Abstract
Objective:
Irritable bowel syndrome (IBS) is a chronic functional disorder of the gastrointestinal tract that causes abdominal pain or discomfort and alters bowel with no organic abnormalities. Treatment options for IBS have increased in number in the past decade, and clinicians should not be limited to use only conventional treatments to cure it. This article is a placebo-controlled clinical trial to assess the therapeutic effects of low-dose bismuth subcitrate on symptoms and the health-related quality of life in adult patients with IBS.
Methods:
This clinical trial was done during July 2015 to January 2016 in Isfahan, Iran. For each of three subtypes (IBS-constipation dominant, IBS-diarrhea dominant [IBS-D], and IBS-mixed), we included patients with IBS aged 18–70 years, diagnosed according to the Rome III criteria. In this study, 129 eligible patients were enrolled, of which 119 continued on the protocol to the end of study. They were allocated in placebo group (Group A) and intervention group (Group B). The medication for Group B was mebeverine and bismuth subcitrate and for Group A was mebeverine and placebo of bismuth subcitrate. Initially, the patients of both groups completed IBS-related questionnaires (IBS-quality of life, IBS-severity scoring system), then given drugs for a 4-week period (1st on-drug period). Then, both groups were given only mebeverine hydrochloride 200 mg capsule for another 4 weeks (off-drug period). At last, Group A and Group B were given medication (2nd on-drug period), the same as 1st on-drug period.
Findings:
With respect to quality of life, the trend of IBS-QOL score changed significantly during the study period in both the intervention and placebo groups; however, no significant differences were observed between the two groups (P < 0.005). In subgroups analysis, quality of life significantly improved in IBS-D during the study from the first measurement to the end of study (P = 0.004). The trends of changes in the severity of pain during the study between the intervention and control group were significantly different (P = 0.018).
Conclusion:
According to our study, IBS-D patients' symptoms improved significantly with bismuth therapy. We found that adding low-dose bismuth to mebeverine in nonresponsive IBS patients in conventional treatment could be helpful.
KEYWORDS: Abdominal pain, Bismuth subcitrate, bloating, constipation, diarrhea, irritable bowel syndrome, Mebeverine
INTRODUCTION
Irritable bowel syndrome (IBS) is a chronic functional disorder of the gastrointestinal tract that causes abdominal pain or discomfort and alters bowel with no organic abnormalities.[1] IBS has four subtypes of constipation predominant (IBS-C), diarrhea-predominant (IBS-D), mixed (IBS-M), and unspecified IBS.[2]
The exact etiology of IBS remains unclear.[3] Treatment of patients with IBS is usually directed to the predominant IBS symptoms.[4,5] There is currently no universally accepted satisfactory effective treatment for IBS. The current therapies of IBS include antispasmodics, peppermint oil, antidepressants, and psychotherapy.[2,6]
Bacterial infectious gastroenteritis is a significant risk factor for the development of IBS.[7] Nearly 10% of patients with an intestinal bacterial infection report postinfectious symptoms up to 10 years after the infectious event.[8] Recent studies have identified the role of bacteria in maintaining normal gut function, including protection against pathogens, metabolism, and absorption.[9] In addition, they demonstrated an increased quantity and alteration in the type and distribution of gut bacterial flora in patients with IBS.[10,11]
Prior studies have shown that broad-spectrum antibiotics, such as tetracycline, amoxicillin-clavulanate, metronidazole, neomycin, and fluoroquinolones, can improve bowel symptoms.[12,13] Bismuth subcitrate displays anti-inflammatory actions and also acts as an anti-acid and mild antibiotic. As an antidiarrheal, its exact mechanism has not been determined. Bismuth subcitrate may exert its antidiarrheal action not only by stimulating absorption of fluid and electrolytes across the intestinal wall (anti-secretory action) but also by inhibiting the synthesis of a prostaglandin responsible for intestinal inflammation and hypermotility.[14,15,16]
We performed a placebo-controlled clinical trial to assess the therapeutic effects of low-dose bismuth subcitrate on symptoms and the health-related quality of life in adult patients with IBS.
