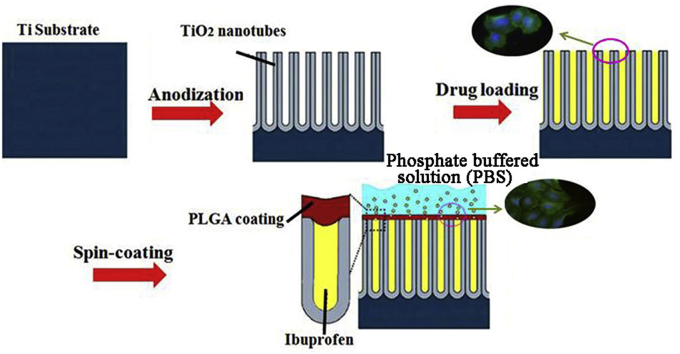
Abstract
Bacterial infection and tissue inflammation are the major causes of early failure of titanium-based orthopedic implants; thus, surgical implants with tunable drug releasing properties represent an appealing way to address some of these problems of bacterial infection and tissue inflammation in early age of orthopedic implants. In this work, a hybrid surface system composed of biodegradable poly(lactic-co-glycolic acid) (PLGA) and titania nanotubes (TNTs) has been successfully constructed on Ti implants with the aim of preventing bacterial infection via long-term drug release. By varying the size of the TNTs and the thickness of the polymer film, the drug release profile can be tuned to achieve the optimal therapeutic action throughout the treatment time. The size of TNTs plays a dominant role in the drug loading dose of TNTs/PLGA hybrid coatings. In this work, TNTs with an average size of 80 nm can achieve the largest loading dose. Depending on the polymer thickness, significant improvement in the drug release characteristics is attained, for instance, reduced burst release (from 84% to 27%) and overall release time extended from 5 to over 40 days. In addition, the PLGA layers may favor the proliferation and osteogenesis of MC3T3-E1 mouse cells at an earlier stage. Therefore, this TNT/PLGA hybrid surface system can be employed as an effective bioplatform for improving both self-antibacterial performance and biocompatibility of Ti-based biomaterials.
Keywords: Antibacterial, Biocompatibility, Coating, Drug delivery, Implants
Graphical abstract
Highlights
-
•
The TNT/PLGA coatings system is successfully constructed on titanium implants.
-
•
TNTs with an average size of 80 nm can achieve the largest loading dose of ibuprofen.
-
•
This system shows reduced burst release (from 84% to 27%).
-
•
This system can achieve long-term release of drugs over 40 days.
-
•
The surface system exhibits good biocompatibility.
1. Introduction
Titanium (Ti) and titanium alloys are used in orthopedic implants because of their desirable mechanical strength, corrosion resistance, and biocompatibility [1], [2]. However, implant failure arising from post-surgery infection remains one of the most serious complications after surgery [3], [4]. Although antibiotics are usually prescribed to prevent complications [3], systemic drug administration, regardless of whether it is intravenous, intramuscular, or topical, suffers from limitations such as low drug solubility, poor biodistribution, lack of selectivity, uncontrolled pharmacokinetics, and serious side effects on non-target tissues [5]. Hence, surface modification, including building a local antibacterial agents delivery system to the implantation site, is preferred [6], [7]. Vancomycin, penicillin, gentamicin, antimicrobial peptides, and indomethacin have been used as drugs for this purpose because they mitigate inflammation and inhibit bacteria growth [8], [9], [10].
Titanium dioxide (TiO2) has attracted much attention since the discovery of its excellent photocatalytic performance in water splitting when illuminated by ultraviolet (UV) light [11], [12], [13], and extensive research on the fabrication, structure, and application of TiO2 nanomaterials has ensued [14], [15]. In 1999, Zwilling and co-workers found that TiO2 porous membranes could be produced on Ti by anodic oxidation in a fluoride electrolyte [16], and in 2001, Gong et al. reported the preparation of even and orderly TiO2 nanotubes (TNTs) on Ti by anodic oxidation in an electrolyte containing hydrofluoric acid (HF) [17]. Since then, uniform TNT arrays with various pore sizes (22–110 nm), lengths (200–6000 nm), and wall thicknesses (7–34 nm) have been produced by adopting different conditions [18], [19]. Due to the low immunogenicity of TNT arrays, they are introduced into biological applications [20]. TNT arrays have also been reported to have the ability to direct stem cell fate [21] and regulation of the behavior of endothelial cells. Furthermore they also can improve osteoblast attachment, function, and proliferation [22], [23], [24]. In addition, targeted delivery of antibiotics and drugs from TNTs has been studied [25], [26], [27], [28], [29]. However, the connection between TNT size and the dose of loaded drugs has not previously been investigated very deeply.
