ABSTRACT
Introduction: Chronic Cough (CC) is common and often associated with significant comorbidity and decreased quality of life. In up to 50% of cases, the cough is refractory despite extensive investigation and treatment trials. It is likely that the key abnormality in refractory CC is dysfunctional, hypersensitive sensory nerves, similar to conditions such as laryngeal hypersensitivity and neuropathic pain.
Areas covered: The aim of this systematic review is to assess drug therapies for refractory CC. The authors review the current management of CC and provide discussion of the similarities between neuropathic pain and refractory CC. They review repurposed and new pharmacological treatments. Several meta-analyses were performed to compare the efficacy of treatments where possible.
Expert opinion: Repurposed pain medications such as gabapentin and pregabalin reduce the frequency of cough and improve quality of life. Along with speech pathology, they are important and alternate treatments for refractory CC. However, more treatments are needed and the P2X3 ion channel receptor antagonists show the most promise. With a better understanding of neuronal activation and sensitisation and their signal processing in the brain, improved animal models of cough, and the use of validated cough measurement tools, more effective treatments will develop.
KEYWORDS: Antitussives, cough hypersensitivity syndrome, ion channel receptor antagonists, neuromodulating drugs, refractory chronic cough
1. Introduction
Chronic cough (CC) is common and is associated with decreased quality of life [1]. Common causes of CC are asthma, gastroesophageal reflux disease (GERD), and upper airway disorders such as postnasal drip syndrome and rhinosinusitis. Cough has been reported to be refractory to guideline-based treatment in approximately 42% of cases [2]. Current guideline-approved treatment options such as gabapentin and speech pathology treatment (SPT) [3] improve cough in many patients but they do not work for all patients and gabapentin and other centrally acting drugs can have undesirable side effects. New and improved treatments are therefore needed. The understanding of the pathophysiology of cough reflex and its connections in the brain has improved and led to the identification of targets for antitussive drug development. There have been numerous phase I and II clinical trials of treatments for refractory CC and some results have been very promising. We reviewed the clinical effectiveness of current and developing drug therapies for refractory CC.
1.1. The management of CC
The classification of cough is given in Table 1. ‘Chronic cough’ is defined as a cough of more than 8 weeks’ duration. [4] CC that persists despite assessment and treatment according to an accepted guideline is termed refractory chronic cough, idiopathic chronic cough, or unexplained cough [2,3,5]. Cough reflex hypersensitivity (CRS) is a key feature of refractory CC involving both peripheral and central sensitization of the cough reflex [6,7]. The term sensory neuropathic cough is now often recognized in cough guidelines. It has overlap with laryngeal hypersensitivity and cough hypersensitivity [7,8] syndromes and is a component of refractory CC [9]. Early systematic evaluation and treatment guidelines for CC by Irwin and colleagues (1977) were based on the anatomic locations of the receptors and afferent pathways involved in the cough reflex [10]. Using such an approach, Irwin and colleagues reported that the cause of CC could be determined in 100% of patients and that subsequent cause-specific treatment was almost always successful. A stepwise diagnostic approach, termed the anatomic-diagnostic protocol (ADP), was recommended by the American College of Chest Physicians (ACCP) in 1998 [5]. The ADP involves a targeted patient history and physical examination to investigate the possible cause/s of their cough. This information is then used to initiate a stepwise treatment management program until resolution of the cough symptoms.
Table 1.
Classification of cough.
| Clinical cough descriptor | Definition |
|---|---|
| Acute | Cough that lasts for <3 weeks. |
| Subacute | Cough that lasts 3 to 8 weeks. |
| Chronic | Cough that lasts >8 weeks |
| Chronic refractory | Cough that does not respond to usual medical treatment such as the ADP. |
| Chronic idiopathic | Cough with no underlying cause even after a thorough systematic review. |
| Specific | A known underlying disease causing the cough. |
| Sensory neuropathic cough | A chronic cough disorder that is thought to have a neurogenic cause.a |
ADP: anatomical diagnostic protocol.
aSometimes referred to as a cough caused by ‘laryngeal sensory neuropathy,’
Symptoms include: Allotussia, cough triggered in response to a nontussive stimulus, e.g. talking; Hypertussia, increased cough sensitivity in response to a known tussigen, e.g. smoke; Laryngeal paresthesia, abnormal throat sensation, e.g. ‘tickle’.
[Adapted from [51] with permission of Taylor & Francis].
Modifications to the ADP sought to simplify the assessment and management of CC [11,12]. Yu et al. [13] evaluated a sequential three-step empirical therapy for CC with an overall success rate of 88%. However, subsequent studies showed that an anatomic diagnostic or empirical approach failed to identify the cause of cough in approximately 40% of cases [2].
The management approaches to CC have been systematized in clinical practice guidelines published in several countries. The ACCP guidelines [14] evaluate each component of the ADP and provide a set of user-friendly guides for clinical practice on the major causes of cough and treatment recommendations. The European Respiratory Society guidelines [15] suggest two pathways, one using an empirical approach or recommended investigations that can be used in parallel. The British Thoracic Society guidelines [16] cover not only CC but also acute cough and the organization of cough clinics. The Australian Cough Guidelines Summary (CICADA) is a clinical guideline for the assessment and management of persistent cough in children and adults. The guideline was developed by a multidisciplinary expert committee (including Allied Health, Otolaryngology, Respiratory and Immunology and Psychology) and is unique as it recognizes conditions such as obstructive sleep apnea and paradoxical vocal fold movement (PVFM)/vocal cord dysfunction as causes of specific cough [17].
1.2. Cough hypersensitivity syndrome
CC has been labeled as a cough hypersensitivity syndrome (CHS) [18,19] with neuroinflammatory mechanisms likely to be the underlying mechanisms. The concept evolved from clinical observations of patients who had no apparent clinical cause for their cough (unexplained/idiopathic cough) or remained refractory to usual cough treatments (refractory CC) and from analogies with chronic neuropathic pain [20].
Functional changes in TRPV1, TRPA1, and P2X3 nerve channels and the development of peripheral and central sensitization are thought to turn cough from being a defensive reflex into a cough hypersensitivity syndrome [21]. Distinct higher brain circuitry for facilitating and suppressing the cough reflex has been visualized by functional magnetic resonance imaging (fMRI) [22]. CHS is associated with hypersensitivity of the larynx and upper airway and is often diagnosed by clinical history [23] and sometimes through quantitative sensory testing such as with hypertonic saline challenge, transnasal laryngoscopy with odor provocation, and cough reflex sensitivity testing with capsaicin [7,8]. Although some features of refractory CC are encompassed by the term CHS, many patients localize symptoms to the larynx [9,24] and therefore laryngeal hypersensitivity syndrome maybe a better description. CHS is known to overlap with other laryngeal hypersensitivity syndromes including PVFM [7] and muscle tension dysphonia. [8] PVFM has been identified in around 56% of subjects with CC. [7] PVFM and refractory CC exhibit overlap in symptomatology, such as cough and dysphonia, and overlap in disease associations, namely asthma, GERD, and rhinosinusitis. Patients with refractory CC or with combined refractory CC and PVFM have marked CRS [7]. The term laryngeal hypersensitivity is often used interchangeably with sensory neuropathic cough [25,26] (Table 1).
1.3. Receptors common to CC and chronic pain
TRP channels are expressed in almost every tissue and cell type and play an important role in the regulation of various cell functions. They are able to sense temperature, noxious stimuli, pain, stretch, and osmolarity, and are involved in various diseases through an increased level of channel expression [27]. TRP ion channels are present in the airways, primary airway sensory neurons, smooth muscle, and epithelial cells [21]. In cough, inflammation in the lungs or esophagus increases the afferent nerve excitation [28] that leads to a referred sensation of irritation in the throat and a reduced cough threshold. The reduced cough threshold in refractory CC is associated with increased expression of TRPV1 receptors on airway nerves [29]. Several highly selective TRPV1 antagonists have advanced into clinical development for the treatment of pain. [30,31] One of these TRPV1 antagonists, SB-705498 has been recently trialed in the treatment of refractory CC [32].
Peptide substance P and its tachykinin receptor, neurokinin-1 (NK1), have also been the focus of considerable research for their role in a variety of both central and peripheral diseases [33]. NK1 receptor antagonists appear able to block behavioral responses to noxious and other stressful sensory pain stimuli at a level detectable in animal tests but fail to provide the level of sensory blockade required to produce clinical analgesia in humans [34]. A variety of reasons have been proposed for the presumed mismatch between the preclinical effects of NK1 receptor antagonists in animal models and their effects in humans including using animal species with different pain pathways to humans and differences in pharmacokinetic parameters [35,36]. Preclinical studies have shown that NK1 receptor antagonists block the neurogenic inflammatory response produced by administration of capsaicin [37,38] and electrical stimulation of the trigeminal ganglion [39]. Most of the interest surrounding the use of NK1 receptor antagonists in cough has been restricted to their involvement in the cough response in asthma [40]. CP-99,994 was found to inhibit capsaicin-induced cough in the guinea pig when administered both subcutaneously and into the ventricles of the brain, suggesting that the compound exerts its effects both centrally and peripherally. While a similar result did not occur in human trials [41] a much more recent trial reported a positive effect on refractory CC patients with the centrally active NK1 antagonist Orvepitant. [42] P2X and P2Y receptors are purinergic cell surface ion channels gated by extracellular ATP [43]. Cellular distress caused by injury or infection often leads to the release of high concentrations of ATP, inducing hypersensitization of nerves and causing chronic or debilitating symptoms, such as CC. P2X3, P2X2/3, P2X4, and P2X7 receptors have received a lot of recent attention as potential targets to treat a variety of conditions that include chronic pain and arthritis [44]. Clinically the P2X7 receptor antagonists CE-224535 and AZD9056 have not demonstrated efficacy in rheumatoid arthritis and it is unknown whether they may be useful in pain indications [45,46]. P2X3 receptor expression changes in animal pain models of P2X3 knock-out mice have shown the development of reduced mechanical allodynia [47] and neuronal P2X3 receptor activation predisposing afferent neurons to inflammatory hyperalgesia [48]. The antitussive actions of the P2X3 antagonist AF-219 occurs in the absence of any effect on capsaicin-induced cough [49], which is consistent with preclinical cough studies in guinea pigs showing that ATP and capsaicin have independent mechanisms of action [50].
N-methyl-D-aspartate (NMDA) receptors are involved in acid-evoked reflexes such as the cough reflex [51]. Ketamine is a commonly used analgesia in pain and has both acute and prolonged effects on chronic neuropathic pain syndromes and symptoms of allodynia and hyperalgesia. Allodynia and hyperalgesia are akin to the clinical characteristics of allotussia and hypertussia found in refractory CC. Unfortunately, NMDA antagonists have variable outcomes in their treatment of neuropathic pain [52] but maybe worth investigating further in refractory CC. In a guinea pig study by Canning and Mori [53], a predominant role for NMDA receptor activation during cough and a modulatory role for non-NMDA receptors was found. A synergistic inhibition of evoked coughing was observed when both NMDA (AP-5) and non-NMDA receptor antagonists (CNQX) were administered simultaneously.
Of the nine subtypes of Nav channels, Nav1.7, Nav1.8, and Nav1.9 are primarily expressed by sensory neuron C-fibers and A-fibers including those from the nodose and jugular ganglia [54]. In response to inflammatory mediators these channels are upregulated increasing cough sensitivity. Unfortunately, inhibition of these Nav channel blockers may anesthetize the airways to any stimuli potentially blocking necessary defensive coughing. Nav1.7 target anesthetics such as lidocaine have been found to be minimally effective at blocking cough. A lot of recent interest has been generated in Nav 1.8 as a target for both inflammatory and neuropathic pain with a few inhibitors being used in preclinical target validation [55–57]. Whether targeting Nav1.8 or Nav1.9 are effective at blocking unhelpful cough remains to be investigated.
1.3.1. Further similarities between CC and chronic pain
The overall prevalence of CC is 9.6% [58] which is similar to the prevalence of neuropathic pain in Australia (8.5%) [59] and in Europe (7–8%) [60]. There are, however, wide regional variations ranging from a high of 18.1% in Oceania down to 2.3% in Africa for CC [58]. Chronic neuropathic pain has been found to be more frequent in women [61], and this is consistent with refractory CC [62,63]. The basic neurobiological mechanisms and pathologies of refractory CC and chronic pain show substantial homologies. Chronic pain may result from disorders of the peripheral nervous system or they may arise from the central nervous system (brain and spinal cord) [64]. ‘Chronic pain’ and ‘chronic cough’ serve as umbrella terms encompassing a wide variety of clinical features such as hyperalgesia/hypertussivity and allodynia/allotussivity, features that can be broken down further into the modality affected.
A key feature of refractory CC is an increased cough reflex sensitivity involving both peripheral and central sensitization of the cough reflex [2,6]. Peripheral sensitization can occur in sensitized areas like the larynx, esophagus, pharynx, and bronchi mediated by the vagus nerve. Inflammatory mediators such as histamine and prostaglandins sensitize cough afferent nerve endings increasing the excitation of afferent nerves [28] and decreasing the threshold for cough. Patients with CC have a fivefold elevation of TRPV1-containing nerves [28] and exposure to low-level tussive stimuli such as smoke results in a hypersensitive cough reflex termed hypertussia [65]. In refractory CC, there is a significant increase in cough reflex sensitivity to capsaicin [7] that explains why hypertussia is common in refractory CC. This is common to hyperalgesia in neuropathic pain where there is pain triggered by low-level exposure to a known painful stimulus [20].
Central sensitization is a state where increased excitability is triggered within the spinal cord by peripheral noxious inputs. When neurons in the dorsal horn spinal cord are subject to central sensitization, they exhibit some or all the following: development of or increases in spontaneous activity, a reduction in the threshold for activation by peripheral stimuli, increased responses to suprathreshold stimulation, and an enlargement of their receptive fields [66]. After peripheral nerve injury, damaged and nondamaged electrophysiological changes particular to central sensitization correlate with the development in human experimental subjects after a noxious conditioning input of allodynia (particularly dynamic tactile or brush-evoked allodynia), the temporal summation of repeated low-intensity stimuli from an innocuous sensation to pain, with ‘after-pain’ on cessation of the stimulus, and widespread secondary hyperalgesia [66]. These changes can be elicited in human volunteers by noxious stimulation of the skin as with topical or intradermal capsaicin or repeated heat stimuli [67], and in the gastrointestinal tract by exposure to low pH solutions [68]. Similar clinical features such as an abnormal laryngeal sensation or throat tickle (laryngeal paresthesia), increased cough sensitivity in response to a known tussigen (hypertussia), and cough triggered in response to non-tussive triggers such as cold air or talking on the phone (allotussia) are seen in refractory CC [65]. The involvement of peripheral sensitization in central sensitization indicates that these are not mutually exclusive phenomenon. This may make it difficult to fully dissect the specific central and peripheral contributions of underlying symptoms for both chronic pain and cough [20].