METHODS
This study is a placebo-controlled clinical trial (registration number: IRCT2017081435691N1) done during July 2015 to January 2016 in two gastrointestinal disorder clinics, Isfahan, Iran. For each of three subtypes (IBS-C, IBS-D, and IBS-M), we included patients with IBS aged 18–70 years, diagnosed according to the Rome III criteria. This study was approved by the Isfahan Regional Bioethics Committee, and informed consent was obtained in all cases before the study.
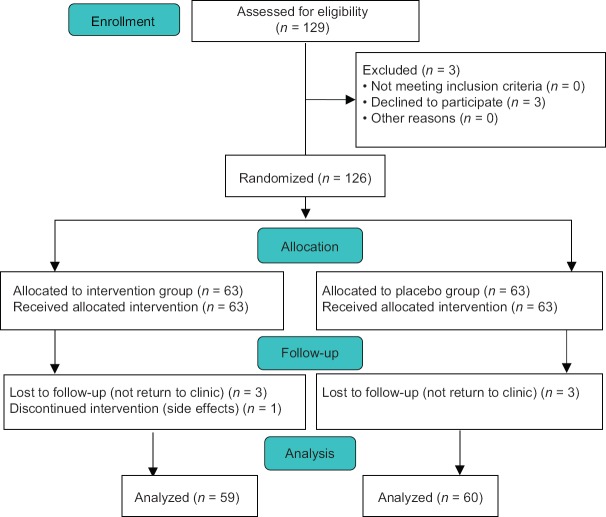
Eligible patients were allocated consecutively (one to the intervention and one patient to the placebo group) by a nontherapist member of research team to placebo and intervention groups. Three subtypes of IBS (IBS-C, IBS-M, and IBS-D) were assessed including 40 patients in each group. They were divided in two groups: A (placebo group) and B (intervention group). In this study, 129 eligible patients were enrolled, of which 119 patients continued the protocol to the end of study. In the intervention group, three patients were lost to follow-up because they did not return to clinic and one patient discontinued intervention because of drug side effects. In the placebo group, one patient was lost to follow-up because of not returning to the clinic and two patients discontinued intervention because of changing their physicians [Figure 1].
Figure 1.
Flow diagram for study enrolment
Exclusion criteria were pregnancy, having a documented chronic heart or liver failure, surgery on the gastrointestinal tract except appendectomy, malabsorption diseases, hyperthyroidism, inflammatory bowel diseases, connective tissue diseases, severely progressive diseases, diabetes mellitus, use of antibiotic drugs within 1 month before the study, significant weight loss, fever, and bloody stools.
The pharmacotherapy regimen for the intervention group was mebeverine hydrochloride 200 mg capsule once daily (before breakfast) and bismuth subcitrate 120 mg tablet twice daily (30 min before breakfast and dinner). For the placebo group, mebeverine hydrochloride 200 mg capsule once daily (before breakfast) and placebo of bismuth subcitrate (that did not have any effective material) 120 mg tablet twice daily (after breakfast and dinner meal) were given. Mebeverine was identical in appearance and was provided from the Irandarou Pharmaceutical Company (Tehran, Iran), and bismuth subcitrate and placebo were identical in appearance and supplied by the Shafa Pharmaceutical Company (Tehran, Iran).
