Abstract
Background
Insulin-degrading enzyme (IDE) is an important regulator for Aβ clearance and diabetes. Although it is indispensable in removing plaques related to onset Alzheimer’s disease (AD) and in degrading insulin related to diabetes, there have been few studies on the dynamic level of IDE in different stages of AD.
Material/Methods
The present study explored the level IDE protein in different stages of APPswe/PS1dE9 mice and their correlations with cognitive decline. The 4-month-old, 10-month-old, and 18-month-old mice were used as the different age stages of mice. Cognitive function was evaluated using the Morris water maze test. We also observed the level of Aβ plaques in brain regions of different stages.
Results
The data revealed that the expression of IDE was dramatically higher than in age-matched wild mice at the age of 10 months and 18 months. In terms of distribution, Aβ plaques were deposited mostly in the cortex and hippocampus, especially in 10-month-old and 18-month-old APPswe/PS1dE9 mice. The cognitive function of 4-month-old APPswe/PS1dE9 mice was not significantly differ in spatial learning. However, the cognitive function, both spatial learning and spatial memory, was dramatically lower in 10-month-old and 18-month-old groups.
Conclusions
There was a positive correlation between the expression of IDE and spatial memory in 10-month-old and 18-month-old APPswe/PS1dE9 mice. The study of this protein may provide reference values for the further study of IDE in Alzheimer’s disease.
MeSH Keywords: Alzheimer Disease, Amyloid beta-Peptides, Insulysin, Mild Cognitive Impairment
Background
Alzheimer’s disease (AD), a progressive neurodegenerative disorder, is already a major focus of research attention. AD causes functional impairment, like loss of cognitive functions and behavioral changes, which has severe effects on AD patients [1,2]. It was reported that over 46 million elderly people have cognitive impairment and memory loss caused by AD [3,4]. Furthermore, this trend may be increased to more than 131 million by 2050, and expenditure on AD patients with dementia is more than $800 billion every year [5,6]. Cognitive decline has become the most important problem with AD patients [7].
Cognitive functions are attributed to different types of cerebral activities, which include learning memory, spatial perception, reasoning, judgement, and evaluation [8]. The brain areas involved in cognitive functions include the amygdala, hypothalamus, hippocampus, cingulate gyrus, and thalamus [9,10]. The decline in these functions, especially in the hippocampus, is considered to be an aggravating factor leading to progression of dementia from the mild cognitive impairment (the MCI phase) phase to Alzheimer’s disease (the DAT phase) [11,12].
Although factors promoting cognitive decline have been identified, such as age and genetic predisposition, the key pathogenic mechanism of AD memory loss is the accumulation of amyloid-β (Aβ) in the brain [13,14]. This compound is a cleavage product of the amyloid precursor protein (APP) by β- and γ-secretase. Aβ-induced neuron apoptosis and autophagy are considered to be the core pathological mechanism underlying neuronal damage in AD [15–17]. Recent research presents the consensus that Aβ42, the abundant cleavage product in the parenchyma plaques, is the primary amyloidogenic form in AD [18]. The lost dynamic balance between Aβ removal and production causes the abnormal accumulation of Aβ in the brain, triggering neuronal dysfunction and cell death [19]. The production of Aβ is believed to be important in the AD pathological process, but its clearance is also useful in maintaining Aβ steady-state levels [20,21].
Insulin-degrading enzyme (IDE) is a thiol-sensitive zinc metallopeptidase that is a major endogenous Aβ-degrading enzyme [22]. The level and enzymatic activity of IDE are negatively correlated with the size of the amyloid plaques and AD pathology [23]. There is evidence showing IDE-KO that mice had a more than 95% reduced rate of degradation of physiological Aβ levels and it also increased the levels of endogenous cerebral Aβ in an IDE-KO animal model [24]. Owing to the dual function of IDE in reducing insulin secretion and the role of plaque removal, it might show the different alteration of expression level compared to ordinary Aβ-degrading enzymes such as the metalloproteases neprilysin (NEP) and endothelin-converting enzyme (ECE) in different stages of the AD pathological process (mature adult, middle age, and old age) [25]. The level of this protein could be affected not only by the gradual accumulation of Aβ plaques, but also by insulin-related intervention. Thus, its expression level might have its own specificity. However, there have been few studies on the actual expression of IDE in different stages (juvenile, adult, old age) in APPswe/PS1dE9 double-transgenic mice and their correlation with cognitive decline.