Pharmacological management remains the most important therapeutic option for chronic neuropathic pain even though the results are often unsatisfactory. There is a similar unmet need in refractory CC. Understanding the role of receptors and mechanisms involved in neuropathic pain and neuropathic cough is important for understanding the mechanism of drug therapy for cough and how drugs designed for the treatment of pain can be repurposed for cough. Pharmacological fMRI is an ideal noninvasive tool that can be used to determine the effects of drugs on brain activation. An fMRI study in humans by Iannetti et al. [69] demonstrated the complex effects of the neuromodulating drug gabapentin on brain activation. The most pronounced effect was a reduction in stimulus-induced brain deactivation following central sensitization. In cough, Mazzone et al. [22] investigated the neural control of cough and cough suppression in healthy humans with capsaicin-evoked cough using fMRI. These studies have confirmed the existence of distinct higher brain circuitry for facilitating and suppressing the cough reflex and provide novel insights into the supramedullary control of cough in humans. This technology could be further utilized in refractory CC medication trials.
1.4. Cough measurement tools
New and repurposed drugs for CC should be evaluated using valid cough measurements including a combination of subjective and objective measures. There has been considerable progress in the development of tools that assess cough in humans (Table 2).
Table 2.
A comprehensive list of cough assessment tools (derived from [73]).
| Subjective Assessments for Cough |
|
| Objective Cough Sensitivity Assessments |
|
| Objective Cough Frequency Assessments |
|
The most widely used tests include the visual analog scale (VAS) for cough [70,71] because it is simple and practical, the Leicester Cough Questionnaire (LCQ) [1] and Cough Quality of Life Questionnaire (CQLQ) [72] are all well validated for assessing the impact of cough on health status [73]. The Cough Severity Index (CSI) is a validated a severity index for CC related to the upper airway [74]. Cough reflex sensitivity (challenge) tests measure the sensitivity of the cough reflex and are better used to determine the mechanism of action of therapy, rather than efficacy. The Leicester Cough Monitor (LCM) [75] and VitaloJak [76] are ambulatory cough monitors that consist of a microphone and recording device to determine cough frequency. They do not correlate strongly with subjective measures of cough as they do not measure the intensity or impact of cough [73].
Cough reflex sensitivity challenge tests are also used in animal models of cough. These tests often require the animal to be anesthetized. However, anesthesia is known to modulate cough, especially C-fiber-dependent cough [77–79]. In addition, once threshold levels of stimulation are attained with acid challenge, the coughing evoked in anesthetized animals becomes stimulus intensity-independent. Changes in threshold sensitivity or on the number of doses evoking repetitive coughing events may be a more appropriate analysis rather than the cumulative number of coughs over a complete challenge [53]. A similar approach has been employed in studying cough evoked by mechanical stimulation of the airway mucosa in allergic dogs [80].
While objective cough counts for human clinical trials offer a better measure of treatment effectiveness this has never been assessed in guinea pig cough models, which rely solely on reflex cough tests. Even in patients the development of devices for accurate objective cough monitoring has been challenging [81]. Stratifying patient groups for disease phenotype is also important. Most cough animal studies use healthy animals to test antitussives in cough challenge tests or do not generate appropriate disease states and treat cohorts with homogeneity [82]. Further, rats and mice do not cough and guinea pigs while being the preferred cough animal model have features driving cough that may not be critical in humans [54]. Few attempts have been made to generate models displaying the features of CHS [83] or the urge to cough [84], which likely drives behavioral coughing. When specific efforts are made disease-specific neurophenotypes can be encapsulated in animal models and have shown consistent outcomes with human phenotypes [82].
2. Systematic review of drug therapies for refractory CC
2.1. Methods
We searched MEDLINE, EMBASE, and Google Scholar from 2005 to May 2017 and included clinical studies of drug therapies for the treatment of CC primarily focusing on Phase II and Phase III studies. The following keywords were used: ‘refractory chronic cough’, ‘idiopathic chronic cough’, and ‘unexplained chronic cough’, AND ‘clinical trials’, ‘cough medications’, or ‘cough treatment’. The reference lists of identified articles were searched to find additional relevant publications. Only articles published in English in adult patients with refractory CC were considered. The definition of refractory CC was dry, persistent cough, greater than 8 weeks duration that had not responded to guideline-directed management and treatments. Only studies in which patients receiving a pharmacological treatment in a cohort study or randomized controlled trial (RCT) and had an assessment of cough with an outcome measure were reviewed (Table 3). Comparable trials were included in the meta-analyses and underwent methodologic assessment with a Cochrane risk bias tool [85].
Table 3.
Publications included in the systematic review.
| Citation | Publication type | Study design | Trialed drug (active) therapy |
|---|---|---|---|
| Bastian et al., 2006 [26] | Full peer-reviewed | Prospective cohort, consecutive patients |
Amitriptyline 10 mg daily for 21 days |
| Jeyakumar et al., 2006 [86]a | Full peer-reviewed | Randomized controlled trial. | Amitriptyline 10 mg daily |
| Norris & Schweinfurth, 2010 [87] | Full peer-reviewed | Retrospective case series |
Amitriptyline 25 mg/day to maximum dose of 100 mg/day. Patients with no response or intolerable side effects prescribed gabapentin (median dose 300 mg/tid) for 2 months. |
| Lee & Woo, 2005 [25] | Full peer-reviewed | Case series | Gabapentin 100–900 mg daily for 4 weeks. Nonresponders stop at 4 weeks, responders continue dose for 3 months. |
| Mintz & Lee, 2006 [101] | Letter peer-reviewed | Case series | Gabapentin 100 mg bid to 1600 mg daily dose for 3 months to 1 year. |
| Ryan et al., 2012 [89]a | Full peer-reviewed | Randomized, double blind, placebo-controlled parallel trial | Gabapentin maximum daily dose of 1800 mg for 10 weeks. |
| Halum et al., 2009 [102] | Full peer-reviewed | Retrospective chart review | Pregabalin 75–150 mg bid for 4 weeks |
| Vertigan et al., 2016 [90]a | Full peer-reviewed | Randomized, double blind, placebo-controlled parallel trial | Pregabalin 300 mg + Speech Pathology for 14 weeks. |
| Morice et al., 2007 [88]a | Full peer-reviewed | Randomized double blind placebo-controlled crossover trial |
Morphine sulfate 5 mg bid for 4 weeks. An open-label extension of 10 mg bid in a subgroup of patients for 3 months. |
| Dion et al., 2017 [112] | Letter peer-reviewed | Prospective case series on consecutive patients | Tramadol 50 mg every 8 h as needed. |
| Young et al., 2010 [117] | Conference Abstract | Randomized, double blind, placebo-controlled parallel trial. | Ketamine low dose single infusion |
| Khalid, S et al., 2014 [32]a | Full peer-reviewed | Randomized, double blind, placebo-controlled crossover trial. | TRPV1 antagonist SB-705498 600 mg. |
| Abdulqawi et al., 2015 [91]a | Full peer-reviewed | Randomized, double blind, placebo-controlled two-period, crossover (phase II) study | P2X3 Antagonist AF-219 600 mg bid. |
| Smith et al., 2017 [92]a | Conference Abstract | Multicenter, randomized placebo-controlled parallel trial (Phase IIb study) | P2X3 Antagonist MK-7264 (formerly AF-219) 7.5 mg, 20 mg, or 50 mg BID for 12 weeks. |
| Smith et al., 2017 [42] VOLCANO-1 Study | Conference Abstract | Pilot, open-label Phase IIa study. | NK1 Antagonist Orvepitant 30 mg once daily for 4 weeks. |
| Yousaf et al., 2010 [93]a | Full peer-reviewed | Randomized, double blind, placebo-controlled parallel trial | Erythromycin 250 mg daily for 12 weeks. |
| Hodgson et al., 2016 [94]a | Full peer-reviewed | Randomized, double blind, placebo-controlled parallel trial | Azithromycin 500 mg/day for 3 days followed by 250 mg tid/week for 8 weeks. |
| Birring et al., 2017 [125]a | Full peer-reviewed | Multicenter, double blind, randomized placebo-controlled, 2-period crossover trial (Phase IIa study). | PA101 Cromolyn sodium 40 mg tid for 2 weeks. |
aThese randomized controlled trials were included in the meta-analyses.
2.1.1. Statistical analysis
Where possible we performed meta-analyses on placebo-controlled randomized trials that measured the effectiveness of a pharmacological treatment on refractory CC patients using RevMan 5 software [Review Manager (RevMan) Version 5.3. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2014].
We compared centrally acting antitussives/neuromodulator RCTs that had a placebo comparator and used a similar cough outcome measurement. This analysis included the the Jeyakumar et al. (Amitriptyline) study [86], the Morice et al. (morphine sulfate) study [88], the Ryan et al. (Gabapentin) study [89], and the Vertigan et al. (Pregabalin + Speech Pathology) study [90]. As the Jeyakumar et al. study used a different measure of cough QOL to the other studies, we converted continuous outcome data into Standardized Mean Difference (SMDs) and presented these with 95% CIs. The Inverse-Variance method was used to calculate the pooled estimate.
We also compared the placebo RCT P2X3 receptor antagonist trials that had used the same cough outcome measure, that is, cough frequency with an automated cough monitor in coughs/h from Abdulqawi et al. (AF-219) study [91] and the Smith et al. (MK-7264) study [92].
Finally, the placebo RCT macrolide antibiotic trials that had also measured cough frequency by automated cough monitor in coughs/h from the Yousaf et al. (Erythromycin) [93] study and the Hodgson et al. (Azithromycin) [94] study were compared. Continuous data was converted into mean differences (MDs) and presented with 95% CI in the meta-analysis. The Inverse-Variance method was used to calculate the pooled estimate.
For the dichotomous data (Y/N for a response to the active medication compared to a Y/N response to the placebo treatment), we calculated effect sizes as risk ratios (RR) with 95% CIs. For the Pregabalin + SPT trial, we contacted the authors to request the required information. Heterogeneity across the studies’ results was examined and quantified with I2, which describes the percentage of total variation across studies that is due to heterogeneity rather than due to chance [95]. A value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity. For comparisons that had high heterogeneity (I2 > 75% and p < 0.01), we used a random effects model for the pooled estimate. We presented the meta-analysis outcomes in Forest Plots using RevMan V5.3 for each comparison.
2.2. Results
We identified 156 studies from which 84 articles were excluded after applying the exclusion criteria to the Title or Abstract. A further 53 articles were excluded after full text screen leaving 19 articles for systematic review (Figure 1) [96] and (Table 3).
Figure 1.
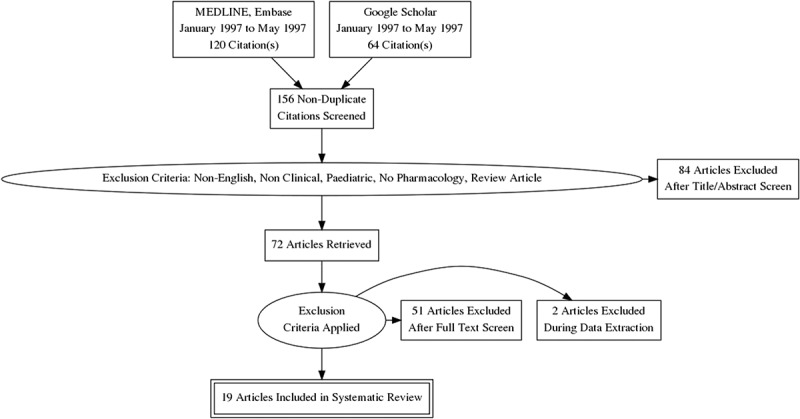
PRISMA flow diagram explaining the screening process of citations and number of articles included in the systematic review.
The study quality of the nine full publication placebo-RCTs was high in five studies (Figure 2). There was significant risk of bias identified in two studies [86,91]. Randomization and concealment of allocation were determined to be of high risk in the Jeyakumar et al. [86] study, specifically patients were randomized by chart numbers (not computer-generated randomization) and there was a predictable allocation sequence (patients with even chart numbers were placed on amitriptyline, odd numbers were placed on codeine/guaifenesin). Blinding of the intervention and incomplete data were determined to be of high risk in the Abdulqawi et al. [91] study. This was due to the notable taste disturbances of AF-219 that led to 25% of patients withdrawing before the end of the trial.
Figure 2.
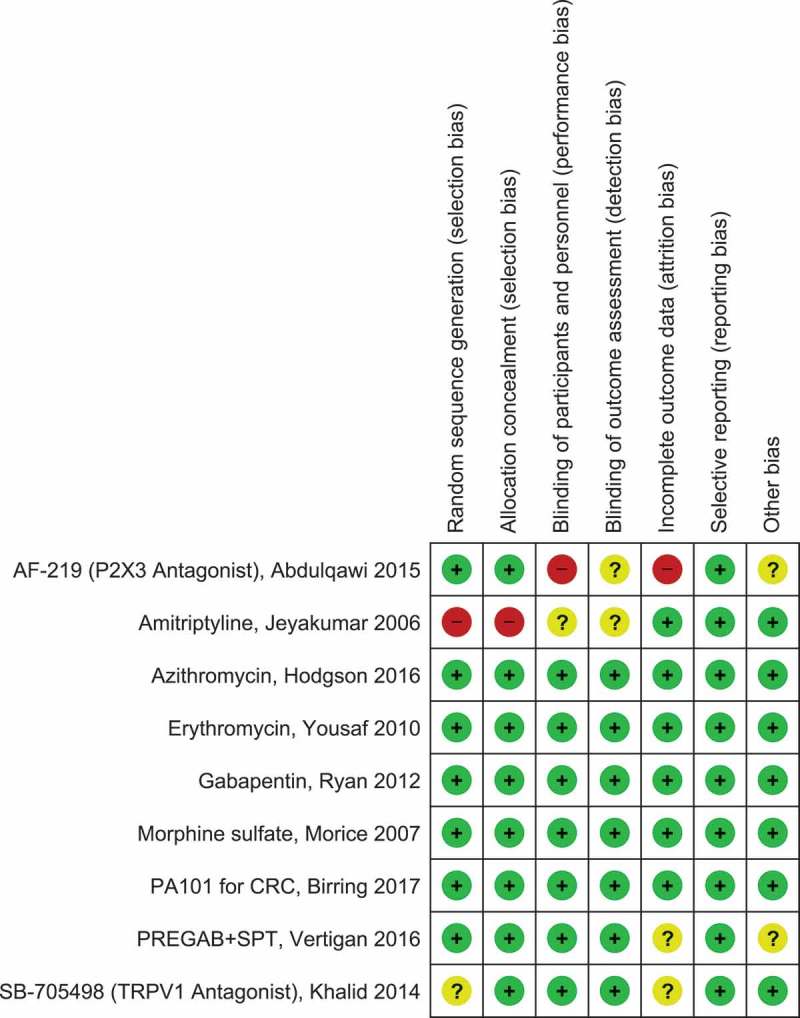
Quality assessment (Cochrane risk of bias tool) for included RCTs.
