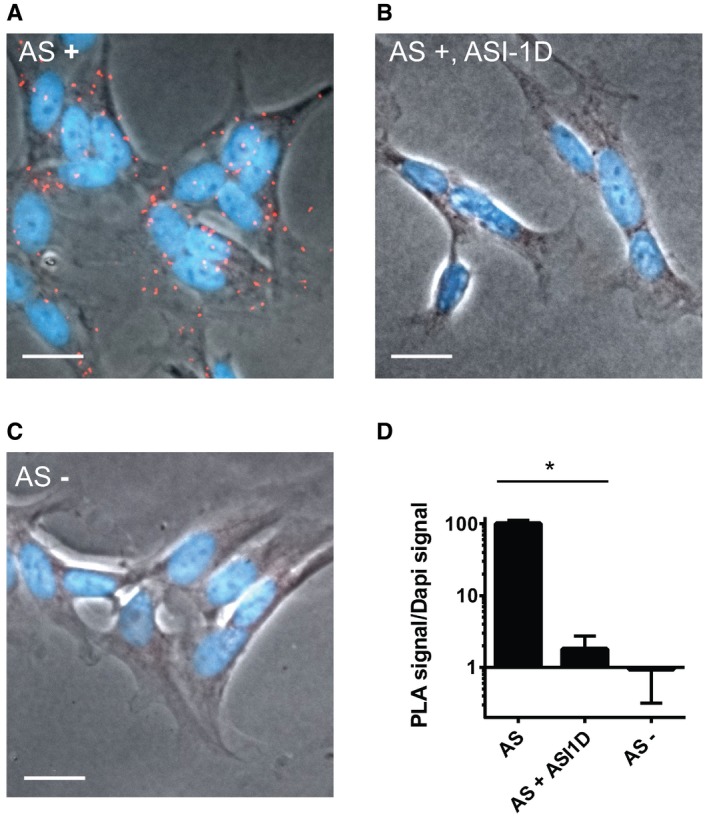
- AS‐expressing SH‐SY5Y cells were analysed by proximity ligation assay to demonstrate an intracellular interaction between SERCA and AS using SERCA2 and syn211 as antibody pair. Positive PLA signals are shown as red staining merged with the phase contrast image and DAPI‐stained nuclei (blue). Scale bar is 20 μm.
- Proximity ligation assays as in (A), but with cells treated with AS aggregation inhibitor, ASI‐1d, from day 3. Scale bar is 20 μm.
- Proximity ligation assay as in (B), but in cells without AS expression. Scale bar is 20 μm.
- The signal from proximity ligation assay was quantification as total red fluorescence signal, divided by the DAPI signal to normalise cellular content. In each experiment, 10 microscopic images containing > 100 cells were quantified. Bars represent mean ± SD signal normalised to AS‐expressing cells from three experiments (one‐way ANOVA multiple comparisons with Sidak post hoc test, *P = 0.0001).
Having demonstrated that AS aggregates can bind SERCA in vitro and shown that the interaction occurs in AS aggregate‐stressed cells, we wanted to test whether AS oligomers can, indeed, activate SERCA and thus cause reduction in cytosolic Ca2+ as observed in AS aggregate‐containing cells. First, we investigated the impact on the enzymatic ATPase activity of SERCA. This measurement revealed a dose‐dependent increase in ATP hydrolysis by SERCA‐containing microsomes when incubated with oligomeric AS in contrast to monomers (Fig 4A). The activation was not due to an inherent ATPase activity in AS oligomers as no hydrolysis was observed when the oligomers were incubated with ATP alone (Fig EV3A). Moreover, phosphate release was due to Ca2+‐ and ATP‐dependent mechanisms, ruling out non‐specific ATPases and mobilisation of precipitated phosphate (Fig EV3A). To investigate the specificity further, we demonstrated that β‐synuclein (β‐syn) and Tau proteins had no effect on the ATPase activity of SERCA (Fig 4A). Insoluble preformed AS filaments (PFF) assembled from wild‐type full‐length AS (wt PFF) also activated SERCA, and this activity was absent in filaments assembled from C‐terminally truncated AS (1–95 PFF; Fig 4B). This suggests that activation relies on folding‐specific structures requiring the acidic C‐terminus and not the core amyloid‐type beta‐sheet structure formed by the N‐terminal 95 residues. To further exclude that a common amyloid‐type core structure contributes to SERCA activation, we tested amyloid fibrils assembled from purified islet amyloid polypeptide (IAPP PFF) and observed no activation (Fig 4B). To validate that the activation was not caused by uncharacterised constituents in the preparations, we pre‐incubated AS oligomers with rabbit polyclonal antibody ASY‐1 targeting AS, and this treatment blocked stimulation in contrast to negative control non‐immune rabbit IgG (Fig 4C). The above functional studies were conducted on SERCA1a from rabbit muscle sarcoplasmic reticulum that represents a specialised form of ER, but AS oligomers could also increase the ATP hydrolysis by cellular ER microsomes from COS cells overexpressing the human SERCA1a (Fig EV3B). Second, we studied the effect of AS oligomers on 45Ca2+ uptake into SERCA‐containing microsomes to determine whether increased ATP hydrolysis is due to increased Ca2+ pumping or an uncoupled ATP hydrolysis as reported for thermogenic drugs 24. AS oligomers, but not monomers, increase 45Ca2+ uptake compared to the buffer control (Fig 4D).
Figure 4. AS oligomers activate SERCA's ATPase activity, calcium pumping and rate of dephosphorylation.
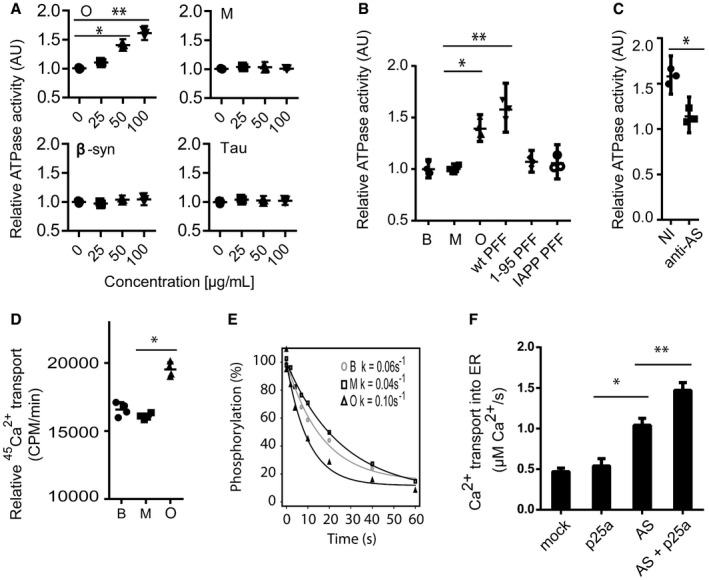
- ATPase activity of SERCA was measured as release of inorganic phosphate from ATP. Sarcoplasmic reticulum (SR) vesicles isolated from rabbit skeletal muscle containing SERCA1a were mixed with AS oligomer (O), AS monomer (M), β‐synuclein (β‐syn) or Tau in the presence of ATP (5 mM), Ca2+ (10 μM) and membrane‐permeating ionomycin (2 μM). The ordinate presents ATP hydrolysis within the first 5 min normalised to buffer controls (0 μg/ml). The abscissa presents the concentration of tested proteins. Bars represent the geometric mean of the relative ATP hydrolysis from three individual experiments ± 95% CI (Wilcoxon signed rank test, *P = 0.0482, **P = 0.0034).
- ATPase activity of SERCA was measured as in (A) compared to buffer control (B), but in presence of 50 μg/ml AS monomer (M), oligomer (O), sonicated insoluble fibrils assembled from full‐length 1–140 amino acid wild‐type AS (wt PFF), sonicated insoluble fibrils assembled from C‐terminally truncated 1–95 amino acid AS (1–95 PFF) or sonicated insoluble fibrils assembled from islet amyloid polypeptide (IAPP PFF) that represent a control amyloid‐type protein aggregate. Bars represent geometric mean of three individual experiments ± 95% CI (Kruskal–Wallis one‐way rank test with Dunn's post hoc test, *P = 0.0161, **P = 0.0016).
- Targeting AS oligomer (50 μg/ml) with rabbit polyclonal ASY‐1 (1 mg/ml) blocked their ATPase‐stimulating activity on SERCA, whereas non‐immune rabbit IgG had no effect. Bars represent the geometric mean of three individual experiments ± 95% CI compared to buffer controls (Wilcoxon signed rank test, *P = 0.048).
- SERCA‐dependent Ca2+ transport across rabbit muscle ER membranes was measured by accumulation of 45Ca2+ in ER vesicles in the presence of 50 μg/ml AS oligomers (O), monomer (M) or buffer control (B). Ordinate represents accumulation of 45Ca2+ in SR vesicles per min measured as counts per minute (CPM). Columns represent the geometric mean of three individual experiments ± 95% CI (Wilcoxon signed rank test, *P = 0.011).
- The effect of AS oligomers on dephosphorylation of the SERCA E1 state. 32P pre‐phosphorylated SERCA was incubated with 50 μg/ml oligomer (O), 50 μg/ml monomer (M) or buffer control in presence of 10 mM EGTA in order to prevent rephosphorylation of SERCA. The residual 32P‐SERCA was quantified over time by phospho‐imaging of SERCA isolated by acid SDS–PAGE. Ordinate axis represents percentage remaining 32P phosphorylated SERCA and abscissa represents the time. The dephosphorylation rate constants were determined to be k = 0.06/s ± 0.003 for buffer (B), k = 0.04/s ± 0.003 for monomer (M), and k = 0.10/s ± 0.006 for oligomer (O). N = 9 (Kruskal–Wallis one‐way rank test with Dunn's post hoc test, B vs. O: P = 0.0038 and M vs. O: P = 0.0002).
- The effect of AS aggregation on Ca2+ transport into ER in living cells was studied in OLN‐t40‐AS transfected with low‐affinity ER‐targeted luminescent aequorin (erAEQ) and co‐expression of p25α to induce aggregation. OLN‐93 cells without AS were used as negative control. The transport of Ca2+ into ER was studied 48 h post‐transfection upon supplementing Ca2+‐depleted cells with 0.5 mM extracellular Ca2+ by measuring increase in luminescence as ER was refilled with Ca2+. Bars represent the mean transport rate of Ca2+ into ER ± SD. N = 15 (one‐way ANOVA multiple comparison with Sidak post hoc test, *P = 0.0001, **P = 0.0023).
Figure EV3. AS oligomers can only stimulate SERCA activity when Ca2+ and ATP are present, and AS oligomer cannot by itself hydrolyse ATP .
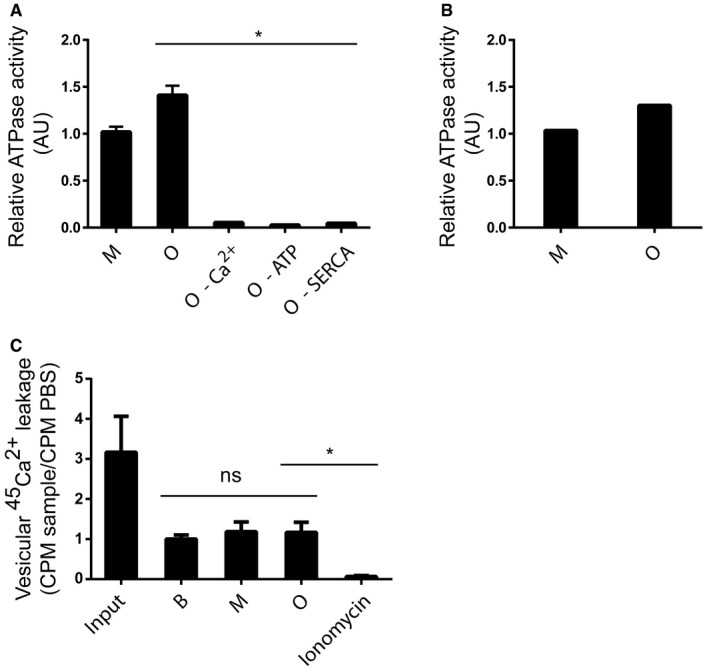
- SERCAs ATPase activity was measured as hydrolysis of ATP and release of inorganic phosphate as in Fig 4A–C. Sarcoplasmic reticulum (SR) vesicles isolated from rabbit skeletal muscle containing SERCA1a were mixed with 50 μg/ml AS monomer (M), AS oligomer (O), AS oligomer without Ca2+ (oligomer–Ca2+), AS oligomer in the absence of ATP (oligomer–ATP) or AS oligomer in the absence of SERCA (O–SERCA). Bars represent the average relative ATP hydrolysis ± SD normalised to buffer (Kruskal–Wallis one‐way rank test with Dunn's post hoc test, *P = 0.01). N = 3.
- Sarcoplasmic reticulum is a highly specialised muscle form of endoplasmic reticulum; and to validate that the activity is not dependent on this, the SERCA ATPase activity was also measured in ER microsomes extracted from COS cells overexpressing SERCA1a in the presence of 50 μg/ml AS monomer (M) or AS oligomer (O). Bars represent the average relative ATP hydrolysis normalised to buffer from three individual experiments (Wilcoxon signed rank test, *P = 0.0286).
