Abstract
Objective
To compare the real-world effectiveness and safety of non-vitamin-K-antagonist oral anticoagulant (NOAC) treatment in atrial fibrillation (AF) patients with a vitamin-K-antagonist (VKA)-based treatment.
Methods
This was a retrospective analysis of an anonymized claims dataset from 3 German health insurance funds covering the period from January 01, 2010 to June 30, 2014, with a minimum observation time of 12 months. All continuously insured patients with at least 2 outpatient AF diagnoses and/or 1 inpatient respective diagnosis who received at least 1 outpatient prescription of a NOAC or VKA were included.
Outcomes and measures
Death, ischemic strokes (IS), non-specified strokes, transient ischemic attacks (TIAs), myocardial infarctions (MIs), arterial embolism (AE), hemorrhagic strokes, severe bleedings, and composite outcomes. Main comparisons were done based on propensity score-matched (PSM) cohorts. Results were reported as incidence rate ratios and hazard ratios (HRs).
Results
We assigned 37,439 AF patients to each PSM cohort (NOAC cohort: mean age 78.2 years, mean CHA2DS2VASc score 2.96, mean follow-up 348.5 days; VKA cohort: mean age 78.2 years, mean CHA2DS2VASc 2.95, mean follow-up 365.5 days). NOAC exposure was associated with significantly higher incidence rate ratios; 95% CI/HRs; 95% CI for the following outcomes: death (1.22; 1.17–1.28/1.22; 1.17–1.28), IS (1.90; 1.69–2.15/1.92; 1.69–2.19), non-specified strokes (2.04; 1.16–3.70/1.93; 1.13–3.32), TIAs (1.52; 1.29–1.79/1.44; 1.21–1.70), MIs (1.26; 1.10–1.15/1.31; 1.13–1.52), AE (1.75; 1.32–2.32/1.81; 1.36–2.34) and severe bleeding (1.92; 1.71–2.15/1.95; 1.74–2.20). Multivariable Cox regression analyses and additional sensitivity analysis, including analysis of PSM-matched NOAC/VKA treatment-naive patients, only confirmed the above results. The study was documented under clinicaltrials.gov (NCT02657616).
Conclusion and relevance
A VKA therapy seems to be more effective and safer than a NOAC therapy in a real-world cohort of German AF patients.
Keywords: atrial fibrillation, AF, anticoagulation, NOAC, VKA, cohort study
Introduction
For nearly 60 years, vitamin-K-antagonists (VKAs) were the only oral anticoagulation (OAC) treatment option for atrial fibrillation (AF) patients.1,2 Since 2011, four non-vitamin-K-antagonist oral anticoagulants (NOACs) have been approved for treatment of AF patients (Dabigatran, Rivaroxaban, Apixaban, Edoxaban). Most randomized controlled trials (RCTs) comparing NOACs with dose-adjusted VKA treatment as well as meta-analyses summarizing these RCTs have shown that NOAC treatment in AF patients is at least as effective and safe as VKA treatment.3–6
However, the real-world effectiveness and safety of OAC treatment in AF patients may differ from the efficacy and safety shown in RCTs. With regard to VKA treatment, several real-world limitations exist, such as a narrow therapeutic index and variability in drug exposure, early bleeding or difficulties in reaching the international normalized ratio targets or food interactions or the required regular coagulation monitoring.7–9 On the other hand, under-dosing or over-dosing of anticoagulants and non-adherence to treatment, which cannot be detected in regular coagulation testing, may be important real-world disadvantages of NOAC treatment.10–12
Observational studies comparing the real-world effectiveness and safety of NOAC treatment with VKA treatment in AF patients have provided conflicting results. These might be explained by different applied methodologies, as different patient inclusion/exclusion criteria (existing comorbidities, previous anticoagulation treatment, low-dosage NOAC therapy) or different definition of follow-up periods. Most of these studies excluded a substantial percentage of AF patients and defined tight criteria for follow-up periods; so, they did not study NOAC and VKA treatment in a real-world scenario but applied eligibility criteria more in line with previous clinical trials.8,9,13–18
The main objective of this paper was to compare the real-world effectiveness and safety of NOAC treatment with VKA treatment in an unselected real-world population of AF patients, based on a follow-up period that included real-world phenomena such as over- or under-dosing of anticoagulants as well as non-adherence of patients.