Green circle with plus sign indicates low risk of bias, yellow circle with question mark indicates unclear risk of bias, red circle with minus sign indicates high risk of bias. RevMan Version 5.3. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2014. Full color available online.
The type of study, drug dose and duration, primary efficacy outcome, primary results, adverse effects of trialed drugs, and any methodological weaknesses are summarized in Table 4 for centrally acting/neuromodulating antitussives, Table 5 for ion channel receptor antagonists, and Table 6 for other medications. Additional study details that were considered unique and/or important are included in the text.
Table 4.
Centrally acting antitussive medications for refractory chronic cough.
| Study author/s | Type of study | N | Drug treatment dose and duration | Primary efficacy outcome | Primary study results and conclusion/s | Adverse/side effects | Methodological weaknesses |
|---|---|---|---|---|---|---|---|
| Bastian et al., 2006 [26] | Prospective cohort, consecutive patients |
12 | 10 mg amitriptyline daily for 21 days |
Patient self-report on 0% to 100% scale |
10 of 12 patients had ≥50% response; 6 of 8 patients ≥50% response >20 days off amitriptyline. One patient had no benefit with amitriptyline but did with subsequent gabapentin (dose not given). | No side effects. | No placebo comparator group. No objective cough measures. Small sample size. |
| Jeyakumar et al., 2006 [86] | Randomized controlled trial. | 28 |
10 mg Amitriptyline daily, n = 15 vs. 10–100 mg/5 ml or 10 ml codeine/guaifenesin every 6 awake hours, n = 13 for 10 days |
Patient self-report % reduction in cough frequency and severity; cough quality-of-life (QOL) questionnaire |
13 of 15 (87%) in amitriptyline group had ≥50% cough improvement compared with 1 of 13 (8%) in codeine/guaifenesin group, NNT = 1.3; improved CQLQ scores were associated with amitriptyline (change in score from baseline 24.5 compared to guaifenesin-codeine of 2.9), p = 0.0007. | None reported. | No objective cough measure, no placebo control group comparator. No power calculation. No baseline characteristics comparison between groups. High risk of selection bias (patients where randomized by chart numbers and presence of nasal allergies). |
| Norris & Schweinfurth, 2010 [87] | Retrospective case series | 10^ | Amitriptyline used as the first-line neuromodulator, n = 5 (median dose 25 mg/day, maximum dose 100 mg/day). Patients with nonresponse or intolerable side effects to amitriptyline were prescribed gabapentin, n = 3 (median dose 300 mg/tid). Gabapentin only, n = 1. No neuromodulator, n = 1. | Patient self-reported subjective improvement (Yes/No). | The mean time from the initiation of therapy to improvement or resolution of symptoms was 2 months (range, 1 to 5 months). 86% of patients with evidence of motor neuropathy responded to the neuromodulator therapy, versus zero response among those without evidence of motor neuropathy. It was thought that the patient’s condition must first be medically optimized with control of laryngopharyngeal reflux disease, postnasal drip, and/or discontinuation of potentially causative medications. | 3 (30%) of patients taking either amitriptyline or gabapentin experienced a dry mouth. One (10%) patient reported fatigue on a dose of 75 mg amitriptyline. | Retrospective chart review. No objective measures of improvement. Small sample size. No comparator group. |
| Lee & Woo, 2005 [25] | Case series | 28 |
Gabapentin 100–900 mg daily over 4 weeks, nonresponders stop at 4 weeks, responders continue dose for 3 months and then taper, intolerant carbamazepine 100 mg tid |
Symptom response (unclear if patient or clinician reported). | Nineteen (68%) patients had a clinically positive response to gabapentin and those patients identified with superior or recurrent laryngeal neuropathy responded even more favorably (80%) In the workup for laryngeal neuropathy videostroboscopy and L-EMG are invaluable tools in diagnosis. | Five patients (17.8%) complained of dizziness or somnolence that caused them to taper or discontinue the medication. | Prior workup included however, not systematic or uniform across patients. Efficacy outcome not clear or objective. No control group for comparison. |
| Mintz & Lee, 2006 [101] | Case series | 6 | Gabapentin 100 mg bid to 1600 mg daily dose | Clinician assessment | 5 of 6 patients had either complete resolution or substantial improvement in cough. One patient relapsed on therapy and gained control by an increase in dose. | Fatigue in 1 (17%), drowsiness for 1 week in 1 (17%). | Treated patients according the ADP and when patients were refractory to this gabapentin was trialed. Prior workup criteria and subsequent treatments not explained. No objective cough measure. No comparator group. Small sample size. |
| Ryan et al., 2012 [89] | Randomized, double-blind, placebo-controlled parallel trial | 62 | Gabapentin 6 capsules (1800 mg) daily (n = 32) versus matching placebo 6 capsules daily (n = 30).* | Primary outcome was cough QOL measured by the LCQ. Secondary outcomes were cough severity (VAS), cough frequency (LCM®). Capsaicin cough reflex sensitivity testing was used to assess treatment mechanism. Other outcomes were urge to cough score and laryngeal dysfunction score (LDQ). |
Gabapentin significantly improved cough-specific quality of life compared with placebo-between group difference in LCQ score during treatment period 1.80, 95% CI 0.56–3.04; p = 0.004; clinical improvement in LCQ score of >1.3 (the smallest change in score regarded as clinically meaningful) for the gabapentin group compared to the placebo group (20/27 [74.1%] versus 12/26 [46.2%]; p = 0.038). Number needed to treat = 3.58. The treatment of refractory chronic cough with gabapentin is both effective and well tolerated. These positive effects suggest that central reflex sensitization is a relevant mechanism in refractory chronic cough. |
Side effects occurred in both groups with the most common in the Gabapentin group being nausea, stomach pain [4/17], dizziness [3/17], and fatigue [3/17]. For the Placebo group most common side effects were dizziness [1/6], nausea, stomach pain [2/6], and fatigue [1/6]. | Non-validated short-duration cough frequency recording during the capsaicin cough reflex sensitivity test rather than 24 h cough frequency recording was used; however, any potential bias was controlled by using a placebo group. No long-term treatment or follow-up of patients. However, gabapentin treatment effect had not reached a plateau by 8 weeks suggesting that longer-term treatment may be required for some patients. A risk versus benefits analysis would need to be done for individual patients. |
| Halum et al., 2009 [102] | Retrospective chart review of consecutive patients prescribed pregabalin for symptoms of LSN including CC. | 5 | Pregabalin 75–150 mg bid | Pre- and posttreatment questionnaires asking patients to rate symptoms on a scale from 0 to 5 | 3 of 5 improved cough severity None of the patients developed drug tolerance effects over time. | Sedation in 4 (80%), of whom half tolerated, half discontinued medication |
Retrospective chart review. No objective measures of improvement. Small sample size. No comparator group. |
| Vertigan et al., 2016 [90] | Randomized, double-blind, placebo-controlled parallel trial | 40 | Speech pathology (SPT) with 300 mg pregabalin (PREG) treatment (n = 20) versus SPT with placebo (PLAC) treatment (n = 20). | Primary outcomes were cough QOL measured by the LCQ, cough severity (VAS), cough frequency (LCM®). Secondary outcomes were capsaicin cough reflex sensitivity, urge to cough score and laryngeal function measures. | Cough severity, cough frequency and cough QOL improved in both groups. The degree of improvement in the LCQ was greater with SPT + PREG (mean difference; 3.5 [95% CI 1.1 to 5.8] than for placebo (SPT + PLAC), p = 0.024. The degree of improvement in the cough severity (VAS) was greater with SPT + PREG (mean difference; 25.1, [95% CI 10.6 to 39.6] than for placebo (SPT+PLAC), p = 0.002. The degree of improvement in cough frequency was greater with SPT+PREG (mean difference; 11.2 [95% CI 2.6 to -19.7] than for placebo (SPT + PLAC), p = 0.013. Combined SPT+PREG treatment is beneficial in reducing symptoms in refractory CC with the benefit continuing for at least 4 weeks after cessation of pregabalin. Results are consistent with sensory hyperresponsiveness being a mechanism of refractory CC. |
Side effects occurred in both groups with the most common in the SPT + PREG group being dizziness [9/20], fatigue [7/20] and cognitive changes [6/20]. These resolved after pregabalin was ceased. For the SPT + PLAC group gastrointestinal [7/20], and fatigue [6/20] were the most common side effects reported. | Low recruitment rate. No comparison to pregabalin alone or placebo alone. More than one primary outcome. Baseline imbalance across groups for cough duration-longer for SPT + Placebo group. |
| Morice et al., 2007 [88] | Randomized double-blind placebo-controlled crossover trial | 27 | 5 mg twice daily slow-release morphine sulfate or matching placebo for 4 weeks of treatment. An open-label extension of the study was done in a subgroup of patients using 10 mg twice daily for 3 months. | Primary efficacy outcome was cough QOL measured by the LCQ. Secondary outcomes were citric acid cough challenge (C2 and C5) and cough severity assessed on a scale of 0 to 9 recorded in a daily diary entry. | There was a significant improvement in LCQ with morphine treatment compared to placebo. Mean score for LCQ was 13.3 (2.5) at baseline, 13.5 (2.7) on placebo (NS) and 15.5 (2.7) on morphine (p < 0.01 vs. baseline, p < 0.02 vs. placebo). The average improvement in total LCQ score was higher than 2.56 which has been demonstrated to be clinically significant. Maximum benefit for responders was achieved by Day 5 and this benefit was sustained through the 4 weeks of the study. There was no change in the citric cough reflex sensitivity test. At the end of 3 months, there was a similar improvement in cough between the 5 and 10 mg groups. | Most common side effects noted were constipation in 10 (40%) and drowsiness in 7 (25%) patients. In the subgroup who increased morphine dose to 10 mg twice daily the incidence of drowsiness doubled. | Not strictly refractory chronic cough cohort, 16 from 27 patients had a productive cough. Studies of psychoactive drugs like morphine cannot be completely blinded as the patient is conscious of the affects a quarter of the patients in this study noted mild and transient sedation. Long-term effects of low-dose morphine in patients with CC were not assessed in this study. Strength of this study was that it was a controlled trial of opiate therapy in clinically significant cough. |
| Dion et al., 2017 [112] | Prospective case series on consecutive patients | 16 | Tramadol 50 mg every 8 h as needed. | Efficacy outcomes were cough severity index (CSI) and Leicester Cough Questionnaire (LCQ) at ≥14 days of treatment. A number of investigations and review by a gastroenterologist or speech-language therapist determined subjects meeting diagnostic criteria for neurogenic cough. | All subjects reported at least some improvement in their cough symptoms. CSI scores improved from 23 to 14 and LCQ scores improved from 74 to 103 (p = 0.003 and p = 0.005, respectively) pre- vs. posttreatment in patients with neurogenic cough. The results of this study suggest that tramadol warrants additional evaluation as a treatment of neurogenic cough. |
Four (25%) patients reported somnolence and were recommended to reduce their dosage or frequency to combat symptoms. | Limitations of a preliminary study including small sample size and no control group for comparison. Variable treatment time from 15 to 1029 days. Used LCQ scoring was not consistent with the validated 19-item LCQ score. Here LCQ was assessed on scale of 0 to 133 with 0 most severe. Some follow-up assessments were short at 2 weeks. |
Table 5.