The patients were assessed at baseline (day 0), the 4th week (day 28), the 8th week (day 56), and the 12th week (day 84) of treatment. Data on the quality of life and IBS symptoms (severity of abdominal pain, severity of bloating and abdominal discomfort, and improvement of stool form) were recorded by completing the IBS questionnaire by the patients. Initially, patients of both groups completed IBS-related questionnaires (IBS-quality of life [IBS-QOL], IBS-severity scoring system [IBS-SSS]). The IBS-QOL is a self-report quality-of-life measure specific to IBS that can be used to assess the impact of IBS and its treatment. The IBS-QOL was developed using a need-based model. Then, they were given drugs for a 4-week period (1st on-drug period). The patients answered the 5-point Likert scaled questions of IBS-QOL questionnaire and then in IBS-SSS questionnaire themselves. The individual responses to the 34 items are summed and averaged for a total score and then transformed to a 0–100 scale for ease of interpretation with higher scores indicating better IBS-specific quality of life. There are also eight subscale scores for the IBS-QOL (dysphoria, interference with activity, body image, health worry, food avoidance, social reaction, sexual, relationships).[17,18] The questionnaire has standardized in the Iranian population and has acceptable reliability and validity (α = 0.93).[19]
The IBS-SSS questionnaires evaluated the severity of abdominal pain and bloating or abdominal discomfort, improvement of stool form, and improvement of overall quality of their life on a scale of 0–100 (0 = no pain/no abdominal distention/very happy with bowel habit/and no effect on life in general/to 100 = very severe pain/very severe abdominal distention/very unhappy with bowel habit/and completely affecting life in general). The questionnaire has acceptable reliability and validity (α = 0.84).[19]
At the end of the 4-week period, the patients returned to the clinic and any unused drugs were returned. They were assessed by similar IBS questionnaires again. Then, both groups were given only mebeverine hydrochloride 200 mg capsule once daily before breakfast. In other words, bismuth and placebo of bismuth subcitrate tablets were not given for another 4 weeks (off-drug period).
At the end of the second 4-week period, the patients returned to the clinic and any unused drugs were returned again. They were assessed by the similar IBS questionnaires for the third time. This time, they were given both mebeverine hydrochloride and bismuth subcitrate or placebo (Group A) similar to the first 4-week period again (2nd on-drug period).
At the end of the third 4-week period, the patients returned to the clinic and any unused drugs were returned again and they were assessed by similar IBS questionnaire for the last time. In this study, if the patients had any problem, they could contact the clinic or their physicians.
Quantitative and qualitative data were presented as mean ± standard deviation and frequency (percentages), respectively. Normality of quantitative data was evaluated using Kolmogorov–Smirnov test and Q-Q plot. Main statistical method for analyzing data was repeated measures ANOVA. Sphericity assumption was evaluated using Mauchly's test. When sphericity assumption was violated, Huynh-Feldt method was used. Qualitative data between study groups were compared using Chi-square test and within group were evaluated using Cochran Q-test. All statistical analyses were conducted using SPSS software, version 15.
RESULTS
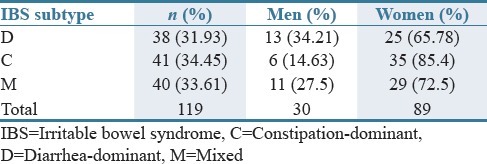
In this study, 129 eligible patients were enrolled, of which 119 patients consisting of 30 (25.21%) men and 89 (74.78%) women continued on the protocol to the end of study. Among 119 included patients, 59 were assigned to bismuth subcitrate group (44 [74.57%] women and 15 [25.42%] men) and 60 to placebo group (44 [73.33%] women and 16 [26.66%] men). The baseline characteristics of the patients were also similar in different subtypes of IBS [Tables 1–3].
Table 1.
Characteristics of the patients in different irritable bowel syndrome subtypes (n=119)
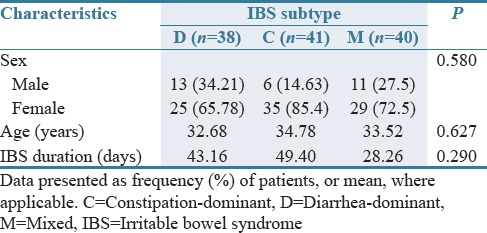
Table 3.
