Abstract
Tenofovir (TFV) disoproxil fumarate and emtricitabine (FTC) are used in combination for HIV treatment and pre-exposure prophylaxis (PrEP). TFV disoproxil fumarate is a prodrug that undergoes diester hydrolysis to TFV. FTC and TFV are nucleoside/nucleotide reverse transcriptase inhibitors that upon phosphorylation to nucleotide triphosphate analogs competitively inhibit HIV reverse transcriptase. We previously demonstrated that adenylate kinase 2, pyruvate kinase, muscle and pyruvate kinase, liver and red blood cell phosphorylate TFV in peripheral blood mononuclear cells (PBMC). To identify the kinases that phosphorylate FTC in PBMC, siRNAs targeted toward kinases that phosphorylate compounds structurally similar to FTC were delivered to PBMC, followed by incubation with FTC and the application of a matrix-assisted laser desorption ionization–mass spectrometry method and ultra high performance liquid chromatography-UV to detect the formation of FTC phosphates. Knockdown of deoxycytidine kinase decreased the formation of FTC-monophosphate, while siRNA targeted toward thymidine kinase 1 decreased the abundance of FTC-diphosphate. Knockdown of either cytidine monophosphate kinase 1 or phosphoglycerate kinase 1 decreased the abundance of FTC-triphosphate. Next-generation sequencing of genomic DNA isolated from 498 HIV-uninfected participants in the HIV Prevention Trials Network 069/AIDS Clinical Trials Group A5305 clinical study, revealed 17 previously unreported genetic variants of TFV or FTC phosphorylating kinases. Of note, four individuals were identified as simultaneous carriers of variants of both TFV and FTC activating kinases. These results identify the specific kinases that activate FTC in PBMC, while also providing further insight into the potential for genetic variation to impact TFV and FTC activation.
Keywords: : kinase, tenofovir, emtricitabine, pharmacogenetics, nucleotide reverse transcriptase inhibitor
Introduction
The combination of oral tenofovir (TFV) disoproxil fumarate and emtricitabine (FTC) comprise the only regimen currently approved by the United States Food and Drug Administration for HIV pre-exposure prophylaxis (PrEP).1,2 TFV disoproxil fumarate is a prodrug that upon hydrolysis is transformed to TFV. TFV and FTC, which are adenosine monophosphate and cytidine analogs, respectively, are nucleoside/nucleotide reverse transcriptase inhibitors that require intracellular phosphorylation to form the nucleotide triphosphate analogs that competitively inhibit HIV reverse transcriptase. As a monophosphate analog, TFV only requires two phosphorylation steps to become pharmacologically active.
We have previously demonstrated that adenylate kinase 2 (AK2) phosphorylates TFV to TFV-monophosphate (TFV-MP) in peripheral blood mononuclear cells (PBMC), vaginal tissue, and colorectal tissue.3 Interestingly, our laboratory found that phosphorylation of TFV-MP is compartment specific in that creatine kinase, muscle (CKM) can phosphorylate TFV-MP to TFV-DP in colon tissue, while pyruvate kinase, muscle (PKM) and pyruvate kinase, liver and red blood cell (PKLR) were observed to catalyze the formation of TFV-DP in PBMC and vaginal tissue.3
The identification of these kinases spurred us to test whether genetic variants of AK2, PKLR, PKM, and/or CKM exist that might impact phosphorylation of TFV and in doing so, several previously unreported genetic variants in the genes that encode these kinases were discovered.3 Using bioinformatics tools, several of these variants were predicted to have an impact on the function of the protein, including a potential decrease or loss of enzymatic activity. This finding put forth the possibility that these genetic variations could result in inter-individual variability in TFV activation, and therefore TFV efficacy, even when adherence to TFV is high.
In contrast to TFV, FTC requires three phosphorylation steps to form the pharmacologically active triphosphate4; however, the kinases that phosphorylate FTC in PBMC have yet to be reported. We hypothesized here that the kinases likely to exhibit activity toward FTC in PBMC include deoxycytidine kinase (DCK), thymidine kinase 1 (TK1), cytidine monophosphate kinase 1 (CMPK1), and phosphoglycerate kinase 1 (PGK1).
DCK has been shown in vitro to phosphorylate FTC to FTC-MP using calf thymus DCK4 while TK1 has been demonstrated to phosphorylate zidovudine and stavudine, which belong to the same drug class as TFV and FTC.5 Although zidovudine and stavudine are thymidine rather than cytidine analogs, both are dideoxynucleosides as FTC is, with all compounds lacking both 2′- and 3′-hydroxyl groups in their sugar ring.
Lamivudine is a cytidine analog structurally similar to emtricitabine, with the only difference between the two being a fluorine atom in emtricitabine at the 5 position of the cytidine base. Previous studies performed using purified CMPK1 demonstrated that this kinase can phosphorylate lamivudine monophosphate to lamivudine diphosphate.6 As such, it can be envisioned that CMPK1 could catalyze the phosphorylation of FTC-MP to FTC-DP.
Finally, we hypothesized that the pharmacologically active compound FTC-TP could be the result of phosphorylation of FTC-DP to FTC-TP by PGK1. The basis for this prediction stems from previously demonstrated phosphorylation of the diphosphorylated anabolite of the deoxynucleoside analog L-Fd4C using PGK1 purified from HepG2 cells.7 This compound is structurally similar to FTC, except for a double bond between the pentose 2′ and 3′ positions rather than a sulfur atom at the 3′ position that FTC exhibits.
The goal of this study was to determine whether a given individual could carry genetic variants of the kinases that activate both TFV and FTC, as this has yet to be investigated. To take the first step toward this, we identified the kinases that activate FTC in PBMC through the use of siRNA, thereby providing the first experimental identification of the cascade of kinases that phosphorylate FTC to FTC-MP, FTC-DP, and the pharmacologically active FTC-TP, in cells relevant to HIV infection. In applying next-generation sequencing of genomic DNA isolated from whole blood collected from HIV Prevention Trials Network study (HPTN) 069/AIDS Clinical Trials Group (ACTG) A5305 clinical study participants,8,9 we detected previously unreported variants in the kinases that activate TFV and in those that activate FTC. Through this work we found that there indeed are individuals carrying variants in both TFV and FTC activating kinases.
Materials and Methods
siRNA knockdown of kinases
PBMC were obtained from Bioreclamation (Westbury, NY) and donor information is as follows: PBMC (n = 5; 54 y.o. male, 61 y.o. male, 61 y.o. female, 54 y.o. male, and 61 y.o. male). All donors were healthy, HIV-uninfected individuals.
PBMC were transfected using siGENOME siRNA (Dharmacon, GE Healthcare Life Sciences, Lafayette, CO) targeted toward CMPK1, DCK, PGK1, and TK1. PBMC transfected with nontargeting siRNA were used as controls. For transfection of PBMC, a Neon™ Transfection System (Life Technologies, Fredrick, MD) was employed. Electroporation conditions were as follows: pulse voltage 2,100 V, pulse width 15 ms, 1 pulse, 1 × 107 cells, and 500 nM siRNA. The electroporation was performed in 0.4 cm cuvettes (Bio-Rad, Hercules, CA) using a Gene Pulser Xcell™ electroporater (Bio-Rad). A square waveform (500 V and 10 ms) and 500 nM of siRNA was used.