Receptor antagonists for the treatment of refractory chronic cough.
| Study author/s | Type of study | N | Drug treatment dose and duration | Efficacy outcome | Study results and conclusion/s | Adverse/side effects | Methodological weaknesses/comments |
|---|---|---|---|---|---|---|---|
| Young et al., 2010 [117] | Randomized, double-blind, placebo-controlled parallel trial. | 24 | 30 min infusion of low dose ketamine or placebo (saline) at each of the 2 visits at least 1 week apart. | Capsaicin cough reflex sensitivity test (C5 and C2) and, objective 24 h cough frequency (Vitalojak) | Ketamine had no significant effect on C5 or C2 over time compared to placebo in either patient group. 24 h cough frequency did not significantly change after ketamine, 13 (16.8) coughs/h compared to placebo, 13.5 (14.9) coughs/h in either patient group. It was concluded that central upregulation of the NMDA receptor did not appear to be responsible for cough reflex hypersensitivity in patients with CC | Side effects were not investigated or reported. | Published Abstract no full-publication peer review. Subjective or central measures of cough not used. Safety and adverse effects of ketamine not investigated, |
| Khalid, S et al. [32] | Randomized, double-blind, placebo-controlled crossover trial. | 21 | Patients received a single dose of 600 mg of SB-705498 (6 × 100 mg) a TRPV1 antagonist or matching placebo with a 4-week washout separating the 2 treatment periods. | Co-primary outcomes were assessed by the capsaicin cough reflex sensitivity test (C5) before dose, 2 h after dose and 24 h after dose and with 24 h cough frequency using the VitaloJAK® cough recorder before and after dose. Secondary outcomes included patient-reported cough severity by VAS, urge to cough by VAS before dose, 2 h and 24 h after dosing and cough specific QOL (CQLQ) before dose and 14 days after dose. Blood samples were collected for pharmacokinetic testing up to 4 h and at 24 h after dosing. | Treatment with SB-705498 produced a significant improvement in cough reflex sensitivity to capsaicin at 2 h and a borderline significant improvement at 24 h compared with placebo (adjusted mean difference of +1.3 doubling doses at 2 h [95% CI, +0.3 to +2.2; p = 0.005] and +0.7 doubling doses at 24 h [95% CI, +0.0 to +1.5; p = 0.026]. 24 h objective cough frequency was not improved GEM [SD] 24.2 [12.9] compared with placebo 23.9 [10.5]. Patient-reported cough severity, urge to cough, and cough-specific QOL were also not improved with SB-705498 when compared with placebo. The average receptor occupancy was estimated at approximately 45% at peak plasma concentrations (2 h after dosing), with a gradual reduction in occupancy to 25% at 24 h after dosing. | No serious adverse events occurred during the study. Ten (48%) nonserious adverse events were reported on placebo treatment and 7 (33%) were reported on SB-705498 treatment (specific AEs not given). Most common AE was headache reported in 3 patients (14%) during placebo treatment and 2 patients (10%) during SB-705498 treatment. | 1-sided statistical test to detect a 2.5% difference between SB-705498 and placebo was used. A two-sided test would have resulted in larger p-values for this study. |
| Abdulqawi et al., 2015 [91] | Randomized, double-blind, placebo-controlled two-period, crossover (phase II) study | 24 | Patients received AF-219 (P2X3 Antagonist), 600 mg or placebo twice a day. After a 2 week washout, patients were then assigned to receive the other treatment. | Primary outcome: Daytime cough frequency at baseline and after 2 weeks of treatment using 24 h ambulatory cough recordings. Secondary outcomes included cough severity VAS, urge to cough VAS and Cough Quality of Life Questionnaire (CQLQ) scores. | Cough frequency was reduced by 75% when patients were allocated to AF-219 compared to when allocated to placebo in the ITT population (p = 0.0003). In the sensitivity analysis (worst case imputation for missing data), the placebo-adjusted reduction in daytime cough frequency during AF-219 treatment was 65% (95% CI 29–82; p = 0.005). Daytime cough frequency fell from a mean 37 coughs/h (SD 32) to 11 (8) coughs/h after AF-219 treatment versus 65 (163) coughs/h to 44 (51) coughs/h after placebo. Daytime cough VAS significantly improved with AF-219 compared with placebo after 2 weeks of treatment in the ITT population: mean change of -25.6 (95% CI -41.5 to -9.6) mm, p = 0.003. Urge to cough VAS, p = 0.035 and CQLQ score for the ITT population also improved, p = 0.018. Antagonists of P2X3 receptors such as AF-219 are a promising new group of antitussives. |
No serious AEs during the trial. All nonserious AEs were either mild or moderate in severity and included nausea in 9/24 (38%) and oropharyngeal pain in 5/24 (21%) of AF-219 patients. Six patients withdrew before the end of the study because of taste disturbances, which were reported by all patients (n = 24) taking AF-219. | All patients had taste disturbances on AF-219 treatment resulting in unblinding. Long-term safety and efficacy needs to be established-Phase II study. Small sample size, missing data for primary outcome. Industry sponsored trial. |
| Smith et al., 2017 [92] | Multicenter, randomized placebo-controlled parallel trial (Phase IIb study) | 253 | 7.5 mg MK-7264 (P2X3 Antagonist, formerly AF-219) n = 64, 20 mg MK-7264 n = 63, 50 mg MK-7264 or Placebo, n = 63 (all BID) for 12 weeks. | Primary outcome was mean change in Awake Cough Frequency (coughs/hour) posttreatment vs. baseline using the VitaloJAK®. Secondary outcome was cough severity (VAS). Subjects had refractory CC and a cough severity VAS ≥ 40 mm. | MK-7264 at a dose of 50 mg significantly reduced the Awake Cough Frequency outcome when compared with placebo, log10 (SD) change for 50 mg MK-7264 was -0.80 (0.11) and for placebo was -0.40 (0.11), p = 0.0027 while the difference from placebo for the 7.5 mg and 20 mg doses were not significantly different. This result was consistent with the patient-reported cough severity VAS. Targeting P2X3 with 50 mg MK-7264 significantly reduced the frequency of cough in patients with refractory CC after 12 weeks of treatment compared with placebo. |
The most common side effect reported was related to taste. These were reported in 4 patients on placebo, 6 patients on 7.5 mg, 31 patients on 20 mg and, 51 patients on 50 mg of MK-7264 respectively. Of these 1 patient on placebo and 6 patients on 50 mg MK-7264 dose discontinued the study due to the taste-related side effect. | Published Abstract no full-publication peer review. Taste disturbances cause unblinding. Industry sponsored trial |
Table 6.
Other medications for refractory chronic cough.
| Study author/s | Type of study | N | Drug treatment dose and duration | Efficacy outcome | Study results and conclusion/s | Adverse/side effects | Methodological weaknesses |
|---|---|---|---|---|---|---|---|
| Yousaf et al., 2010 [93] | Randomized, double-blind, placebo-controlled parallel trial | 30 | Erythromycin stearate capsules (250 mg daily), n = 15 or matching placebo capsules (one/day), n = 15 for 12 weeks. | Primary outcome was a change in log 24 h cough frequency from baseline to 12 weeks. Secondary endpoints were changes in induced sputum neutrophils differential cell count, sputum bacterial colonies, log C2, C5, cough VAS, and LCQ. | Twenty-eight patients completed the study (erythromycin n = 13, placebo n = 15); two withdraw due to personal reasons. After adjusting for baseline differences there was no difference in the change in cough frequency between erythromycin and placebo groups at 12 weeks (mean difference in fold change 1.1; 95% CI 0.7 to 1.5; p = 0.585). There was a statistically significant between-treatment difference in the change n sputum neutrophils at 12 weeks (mean difference 16.8%; 95% CI 1.6 to 32.1%; p = 0.03). There was no difference in the change in LCQ, cough VAS, log C2, or log C5 between treatments. Log bacterial colony forming units was unchanged with active and placebo treatment. IL-8 was measured in six active treatment patients and 10 placebo treatment patients resulting in a mean difference in fold change 2.9; 95% CI 0.9 to 9.5; p = 0.08). |
Two patients in the placebo group reported abdominal discomfort at the 6 week visit, which had resolved by the 12 week visit. One patient in the erythromycin group reported dizziness at the 6 week visit, which resolved within a week. | |
| Hodgson et al., 2016 [94] | Randomized, double-blind, placebo-controlled parallel trial | 44 |
Azithromycin capsules, 500 mg daily for 3 days followed by 250 mg three times a week for 8 weeks, n = 21. The control intervention was lactose-containing placebo capsules n = 21, taken according to the same dosing schedule. |
Primary outcome measure was change in LCQ score from baseline to end of treatment at week 8. Secondary outcome measures were cough severity score by VAS and FENO by (NIOX). | There was a clinically important improvement in LCQ score with azithromycin (mean change, 2.4; 95% CI, 0.5 to 4.2) but not placebo (mean change, 0.7; 95% CI, -0.6 to 1.9), but the between-group difference was not statistically significant (p = 0.12). 11 of 21 (52%) subjects in the azithromycin group had a clinically significant improvement in their LCQ score. There was a large and significant improvement in LCQ score in patients with chronic cough and a concurrent diagnosis of asthma who were treated with azithromycin (mean change, 6.19; 95% CI, 4.06 to 8.32). Treatment with low-dose azithromycin for 8 weeks did not significantly improve LCQ score compared with placebo. The use of macrolides for treatment-resistant cough cannot be recommended from this study, but they may have a place in the treatment of chronic cough associated with asthma with a large and significant improvement in LCQ. |
Side effects occurred in both groups including GI effects such as diarrhea with azithromycin [4] and placebo [2]; heartburn [1], [1]; abdominal pain [2], [1]; and nausea [1], [1] respectively. Less common effects such as musculoskeletal occurred in the placebo group only. | Patients with other comorbidities such as asthma, rhinitis, or reflux were not excluded as for other refractory CC studies. The study was not designed to detect subgroup effects, so significant improvement in LCQ for the CC with asthma subgroup maybe a chance finding rather than a true treatment effect. No objective cough measurement used. |
| Birring et al., 2017 [125] | Multicenter, double-blind, randomized placebo-controlled, 2-period cross-over trial (Phase IIa study) in patients with IPF and chronic cough and a parallel study of similar design in patients with refractory CC. | 27 | PA101 cromolyn sodium formulation (40 mg) or matching placebo three times a day via oral inhalation for 2 weeks, followed by a 2 week washout, and then crossed over to the other arm. | Primary efficacy outcome was change from baseline in objective daytime cough frequency (from 24 h acoustic recording, Leicester Cough Monitor) to posttreatment. Secondary cough outcomes included the LCQ, and cough severity (VAS). | In patients with IPF, PA101 significantly reduced daytime cough frequency by 31.1% at day 14 compared with placebo (ratio of least-squares [LS] means 0.67, 95% CI 0.48–0.94, p = 0.0241). In the refractory CC cohort the daytime average cough count decreased by 12 coughs/h at Day 14 with PA101 treatment and by 9 coughs/h at Day 14 for placebo treatment, p = 0.319. The subjective endpoints of LCQ and VAS were also comparable between the two groups. Inhaled PA101 was therefore no better than placebo in the treatment of patients with refractory CC. However, PA101 was effective in the treatment of IPF patients with CC suggesting that the mechanism of cough in IPF might be disease specific. This is an important finding. | Adverse effects were comparable to placebo. Four patients (two placebo and two PA101) discontinued the study due to relatively mild side effects such as headache and cough. There were no serious AE’s reported. | Small number of patients investigated and the study was underpowered to assess subjective measures. Duration of treatment was brief. Industry sponsored trial. The sponsor participated in the study design, study oversight, medical monitoring, data management, analysis, and reporting of the data. However, an independent statistician analyzed the data. |
2.2.1. Centrally acting medications for refractory CC
Centrally acting neuromodulators act on enhanced neural sensitization – a key component of refractory CC. The suppression of cough with opiates have long been advocated [97,98]; however, there are few quality trial data to support this recommendation. A recent placebo-controlled crossover trial that used objective and subjective cough measures suggested an antitussive effect similar to that of placebo in COPD patients [99].
Each of the centrally acting neuromodulators (amitriptyline, gabapentin, pregabalin, morphine, and tramadol) that we reviewed had positive effects on cough-specific quality of life and/or cough severity in patients with refractory CC or CC associated with a neuropathic disease.
2.2.1.1. Amitriptyline
Amitriptyline is a tricyclic antidepressant and inhibitor of serotonin reuptake that has been successfully used in the treatment of sensory and laryngeal neuropathic cough [26]. Jeyakumar et al. [86] investigated amitriptyline in the treatment of refractory CC resulting from postviral vagal neuropathy. Primary outcomes were patient self-report percent reduction in cough frequency and severity, and CQLQ. Improved CQLQ scores were associated with amitriptyline (calculated^ mean (SD) change in score from baseline was 24.5 (5.0) compared to mean (SD) change in score from baseline of 2.9 (3.8) for placebo [meta-analysis, Figure 3(a)]. Thirteen from 15 (87%) patients in the amitriptyline group had ≥50% cough improvement compared to one from 13 (8%) patients in the codeine/guaifenesin group [meta-analysis, Figure 4], number needed to treat (NNT) = 1.3. Combined NNT for 15 studies assessing amitriptyline (25–150 mg/day) for neuropathic pain is 3.6 (95% CI 3.0–4.4) [52]. There were no adverse effects of amitriptyline reported for this study (Table 4) but there was high risk of bias (Figure 2).
Figure 3.
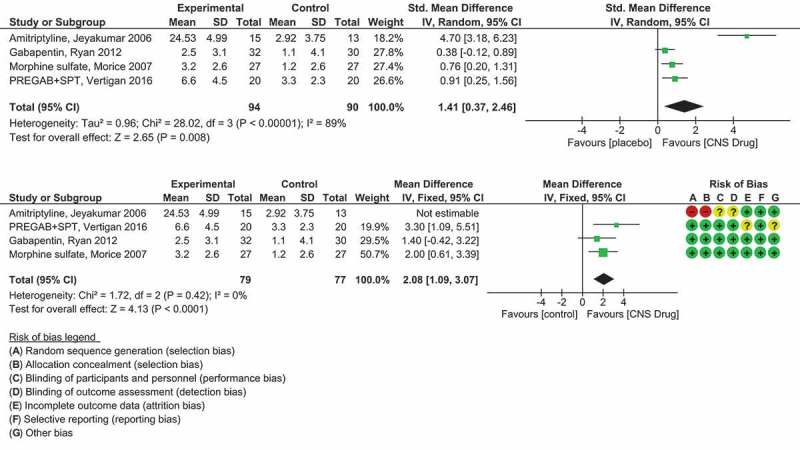
(a) Forest plot of a meta-analysis on CNS/neuromodulating medication vs. placebo medication on cough QOL. As the Jeyakumar et al study used a different cough QOL measurement (the CQLQ) compared to the LCQ the standardised mean difference (SMDs) were calculated. (b) Forest plot of a meta-analysis on CNS/neuromodulating medication vs. placebo medication on cough QOL. The study under high risk of selection bias (Jeyakumar et al.) was removed in a sensitivity analysis. The remaining three studies all used the same cough QOL measurement (LCQ) so the mean differences (MDs) were calculated.
The green squares and black horizontal lines represent the SMD or MD and 95% CI for each study. The larger the green square the more weight that study contributes to the overall pooled estimate (black diamond). Risk of bias summary has also been included for each study (top right). RevMan Version 5.3. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2014. Full color available online.
Figure 4.
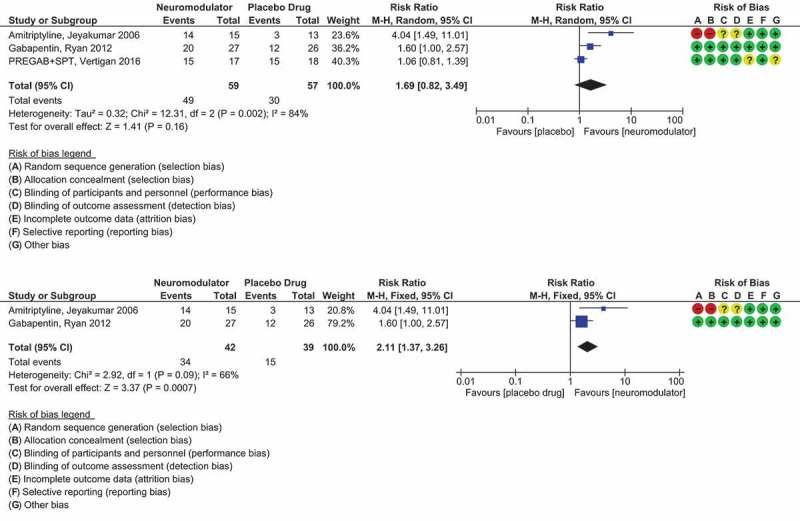
(a) Forest plot comparing responders to non-responders for the CNS/neuromodulating medications of amitriptyline, gabapentin and pregabalin placebo-controlled randomised trials. (b) Forest plot comparing responders to non-responders for gabapentin and amitriptyline placebo-controlled randomised trials only.