Alleviating pain and reducing inflammation after surgeries are important. Ibuprofen, a nonsteroidal chemical, is used as an analgesic, antipyretic, and anti-inflammatory drug. However, it has a fairly short action time because of a limited half-life of only 1–3 h, thus requiring frequent oral or parenteral administration [30]. Ibuprofen release time must therefore be prolonged; the use of a polymer coating to control the elution kinetics of ibuprofen is the main objective of this piece of work. In this work, TNT arrays are produced on Ti in an ethylene glycol electrolyte containing ammonium fluoride, and poly (lactic-co-glycolic acid) (PLGA), a biodegradable and antibacterial polymer [31], [32], is coated onto the TNT arrays. Additionally, PLGA has been reported to have good biocompatibility for cell attachment and proliferation [33]. The effects of the thickness of the PLGA layer on the release behavior of ibuprofen from coatings are explored and discussed. Furthermore, the biocompatibility of samples is explored via in vitro test.
2. Experimental section
2.1. Materials
Titanium disks (99.6% Ti) with a thickness of 0.25 mm and diameter of 6 mm were supplied by Baosteel Group Corp (Shanghai, China). Ethylene glycol (EG) and ammonium fluorides (NH4F) were obtained from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). The poly (D, L-lactide-co-glycolide) (PLGA) co-polymer with a molar ratio of LA:GA of 75:25 and low molecular weight with an inherent viscosity of 0.61 dL/g was provided by Daigang BIO Engineer Limited Co. (Jinan, China). Ibuprofen was purchased from Zhengzhou Sainuokang Chemical Products Co., Ltd. (China).
2.2. Synthetic methods
2.2.1. Fabrication of TNT arrays on Ti
The Ti disks were cleaned by acetone, ethanol, and deionized water using an ultrasonic cleaner. Then they were etched in solution containing HF, HNO3, and H2O at a ratio of 1:4:5 [34]. TNT arrays were synthesized by a two-electrode DC anodization system. The electrolyte contained 0.3% NH4F, ethylene glycol, and H2O with volume ratio of 3:97 between ethylene glycol and water. The effects of the reaction temperature, anodization voltage, reaction time and gravity on the microtopography of the TNTs were studied. The anodization voltage was varied from 10 to 60 V, reaction time from 10 to 180 min, and reaction temperature from 0 to 100 °C.
2.2.2. Drug loading and release study
In order to aviod capillarity, the prepared TNTs were cleaned by deionized water and dried in vacuum oven at 60 °C for 24 h, and then they were immersed in ibuprofen solution (100 mg/mL) for 2 days [35].
The PLGA solution of chloroform was then prepared. The concentrations of PLGA solution [0.5%, 1%, and 2% (w/v)] was pipetted onto the drug loaded TNTs, spin coated, and dried in air. The steps were repeated if necessary.
The amount of drug release from TNT/PLGA samples was determined using ultraviolet–visible (UV–Vis) spectrophotometry [36]. UV–Vis measurements were taken at short intervals during the first 6 h after preparation to monitor the initial burst release of ibuprofen, followed by testing every 24 h to observe the long-term release behavior until all the drug had been released to the phosphate buffered solution (PBS). The percentage of drug release was calculated by dividing the accumulated amount of released drug by the total loaded amount. The total amount of drug loading was detected when the UV–Vis absorption spectra exhibited no further changes.
2.3. In vitro study
2.3.1. Cell viability
The MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide) assay could be used to determine the cell viability of the samples. First, 350 μL 1 × 105 mL−1 mouse MC3T3-E1 cells (Tongji Hospital, Wuhan, China) were cultured at 48-well plate with samples at the bottom at 37 °C in a humidified atmosphere of 5% CO2 for 1, 3, and 7 days. After removing the medium, 350 μL MTT (0.5 mg/mL) solution was dissolved into the sterilized PBS (pH 7.4), and then incubated in a 5% CO2 incubator for 4 h. After incubation, the medium was removed and 350 μL dimethyl sulfoxide (DMSO) was added into the well, followed by incubation of the color reaction for 15 min in an incubator. After that, the samples were removed and the cultured medium was measured by the microplate reader at wavelengths of 490 nm and 570 nm. The cell viability is determined from the absorbance readings and calculated by dividing the values of samples to those of the control.
2.3.2. Alkaline phosphatase activity
An alkaline phosphatase (ALP) assay was used to determine the osteogenic differentiation on the samples after culturing for 3, 7, and 14 days. After incubation at 48-well plate with samples at the bottom at 37 °C in a humidified atmosphere of 5% CO2, the medium was removed and 500 μL of Triton X-100 (1%) was added into the 48-well plates. After shaking 5 min, the 48-well plate with samples was incubated in a water bath at 37 °C for 1 h. Then, 30 μL supernatant was tested by using an AKP ELISA kit at the wavelength of 520 nm by the microplate reader.
2.3.3. Cell morphology
Mouse MC3T3-E1 cells (1 × 104 cell/mL) were seeded into the 48-well plates containing pre-sterilized samples. After 8 h of incubation at 37 °C in a humidified atmosphere of 5% CO2, samples were washed with PBS (37 °C) three times and then samples were immersed into a 4% formaldehyde solution for 10 min. After that, samples were washed with PBS an additional three times. Subsequently, the samples were immersed in FITC (YiSen, Shanghai, China) for 30 min in the absence of light at room temperature, and then washed with PBS three times. The samples were then successively stained with DAPI (YiSen, Shanghai, China) at room temperature in the dark for just 30 s. After washing with PBS three additional times, the cell morphology was examined using an inverted fluorescence microscope (IFM, Olympus, IX73).