Methods
Dataset and defined AF patient cohorts
We used an anonymized claims dataset from 3 German health funds (AOK PLUS, AOK Bayern and AOK Baden-Württemberg; 11.1 million insured) which initially included all continuously insured patients with at least 2 outpatient AF diagnoses and/or 1 inpatient AF diagnosis (ICD-10 code I48.1 from 2010 to 2012; I48.0, I48.1, I48.2, I48.9 since January 01, 2013). Patients with an additional diagnosis of atrial flutter (ICD-10 code I48.0 from 2010 to 2012; I48.3, I48.4 since January 01, 2013) remained in the sample. The dataset covered the period from January 01, 2010 to June 30, 2014.
The inclusion period ranged from January 01, 2011 to June 30, 2014 (AOK Baden-Württemberg, due to missing mortality data: January 01, 2012–June 30, 2014). We excluded all patients who died before or were not aged at least 18 years at index date and had no CHA2DS2VASc (congestive heart failure, hypertension, age, diabetes, previous stroke/transient ischemic attack, and Vascular disease) score of >1 based on the observed patient characteristics in the 12 months before index date.
Two cohorts were defined: 1) AF patients who received NOAC treatment with either Dabigatran, Rivaroxaban or Apixaban (Edoxaban was not available during the inclusion period of this study), 2) AF patients who received VKA treatment.
Patients in the NOAC/VKA cohorts needed to receive at least 1 outpatient prescription of a NOAC (Rivaroxaban ATC: B01AX06/B01AF01, inclusion of Rivaroxaban patients started with the AF approval of that drug on December 01, 2012; Dabigatran ATC: B01AE07, inclusion started on January 01, 2011; Apixaban ATC B01AX08/B01AF02, inclusion started on January 01, 2013) or a VKA (Warfarin ATC: B01AA03, Phenprocoumon ATC: B01AA04) after at least 1 observed inpatient diagnosis and/or at least 2 observed outpatient AF diagnoses. As index date, we defined the date of the first observed NOAC prescription (NOAC cohort) or VKA prescription (VKA cohort). Observation of a patient ended in case of the following events: no follow-up prescription of the agent that was first prescribed at index date in the 180 days after the last prescription, or any prescription of another NOAC or VKA agent different from the agent that was initially prescribed at index date, death of the patient, or end of data availability (30/06/2014). In case a patient qualified for inclusion into both cohorts, he/she was assigned to the NOAC cohort.
Study outcomes
All observed patients were followed with regard to the following events:
-
a. all-cause death;
and acute hospitalizations with the following events as main hospital admission diagnosis (ICD-10 codes):
b. ischemic stroke (IS; I63.x)
c. non-specified stroke (I64.x)
d. transient ischemic attack (TIA; G45.x)
e. myocardial infarction (MI; I21.x/I22.x)
f. arterial embolism (AE; H34.x/I26.x/K55.0)
g. hemorrhagic stroke (I60.x/I61.x/I62.x)
h. severe bleeding (ICD-10 codes: K92.2, K62.5, K55.22, R04.x, K25.0, K25.2, K25.4, K25.6, K26.0, K26.2, K26.4, K26.6, K27.0, K27.2, K27.4, K27.6, K28.0, K28.2, K28.4 or K28.6).
Above outcomes were aggregated to 3 composite outcomes (CO): effectiveness CO1 covering outcomes a–f; safety CO2 covering outcomes g and h; and a general CO3 covering all outcomes a–h.
Statistical analysis
The following numbers were separately reported for event categories a–h as well as for CO1–CO3: First, based on the number of events per observed 100 patient years, incidence rate ratios (IRRs) for the inclusion of a patient to NOAC versus VKA cohort were calculated. In the respective composite outcome calculations, each event was counted separately, as these events caused different hospitalizations. Multiple events per patient were possible in this analysis.