Immunoblotting of kinases
Following electroporation, PBMC were cultured for 48 h in DMEM supplemented with 10% FBS before homogenization. For immunoblots, 10 μg of homogenized cells and tissues were resolved by SDS-PAGE. Proteins were transferred onto a nitrocellulose membrane and blotted with antibodies for CMPK1, DCK, PGK1, and TK1 (Thermo Fisher Scientific, Waltham, MA). Anti-β-actin was used for normalization (Cell Signaling, Danvers, MA). Homogenized cells and tissues were then immunoblotted for protein expression and intracellular FTC phosphates were detected using uHPLC-UV and MALDI-MS.
Detection of FTC phosphates
To first confirm the formation of FTC phosphates in PBMC a MALDI-MS method was developed. Matrix solutions included α-cyano-4-hydroxycinnamic acid (CHCA) for the detection of FTC-MP and 9-aminoacridine (9AA) for the detection of FTC-DP and FTC-TP. CHCA matrix was 10 mg/mL solution in 50% water, 50% acetonitrile with 0.1% trifluoroacetic acid. The 9AA matrix was a 15 mg/mL solution in 75% acetone, 25% methanol with 0.1% trifluoroacetic acid.
The samples were prepared as 2 μL PBMC lysate per 8 μL of matrix solution, spotted in 2 μL aliquots onto the wells of steel 96 spot plate. Samples were detected in negative ion mode: 325.066 m/z, 405.900 m/z, and 485.939 m/z for FTC-MP, FTC-DP, and FTC-TP, respectively. Detection parameters included 90 microscans over 15 min per spot, divided over three scan events (full scan, parent ion, MS2 fragment ions). The MS2 transitions were as follows: FTC-MP 325.066 > 259.068 m/z (55 eV), FTC-DP 405.900 > 311.911 (30 eV), and FTC-TP 485.939 > 387.960 m/z (35 eV) (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/aid). The average MS2 spectra per scan event was utilized to generate relative ion abundance for comparison across samples.
For uHPLC-UV detection, 20 μL PBMC lysates were extracted with 100 μL of 70% MeOH: 30% water at room temperature for 10 min. Samples were centrifuged at 12,000 g for 10 min at 4°C. The supernatant was dried and reconstituted with 50 μL of HPLC mobile phase A. HPLC mobile phase A contained: 95% water, 5% MeOH, and 5 mM dimethylhexylamine at a pH of 7. Mobile phase B contained: 20% water, 80% MeOH, and 5 mM dimethylhexylamine. The isocratic gradient was 0.0–8.0 min, 0%–45% mobile phase B; 8.0–8.5 min 45%–100% mobile phase B; 8.5–10.0 min, 100% mobile phase B, 10.0–10.5 min, 100%–0.0% mobile phase B, 10.5–12.5 min, 0% mobile phase B. Separations were performed on a HALO C18 reverse phase column, 2.1 × 100 mm, with a 2.7 μm particle. The injection volume was 10 μL and all conditions were at room temperature.
The UV detector utilized was a multichannel diode array detector at 280 nm λ, determined to be the optimal wavelength for FTC, FTC-MP, FTC-DP, and FTC-TP. A standard mixture of FTC and each phosphorylated metabolite at 10 μM in MeOH was injected every 10 samples, with a UV peak area%RSD of 2.73, 1.01, 11.86, and 4.94 for each of FTC, FTC-MP, FTC-DP, and FTC-TP, respectively, throughout the sample sequence. The lower limit of detection, determined from 10 μL injections of 0.5, 1, 5, 10, 20, and 50 μM standard were found to be 10, 5, 10, and 50 fmol for FTC, FTC-MP, FTC-DP, and FTC-TP, respectively. The resulting interpolated concentrations of analytes detected in PBMC samples were normalized to protein concentration.
Clinical study sites and sample collection
Whole blood was obtained from HIV-uninfected individuals (n = 498) enrolled in the HPTN 069/ACTG A5305 clinical study across 13 clinical research sites (CRS) located within the United States: Bridge HIV, San Francisco, CA; Case Western, Cleveland, OH; UNC Chapel Hill, Chapel Hill, NC; Fenway Health CRS, Boston, MA; George Washington University CRS, Washington, DC; Hospital of University of Pennsylvania, Philadelphia, PA; Johns Hopkins University CRS, Baltimore, MD; New Jersey Medical School CRS, Newark, NJ; Pitt CRS, Pittsburgh, PA; Puerto Rico AIDS CRS, San Juan, PR; UCLA Care Center CRS, Los Angeles, CA; University of Washington AIDS CRS, Seattle, WA and Weill Cornell CRS, New York, NY.
The study protocol was reviewed and approved by the local site institutional review boards. Participants contributing samples to this substudy all provided written informed consent including consent to genetic testing. These participants were cisgender men and women and transgender women who have sex with men in locations across the United States.
Genomic DNA isolation from HPTN 069/ACTG A5305 whole blood samples
Genomic DNA was isolated from 200 μL of whole blood from each individual using a QIAamp 96 DNA Blood Kit (QIAGEN, Valencia, CA). Genomic DNA was extracted following protocol “Purification of DNA from Whole Blood, Plasma, Serum, or Body Fluids–(EN)” from QIAGEN. Purified DNA was eluted using 150 μL DEPC-treated, nuclease-free water (Quality Biological, Inc., Gaithersburg, MD) and concentrated using a ZR-96 DNA Clean-up KitTM (Zymo Research, Irvine, CA). Resulting concentrated and purified genomic DNA was eluted in 12 μL DEPC-treated, nuclease-free water.
Next-generation sequencing target design, sample preparation, and analysis
Sequencing of the kinases that have been shown to phosphorylate TFV to TFV-DP, AK2, CKM, PKM, and PKLR, in addition to the kinases proposed to phosphorylate FTC to FTC-TP, CMPK1, DCK, PGK1, and TK1, was performed using the Illumina TruSeq Custom Amplicon kit v1.5 (Illumina, San Diego, CA). The same genomic DNA was used to sequence for all kinases. Probes for sequencing the combination of these seven kinases were designed using Illumina DesignStudio software as previously described.3
Genomic DNA isolated from clinical samples was processed following the Illumina TruSeq Custom Amplicon Library Preparation Guide (Part Number 15027983 Rev. C, August 2013). DNA concentration was measured using a Qubit® 3.0 Fluorimeter (Thermo Scientific, New York, NY). A plasmid containing a known sequence for AK2 was used as an additional sequencing control. Fifty ng of DNA input were used per DNA sample sequenced. The resulting prepared DNA library (6 μL) was diluted in 594 μL HT1 buffer containing 1% PhiX sequencing control.