3. Results and discussion
3.1. Characterization
The structure and morphology of the TNTs can be controlled by changing the synthesis parameters such as the oxidation voltage, time, and temperature. Here, a series of experiments is performed to explore the impact of each parameter on the TNT morphology, which is quite important for the subsequent drug loading of TNT/PLGA hybrid coatings.
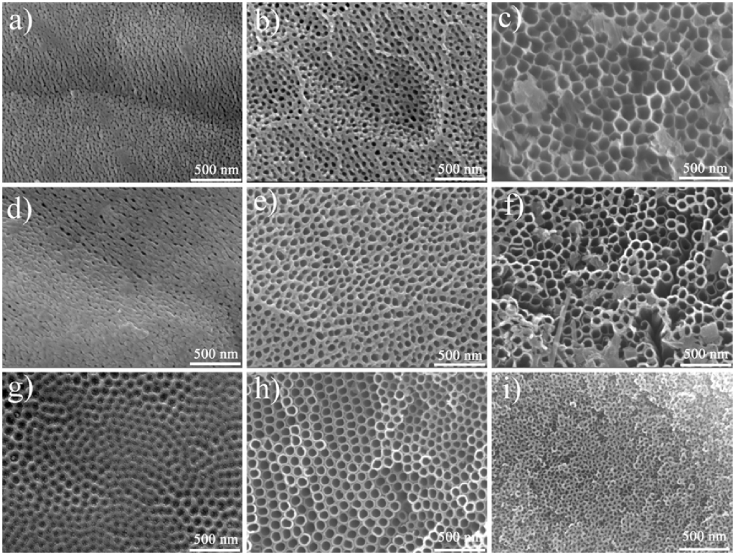
Field emission scanning electron microscope (FE-SEM) images are shown in Fig. 1a–c of the samples anodized for 1 h at 20 °C and different voltages of 10 V, 30 V, and 60 V, respectively. The diameter of the TNTs is relatively small when the voltage is low. The tubular structure is not obvious and is entangled. As the voltage increases, the tubular structure becomes clearer and the inner diameter increases gradually, as shown in Fig. 1b. At a voltage of 60 V, the inner diameter of the TNTs is 120 nm, as shown in Fig. 1c. These results confirm that a larger oxidation voltage is positively correlated to the formation and morphology of TNT diameters. The size of the inner core of the TNTs increase with increasing voltage.
Fig. 1.
The FESEM figures of TNTs prepared at 20 °C for 1 h under different oxidation voltages (a) 10 V (b) 30 V and (c) 60 V. The FESEM figures of TNTs prepared at 20 °C under 30 V for (d) 10 min (e) 60 min (f) 180 min. The FESEM figures of TNTs prepared under 30 V for 1 h at different reaction temperature (g) 0 °C (h) 60 °C (i) 100 °C.
Fig. 1d–f shows the FE-SEM images of the samples anodized at 30 V and 20 °C for different times (10 min, 60 min, and 180 min). After 10 min, only holes without TNTs are observed on the surface, as shown in Fig. 1d. When oxidation time increases to 60 min, nanotubes begin to emerge and gradually become more sequential (Fig. 1e). However, because the fluorine ions can corrode the TNTs, when oxidation time reaches 180 min, the structure of the TNTs is disrupted. (Fig. 1f).
The pore size of the TNTs can be controlled via the reaction temperature. Fig. 1g–i shows images of the samples prepared at 0 °C, 60 °C, and 100 °C. When the temperature is 0 °C, myrids of tubes with small size can be observed, as shown in Fig. 1g. When the temperature is raised to 60 °C, tube size increases, as shown in Fig. 1h. This can be attributed to the fact that as the temperature increases, the viscosity of the electrolyte falls and the F− ions in the electrolyte are more motile. However, when temperature increases too much, to 100 °C, the diameter of the TNTs decreases and the structure begins to collapse (Fig. 1i).
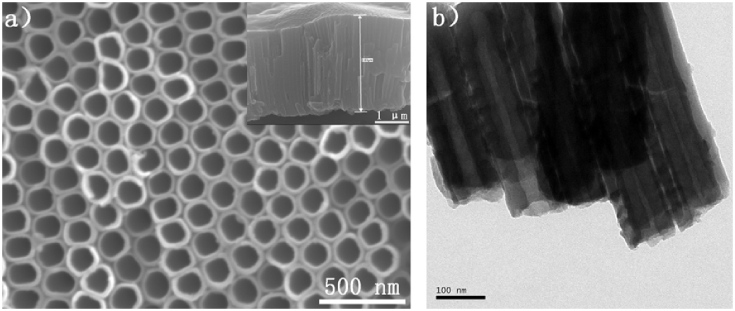
Fig. 2 depicts the FE-SEM images of the TNTs prepared in glycol electrolyte containing 0.3 wt% NH4F and 3 vol% H2O at 60 °C at a voltage of 30 V for 1 h. As shown in Fig. 2a, the TNTs are orderly and uniform with an inner diameter of about 80 nm. The inset figure shows a fractured surface of nanotubes with lengths about 2.83 μm. A transmission electron microscope (TEM) image of the TNTs is displayed in Fig. 2b. The nanotubes have a tubular structure with diameters of about 84 nm.