Second, unadjusted hazard ratios (HRs) based on Cox regression models were assessed. Third, percentage of event-free patients over time with regard to CO1–CO3 was depicted in the Kaplan–Meier (KM) curves using the Log-Rank tests for testing statistical significance of the differences between observed cohorts. In all time-to-event analyses, only the first event was taken into account.
Main event rate comparisons were done based on propensity-score matched (PSM) samples. Propensity scores were calculated using logistic regression estimation (group affiliation as dependent variable) including age, gender, and Charlson Comorbidity Index (CCI) as fixed independent variables. Furthermore, 32 different variables potentially describing the thromboembolic/bleeding risk or the general comorbidity profile of an AF patient (related to the 12 months prior to the index date) were principally included as independent variables (backward elimination), among them the CHA2DS2VASc score. All other variables are listed in Table S1. Each PSM matching was done using a 1:1 matching approach within the specific 5-year age and gender classes, and a maximum accepted difference in propensity scores of 0.0001.
Additionally, 3 multivariable Cox regression analyses were carried out, including all patients assigned to the cohorts; results were reported as adjusted HRs (aHRs). As dependent variables, we used time-to-first event related to CO1–CO3. In addition to the exposure to the different cohorts, all variables included in the PSM procedure were principally included in these models as independent variables. Furthermore, to cover cancer as an additional cause of thromboembolic or bleeding events, or death that was not related to a previously initiated anticoagulation therapy, we included an independent variable that described whether any cancer diagnosis during the follow-up period was documented for a patient (all ICD-10 C-diagnoses and any diagnosis code D00.x until D09.x).
All reported p-values were two-sided, and 95% CIs were calculated for IRRs, HRs, and aHRs by applying independent t-tests or Wilcoxon rank-sum tests, where applicable. For categorical variables, the Chi-squared test was performed. Significance level was set at a p-value <0.05. All descriptive analyses were performed with Microsoft SQL Server 2008 and Microsoft Excel 2016. All other statistical analyses were performed with STATA/MP 13.1 and SPSS 17.0.
Sensitivity analyses
We additionally carried out 3 sensitivity analyses. In the first, we included patients only if they had not received any VKA or NOAC anticoagulation therapy in the 12 months before the index date (NOAC/VKA therapy-naive cohorts). In the second, we redid our analysis by limiting the follow-up period for patients in the respective cohorts in case a defined daily dosage (DDD)-based treatment gap >30 days was observed. The DDD is the average daily dosage of a drug a patient should receive as reported by the World Health Organization and, for Germany, by the Wissenschaftliches Institut der AOK.19,20 In the third sensitivity analysis, we divided the NOAC group into 2 subgroups: patients who received a low-dosage NOAC therapy only (patients only received prescriptions of Rivaroxaban 15/10 mg or Dabigatran 110/75 mg or Apixaban 2.5 mg) and patients who received at least 1 prescription of a full-dosage NOAC (Rivaroxaban 20 mg or Dabigatran 150 mg or Apixaban 5 mg). All defined outcomes were reported for these subgroups, and compared with their respective VKA siblings.
Regulatory aspects
Because of the non-interventional, retrospective nature of this study and because our analysis involved an anonymized dataset, no ethical review was required. Because of the anonymized and retrospective nature of the data, an informed consent was not obtained from the patients. Although claims data is not publicly available, data protection measures were established and reviewed by the respective insurance funds which only release partial datasets based on the agreed-upon conditions in the respective study protocol. The study protocol was reviewed by a scientific steering committee to which all the authors belonged. The study was documented under clinicaltrials.gov (NCT02657616).
Patient involvement
Since retrospective anonymized claims data from a German sickness fund were analyzed in this study, no patient involvement took place. The research question, however, was developed in accordance with all authors of this study and patient recruitment was not needed because of the nature of the analyzed data.