Illumina VariantStudio software was used to annotate and analyze variant read quality as previously described.3 Identified genetic variants have been deposited into dbSNP under the submitter handle “BUMPUSLAB.” Predictions regarding the phenotypic consequence of missense variants were determined using SIFT (sorts intolerant from tolerant substitutions; J. Craig Venter Institute online tool) and PolyPhen (polymorphism phenotyping; Harvard University online tool) in silico prediction tools. The functional consequences of resulting amino acid substitutions were predicted using SIFT and PolyPhen in silico tools. A SIFT score <0.05 resulted in a prediction that an amino acid substitution was possibly deleterious and >0.05 a tolerated substitution. A PolyPhen score >0.908 was suggestive of a probably damaging, 0.447–0.908 a possibly damaging, or <0.447 a benign amino acid substitution.10,11
Statistical analysis
Statistical analyses were performed using GraphPad Prism (San Diego, CA). Two-tailed unpaired t tests were performed and significance was denoted as follows: *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.
Results
Kinase Activation of FTC in PBMC
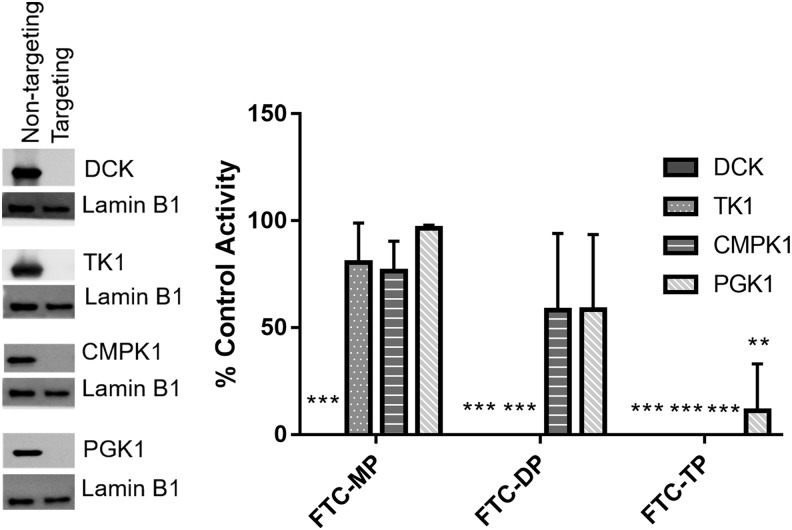
To identify the kinases that activate FTC in PBMC we first immunoblotted for kinases DCK, TK1, CMPK1, and PGK1 in PBMC protein lysate. As shown in Figure 1, each these of kinases were readily detectable in PBMC; thus, to test whether these kinases exhibit activity toward FTC in PBMC we knocked down the expression of each kinase individually using siRNA (Fig. 1). The nontargeting siRNA lane in the representative immunoblot is commensurate with basal expression of each. Following incubation of these PBMC with FTC, the formation of FTC-MP, FTC-DP, and FTC-TP was detected using MALDI-MS to confirm the identities of these analytes (Supplementary Fig. S1). Quantitation of analyte formation was then performed using uHPLC-UV since linearity of detection was not achievable using MALDI-MS.
FIG. 1.
Targeted siRNA knockdown of kinases in PBMC resulting impact on FTC-MP, FTC-DP and FTC-TP intracellular formation. PBMC were electroporated with 500 nM nontargeting siRNA or siRNA targeting DCK, TK1, CMPK1, or PGK1 and incubated for 48 h. Representative immunoblots demonstrate decreased kinase expression with each targeted siRNA treatment relative to the nontargeting siRNA control. siRNA treated PBMC were incubated with 10 μM FTC for 12 h (n = 5). Intracellular anabolites were extracted and FTC-MP, FTC-DP, and FTC-TP were measured using uHPLC-UV as depicted in the corresponding bar graphs showing mean ± standard deviation for each treatment. Statistical analyses were performed using a two-tailed unpaired t test to compare relative levels of anabolite production between nontargeted and targeted siRNA conditions; **p ≤ 0.01; ***p ≤ 0.001. FTC, fumarate and emtricitabine; PBMC, peripheral blood mononuclear cell.
Knockdown of DCK expression decreased the formation of FTC-MP, FTC-DP, and FTC-TP while knockdown of TK1 decreased the abundance of FTC-DP and FTC-TP (Fig. 1). Delivery of siRNA targeted toward either CMPK1 or PGK1 to PBMC resulted in lower level of production of FTC-TP only compared with the control PBMC (Fig. 1).
Sequencing of participants for genetic variants in FTC-activating kinases DCK, CMPK1, TK1, and PGK1
Of the 498 individuals genotyped, 34 exhibited variants in CMPK1, 12 exhibited variants in DCK, and 2 individuals displayed variants for the PGK1 gene. The 34 individuals with detected variants in CMPK1 indicate a 6.8% (34/498) frequency of individuals carrying variants in this population. For the DCK gene, 12 individuals carrying variants yielded a 2.4% (12/498) frequency. In PGK1, two of 498 individuals exhibited variants in this gene (0.4% frequency, 2/498 individuals). The kinase TK1 did not have any individuals with variants detected to affect the amino acid level of this protein. Distribution of variants among individuals is depicted in a Venn diagram in Figure 2.
FIG. 2.
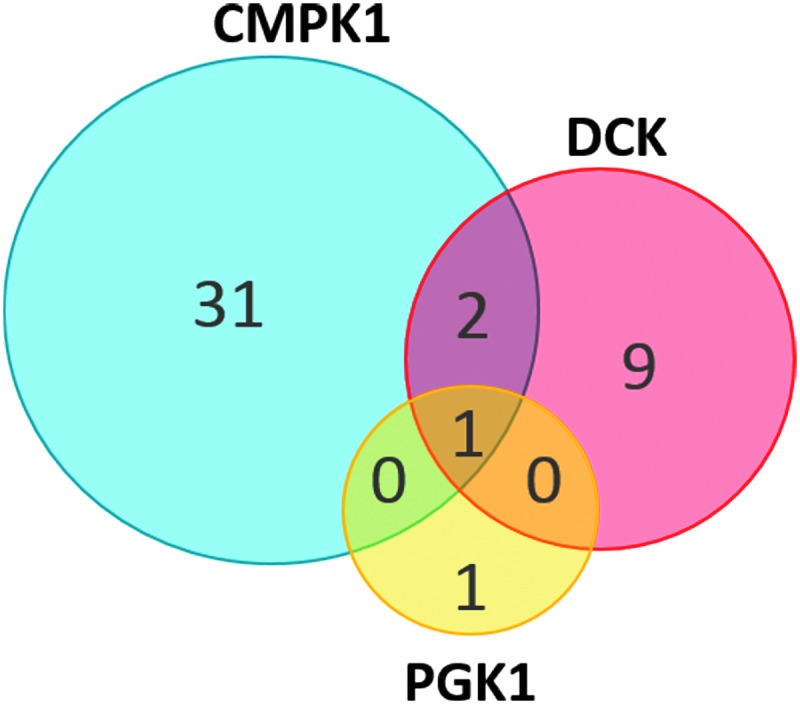
Distribution of genetic variants in FTC-activating kinases. Each circle represents a nucleotide kinase that was sequenced in each of the 498 participants, with CMPK1 in blue, DCK in red, and PGK1 in yellow. Numerical values indicate the number of individuals detected to carry a single nucleotide variation or deletion. Overlapping regions of each circle indicate the number of individuals with genetic variants in more than one kinase.