In the present study, 4-month-old, 10-month-old, and 18-month-old APP/PS1 AD mice were used to explore the dynamic alteration of IDE in the AD pathophysiological process. In the AD animal model, the age span phases in C57BL/6J mice is divided into 3 stages, which include 3–6 months of age (mature adult), 10–15 months of age (middle age), and 18–24 months of age (old age) [26]. Meanwhile, APP/PS1 mice show amyloid plaques formation at around 4 months of age [27]. Thus, 4-, 10-, and 18-month-old mice can stand for the AD model mice stage as mature adult, middle age, and old phases, respectively. Furthermore, we also detected the cognitive function of AD model mice and Aβ plaque accumulated in the brain areas in charge of the cognitive functions (hippocampus and cortex). Meanwhile, the correlation between cognition and IDE was also analyzed.
Material and Methods
Animal Model
APPswe/PS1dE9 (Jackson Laboratory, stock No. 004462, n=30, male) were purchased from Nanjing Biomedical Research Institute of Nanjing University. All animal experiments conformed to the National Institutes of Health guidelines. All animal procedures were approved by the Ethics Committee of Xuanwu Hospital of Capital Medical University. The model mice were kept with accessible water and fed under a 12-h light-dark cycle. In the AD animal model, the age span phases in C57BL/6 J mice is divided into 3 stages: 3–6 months of age (mature adult), 10–15 months of age (middle age), and 18–24 months of age (old age) [26]. Thus, 4-, 10-, 18-month-old mice can represent the AD model mouse stage of mature adult, middle age, and old phases respectively [26,28]. We separated these mice into 3 groups: a 4-month-old group (AD, n=10), a 10-month-old group (AD, n=10), and an 18-month-old (AD, n=10). We used C57BL/6J mice of corresponding ages as wild-type groups (WT, n=10 per group).
Western blot
The hippocampus was taken out together with the inferior temporal cortex and prefrontal cortex (abbreviated hereafter as cortex). Then, we homogenized the tissue in neuronal Protein Extraction Reagent (Thermo Scientific, 87792) containing a cocktail of protease and phosphatase inhibitors (Thermo Scientific, 87786). Protein concentrations were determined using BCA protein assay (Thermo Scientific, 23227). Equal amounts of total protein were analyzed by 12% SDS-PAGE gels. Then, we transferred it to nitrocellulose (NC) membranes (Solarbio, Beijing, China). The primary antibodies rabbit anti-IDE (Abcam, 1: 1000) were used. Image Lab (Bio-Rad) was utilized for protein band densitometry.
Immunohistochemistry analysis
Immunohistochemical examinations were checked to determine the level of IDE in hippocampus and cortex of model mice. We separated 4 mice per group and primed them with saline solution. Then, perfusion was conducted with 4% paraformaldehyde. Brain tissues were removed and cut through the mid-sagittal plane. Hemispheres of the brain were fixed with 4% paraformaldehyde overnight, then we embedded them with paraffin. The paraffin sections were dried for 1 h at 60°C and dewaxed with xylene. After a graded series of ethanol solutions, antigen repair, incubation with 3% H2O2, and blocking (5% BSA), slides were incubated with primary antibody (anti-IDE,1: 50) overnight at 4°C, then rinsed with PBS and incubated with secondary antibody for 20 min at room temperature [29]. We visualized them with diaminobenzidine. The mean integral optical density (IOD) was calculated in 3 fields of the hippocampus and cortex for each slide. Each field was imaged at 400× using a microscope (Leica, DM3000) equipped with Image-Pro Plus 6.0 (Media Cybernetics, Rockville, MD, USA).
Thioflavin T assay
Brain tissue sections were washed 3 times with TBS for 5 min each time. Then, we incubated them for 10 min at room temperature with 10 μg/ml thioflavin T (ThT, Sigma, No. T3516). The sections were washed with 50% C2H5OH 3 times for 5 min each time. After that, we washed them with TBS 1 time for 5 min and kept them in the dark during the entire procedure. This reagent was used to stain amyloid Aβ deposits and must be freshly prepared and filtered. In the presence of amyloid fibrils, there is a bright fluorescence with this dye at the excitation and emission maxima of 450 and 482 nm, respectively [30].
Morris water maze
Hippocampal-dependent spatial learning and memory were evaluated using the Morris water maze, as described previously [28]. All mice in groups were individually coded. The measurements were performed by investigators blinded to group designations. Each group of mice (4-month-old, n=10;10-month-old, n=10; 18-month-old, n=10 WT and APPswe/PS1dE9 mice) received 5 consecutive days of training to find the submerged platform (in the second quadrant). Before the training, we kept the mice in the water for adaptation and carefully observed the movement and trajectory of each mouse. We excluded mice that were visually impaired or weak according to the observation of swim speed, swim path to a visible platform, and behavioral phenotypes that related to visual and motor functions. The experimental environment remained constant during behavioral testing. Swimming trajectories were videotaped. Escape latency and time spent in the target quadrant during probe trials were analyzed. After that, we put all the mice back for a 7-day rest. Then, the brains were removed for subsequent experiments.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software (version 5.01; CA, USA). The data presented for each experimental condition were obtained from at least 3 independent experiments. All data are presented as the mean ±S.D. Statistical significance was analyzed by the t test. The group differences in the escape latency were analyzed using two-way ANOVA in the Morris water maze test. A 2-tailed t test was used to evaluate and compare the 2 groups for time spent in the target quadrant during the last day. Linear regression analysis was carried out to evaluate correlations between the levels of IDE and spatial memory. Statistical significance was set at p<0.05.