- AS oligomers have previously been proposed as forming pores in membranes leading to a leakage of Ca2+. The ability of AS oligomers to form Ca2+ permeable pores was studied in intact microsomes from extracted rabbit muscle preloaded with 45Ca2+. The microsomes were incubated with PBS (B), monomer (M) or oligomer (O) for 1 min before analysis. Ionomycin‐treated microsomes served as positive control for completely permeabilised membranes. No difference in 45Ca2+ efflux could be demonstrated between PBS and the AS preparations in contrast to ionomycin that caused release of all 45Ca2+ from the microsomes. Bars represent the average 45Ca2+ CPM ± SD remaining after 5 min normalised to PBS from three individual experiments (Kruskal–Wallis one‐way rank test with Dunn's post hoc test, *P = 0.0427).
To exclude that the vesicular 45Ca2+ uptake was due to oligomers making the membrane permeable to Ca2+, we conducted the following control experiment. First, SERCA1a‐containing microsomes from rabbit muscles were loaded with 45Ca2+ and treated with PBS, AS monomers or oligomers for 5 min before the remaining intraluminal 45Ca2+ was quantified. Ionomycin‐treated microsomes served as positive control (Fig EV3C). No difference in 45Ca2+ efflux could be demonstrated between PBS and the AS preparation in contrast to ionomycin that caused release of all 45Ca2+ from the microsomes. This strongly argues against AS oligomers forming Ca2+‐permeable pores in the biological membranes used in our uptake assay. The Ca2+‐transport cycle of SERCA consists of several intermediary steps where dephosphorylation is rate limiting. We studied the effect of AS oligomers on this step by measuring the rate of breakdown of 32P‐labelled SERCA after blocking further phosphorylation with EGTA. We thereby demonstrated that AS oligomers stimulate the rate of dephosphorylation in contrast to monomer and buffer control (Fig 4E). We concluded that AS oligomers increase three functional characteristics of SERCA: ATPase activity, transmembrane Ca2+ pumping and dephosphorylation.
To corroborate the finding that intracellular AS aggregates do, indeed, activate ER‐resident SERCA, we turned to the AS‐expressing OLN‐93 model where co‐expression with p25α stimulates intracellular AS aggregation 15. OLN‐T40‐AS cells that stably express human AS were used with the parental OLN‐93 line without any AS expression as negative control. ER Ca2+ levels were quantified by the ER‐targeted low‐affinity Ca2+‐sensing protein aequorin (erAEQ) 25. Cells were transfected with p25α or an empty expression vector for 48 h, after which cellular ER Ca2+ content was depleted by incubation with ionomycin in the EGTA‐supplemented medium. The SERCA‐mediated Ca2+ uptake rate into the Ca2+‐depleted ER was determined by measuring the increase in Ca2+‐dependent aequorin luminescence after supplementing the medium with 0.5 mM Ca2+ (Fig 4F). The presence of p25α‐induced AS aggregates significantly increased the rate of ER filling compared to cells only expressing AS. P25α expression alone did not increase uptake in ER, but OLN‐T40‐AS cells displayed a greater uptake than the parental OLN‐93 cells. It is unclear whether this is due to clonal variation or small amounts of spontaneously forming oligomers. However, induction of AS aggregation in the OLN‐t40‐AS line by p25α significantly increases ER Ca2+ uptake, supporting our hypothesis of intracellular activation of SERCA by AS oligomers. To corroborate the link between SERCA activation and the biphasic cytosolic calcium response, we treated non‐mitotic wt SH‐SY5Y cells with 10 μM of the SERCA activator XCT 790 26 in order to mimic the AS aggregate‐dependent stimulation. Figure EV4A demonstrates XCT 790 caused an early reduction in cytosolic Ca2+ after 4 h followed by a persistent increase in cytosolic Ca2+ after 24. This biphasic response is accelerated compared to the AS aggregate‐dependent response (Fig 1B) indicating XCT 790 in this concentration is a more potent SERCA activator than aggregated AS.
Taken together, the biochemical demonstration of AS oligomers stimulating SERCA suggests that early reduction in cytosolic Ca2+ during AS‐dependent degeneration is caused by SERCA stimulation. We further hypothesise that early Ca2+ reduction represents a decisive signalling point in the degenerative process. To test this hypothesis in cells, we used the reversible SERCA inhibitor CPA 27, 28, 29, 30. At concentrations around 6 μM, CPA induces mild ER stress with enhanced levels of apoptosis 31, and upon titration, we identified the 10‐fold lower 0.5 μM CPA as the minimum concentration that could normalise the reduced Ca2+ level in non‐mitotic SH‐SY5Y cells after AS expression for 5 days. This concentration did not affect the Ca2+ levels in the b‐gal‐expressing control cells (Fig 5A). The cells were treated with CPA from day 3 after transgene expression had been induced by removal of doxycycline as described for ASI‐1D treatment in Fig 1B. Surprisingly, CPA treatment by antagonising SERCA‐dependent removal of Ca2+ from cytosol normalised the abnormally increased cytosolic Ca2+ level in the SH‐SY5Y after 10 days of transgene expression without any detrimental effects on Ca2+ in the controls cells (Fig 5A). Normalising cytosolic Ca2+ levels by CPA treatment was beneficial because this treatment significantly reduced AS‐dependent cell loss when compared to the b‐gal expressing control cells that were unaffected by the treatment (Fig 5B). Normalising cytosolic calcium by CPA treatment also reduced the cell stress in the OLN‐T40‐AS model after 24 h expression of aggregation‐inducing p25α as evidenced by an approximately 50% reduction in nuclear translocation of NF‐κB) that compares to the total reduction by treatment with the aggregation inhibitor ASI‐1D (Fig EV4B). The biphasic cytosolic Ca2+ response in primary hippocampal neurons from AS‐transgenic mice was also normalised by CPA treatment from day 3 without affecting Ca2+ levels in cultures from wt littermates (Fig 5C). To evaluate the protective effect of CPA treatment on the neuron cultures, we developed a survival assay based on comparing neuron numbers after 6 and 14 days of culture (Fig 5D). Because primary cultures also contain glial cells we used immunostaining of NeuN and MAP2 as neuronal markers (Figs 5D and EV4C). During the period from 6 to 14 days of culture, we saw no significant reduction in non‐transgenic primary hippocampal neurons, which contrasts with the approximately 40% loss measured by NeuN and 30% measured by MAP2 in the AS‐expressing cultures (Figs 5D and EV4C). The CPA treatment completely protected against AS‐induced neuron loss and had no adverse effects on the cultures of non‐transgenic littermates (Fig 5D). Treatment with 0.5 μM CPA reduced the interaction between AS and SERCA by approx. 50% (Fig 5E) and reduced total AS levels with 10% (Fig 5F) suggesting the reduced Ca2+ level may compromise proteostatic mechanisms.
Figure 5. Inhibition of SERCA by cyclopiazonic acid normalises both the early decrease and late increase in cytosolic calcium and enhances the viability in differentiated SH‐SY5Y cells and primary neurons.
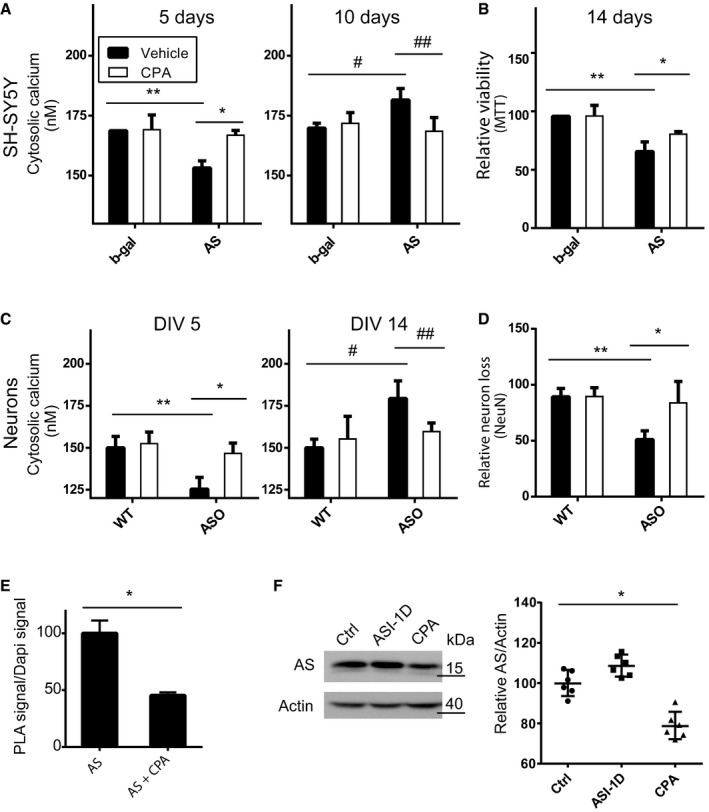
- Non‐mitotic SH‐SY5Y cells were treated with 0.5 μM CPA beginning after 3 days of AS/b‐gal expression started using DMSO as solvent control. Bars display Ca2+ concentrations as mean ± SD, N = 3 (one‐way ANOVA multiple comparisons with Sidak post hoc test, *P = 0.014, **P = 0.0031, # P = 0.0254, ## P = 0.0145). The average Ca2+ level was calculated by measuring > 200 randomly selected cells in each experiment.
- Viability of the SHSY5Y cells was measured by the MTT assay for mitochondrial oxidoreductase activity after 14 days of AS or b‐gal expression with CPA (0.5 μM) treatment from day 3. Bars represent relative viability normalised to vehicle‐treated b‐gal cells displayed as mean ± SD. N = 3 (one‐way ANOVA multiple comparisons with Sidak post hoc test, *P = 0.0002, **P = 0.0304).
- Primary hippocampal neurons from P0 mice expressing human AS (ASO) under the mThy1 promoter and wild‐type (wt) littermates were treated with 0.5 μM CPA from day 3 in vitro (DIV 3). Cytosolic Ca2+ was measured at DIV 5 and DIV 14. Bars represent Ca2+ concentrations as mean ± SD, N = 3 (one‐way ANOVA multiple comparisons with Sidak post hoc test, *P = 0.0041, **P = 0.0095, # P = 0.0081, ## P = 0.0461). The average Ca2+ level was calculated by measuring > 500 randomly selected neurons in each experiment.
- Survival of neurons from day 6 to day 14 was quantified by counting NeuN‐positive nuclei. Bars represent remaining Neu+ neurons at day 14 normalised to the number present at day 6, presented as means ± SD of four individual experiments with > 400 neurons (one‐way ANOVA multiple comparisons with Sidak post hoc test, *P = 0.0033, **P = 0.0137).
- To test the effect of CPA treatment on the interaction between SERCA and AS aggregates, non‐mitotic SH‐SY5Y cells expressing AS for 5 days were treated with 0.5 μM CPA during the last 2 days. The cells were subjected to AS‐SERCA PLA using SERCA2 and syn211 as antibody pair as described in Fig 3. The total red fluorescence signal from the PLA was divided by the DAPI signal to normalise for cellular content. In each experiment, 10 microscopic images containing > 100 cells were quantified. Data are presented as normalised to the signal from non‐CPA‐treated cells. Bars represent mean ± SD signal normalised to AS‐expressing cells from three experiments (one‐way ANOVA multiple comparisons with Sidak post hoc test *P = 0.0001).
- α‐syn levels in differentiated SH‐SY5Y cells expressing α‐syn for 5 days, and treated the last 2 days with 20 μM ASI‐1D or 0.5 μM CPA, were measured by immunoblotting. Representative immunoblot is presented. Quantification of α‐syn normalised to the actin loading control is presented as geometric mean ± 95% CI for six independent experiments (Wilcoxon signed rank test, *P = 0.0313).
To test whether SERCA inhibition also is neuroprotective in vivo, we took advantage of a C. elegans model overexpressing both AS and GFP in dopaminergic neurons under the dat‐1 promoter (Pdat‐1::GFP/AS). This model displays an AS‐dependent degeneration of anterior deirid (ADE) and cephalic (CEP) neurons compared to C. elegans which expresses only GFP (Pdat‐1::GFP) 32, 33, 34. Dendrites of the 4 dopaminergic CEP neurons project towards the anterior mouth region and are easily quantifiable by fluorescence microscopy of fixed C. elegans 32, 33, 34. We scored absent, discontinuous and beaded dendrites as unhealthy. Examples of healthy and unhealthy C. elegans with 4 and 1 intact dendrite are presented in 3D‐projected images in Fig 6A. Quantification of healthy CEP dendrites of 8‐day‐old C. elegans is presented in Fig 6B. Control pdat‐1::GFP C. elegans displayed an age‐dependent dendritic loss with an average of 2.1 dendrites remaining. This loss is significantly increased in AS‐expressing pdat1 AS/GFP worms with only 1.5 dendrites left after 8 days (Fig 6B) representing a 40% larger loss than in control worms. Inhibition of SERCA was initiated on 3‐day‐old C. elegans by transferring them to new bacterial lawns onto which 70 μl 10 mM CPA was evenly distributed. New CPA‐covered bacterial lawn was applied at day 6, and worms were fixed at day 8 prior to quantifying their dendrites. CPA treatment of AS‐transgenic worms rescued survival of CEP neurons to the level seen in control worms, but did not affect age‐dependent CEP loss in control C. elegans (Fig 6B). This indicates that SERCA inhibition by CPA specifically rescues the AS‐dependent, but not age‐dependent, dendrite loss which supports the hypothesis of SERCA inhibition as a therapeutic strategy in α‐synucleinopathies (Fig 8).