Characteristics of the patients and the baseline in different subtypes of irritable bowel syndrome
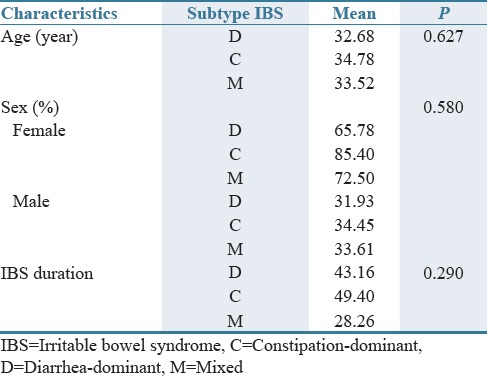
Table 2.
Frequency (%) of patients with different irritable bowel syndrome subtypes
With respect to quality of life, the trend of IBS-QOL score changed significantly during the study period in both intervention and placebo groups, but no significant differences were observed between two groups (P < 0.005). In subgroups analysis, quality of life significantly improved in type D during the study from the first measurement to the end of study (P = 0.004).
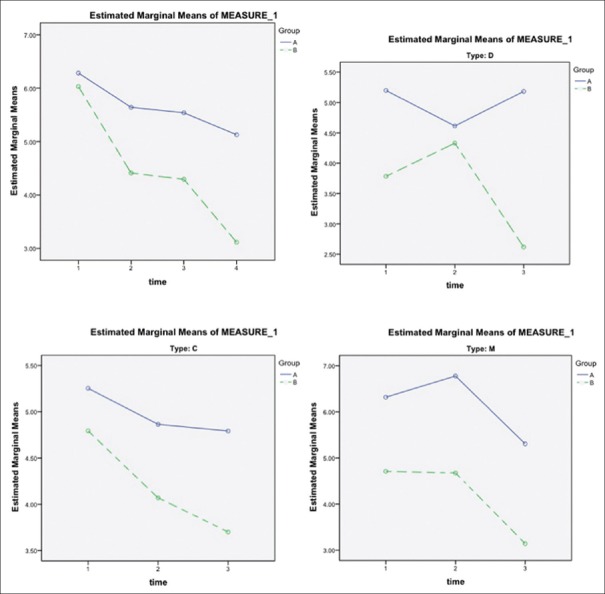
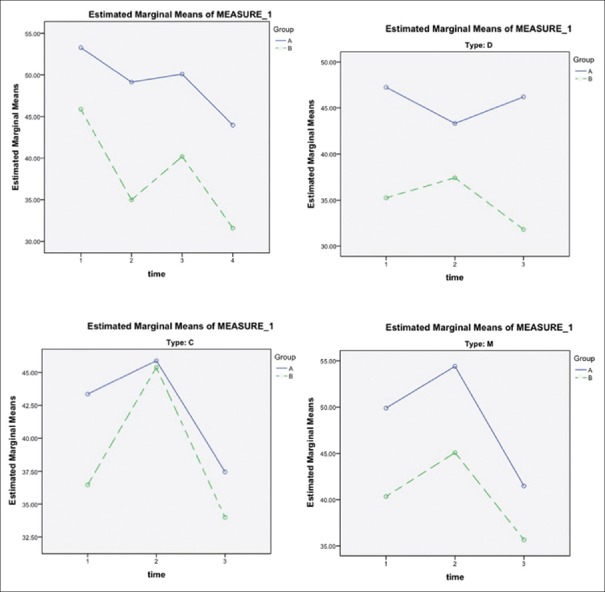
The trends of changes in the severity of pain during the study among the intervention and control group were significantly different (P = 0.018). In the intervention group, pain severity mean score reduced from 55 to 32, whereas in the control group, it changed from 57 to 53; however, the differences were not significant when analysis was repeated in subgroups (IBS-C, IBS-M, and IBS-D) [Figure 2].
Figure 2.