The blue squares and black horizontal lines represent the Risk Ratio and 95% CI for each study. The larger the blue square the more weight that study contributes to the overall pooled estimate (black diamond). Summary risk of bias has also been included for each study. RevMan Version 5.3. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2014. Full color available online.
2.2.1.2. Gabapentin
Gabapentin acts by blocking a subset of central voltage-gated calcium channels and has recently been recommended as a treatment option for refractory CC by the CHEST Guideline and Expert Panel Report [3]. The effectiveness of gabapentin treatment for refractory CC was investigated in a placebo-controlled randomized trial by Ryan et al. [89] (Table 4). The primary efficacy outcome was cough quality of life measured by the LCQ. The change in LCQ score from baseline was mean (SD) 2.5 (3.1) for gabapentin and 1.1 (4.1) for placebo, p = 0.004 [meta-analysis Figure 3(a,b)].
Significantly more participants in the gabapentin group who had remained in the study at week 8 had a clinical improvement in LCQ score of greater than 1.3 (the smallest change in score regarded as clinically meaningful [100]) than did those in the placebo group (20 [76.9%] of 26 vs. 12 [46.2%] of 27; p = 0.038) [meta analysis, Figure 4]. This corresponds to a NNT of 3.6. The combined NNT for 14 RCTs of gabapentin (900–3600 mg/day) for neuropathic pain is 6.3 (95% CI 5.0–8.3) [52].
An important aspect of this study was the defining of patients with central sensitization of the cough reflex. This was done on the basis of CRS (defined in patients for whom a concentration of <134.8 μM/L of capsaicin stimulated five or more coughs [C5]) and a history of specific cough characteristics. These included cough triggered by laryngeal paresthesia (throat irritation/sensation), nontussive triggers such as talking on the phone or air conditioning (allotussia), and tussive triggers such as smoke and fumes (hypertussia).
Ten from 32 (31%) patients assigned gabapentin had one or more adverse effects compared with three from 30 (10%) assigned placebo (p = 0.059). Adverse effects were managed by temporarily reducing the dose (in six [19%] in the gabapentin group versus three [10%] in the placebo group), or by withdrawing patients from the study (one [3%] vs. one [3%]). At the primary outcome meassurement of 8 weeks participants with central sensitization (n = 39) had an enhanced response to gabapentin, Baseline LCQ Mean (SD) 13.5 (8.6), Week 8 LCQ Mean (SD) 17.1 (10.6) compared to those without central sensitization (n = 23) Baseline, LCQ Mean (SD) 13.9 (9.5), Week 8 LCQ Mean (SD) 15.3 (8.7), p = 0.001.
After withdrawal of the gabapentin, there was reduced effectiveness further supporting its antitussive effect. Peripheral cough reflex sensitivity to capsaicin did not change significantly, suggesting that gabapentin did not act by reducing peripheral sensitization. The treatment effect had not reached a plateau by 8 weeks justifying longer-term treatment for some individuals. This has been shown in case studies before [25,101]; however, further placebo-RCTs would need to be conducted to confirm how long a patient with refractory CC needs to remain on gabapentin for cough resolution.
2.2.1.3. Pregabalin
Pregabalin has a similar structure to gabapentin. It acts on central nervous system calcium channels, leading to decreased release of neurotransmitters such as glutamate, noradrenaline, and substance P. Halum et al. [102] showed that pregabalin was effective in the treatment of a small number of patients with laryngeal sensory neuropathy. In 2016, Vertigan et al. [90] compared a combination treatment of pregabalin (PREG) and SPT to a matching placebo medication (PLAC) and SPT for patients with refractory CC (Table 3). The change in LCQ score from baseline for the PREG + SPT group was mean (SD) 6.6 (4.5) compared to the change in LCQ score from baseline for the PLAC + SPT group, mean (SD) 3.3 (2.3), p = 0.024 [meta-analysis, Figure 3(a,b)]. Importantly there was a sustained effect from the treatment after cessation of the pregabalin. Fifteen from 17 (88%) patients that completed treatment in the PREG + SPT group had a minimally important change (MIC) in their LCQ of greater than 1.3, and 15/18 (83%) patients that completed treatment in the PLAC + SPT group had an MIC in their LCQ of greater than 1.3, NNT = 20 [meta-analysis, Figure 4]. This large NNT would be attributable to the SPT component used in both groups. It would be expected that there would be a much smaller NNT if the placebo group did not include the SPT component. Pregabalin in neuropathic pain has a combined NNT of 7.7 [52]. Capsaicin cough sensitivity also improved for both treatment groups but was not statistically significant between groups.
Uniquely, the effects on voice and laryngeal symptoms by the intervention were also investigated. Laryngeal hypersensitivity (LHQ) scores were found to improve with treatment in both groups but the change in LHQ score was significantly higher with the PREG + SPT group (p = 0.02) and this effect was maintained at follow-up (4 weeks after cessation of treatment). Notably, laryngeal hypersensitivity outcomes were similar to the self-reported cough outcomes implying laryngeal hypersensitivity may be relevant in the concept of central mechanisms in CHS and refractory CC. The proposed central action of pregabalin was further supported by no change on phonation between the two treatment groups. SPT targets vocal function suggesting that the combined treatment of PREG + SPT target specific elements in the treatment of cough.
Adverse effects of the treatment were high, 75% of participants reported an AE. Blurred vision, cognitive changes, dizziness, and weight gain were significantly greater in the PREG + SPT group while sleep disturbance and headache were significantly greater for the PLAC + SPT group. The incidence of AEs for this study was much higher than the gabapentin study (31%) [89]. The risk/benefit of pregabalin versus gabapentin for the treatment of refractory CC needs to be carefully considered. The magnitude of change in LCQ and cough severity in the pregabalin study was greater than the gabapentin study; however, adverse effects were higher. Pregabalin has greater abuse potential compared to gabapentin most likely due to its more rapid absorption and faster onset of action [103].
This combination treatment study further supports the effect of SPT on refractory CC [104].
2.2.1.4. Morphine
In a placebo-controlled randomized crossover study, Morice et al. [88] investigated the treatment of refractory CC with the opiate morphine sulfate compared to placebo. Cough was reported to be productive in 16 of 27 (59.2%) patients. Adverse effects were elicited at each visit by enquiring about the known side effects of opiate therapy from a symptom checklist.
Similar to the Gabapentin study [89], there was no significant difference between morphine and placebo for the citric acid cough challenge test supporting its central mechanism.
The study demonstrated a favorable benefit of morphine, mean (SD) change in LCQ score from baseline of 3.2 (2.6) over placebo, mean (SD) change in LCQ score from baseline of 1.2 (2.6), p = 0.02 [meta-analysis, Figure 3(a,b)]. The distribution of response seemed to segregate into responders and nonresponders (shown graphically by the author [88]). Response to treatment occurred rapidly, with maximum benefit being achieved by Day 5 in those who responded. The patients who had a subtherapeutic response requested an increase in dose to 10 mg twice daily in an extension phase of the study. This brought about a further amelioration of cough scores, suggesting that the optimum dose of morphine in the suppression of CC lies between 5 and 10 mg twice daily. However, with dose escalation, the incidence of drowsiness also doubled.
2.2.1.5. Tramadol
Tramadol is a centrally acting analgesic structurally related to codeine and morphine. Tramadol has two enantiomers, both of which contribute to analgesic activity via different mechanisms. Tramadol has been associated with serotonin syndrome when taken with serotonergic medications in case reports [105–107] although in a large recent study this was found to be very unlikely even in overdose [108]. There have been significant adverse effects such as seizures reported suggesting that the decision to prescribe tramadol should be carefully considered [108,109]. A recent Cochrane Systematic review found that there is only modest information to support tramadol use in neuropathic pain [110]. Interestingly, in a trial by Sindrup et al. [111], there was a significant therapeutic effect of tramadol on paresthesia, allodynia, and touch-evoked pain – similar characteristics to those described in neuropathic CC.
To date, there are no RCTs on the use of tramadol for neurogenic or refractory CC. However, due to tramadol’s effects on neuropathic pain it may be considered a developing therapy for refractory CC. Dion et al. [112] investigated the treatment of neurogenic cough with tramadol at a tertiary care laryngology practice (Table 3). The LCQ and the CSI [74] (which quantifies patients’ upper airway cough symptoms) were the primary outcomes. The LCQ scoring system differed to the usual LCQ score as the investigators did not calculate the domain scores. There was a change in LCQ score from baseline to posttreatment for tramadol (improved from 74 to 103, p = 0.005). Subsequent long-term placebo-controlled trials could further elucidate the duration, effectiveness, and safety of tramadol treatment for neurogenic cough.
2.2.1.6. Meta-analysis of CNS/neuromodulator drugs for refractory CC
Figure 3(a) shows the results from a meta-analysis on the centrally acting antitussives/neuromodulators versus placebo medication that had a continuous outcome measurement of cough QOL. As three of the studies used the LCQ and the Jeyakumar et al. [86] study used a CQLQ, the data was converted to SMDs for comparison. There was high heterogeneity (I2 = 89%, p < 0.00001) between the studies so a random effects model was used for the pooled analysis. There is an overall significant pooled effect from the CNS/neuromodulating treatment (SMD 1.41, 95% CI 0.37 to 2.46, p = 0.008) (Figure 3(a)).
In a sensitivity analysis, we removed the study under high risk of selection bias (Jeyakumar et al. [86]) and the effect for the remaining three studies was increased (MD 2.08, 95% CI 1.09 to 3.07, p < 0.0001) (Figure 3(b)).
Figure 4(a) shows the results from a meta-analysis on the centrally acting/neuromodulators that had a dichotomous outcome of treatment response. There was moderately to high heterogeneity (I2 = 84%, p = 0.002) between the studies so a random effects model was used for the pooled analysis. There is an overall nonsignificant pooled effect from the active treatment (RR 1.69, 95% CI 0.82 to 3.49, p = 0.16). This is likely due to the positive effect on refractory CC by the SPT + placebo treatment where response rates where very similar (83%) compared to the Pregabalin + SPT response rate of 88%.
If we remove the Vertigan et al. [90] study from the meta-analysis due to the effect of speech pathology on cough outcomes, there is now a significant pooled-effect from the remaining neuromodulators, gabapentin and amitriptyline (RR 2.11, 95% CI 1.37 to 3.26, p = 0.0007) (Figure 4(b)). There is less effect from the gabapentin treatment but there is more risk of bias for the amitriptyline study.
If the RR for these two studies is converted to NNT, amitriptyline has a NNT of 1.3, and for gabapentin, NNT is 3.6. Most meta-analyses of effective analgesic treatments generally report a NNT of 2–4. If we compare commonly used analgesics such as amitriptyline and gabapentin for neuropathic pain, the combined NNT to achieve more than 50% pain relief are NNT = 3.6 for tricyclics and NNT = 6.3 for gabapentin [52]. Our analyses also suggest that amitriptyline and gabapentin are also good candidates for treating neuropathic/refractory CC.
2.2.2. Receptor antagonist treatments for pain and refractory CC
Transient receptor potential (TRP) ion channels and acid sensing ion channels (ASICs) are important in pain and cough. From the TRP superfamily, the main triggers in the airways are the TRPV and the TRPA ion channels, expressed on the C-fibers of sensory nerves [27,113–115]. Nociceptive sensory neurons also take part in protective reflexes, including the cough and sneeze reflexes, and release inflammatory neuropeptides in the periphery upon stimulation by different environmental stimuli [113]. The TRPA1 receptor antagonist GRC17536 has shown a statistically significant and clinical response in a Phase IIa clinical trial for the treatment of diabetic peripheral neuropathy [116]; however, an unpublished placebo-controlled study of GRC17536 in CC did not reduce 24 h cough frequency, cough VAS, or citric acid cough reflex sensitivity [21].
2.2.2.1. The NMDA receptor antagonist ketamine
Only a few ion channels expressed in the primary sensory nerves are known to be directly gated by acid and lead to sensory activation. These include TRPV1 and ASICs. Acid can modulate cough pathways triggering or sensitizing cough. NMDA receptors are involved in acid-evoked reflexes such as the cough reflex [51].
In a recent study assessing the effects of low dose ketamine on refractory CC, ketamine was found to have no significant effect on capsaicin cough reflex sensitivity or 24 h objective cough counts in patients with cough or in healthy control subjects [117] (Table 5). Change in 24 h cough frequency for cough patients was baseline, median (IQR) 13.0 (16.8) to post-ketamine median (IQR) 13.5 (14.9), p = 0.52 coughs/h compared to placebo baseline, median (IQR) of 4.7 (2.8) to post-placebo saline median (IQR) of 4.4 (2.4), p = 0.44 coughs/h. This implies that not all NMDA receptor antagonists are equivalent in their antitussive effects; perhaps as a consequence of the involvement of receptor subtypes or that the cough outcomes used in some studies may not be specific measures of central effects.
NMDA receptors are present in both central and peripheral tissues. In the gabapentin [89] and morphine [88], RCTs reviewed earlier there was also no change in cough reflex sensitivity assessed with capsaicin suggesting a central mechanism of action. Conversely in a guinea pig study [118], memantine was found to significantly inhibit capsaicin-induced cough suggesting that the memantine site of action was peripheral. Further, capsaicin cough reflex sensitivity and objective cough frequency outcomes significantly decreased in both the pregabalin with SPT and the placebo with SPT groups from the Vertigan et al. [90] study suggesting that speech pathology has an effect on the peripheral component of refractory cough.