Figure 6. Inhibition of SERCA by cyclopiazonic acid protects against human AS stress‐dependent loss of dopaminergic neurons in C. elegans .
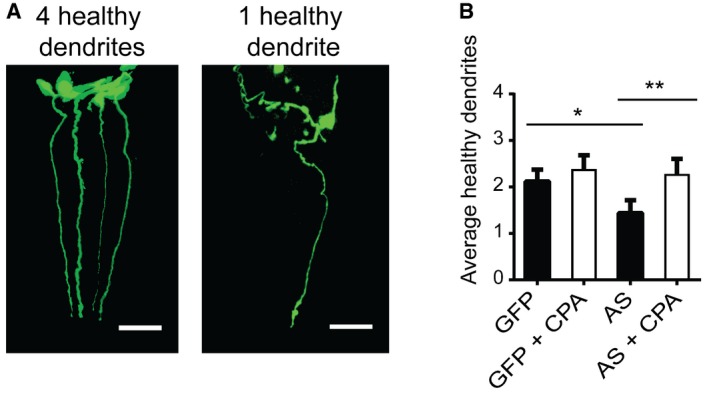
- Representation of an 8‐day‐old Pdat‐1::GFP worm with four intact CEP neurons determined by the presence of intact anterior projecting dendrites (left) and an 8‐day‐old Pdat‐1::GFP/AS worm with one remaining dendrite (right). Images are 3D reconstructions of z‐stacks obtained at 63× magnification. Scale bar is 20 μm.
- Quantification of average number of intact CEP neurons per C. elegans upon CPA treatment. CPA treatment of C. elegans started at day 3. Bars represent the average number of healthy dendrites projecting from CEP neurons ± SD. N = 4 (one‐way ANOVA multiple comparisons with Sidak post hoc test, *P = 0.0146, **P = 0.0008). > 35 worms per experiment.
To investigate if AS and SERCA interact in human brain, we conducted the AS‐SERCA PLA assay on brain sections of occipital cortex from a neurological intact control patient and frontal cortex from a patient affected by DLB (Fig 7A and B). Inspection of images captured from the control patient revealed a low level of PLA‐positive signals that always were present as single spots or a few in proximity. By contrast, the DLB patient displayed a large number of labelled cells that often possessed clusters of many PLA signals indicative of larger amounts of complexes in individual cells. This was evident by the significantly increased area covered by PLA signals and also the increased intensity of the signals (Fig 7B). It should be noted that there is a large variation in the amount of PLA signals within DLB tissue suggesting the disease process is heterogeneously affecting the tissue being studied.
Figure 7. AS and SERCA co‐occur in synucleinopathies.
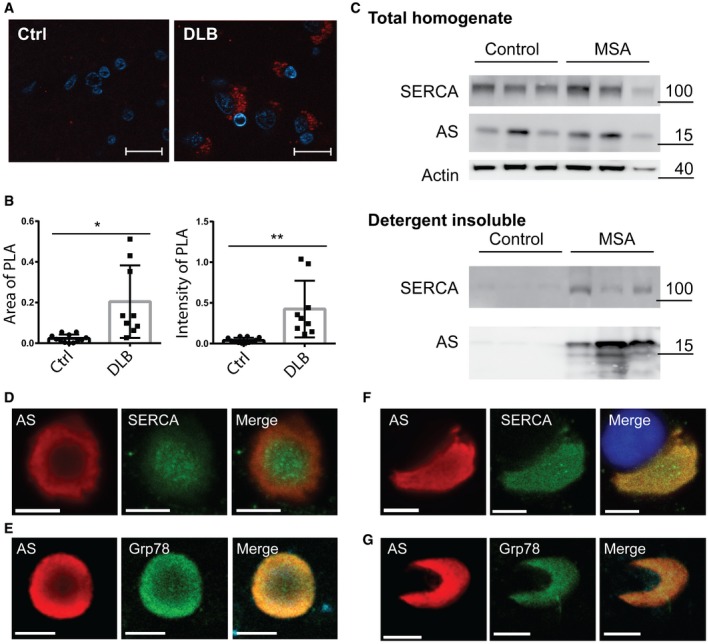
- Representative image of proximity ligation assay using SERCA2 and syn211 as antibody pair on 5‐μm sections paraffin‐embedded human frontal cortex from DLB case (DLB) and occipital cortex from neurological healthy case (Ctrl). Scale bar is 20 μm.
- The proximity ligation signal was quantified in 10 randomly distributed microscopic images using ImageJ as mean area ± SD covered by PLA signal (left graph) and mean PLA intensity ± SD in the covered area (right graph) of signal above 3× the background signal (Student's t‐test, *P = 0.0023, **P = 0.0011).
- Immunoblot analysis of extracts from human cerebellar dentate nucleus from three neurological healthy cases (Control) and three cases with multiple system atrophy (MSA). Presented is the total homogenate and the detergent‐insoluble fraction solubilised in 5% SDS, 8 M urea prior to immonoblotting analysis (detergent insoluble). The molecular markers for the individual immunoblots are presented to the right of the panels.
- Immunostaining of isolated Lewy body (LB) from a PD patient labelled for AS and SERCA2 immunoreactivity. Scale bar is 5 μm.
- Immunostaining of isolated LB from a patient with DLB labelled for AS and ER marker GRP78. GRP78 reactivity is present in the inclusion. Scale bar is 7.5 μm.
- Immunostaining of isolated glial cytoplasmic inclusion (GCI) from MSA patient labelled for AS and SERCA. DAPI stains a co‐isolated oligodendrocyte nucleus. Scale bar is 5 μm.
- Immunostaining of isolated GCI from MSA patient labelled for AS and GRP78. Scale bar is 7.5 μm.
The hallmark glial cytoplasmic inclusions (GCI) in MSA presents with insoluble AS fibrils in oligodendroglia. We investigated whether SERCA cosegregates with insoluble AS fibrils in cerebellar extracts from three cases diagnosed with MSA and compared them to three neurological healthy cases (control) where is expected to be detergent soluble. Figure 7C demonstrates the presence of SERCA along with AS in the detergent‐insoluble fractions in MSA cases whereas this fraction i in the control cases was devoid of SERCA and AS. This contrasts the total homogenates that displayed similar amounts of SDS‐extractable SERCA.
To further investigate the association of SERCA to insoluble AS fibrils in Lewy bodies (LB) and GCI we isolated from the midbrain of a PD patient and glial cytoplasmic inclusions (GCI) from temporal white matter of an MSA patient. We used this purified preparation because they are rich in AS aggregates and devoid of the strong ubiquitous staining that arises from ER present in all brain cells. Immunofluorescence microscopical analysis revealed SERCA epitopes in both midbrain LB and MSA‐derived GCI (Fig 7D and F). In LBs, SERCA is present as large granular structures in the core where AS is less abundant, whereas SERCA in GCI displays a more even and less granular presence largely co‐localising with AS (Fig 7D and F). We also stained inclusions for the ER protein GRP78 (also known as BiP) to evaluate if the SERCA protein may be embedded in ER when present in the AS‐containing inclusion (Fig 7E and G) and could confirm GRP78 is present in both types of inclusions.
Discussion
To the best of our knowledge, we are the first to demonstrate a prolonged cellular state characterised by a reduced level of cytosolic Ca2+ in neurons elicited by a progressive build‐up of intracellular AS aggregates. The reduced Ca2+ level is observed in cell models at an early stage of the degenerative process where cells appear unaffected. Quantitatively, it amounts to an approximately 20% reduction in the resting level in primary hippocampal neurons from 150 to 125 nM. Our observation is corroborated by an X‐ray fluorescence study that observed a reduced amount of Ca2+ in frozen cultured neurons expressing transgenic human AS, albeit this analysis did not allow for specific assignment of the reduced Ca2+ to cytosol 35. The apparently unaffected or “healthy” phenotype at this early stage may be the reason for the phase staying unrecognised, but another reason is that studies of AS in relation to Ca2+ have focussed on dynamic effects like Ca2+‐influx induced by depolarisation or effects of applying extracellular AS on stimulation by neurotransmitters 36, 37. The significance of an early degenerative phase was first noted in our OLN cell model 15, where the expression of certain genes was increased early after induction of AS aggregation, which is where we observe decreased cytosolic Ca2+ 10. Silencing one of these genes protected the cells and motivated us to study this phase in more depth 10. Increases in cytosolic Ca2+ can be considered a cellular default response to a range of noxious stimuli that compromises the active and coordinated ion transport processes maintaining the steep gradient of Ca2+ ions across membranes. Such increases are hypothesised to represent pathogenic drivers in a range of neurodegenerative states like AD and PD 38, 39.
Apart from the study of Ramonet et al, where the cation ATPase, ATP13A2 encoded by PARK9 gene was overexpressed in mouse cortical neurons 40, reduced cytosolic Ca2+ for prolonged periods has not been observed in known disease models. However, this can be obtained for limited periods of time by infusion of Ca2+ chelators, for example BAPTA, through patch pipettes that elicit acute electrophysiological effects 41, 42. Increased expression of Ca2+‐binding proteins has been used to lower Ca2+ levels, but this strategy is not optimal for lowering steady‐state Ca2+ levels. However, it allows changing of Ca2+ transients, for example in the study of the effect of glutamate on Ca2+‐dependent genes by nuclear targeting of Ca2+‐buffering parvalbumin 43. The reduction of cytosolic Ca2+ by intracellular AS aggregates comes at the cost of energy as Ca2+ has to be actively transported. Mechanistically, this process can be facilitated by ion exchangers like sodium–Ca2+ exchanger (NCX) or by Ca2+ pumps. The low basal cytosolic Ca2+ level in our models suggests that high‐affinity mechanisms are operating in contrast to low affinity but high‐capacity NCX transporters. Ca2+ pumps are optimal for this process as they have evolved to fine‐tune cytosolic Ca2+ levels in the nanomolar range 44, 45, 46. We hypothesised that aggregated AS binds directly to and stimulates the predominant Ca2+ pumps in the plasma membrane and endoplasmic reticulum, PMCA and SERCA, because total AS levels remain fairly stable in our cell models and the decrease in cytosolic Ca2+ is prevented by the aggregation inhibitor ASI‐1D. Co‐immunoprecipitation experiments followed by proteomic techniques have demonstrated that PMCA from porcine brain binds both monomer and oligomeric AS 20. Likewise, SERCA was found to bind AS by pull‐down experiments; however, pronounced selectivity was seen with strong binding of aggregated AS to SERCA in contrast to very low binding of monomeric AS. The interaction seems to rely on specific structural features of SERCA because the Ca2+‐bound E1 state demonstrated stronger interaction with AS than the Ca2+‐free thapsigargin‐stabilised E2 state. During the E1‐E2 transition, the largest structural change in the cytosolic part of SERCA occurs in the actuator domain 47, 48, suggesting that this region may be a candidate for binding AS aggregates. The binding of AS oligomers to SERCA resulted in activation of three functional characteristics: Ca2+ pumping, ATP hydrolysis and SERCA dephosphorylation. This suggests that AS aggregates bind to a defined part of SERCA that promotes its overall functionality. In fact, the actuator domain undergoes major structural rearrangements during the E1P‐E2P‐E2 partial reaction steps involved in dephosphorylation 49, and this reaction sequence is rate limiting for Ca2+ pumping and ATP hydrolysis. It is thus plausible that interaction of AS aggregates with the actuator domain activates Ca2+ pumping and ATP hydrolysis by facilitating the E1P‐E2P‐E2 steps. The existence of protein‐based regulators of SERCA pumps is not unprecedented. Under normal conditions, SERCA activity is inhibited by phospholamban for SERCA1 and ‐2 22 and sarcolipin for SERCA1 in skeletal muscle 50. In contrast, presenilin‐1 and ‐2 have been shown to activate SERCA2b 51, linking SERCA activation to Alzheimer's disease. We studied SERCA1a in most biochemical assays. However, to exclude that the AS oligomer interaction with SERCA studied relies on muscle‐specific SERCA properties, we also demonstrated that oligomers stimulate human SERCA1a from microsomes of COS‐1 cells overexpressing human SERCA1a and that they bind to SERCA protein in extracts from mouse and porcine brains as determined by immunoprecipitation.