Of note, within this population, three clinical trial participants exhibited variants in more than one kinase. CMPK1 and DCK variants were detected in two individuals, while one individual exhibited variants in all three kinases with detected variants at the amino acid level.
Identification of DCK, CMPK1, TK1, and PGK1 genetic variants
In sequencing the participants for DCK (NM_000788.2), we found that 12 individuals were detected to carry six unique genetic variants predicted to result in an amino acid substitution (Table 1 and Supplementary Table S1). All individuals carrying variants exhibited heterozygosity for these single nucleotide variants. Of these six genetic variants, two were previously unreported, with one variant with the amino acid substitution S93F predicted to be deleterious and the other variant resulting in a stop codon. The three previously reported variants are accessible in the SNP database and are listed as follows: rs67437265 (NM_000788.2:c.364C>T) was detected in six individuals, rs66878317 (NM_000788.2:c.70A>G) was detected in one individual, and the SNP rs144479260 (NM_000788.2:c.261G>A) was detected in one individual. Across all individuals carrying variants for DCK, only one individual presented a variant predicted to be deleterious, resulting in a 0.2% (1/498) frequency.
Table 1.
Missense Variants Detected Across Study Participants in TFV- and FTC-Activating Kinases
| Racial and ethnic self-identification | Participant sex at birth | Gene | Protein position | Amino acid substitution (ref.>alt.) | SIFT prediction | PolyPhen prediction | Frequency |
|---|---|---|---|---|---|---|---|
| Asian | Male | DCK | 93 | S > F | Deleterious (0) | Probably_damaging (0.969) | 0.2% (1/498) |
| Black or African | Female | DCK | 151 | E>* | N/A | N/A | 0.6% (3/498) |
| Black or African | Female | DCK | 87 | E > D | Tolerated (0.61) | Benign (0) | 0.2% (1/498) |
| Multiracial | Male | DCK | 24 | I > V | Tolerated (0.65) | Benign (0.002) | 0.2% (1/498) |
| White | Male | DCK | 122 | P > S | Tolerated (0.06) | Probably_damaging (1) | 0.8% (4/498) |
| Other | Male | DCK | 122 | P > S | Tolerated (0.06) | Probably_damaging (1) | 0.2% (1/498) |
| Multiracial | Male | DCK | 122 | P > S | Tolerated (0.06) | Probably_damaging (1) | 0.2% (1/498) |
| Multiracial | Male | CMPK1 | 166 | R > S | Deleterious (0) | Probably_damaging (0.999) | 0.2% (1/498) |
| White | Male | CMPK1 | 80 | Q > H | Deleterious (0) | Benign (0.31) | 0.2% (1/498) |
| Other | Male | CMPK1 | 80 | Q > H | Deleterious (0) | Benign (0.31) | 0.2% (1/498) |
| Black or African | Female | CMPK1 | 80 | Q > H | Deleterious (0) | Benign (0.31) | 0.2% (1/498) |
| White | Male | CMPK1 | 183 | R > I | Deleterious (0) | Probably_damaging (0.998) | 0.2% (1/498) |
| Black or African | Male | CMPK1 | 74 | R > S | Deleterious (0.03) | Benign (0.374) | 0.2% (1/498) |
| Multiracial | Male | CMPK1 | 80 | Q > H | Deleterious (0) | Benign (0.31) | 0.2% (1/498) |
| White | Male | CMPK1 | 80 | Q > H | Deleterious (0) | Benign (0.31) | 0.6% (3/498) |
| White | Male | CMPK1 | 74 | R > S | Deleterious (0.03) | Benign (0.374) | 0.2% (1/498) |
| Other | Male | CMPK1 | 80 | Q > H | Deleterious (0) | Benign (0.31) | 0.4% (2/498) |
| Black or African | Male | CMPK1 | 80 | Q > H | Deleterious (0) | Benign (0.31) | 0.4% (2/498) |
| Multiracial | Male | CMPK1 | 107 | E > K | Deleterious (0.01) | Benign (0.023) | 0.2% (1/498) |
| Multiracial | Male | CMPK1 | 115 | N > S | Tolerated (0.84) | Benign (0.001) | 0.4% (2/498) |
| Black or African | Male | CMPK1 | 115 | N > S | Tolerated (0.84) | Benign (0.001) | 0.2% (1/498) |
| White | Male | CMPK1 | 115 | N > S | Tolerated (0.84) | Benign (0.001) | 0.2% (1/498) |
| White | Male | CMPK1 | 66 | A > S | Deleterious (0) | Possibly_damaging (0.901) | 1.0% (5/498) |
| Black or African | Male | CMPK1 | 66 | A > S | Deleterious (0) | Possibly_damaging (0.901) | 0.6% (3/498) |
| White | Female | CMPK1 | 66 | A > S | Deleterious (0) | Possibly_damaging (0.901) | 0.2% (1/498) |
| Black or African | Female | CMPK1 | 66 | A > S | Deleterious (0) | Possibly_damaging (0.901) | 0.2% (1/498) |
| Black or African | Female | CMPK1 | 74 | R > S | Deleterious (0.03) | Benign (0.374) | 0.4% (2/498) |
| White | Female | CMPK1 | 74 | R > S | Deleterious (0.03) | Benign (0.374) | 0.2% (1/498) |
| White | Male | PGK1 | 145 | A > S | Tolerated (0.16) | Benign (0.023) | 0.2% (1/498) |
| White | Female | PGK1 | 145 | A > S | Tolerated (0.16) | Benign (0.023) | 0.2% (1/498) |
| Multiracial | Male | PGK1 | 61 | M > I | Tolerated (0.1) | Benign (0.062) | 0.2% (1/498) |
| White | Male | AK2 | 157 | N > S | Tolerated (0.15) | Benign (0.001) | 0.2% (1/498) |
| White | Male | AK2 | 17 | R > G | Deleterious (0) | Possibly_damaging (0.514) | 0.2% (1/498) |
| White | Male | AK2 | 209 | A > T | Tolerated (0.5) | Benign (0.056) | 0.4% (2/498) |
| Native Hawaiian or Pacific Islander | Male | AK2 | 129 | S > N | Tolerated (0.49) | Benign (0.001) | 0.2% (1/498) |
| White | Male | AK2 | 129 | S > N | Tolerated (0.49) | Benign (0.001) | 0.2% (1/498) |
| White | Male | CKM | 35 | T > I | Deleterious (0) | Probably_damaging (0.914) | 0.2% (1/498) |
| Multiracial | Male | CKM | 251 | R > P | Tolerated (0.09) | Benign (0.129) | 0.2% (1/498) |
| White | Male | CKM | 83 | E > G | Deleterious (0.01) | Possibly_damaging (0.467) | 0.4% (2/498) |
| Black or African | Male | CKM | 83 | E > G | Deleterious (0.01) | Possibly_damaging (0.467) | 0.2% (1/498) |
| Multiracial | Male | CKM | 43 | R > Q | Tolerated (0.07) | Benign (0.288) | 0.2% (1/498) |
| White | Female | CKM | 4 | G > C | Tolerated (0.1) | Probably_damaging (0.998) | 0.2% (1/498) |
| Black or African | Female | CKM | 52 | T > N | Deleterious (0.04) | Probably_damaging (0.972) | 0.2% (1/498) |
| Multiracial | Male | PKLR | 272 | L > V | Deleterious (0) | Probably_damaging (0.974) | 0.2% (1/498) |