Results
Age-dependent changes of IDE in different stages of APPswe/PS1dE9 mice
Increasing evidence shows that Aβ degrading enzymes contribute to the AD pathological process [31]. The levels of IDE in the hippocampus and cortex, which are the regions vulnerable to amyloid β protein accumulation, of all mice were first detected by Western blot analysis (Figure 1). The level of IDE in APPswe/PS1dE9 double-transgenic mice dramatically increased compared with age-matched control mice at the age of 10 months (p<0.01) and 18 months (p<0.05), but not at 4 months (Figure 1A, 1D). There were significant age-dependent group differences in the levels of IDE between 4-month-old and 10-month-old groups, showing the phases of the AD pathological development process in AD model mice (Figure 1B, p<0.01). However, no obvious changes were found among the 3 age groups of control mice (Figure 1C). All these data indicated that the expression level of IDE was associated with AD, especially in the aged phases. IDE protein expression was up-regulated with age in APP/PS1 mice (Figure 1B, p<0.01).
Figure 1.
The expression level of IDE in the cortex and hippocampus of APPswe/PS1dE9 mice at different ages. (A) Representative bands of IDE in the cortex and hippocampus of APPswe/PS1dE9 AD model mice included 4-month-old (4 M), 10-month-old (10 M), and 18-month-old (18 M) mice. T stand for APP/PS1 mouse groups. WT stand for wild-type groups. (B) The relative expression of IDE in the cortex and hippocampus of APPswe/PS1dE9 AD model mice. (C) The relative expression of IDE in the cortex and hippocampus of wild-type mice. (D) The relative expression of IDE in the cortex and hippocampus at different ages (* p<0.05, ** p<0.01).
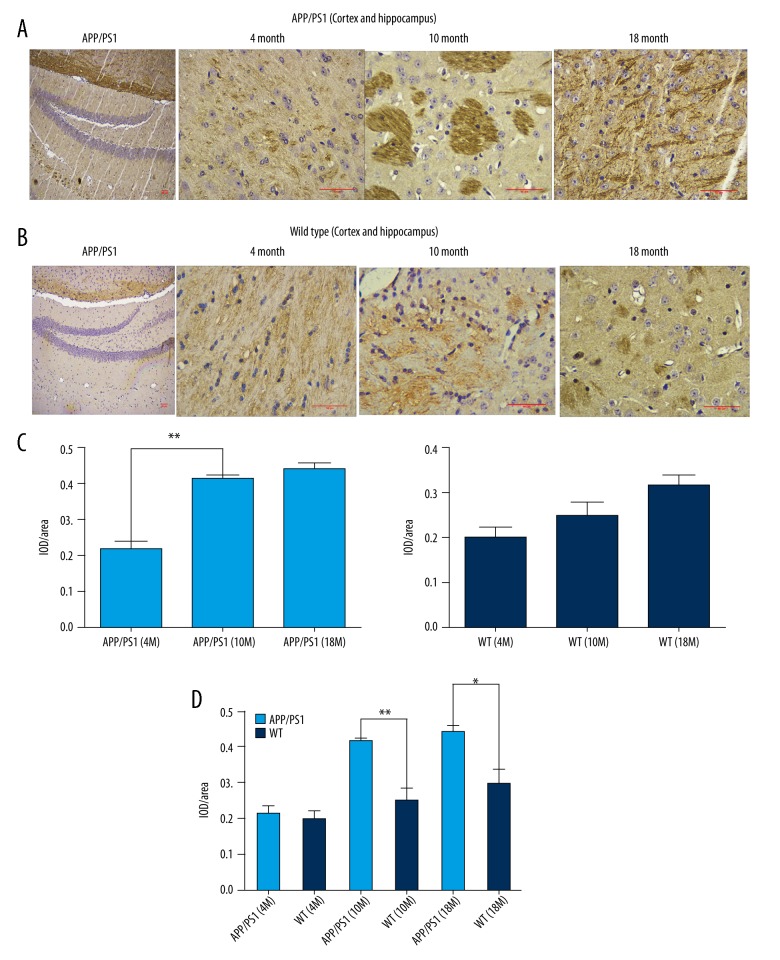
The expression level of IDE has obvious discrepancy in the different areas of the brain in APPswe/PS1dE9 mice
We continued to confirm the detailed and specific relationship of IDE with AD pathogenesis by immunohistochemistry (IHC) using paraffin sections of the APP/PS-1 mouse brain tissues (Figure 2). Expression of this protein in 10-month-old (p<0.01) and 18-month-old (p<0.05) mice were also higher than that in the age-matched mice in the control group, especially in the hippocampus and cortex (Figure 2A, 2B, 2D). Meanwhile, there were obviously differences in expression distribution of IDE in different brain regions. The level of IDE was increased significantly in the hippocampus and cortex of APPswe/PS1dE9 mice in the 10-month-old group compared to 4-month-old AD mice (Figure 2C, p<0.01). This evidence shows that IDE expression changes with age, especially in the hippocampus and cortex.