The soluble oligomeric AS aggregates used for binding and functional studies are chemically unmodified 52 in contrast to other in vitro produced oligomers that rely on chemical modifications 53, 54. Our oligomers display a folding pattern that differs from insoluble filaments based on HDX‐MS investigations 19, but share functional properties with amyloid‐type AS filaments like inhibition of 20S proteasomes 21, binding of aggregate‐specific antibody FILA‐1 55 and stimulations of SERCA activity (Fig 4B). To visualise the oligomers, we immobilised them on EM grids immediately upon their elution from the gel filtration column. The oligomers displayed an underlying structure of twisted ribbons with a tendency to form closed and open circular structures with a diameter of around 40 nm. These structures resembled the annular structures formed between wild‐type and A53T mutant AS that display a diameter in the 50 nm range and with regular height fluctuations 56. Our oligomer preparation displays greater structural resolution than those previously reported 52, 56. The reason may be our rapid immobilisation on the EM grid, which contrasts with previous studies where samples were stored at 4°C for varying stretches of time before analysis by TEM and analytical ultracentrifugation 52, 56. Low temperatures may adversely affect structural studies of AS oligomers because they dissociate into monomers at 4°C with 15% released after 3 h and 95% after 14 days along with loss of epitopes for conformational‐specific antibody FILA‐1 19; and further cooling to −13°C dissociates even stable amyloid AS fibrils 57. Our well‐characterised oligomer preparation will form a good starting point for generating complexes with purified SERCA for analysis by high‐resolution single particle cryo‐electron microscopy 58, as such complexes will allow the characterisation of the interphase between the well‐characterised SERCA surface and the active surface of the yet uncharacterised AS oligomers. Insoluble fragments of AS filaments may also be used for such studies because they engage SERCA and activate its ATPase activity, but are easier to generate and are more stable than oligomers. AS oligomers have been proposed to function as pores in biological membranes 59, 60, 61. However, most of these reports are based on studies of artificial phospholipid membranes and use high concentrations of oligomers. We recently demonstrated that our AS oligomer preparation is toxic to OLN‐93 cells when applied at 70 μg/ml 62. However, only 15% of the cells died within 24 h, indicating the toxicity was not based on formation of non‐specific pores in plasma membranes, but rather caused a stress‐triggering toxic response from within the cell, for example by Ca2+ entering voltage‐gated Ca2+ channels 37. We tested directly if our AS oligomer preparation permeates the microsomes used for the 45Ca2+ uptake assay at concentrations of 50 μg/ml and found no increased 45Ca2+ efflux from preloaded microsomes treated with oligomers compared to monomers or buffer (Fig EV3C). We therefore feel confident that the oligomer‐stimulated 45Ca2+ uptake is due to SERCA activation.
The specific SERCA inhibitor CPA can antagonise SERCA activation and was used to investigate the significance of the reduced cytosolic Ca2+. Dose–response experiments demonstrated that chronic treatment with 0.5 μM CPA abrogated the early reduction in cytosolic Ca2+ observed in cell models and primary neurons and prevented subsequent Ca2+ increase and ensuing AS aggregation‐dependent cell death. The effective CPA concentration of 0.5 nM was low compared to what is used to induce unfolded protein stress in cell models 63, 64, and it was not toxic to our cell models even after treatment for more than 2 weeks. This was expected because low‐dose long‐term pharmacological inhibition of SERCA with thapsigargin that causes a sustained increase in cytosolic Ca2+ protects against neuronal cell death due to growth factor removal 65. The prevention of the later phase with increased cytosolic Ca2+ is surprising because CPA acts by preventing SERCA pumping Ca2+ from cytosol into ER which should thus favour an increase in cytosolic Ca2+. This suggests that the secondary Ca2+ increase represents a breakdown of compensatory processes leading to an unbalanced influx from Ca2+ stores, for example enhanced efflux from ER due to sensitisation of ryanodine or inositol‐1,4,5 tris‐phosphate receptors, Ca2+ channels or from extracellular space via dysregulated Ca2+ influx channels or exchangers. The protective effect of CPA demonstrates that pivotal degenerative processes are activated during the early phase are amenable to pharmacological intervention. Their nature remains largely unknown but the stress that elicits the cytoprotective NF‐κB AS aggregates 10 is reduced in our OLN model upon inhibition of SERCA by CPA (Fig EV4B). NF‐κB is increased in PD as determined by accumulation of p65 in midbrain dopaminergic neurons and Lewy body inclusions in PD and DLB 66, 67, 68, 69, 70 and it is also activated by disease‐causing mutations in LRRK‐2 when studied in iPSC‐derived neurons 71. Inhibiting NF‐κB signalling in c‐REL‐deficient mice induces a late onset parkinsonism also comprising accumulation of aggregated AS 72 and the NF‐κB inhibitor IκBiα is increased early in anterior cingulate in PD 73 like we demonstrate in our cell model 10. This suggests protective NF‐κB signalling may be of particular importance for the nerve cell populations being vulnerable in PD.
The reduced Ca2+ levels do also directly affect the cellular handling of AS as demonstrated by CPA treatment reducing the interaction between AS aggregates and SERCA by PLA assay and lowering levels of total cellular AS suggesting involvement of chaperone and protein catabolic pathways (Fig 5E and F). How this mechanistically is carried out is uncertain. Several investigations have studied direct effects of Ca2+ on AS aggregation in vitro. They demonstrate a proaggregatory effect of increased Ca2+ levels but often with fluorescently labelled AS proteins 74 and those using non‐modified proteins used high Ca2+ concentrations 23 because the C‐terminal Ca2+‐binding site in AS has an IC50 around 200 μM) 75. However, changed Ca2+ levels have potential for affecting a range of pathways regulating intracellular AS levels and specific candidates for rescue by CPA treatment are chaperone‐mediated autophagy 76, USP‐19‐dependent excretion of aggregated AS 77 and changed gene expression regulating cytoprotective so‐called neuroprotective shield genes that depend on nuclear Ca2+ concentration 43. The neuroprotective effect of CPA against AS aggregate toxicity and thapsigargin against growth factor withdrawal‐dependent neuron death 65 demonstrates that SERCA represents a potentially neuroprotective target, but its ubiquitous expression raises concerns regarding adverse effects in other organ systems. However, there is precedence for targeting ubiquitously expressed ion transporting ATPases as exemplified by the cardiotonic steroids targeting Na‐K‐ATPase, which is used in congestive heart failure with a narrow therapeutic window 78, 79. Importantly, structural studies of the interaction between AS aggregates and SERCA may allow identification of compounds that block this abnormal interaction. In principle, such compounds could target surfaces of the AS aggregate. Although the active surfaces of AS aggregates are unknown, we show that AS requires C‐terminal 45 residues in its monomeric building blocks as demonstrated by absent stimulatory activity of filaments formed by C‐terminally truncated AS‐1‐95. This is analogous to what was required to exhibit increased AS aggregate affinity towards p25α protein 21. This highly negatively charged and proline‐rich region of AS has never been resolved in structural studies and the recently described Greek‐key type beta‐sheet domain resolved in AS filaments terminated around residue 98 80, so it remains unknown how it contributes to aggregate‐dependent functions. Rather than targeting SERCA and AS aggregates, more tractable down‐stream targets may be uncovered by studying the degenerative signalling pathways activated during the early low‐Ca2+ phase, for example protective nuclear NFkB translocation that is attenuated upon CPA treatment (Fig EV4B). Such AS aggregate‐dependent dysfunctions may radiate from the endoplasmic reticulum, as this organelle contributes to a multitude of cellular functions including protein sorting, unfolded protein response, excretion of misfolded cytosolic proteins during proteasomal stress, uptake of Ca2+ via store‐operated Ca2+ entry from the plasma membrane and Ca2+ loading into mitochondria via mitochondria‐associated membranes (MAMS) 81. The endoplasmic reticulum permeates all neuronal compartments from pre‐ to post‐synaptic structures, and AS aggregates appear to have a preference for targeting endoplasmic reticulum in AS‐transgenic mouse and PD brain tissue 17. The SERCA affinity of oligomers can represent one mechanism whereby they become concentrated at the ER surface, and the activation of its Ca2+ transporting activity can affect ER functions including protein folding and Ca2+ signalling, for example via ligand‐gated ryanodine or inositol‐1,4,5 tris‐phosphate Ca2+ channels. We demonstrate the SERCA activator XCT 790 qualitatively can mimic the AS aggregate effect by first causing a reduction in cytosolic Ca2+ that subsequently is followed by an increased Ca2+ level that likely reflects a breakdown of compensatory mechanisms (Fig EV4A). Although the biphasic process caused by 10 μM XCT 790 is accelerated, does it suggest that studies of cellular responses to SERCA activators may be used to disentangle the complex response induced by intracellular AS aggregates and which may arise from several AS aggregate‐induced dysfunctions, for example caused by low Ca2+ stress, ER overload stress, mitochondrial stress and dysfunctional autophagy.
The duration of the period where a neuron encounters reduced average cytosolic Ca2+ will likely influence its contribution to the symptomatology of synucleinopathies where the effect of neuronal dysfunction on circuitries may differ from a frank loss of the cell. In the non‐mitotic SH‐SY5Y model, cells exhibit progressive degeneration where approximately 30% of the cells are lost after 14 days in accordance with previous reports 13. These cells experienced reduced Ca2+ for 6 days of the 2 weeks period, which is a significant part of these non‐mitotic cells’ life span. According to the Braak hypothesis of prion‐like spreading of misfolded AS cytopathology, individual neurons likely encounter a gradual build‐up of pathogenic AS species that may last for years before the cells ultimately die. In this process, they may have passed on prion‐like AS species to connected neurons and contributed to symptomatology by changing circuitry properties that are modulated by low cytosolic Ca2+. The hypothesis of prion‐like spreading of AS cytopathology in the nervous system posits that small amounts of misfolded AS seeds are released from donor cells and taken up in recipient cells. Here, they start a process of templated aggregation of the native AS that would result in a gradual build‐up of intracellular aggregates that may span from early oligomeric species to mature amyloid‐type fibrils. The time‐course for this build‐up is unknown, but it can be obtained in transgenic mouse models by inoculation of preformed fibrils or brain extracts from diseased animals, which initiate a neurodegenerative process that traverse several neurons within months 82. In humans, the process is expected to be much slower. In relation to the Braak staging of PD, RBD is hypothesised as representing early manifestation with LB development in brainstem regions preceding the spread to midbrain dopaminergic neurons where actual parkinsonism evolve 83. More than 70% of RBD patients develop PD within 7 years, which suggests that migration of symptom‐causing misfolded AS species from the lower brainstem to the midbrain can take several years. To investigate the hypothesis that AS aggregate‐dependent effects on SERCA may occur in brain regions not commonly recognised as affected by Lewy body pathology, we studied the frontal cortex from a DLB patient and occipital cortex from an age‐matched control using the AS‐SERCA PLA assay. Figure 7A and B demonstrates nearly absent signals in the controls brain suggesting no interactions between SERCA and AS aggregates are taking place. By contrast, the DBL brain exhibits strongly increased signal in terms of area covered with PLA signal and intensity of the signals. This strongly corroborates our hypothesis that interactions between SERCA and AS aggregates develop in tissue from patient affected by synucleinopathies. Our data merely serve as proof of concept, but will justify proper clinical studies of selected brain regions from larger patient cohorts affected by different synucleinopathies. The large microheterogeneity observed in the AS‐SERCA PLA signal on the stained tissue section calls for further studies in affected cell types and their relation to putative structural differences and tissue responses like neuroinflammation (Fig 7B). To further substantiate the interaction between aggregated AS species and SERCA, we analysed the extracts from the cerebellar dentate nucleus from three MSA patients and three controls. The tissue was subjected to differential extraction to investigate if the normally detergent‐soluble SERCA in the MSA cases will co‐fractionate with the detergent‐insoluble AS aggregates. Figure 7C demonstrates the presence of detergent‐insoluble AS in the MSA cases in contrast to the controls and SERCA cosediment with the insoluble AS aggregates. To further describe the relation between aggregated AS and SERCA within the AS fibrils intracellular inclusions, we isolated Lewy bodies from patients affected by PD and DLB and also GCI from a patient affected by MSA. SERCA was preferentially present in the core of the brainstem‐type Lewy bodies from the PD patient whereas it was more diffusely distributed along with the fibrillar AS in the less well organised DLB‐type Lewy bodies and the GCI. In the inclusions, SERCA colocalised with the ER marker, Grp78, suggesting ER structures may be trapped in the inclusions, which may contribute to ER dysfunctions.
How may these experimental findings reconcile with first previous epidemiological and genetic data on protective effects against PD by L‐type Ca2+ channel antagonists 2, 3 and second the pathoanatomical data on selective vulnerability of neuronal populations in PD that in substantia nigra pars compacta neurons exhibit large Ca2+ driven by L‐type Ca2+ channels 84, 85? First, it should be kept in mind that both the early phase with reduced cytosolic Ca2+ and the following phase with increased Ca2+ both are inhibited by inhibiting SERCA with CPA. Hence, treatment strategies targeting the high Ca2+ phase may still contribute some cellular relief despite not targeting the underlying mechanism. The largest epidemiological study confirmed a protective effect of ongoing treatment with L‐type Ca2+ channel antagonists but there was no effect of past use, which made the authors suggest the effect could represent a symptomatic relief rather than a true disease modification 3.