| White | Male | PKLR | 569 | R > Q | Deleterious (0.04) | Benign (0.399) | 0.2% (1/498) |
| Black or African | Male | PKLR | 569 | R > Q | Deleterious (0.04) | Benign (0.399) | 0.2% (1/498) |
| Black or African | Male | PKLR | 272 | L > V | Deleterious (0) | Probably_damaging (0.974) | 0.2% (1/498) |
| White | Male | PKLR | 486 | R > W | Deleterious (0) | Probably_damaging (0.996) | 0.2% (1/498) |
| Black or African | Male | PKLR | 406 | G > W | Deleterious (0) | Probably_damaging (1) | 0.2% (1/498) |
| White | Male | PKLR | 133 | N > K | Deleterious (0.03) | Probably_damaging (0.999) | 0.2% (1/498) |
| White | Male | PKM | 178 | L > I | N/A | N/A | 0.4% (2/498) |
| White | Male | PKM | 5 | S>* | N/A | N/A | 0.6% (3/498) |
| White | Male | PKM | 36 | T > S | N/A | N/A | 0.2% (1/498) |
| White | Male | PKM | 132 | V > A | N/A | N/A | 0.2% (1/498) |
| White | Male | PKM | 36 | T > S | N/A | N/A | 0.2% (1/498) |
| White | Female | PKM | 229 | N > S | N/A | N/A | 0.2% (1/498) |
The demographic information for the participants carrying each genetic variant detected are shown for all of the individual kinase-encoding genes that were investigated. The frequency reflects the number of individuals sharing the same racial/ethnic self-identification and sex assigned at birth who were found to carry the indicated variant. The functional consequence of resulting amino acid exchanges were predicted using SIFT and PolyPhen in silico tools. A SIFT score <0.05 was suggestive of a damaging amino acid substitution and >0.05 a tolerated substitution. A PolyPhen score >0.908 was suggestive of a probably damaging, 0.447–0.908 a possibly damaging, or <0.447 a benign amino acid substitution. N/A indicates that in silico tools were not able to predict a functional impact of the amino acid substitution.
Using the reference sequence NM_016308.2 for CMPK1, seven variants across 34 individuals were detected (Table 1 and Supplementary Table S1). All individuals were heterozygous for the variants they exhibited. Of these seven variants, three were previously unreported.
An unreported variant translating to an arginine to serine exchange at amino acid 74 of the protein was predicted to be deleterious, with one individual exhibiting this variant. A second unreported variant was also predicted to be deleterious, with an amino acid substitution from arginine to isoleucine at residue 183. One individual in this study was observed to carry this variant. The third unreported CMPK1 missense variant was detected in five individuals and is predicted to affect amino acid 192 of the protein, substituting an asparagine residue for a lysine residue. This variant was predicted to be tolerated.
The remaining four variants detected in individuals for this kinase were listed in the SNP database. The variant rs80046781 (NM_016308.2:c.196G>T) was detected in nine individuals and is predicted to be deleterious, yielding a 1.8% (9/498) frequency of this variant. A second previously reported variant, rs35687416 (NM_016308.2:c.240G>T) was also predicted to be deleterious, with 11 individuals exhibiting this variant (2.2% frequency, 11/498). The variant rs139782187 (NM_016308.2:c.498G>T) was detected in one individual and was predicted to be deleterious. Finally, the fourth variant rs72553947 (NM_016308.2:c.344A>G) was predicted to be tolerated and was found in four individuals. Overall, the frequency of individuals exhibiting deleterious variants for CMPK1 was 5.0% (25/498).
Interestingly, for TK1 (NM_003258.4), no exonic, or missense variants were detected in any of the participants. However, a previously reported intron variant rs2661681 (NM_003258.4:c.394-4G>A) was detected in five individuals, yielding a 1% (5/498) frequency for the only variant detected for this kinase in this study. Because this variant is in the intron, there was no functional impact prediction available. In addition, for PGK1 we detected one previously unreported variant and one previously reported variant in PGK1 using reference sequence NM_000291.3 (Table 1 and Supplementary Table S1). Neither variant was predicted to be deleterious.
Sequencing of participants for genetic variants in TFV-activating kinases AK2, CKM, PKM, and PKLR
To determine whether any individuals carrying genetic variants of the kinases that activate FTC also carried variants of TFV-activating kinases, a targeted assay to sequence the exonic regions of the genes AK2, CKM, PKM, and PKLR was applied.
Of the individuals genotyped, nine were observed to carry a single nucleotide variant in the AK2 gene, yielding a 1.8% frequency (9/498 individuals) of AK2 variants within this population. One individual carrying one variant that was predicted to be deleterious (0.2%, 1/498 individuals) was detected among the nine individuals with observed AK2 variants.
In the CKM gene, eight individuals were found to carry variants, showing 1.6% (8/498 individuals) of individuals carrying variants in this kinase. Of these individuals, five were predicted to carry deleterious variants, yielding a frequency of 1% (5/498 individuals).
The PKM gene sequenced across all individuals detected 11 participants to have variants (2.2% frequency, 11/498 individuals).
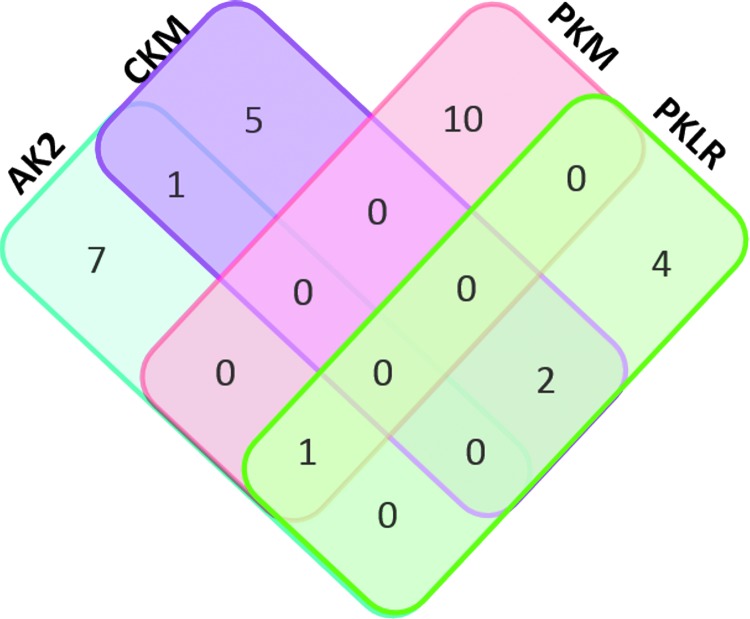
The in silico tools SIFT and Polyphen were not able to predict the functional impact of PKM variants due to a lack of sequence diversity that is required to facilitate such predictions. Sequencing the kinase PKLR yielded 11 individuals predicted to carry genetic variants resulting in amino acid sequence alterations. All 11 individuals were observed to carry variants that were predicted to have a deleterious functional impact. These 11 individuals make up the observed frequency of 2.2% (11/498 individuals). Variant distributions of the TFV-activating kinases AK2, CKM, PKM, and PKLR in individuals are demonstrated using a Venn diagram in Figure 3.