Figure 2.
The expression of IDE has obvious discrepancy in the different areas of the brain in APPswe/PS1dE9 mice. (A) Immunohistochemically stained paraffin section of the APPswe/PS1dE9 mouse brain tissues (n=4 per group). Scale bars, 50 μm. (B) Immunohistochemical stained paraffin section of the wild-type mouse brain tissue. (C, D) The relative expression of IDE in the cortex and hippocampus tested by immunohistochemical staining (* p<0.05, ** p<0.01).
Distribution of increased Aβ plaques in brain regions of different stages in APPswe/PS1dE9 mice
The deposition of Aβ plaques plays a very important role in the occurrence and development of AD [32]. The previous results have demonstrated that the level of IDE increased with age. IDE is one of the most important Aβ protein-degrading enzymes in AD; therefore, we sought to confirm the plaque formation at different ages in APPswe/PS1dE9 mice. The Aβ plaques in the brains of APPswe/PS1dE9 mice at 4, 10, and 18 months of age and the age-matched wild-type mice were analyzed with thioflavin T staining. The number of Aβ plaques were observed after staining, which revealed dense core amyloid plaques [33] (Figure 3). As illustrated in Figure 3A and 3B, the APPswe/PS1dE9 mice displayed greater numbers and area of Aβ plaques with age. There were significant age-dependent group differences in the numbers and area of Aβ plaques among the 3 groups of APPswe/PS1dE9 mice (Figure 3C, p<0.01). Furthermore, there were more plaques deposited in the cortex and hippocampus, especially in 10-month-old and 18-month-old groups of APPswe/PS1dE9 mice (Figure 3A, 3C, 3D, p<0.01).
Figure 3.
Distribution of increased Aβ plaques in brain regions (cortex and hippocampus) at different stages. (A, B) Representative epifluorescence microphotographs showing the β amyloid plaques density between APPswe/PS1dE9 and wild-type brains stained with thioflavin T, scale bars 50 μm (n=4 per group). (C, D) Quantification of the number of thioflavin-positive plaques in the cortex and hippocampus at different ages (* p<0.05, ** p<0.01).
APPswe/PS1dE9 double-transgenic mice dramatically decreased cognitive ability with age
Previous studies have found that Aβ plaques can induce memory loss [34,35]. To evaluate spatial memory, we performed behavioral testing with the 3 age groups of mice using the Morris water maze, which can assess spatial learning and memory associated with the hippocampus [28]. The analysis of mean escape latencies during the 5 days of training are exhibited in Figure 4A–4C. The data showed longer escape latencies in the 10-month-old (Figure 4B, group factor effect: F(1, 18)=5.64, p<0.05) and 18-month-old groups (Figure 4C, group factor effectL F(1, 18)=14.94, p<0.01) groups of APPswe/PS1dE9 mice compared with age-matched WT mice. However, there were no obvious differences between the 4-month-old APPswe/PS1dE9 mice and WT mice (Figure 4A, group factor effect: F(1, 18)=3.76, p>0.05). Meanwhile, there was no significant difference in swimming speed of APPswe/PS1dE9 double-transgenic mice compared with corresponding control mice (4 M: 11.11±1.77 vs. 10.19±2.16, p>0.05; 10 M: 8.28±1.62 vs. 9.58±1.76, p>0.05; 18 M: 7.35±1.14 vs. 7.50±1.78, p>0.05). This implies the escape latency differences in the water maze test were not due to motor deficits. Moreover, the visible platform trials in the water maze also suggested there were no significant differences in visual acuity and swimming speed between APP/PS1 mice and control mice at the 3 age groups.
Figure. 4.
Spatial learning and memory declined in APPswe/PS1dE9 mice. (A) The escape latency showed spatial learning in 4-month-old mice. (B) Spatial learning ability of 10-month-old mice. (C) Spatial learning ability of 18-month-old mice. (D) Time spent in the target quadrant (the second quadrant) tested by probe trial (n=10 per group, * p<0.05, ** p<0.01).