The trends of changes in the severity of pain during the study among the intervention and control group. Group A: placebo group; Group B: intervention group. C = Constipation-dominant, D = Diarrhea-dominant, M = Mixed. Time 1: Assessed at baseline (day 0); Time 2: The 4th week (day 28) of treatment, 1st on-drug period; Time 3: The 8th week (day 56) of treatment, off-drug period; Time 4: the 12th week (day 84) of treatment, 2nd on-drug period
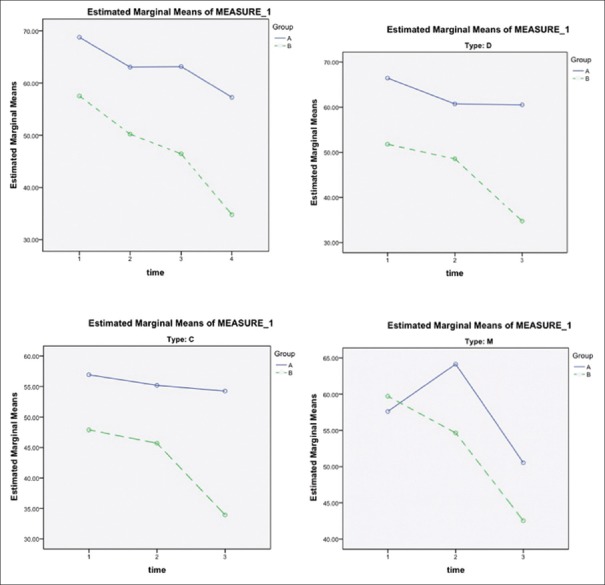
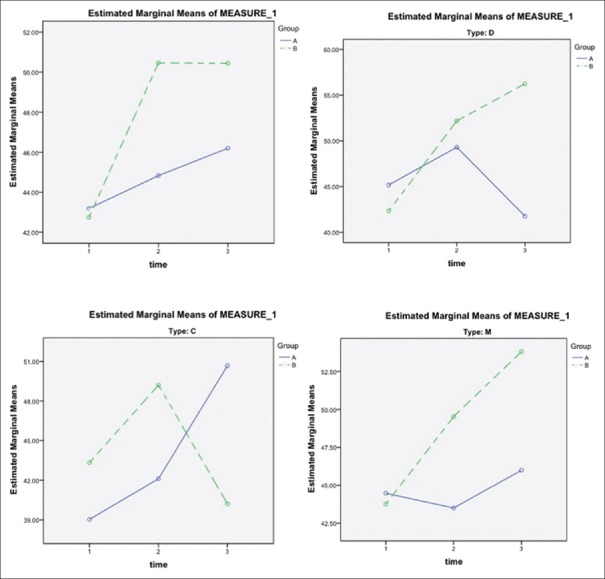
The trend of numbers of days with pain was significantly different (P = 0.021) in both intervention and control groups; the intervention group suffered fewer days of pain. However, subgroup analysis showed that there was no significant difference in subtype (C) [Figure 3].
Figure 3.
The trend of numbers of days with pain during the study among the intervention and control group. Group A, placebo group; Group B, intervention group. C = Constipation-dominant, D = Diarrhea-dominant, M = Mixed. Time 1: Assessed at baseline (day 0); Time 2: The 4th week (day 28) of treatment, 1st on-drug period; Time 3: The 8th week (day 56) of treatment, off-drug period; Time 4: The 12th week (day 84) of treatment, 2nd on-drug period
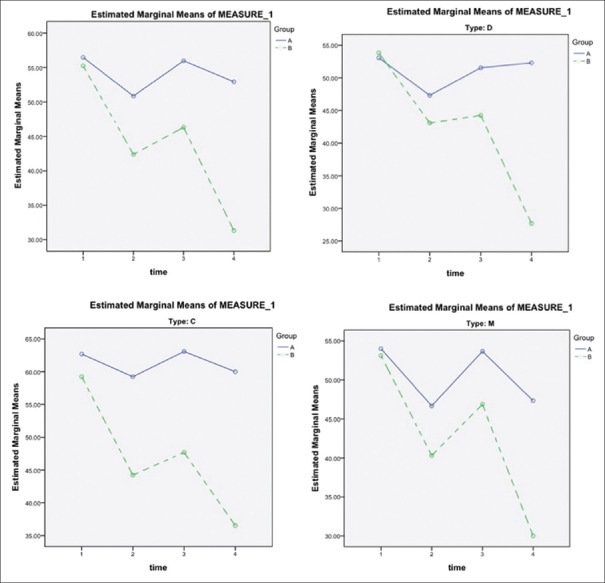
The trend of changes in severity of bloating during the study inpatients who complained about bloating was significantly different between the intervention and placebo groups. Improvement was seen in the intervention group with respect to bloating. In subgroup analysis, the trends of changes in severity of bloating and the frequency of abdominal bloating were significantly different in subtype D in all stages of the investigation in both intervention and control groups [Figure 4].