In our proposed paradigm of a biphasic basal Ca2+ change in neurons experiencing a build‐up of AS aggregates, L‐type Ca2+ channel antagonists may retard or attenuate the second phase with increased cytosolic Ca2+ that is associated with development of cell death. An action on the early phase can also be envisioned if expression of CaV1.3 channels partly is regulated by negative feedback from average cytosolic calcium levels. The increased levels of CaV1.3 antigen and mRNA in cortical tissue with no Lewy body pathology from early PD patients 4, 5 could represent a response to soluble AS oligomers activating SERCA and reducing average Ca2+ in neurons not exhibiting spontaneous pace making changes in Ca2+ as in dopaminergic neurons of substantia nigra pars compacta. The ongoing clinical study of early PD patients treated with L‐type Ca channel antagonist isradipine may inform about these issues in the near future (ClinicalTrials.gov Identifier: NCT02168842).
The selective vulnerability of neuronal populations lost in PD has allowed certain characteristics of these neurons to be proposed 84. Areas affected by Lewy body pathology and substantial neuron loss in PD can be exemplified by substantia nigra pars compacta, locus coeruleus, median raphe nucleus. These neuronal populations display diffuse axonal projections and often possess very large numbers of presynaptic terminals 84. AS is a protein that normally resides in presynapses in high concentrations and its aggregation process is concentration dependent. This makes presynapses likely to be the first sites to experience the build‐up of AS aggregates, as supported by proteinase K blotting experiments on cortical tissue affected by dementia with Lewy bodies 86, that secondarily will activate SERCA, increase ATP consumption and reduce cytosolic calcium levels at these sites. A local aggregatory process in terminals may spread within the terminal field of individual neurons as AS readily disperses between terminals of single neurons in a process affected by neuronal activity 87. The engagement of SERCA pumps in the large terminal fields of vulnerable neurons will increase ATP consumption putting a stress on their mitochondria and the potentially increase oxidative stress. This process be further complicated because the reduced average cytosolic Ca2+ may compromise activity‐dependent calcium loading into mitochondria thereby compromising their oxidative phosphorylation 88. When aggregation increases and AS aggregates are transported from terminals into axons and cell bodies then different mechanisms may come into play caused by the SERCA activation both due to lowered calcium, for example in the nucleus 89 and potentially also ER Ca2+ overload if not relieved by overload channels 90. How these mechanisms driven by a reduced average cytosolic Ca2+ interacts with cell type‐specific effects in vulnerable substantia nigra dopaminergic neurons will have to be tested? They may affect the Ca2+‐induced Ca2+‐release from ER facilitated by the oscillating in Ca2+ driven by the L‐type Ca2+ channels and thereby the well‐described filling of Ca2+ into mitochondria 91. However, the recently described ER overload channel TMCO1 may well add another layer of complexity in conditions where SERCA activation will cause Ca2+ overload in ER 90. However, irrespective of vulnerable areas in PD it should be kept in mind that AS aggregate pathology has potential to affects brain cells on a broader scale. This has been demonstrated by the clinical studies of families with triplications in the SNCA gene that express increased levels of normal AS protein as reviewed 92. These families often presented with parkinsonism caused by degeneration of the vulnerable substantia nigra pars compacta but often displayed dementia and other non‐motor symptoms at the time of diagnosis or shortly after. This was due to widespread accumulation of aggregated AS in neurons but also glial cells.
Conclusively our demonstration of AS aggregates activating SERCA and causing dysregulation of cytosolic Ca2+ holds potential for affecting the above‐described PD‐associated mechanisms apart from yet undescribed neuronal functions that potentially are tractable (Fig 8). The specific nature of such dysfunctions, their signalling pathways and brain regions affected in the different synucleinopathies merits further investigations.
Materials and Methods
Cell culture and assays
OLN‐T40‐AS cells 93, 94 were transfected with pcDNA3.1 zeo(−) plasmid‐expressing AS aggregation‐promoting protein, p25α and TdTomato as transfection marker, or mock control (empty vector) 10, 15, 21, 95 by either electroporation by AMAXA Nucleofector®II Kit L using pre‐set program A‐033 (Life Technologies), or FuGENE® 6 Transfection Reagent (Promega) according to the manufacturer's instructions. SH‐SY5Y cells with inducible expression of AS and β‐galactosidase (b‐gal) were kind gifts from Professors Leonidas Stefani and Kostas Vekrellis 13. Cells were differentiated using 20 μM all‐trans retinoic acid (Molecular Probes/Invitrogen) for 2 days prior to expression of AS and b‐gal, which were induced by removal of doxycycline. Primary hippocampal neurons were cultured from new‐born wt and Thy1‐a‐Syn Line 61 (ASO 96) mice (P0) 97. Male ASO neurons were compared to neurons prepared from wild‐type males from same litter. Neurons isolated from male pubs were only used, due to transgene insertion into X‐chromosome and to random X‐chromosome inactivation in females. Hippocampi were dissected in ice‐cold Hank's balanced salt solution, dissociated in 20 U/ml papain in Leibovitz's L15 medium (L15, Gibco) for 20 min at 37°C, washed twice in L15 medium and titurated in plating medium [MEM (Gibco) supplemented with 5 g/l glucose, 0.2 g/l NaHCO3, 0.1 g/l transferrin, 0.25 g/l insulin, 0.3 g/l l‐glutamine and 10% foetal bovine calf serum (heat‐inactivated)]. Hippocampal neurons were seeded on Matrigel® matrix (Corning ®)‐coated coverslips. After 24 h, the medium was changed to growth medium [MEM supplemented with 5 g/l glucose, 0.2 g/l NaHCO3, 0.1 g/l Transferrin, 0.3 g/l l‐glutamine, 1× B‐27 supplement, 2 μM cytosine arabinoside and 5% foetal bovine calf serum (heat‐inactivated)]. Primary neurons were genotyped for transgenic AS and gender using the primers ASO‐sense: 5′‐GACGGGTGTGACAGCAGTAGCC‐3′, ASO‐antisense: 5′‐GATGATGGCATGCAGCACTGG‐3′ and SRY F: 5′‐TTGTCTAGAGAGCATGGAGGGCCATGTCAA‐3′, SRY R: 5′‐CCACTCCTCTGTGACACTTTAGCCCTCCGA‐3′. Neurons and cell lines were kept at 37°C under 5% CO2, and cell lines were tested for mycoplasma every month. Cells and neurons were treated with either 20 μM aggregation inhibitor ASI‐1D (> 98% pure H‐RGGAVVTGRRRRRR‐NH2 (Schafer‐N, Copenhagen, DK), 10 mM stock in 98% ethanol 16) or 0.5 μM CPA (Sigma‐Aldrich, #C1530, 10 mM stock in DMSO).
The viability of SH‐SY5Y cells was evaluated by metabolic activity in an MTT assay (Life Technologies). Viability of primary hippocampal neurons was evaluated as the ratio of NeuN‐positive immunostained neuronal nuclei between DIV 14 and DIV 6 or ratio of Map‐2 immunostained neuronal cell bodies between DIV 14 and DIV 6. Images were obtained using a Zeiss Observer Z1 inverted microscope equipped with ApoTome.2. Neuronal cells were blindly counted in ten randomly distributed fields throughout the coverslip, > 500 neurons on three coverslips were included in each individual experiment (total of > 1,500 neurons).
Absolute cytosolic Ca2+ levels in OLN‐T40‐AS, SH‐SY5Y and primary neurons were determined using the Ca2+‐sensitive fluorescent indicator Fura‐2‐AM (Molecular Probes/Invitrogen) and an Olympus Scan^R high‐content fluorescence microscope equipped with Fura‐2 filters. Cells were loaded with Fura‐2 in sterile filtered HEPES‐buffered saline (HBS: 20 mM HEPES, 150 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM glucose, pH 7.4) containing 2.5 μM Fura‐2 AM, 0.04% pluronic acid, F127 for 30 min at 37°C, 5% CO2. The Fura‐2‐containing medium was replaced with fresh HBS without Fura‐2 and incubated additionally for 30 min. The fluorescence was measured on an Olympus Scan^R high‐content microscope using excitation wavelengths at 340 and 380 nm and emission at 510 nm. The ratio of the fluorescence signal at 510 nm upon excitation at 340 nm and excitation at 380 nm (FL_ex340/FL_ex380) gives a relative Ca2+ estimate independent of cell morphology. The cytosolic Ca2+ levels in single cells were measured by placing a region of interest (ROI) outside the nucleus. The size of the ROI was set to be 8 μm2. For OLN cells, at least 50 transfected cells were measured in three independent replicates. For the inducible SH‐SY5Y cells and primary neurons, more than 300 cells were measured in three independent experiments. The Fura‐2 ratios were converted to molar concentration using Fura‐2 Calcium Imaging Calibration Kit (Molecular Probes, F6774), generating a standard curve from 0 to 350 nM Ca2+.
Transport of Ca2+ into ER in cells was measured using the OLN cell line as described in Ref. 25. Cells were plated in a six‐well plate and after 8 h transfected by lipofectamine with 1.5 μg plasmid encoding low‐affinity ER‐targeted aequorin (erAEQ) and 2.5 μg of p25α or empty vector. Twenty‐four hour post‐transfection, cells were washed, collected and replated on a 96‐well plate. After an additional 24 h (48 h after transfection), cells were incubated for 1.5 h at 4°C in Krebs Ringer modified buffer (KRB: 125 mM NaCl, 5 mM KCl, 1 mM Na3PO4, 1 mM MgSO4, 5.5 mM glucose, 20 mM HEPES, pH 7.4 at 37°C), supplemented with the 5 μM ionomycin (A23187), 600 μM EGTA and 5 μM coelenterazine n (Invitrogen) in order to deplete ER Ca2+ and functionally reconstitute erAEQ. Afterwards, cells were extensively washed with KRB supplemented with 2% bovine serum albumin and 1 mM EGTA. Refilling of the empty ER was obtained by placing the cells in KRB supplemented with 100 μM EGTA and CaCl2 to reach the final concentration of 0.5 mM CaCl2, and the generated light was measured in a Perkin‐Elmer Envision plate reader equipped with a two‐injector unit. At the end of the experiment, a hypotonic, Ca2+‐rich, digitonin‐containing solution was added to discharge the remaining aequorin pool. Output data were analysed and calibrated with a custom‐made macro‐enabled Excel workbook. ER Ca2+ uptake speed was calculated as the first derivative using the slope excel function and smoothed for three time points.
Immunostaining
Cells were fixed in 4% paraformaldehyde for 30 min at room temperature (RT) followed by a wash in PBS and 10 min permeabilisation in 0.1% Triton X‐100, 50 mM glycine, 3 mM CaCl2, 2 mM MgCl2, pH 7.4. Unspecific binding was blocked by 3% bovine serum albumin in PBS for 1 h followed by incubation with the primary antibody for 1.5 h [anti‐AS (1 μg/ml, ASY‐1 15, 21), anti‐SERCA (1 μg/ml, Abcam, ab3625), anti‐p65 (NF‐κB) (2.5 μg/ml, Cell Signaling Technology, (D14E12) #8242)]. Unbound antibodies were removed by washing in PBS followed by incubation with Alexa Fluor‐conjugated secondary antibodies (Life Technologies). Unbound secondary antibodies were removed by washing in PBS before mounting in Prolong Antifade Gold Mountant (Life Technologies). For Duolink® PLA, the previously described ICC protocol was followed until washing after incubation with primary antibodies [anti‐AS (1 μg/ml, syn211, Abcam, ab80627) and anti‐SERCA2 (1 μg/ml, Abcam, ab3625)] and continued according to the manufacturer's instructions with the Duolink® In Situ Red Starter Kit Mouse/Rabbit (Duolink®, Sigma‐Aldrich). All PLA experiments were stained and evaluated blinded. Human tissue used for PLA was obtained from brain donors recruited with informed consent through a regional brain donor programme with ethics approval from the Human Research Ethics Committees of South Eastern Sydney Local Health District and the Universities of Sydney and New South Wales (Sydney) which complies with the statement of human experimentation issued by the National Health and Medical Research Council of Australia. Immunohistochemical stainings were conducted on isolated LB and GCIs from fresh‐frozen brains from patients with DLB/PD and MSA as described 98, 99, 100 using anti‐SERCA (1 μg/ml, Abcam, ab3625), anti‐AS (ASY‐1) and anti‐GRP78 (BD Transduction Laboratories™, # 610979).
C. elegans strains and culture
All C. elegans strains were maintained at 20°C on standard nematode growth medium (NGM) plates spotted with Escherichia coli strain OP50 as the food source. The strain UA44 [baInl1; Pdat‐1::AS, Pdat‐1::GFP] expressing AS and GFP in the dopaminergic neurons was a kind gift from the Caldwell laboratory 32, 33, 34. The control strain BZ555 [egIs1(dat‐1::GFP)] that only expresses GFP in the dopaminergic neurons was obtained from the Caenorhabditis Genetics Center. For the CPA treatment, 35‐mm NGM plates with a fixed volume of 4 ml media were used. About 70 μl of CPA in DMSO (10 μM) or 70 μl DMSO as control was spotted on top of the OP50. The plates were set aside for 4 h to allow the CPA to disperse before 30–40 three‐day‐old worms were transferred to the plates. The worms were moved to fresh plates every other day to provide fresh CPA and avoid cross‐contamination with progeny. Eight‐day‐old worms were fixed overnight at RT in 2% paraformaldehyde, washed three times in PBS and mounted in fluorescence‐mounting media (Dako, # S302380‐2). The worms were assessed for degeneration of the dopaminergic neurons by fluorescence microscopy on a Zeiss Observer Z1 inverted microscope equipped with Apotome.2. Images were obtained using Pln Apo 63×/1.4 Oil DIC II objective as 3D‐projected images of 40 z‐stage sections, each 0.3 μm thick, giving a total image depth of 12 μm.