Additionally, spatial memory was evaluated using the probe trial at the sixth day to check the memory retention. As expected, all 3 age groups of APPswe/PS1dE9 mice spent less time in the target quadrant compared with age-matched controls (Figure 4D, p<0.01). Collectively, these data indicate that 10-month-old and 18-month-old APPswe/PS1dE9 mice had obvious cognitive deficits when compared with the age-matched WT mice.
Expression of IDE is inversely correlated with cognitive function
The potential role of IDE in the cognitive deficits was analyzed by correlation analysis. The time spent in the target quadrant in the Morris water maze was used to evaluate spatial memory ability. The correlations between the expression of IDE and spatial memory are shown in Figure 5.
Figure 5.
Relationship between the level of IDE and cognitive impairment in APPswe/PS1dE9 mice. (A) Correlation between the expression of IDE and spatial memory function was observed in the cortex and hippocampus of 4-month-old APPswe/PS1dE9 mice. (r2=0.07729, p>0.05). (B) Correlation between the expression of IDE and spatial memory function of 10-month-old APPswe/PS1dE9 mice (r2=0.6264, p<0.01). (C) Correlation between the expression of IDE and spatial memory function of 18-month-old APPswe/PS1dE9 mice (r2=0.5484, p<0.05).
There was a significant positive correlation between the expression of IDE and spatial memory in 10-month-old (Figure 5B, r2=0.6264, p<0.01) and 18-month-old (Figure 5C, r2=0.5484, p<0.05) APPswe/PS1dE9 mice. However, the level of IDE in 4-month-old APPswe/PS1dE9 mice did not significantly correlate with spatial memory performance (Figure 5A, p>0.05). These data indicate that the expression of IDE is inversely correlated with cognitive function in APPswe/PS1dE9 mice.
Discussion
Aβ plaques are one of the most important risk factors of advanced cognitive impairment in the pathogenesis of AD. They can be produced by neuronal cells and peripheral cells [36]. Almost all patients with Alzheimer’s disease, which includes familial AD and sporadic AD, have Aβ plaques deposited in brain tissue. Greater Aβ plaques accumulation also causes enhanced neuronal loss and leads to cognitive impairment [1]. Therefore, reducing the production of Aβ and promoting Aβ degradation have become one of ways to control the progression of AD.
Aβ oligomers could be degraded by various enzymes, which include neutral endopeptidase (NEP), endothelin-converting enzyme (ECE), and insulin-degrading enzyme (IDE). It was reported that NEP, which is located on the cell surface, plays a more important role in resisting Aβ accumulation in infant and juvenile mice than does endothelin-converting enzyme (ECE) [37]. Compared with other Aβ-degrading enzymes, IDE not only affects the pathogenesis of AD, but also is associated with type 2 diabetes [38], and in-depth study may help understand the actual pathological changes and the prognosis of related senile diseases. The affinity of IDE for insulin is much higher than that for Aβ, but its rate of hydrolysis of insulin is very slow. Because IDE has a strong affinity for insulin, the scientific community has been paying more attention to its role in diabetes. It has begun to find that IDE actually has its own unique effect on AD in recent years. Meanwhile, the scientific community has found that patients with type 2 diabetes have an accelerated decline in cognitive function, and the risk of dementia is increased by 1.3 to 1.4 times [39]. Because of this close relationship between diabetes and AD, IDE is also a substance that might play a role in both diseases. This protein could affect the pathogenic core factors (insulin and Aβ) in both diseases. So, the actual changes in the expression level of IDE might be more complex than the changes in other Aβ-degrading enzymes, but few studies have reported on this.
IDE, composed of 4 similar domains, is a zinc metallopeptidase that can degrade many physiological peptides. Many studies have reported that IDE is secreted in exosomes from astrocytes in the brain [38]. In this study, we demonstrated that the expression of IDE was dramatically higher with increased age in APPswe/PS1dE9 AD model mice (Figure 1). The same trend was also found in paraffin sections of the mouse brain tissue (Figure 2A, 2B). The AD susceptibility genes APP, PS-1, and PS-2 have all been found to increase Aβ production [40]. Our team used APPswe/PS1dE9 double-transgenic mice, which showed amyloid plaques formation at around 4 months of age [27]. Results obtained using the Western blot-extracted protein from the hippocampus and cortex of model mice and those obtained through the immunohistochemical method that used paraffin sections of the mouse brain tissue showed that IDE is up-regulated at different stages of APPswe/PS1dE9 mice, depending on age (Figure 1A, 1B). A possible mechanism for this trend might be that neurotoxicity damage caused by Aβ causes cellular dysfunction or cell apoptosis in which a small percentage of astrocytes become lysed. This mechanism might increase the release of IDE induced by altering membrane integrity.