Results
Patient and OAC treatment characteristics
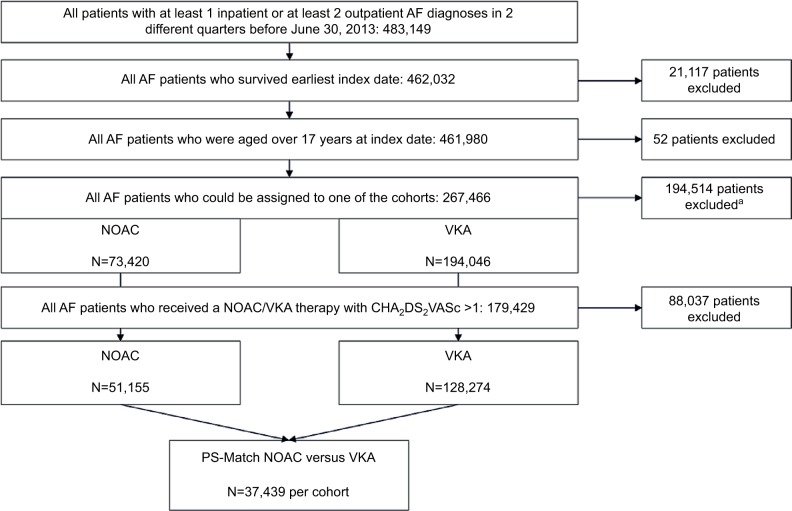
Based on 483,149 AF patients, we finally assigned 51,155 AF patients to the NOAC cohort (13,476 patients Dabigatran, 34,233 Rivaroxaban, 3,446 Apixaban) and 128,274 patients to the VKA cohort (127,521 Phenprocoumon, 753 Warfarin) (Figure 1). Patients who received VKA therapy were younger (mean age 76.6 years) than those receiving NOAC therapy (mean age 78.2 years) (Table 1). Mean follow-up period since index date was 341.2 days for NOAC cohort and 393.1 days for VKA cohort; 19.4% (NOAC) and 45.9% (VKA) of the patients received only 1 respective OAC prescription. The reasons for this were early discontinuation of therapy defined as treatment gap of 180 days (5.7%/41.3% of NOAC/VKA patients), prescription of another OAC agent as prescribed at index date (5.7%/0.0%), end of observational period (4.4%/0.7%) or death of the patient (3.6%/3.9%).
Figure 1.
Defined patient samples.
Notes: aNo possible assignment to 1 of the 2 defined cohorts because of prescription of 2 different agents at index date, death at index date, prescription of an OAC agent 6 month before index date but no follow-up prescription after index date, or no OAC therapy at all.
Abbreviations: AF, atrial fibrillation; NOAC, non-vitamin K antagonist oral anticoagulants; OAC, oral anticoagulation; PS, propensity score; VKA, vitamin-K-antagonist.
Table 1.
Characteristics of observed AF patient samples
| Characteristics | All observed AF-patients | Unmatched
|
PS-matched
|
||||
|---|---|---|---|---|---|---|---|
| Cohort 1 (NOAC) | Cohort 2 (VKA) | Cohort 1 versus 2 (p-value) | Cohort 1 (NOAC) | Cohort 2 (VKA) | Cohort 1 versus 2 (p-value) | ||
| N | 483,149 | 51,155 | 128,274 | – | 37,439 | 37,439 | – |
| Mean follow-up time since index date (SD); median | – | 341.2 days (245.29); 290 | 393.1 days (311.30); 239 | p<0.001 | 348.5 days (247.57); 299 | 365.5 days (290.74); 197 | p>0.100 |
| Mean age in years (SD) | 76.24 (13.33) | 78.21 (8.56) | 76.61 (8.34) | p<0.001 | 78.21 (7.40) | 78.16 (7.37) | p>0.100 |
| Gender; Female N (%) | 253,750 (52.52) | 26,519 (51.84) | 66,112 (51.54) | p>0.100 | 19,659 (52.51) | 19,622 (52.41) | p>0.100 |
| Mean CCI without age factor (SD) | 3.56 (2.79) | 5.09 (2.74) | 4.45 (2.46) | p<0.001 | 4.78 (2.60) | 4.80 (2.55) | P<0.500 |
| Mean CHA2DS2 VASc score (SD) | 2.47 (1.54) | 3.09 (1.09) | 2.88 (1.00) | p<0.001 | 2.96 (1.04) | 2.95 (1.03) | p>0.100 |
| Prescribed DDDs of study medicationa | NA | 1.21 (1.97) | 0.81 (1.67) | – | 1.19 (1.87) | 0.82 (1.80) | – |
| Prescribed DDDs of Heparins or Clopidogrelb | NA | 3.27 (21.20) | 3.32 (13.12) | p<0.001 | 3.16 (22.22) | 3.67 (16.66) | p<0.001 |
Notes: The table lists sociodemographic characteristics for the different observed AF patient samples. These data refer to patient-specific index dates for age/gender and to the 12-month baseline period before index date. The date of the first prescription of a NOAC/VKA agent in the inclusion period was used as the index date.