Protein preparations
Recombinant human AS was produced as described 101. AS oligomers were formed by incubation of 10 mg/ml monomeric AS on ice for 30 min, followed by gel filtration separation of oligomers and monomers according to Lashuel et al 52. A phosphor‐free elution buffer, TBS (10 mM Tris–HCl, 140 mM KCl), was used for transmission electron microscopy and ATPase activity assay. AS oligomers were concentrated using Amicon Ultra‐15, Ultracell‐10k filter device (Millipore). The concentration of AS oligomer was determined by bicinchoninic acid (BCA) protein concentration assay (Pierce).
Fresh brain tissue from C57BL/6 OlaHsd (ASdel) mice, which does not express AS, was dounce‐homogenised in threefold excess volume extraction buffer [25 mM MES, 5 mM DTT, 2 mM EDTA, 9% sucrose, Complete Mini Protease Inhibitor (Roche)] compared to the weight of tissue and centrifuged 1,700 g at 4°C for 15 min to remove debris and nuclei. The resulting supernatant was further centrifuged at 100,000 g for 1 h at 4°C, and the pellet was dounce‐homogenised in an extraction buffer containing 0.5% Triton X‐100. The homogenate was centrifuged 100,000 g for 1 h at 4°C and used as brain membrane extract for co‐immunoprecipitation. SERCA1a was prepared as sarcoplasmic reticuli (SR) microsomes by deoxycholate extracts from rabbit muscle sarcoplasmic reticuli 102, 103. Samples were stored at −80°C and thawed on ice immediately before the experiment. The SR microsomes were dissolved in PBS, 1% Triton X‐100 for 30 min on ice and subsequently centrifuged at 100,000 g for 1 h prior to co‐immunoprecipitation.
Human tissue was obtained with informed consent and the experiments conformed to the principles set out in the WMA Declaration of Helsinki and the Department of Health and Human Services Belmont Report. Human cerebellum dentate nuclei (~0.05 g) were dissected from frozen brain slices of three cases of MSA and three neurological healthy cases donated from the Brain Bank at Bispebjerg‐Frederiksberg University Hospital (University Hospital of Copenhagen, DNK; approved by the Danish Data Protection Agency, j.no. BBH‐2010‐06, I‐suite 00971). The use of the brain tissue was approved by the regional ethical committee of Region Hovedstaden (DNK), journal no. H‐16025210. MSA patients were diagnosed according to the current MSA consensus guidelines 104. Detailed neuropathological post‐mortem evaluations were performed on each MSA brain 105. The normal controls were free of neurological disorders. The tissue was processed according to Dickson et al 106 with minor changes. The tissue was homogenised in 20 volumes (weight/volume) PBS+ pH 7.4, supplemented with complete protease inhibitor mix (Roche) and phosphatase inhibitors (25 mmol/l NaF, 25 mmol/l β‐glycerophosphate, 0.1 mmol/l Na‐vanadate). Debri was removed from the homogenate by centrifugation at 1,000 g for 10 min. The supernatant (S1) was diluted to a final protein concentration of 1 mg/ml. Equal amounts of S1 were centrifuged at 100,000 g for 1 h. The resulting pellets were washed twice in PBS+, extracted in PBS+ with 1% Triton X‐100 with 0.5% deoxycholate and 0.1% SDS and centrifuged at 100,000 g for 1 h. The resulting pellet was washed twice with PBS+ with 1% Triton X‐100 with 0.5% deoxycholate and 0.1% SDS, before complete extraction in PBS+ with 8 M urea and 5% SDS.
Electron microscopy
Fresh EM samples were prepared as previously described 107. Specimens were applied on previously glow‐discharged homemade carbon‐coated copper girds and incubated for 10 s. Subsequently, specimens were stained three times with 1% uranyl formate solution. Raw micrographs were acquired automatically at 42,000 magnification at specimen level using the Leginon system 108 operated on a Tecnai Spirit BioTWIN TEM at 120 kV acceleration voltage and a Tietz 416 CMOS detector.
Co‐immunoprecipitation of AS and SERCA
Detergent extract of SR microsomes from rabbit muscle was used as the source for SERCA, and its conformations were stabilised in E1 conformation by 5 μM Ca2+ or in E2 conformation by 1 μM thapsigargin (Sigma‐Aldrich, # T9033). AS oligomer or monomer (2 μg/ml) was mixed with 0.5 mg/ml ASdel mouse brain detergent extract of membrane fraction, or 4 μg/ml SERCA 1A detergent extract of SR microsomes from rabbit muscle t in PBS, 0.5% Triton X‐100 and incubated overnight at 4°C. AS binding (ASY‐1) or control (non‐immune IgG) sepharose beads were added in 5× molar excess compared to AS. The samples were incubated for 2 h with rotation. Sepharose beads were isolated by centrifugation in a tabletop centrifuge at 800 g for 1 min and washed twice in PBS, 0.5% Triton X‐100. The bound protein was extracted in SDS Laemmli buffer without reducing dithiothreitol for 30 min at 37°C. Extracted immunoprecipitated proteins were separated on 10–16% SDS–PAGE, electroblotted onto PVDF membranes; immunoreactivity to AS was evaluated by anti‐AS (ASY‐1) and SERCA was evaluated by polyclonal rabbit anti‐SERCA (raised against the peptide of amino acid 809‐827 109, a kind gift from Professor J.V. Møller, University of Aarhus, DK).
Functional studies of SERCA
The ATPase activity of SERCA was studied by monitoring the release of inorganic phosphate 110. Transport Ca2+ by SERCA was measured as accumulation of 45Ca2+ in microsomal vesicles, which were quenched with LaCl3 followed by capture by filtration 111. Combined assays to simultaneously measure Ca2+‐activated ATP hydrolysis and ATP‐driven Ca2+ uptake were conducted with SR vesicles at 37°C in a medium containing 20 mM MOPS/Tris, pH 6.8, 100 mM KCl, 5 mM MgCl3, 5 mM potassium oxalate, 0.5 mM EGTA and 0.45 mM CaCl2 (free Ca2+ approx. 10 μM). The medium used for Ca2+‐uptake measurements also contained 45Ca2+ (approx. 0.1 MBq/ml) to trace Ca2+ accumulation in the vesicles. The reaction was initiated by addition of 5 mM ATP. This system was also used to investigate leakage of 45Ca2+ from preloaded microsomes with the exception of the absence of oxalate. In brief, the microsomes were preloaded with 45Ca2+ overnight at RT and then incubated at 37°C with AS, buffer or ionomycin for 5 min before quenching by LaCl3 and capturing on filter. Furthermore, dephosphorylation of 32P pre‐phosphorylated SERCA was followed by serial time interval quantification of acid‐quenched SERCA on SDS–PAGE 102, 111. Pre‐phosphorylation of SERCA was conducted at 0°C for 15 s in the presence of 5 μM [γ‐32P]ATP and 40 mM MOPS/Tris, pH 7.0, 122 mM KCl, 5 mM MgCl2, 1 mM EGTA and CaCl2 to give a free Ca2+ concentration of 10 μM, and terminated by addition of 10 mM EGTA to chelate Ca2+. The rate of dephosphorylation was followed over time by acid‐quenching the reaction at serial time intervals, and the phosphoenzyme in the quenched samples was isolated by acidic SDS–PAGE and quantified using the Packard Cyclone™ storage phosphor system 111. The dephosphorylation rate constants were determined as mono‐exponential decay function using the SigmaPlot program (SPSS, Inc.).
Enzyme‐linked immunosorbent assay
A 96‐well maxisorb plate was coated with Syn211 (1 μg/ml) and incubated for 2 h at RT. The plate was washed with TBS‐T and blocked in 10% foetal calf serum for 2 h at RT followed by incubation with 0.7 nM biotinylated AS overnight at 4°C with or without increasing amounts of non‐biotinylated AS (3.5, 7 or 35 nM) or ASI‐1D (5, 10 or 20 μM). After washing, streptavidin‐conjugated HRP was added for 2 h at RT followed by addition of TMB. The reaction was stopped by addition of 1 M phosphoric acid, and absorbance was measured at 450 nm.
Statistical analysis
Statistical evaluation of results was performed using parametric tests for data sets following normal distribution by comparing means ± SD. For smaller data sets where normal distribution could not be proven, statistical evaluation was conducted by nonparametric tests and data presented as geometric mean ± 95% confidence interval. In order to compare one mean to another mean, Student's t‐test was conducted as parametric test and Wilcoxon signed rank test was used as nonparametric test. In order to test the null‐hypothesis in multiple comparisons, when comparing several groups to one mean, one‐way ANOVA combined with Sidak post hoc test was applied as parametric test and Kruskal–Wallis one‐way rank test with Dunn's post hoc test was applied as nonparametric test to obtain an adjusted P‐value using the GraphPad Prism program (GraphPad Software). P‐values below 0.05 are considered to be statistically significant.
Author contributions
CB planned, executed and analysed most experiments and wrote the manuscript. LBL, RHK, LR, EG and JZ contributed critically to aspects of the project. W‐PG and TCh isolated Lewy bodies and glial cytoplasmic inclusions from human brain material. AM performed negative staining electron microscopy. MB and TCa performed the experiments on alpha‐synuclein stimulated Ca2+ uptake in ER. GH and YF performed the immunohistochemical analyses on human brain tissue. TB, SA and BP isolated the tissue from frozen MSA and control brains. JPA supervised and assisted in the in vitro analysis of SERCA functions. AO conducted the C. elegans experiments. PHJ was part of all planning and supervised analyses and final writing of manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Expanded View Figures PDF
Review Process File
Acknowledgements
The authors acknowledge the Caldwell laboratory at University of Alabama, USA, for sharing the C. elegans model overexpressing AS. The original C. elegans strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). In addition, the authors wish to thank Prof. Daniel Otzen at iNano, Aarhus University, Denmark, for generously donating islet amyloid polypeptide and Drs Tie‐Shan Tang and Aimin Zhou etc. for valuable discussion on ER Ca2+ overload. The authors also acknowledge Dr. Bo Martin Bibby, Department of Biostatistics, Aarhus University for the statistical assistance. The Lundbeckfoundation, Dandrite, Aarhus University, the Michael J. Fox Foundation and Parkinsonforeningen are thanked for generous support.