The expression of APPswe/PS1dE9 promotes the rapid accumulation of Aβ plaques in the brain of AD model mice. Aβ can form senile plaques outside the cell and produce strong cytotoxicity, leading to neuronal apoptosis in the cells. Owing to this cytotoxicity, the neurons in important brain regions associated with cognitive functions are gradually lost [41]. The data showed the APPswe/PS1dE9 mice displayed strongly increased numbers and area of Aβ plaques with age. Furthermore, there were more plaques deposited in the cortex and hippocampus, especially in 10-month-old and 18-month-old groups of APPswe/PS1dE9 mice (Figure 3A, 3C, 3D).
The cortex and hippocampus are considered to be important regions for AD pathological variation [42]. We found region- and age-related changes in the level of IDE in the cerebral cortex and hippocampus. The protein of IDE in the cerebral cortex and hippocampus was significantly increased in the APPswe/PS1dE9 mice older than 10 months of age (Figure 1A, 1B, 2A, 2B), which strongly matched the increase in Aβ plaques indicated by thioflavin T staining (Figure 3A, 3B).
Cognitive impairment is one of the main clinical manifestations of Alzheimer’s disease and seriously decreases the quality of life of people with AD [42]. According to the Morris water maze test results, 10-month-old and 18-month-old APPswe/PS1dE9 mice showed obvious cognition deficits, revealing significantly reduced time spent in the target quadrant compared with control mice (Figure 4D). These results also matched the trend of Aβ plaques accumulation detailed above.
Results of the present study demonstrate that the level of IDE is associated with AD pathogenesis, especially with Aβ plaques, which were known to be one of the causes of cognitive decline [43]. Our evaluation of the relationships between cognitive decline and expression of IDE showed the expression of IDE is inversely correlated with cognitive function in 10-month-old (Figure 5B) and 18-month-old (Figure 5C) APPswe/PS1dE9 mice. A possible mechanism for this might be associated with increased IDE in the specific brain regions in which Aβ accumulated. On the one hand, the increased expression of IDE makes more Aβ be decomposed in part of cortex and hippocampus. This trend reduced the neurotoxicity effect induced by Aβ in these areas. More neurons survived owing to the elevated level of IDE. On the other hand, IDE, as the insulin-degrading enzyme, reduces the regional brain insulin level. Insulin is the most important hormone that lowers the level of blood glucose. Because of the decreased level of insulin, the level of brain blood glucose increased. The elevated blood glucose could help more neurons survive Aβ-induced neurotoxicity.
Although the association of IDE and cognitive decline was shown in our study, our results do not prove a causal relationship. It should be noted that this study examined only APPswe/PS1dE9 AD model mice, and our results do not suggest that the same applies to clinical AD patients in the corresponding age stages. Further research is needed to elucidate the relationship between IDE and cognitive function in the AD pathological process. Our findings suggest that this protein might provide a reference value for earlier diagnosis of cognitive impairment and timely therapy to correct Aβ plaques accumulation.
Conclusions
These observations, together with those of alterations, suggest that IDE affected AD pathogenesis in an age-dependent manner. In summary, the expression of IDE is negatively associated with cognitive decline. The study of this protein may provide reference values for the further study of IDE in Alzheimer’s disease.
Footnotes
Conflicts of interest
None.
Source of support: The present study was supported by the National Natural Science Foundation of China (No. 81472007)
References
- 1.Day GS, Lim TS, Hassenstab J, et al. Differentiating cognitive impairment due to corticobasal degeneration and Alzheimer disease. Neurology. 2017;88(13):1273–81. doi: 10.1212/WNL.0000000000003770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandez MV, Kim JH, Budde JP, et al. Analysis of neurodegenerative Mendelian genes in clinically diagnosed Alzheimer Disease. PLoS Genet. 2017;13(11):e1007045. doi: 10.1371/journal.pgen.1007045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brookmeyer R, Abdalla N, Kawas CH, et al. Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimers Dement. 2018;14(2):121–29. doi: 10.1016/j.jalz.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vebraite E, Morkuniene V, Petrikonis K, et al. Cognitive failure evaluation and therapy based on pharmacy practice – utilization of anti-dementia drugs and food supplements in Lithuania. Int J Clin Pharmacol Ther. 2013;51(4):323–31. doi: 10.5414/CP201848. [DOI] [PubMed] [Google Scholar]