2.2.2.2. The TRPV1 receptor antagonist SB-705498
Khalid et al. [32] assessed the antitussive effects of the TRPV1 receptor antagonist SB-705498 in patients with refractory CC (Table 5). Patients who did not cough at least five times [C5] after capsaicin inhalation up to a concentration of 250 µmol/L were also excluded; this is an extra cough criterion not used by other studies. Co-primary outcomes were assessed by the capsaicin cough reflex sensitivity test (C5) before dose, 2 h after dose, and 24 h after dose and with 24-h cough frequency using the VitaloJAK® cough recorder before and after dose. Data for the two primary endpoints were analyzed using a mixed-effects model with a power to detect a 1-sided 2.5% difference between SB-705498 and placebo. A significant improvement in C5 values with SB-705498 treatment was reported at 2 h (p = 0.005) and borderline significant at 24 h (p = 0.026) when compared to placebo treatment. Patients coughing on placebo were accounted for in the analysis by imputing the next concentration of capsaicin with no change in significance. Twenty-four hour objective cough frequency was not improved GEM (SD) 24.2 (12.9) compared with placebo 23.9 (10.5). Ten nonserious AEs were reported for placebo and seven were reported for SB-705498 treatment. There were no significant changes in tympanic temperature – an important finding as clinical studies on TRPV1 blockade in pain have shown effects on core body temperature [116]. This study highlights the importance of using both objective and subjective cough measures when testing the antitussive effects of medicines.
2.2.2.3. The P2X3 receptor antagonist AF-219
Abdulqawi et al. [91] investigated the efficacy of AF-219 on 24 patients with refractory CC (six [25%] patients had a productive cough) (Table 5). Patients taking ACE inhibitors, opioids, neuromodulators or any other treatment that might modulate cough were excluded. On the basis of new safety data for AF-219, the protocol was amended once during recruitment. Safety was assessed through monitoring adverse events, physical examinations, vital signs, ECGs, blood and urine analysis, and urinary-tract ultrasound scans. Primary analysis included all randomized patients who took at least one dose of the study drug (intent to treat [ITT] analysis). Only observed data were included (no imputation for missing data). A per-protocol population analysis was also completed for end-of-treatment cough assessment for both study periods and for those that did not deviate from the protocol in a way that could have affected efficacy results.
The primary efficacy outcome measure was daytime cough frequency at baseline and after 2 weeks of treatment using the VitaloJAK® 24 h ambulatory cough recorder.
Daytime cough frequency fell from a mean (SD) of 37 (32) coughs/h to 11 (8) coughs/h after AF-219 treatment versus 65 (163) coughs/h to 44 (51) coughs/h after placebo, p = 0.0003 (Table 5) Mean (SD) change in coughs/h were calculated by the author (NMR) for meta-analysis, Figure 5. Patients with the highest cough frequency had the greatest improvements. When receiving AF-219, 11 of 22 patients selected the three most improved ratings (‘a very great deal better’, ‘a great deal better’, or ‘a good deal better’) compared with 1 of 22 patients during placebo treatment in the ITT population. Interestingly, for the 14 patients assessed in the same way in the per-protocol population, 8 of 14 (57%) fell into the top three categories while 6 of 14 (43%) patients said that their cough was ‘a little better’, the ‘same’, ‘a little worse’, ‘a good deal worse’, or ‘a very great deal worse’.
Figure 5.
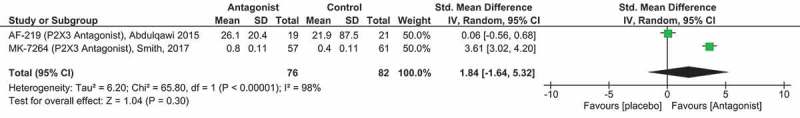
Forest plot of the P2X3 receptor antagonist AF-219/MK-7264 compared to placebo medication.
The green squares and black horizontal lines represent the SMDs and 95% CI for each study. The larger the green square the more weight that study contributes to the overall pooled estimate (black diamond). Risk of bias assessment not included as the Smith et al [92] study is a published Abstract only. Full color available online.
This study and some of the authors were industry sponsored. Declarations of interests were noted for study design, medical monitoring, trial oversight, trial monitoring, data management, analysis, and reporting of the study. The authors concluded that AF-219 was associated with improvements in both objective and subjective measures of cough suggesting that P2X3 antagonists may have a role in mediation of neuronal hypersensitivity and the treatment of refractory CC.
In a more recent Phase IIb clinical trial, Smith et al. [92] further investigated the role of MK-7264 (formerly AF-219) in the treatment of patients with refractory CC. This was a larger clinical trial to further evaluate safety, efficacy, and the therapeutic dose range of MK-7264. Primary outcome was mean change in Awake Cough Frequency (coughs/h) posttreatment versus baseline using the VitaloJAK®. MK-7264 at a dose of 50 mg significantly reduced the Awake Cough Frequency outcome Mean log10 (SD) -0.80 (0.11) compared to placebo Mean log10 (SD) -0.40 (0.11), p = 0.0027 (Table 5) [meta-analysis, Figure 5]. At this stage only a published abstract is available for review hence details of the statistical methods and full results were not available. Dysgeusia was reported to be the most common adverse effect with 81% of patients on the 50 mg BID active dose reporting AEs relating to taste.
2.2.2.4. The NK1 receptor antagonist Orvepitant
The VOLCANO-1 study by Smith et al. [42] assessed the efficacy and safety of the centrally active NK1 antagonist Orvepitant in patients with refractory CC. The primary efficacy outcome measurement was objectively measured daytime cough frequency (coughs/h) using the VitaloJAK® after 4 weeks of treatment. There was a statistically significant improvement in daytime cough frequency at Week-4: mean reduction of 18.9 coughs/h [95% CI 28.3 to 9.6), p < 0.001, a decrease of 26% from baseline. At the 8-week follow-up, the reduction in daytime cough frequency was sustained (20.4 coughs/h [95% CI 37.5 to 3.2], p = 0.02), a decrease of 28% from baseline cough frequency suggesting a ‘normalizing’ effect on the hypersensitized cough reflex. Orvepitant was safe and well-tolerated suggesting that it may be a promising antitussive for both peripheral and central CRS in refractory CC. However, as this is a published abstract, results on peripheral cough measurements were not given. These results would also need to be confirmed through placebo-controlled randomized studies.
2.2.2.5. Meta-analysis of P2X3 receptor antagonist AF-219/MK-7264 for refractory CC
Figure 5 shows the results from a meta-analysis comparing the continuous outcome measure of cough frequency (coughs/h) for P2X3 receptor antagonist AF-219/MK7264 compared to placebo. Data from the Smith et al. MK7264 study is in a log10 scale so the data were converted to SMDs for comparison. There was very high heterogeneity (I2 = 98%, p < 0.00001) between the studies, so a random effects model was used for the pooled analysis. The overall pooled effect of the P2X3 antagonist on cough frequency was nonsignificant, SMD [95% CI] 1.84 [-1.64 to 5.32], p = 0.30. This is likely due to the extreme differences in the data for these two studies. The mean and SD from the Smith et al. study (although confirmed with study author) is highly different to that from the Abdulqawi et al. study. Further confirmation of this result will not be possible until full publication and disclosure by the sponsor occurs.
2.2.3. Other medication treatments for refractory CC
2.2.3.1. Macrolide antibiotics for refractory CC
Jatakanon et al. [119] found that patients with refractory CC have induced sputum neutrophilia and raised concentration of mediators associated with neutrophilic airway inflammation, including interleukin 8 (IL-8), tumor necrosis factor alpha and prostaglandin E2 (PGE2). Yousaf et al. [120] suggested that there might be a causal link between neutrophilic airway inflammation and cough when noting a significant, independent association between the induced sputum neutrophil count and 24 h cough frequency. Long-term low-dose macrolides have been shown to reduce induced sputum neutrophil count in neutrophilic inflammation of the airways [121,122].
2.2.4. Erythromycin
In a randomized double-blind placebo controlled trial, Yousaf et al. [93] tested the hypothesis that erythromycin given for 12 weeks would reduce neutrophilic airway inflammation and 24 h cough frequency in patients with unexplained CC. Importantly, this study used a wide range of objective and subjective measures of cough severity to determine treatment effect. Low-dose erythromycin treatment for 12 weeks was found to reduce the induced sputum neutrophil count but not cough frequency or cough severity in patients with refractory CC (Table 6). An important and unexpected finding of this study was the large reduction in 24 h cough frequency and improvement in LCQ seen with the placebo treatment. The authors make an important point that this result supports suggestions that traditional uncontrolled treatment trials evaluating patients with CC may be flawed, particularly if 24 h cough frequency is used to assess treatment response.
2.2.5. Azithromycin
Hodgson et al. [94] also sought to explore potential effects of the macrolide azithromycin on cough symptoms in patients with refractory CC (Table 6). This study compared 250 mg of azithromycin or matching placebo 3 times a week for 8 weeks. The LCQ was the primary outcome measure with cough VAS and exhaled nitric oxide measured as secondary outcomes. There was a clinically important improvement in LCQ score with azithromycin mean change [95% CI] 2.4 [0.5 to 4.2] but not with placebo, mean change [95% CI] 0.7 [-0.6 to 1.9]. However, the between-group difference was not statistically significant (p = 0.12). Eleven of the 21 (52%) subjects in the azithromycin group had a clinically significant improvement in their LCQ score. In patients with CC and a concurrent diagnosis of asthma, there was a large and significant improvement in LCQ score (mean change [95% CI] 6.19 [4.06 to 8.32]) when treated with azithromycin. The results did not support the routine use of low-dose macrolides in patients with refractory CC, but for CC in association with asthma the results suggested that further investigation was warranted. There were no objective measures of cough frequency or cough severity used in this study.
2.2.5.1. Meta-analysis of macrolide antibiotic drugs for refractory CC
As the erythromycin study [93] assessed LCQ as a secondary outcome, a meta-analysis on the two macrolide antibiotics could be performed (Figure 6). It can be seen that there is a small effect from azithromycin over placebo treatment on LCQ in refractory CC patients, MD [95% CI] 1.70 [-0.66 to 4.06]. There was no effect of erythromycin over placebo on LCQ in patients with refractory CC, MD [95% CI] 0 [-2.72 to 2.72], therefore the overall pooled estimate of effect was not significant, MD [95% CI] of 0.97 [-0.81 to 2.75], p = 0.29. Both studies were assessed to have a very low risk of bias (Figure 2).
Figure 6.
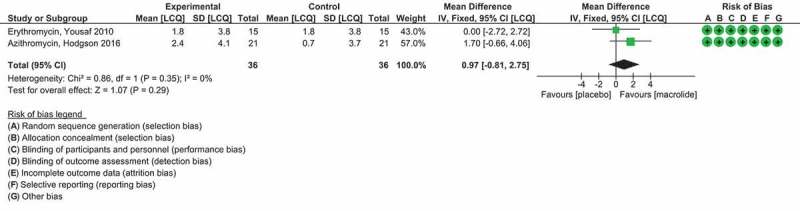
Forest plot of macrolide antibiotics erythromycin and azithromycin compared to placebo medication.
The green squares and black horizontal lines represent the Risk Ratio and 95% CI for each study. The larger the green square the more weight that study contributes to the overall pooled estimate (black diamond). Summary risk of bias has also been included for each study. Full color available online.
2.2.5.2. PA101 cromolyn sodium formulation for refractory CC
PA101 is a novel formulation of cromolyn sodium delivered via a high-efficiency eFlow nebulizer (PARI, Germany) that achieves significantly higher lung deposition compared to previous formulations. Cromolyn blocks calcium ion influx into mast cells preventing the degranulation of mast cells in the lungs. Inhaled sodium cromoglicate has been shown to inhibit both allergen-induced early and late asthmatic responses and exercise-induced asthma [123,124]. Birring et al. [125] very recently assessed the efficacy and safety of inhaled PA101 in a randomized placebo-controlled trial in patients with idiopathic pulmonary fibrosis (IPF) and CC and a parallel study of similar design in patients with refractory CC. The primary efficacy endpoint was change from baseline to posttreatment daytime cough frequency using the LCM. Twenty-four participants with IPF were randomly assigned to treatment groups. In patients with IPF, PA101 reduced daytime cough frequency by 31.1% at day 14 compared with placebo. Daytime cough frequency with PA101 treatment fell from a mean (SD) of 55 (55) coughs/h at baseline to 39 (29) coughs/h at day 14 versus 51 (37) coughs/h at baseline to 52 (40) cough/h following placebo treatment (ratio of least-squares [LS] means 0.67, 95% CI 0.48 to 0.94, p = 0.024).
Twenty-eight participants with refractory CC were enrolled during the same period and 27 received study treatment. By contrast, no treatment benefit for PA101 was observed in the refractory CC cohort; mean reduction in daytime cough frequency was 25.9% with PA101 and 19.7% with placebo (6.2% greater mean reduction for PA101 when adjusted for placebo); ratio of LS means 1.27, 0.78 to 2.06, p = 0.31 (Table 6).
PA101 was well tolerated in both cohorts. The incidence of adverse effects was similar between PA101 and placebo treatments, mild in severity, and no severe adverse effects were reported. Four patients in the refractory CC cohort discontinued the study due to AE’s: two patients during placebo treatment (headache, cough, and oropharyngeal pain; dizziness), and two patients during PA101 treatment (angioedema and sinus tachycardia; pharyngeal hypoesthesia, cough, and dyspnea) [125]. The results of this study suggest that the mechanism of cough in IPF might be disease specific as it responds to PA101 treatment while refractory CC without IPF does not respond to PA101 treatment.
2.3. Expert opinion
CC is common and refractory CC is very difficult to treat, as effective antitussives to control cough are currently limited. The similarity between mechanisms in pain and cough led to trials of neuromodulators such as amitriptyline, gabapentin and pregabalin that demonstrated an antitussive effect. The importance of central mechanisms regulating cough has been reported in fMRI studies. Gabapentin and Speech Pathology Treatment are effective therapies that are now incorporated into the treatment guidelines for unexplained or refractory CC but novel and other antitussive treatments are still needed. Drugs that target peripheral neuronal sensory receptors have recently been investigated; the P2X3 receptor antagonists show most promise. Further understanding of the mechanisms of cough is required to develop new treatments and this could be enhanced through the development of appropriate and specific disease state animal models.
The primary available therapies for refractory CC include gabapentin, speech pathology, and morphine. However, the current options do not always work and some have undesirable side effects. The goal is to develop effective cough treatments that reduce but not completely suppress cough and avoid CNS side effects such as sedation. We need a better understanding of cough mechanisms, for example, what is the relative importance of peripheral sensitization, central sensitization, and inhibitory pathways? It is also important to establish whether there are disease-specific differences or phenotypes of cough, as this will dictate the development of therapies.