Fig. 2.
FESEM images of TNTs (a) top view and the inset is fracture surface. (b)Transmission electron microscopy images of TiO2 nanotubes.
Concentration of PLGA can influence the samples' morphology. The surface morphologies of one layer of PLGA coated on TNT implants at different concentrations of PLGA solution [0.5%, 1%, and 2% (w/v)] are shown in Fig. S1. Lower polymer concentrations or higher polymer concentrations lead to a uniform coating on the TNTs, as shown in Figs. S1a and S1c. The best coating is achieved at a concentration of PLGA solution of 1% (w/v), at which the polymer coatings on the TNTs are relatively complete and uniform, as shown in Fig. S1b. This is because fewer concentration of PLGA could not form bioplatform well to cover TNTs after drying, however larger concentration of PLGA could form a bioplatform much too thickness and uneven.
3.2. Release behavior from samples
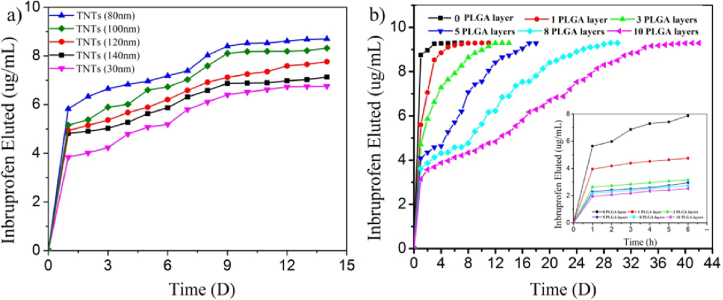
To explore the effect of different sizes of TNTs on the dose loaded in the TNT/PLGA coating samples, five samples with different tube size are explored. Three layers of PLGA were applied to the TNTs. The ibuprofen release behavior from the TNT/PLGA hybrid film–coated samples with different tubular sizes are presented in Fig. 3a. At the earliest stage (within 6 h), all samples display almost the same release behavior, while the release doses are quite different depending on tube size. TNTs with diameters of 80 nm show the largest release dose of 4.79 μg/mL among the five samples. The corresponding release dose from TNTs with diameters of 140 nm, 120 nm, 100 nm, and 80 nm are 4.59, 4.22, 4.02, and 3.43 μg/mL, respectively. Extending the time to 14 days, the release rate falls almost to zero, in other words, the amount of release from samples reflects the dose of drugs loaded in the samples. As show in Fig. 3a, the loading dose is almost up to 8.8 μg/mL, 8.2 μg/mL, 7.8 μg/mL, 6.8 μg/mL, and 6.2 μg/mL of the TNTs of 80 nm, 100 nm, 120 nm, 140 nm, and 30 nm, respectively. When the size of the TNTs decreases from 140 nm to 80 nm, the loading dose increases gradually as the surface area increases. However, if these TNTs are too small (below 30 nm in this work), they can prevent sufficient ibuprofen solution from flowing inside due to capillarity. It is clearly demonstrated that the diameter of TNTs plays an important role in the drug dose. Therefore, it is important for TNT/PLGA hybrid–coated Ti implants to obtain an optimal tube size to achieve the largest amount of drug loading. TNTs with diameters that are too large or too small are not appropriate for the largest loading dose of ibuprofen. However, if the loading drug changed, the choice of TNT size should be changed likewise.
Fig. 3.
a) In vitro ibuprofen release profiles in PBS from different size of nanotubers with 3 layers of PLGA. b) In vitro ibuprofen release profiles in PBS from nanotubes with different layers of PLGA.
To investigate the effects of PLGA thickness on the release behavior of ibuprofen from TNT/PLGA hybrid–coated samples, titanium samples with the same TNT size of 80 nm, which can hold a much large dose of drugs, are chosen. As shown in Fig. 3b, the drug release profiles are quite different for these TNT/PLGA hybrid film–coated samples with different numbers of PLGA layers (Table 1). When no layer of PLGA is present, the samples show a burst release behavior, as shown in Fig. 3b, and the release concentration is up to about 6 μg/mL and 8 μg/mL within 1 h and 6 h, respectively. The release dose reaches 94% and 100% within 1 day and 7 days, respectively. Compared to the burst release from the sample with no layer of PLGA, the release rate of ibuprofen from TNT/PLGA-coated samples is significantly smaller. The corresponding concentration of ibuprofen released from TNT/PLGA hybrid film coated–samples with1 layer of PLGA is 3.9 and 4.2 μg/mL within 1 h and 6 h, respectively, and the release amount is 51% and 60% within 6 h and 1 day, respectively. However, the leaching amount is up to 99% within 7 days (Table 1). The swelling and hydrolysis of PLGA may be responsible for this release behavior. At the earlier stage, 1 layer of PLGA can slow the release rate. However, due to the rapid swelling and hydrolysis rate of the PLGA, the ibuprofen will release rapidly from the TNTs in 9 days. The release time of ibuprofen from TNT/PLGA hybrid film–coated samples with 3 layers of PLGA can last about 12 days. When the TNTs are further covered by 10 layers of PLGA, ibuprofen release time lasts up to about 40 days (Fig. 3b) due to restriction of the drug molecule movement by the polymer chains and lower diffusion due to polymer swelling [37]. It can be clearly observed from the cross-section images (Figs. S2a and S2e) that the thickness of PLGA increases as the number of spin-coated layers of PLGA increases (Fig. S2), from about 0.44 μm for 1 layer of PLGA to 4.08 μm for 10 layers. The above results prove that the thicker layer can inhibit the swelling and hydrolysis rate of the PLGA, which may ultimately limit the release of the ibuprofen from the samples.