- 5.Neu SC, Crawford KL, Toga AW. Sharing data in the global Alzheimer’s Association interactive network. Neuroimage. 2016;124(Pt B):1168–74. doi: 10.1016/j.neuroimage.2015.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wimo A, Guerchet M, Ali GC, et al. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement. 2017;13(1):1–7. doi: 10.1016/j.jalz.2016.07.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allone C, Lo Buono V, Corallo F, et al. Cognitive impairment in Parkinson’s disease, Alzheimer’s dementia, and vascular dementia: The role of the clock-drawing test. Psychogeriatrics. 2018;18(2):123–31. doi: 10.1111/psyg.12294. [DOI] [PubMed] [Google Scholar]
- 8.Larrabee GJ. The multiple validities of neuropsychological assessment. Am Psychol. 2015;70(8):779–88. doi: 10.1037/a0039835. [DOI] [PubMed] [Google Scholar]
- 9.Kilts CD, Kelsey JE, Knight B, et al. The neural correlates of social anxiety disorder and response to pharmacotherapy. neuropsychopharmacol. 2006;31(10):2243–53. doi: 10.1038/sj.npp.1301053. [DOI] [PubMed] [Google Scholar]
- 10.Pike NA, Roy B, Gupta R, et al. Brain abnormalities in cognition, anxiety, and depression regulatory regions in adolescents with single ventricle heart disease. J Neurosci Res. 2018 doi: 10.1002/jnr.24215. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henry-Feugeas MC. MRI of the ‘Alzheimer syndrome’. J Neuroradiol. 2007;34(4):220–27. doi: 10.1016/j.neurad.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Henry-Feugeas MC. Assessing cerebrovascular contribution to late dementia of the Alzheimer’s type: The role of combined hemodynamic and structural MR analysis. J Neurol Sci. 2009;283(1–2):44–48. doi: 10.1016/j.jns.2009.02.325. [DOI] [PubMed] [Google Scholar]
- 13.Han P, Nielsen M, Song M, et al. The impact of aging on brain pituitary adenylate cyclase activating polypeptide, pathology and cognition in mice and rhesus macaques. Front Aging Neurosci. 2017;9:180. doi: 10.3389/fnagi.2017.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mormino EC, Sperling RA, Holmes AJ, et al. Polygenic risk of Alzheimer disease is associated with early- and late-life processes. Neurology. 2016;87(5):481–88. doi: 10.1212/WNL.0000000000002922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calvo-Rodriguez M, de la Fuente C, Garcia-Durillo M, et al. Aging and amyloid beta oligomers enhance TLR4 expression, LPS-induced Ca(2+) responses, and neuron cell death in cultured rat hippocampal neurons. J Neuroinflammation. 2017;14(1):24. doi: 10.1186/s12974-017-0802-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaerzadeh F, Motamedi F, Minai-Tehrani D, et al. Monitoring of neuronal loss in the hippocampus of Abeta-injected rat: Autophagy, mitophagy, and mitochondrial biogenesis stand against apoptosis. Neuromolecular Med. 2014;16(1):175–90. doi: 10.1007/s12017-013-8272-8. [DOI] [PubMed] [Google Scholar]
- 17.Singh AK, Bissoyi A, Kashyap MP, et al. Autophagy activation alleviates amyloid-beta-induced oxidative stress, apoptosis and neurotoxicity in human neuroblastoma SH-SY5Y cells. Neurotox Res. 2017;32(3):351–61. doi: 10.1007/s12640-017-9746-5. [DOI] [PubMed] [Google Scholar]
- 18.Petrasek T, Skurlova M, Maleninska K, et al. A rat model of Alzheimer’s disease based on Abeta42 and pro-oxidative substances exhibits cognitive deficit and alterations in glutamatergic and cholinergic neurotransmitter systems. Front Aging Neurosci. 2016;8:83. doi: 10.3389/fnagi.2016.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye X, Feng T, Tammineni P, et al. Regulation of synaptic amyloid-beta generation through BACE1 retrograde transport in a mouse model of Alzheimer’s disease. J neurosci. 2017;37(10):2639–55. doi: 10.1523/JNEUROSCI.2851-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mawuenyega KG, Sigurdson W, Ovod V, et al. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330(6012):1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harach T, Jammes F, Muller C, et al. Administrations of human adult ischemia-tolerant mesenchymal stem cells and factors reduce amyloid beta pathology in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2017;51:83–96. doi: 10.1016/j.neurobiolaging.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Zhao L, Yao J, Mao Z, et al. 17beta-Estradiol regulates insulin-degrading enzyme expression via an ERbeta/PI3-K pathway in hippocampus: Relevance to Alzheimer’s prevention. Neurobiol Aging. 2011;32(11):1949–63. doi: 10.1016/j.neurobiolaging.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vekrellis K, Ye Z, Qiu WQ, et al. Neurons regulate extracellular levels of amyloid beta-protein via proteolysis by insulin-degrading enzyme. J Neurosci. 2000;20(5):1657–65. doi: 10.1523/JNEUROSCI.20-05-01657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farris W, Mansourian S, Chang Y, et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci USA. 2003;100(7):4162–67. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miners JS, Baig S, Palmer J, et al. Abeta-degrading enzymes in Alzheimer’s disease. Brain Pathol. 2008;18(2):240–52. doi: 10.1111/j.1750-3639.2008.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schatten H, Constantinescu GM. Animal models and human reproduction. John Wiley & Sons Inc; 2017. [Google Scholar]