EMBO Reports (2018) 19: e44617
References
- 1. Nussbaum RL (2017) Genetics of synucleinopathies. Cold Spring Harb Perspect Med https://doi.org/10.1101/cshperspect.a024109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ritz B, Rhodes SL, Qian L, Schernhammer E, Olsen JH, Friis S (2010) L‐type calcium channel blockers and Parkinson disease in Denmark. Ann Neurol 67: 600–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pasternak B, Svanstrom H, Nielsen NM, Fugger L, Melbye M, Hviid A (2012) Use of calcium channel blockers and Parkinson's disease. Am J Epidemiol 175: 627–635 [DOI] [PubMed] [Google Scholar]
- 4. Hurley MJ, Brandon B, Gentleman SM, Dexter DT (2013) Parkinson's disease is associated with altered expression of CaV1 channels and calcium‐binding proteins. Brain 136: 2077–2097 [DOI] [PubMed] [Google Scholar]
- 5. Hurley MJ, Gentleman SM, Dexter DT (2015) Calcium CaV1 channel subtype mRNA expression in Parkinson's disease examined by in situ hybridization. J Mol Neurosci 55: 715–724 [DOI] [PubMed] [Google Scholar]
- 6. Surmeier DJ, Schumacker PT, Guzman JD, Ilijic E, Yang B, Zampese E (2017a) Calcium and Parkinson's disease. Biochem Biophys Res Commun 483: 1013–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lieberman OJ, Choi SJ, Kanter E, Saverchenko A, Frier MD, Fiore GM, Wu M, Kondapalli J, Zampese E, Surmeier DJ et al (2017) Alpha‐synuclein dependent calcium entry underlies differential sensitivity of cultured SN and VTA dopaminergic neurons to a Parkinsonian neurotoxin. eNeuro 4: ENEURO.0167‐17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 24: 197–211 [DOI] [PubMed] [Google Scholar]
- 9. Bezprozvanny IB (2010) Calcium signaling and neurodegeneration. Acta Naturae 2: 72–82 [PMC free article] [PubMed] [Google Scholar]
- 10. Kragh CL, Gysbers AM, Rockenstein E, Murphy K, Halliday GM, Masliah E, Jensen PH (2014) Prodegenerative IkappaBalpha expression in oligodendroglial alpha‐synuclein models of multiple system atrophy. Neurobiol Dis 63: 171–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hamza TH, Chen H, Hill‐Burns EM, Rhodes SL, Montimurro J, Kay DM, Tenesa A, Kusel VI, Sheehan P, Eaaswarkhanth M et al (2011) Genome‐wide gene‐environment study identifies glutamate receptor gene GRIN2A as a Parkinson's disease modifier gene via interaction with coffee. PLoS Genet 7: e1002237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamada‐Fowler N, Fredrikson M, Soderkvist P (2014) Caffeine interaction with glutamate receptor gene GRIN2A: Parkinson's disease in Swedish population. PLoS One 9: e99294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vekrellis K, Xilouri M, Emmanouilidou E, Stefanis L (2009) Inducible over‐expression of wild type alpha‐synuclein in human neuronal cells leads to caspase‐dependent non‐apoptotic death. J Neurochem 109: 1348–1362 [DOI] [PubMed] [Google Scholar]
- 14. Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, Masliah E (2002) Differential neuropathological alterations in transgenic mice expressing alpha‐synuclein from the platelet‐derived growth factor and Thy‐1 promoters. J Neurosci Res 68: 568–578 [DOI] [PubMed] [Google Scholar]
- 15. Kragh CL, Lund LB, Febbraro F, Hansen HD, Gai WP, El‐Agnaf O, Richter‐Landsberg C, Jensen PH (2009) Alpha‐synuclein aggregation and Ser‐129 phosphorylation‐dependent cell death in oligodendroglial cells. J Biol Chem 284: 10211–10222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. El‐Agnaf OM, Paleologou KE, Greer B, Abogrein AM, King JE, Salem SA, Fullwood NJ, Benson FE, Hewitt R, Ford KJ et al (2004) A strategy for designing inhibitors of alpha‐synuclein aggregation and toxicity as a novel treatment for Parkinson's disease and related disorders. FASEB J 18: 1315–1317 [DOI] [PubMed] [Google Scholar]
- 17. Colla E, Jensen PH, Pletnikova O, Troncoso JC, Glabe C, Lee MK (2012) Accumulation of toxic alpha‐synuclein oligomer within endoplasmic reticulum occurs in alpha‐synucleinopathy in vivo . J Neurosci 32: 3301–3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu R, Hinkle PM (2000) Rapid turnover of calcium in the endoplasmic reticulum during signaling. Studies with cameleon calcium indicators. J Biol Chem 275: 23648–23653 [DOI] [PubMed] [Google Scholar]
- 19. Mysling S, Betzer C, Jensen PH, Jorgensen TJ (2013) Characterizing the dynamics of alpha‐synuclein oligomers using hydrogen/deuterium exchange monitored by mass spectrometry. Biochemistry 52: 9097–9103 [DOI] [PubMed] [Google Scholar]
- 20. Betzer C, Movius AJ, Shi M, Gai WP, Zhang J, Jensen PH (2015) Identification of synaptosomal proteins binding to monomeric and oligomeric alpha‐synuclein. PLoS One 10: e0116473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lindersson E, Beedholm R, Hojrup P, Moos T, Gai W, Hendil KB, Jensen PH (2004) Proteasomal inhibition by alpha‐synuclein filaments and oligomers. J Biol Chem 279: 12924–12934 [DOI] [PubMed] [Google Scholar]
- 22. Brini M, Carafoli E (2009) Calcium pumps in health and disease. Physiol Rev 89: 1341–1378 [DOI] [PubMed] [Google Scholar]
- 23. Nielsen MS, Vorum H, Lindersson E, Jensen PH (2001) Ca2+ binding to alpha‐synuclein regulates ligand binding and oligomerization. J Biol Chem 276: 22680–22684 [DOI] [PubMed] [Google Scholar]
- 24. Mahmmoud YA, Gaster M (2012) Uncoupling of sarcoplasmic reticulum Ca(2)(+)‐ATPase by N‐arachidonoyl dopamine. Members of the endocannabinoid family as thermogenic drugs. Br J Pharmacol 166: 2060–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cali T, Ottolini D, Negro A, Brini M (2012) alpha‐Synuclein controls mitochondrial calcium homeostasis by enhancing endoplasmic reticulum‐mitochondria interactions. J Biol Chem 287: 17914–17929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gruber SJ, Cornea RL, Li J, Peterson KC, Schaaf TM, Gillispie GD, Dahl R, Zsebo KM, Robia SL, Thomas DD (2014) Discovery of enzyme modulators via high‐throughput time‐resolved FRET in living cells. J Biomol Screen 19: 215–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thastrup O, Dawson AP, Scharff O, Foder B, Cullen PJ, Drobak BK, Bjerrum PJ, Christensen SB, Hanley MR (1994) Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. 1989. Agents Actions 43: 187–193 [DOI] [PubMed] [Google Scholar]
- 28. Seidler NW, Jona I, Vegh M, Martonosi A (1989) Cyclopiazonic acid is a specific inhibitor of the Ca2+‐ATPase of sarcoplasmic reticulum. J Biol Chem 264: 17816–17823 [PubMed] [Google Scholar]
- 29. Foskett JK, Wong D (1992) Calcium oscillations in parotid acinar cells induced by microsomal Ca(2+)‐ATPase inhibition. Am J Physiol 262: C656–C663 [DOI] [PubMed] [Google Scholar]
- 30. Takahashi M, Kondou Y, Toyoshima C (2007) Interdomain communication in calcium pump as revealed in the crystal structures with transmembrane inhibitors. Proc Natl Acad Sci USA 104: 5800–5805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miani M, Barthson J, Colli ML, Brozzi F, Cnop M, Eizirik DL (2013) Endoplasmic reticulum stress sensitizes pancreatic beta cells to interleukin‐1beta‐induced apoptosis via Bim/A1 imbalance. Cell Death Dis 4: e701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cao S, Gelwix CC, Caldwell KA, Caldwell GA (2005) Torsin‐mediated protection from cellular stress in the dopaminergic neurons of Caenorhabditis elegans . J Neurosci 25: 3801–3812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tucci ML, Harrington AJ, Caldwell GA, Caldwell KA (2011) Modeling dopamine neuron degeneration in Caenorhabditis elegans . Methods Mol Biol 793: 129–148 [DOI] [PubMed] [Google Scholar]
- 34. Dexter PM, Caldwell KA, Caldwell GA (2012) A predictable worm: application of Caenorhabditis elegans for mechanistic investigation of movement disorders. Neurotherapeutics 9: 393–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ducic T, Carboni E, Lai B, Chen S, Michalke B, Lazaro DF, Outeiro TF, Bahr M, Barski E, Lingor P (2015) Alpha‐synuclein regulates neuronal levels of manganese and calcium. ACS Chem Neurosci 6: 1769–1779 [DOI] [PubMed] [Google Scholar]
- 36. Hettiarachchi NT, Parker A, Dallas ML, Pennington K, Hung CC, Pearson HA, Boyle JP, Robinson P, Peers C (2009) alpha‐Synuclein modulation of Ca2+ signaling in human neuroblastoma (SH‐SY5Y) cells. J Neurochem 111: 1192–1201 [DOI] [PubMed] [Google Scholar]
- 37. Melachroinou K, Xilouri M, Emmanouilidou E, Masgrau R, Papazafiri P, Stefanis L, Vekrellis K (2013) Deregulation of calcium homeostasis mediates secreted alpha‐synuclein‐induced neurotoxicity. Neurobiol Aging 34: 2853–2865 [DOI] [PubMed] [Google Scholar]
- 38. Mattson MP (2007) Calcium and neurodegeneration. Aging Cell 6: 337–350 [DOI] [PubMed] [Google Scholar]
- 39. Bezprozvanny I (2009) Calcium signaling and neurodegenerative diseases. Trends Mol Med 15: 89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ramonet D, Podhajska A, Stafa K, Sonnay S, Trancikova A, Tsika E, Pletnikova O, Troncoso JC, Glauser L, Moore DJ (2012) PARK9‐associated ATP13A2 localizes to intracellular acidic vesicles and regulates cation homeostasis and neuronal integrity. Hum Mol Genet 21: 1725–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Konnerth A, Dreessen J, Augustine GJ (1992) Brief dendritic calcium signals initiate long‐lasting synaptic depression in cerebellar Purkinje cells. Proc Natl Acad Sci USA 89: 7051–7055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roussel C, Erneux T, Schiffmann SN, Gall D (2006) Modulation of neuronal excitability by intracellular calcium buffering: from spiking to bursting. Cell Calcium 39: 455–466 [DOI] [PubMed] [Google Scholar]
- 43. Mauceri D, Hagenston AM, Schramm K, Weiss U, Bading H (2015) Nuclear calcium buffering capacity shapes neuronal architecture. J Biol Chem 290: 23039–23049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brini M, Carafoli E, Cali T (2017) The plasma membrane calcium pumps: focus on the role in (neuro)pathology. Biochem Biophys Res Commun 483: 1116–1124 [DOI] [PubMed] [Google Scholar]
- 45. Vandecaetsbeek I, Vangheluwe P, Raeymaekers L, Wuytack F, Vanoevelen J (2011) The Ca2+ pumps of the endoplasmic reticulum and Golgi apparatus. Cold Spring Harb Perspect Biol 3: a004184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Giladi M, Tal I, Khananshvili D (2016) Structural features of ion transport and allosteric regulation in sodium‐calcium exchanger (NCX) proteins. Front Physiol 7: 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Toyoshima C, Nomura H (2002) Structural changes in the calcium pump accompanying the dissociation of calcium. Nature 418: 605–611 [DOI] [PubMed] [Google Scholar]
- 48. Sorensen TL, Moller JV, Nissen P (2004) Phosphoryl transfer and calcium ion occlusion in the calcium pump. Science 304: 1672–1675 [DOI] [PubMed] [Google Scholar]
- 49. Toyoshima C (2009) How Ca2+‐ATPase pumps ions across the sarcoplasmic reticulum membrane. Biochim Biophys Acta 1793: 941–946 [DOI] [PubMed] [Google Scholar]
- 50. Odermatt A, Becker S, Khanna VK, Kurzydlowski K, Leisner E, Pette D, MacLennan DH (1998) Sarcolipin regulates the activity of SERCA1, the fast‐twitch skeletal muscle sarcoplasmic reticulum Ca2+‐ATPase. J Biol Chem 273: 12360–12369 [DOI] [PubMed] [Google Scholar]
- 51. Green KN, Demuro A, Akbari Y, Hitt BD, Smith IF, Parker I, LaFerla FM (2008) SERCA pump activity is physiologically regulated by presenilin and regulates amyloid beta production. J Cell Biol 181: 1107–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lashuel HA, Petre BM, Wall J, Simon M, Nowak RJ, Walz T, Lansbury PT Jr (2002) Alpha‐synuclein, especially the Parkinson's disease‐associated mutants, forms pore‐like annular and tubular protofibrils. J Mol Biol 322: 1089–1102 [DOI] [PubMed] [Google Scholar]
- 53. Danzer KM, Haasen D, Karow AR, Moussaud S, Habeck M, Giese A, Kretzschmar H, Hengerer B, Kostka M (2007) Different species of alpha‐synuclein oligomers induce calcium influx and seeding. J Neurosci 27: 9220–9232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nasstrom T, Fagerqvist T, Barbu M, Karlsson M, Nikolajeff F, Kasrayan A, Ekberg M, Lannfelt L, Ingelsson M, Bergstrom J (2011) The lipid peroxidation products 4‐oxo‐2‐nonenal and 4‐hydroxy‐2‐nonenal promote the formation of alpha‐synuclein oligomers with distinct biochemical, morphological, and functional properties. Free Radic Biol Med 50: 428–437 [DOI] [PubMed] [Google Scholar]
- 55. Paleologou KE, Kragh CL, Mann DM, Salem SA, Al‐Shami R, Allsop D, Hassan AH, Jensen PH, El‐Agnaf OM (2009) Detection of elevated levels of soluble alpha‐synuclein oligomers in post‐mortem brain extracts from patients with dementia with Lewy bodies. Brain 132: 1093–1101 [DOI] [PubMed] [Google Scholar]
- 56. Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT Jr (2000) Acceleration of oligomerization, not fibrillization, is a shared property of both alpha‐synuclein mutations linked to early‐onset Parkinson's disease: implications for pathogenesis and therapy. Proc Natl Acad Sci USA 97: 571–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kim HY, Cho MK, Kumar A, Maier E, Siebenhaar C, Becker S, Fernandez CO, Lashuel HA, Benz R, Lange A et al (2009) Structural properties of pore‐forming oligomers of alpha‐synuclein. J Am Chem Soc 131: 17482–17489 [DOI] [PubMed] [Google Scholar]