(NOAC/VKA) per observed patient day during follow-up period;
Per observed patient month. CHA2DSs Vasc (congestive heart failure, hypertension, age, diabetes, previous stroke/transient ischemic attack, and Vascular disease). “–” indicates not calculated.
Abbreviations: CCI, Charlson comorbidity index; DDD, defined daily dosage; NA, not applicable; NOAC, non-vitamin K antagonist oral anticoagulants; PS, propensity score; VKA, vitamin-K-antagonist.
In the NOAC cohort, 64.8% of the observed patients received at least 1 low-dosage prescription of Rivaroxaban (15 mg: 53.6% of Rivaroxaban patients; 10 mg: 9.7% of Rivaroxaban patients), Dabigatran (110 mg: 75.9% of Dabigatran patients; 75 mg: 6.7% of Dabigatran patients), or Apixaban (2.5 mg: 62.2% of Apixaban patients); 52.1% of the observed NOAC-patients received a low-dosage therapy only. Observed NOAC-patients who received a low-dosage therapy were older than all patients in the NOAC cohort (80.7 versus 78.2 years; p<0.001). For 49.4% of these patients, no renal insufficiency or renal failure (ICD-10 N17.-/N18.-/N19.-) was diagnosed in the 12 months before index date.
Finally, 74,878 patients (37,439 patients in each cohort) could be assigned to the PSM cohorts (mean age of both cohorts was 78.2 years, 52.5%–52.4% female, mean CHA2DS2VASc score 2.96–2.95; Table 1).
Event rate comparisons
Table 2 summarizes results of a comparison of event rates between the PSM-matched cohorts (crude event rates, based on overall samples, reported in Table S2). Exposure to the NOAC treatment, when compared to VKA treatment, was associated with significantly higher IRRs for death (1.22; 95% CI: 1.17–1.28), IS (1.90; 95% CI: 1.69–2.15), non-specified strokes (2.04; 95% CI: 1.16–3.70), TIAs (1.52; 95% CI: 1.29–1.79), MIs (1.26; 95% CI: 1.10–1.45), AE (1.75; 95% CI: 1.32–2.32), and severe bleeding (1.92; 95% CI: 1.71–2.15) as well as for the 3 composite outcomes (CO1: 1.32; 95% CI: 1.27–1.37; CO2: 1.65; 95% CI: 1.49–1.82; CO3: 1.36; 95% CI: 1.31–1.41). Only for hemorrhagic strokes were no significant IRR differences observed (Table 2).
Table 2.