A key challenge for cough research is a need for better understanding of the cough neural pathways. This is difficult to study in vivo and therefore better animal cough models that reflect human cough are needed.
During the next 5 years, clinical trials in CC are planned with medications that target P2X3, NK1, TRPV4, TRPM8, and ALPHA7-Nicotinic receptors. Current research into central cough inhibition pathways with fMRI imaging techniques have highlighted areas in the brain for central sensitization and therapies that target this are worth investigating.
Funding Statement
NM Ryan is supported by an NHMRC Early Career Fellowship Award ID 1072056.
Article highlights
Refractory chronic cough is common and difficult to treat.
Increased understanding of the similarities in refractory chronic cough and neuropathic pain have resulted in more treatments being available but they do not work for everyone.
Gabapentin and speech pathology have recently been incorporated into treatment guidelines for refractory chronic cough.
Novel treatments that target peripheral and central neural receptors are being tested for refractory chronic cough with the P2X3 receptor anatgonists showing the most promise.
Functional brain imaging, phenotypic cough animal models, and the use of appropriate cough measurement tools will facilitate the development of novel antitussive drugs.
This box summarizes key points contained in the article.
Declaration of interest
S Birring has received fees for scientific advisory work for the development of therapies for refractory cough from Patara, Merck & Co., and Bayer Healthcare. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Birring SS, Prudon B, Carr AJ, et al. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax. 2003;58:339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haque RA, Usmani OS, Barnes PJ.. Chronic idiopathic cough: a discrete clinical entity? CHEST J. 2005;127:1710–1713. [DOI] [PubMed] [Google Scholar]
- 3.Gibson P, Wang G, McGarvey L, et al. Treatment of unexplained chronic cough: CHEST guideline and expert panel report. CHEST J. 2016;149:27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]; • These recent cough guidelines recommend that speech pathology treatment or a trial of gabapentin be trialed for those patients with unexplained cough or to those that remain refractory to usual cough treatments.
- 4.Chung KF, Pavord ID.. Prevalence, pathogenesis, and cause of chronic cough. Lancet. 2008;371:1364–1374. [DOI] [PubMed] [Google Scholar]
- 5.Irwin RS, Boulet LP, Cloutier MM, et al. Managing cough as a defence mechanism and as a symptom. A consensus panel report of the American College of Chest Physicians. Chest. 1998;114:1269–1275. [DOI] [PubMed] [Google Scholar]
- 6.Chung KF. Approach to chronic cough: the neuropathic basis for cough hypersensitivity syndrome. J Thorac Dis. 2014October;6:S699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan NM, Gibson PG. Characterization of laryngeal dysfunction in chronic persistent cough. Laryngoscope. 2009;119:640–645. [DOI] [PubMed] [Google Scholar]; • Laryngeal dysfunction which manifests as paradoxical vocal cord movement and extrathoracic airway hyperresponsiveness is common in chronic cough. Laryngeal hypersensitivity is the common mechanism and it responds to speech pathology treatment.
- 8.Vertigan AE, Bone SL, Gibson PG. Laryngeal sensory dysfunction in laryngeal hypersensitivity syndrome. Respirology. 2013;18:948–956. [DOI] [PubMed] [Google Scholar]
- 9.Gibson PG, Vertigan AE. Management of chronic refractory cough. BMJ. 2015;351:h5590. [DOI] [PubMed] [Google Scholar]
- 10.Irwin RS, Rosen MJ, Braman SS. Cough. A comprehensive review. Arch Intern Med. 1977September;137:1186–1191. [DOI] [PubMed] [Google Scholar]
- 11.Kastelik J, Aziz I, Ojoo J, et al. Investigation and management of chronic cough using a probability-based algorithm. Eur Respir J. 2005;25:235–243. [DOI] [PubMed] [Google Scholar]
- 12.Pratter MR, Brightlin CE, Boulet LP, et al. An empiric integrative approach to the management of cough. Chest. 2006;129:222S-231S. [DOI] [PubMed] [Google Scholar]
- 13.Yu L, Qiu Z, Lue H, et al. Clinical benefit of sequential three‐step empirical therapy in the management of chronic cough. Respirology. 2008;13:353–358. [DOI] [PubMed] [Google Scholar]
- 14.Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:1S–23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morice AH, Fontana GA, Belvisi MG, et al. ERS guidelines on the assessment of cough. Eur Respir J. 2007;29:1256–1276. [DOI] [PubMed] [Google Scholar]
- 16.Morice AH, McGarvey L, Pavord I. British Thoracic Society Cough Guideline Group: recommendations for the management of cough in adults. Thorax. 2006;61:i1–i24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibson PG, Chang AB, Glasgow NJ, et al. CICADA: cough in children and adults: diagnosis and assessment. Australian cough guidelines summary statement. Med J Aust. 2010;192:265–271. [DOI] [PubMed] [Google Scholar]; • CICADA is a clinical guideline for the assessment and management of persistent cough in children and adults developed by a multidisciplinary expert committee after a needs assessment by clinicians.
- 18.Chung KF. Chronic ‘cough hypersensitivity syndrome’: a more precise label for chronic cough. Pulm Pharmacol Ther. 2011;24:267–271. [DOI] [PubMed] [Google Scholar]
- 19.Morice A, Faruqi S, Wright C, et al. Cough hypersensitivity syndrome: a distinct clinical entity. Lung. 2011;189:73–79. [DOI] [PubMed] [Google Scholar]; • The authors postulate that most patients with chronic cough have a single discrete clinical entity that they term ‘Cough Hypersensitivity Syndrome’. The unifying characteristic is afferent hypersensitivity and by regarding chronic cough as a single clinical entity it allows insight into the diagnosis, treatment, and future research of this characterized group of patients.
- 20.O’Neill J, McMahon SB, Undem BJ. Chronic cough and pain: Janus faces in sensory neurobiology? Pulm Pharmacol Ther. 2013October;26:476–485. [DOI] [PubMed] [Google Scholar]
- 21.Chung KF. Advances in mechanisms and management of chronic cough: The Ninth London International Cough Symposium 2016. Pulm Pharmacol Ther. 2017;47:2–8. [DOI] [PubMed] [Google Scholar]
- 22.Mazzone SB, Cole LJ, Ando A, et al. Investigation of the neural control of cough and cough suppression in humans using functional brain imaging. J Neurosci. 2011;31:2948–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The authors provide novel insights into the supramedullary control of cough and cough suppression by using functional brain imaging in healthy humans with capsaicin-evoked cough. The data eloquently show the existence of distinct higher brain circuitry for facilitating and suppressing the cough reflex.
- 23.Morice AH, Millqvist E, Belvisi MG, et al. Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. Eur Respir J. 2014;44:1132–1148. [DOI] [PubMed] [Google Scholar]
- 24.Chung KF, McGarvey L, Mazzone SB. Chronic cough as a neuropathic disorder. Lancet Respir Med. 2013;1:414–422. [DOI] [PubMed] [Google Scholar]
- 25.Lee B, Woo P. Chronic cough as a sign of laryngeal sensory neuropathy: diagnosis and treatment. Ann Otology Rhinology Laryngol. 2005;114:253–257. [DOI] [PubMed] [Google Scholar]
- 26.Bastian RW, Vaidya AM, Delsupehe KG. Sensory neuropathic cough: a common and treatable cause of chronic cough. Otolaryngology—Head Neck Surgery. 2006;135:17–21. [DOI] [PubMed] [Google Scholar]
- 27.Nilius B, Owsianik G, Voets T, et al. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. [DOI] [PubMed] [Google Scholar]
- 28.Canning BJ, Chang AB, Bolser DC, et al. Anatomy and neurophysiology of cough: CHEST Guideline and Expert Panel report. Chest. 2014;146:1633–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groneberg DA, Niimi A, Dinh QT, et al. Increased expression of transient receptor potential vanilloid-1 in airway nerves of chronic cough. Am J Respir Crit Care Med. 2004December15;170:1276–1280. [DOI] [PubMed] [Google Scholar]
- 30.Rami HK, Thompson M, Stemp G, et al. Discovery of SB-705498: a potent, selective and orally bioavailable TRPV1 antagonist suitable for clinical development. Bioorg Med Chem Lett. 2006June15;16:3287–3291. [DOI] [PubMed] [Google Scholar]
- 31.Gavva NR, Bannon AW, Hovland DN Jr., et al. Repeated administration of vanilloid receptor TRPV1 antagonists attenuates hyperthermia elicited by TRPV1 blockade. J Pharmacol Exp Ther. 2007;323:128–137. [DOI] [PubMed] [Google Scholar]
- 32.Khalid S, Murdoch R, Newlands A, et al. Transient receptor potential vanilloid 1 (TRPV1) antagonism in patients with refractory chronic cough: a double-blind randomized controlled trial. J Allergy Clin Immunol. 2014;134:56–62. [DOI] [PubMed] [Google Scholar]
- 33.Duffy RA. Potential therapeutic targets for neurokinin-1 receptor antagonists. Expert Opin Emerg Drugs. 2004May;9:9–21. [DOI] [PubMed] [Google Scholar]
- 34.Hill R. NK1 (substance P) receptor antagonists–why are they not analgesic in humans? Trends Pharmacol Sci. 2000July;21:244–246. [DOI] [PubMed] [Google Scholar]
- 35.Dionne RA, Max MB, Gordon SM, et al. The substance P receptor antagonist CP-99,994 reduces acute postoperative pain. Clin Pharmacol Ther. 1998;64:562–568. [DOI] [PubMed] [Google Scholar]
- 36.Urban LA, Fox AJ. NK1 receptor antagonists–are they really without effect in the pain clinic? Trends Pharmacol Sci. 2000December;21:462–464. [DOI] [PubMed] [Google Scholar]
- 37.Lee WS, Moussaoui SM, Moskowitz MA. Blockade by oral or parenteral RPR 100893 (a non-peptide NK1 receptor antagonist) of neurogenic plasma protein extravasation within guinea-pig dura mater and conjunctiva. Br J Pharmacol. 1994July;112:920–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moussaoui SM, Philippe L, Le Prado N, et al. Inhibition of neurogenic inflammation in the meninges by a non-peptide NK1 receptor antagonist, RP 67580. Eur J Pharmacol. 1993July20;238:421–424. [DOI] [PubMed] [Google Scholar]
- 39.Shepheard SL, Williamson DJ, Williams J, et al. Comparison of the effects of sumatriptan and the NK1 antagonist CP-99,994 on plasma extravasation in Dura mater and c-fos mRNA expression in trigeminal nucleus caudalis of rats. Neuropharmacology. 1995;34:255–261. [DOI] [PubMed] [Google Scholar]
- 40.El-Hashim AZ, Wyss D, Lewis C. Effect of a novel NK1 receptor selective antagonist (NKP608) on citric acid induced cough and airway obstruction. Pulm Pharmacol Ther. 2004;17:11–18. [DOI] [PubMed] [Google Scholar]
- 41.Fahy JV, Wong HH, Geppetti P, et al. Effect of an NK1 receptor antagonist (CP-99,994) on hypertonic saline-induced bronchoconstriction and cough in male asthmatic subjects. Am J Respir Crit Care Med. 1995;152:879–884. [DOI] [PubMed] [Google Scholar]
- 42.Smith JA, Allman D, Badri H, et al. The neurokinin-1 receptor antagonist orvepitant is a novel anti-tussive therapy for chronic refractory cough: results from a phase 2 study (VOLCANO-1). Am J Respir Crit Care Med. 2017;A2672 (Abstract).. [DOI] [PubMed] [Google Scholar]
- 43.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006August03;442:527–532. [DOI] [PubMed] [Google Scholar]
- 44.Guile SD, Ince F, Ingall AH, et al. The medicinal chemistry of the P2 receptor family. Prog Med Chem. 2001;38:115–187. [DOI] [PubMed] [Google Scholar]
- 45.Keystone EC, Wang MM, Layton M, et al. Clinical evaluation of the efficacy of the P2X7 purinergic receptor antagonist AZD9056 on the signs and symptoms of rheumatoid arthritis in patients with active disease despite treatment with methotrexate or sulphasalazine. Ann Rheum Dis. 2012;71:1630–1635. [DOI] [PubMed] [Google Scholar]
- 46.Stock TC, Bloom BJ, Wei N, et al. Efficacy and safety of CE-224,535, an antagonist of P2X7 receptor, in treatment of patients with rheumatoid arthritis inadequately controlled by methotrexate. J Rheumatol. 2012;39:720–727. [DOI] [PubMed] [Google Scholar]
- 47.Cockayne DA, Dunn PM, Zhong Y, et al. P2X(2) knockout mice and P2X(2)/P2X(3) double knockout mice reveal a role for the P2X(2) receptor subunit in mediating multiple sensory effects of ATP. J Physiol. 2005;567:621–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prado FC, Araldi D, Vieira AS, et al. Neuronal P2X3 receptor activation is essential to the hyperalgesia induced by prostaglandins and sympathomimetic amines released during inflammation. Neuropharmacology. 2013;67:252–258. [DOI] [PubMed] [Google Scholar]
- 49.Smith J, Kitt M, Butera P, et al. S27 The effect of P2X3 antagonism (AF–219) on experimentally evoked cough in healthy volunteers and chronic cough patients. Thorax. 2016;71:A17.1–A17. [Google Scholar]
- 50.Kamei J, Takahashi Y, Yoshikawa Y, et al. Involvement of P2X receptor subtypes in ATP-induced enhancement of the cough reflex sensitivity. Eur J Pharmacol. 2005;528:158–161. [DOI] [PubMed] [Google Scholar]
- 51.Gibson PG, Ryan NM. Cough pharmacotherapy: current and future status. Expert Opin Pharmacother. 2011August;12:1745–1755. [DOI] [PubMed] [Google Scholar]
- 52.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015February;14:162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The authors provide an updated therapeutic algorithm for neuropathic pain based on GRADE resulting in changes to first-, second-, and third-line recommended treatments for neuropathic pain. Tricyclic antidepressants, pregabalin, gabapentin, gabapentin extended release or enacarbil, and duloxetine are first line based on strong GRADE recommendations for use. Lidnocaine patches are no longer first line because of weak quality of evidence. Strong opioids are now recommended as third line, primarily due to the potential risk of abuse.