Table 1.
Drug release characteristics of prepared TNT/Ti implants loaded with ibuprofen (model drug) and modified with different layers of PLGA films (Three samples of each group).
| Coating on drug loaded TNT | Polymer thickness (μm) | Drug release (%) |
Drug totally released (number of days) | ||
|---|---|---|---|---|---|
| 6 h | 1 day | 7 days | |||
| Pure NT | – | 84.23 | 94.47 | 100.00 | 7 ± 1 |
| 1 layer | 0.44 | 51.43 | 60.39 | 99.23 | 9 ± 2 |
| 3 layers | 0.98 | 34.56 | 50.64 | 89.48 | 12 ± 2 |
| 5 layers | 2.02 | 31.27 | 44.69 | 66.74 | 16 ± 1 |
| 8 layers | 2.84 | 29.59 | 39.31 | 49.42 | 30 ± 2 |
| 10 layers | 4.08 | 27.46 | 33.72 | 45.32 | 40 ± 2 |
3.3. In vitro studies
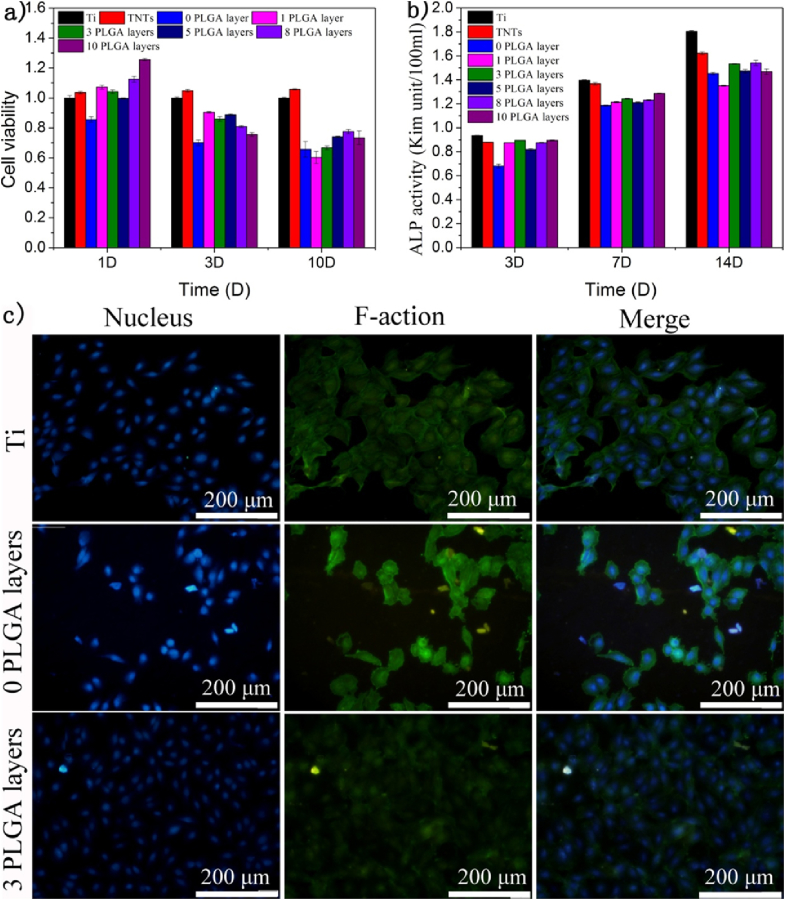
Fig. 4a shows the viability of MC3T3-E1 on the samples measured via MTT assay. Obviously, no significant inhibition of cell proliferation is exhibited on the sample with no PLGA compared with the TNTs without drugs, indicating that the ibuprofen has some cytotoxicity at high doses [38]. However, when 1 layer of PLGA is coated on the samples of TNT loaded with drugs, the cell proliferation on the samples improves significantly because the synergistic effect from PLGA and TNTs can offer more bioactive receptor binding sites for the attachment of filopodia of MC3T3-E1. Once the culture time extends to 10 days, the cell proliferation on samples with 1 layer of PLGA decreases, which demonstrates that the PLGA has degraded, allowing release of the ibuprofen and thus decreasing cell viability. In addition, the swelling of PLGA also has some negative influence on the adhesion and proliferation of cells on the samples. However, for the samples coated with 3 layers of PLGA, cell proliferation on samples after culturing for 10 days was nearly the same as with no layers of PLGA, indicating that the PLGA had degraded almost completely. But with additional PLGA layers, cell proliferation showed no change, suggesting that TNTs after loading drugs with 3 layers of PLGA is adequate for cell proliferation of MC3T3-E1.