Event rates, IRRs and HRs (time to first event) in compared PSM-AF patient cohorts; PSM NOAC versus VKA
| Observed outcome (events per 100 patient years)a
|
Observed outcome (time-to-first event)b
|
|||
|---|---|---|---|---|
| PSM cohort 1 (NOAC) N=37,439 patients | PSM cohort 2 (VKA) N=37,439 patients | IRR (95% CI; p-value) cohort 1 versus 2 | PSM-HR (95% CI; p-value) cohort 1 versus 2 | |
| a: Death | 11.28 | 9.23 | 1.22 (1.17–1.28; p<0.001) | 1.22 (1.17–1.28; p<0.001) |
| b: IS | 2.18 | 1.15 | 1.90 (1.69–2.15; p<0.001) | 1.92 (1.69–2.19; p<0.001) |
| c: Non-specified stroke | 0.11 | 0.05 | 2.04 (1.16–3.70; p<0.010) | 1.93 (1.13–3.32; p<0.050) |
| d: TIA | 0.99 | 0.65 | 1.52 (1.29–1.79; p<0.001) | 1.44 (1.21–1.70; p<0.001) |
| e: MI | 1.33 | 1.06 | 1.26 (1.10–1.45; p<0.001) | 1.31 (1.13–1.52; p<0.001) |
| f: AE | 0.39 | 0.22 | 1.75 (1.32–2.32; p<0.001) | 1.81 (1.36–2.34; p<0.001) |
| g: Hemorrhagic stroke | 0.47 | 0.50 | 0.94 (0.76–1.17; p=0.573) | 0.95 (0.76–1.21; p=0.695) |
| h: Severe bleeding | 2.47 | 1.29 | 1.92 (1.71–2.15; p<0.001) | 1.95 (1.74–2.20; p<0.001) |
| CO1 (a–f) | 16.28 | 12.36 | 1.32 (1.27–1.37; p<0.001) | 1.31 (1.26–1.36; p<0.001) |
| CO2 (g and h) | 2.94 | 1.78 | 1.65 (1.49–1.82; p<0.001) | 1.70 (1.53–1.88; p<0.001) |
| CO3 (a–h) | 19.22 | 14.15 | 1.36 (1.31–1.41; p<0.001) | 1.35 (1.30–1.41; p<0.001) |
| Sum observed patient years | 35,746.97 | 37,486.99 | – | – |
Notes:
The table lists results of the event rate analyses based on PSM cohorts. Results are reported as IRRs for events per 100 observed patient years;
The table lists the results of Cox regression analyses based on PSM cohorts. Results are reported as HRs for time-to-first event. “–” indicates not calculated. a. All-cause death; b. Ischemic stroke (I63.x); c. Non-specified stroke (I64.x); d. Transient ischemic attack (G45.x); e. Myocardial infarction (I21.x/I22.x); f. Arterial embolism (H34.x/I26.x/K55.0); g. Hemorrhagic stroke (I60.x/I61.x/I62.x); h. Severe bleeding (ICD-10 codes: K92.2, K62.5, K55.22, R04.x, K25.0, K25.2, K25.4, K25.6, K26.0, K26.2, K26.4, K26.6, K27.0, K27.2, K27.4, K27.6, K28.0, K28.2, K28.4 or K28.6).
Abbreviations: AE, arterial embolism; AF, atrial fibrillation; CO1, effectiveness composite outcome 1; CO2, safety composite outcome 2; CO3, general composite outcome 3; HR, hazard ratio; IRR, incidence rate ratio; IS, ischemic stroke; MI, myocardial infarction; NOAC, non-vitamin K antagonist oral anticoagulants; PSM, propensity score-matched; TIA, transient ischemic attack; VKA, vitamin-K-antagonist.