FIG. 3.
Distribution of genetic variants in TFV-activating kinases. Each rectangle represents a nucleotide kinase that was sequenced in each of the 498 participants, with AK2 in blue, CKM in pink, PKM in orange, and PKLR depicted in green. Numerical values indicate the number of individuals detected to carry a single nucleotide variation or deletion. Overlapping regions of each rectangle indicate the number of individuals with genetic variants in more than one kinase. TFV, tenofovir.
Analysis of AK2, CKM, PKLR, and PKM genetic variants
For the DNA reference sequence corresponding to AK2 (NM_001625.3), we observe two previously unreported variants and three previously reported variants. These five variants were detected across nine individuals (1.8% frequency, 9/498 individuals). Variants observed are shown in Table 1 and detailed in Supplementary Table S1. All individuals with detected variants were observed to be heterozygous for these variants. Both unreported variants were predicted to be tolerated, with one variant resulting in a N157S exchange and the other resulting in a P131T amino acid substitution.
Three variants previously listed in the single nucleotide polymorphism database (dbSNP) were detected and are listed as follows: rs138577419 (NM_001625.3:c.49C>G) was detected in one individual, rs12116440 (NM_001625.3:c.625G>A) was detected in two individuals, with the variant rs61750965 (NM_001625.3:c.386G>A) also observed in two individuals. Of the individuals exhibiting genetic variants for AK2, only one individual was observed to carry a predicted deleterious variant, resulting in a 0.2% frequency.
Comparison to the DNA reference sequence for CKM (NM_001824.4), through sequencing the exon region of this gene, yielded four previously unreported variants and two variants found in the SNP database. These six variants were detected across eight individuals, presenting a 1.6% (8/498) frequency of individuals carrying variants (Table 1 and Supplementary Table S1). All individuals with CKM genetic variants were observed to be heterozygous for those variants.
The four previously unreported variants resulted in a predicted deleterious T35I amino acid substitution, a predicted deleterious T53 N exchange, and two predicted tolerated amino acid substitutions at R43Q and G4C. Of the six variants detected for CKM in this population, two were found in dbSNP. The variant rs149354459 (NM_001824.4:c.752G>C) was observed in one individual, while the variant rs11559024 (NM_001824.4:c.248A>G) was observed in two individuals. Across all variants detected in the CKM gene sequenced, five individuals exhibited variants that were predicted to be deleterious (1% frequency, 5/498).
For PKM (reference sequence NM_001206796), 11 individuals were found to carry variants at the amino acid level (2.2% frequency, 11/498). Across these 11 individuals, five total variants were observed, with three previously unreported variants and two variants found in the SNP database. These variants are presented in Table 1 and detailed in Supplementary Table S1.
Variants detected in PKM did not have functional predictions at the protein level because the in silico prediction tools PolyPhen and SIFT did not have sufficient sequence diversity information in the multiple alignments used to predict variant functional impact. The two previously reported genetic variants for PKM were rs180716407 (NM_001206796.2:c.14C>G) and rs201533100 (NM_001206796.2:c.395T>C). The variant rs180716407 was detected in five individuals while the variant rs201533100 was detected in one individual. Interestingly, study participants exhibiting variants in the kinase PKM all self-identified as White and all except for one female were assigned the sex male at birth.
In sequencing PKLR (reference sequence NM_000298.5), five variants comprised of single nucleotide variants and deletions that are predicted to be reflected in the amino acid sequence were detected across seven individuals (Table 1 and Supplementary Table S1). Two previously unreported variants were predicted to have a deleterious impact on protein function. The amino acid substitution of tryptophan for glycine at the protein position 406 is predicted to be deleterious as is the previously unreported N133K exchange.
Of the five variants detected in this population, three had been previously reported and are in the dbSNP. The variant rs147659527 (NM_000298.5:c.814C>G) was detected in two individuals as was the variant rs61755431 (NM_000298.5:c.1706G>A). The variant rs116100695 (NM_000298.5:c.1456C>T) was detected in one individual. Of all the individuals carrying variants in the PKLR gene, all seven individuals are predicted to be carrying deleterious variants. This results in a 1.4% frequency (7/498) of individuals exhibiting deleterious variants.
Missense variants in both TFV- and FTC- activating kinases are detectable in the same participant and demographics of subjects carrying variants
Having identified variants in all of the kinases that we have found to activate TFV and FTC, we analyzed our data to determine whether there were any individuals in our study that simultaneously carried missense variants in both TFV- and FTC-activating kinases. Four study participants, all male at birth, were found to have variants in kinases that activated both TFV and FTC (Table 2). Of these, one participant carried variants that were predicted to result in a decrease or loss of function in kinase activity toward both TFV and FTC. The variants detected for this individual were in the genes encoding PKLR and CMPK1.
Table 2.
Study Participants Exhibiting Missense Variants in Both TFV and FTC Activating Kinases
| Racial and ethnic self-identification | Sex assigned at birth | Drug activated | Kinase | Protein position | Amino acid substitution (ref.>alt.) | SIFT prediction | PolyPhen prediction |
|---|---|---|---|---|---|---|---|
| White | Male | TFV | AK2 | 157 | N > S | Tolerated (0.15) | Benign (0.001) |
| FTC | CMPK1 | 66 | A > S | Deleterious (0) | Possibly_damaging (0.901) | ||
| Black or African | Male | TFV | PKLR | 406 | G > W | Deleterious (0) | Probably_damaging (1) |
| FTC | CMPK1 | 74 | R > S | Deleterious (0.03) | Probably_damaging (0.999) | ||
| FTC | CMPK1 | 66 | A > S | Deleterious (0) | Possibly_damaging (0.901) | ||
| Multiracial | Male | TFV | PKLR | 272 | L > V | Deleterious (0) | Probably_damaging (0.974) |
| FTC | DCK | 24 | I > V | Tolerated (0.65) | Benign (0.002) | ||
| FTC | DCK | 122 | P > S | Tolerated (0.06) | Probably_damaging (1) | ||
| FTC | CMPK1 | 166 | R > S | Deleterious (0) | Probably_damaging (0.999) | ||
| FTC | PGK1 | 61 | M > I | Tolerated (0.1) | Benign (0.062) | ||
| White | Male | TFV | AK2 | 209 | A > T | Tolerated (0.5) | Benign (0.056) |
| TFV | PKM | 36 | T > S | N/A | N/A | ||
| TFV | PKLR | 133 | N > K | Deleterious (0.03) | Probably_damaging (0.999) | ||
| FTC | CMPK1 | 115 | N > S | Tolerated (0.84) | Benign (0.001) |
Four study participants were found to simultaneously carry variants in the kinases that activate TFV and FTC. The functional consequence of resulting amino acid exchanges were predicted using SIFT and PolyPhen in silico tools. A SIFT score <0.05 was suggestive of a damaging amino acid substitution and >0.05 a tolerated substitution. A PolyPhen score >0.908 was suggestive of a probably damaging, 0.447–0.908 a possibly damaging, or <0.447 a benign amino acid substitution.