Fig. 4.
a) Cell viability of MC3T3-E1 pre-osteoblasts cultured. The absorbance was detected at wavelength 490 nm and 570 nm b) ALP activities of MCM3T3-E1 pre-osteoblasts cultured after 3,7 and 14 days. The absorbance was detected at the wavelength of 520 nm c) Microscopic view of MCM3T3-E1 cultured on the samples a)Ti and b) 0 PLGA layer c) 3 PLGA layers after 8 h.
ALP activity is an important factor in the expression of bone mineral formation and osteoblastic differentiation on biomaterials in vitro [39]. Fig. 4b shows ALP activity of samples of Ti, TNTs, and samples with no layer of PLGA and samples with different numbers of layers of PLGA after 3, 7, and 14 days. Lower ALP activities are shown on the samples with no layer of PLGA after culturing for 3, 7, and 14 days, indicating that ibuprofen has no osteoblastic differentiation. However, after coating with PLGA, samples present a much higher ALP activity, demonstrating that PLGA can improve the facilitation of osteogenesis. However, 1 layer of PLGA can be degraded very quickly, causing an improvement of ALP activity before 7 days but lowered ALP activities after 14 days. Furthermore, when 3 layers of PLGA are coated onto the TNTs after loading with drugs, the good bioactivity facilitates the osteogenesis, which means that the sample with 3 layers of PLGA favors osteogenetic differentiation. Additional improvement of ALP activity with more layers of PLGA is not obvious. It is believed that 3 layers of PLGA are enough for osteogenetic differentiation, which is in accordance with the MTT results shown in Fig. 4a.
As shown in Fig. 4c, mitosis phase cells can be observed on the sample of Ti, the sample with no layer of PLGA and the samples with 3 layers of PLGA after 8 h of culturing. Obviously, the amount of mitosis phase cells on the sample of TNTs loaded with drugs is far less than on TNTs, further indicating the cytotoxicity of ibuprofen [40]. The amount of cells on the samples with 3 layers of PLGA is much higher than on the samples with no layer of PLGA, indicating that PLGA has good biocompatibility and can decrease the release of drugs because the degradation products of PLGA are not harmful to body, and even preferable for cell adhesion. which is consistent with the results presented in Fig. 4a and b. Furthermore, PLGA layers can also slow down release rate of drugs from samples, and thus releasing acceptable level of drugs, which can be biocompatible to surrounding tissues and cells.
4. Conclusions
This study describes the controlled preparation, characteristics, and ibuprofen release profiles of TNTs/PLGA. We discuss the effects of TNT size on the loading dose of drugs via TNT/PLGA hybrid coatings. The optimal size of TNTs to obtain the largest loading dose of ibuprofen is about 80 nm. Furthermore, varying the polymer thickness significantly improves the drug release characteristics, as evidenced by the reduced burst release (from 84% to 27%) and prolonged release (from 5 days to more than 40 days). In addition, by optimizing the diameter of the titanium nanotubes and the thickness of the polymer film, the drug release profile can be tuned to cater to the specific therapeutic requirements. In vitro tests reveal that the PLGA coating can improve the biocompatibility of implants and favors the attachment and proliferation of osteoblasts on the samples, making this TNT/PLGA system promising for a wide range of applications in a variety of Ti-based biomedical implants and with different drugs, especially orthopedic applications, such as the treatment of bone infection, local delivery of bone morphogenetic protein (BMPs) for bone repair, and targeted treatment of bone cancer and osteomyelitis.
Acknowledgements
This work is jointly supported by Special Prophase Program for Key Basic Research of the Ministry of Science and Technology of China (973 Program) No. 2014CB660809, The National Key Research and Development Plan of China No. 2016YFC1100604, the National Natural Science Foundation of China, Nos. 51422102, 81271715, and 51671081, as well as the Shenzhen Knowledge Innovation Program of Basic Research Items of Guangdong Province (Grant No. JCYJ20140414090541811), China.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.bioactmat.2017.02.001.
Contributor Information
Xiangmei Liu, Email: liuxiangmei1978@163.com.