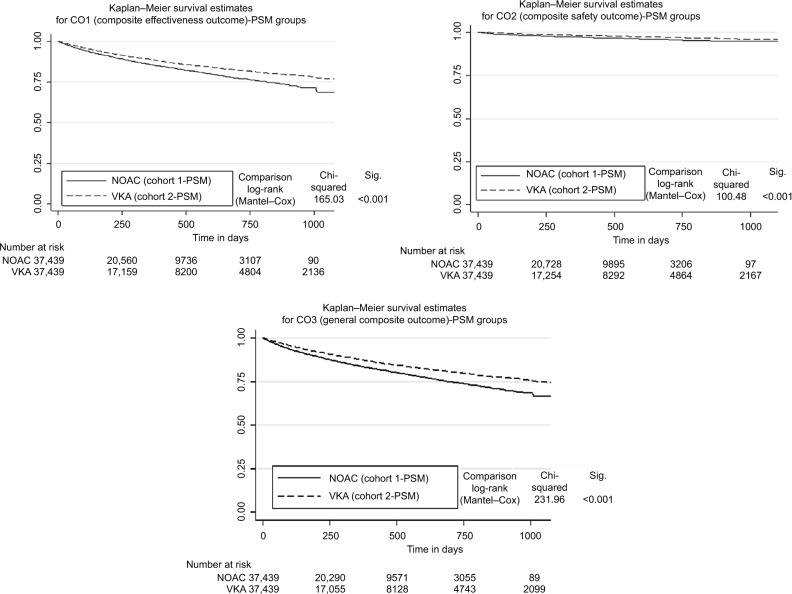
PSM-based HRs for time-to-first event associated with NOAC treatment in comparison to VKA treatment were statistically different from 1 for death (1.22; 95% CI: 1.17–1.28), IS (1.92; 95% CI: 1.69–2.19), non-specified strokes (1.93; 95% CI: 1.13–3.32), TIAs (1.44; 95% CI: 1.21–1.70), MIs (1.31; 95% CI: 1.13–1.52), AE (1.81; 95% CI: 1.36–2.34), and severe bleeding (1.95; 95% CI: 1.74–2.20) as well as for the 3 composite outcomes (CO1: 1.31; 95% CI: 1.26–1.36; CO2: 1.70; 95% CI: 1.53–1.88; CO3: 1.35; 95% CI: 1.30–1.41; Table 2). In accordance with the above results, the Kaplan–Meier curve analysis showed superiority of VKA therapy over NOAC therapy for all composite outcomes: CO1 (effectiveness; p<0.001), CO2 (safety; p<0.001) and CO3 (all events; p<0.001; Figure 2). The above results were mainly confirmed for the effectiveness outcomes in a comparison of PSM-matched patients who received specific NOAC agents (either Dabigatran, Rivaroxaban or Apixaban) with their respective PSM-statistical siblings in the VKA cohort (Table S3); for the safety outcomes, the results could only be confirmed for the Rivaroxaban cohort (Table S3).
Figure 2.
Kaplan–Meier curves (time to event: CO1, CO2, CO3) for PSM-matched Cohorts 1 and 2.
Abbreviations: CO1: effectiveness composite outcome 1; CO2: safety composite outcome 2; CO3: general composite outcome 3; NOAC, non-vitamin K antagonist oral anticoagulants; PSM, propensity score matching; VKA, vitamin-K-antagonist.
In the 3 multivariable Cox regression analyses using composite outcomes CO1–CO3 as dependent variables and including all AF patients assigned to either cohort 1 or 2, most of the included independent variables showed a significant association with the dependent variables, among them most prominently a high level of care, previous acute cardiovascular events, alcohol abuse, or severe kidney disease. Additionally, in all the 3 models, assignment to the NOAC cohort was associated with a higher event risk (Figure S1).
Table 3 compares identified IRRs, HRs, and aHRs for exposure to NOACs against VKAs in our study with those known from the 3 large NOAC-clinical registration trials.8,9,15 In contrast to the RCTs, VKA therapy was more effective than NOAC therapy in our real-world analysis, whereas the analysis of safety outcomes led to inconclusive results.
Table 3.