- 53.Canning BJ, Mori N. Encoding of the cough reflex in anesthetized guinea pigs. Am J Physiol Regulatory, Integr Comp Physiol. 2011;300:R369–R377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keller JA, McGovern AE, Mazzone SB. Translating cough mechanisms into better cough suppressants. Chest. 2017;152:833–841. [DOI] [PubMed] [Google Scholar]
- 55.Jarvis MF, Honore P, Shieh CC, et al. A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc Natl Acad Sci USA. 2007May15;104:8520–8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kemp MI. Structural trends among second-generation voltage-gated sodium channel blockers. Prog Med Chem. 2010;49:81–111. [DOI] [PubMed] [Google Scholar]
- 57.Scanio MJC, Shi L, Drizin I, et al. Discovery and biological evaluation of potent, selective, orally bioavailable, pyrazine-based blockers of the Nav1.8 sodium channel with efficacy in a model of neuropathic pain. Bioorg Med Chem. 2010;18:7816–7825. [DOI] [PubMed] [Google Scholar]
- 58.Song W-J, Chang Y-S, Faruqi S, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J. 2015Letter;45:1479–1481. [DOI] [PubMed] [Google Scholar]; •• This is the first systematic analysis to estimate the epidemiological burden of chronic cough. There were inconsistencies in the existing literature suggesting an urgent need for global standardization. The high epidemiological burden of chronic cough further identified a major unmet clinical need.
- 59.Pollack A, Harrison C, Henderson J, et al. Neuropathic pain. Aust Fam Physician. 2013;42:91. [PubMed] [Google Scholar]
- 60.Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nature Reviews Disease Primers. 2017;3:Article Number:17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bouhassira D, Lanteri-Minet M, Attal N, et al. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136:380–387. [DOI] [PubMed] [Google Scholar]
- 62.Birring SS, Murphy AC, Scullion JE, et al. Idiopathic chronic cough and organ specific autoimmune diseases: a case control study. Respir Med. 2004;98:242–246. [DOI] [PubMed] [Google Scholar]
- 63.McGarvey L. Idiopathic chronic cough: a real disease or a failure of diagnosis? Cough. 2005September23;1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ghosh AK, Ghosh A, Kunda A, et al. Comparative study of efficacy and safety of pregabalin and gabapentin in neuropathic pain. Asian J Pharm Life Sci. 2012;2:64–71. [Google Scholar]
- 65.Vertigan AE, Gibson PG. Chronic refractory cough as a sensory neuropathy: evidence from a reinterpretation of cough triggers. J voice. 2011September;25:596–601. [DOI] [PubMed] [Google Scholar]
- 66.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J pain. 2009;10:895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The authors review the major triggers that initiate and maintain central sensitization in healthy individuals in response to nociceptor input and in patients with inflammatory and neuropathic pain, emphasizing the fundamental contribution and multiple mechanisms of synaptic plasticity.
- 67.Torebjork HE, Lundberg LE, LaMotte RH. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J Physiol. 1992March;448:765–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sarkar S, Aziz Q, Woolf CJ, et al. Contribution of central sensitisation to the development of non-cardiac chest pain. Lancet. 2000September;30(356):1154–1159. [DOI] [PubMed] [Google Scholar]
- 69.Iannetti GD, Zambreanu L, Wise RG, et al. Pharmacological modulation of pain-related brain activity during normal and central sensitization states in humans. Proc Natl Acad Sci USA. 2005December13;102:18195–18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Birring SS, Parker D, Brightling CE, et al. Induced sputum inflammatory mediator concentrations in chronic cough. Am J Respir Crit Care Med. 2004;169:15–19. [DOI] [PubMed] [Google Scholar]
- 71.Lee KK, Matos S, Evans DH, et al. A longitudinal assessment of acute cough. Am J Respir Crit Care Med. 2013;187:991–997. [DOI] [PubMed] [Google Scholar]
- 72.French CT, Irwin RS, Fletcher KE, et al. Evaluation of a cough-specific quality-of-life questionnaire. Chest. 2002;121:1123–1131. [DOI] [PubMed] [Google Scholar]
- 73.Spinou A, Birring SS. An update on measurement and monitoring of cough: what are the important study endpoints? J Thorac Dis. 2014;6:S728–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The authors discuss validated subjective and other objective measurement tools for assessing cough. As each test measures a different component of cough, a combination of subjective and objective tools are strongly advised.
- 74.Shembel AC, Rosen CA, Zullo TG, et al. Development and validation of the cough severity index: a severity index for chronic cough related to the upper airway. Laryngoscope. 2013;123:1931–1936. [DOI] [PubMed] [Google Scholar]
- 75.Matos S, Birring SS, Pavord ID, et al. An automated system for 24-h monitoring of cough frequency: the Leicester Cough Monitor. IEEE Trans Biomed Eng. 2007;54:1472–1479. [DOI] [PubMed] [Google Scholar]
- 76.McGuinness K, Holt K, Dockry R, et al. P159 Validation of the VitaloJAK™ 24 hour ambulatory cough monitor. Thorax. 2012;67:A131–A31. [Google Scholar]
- 77.Canning BJ, Farmer DG, Mori N. Mechanistic studies of acid-evoked coughing in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol. 2006August;291:R454–63. [DOI] [PubMed] [Google Scholar]
- 78.Canning BJ, Mazzone SB, Meeker SN, et al. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol. 2004;557:543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chou YL, Scarupa MD, Mori N, et al. Differential effects of airway afferent nerve subtypes on cough and respiration in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1572–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.House A, Celly C, Skeans S, et al. Cough reflex in allergic dogs. Eur J Pharmacol. 2004May;25(492):251–258. [DOI] [PubMed] [Google Scholar]
- 81.Smith J. Ambulatory methods for recording cough. Pulm Pharmacol Ther. 2007;20:313–318. [DOI] [PubMed] [Google Scholar]
- 82.Belvisi MG, Birrell MA, Khalid S, et al. Neurophenotypes in airway diseases. Insights from translational cough studies. Am J Respir Crit Care Med. 2016;193:1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Clay E, Patacchini R, Trevisani M, et al. Ozone-induced hypertussive responses in rabbits and guinea pigs. J Pharmacol Exp Ther. 2016;357:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Canning BJ. The cough reflex in animals: relevance to human cough research. Lung. 2008;186:S23–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jeyakumar A, Brickman TM, Haben M. Effectiveness of amitriptyline versus cough suppressants in the treatment of chronic cough resulting from postviral vagal neuropathy. Laryngoscope. 2006;116:2108–2112. [DOI] [PubMed] [Google Scholar]
- 87.Norris BK, Schweinfurth JM. Management of recurrent laryngeal sensory neuropathic symptoms. Annals of Otology, Rhinology & Laryngology. 2010;119:788–791. [DOI] [PubMed] [Google Scholar]
- 88.Morice AH, Menon MS, Mulrennan SA, et al. Opiate therapy in chronic cough. Am J Respir Crit Care Med. 2007;175:312–315. [DOI] [PubMed] [Google Scholar]
- 89.Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. The Lancet. 2012;380:1583–1589. [DOI] [PubMed] [Google Scholar]; •• First placebo-controlled randomized clinical trial to show that gabapentin is efficacious in the treatment of refractory chronic cough particularly in those patients with central sensitization.
- 90.Vertigan AE, Kapela SL, Ryan NM, et al. Pregabalin and speech pathology combination therapy for refractory chronic cough: a randomized controlled trial. CHEST J. 2016;149:639–648. [DOI] [PubMed] [Google Scholar]; • The authors goal was to determine whether combined pregabalin and speech pathology treatment was more effective than speech pathology treatment alone. This was based on previous research which showed that speech pathology treatment improves symptoms but resolution is incomplete and that centrally acting neuromodulators improve cough symptoms, but not cough reflex sensitivity, and the effect is short-lived. The combined treatment was found to be more effective.
- 91.Abdulqawi R, Dockry R, Holt K, et al. P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. The Lancet. 2015;385:1198–1205. [DOI] [PubMed] [Google Scholar]; •• This placebo-controlled randomized crossover clinical trial showed that antagonists of P2X3 receptors such as AF-219 is a promising new group of antitussives. AF-219 was found to significantly reduce cough frequency compared to placebo.
- 92.Smith J, Kitt M, Morice A, et al. MK-7264, a P2X3 Receptor Antagonist, reduces cough frequency in patients with refractory chronic cough: results from a randomized, controlled, Phase 2b clinical trial. Am J Respir Crit Care Med. 2017. (Abstract):A7608. [Google Scholar]
- 93.Yousaf N, Monteiro W, Parker D, et al. Long-term low-dose erythromycin in patients with unexplained chronic cough: a double-blind placebo controlled trial. Thorax. 2010;65:1107–1110. [DOI] [PubMed] [Google Scholar]
- 94.Hodgson D, Anderson J, Reynolds C, et al. The effects of azithromycin in treatment-resistant cough. Chest. 2016;149:1052–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chung K. Drugs to suppress cough. Expert Opin Investig Drugs. 2005;14:19–27. [DOI] [PubMed] [Google Scholar]
- 98.Chung KF. Current and future prospects for drugs to suppress cough. IDrugs. 2003;6:781–786. [PubMed] [Google Scholar]
- 99.Smith J, Owen E, Earis J, et al. Effect of codeine on objective measurement of cough in chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2006;117:831–835. [DOI] [PubMed] [Google Scholar]
- 100.Raj A, Pavord D, Birring S. Clinical cough IV: what is the minimal important difference for the Leicester Cough Questionnaire? Hand Exp Pharmacol. 2009;187:311–320. [DOI] [PubMed] [Google Scholar]
- 101.Mintz S, Lee JK. Gabapentin in the treatment of intractable idiopathic chronic cough: case reports. Am J Med. 2006;119:e13–e15. [DOI] [PubMed] [Google Scholar]
- 102.Halum SL, Sycamore DL, McRae BR. A new treatment option for laryngeal sensory neuropathy. Laryngoscope. 2009;119:1844–1847. [DOI] [PubMed] [Google Scholar]
- 103.Ryan NM. A review on the efficacy and safety of gabapentin in the treatment of chronic cough. Expert Opin Pharmacother. 2015;16:135–145. [DOI] [PubMed] [Google Scholar]
- 104.Vertigan AE, Theodoros DG, Gibson PG, et al. Efficacy of speech pathology management for chronic cough: a randomised placebo controlled trial of treatment efficacy. Thorax. 2006;61:1065–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This study showed that speech pathology is an effective management intervention for chronic cough and viable alternative for patients who do not respond to medical treatment.
- 105.Garrett PM. Tramadol overdose and serotonin syndrome manifesting as acute right heart dysfunction. Anaesth Intensive Care. 2004;32:575–577. [DOI] [PubMed] [Google Scholar]
- 106.Houlihan DJ. Serotonin syndrome resulting from coadministration of tramadol, venlafaxine, and mirtazapine. Ann Pharmacother. 2004;38:411–413. [DOI] [PubMed] [Google Scholar]
- 107.Nelson EM, Philbrick AM. Avoiding serotonin syndrome: the nature of the interaction between tramadol and selective serotonin reuptake inhibitors. Ann Pharmacother. 2012;46:1712–1716. [DOI] [PubMed] [Google Scholar]
- 108.Ryan NM, Isbister GK. Tramadol overdose causes seizures and respiratory depression but serotonin toxicity appears unlikely. Clin Toxicol. 2015;53:545–550. [DOI] [PubMed] [Google Scholar]
- 109.Kaye K. Trouble with tramadol. Aust Prescriber. 2004;27:26–27. [Google Scholar]
- 110.Duehmke RM, Derry S, Wiffen PJ , et al. Tramadol for neuropathic pain in adults. Cochrane Database Sys Rev. 2017(6):CD003726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sindrup SH, Madsen C, Brøsen K, et al. The effect of tramadol in painful polyneuropathy in relation to serum drug and metabolite levels. Clin Pharmacol Ther. 1999;66:636–641. [DOI] [PubMed] [Google Scholar]
- 112.Dion GR, Teng SE, Achlatis E, et al. Treatment of neurogenic cough with tramadol: a pilot study. Otolaryngology–Head Neck Surgery. 2017;157:77–79. [DOI] [PubMed] [Google Scholar]
- 113.Bessac BF, Jordt SE. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda, MD). 2008December;23:360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Birrell MA, Belvisi MG, Grace M, et al. TRPA1 agonists evoke coughing in guinea pig and human volunteers. Am J Respir Crit Care Med. 2009December1;180:1042–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Skerratt SE, West CW. Ion channel therapeutics for pain. Channels. 2015;9:344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This review focused on the current status of ion channel modulators and their application toward pain relief. It also discussed some of the drawbacks of current therapies and potential directions for improved treatment of the human pain condition.
- 117.Young EC, Sumner H, Decalmer S, et al. Does central up-regulation of the N-methyl-D-aspartate receptor contribute to cough reflex hypersensitivity? Am J Respir Crit Care Med. 2010;181:A5906–A06. [Google Scholar]
- 118.Smith JA, Hilton ECY, Saulsberry L, et al. Antitussive effects of memantine in guinea pigs. Chest. 2012;141:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jatakanon A, Lalloo UG, Lim S, et al. Increased neutrophils and cytokines, TNF-α and IL-8, in induced sputum of non-asthmatic patients with chronic dry cough. Thorax. 1999;54:234–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yousaf N, Matos S, Birring S, et al. Factors affecting cough frequency in a mixed population. Thorax. 2009;64:A151. [Google Scholar]
- 121.Seemungal TA, Wilkinson TM, Hurst JR, et al. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178:1139–1147. [DOI] [PubMed] [Google Scholar]
- 122.Simpson JL, Powell H, Boyle MJ, et al. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am J Respir Crit Care Med. 2008;177:148–155. [DOI] [PubMed] [Google Scholar]
- 123.Edwards AM. The discovery of cromolyn sodium and its effect on research and practice in allergy and immunology. J Allergy Clin Immunol. 2005;115:885–888. [DOI] [PubMed] [Google Scholar]
- 124.Holgate ST. Stratified approaches to the treatment of asthma. Br J Clin Pharmacol. 2013;76:277–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Birring SS, Wijsenbeek MS, Agrawal S, et al. A novel formulation of inhaled sodium cromoglicate (PA101) in idiopathic pulmonary fibrosis and chronic cough: a randomised, double-blind, proof-of-concept, phase 2 trial. Lancet Respir Med. 2017;5:806–815. [DOI] [PubMed] [Google Scholar]; •• The results of this study suggest that the mechanism of cough in IPF might be disease specific as it responds to PA101 treatment while refractory CC without IPF does not respond to PA101 treatment.