Figure 4.
The trend of changes in severity of bloating during the study among the intervention and control group. Group A, placebo group; Group B, intervention group. C = Constipation-dominant, D = Diarrhea-dominant, M = Mixed. Time 1: assessed at baseline (day 0); Time 2: The 4th week (day 28) of treatment, 1st on-drug period; Time 3: The 8th week (day 56) of treatment, off-drug period; Time 4: The 12th week (day 84) of treatment, 2nd on-drug period
The trends of changes in the patients' satisfaction on bowel habits were not statistically different in all phases of study between two groups and subgroup analysis showed the same results [Figure 5]. In contrast, the trend of changes regarding the impact on daily life in all stages of the study was significantly different. The bismuth group had a better improvement. Subgroups analysis showed that in subtype (D), the trend of changes based on the impact of the disease on daily life in all stages of the study was statistically significant [Figure 6].
Figure 5.
The trends of changes in the patients' satisfaction on bowel habits during the study among the intervention and control group. Group A, placebo group; Group B, intervention group. C = Constipation-dominant, D = Diarrhea-dominant, M = mixed. Time 1: Assessed at baseline (day 0); Time 2: The 4th week (day 28) of treatment, 1st on-drug period; Time 3: The 8th week (day 56) of treatment, off-drug period; Time 4: The 12th week (day 84) of treatment, 2nd on-drug period
Figure 6.
The trend of changes of the impact on daily life during the study among the intervention and control group. Group A, placebo group; Group B, intervention group. C = Constipation-dominant, D = Diarrhea-dominant, M = Mixed. Time 1: Assessed at baseline (day 0); Time 2: The 4th week (day 28) of treatment, 1st on-drug period; Time 3: The 8th week (day 56) of treatment, off-drug period; Time 4: The 12th week (day 84) of treatment, 2nd on-drug period
DISCUSSION
IBS is a common disorder around the world. Symptoms of IBS are commonly seen as abdominal pain and changing in bowel habits and can affect quality of life. The exact pathogenesis of IBS is unknown, but some evidence that shows changes in gut microflora may be considered as a contributing etiological factor in IBS pathogenesis.
Bismuth subcitrate has antibacterial, antacid, and anti-inflammatory effects. Based on previous studies, bismuth subcitrate decreases the growth of bacteria. Short-term treatment with antibiotic (rifaximin) leads to improve signs and symptoms of the patients with IBS.[20] Hence, based on the fact that bacterial overgrowth may be a contributing factor in the pathogenesis of IBS, bismuth subcitrate can be used as an adjunct to IBS therapy. We studied the effect of bismuth subcitrate in a group of patients with diarrhea- and constipation-predominant symptoms and mixed (diarrhea and constipation) subtypes and compared its effectiveness to placebo.
In the open prospective study of de-nol efficacy and safety, patients of the main group (n = 20) were given 120 mg of de-nol thrice daily in combination with meteospasmyl (a spasmolytic and antifoaming agent), and control patients (n = 10) received aluminum phosphate with meteospasmyl (1 capsule thrice daily). Duration of the treatment was 3 weeks. Before the study, all patients had abdominal pain, diarrhea, meteorism, altered composition of fecal bacteria, their excessive growth in the intestines, and morphological signs of chronic inflammation. By the end of therapy, abdominal pain was eliminated in 90 and 60%, meteorism was absent in 80 and 40%, diarrhea in 75 and 50%, excessive bacterial growth in small intestine in 75 and 30%, changes of fecal microflora persisted in 20 and 70%, histological signs of active mucosal inflammation remained in 40 and 85.7% of the patients of the main and control groups, respectively. Treatment with de-nol and spasmolytics for 3 weeks effectively eliminated clinical manifestations of the disease, restored normal composition of intestinal microflora, normalized feces properties, and resolved active inflammation.[21]
In this study, we used the ROME III criteria for diagnosing IBS. Our findings showed that the quality of life in IBS-D increased significantly in the intervention group. No significant difference was seen in the abdominal pain in both groups.