Shuilin Wu, Email: shuilin.wu@gmail.com.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Long M., Rack H.J. Titanium alloys in total joint replacement—a materials science perspective. Biomaterials. 1998;19:1621–1639. doi: 10.1016/s0142-9612(97)00146-4. [DOI] [PubMed] [Google Scholar]
- 2.Wu S., Liu X., Yeung K.W., Liu C., Yang X. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R Rep. 2014;80:1–36. [Google Scholar]
- 3.Popat K.C., Eltgroth M., LaTempa T.J., Grimes C.A., Desai T.A. Decreased Staphylococcus epidermis adhesion and increased osteoblast functionality on antibiotic-loaded titania nanotubes. Biomaterials. 2007;28:4880–4888. doi: 10.1016/j.biomaterials.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 4.SinnáAw M. A multi-drug delivery system with sequential release using titania nanotube arrays. Chem. Commun. 2012;48:3348–3350. doi: 10.1039/c2cc17690d. [DOI] [PubMed] [Google Scholar]
- 5.Simchi E., Tamjid F. Pishbin, Boccaccini A.R. Recent progress in inorganic and composite coating with bactericidal capability for orthopaedic applications. Nanomed. Nanotechnol. Biol. Med. 2011;7:22–39. doi: 10.1016/j.nano.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Li M., Liu X.M., Xu Z.Q., Yeung K.W.K., Wu S.L. Dopamine Modified Organic–inorganic hybrid coating for antimicrobial and osteogenesis. ACS Appl. Mater. Interfaces. 2016;8:33972–33981. doi: 10.1021/acsami.6b09457. [DOI] [PubMed] [Google Scholar]
- 7.Zhou T., Zhu Y., Li X., Liu X., Yeung K.W., Wu S., Wang X., Cui Z., Yang X., Chu P.K. Surface functionalization of biomaterials by radical polymerization. Prog. Mater. Sci. 2016;83:191–235. [Google Scholar]
- 8.Yao, Webster T.J. Prolonged antibiotic delivery from anodized nanotubular titanium using a co-precipitation drug loading method. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009;91:587–595. doi: 10.1002/jbm.b.31433. [DOI] [PubMed] [Google Scholar]
- 9.Ma M., Kazemzadeh Narbat M., Hui Y., Lu S., Ding C., Chen D.D., Hancock R.E., Wang R. Local delivery of antimicrobial peptides using self-organized TiO2 nanotube arrays for peri-implant infections. J. Biomed. Mater. Res. Part A. 2012;100:278–285. doi: 10.1002/jbm.a.33251. [DOI] [PubMed] [Google Scholar]
- 10.Chao K., Liu W., Tung S., Chen D. Liu, Chang Y. Bioactive TiO2 ultrathin film with worm-like mesoporosity for controlled drug delivery. Microporous Mesoporous Mater. 2012;152:58–63. [Google Scholar]
- 11.Fujishima Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972;238:37–38. doi: 10.1038/238037a0. [DOI] [PubMed] [Google Scholar]
- 12.Tryk D.A., Fujishima A., Honda K. Recent topics in photoelectrochemistry: achievements and future prospects. Electrochim. Acta. 2000;45:2363–2376. [Google Scholar]
- 13.Weng Z., Guo H., Liu X., Wu S., Yeung K., Chu P.K. Nanostructured TiO2 for energy conversion and storage. Rsc Adv. 2013;3:24758–24775. [Google Scholar]
- 14.Buot F.A. Mesoscopic physics and nanoelectronics: nanoscience and nanotechnology. Phys. Rep. 1993;234:73–174. [Google Scholar]
- 15.Whitesides G.M. Nanoscience, nanotechnology, and chemistry. Small. 2005;1:172–179. doi: 10.1002/smll.200400130. [DOI] [PubMed] [Google Scholar]
- 16.Zwilling V., Aucouturier M., Darque-Ceretti E. Anodic oxidation of titanium and TA6V alloy in chromic media. An electrochemical approach. Electrochim. Acta. 1999;45:921–929. [Google Scholar]
- 17.Gong C.A., Grimes O.K., Varghese W., Hu R.S., Singh Z. Chen, Dickey E.C. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001;16:3331–3334. [Google Scholar]
- 18.Mor G.K., Varghese O.K., Paulose M., Shankar K., Grimes C.A. A review on highly ordered, vertically oriented TiO2 nanotube arrays: fabrication, material properties, and solar energy applications. Sol. Energy Mater. Sol. Cells. 2006;90:2011–2075. [Google Scholar]
- 19.Wang J., Lin Z. Freestanding TiO2 nanotube arrays with ultrahigh aspect ratio via electrochemical anodization. Chem. Mater. 2008;20:1257–1261. [Google Scholar]
- 20.Ainslie K.M., Tao S.L., Popat K.C., Daniels H., Hardev V., Grimes C.A., Desai T.A. In vitro inflammatory response of nanostructured titania, silicon oxide, and polycaprolactone. J. Biomed. Mater. Res. Part A. 2009;91:647–655. doi: 10.1002/jbm.a.32262. [DOI] [PubMed] [Google Scholar]
- 21.Park J., Bauer S., von der Mark K., Schmuki P. Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 2007;7:1686–1691. doi: 10.1021/nl070678d. [DOI] [PubMed] [Google Scholar]