- 58. Vinothkumar KR (2015) Membrane protein structures without crystals, by single particle electron cryomicroscopy. Curr Opin Struct Biol 33: 103–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tsigelny IF, Sharikov Y, Wrasidlo W, Gonzalez T, Desplats PA, Crews L, Spencer B, Masliah E (2012) Role of alpha‐synuclein penetration into the membrane in the mechanisms of oligomer pore formation. FEBS J 279: 1000–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fecchio C, De Franceschi G, Relini A, Greggio E, Dalla Serra M, Bubacco L, Polverino de Laureto P (2013) alpha‐Synuclein oligomers induced by docosahexaenoic acid affect membrane integrity. PLoS One 8: e82732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Di Scala C, Yahi N, Boutemeur S, Flores A, Rodriguez L, Chahinian H, Fantini J (2016) Common molecular mechanism of amyloid pore formation by Alzheimer's beta‐amyloid peptide and alpha‐synuclein. Sci Rep 6: 28781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lorenzen N, Nielsen SB, Yoshimura Y, Vad BS, Andersen CB, Betzer C, Kaspersen JD, Christiansen G, Pedersen JS, Jensen PH et al (2014) How epigallocatechin gallate can inhibit alpha‐synuclein oligomer toxicity in vitro . J Biol Chem 289: 21299–21310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liang SH, Zhang W, McGrath BC, Zhang P, Cavener DR (2006) PERK (eIF2alpha kinase) is required to activate the stress‐activated MAPKs and induce the expression of immediate‐early genes upon disruption of ER calcium homoeostasis. Biochem J 393: 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kirkpatrick CL, Wiederkehr A, Baquie M, Akhmedov D, Wang H, Gauthier BR, Akerman I, Ishihara H, Ferrer J, Wollheim CB (2011) Hepatic nuclear factor 1alpha (HNF1alpha) dysfunction down‐regulates X‐box‐binding protein 1 (XBP1) and sensitizes beta‐cells to endoplasmic reticulum stress. J Biol Chem 286: 32300–32312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lampe PA, Cornbrooks EB, Juhasz A, Johnson EM Jr, Franklin JL (1995) Suppression of programmed neuronal death by a thapsigargin‐induced Ca2+ influx. J Neurobiol 26: 205–212 [DOI] [PubMed] [Google Scholar]
- 66. Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, Pahan K (2007) Selective inhibition of NF‐kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson's disease. Proc Natl Acad Sci USA 104: 18754–18759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hunot S, Brugg B, Ricard D, Michel PP, Muriel MP, Ruberg M, Faucheux BA, Agid Y, Hirsch EC (1997) Nuclear translocation of NF‐kappaB is increased in dopaminergic neurons of patients with Parkinson disease. Proc Natl Acad Sci USA 94: 7531–7536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Noda K, Kitami T, Gai WP, Chegini F, Jensen PH, Fujimura T, Murayama K, Tanaka K, Mizuno Y, Hattori N (2005) Phosphorylated IkappaBalpha is a component of Lewy body of Parkinson's disease. Biochem Biophys Res Commun 331: 309–317 [DOI] [PubMed] [Google Scholar]
- 69. Soos J, Engelhardt JI, Siklos L, Havas L, Majtenyi K (2004) The expression of PARP, NF‐kappa B and parvalbumin is increased in Parkinson disease. NeuroReport 15: 1715–1718 [DOI] [PubMed] [Google Scholar]
- 70. Togo T, Iseki E, Marui W, Akiyama H, Ueda K, Kosaka K (2001) Glial involvement in the degeneration process of Lewy body‐bearing neurons and the degradation process of Lewy bodies in brains of dementia with Lewy bodies. J Neurol Sci 184: 71–75 [DOI] [PubMed] [Google Scholar]
- 71. Lopez de Maturana R, Lang V, Zubiarrain A, Sousa A, Vazquez N, Gorostidi A, Aguila J, Lopez de Munain A, Rodriguez M, Sanchez‐Pernaute R (2016) Mutations in LRRK2 impair NF‐kappaB pathway in iPSC‐derived neurons. J Neuroinflammation 13: 295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Baiguera C, Alghisi M, Pinna A, Bellucci A, De Luca MA, Frau L, Morelli M, Ingrassia R, Benarese M, Porrini V et al (2012) Late‐onset Parkinsonism in NFkappaB/c‐Rel‐deficient mice. Brain 135: 2750–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Murphy KE, Gysbers AM, Abbott SK, Spiro AS, Furuta A, Cooper A, Garner B, Kabuta T, Halliday GM (2015) Lysosomal‐associated membrane protein 2 isoforms are differentially affected in early Parkinson's disease. Mov Disord 30: 1639–1647 [DOI] [PubMed] [Google Scholar]
- 74. Nath S, Goodwin J, Engelborghs Y, Pountney DL (2011) Raised calcium promotes alpha‐synuclein aggregate formation. Mol Cell Neurosci 46: 516–526 [DOI] [PubMed] [Google Scholar]
- 75. Lowe R, Pountney DL, Jensen PH, Gai WP, Voelcker NH (2004) Calcium(II) selectively induces alpha‐synuclein annular oligomers via interaction with the C‐terminal domain. Protein Sci 13: 3245–3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Vogiatzi T, Xilouri M, Vekrellis K, Stefanis L (2008) Wild type alpha‐synuclein is degraded by chaperone‐mediated autophagy and macroautophagy in neuronal cells. J Biol Chem 283: 23542–23556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lee JG, Takahama S, Zhang G, Tomarev SI, Ye Y (2016) Unconventional secretion of misfolded proteins promotes adaptation to proteasome dysfunction in mammalian cells. Nat Cell Biol 18: 765–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Eichhorn EJ, Gheorghiade M (2002) Digoxin. Prog Cardiovasc Dis 44: 251–266 [DOI] [PubMed] [Google Scholar]
- 79. Wasserstrom JA, Aistrup GL (2005) Digitalis: new actions for an old drug. Am J Physiol Heart Circ Physiol 289: H1781–H1793 [DOI] [PubMed] [Google Scholar]
- 80. Tuttle MD, Comellas G, Nieuwkoop AJ, Covell DJ, Berthold DA, Kloepper KD, Courtney JM, Kim JK, Barclay AM, Kendall A et al (2016) Solid‐state NMR structure of a pathogenic fibril of full‐length human alpha‐synuclein. Nat Struct Mol Biol 23: 409–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Martinez G, Duran‐Aniotz C, Cabral‐Miranda F, Vivar JP, Hetz C (2017) Endoplasmic reticulum proteostasis impairment in aging. Aging Cell 16: 615–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sacino AN, Brooks M, Thomas MA, McKinney AB, Lee S, Regenhardt RW, McGarvey NH, Ayers JI, Notterpek L, Borchelt DR et al (2014) Intramuscular injection of alpha‐synuclein induces CNS alpha‐synuclein pathology and a rapid‐onset motor phenotype in transgenic mice. Proc Natl Acad Sci USA 111: 10732–10737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Boeve BF, Silber MH, Saper CB, Ferman TJ, Dickson DW, Parisi JE, Benarroch EE, Ahlskog JE, Smith GE, Caselli RC et al (2007) Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain 130: 2770–2788 [DOI] [PubMed] [Google Scholar]
- 84. Surmeier DJ, Obeso JA, Halliday GM (2017b) Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci 18: 101–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ (2007) ‘Rejuvenation’ protects neurons in mouse models of Parkinson's disease. Nature 447: 1081–1086 [DOI] [PubMed] [Google Scholar]
- 86. Kramer ML, Schulz‐Schaeffer WJ (2007) Presynaptic alpha‐synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J Neurosci 27: 1405–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fortin DL, Nemani VM, Voglmaier SM, Anthony MD, Ryan TA, Edwards RH (2005) Neural activity controls the synaptic accumulation of alpha‐synuclein. J Neurosci 25: 10913–10921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Griffiths EJ, Rutter GA (2009) Mitochondrial calcium as a key regulator of mitochondrial ATP production in mammalian cells. Biochim Biophys Acta 1787: 1324–1333 [DOI] [PubMed] [Google Scholar]
- 89. Bading H (2013) Nuclear calcium signalling in the regulation of brain function. Nat Rev Neurosci 14: 593–608 [DOI] [PubMed] [Google Scholar]
- 90. Wang QC, Zheng Q, Tan H, Zhang B, Li X, Yang Y, Yu J, Liu Y, Chai H, Wang X et al (2016) TMCO1 is an ER Ca(2+) load‐activated Ca(2+) channel. Cell 165: 1454–1466 [DOI] [PubMed] [Google Scholar]
- 91. Jackson JG, Thayer SA (2006) Mitochondrial modulation of Ca2+ ‐induced Ca2+ ‐release in rat sensory neurons. J Neurophysiol 96: 1093–1104 [DOI] [PubMed] [Google Scholar]
- 92. Devine MJ, Gwinn K, Singleton A, Hardy J (2011) Parkinson's disease and alpha‐synuclein expression. Mov Disord 26: 2160–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Goldbaum O, Oppermann M, Handschuh M, Dabir D, Zhang B, Forman MS, Trojanowski JQ, Lee VM, Richter‐Landsberg C (2003) Proteasome inhibition stabilizes tau inclusions in oligodendroglial cells that occur after treatment with okadaic acid. J Neurosci 23: 8872–8880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Uryu K, Richter‐Landsberg C, Welch W, Sun E, Goldbaum O, Norris EH, Pham CT, Yazawa I, Hilburger K, Micsenyi M et al (2006) Convergence of heat shock protein 90 with ubiquitin in filamentous alpha‐synuclein inclusions of alpha‐synucleinopathies. Am J Pathol 168: 947–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kragh CL, Fillon G, Gysbers A, Hansen HD, Neumann M, Richter‐Landsberg C, Haass C, Zalc B, Lubetzki C, Gai WP et al (2013) FAS‐dependent cell death in alpha‐synuclein transgenic oligodendrocyte models of multiple system atrophy. PLoS One 8: e55243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chesselet MF, Richter F, Zhu C, Magen I, Watson MB, Subramaniam SR (2012) A progressive mouse model of Parkinson's disease: the Thy1‐aSyn (“Line 61”) mice. Neurotherapeutics 9: 297–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC (2010) Alpha‐synuclein promotes SNARE‐complex assembly in vivo and in vitro . Science 329: 1663–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gai WP, Yuan HX, Li XQ, Power JT, Blumbergs PC, Jensen PH (2000) In situ and in vitro study of colocalization and segregation of alpha‐synuclein, ubiquitin, and lipids in Lewy bodies. Exp Neurol 166: 324–333 [DOI] [PubMed] [Google Scholar]
- 99. Jensen PH, Islam K, Kenney J, Nielsen MS, Power J, Gai WP (2000) Microtubule‐associated protein 1B is a component of cortical Lewy bodies and binds alpha‐synuclein filaments. J Biol Chem 275: 21500–21507 [DOI] [PubMed] [Google Scholar]
- 100. Gai WP, Power JH, Blumbergs PC, Culvenor JG, Jensen PH (1999) Alpha‐synuclein immunoisolation of glial inclusions from multiple system atrophy brain tissue reveals multiprotein components. J Neurochem 73: 2093–2100 [PubMed] [Google Scholar]
- 101. Huang C, Ren G, Zhou H, Wang CC (2005) A new method for purification of recombinant human alpha‐synuclein in Escherichia coli . Protein Expr Purif 42: 173–177 [DOI] [PubMed] [Google Scholar]
- 102. De Meis L, Hasselbach W (1971) Acetyl phosphate as substrate for Ca2+ uptake in skeletal muscle microsomes. Inhibition by alkali ions. J Biol Chem 246: 4759–4763 [PubMed] [Google Scholar]
- 103. Meissner G, Conner GE, Fleischer S (1973) Isolation of sarcoplasmic reticulum by zonal centrifugation and purification of Ca2+ ‐pump and Ca2+ ‐binding proteins. Biochim Biophys Acta 298: 246–269 [DOI] [PubMed] [Google Scholar]
- 104. Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Durr A, Fowler CJ et al (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71: 670–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Trojanowski JQ, Revesz T, Neuropathology Working Group on MSA (2007) Proposed neuropathological criteria for the post mortem diagnosis of multiple system atrophy. Neuropathol Appl Neurobiol 33: 615–620 [DOI] [PubMed] [Google Scholar]
- 106. Dickson DW, Liu W, Hardy J, Farrer M, Mehta N, Uitti R, Mark M, Zimmerman T, Golbe L, Sage J et al (1999) Widespread alterations of alpha‐synuclein in multiple system atrophy. Am J Pathol 155: 1241–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Tao H, Lee SC, Moeller A, Roy RS, Siu FY, Zimmermann J, Stevens RC, Potter CS, Carragher B, Zhang Q (2013) Engineered nanostructured beta‐sheet peptides protect membrane proteins. Nat Methods 10: 759–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Suloway C, Pulokas J, Fellmann D, Cheng A, Guerra F, Quispe J, Stagg S, Potter CS, Carragher B (2005) Automated molecular microscopy: the new Leginon system. J Struct Biol 151: 41–60 [DOI] [PubMed] [Google Scholar]
- 109. Moller JV, Ning G, Maunsbach AB, Fujimoto K, Asai K, Juul B, Lee YJ, Gomez de Gracia A, Falson P, le Maire M (1997) Probing of the membrane topology of sarcoplasmic reticulum Ca2+‐ATPase with sequence‐specific antibodies. Evidence for plasticity of the c‐terminal domain. J Biol Chem 272: 29015–29032 [DOI] [PubMed] [Google Scholar]
- 110. Baginski ES, Foa PP, Zak B (1967) Microdetermination of inorganic phosphate, phospholipids, and total phosphate in biologic materials. Clin Chem 13: 326–332 [PubMed] [Google Scholar]
- 111. Vilsen B, Andersen JP, Clarke DM, MacLennan DH (1989) Functional consequences of proline mutations in the cytoplasmic and transmembrane sectors of the Ca2(+)‐ATPase of sarcoplasmic reticulum. J Biol Chem 264: 21024–21030 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded View Figures PDF
Review Process File