TFV, tenofovir; FTC, fumarate and emtricitabine.
Discussion
This study identifies the kinases that activate FTC as DCK (phosphorylation of FTC to FTC-MP), TK1 (phosphorylation of FTC-MP to FTC-DP), and both CMPK1 and PGK1 (phosphorylation of FTC-DP to FTC-TP, the pharmacologically active anabolite of FTC). In doing so, we performed direct measurement of FTC anabolite levels in PBMC that were treated with either nontargeting siRNA or siRNA targeted specifically to the abovementioned kinases individually, using mass spectrometry.
Phosphorylation of FTC to FTC-MP by DCK was reflected in the lack of FTC anabolites resulting from siRNA knockdown of DCK. This indicates the role DCK plays in phosphorylating FTC and could also serve as a model of FTC activation given impaired DCK function resulting from deleterious variants. Similarly, knockdown of TK1 results in a lack of phosphorylation of FTC-MP to FTC-DP, while knockdown of either CMPK1 or PGK1 abrogates or markedly decreases, respectively, phosphorylation of FTC-DP to FTC-TP, the pharmacologically active anabolite of FTC.
Taken together, this information demonstrates a direct measurement of drug levels indicative of kinase activity. Further, these knockdowns could simulate conditions in which deleterious variants in the kinases DCK, TK1, CMPK1, and PGK1 affect FTC activation. In addition, as noted, the PBMC donors in this study were all over the age of 50; however, in validating the use of these cells we did not find a difference in kinase expression or activity in the PBMC from these donors versus younger donors used in our previous study3 (data not shown).
Further, four out of the five donors in this study were male. While it would be interesting to perform future studies to test mechanistically for potential sexual dimorphisms in FTC phosphorylation, sex differences in the activity of these kinases has not been reported in the literature nor was it observed by our laboratory.
We detected six previously unreported variants across these FTC-phosphorylating kinases. Building further upon a previous study from prior work in our laboratory,3 more unreported variants in kinases identified to phosphorylate TFV were also detected in this work. In the genes that encode the TFV-activating kinases AK2, CKM, PKM, and PKLR, we detected 11 previously unreported variants across these kinases.
Ultimately, we sought to determine whether there are individuals who simultaneously carry variants in the genes that encode the kinases that activate both TFV and FTC, which is of interest since these drugs are currently co-prescribed for both HIV treatment and prevention. The analysis implemented in this study conformed to the American College of Medical Genetics and Genomics clinical laboratory standards for next-generation sequencing to increase confidence in the variants we detected.12 Indeed, we were able to identify four such individuals, all of which were male, in this study (Table 2).
Out of four individuals exhibiting missense variants in both TFV- and FTC-activating kinases, only one individual was detected to carry variants that were deleterious for both FTC and TFV activation by the identified kinases. This individual, with a deleterious variant for the gene PKLR, could be impaired in activating TFV-MP to the pharmacologically active TFV-DP. Regarding FTC activation, this same individual exhibits two different deleterious variants in the CMPK1 gene, possibly hindering formation of FTC-DP from FTC-MP as shown in previous studies with lamivudine monophosphate.6 The decreased amount of FTC-DP would lead to a decreased amount of FTC-TP, potentially lowering protection against HIV infection since FTC-TP is the active anabolite.
In DCK, a gene encoding the enzyme that our data suggest can phosphorylate FTC to FTC-MP, three variants that we detected here have been previously reported in the SNP database while one was a previously unreported variant that was predicted to be deleterious. Translation of this latter gene variant results in an amino acid exchange at residue 93 of the protein, reported to be located in an alpha helix and four amino acids away from the substrate binding site at the 97th amino acid.13 The substitution from serine to phenylalanine reported at this position (S93F), changes the residue from a polar, uncharged, and relatively small side chain on the serine to a large, hydrophobic benzene ring on the resulting phenylalanine. This substitution coupled with the proximity to the reported substrate binding site could impact kinase function.
Four of the variants found in the gene encoding CMPK1 were previously reported in the SNP database. Interestingly, while the variant rs80046781 was detected in 9 individuals in this study, no reported frequency was available in the Exome Aggregation Consortium (ExAC) database, a large-scale aggregation of human genomic data including over 60,000 individuals.14 Predicted to result in a deleterious functional impact for this kinase, the genetic variant rs80046781 results in an amino acid A66S substitution. This position is three amino acids away from the nucleotide monophosphate binding domain15 and the amino acid substitution results in a change from a hydrophobic side chain in alanine to a polar side chain in serine.
A second previously reported variant rs35687416 was also predicted to be deleterious, with 11 individuals exhibiting this variant (2.2% frequency, 11/498). Comparing this frequency to the frequency of this variant as reported in the ExAC database, the two subpopulations with the highest frequency for this variant, were East Asian, with 12% frequency and Finnish European, with 6.7% frequency.
The variant rs72553947 was detected in four individuals, resulting in a 0.8% frequency (4/498). This frequency is close to the overall frequency reported in the ExAC database, which reported a frequency across all populations at 0.6%. The fourth previously reported variant for CMPK1 was detected in one individual in our study and was reported in the ExAC database at a frequency of less than 0.001%. Only one subpopulation included in the database reported frequencies for this variant, with the African subpopulation presenting a 0.001% frequency for this reported variant. Interestingly, TK1, which we also found plays a role in the phosphorylation of FTC-MP to FTC-DP did not exhibit any exon variants in our study. As such, larger sample size is needed to determine the potential frequency of TK1 variants.
A kinase found to phosphorylate FTC-DP to FTC-TP, PGK1, exhibited one genetic variant that is denoted as rs11541568 in the SNP database. In our study, this variant was detected in one individual was not predicted to be deleterious, likely because residue 61 is not near either the catalytic or nucleotide binding site.16 In contrast, AK2, the kinase we have previously demonstrated to phosphorylate TFV to TFV-MP, displayed three previously reported genetic variants upon sequencing of this gene.