- 27.Aso E, Lomoio S, Lopez-Gonzalez I, et al. Amyloid generation and dysfunctional immunoproteasome activation with disease progression in animal model of familial Alzheimer’s disease. Brain Pathol. 2012;22(5):636–53. doi: 10.1111/j.1750-3639.2011.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crawley JN. What’s wrong with my mouse? behavioral phenotyping of transgenic and knockout mice. John Wiley & Sons Inc; 2007. [Google Scholar]
- 29.Ba L, Chen XH, Chen YL, et al. Distinct Rab7-related endosomal-autophagic-lysosomal dysregulation observed in cortex and hippocampus in APPswe/PSEN1dE9 mouse model of Alzheimer’s disease. Chin Med J (Engl) 2017;130(24):2941–50. doi: 10.4103/0366-6999.220311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baranger K, Marchalant Y, Bonnet AE, et al. MT5-MMP is a new pro-amyloidogenic proteinase that promotes amyloid pathology and cognitive decline in a transgenic mouse model of Alzheimer’s disease. Cell Mol Life Sci. 2016;73(1):217–36. doi: 10.1007/s00018-015-1992-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Devi L, Alldred MJ, Ginsberg SD, et al. Mechanisms underlying insulin deficiency-induced acceleration of beta-amyloidosis in a mouse model of Alzheimer’s disease. PLoS One. 2012;7(3):e32792. doi: 10.1371/journal.pone.0032792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sevigny J, Chiao P, Bussiere T, et al. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature. 2016;537(7618):50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 33.Kung MP, Hou C, Zhuang ZP, et al. IMPY: an improved thioflavin-T derivative for in vivo labeling of beta-amyloid plaques. Brain Res. 2002;956(2):202–10. doi: 10.1016/s0006-8993(02)03436-4. [DOI] [PubMed] [Google Scholar]
- 34.Varga E, Juhasz G, Bozso Z, et al. Amyloid-beta 1–42 disrupts synaptic plasticity by altering glutamate recycling at the synapse. J Alzheimers Dis. 2015;45(2):449–56. doi: 10.3233/JAD-142367. [DOI] [PubMed] [Google Scholar]
- 35.Howlett DR, Richardson JC, Austin A, et al. Cognitive correlates of Abeta deposition in male and female mice bearing amyloid precursor protein and presenilin-1 mutant transgenes. Brain Res. 2004;1017(1–2):130–36. doi: 10.1016/j.brainres.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 36.Park S, Huh J, Eom T, et al. Effects of newly synthesized recombinant human amyloid-beta complexes and poly-amyloid-beta fibers on cell apoptosis and cognitive decline. J Microbiol Biotechn. 2017;27(11):2044–51. doi: 10.4014/jmb.1707.07003. [DOI] [PubMed] [Google Scholar]
- 37.Zhou L, Liu J, Dong D, et al. Dynamic alteration of neprilysin and endothelin-converting enzyme in age-dependent APPswe/PS1dE9 mouse model of Alzheimer’s disease. Am J Transl Res. 2017;9(1):184–96. [PMC free article] [PubMed] [Google Scholar]
- 38.Li H, Yang S, Wu J, et al. cAMP/PKA signaling pathway contributes to neuronal apoptosis via regulating IDE expression in a mixed model of type 2 diabetes and Alzheimer’s disease. J Cell Biochem. 2018;119(2):1616–26. doi: 10.1002/jcb.26321. [DOI] [PubMed] [Google Scholar]
- 39.Strachan MWJ, Reynolds RM, Marioni RE, et al. Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat Rev Endocrinol. 2011;7(2):108–14. doi: 10.1038/nrendo.2010.228. [DOI] [PubMed] [Google Scholar]
- 40.Scheuner D, Eckman C, Jensen M, et al. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med. 1996;2(8):864–70. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 41.Lillehaug S, Syverstad GH, Nilsson LNG, et al. Brainwide distribution and variance of amyloid-beta deposits in tg-ArcSwe mice. Neurobiol Aging. 2014;35(3):556–64. doi: 10.1016/j.neurobiolaging.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 42.Shepherd CE, Yang Y, Halliday GM. Region- and cell-specific aneuploidy in brain aging and neurodegeneration. Neuroscience. 2018;374:326–34. doi: 10.1016/j.neuroscience.2018.01.050. [DOI] [PubMed] [Google Scholar]
- 43.Ou Z, Kong X, Sun X, et al. Metformin treatment prevents amyloid plaque deposition and memory impairment in APP/PS1 mice. Brain Behav Immun. 2018;69:351–63. doi: 10.1016/j.bbi.2017.12.009. [DOI] [PubMed] [Google Scholar]