In subtype group analysis in patients who expressed pain, clinically significant difference was observed, but it seems that this difference could not reach statistical meaningfulness probably due to the fact that the number of patients in each IBS subtype group was small. Subgroup analysis regarding the number of days that patients had pain showed meaningful differences in subtypes M and D.
With respect to bloating, the general trend of changes in the severity of bloating during the study in those who complained about bloating was significantly different between bismuth and placebo group and bismuth group showed more improvement.
In subtype group analysis, the general trends of changes in the severity of bloating were significantly different in subtype D in all stages of the investigation in both intervention and control groups; however, in constipation and mixed subtypes, it was not statistically significant. We postulate that it is likely due to sample size of our subgroups. The frequency of abdominal bloating in these two subtypes (C and M) was clinically different and in bismuth group showed a slight improvement.
About satisfaction on bowel habits, subgroup analysis showed that in all phases of study, the general trends of changing of this variable were neither statistically nor clinically significant. In terms of impact on daily life, the general trend of frequency in all stages of the study was significantly different statistically and bismuth group had a better status. Furthermore, subgroups analysis showed both clinical and statistical difference in diarrheal subtype, but in the two other subtypes, the general trends of changing of this variable were not significant.
The strength of this study is that included three common type of IBS and evaluated therapeutic effects of bismuth subcitrate (that is a reachable and safe drug with antibiotic effects) in this common types. This clinical trial contains acceptable number of patients, of any culture, age, and sex to evaluate the therapeutic effects of intervention in those groups of patients. In this clinical trial, we follow up patients intensively and evaluate them in four periods of time that have 1 month drug-free time to know better about the effects of our intervention.
In addition, this clinical trial has drawbacks, such as the time length of study, and in few cases side effects of placebo and method of blindness in this study.
In general, according to our study, IBS-D symptoms (abdominal pain, bloating, quality of life, impact of daily life, number of days having pain, and satisfaction on bowel habit) improved significantly with bismuth therapy (bismuth subcitrate 120 mg tablet twice daily, 30 min before breakfast and dinner). We found that adding low dose bismuth to mebeverine in patients with nonresponsive IBS could be helpful.
AUTHORS' CONTRIBUTION
Dr. Hamed daghaghzadeh and Dr. Payman adibi are desinged the study. Ardalan Memar, Yasaman Mohamadi, Nooshin Rezakhani, Parastoo Safazadeh, Sarina Aghaha, collected and analyzed the data and write the original article also Ardalan memar is uploader and updater and final recheck the study in IRCT and JRPP website.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 2.American College of Gastroenterology Task Force on Irritable Bowel Syndrome. Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, et al. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104(Suppl 1):S1–35. doi: 10.1038/ajg.2008.122. [DOI] [PubMed] [Google Scholar]
- 3.Horwitz BJ, Fisher RS. The irritable bowel syndrome. N Engl J Med. 2001;344:1846–50. doi: 10.1056/NEJM200106143442407. [DOI] [PubMed] [Google Scholar]
- 4.Camilleri M. Management of the irritable bowel syndrome. Gastroenterology. 2001;120:652–68. doi: 10.1053/gast.2001.21908. [DOI] [PubMed] [Google Scholar]