The variant rs138577419 was detected in one individual in this study, yielding a frequency of 0.2% (1/498). ExAC reports a frequency of 0.2% throughout all the populations included in their aggregation. Four of the seven subpopulations reported frequencies, with the non-Finnish European reporting 0.4%, African reporting 0.1%, Latino reporting 0.02%, and the subpopulation Other reporting 0.5% frequency of this variant.
A second previously reported variant, rs12116440, was detected in two individuals in this study, exhibiting a 0.4% frequency. This variant is reported in the ExAC database, to have a frequency of 0.7% and to be present in all subpopulations except for the East Asian subpopulation, which showed no frequency for this variant. This variant was also detected in one individual in a previous genotyping study carried out by our laboratory.3
The third variant detected for AK2 that has been previously reported, rs61750965 also displayed a 0.4% (2/498) frequency in this study. A previous study sequencing this kinase carried out by our laboratory also detected this variant in one individual.3 This variant is reported at an overall frequency of 0.4% in the ExAC database, with all subpopulations reporting frequencies for this variant. Of the subpopulation frequencies reported for this variant, the highest reported is in the South Asian population, with 0.7% and the lowest reported is in the East Asian population, with a 0.01% frequency.
Due to the low frequency of the genetic variants detected within the HPTN 069/ACTG A5305 participants, we were unable to make strong correlations between study drug level data and our genotyping data, although an attempt was made to do so resulting in a nonstatistically significant trend toward decreased phosphate levels in individuals carrying variants predicted to be deleterious (data not shown). Additionally, adherence is a confounding issue for HIV PrEP studies making it particularly difficult to definitively determine the impact of genotype on drug phosphorylation since parent drug levels can be highly variable between subjects. To perform a powerful analysis, a pharmacokinetic study design using directly observed therapy is required in which healthy volunteers would be enrolled based on genotype in the same manner used for our recent study of the anti-HIV drug maraviroc.17 Further, to validate the biochemical activity of these variants, an important future study would be to express and purify each individual variant protein and test it directly for activity toward TFV or FTC.
In conclusion, the present study demonstrates that FTC, in addition to TFV may be sensitive to genetic variability in the activities of their respective activating kinases. Identification of the kinases responsible for FTC activation in PBMC enabled us to perform targeted sequencing of the genes encoding these kinases using clinical samples from the HIV Prevention Trials Network 069/AIDS Clinical Trials Group A5305 study. We detected genetic variants in these kinases predicted to have deleterious and damaging phenotypes, suggesting that there could be a genetic basis for inter-individual variation in levels of FTC and phosphorylated FTC anabolites. Of note, individuals were identified who concurrently carry genetic variants in the kinases that activate TFV and also in those that activate FTC. These data add another element that could contribute to the variation in efficacy of TFV and FTC when used as PrEP, despite adherence.
Supplementary Material
Acknowledgments
The authors thank the HPTN 069/ACTG A5305 study team, study participants, all study site personnel, and laboratory staff. This work was supported by the NIH grants UM1 AI068613 (HIV Prevention Trials Network), UM1 AI068636 (AIDS Clinical Trials Group), U19 AI113127, and R01 AI128781. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. Dr. Wilkin has received grant support (paid to Weill Cornell Medicine) from Bristol Myers Squibb, Gilead Sciences, and GlaxoSmithKline/ViiV Healthcare. Dr. Wilkin has served as an ad hoc consult for GlaxoSmithKline/ViiV Healthcare. Dr. Landovitz has received personal fees and nonfinancial support from Gilead Sciences outside the submitted work. Dr. Hendrix has received grant support from ViiV Healthcare/GlaxoSmithKline outside the submitted work.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Fonner VA, Dalglish SL, Kennedy CE, et al. : Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. Aids 2016;30:1973–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mugwanya KK, Baeten JM: Safety of oral tenofovir disoproxil fumarate-based pre-exposure prophylaxis for HIV prevention. Expert Opin Drug Saf 2016;15:265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lade JM, To EE, Hendrix CW, Bumpus NN: Discovery of genetic variants of the kinases that activate tenofovir in a compartment-specific manner. EBioMedicine 2015;2:1145–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furman PA, Davis M, Liotta DC, et al. : The anti-hepatitis B virus activities, cytotoxicities, and anabolic profiles of the (-) and (+) enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Antimicrob Agents Chemother 1992;36:2686–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balzarini J, Herdewijn P, De Clercq E: Differential patterns of intracellular metabolism of 2',3'-didehydro-2',3'-dideoxythymidine and 3'-azido-2',3'-dideoxythymidine, two potent anti-human immunodeficiency virus compounds. J Biol Chem 1989;264:6127–6133 [PubMed] [Google Scholar]
- 6.Pasti C, Gallois-Montbrun S, Munier-Lehmann H, Veron M, Gilles AM, Deville-Bonne D: Reaction of human UMP-CMP kinase with natural and analog substrates. Eur J Biochem 2003;270:1784–1790 [DOI] [PubMed] [Google Scholar]
- 7.Krishnan P, Fu Q, Lam W, Liou JY, Dutschman G, Cheng YC: Phosphorylation of pyrimidine deoxynucleoside analog diphosphates: Selective phosphorylation of L-nucleoside analog diphosphates by 3-phosphoglycerate kinase. J Biol Chem 2002;277:5453–5459 [DOI] [PubMed] [Google Scholar]
- 8.Gulick RM, Wilkin TJ, Chen YQ, et al. : Phase 2 study of the safety and tolerability of maraviroc-containing regimens to prevent HIV infection in men who have sex with men (HPTN 069/ACTG A5305). J Infect Dis 2017;215:238–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulick RM, Wilkin TJ, Chen YQ, et al. : Safety and tolerability of maraviroc-containing regimens to prevent HIV infection in women: A phase 2 randomized trial. Ann Intern Med 2017:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng PC, Henikoff S: Predicting deleterious amino acid substitutions. Genome Res 2001;11:863–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramensky V, Bork P, Sunyaev S: Human non-synonymous SNPs: Server and survey. Nucleic Acids Res 2002;30:3894–3900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rehm HL, Bale SJ, Bayrak-Toydemir P, et al. : ACMG clinical laboratory standards for next-generation sequencing. Genet Med 2013;15:733–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabini E, Hazra S, Ort S, Konrad M, Lavie A: Structural basis for substrate promiscuity of dCK. J Mol Biol 2008;378:607–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lek M, Karczewski KJ, Minikel EV, et al. : Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016;536:285–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Rompay AR, Johansson M, Karlsson A: Phosphorylation of deoxycytidine analog monophosphates by UMP-CMP kinase: Molecular characterization of the human enzyme. Mol Pharmacol 1999;56:562–569 [DOI] [PubMed] [Google Scholar]
- 16.Michelson AM, Blake CC, Evans ST, Orkin SH: Structure of the human phosphoglycerate kinase gene and the intron-mediated evolution and dispersal of the nucleotide-binding domain. Proc Natl Acad Sci U S A 1985;82:6965–6969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu Y, Fuchs EJ, Hendrix CW, Bumpus NN: CYP3A5 genotype impacts maraviroc concentrations in healthy volunteers. Drug Metab Dispos 2014;42:1796–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.