- 22.Peng L., Eltgroth M.L., LaTempa T.J., Grimes C.A., Desai T.A. The effect of TiO2 nanotubes on endothelial function and smooth muscle proliferation. Biomaterials. 2009;30:1268–1272. doi: 10.1016/j.biomaterials.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Popat K.C., Leoni L., Grimes C.A., Desai T.A. Influence of engineered titania nanotubular surfaces on bone cells. Biomaterials. 2007;28:3188–3197. doi: 10.1016/j.biomaterials.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Wu S., Weng Z., Liu X., Yeung K., Chu P. Functionalized TiO2 based nanomaterials for biomedical applications. Adv. Funct. Mater. 2014;24:5464–5481. [Google Scholar]
- 25.Brammer K.S., Oh S., Gallagher J.O., Jin S. Enhanced cellular mobility guided by TiO2 nanotube surfaces. Nano Lett. 2008;8:786–793. doi: 10.1021/nl072572o. [DOI] [PubMed] [Google Scholar]
- 26.Peng L., Mendelsohn A.D., LaTempa T.J., Yoriya S., Grimes C.A., Desai T.A. Long-term small molecule and protein elution from TiO2 nanotubes. Nano Lett. 2009;9:1932–1936. doi: 10.1021/nl9001052. [DOI] [PubMed] [Google Scholar]
- 27.Aninwene G.E., Y. C., II, Webster T.J. Enhanced osteoblast adhesion to drug-coated anodized nanotubular titanium surfaces. Int. J. Nanomed. 2008;3:257. doi: 10.2147/ijn.s2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasilev K., Poh Z., Kant K., Chan J., Michelmore A., Losic D. Tailoring the surface functionalities of titania nanotube arrays. Biomaterials. 2010;31:532–540. doi: 10.1016/j.biomaterials.2009.09.074. [DOI] [PubMed] [Google Scholar]
- 29.Alpaslan B. Ercan, Webster T.J. Anodized 20 nm diameter nanotubular titanium for improved bladder stent applications. Int. J. Nanomed. 2011;6:219. doi: 10.2147/IJN.S15816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia H., Kerr L.L. Sustained ibuprofen release using composite poly (lactic-co-glycolic acid)/titanium dioxide nanotubes from Ti implant surface. J. Pharm. Sci. 2013;102:2341–2348. doi: 10.1002/jps.23580. [DOI] [PubMed] [Google Scholar]
- 31.Smith L.J., Swaim J.S., Yao C., Haberstroh K.M., Nauman E.A., Webster T.J. Increased osteoblast cell density on nanostructured PLGA-coated nanostructured titanium for orthopedic applications. Int. J. Nanomed. 2007;2:493. [PMC free article] [PubMed] [Google Scholar]
- 32.Webster T.J., Smith T.A. Increased osteoblast function on PLGA composites containing nanophase titania. J. Biomed. Mater. Res. Part A. 2005;74:677–686. doi: 10.1002/jbm.a.30358. [DOI] [PubMed] [Google Scholar]
- 33.Pan H., Jiang H., Chen W. A fibroblast/macrophage co-culture model for in vitro evaluation of material biocompatibility and biodegradability. Annu. Northeast Bioeng. Conf. 2007;33:215–216. [Google Scholar]
- 34.Narayanan R., Kwon T., Kim K. TiO2 nanotubes from stirred glycerol/NH4F electrolyte: roughness, wetting behavior and adhesion for implant applications. Mater. Chem. Phys. 2009;117:460–464. [Google Scholar]
- 35.Gulati K., Ramakrishnan S., Aw M.S., Atkins G.J., Findlay D.M., Losic D. Biocompatible polymer coating of titania nanotube arrays for improved drug elution and osteoblast adhesion. Acta Biomater. 2012;8:449–456. doi: 10.1016/j.actbio.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Aw M.S., Simovic S., Addai-Mensah J., Losic D. Polymeric micelles in porous and nanotubular implants as a new system for extended delivery of poorly soluble drugs. J. Mater. Chem. 2011;21:7082–7089. [Google Scholar]
- 37.Avgoustakis K., Nixon J.R. Biodegradable controlled release tablets: III. Effect of polymer characteristics on drug release from heterogeneous poly (lactide-co-glycolide) matrices. Int. J. Pharm. 1993;99:247–252. [Google Scholar]
- 38.Smolinske S.C., Hall A.H., Vandenberg S.A., Spoerke D.G., McBride P.V. Toxic effects of nonsteroidal anti-inflammatory drugs in overdose. Drug Saf. 1990;5:252–274. doi: 10.2165/00002018-199005040-00003. [DOI] [PubMed] [Google Scholar]
- 39.Xu Z., Li M., Lia X., Liu Xiangmei, Ma Fei, Wu Shuilin, Yeung K.W.K., Han Yong, Chu Paul. K. Antibacterial activity of silver doped titanate nanowires on Ti implants. ACS Appl. Mater. Interfaces. 2016;8:16584–16594. doi: 10.1021/acsami.6b04161. [DOI] [PubMed] [Google Scholar]
- 40.Hall A.H., Smolinske S.C., Conrad F.L., Wruk K.M., Kulig K.W., Dwelle T.L., Rumack B.H. Ibuprofen overdose: 126 cases. Ann. Emerg. Med. 1986;15:1308–1313. doi: 10.1016/s0196-0644(86)80617-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.