- 5.Clouse RE. Managing functional bowel disorders from the top down: Lessons from a well-designed treatment trial. Gastroenterology. 2003;125:249–53. doi: 10.1016/s0016-5085(03)00808-4. [DOI] [PubMed] [Google Scholar]
- 6.Ford AC, Moayyedi P, Lacy BE, Lembo AJ, Saito YA, Schiller LR, et al. American college of gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(Suppl 1):S2–26. doi: 10.1038/ajg.2014.187. [DOI] [PubMed] [Google Scholar]
- 7.Futagami S, Itoh T, Sakamoto C. Systematic review with meta-analysis: Post-infectious functional dyspepsia. Aliment Pharmacol Ther. 2015;41:177–88. doi: 10.1111/apt.13006. [DOI] [PubMed] [Google Scholar]
- 8.Schwille-Kiuntke J, Enck P, Zendler C, Krieg M, Polster AV, Klosterhalfen S, et al. Postinfectious irritable bowel syndrome: Follow-up of a patient cohort of confirmed cases of bacterial infection with Salmonella or Campylobacter. Neurogastroenterol Motil. 2011;23:e479–88. doi: 10.1111/j.1365-2982.2011.01779.x. [DOI] [PubMed] [Google Scholar]
- 9.Bolino CM, Bercik P. Pathogenic factors involved in the development of irritable bowel syndrome: Focus on a microbial role. Infect Dis Clin North Am. 2010;24:961–75, ix. doi: 10.1016/j.idc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Esposito I, de Leone A, Di Gregorio G, Giaquinto S, de Magistris L, Ferrieri A, et al. Breath test for differential diagnosis between small intestinal bacterial overgrowth and irritable bowel disease: An observation on non-absorbable antibiotics. World J Gastroenterol. 2007;13:6016–21. doi: 10.3748/wjg.v13.45.6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. A double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412–9. doi: 10.1111/j.1572-0241.2003.07234.x. [DOI] [PubMed] [Google Scholar]
- 13.Pimentel M, Chatterjee S, Chow EJ, Park S, Kong Y. Neomycin improves constipation-predominant irritable bowel syndrome in a fashion that is dependent on the presence of methane gas: Subanalysis of a double-blind randomized controlled study. Dig Dis Sci. 2006;51:1297–301. doi: 10.1007/s10620-006-9104-6. [DOI] [PubMed] [Google Scholar]
- 14.Carrara M, Desideri S, Azzurro M, Bulighin GM, Di Piramo D, Lomonaco L, et al. Small intestine bacterial overgrowth in patients with irritable bowel syndrome. Eur Rev Med Pharmacol Sci. 2008;12:197–202. [PubMed] [Google Scholar]
- 15.Lauritano EC, Gabrielli M, Scarpellini E, Ojetti V, Roccarina D, Villita A, et al. Antibiotic therapy in small intestinal bacterial overgrowth: Rifaximin versus metronidazole. Eur Rev Med Pharmacol Sci. 2009;13:111–6. [PubMed] [Google Scholar]
- 16.Roalfe AK, Roberts LM, Wilson S. Evaluation of the Birmingham IBS symptom questionnaire. BMC Gastroenterol. 2008;8:30. doi: 10.1186/1471-230X-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: Development and validation of a new measure. Dig Dis Sci. 1998;43:400–11. doi: 10.1023/a:1018831127942. [DOI] [PubMed] [Google Scholar]
- 18.Drossman DA, Patrick DL, Whitehead WE, Toner BB, Diamant NE, Hu Y, et al. Further validation of the IBS-QOL: A disease-specific quality-of-life questionnaire. Am J Gastroenterol. 2000;95:999–1007. doi: 10.1111/j.1572-0241.2000.01941.x. [DOI] [PubMed] [Google Scholar]
- 19.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 20.Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32. doi: 10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
- 21.Iakovenko EP, Agafonova NA, Pokhal'skaia OIu, Kolganova AV, Nazarbekova RS, Ivanov AN, et al. The use of bismuth tripotassium dicitrate (De-nol), a promising line of pathogenetic therapy for irritated bowel syndrome with diarrhea. Klin Med (Mosk) 2008;86:47–52. [PubMed] [Google Scholar]