IRRs, HRs in PSM cohorts and aHRs for patients assigned to cohort 1 (NOAC) versus cohort 2 (VKA) compared with HRs reported in clinical trials
| Events | Own analysis
|
Known major clinical trials
|
|||||
|---|---|---|---|---|---|---|---|
| PSM-IRRs (95% CI; p-value) | PSM-HRs (95% CI; p-value) | aHRs (95% CI; p-value) | PSM-HRs (95% CI; p-value) for therapy-naive patients | HRs (95% CI; p-value) – RE-LY8 | HRs (95% CI; p-value) – ROCKET AF9 | HRs (95% CI; p-value) – ARISTOTLE15 | |
| Death | 1.22**** (1.17–1.28; p<0.001) | 1.22**** (1.17–1.28; p<0.001) | 1.10**** (1.06–1.15; p<0.001) | 1.11** (1.03–1.20; p<0.010) | 0.88 (0.77–0.00; NSR) | 0.85 (0.70–1.02; NSR) | 0.89* (0.80–1.00; p<0.050) |
| IS | 1.90**** (1.69–2.15; p<0.001) | 1.92**** (1.69–2.19; p<0.001) | 1.52**** (1.37–1.69; p<0.001) | 1.175**** (1.42–2.17; p<0.001) | 0.76* (0.60–0.98; p<0.050) | 0.94 (0.75–1.17; NSR) | 0.92 (0.74–1.13; NSR) |
| Hemorrhagic stroke | 0.94 (0.76–1.17; NSR) | 0.95 (0.76–1.21; NSR) | 0.68**** (0.56-0.82; p<0.001) | 0.76 (0.52–1.11; NSR) | 0.26**** (0.14–0.49; p<0.001) | 0.59* (0.37–0.93; p<0.050) | 0.51**** (0.35–0.75; p<0.001) |
| Severe bleedings | 1.92**** (1.71–2.15; p<0.001) | 1.95**** (1.74–2.20; p<0.001) | 1.57**** (1.43–1.73; p<0.001) | 1.58 (1.30–1.92; NSR) | 0.93 (0.81–1.07; NSR) | 1.03 (0.90–1.18; NSR) | 0.71**** (0.61–0.81; p<0.001) |
Notes: aHR is based on a multivariable Cox regression analysis; PSM-HR represents hazard ratio based on Cox regression analysis of time-to-first event with regard to PSM cohorts; PSM-IRR represents incidence rate ratio based on comparison of PSM cohorts.
p<0.050;
p< 0.010;
p<0.001.
Abbreviations: AF, atrial fibrillation; aHR, adjusted hazard ratio; HR, hazard ratio; IS, ischemic stroke; NOAC, non-vitamin K antagonist oral anticoagulants; IRR, incidence rate ratio; NSR: non-significant results; PSM, propensity score matching; VKA, vitamin-K-antagonist.
Sensitivity analyses
The first sensitivity analysis included 13,366 therapy-naive patients in each PSM cohort. Here, as in all previous analyses, NOAC therapy was associated with an IRR>1 in comparison to VKA therapy regarding all 3 composite outcomes: CO1: 1.16 (95% CI 1.09–1.24); CO2: 1.27 (95% CI 1.08–1.49); CO3: 1.18 (95% CI 1.11–1.25; Table S4).
In the second sensitivity analysis, follow-up period for each patient in either PSM-matched NOAC or VKA cohort ended if a NOAC or VKA treatment gap of >30 days (instead of >180 days) was observed. Event rates for the effectiveness outcome CO1 in both cohorts were generally lower in this analysis compared with the main analysis, whereas event rates for the safety outcome CO2 were generally higher (Table S4). Nevertheless, IRRs for the composite outcomes CO1 (1.19; 95% CI 1.09–1.29), CO2 (1.33; 95% CI 1.19–1.49), and CO3 (1.24; 95% CI 1.16–1.32) also confirmed the results of the main analysis (Table S4).
In the third sensitivity analysis, 18,343 of the PSM-NOAC patients were assigned to the high-dosage group, whereas 19,096 patients received a low-dosage therapy only (patients only received prescriptions of Rivaroxaban 15/10 mg or Dabigatran 110/75 mg or Apixaban 2.5 mg). In a comparison of the lower NOAC dosage patients to the respective VKA siblings, IRRs for the composite outcomes showed higher thromboem-bolic event risk of NOACs in comparison to the main analysis (CO1: 1.46; 95% CI 1.39–1.54) with similar safety in comparison to the main analysis (CO2: 1.61; 95% CI 1.41–1.84; CO3: 1.48; 95% CI 1.41–1.56), whereas IRRs for CO1 (1.12; 95% CI 1.05–1.19), CO2 (1.68; 95% CI 1.45–1.94), and CO3 (1.19; 95% CI 1.13–1.26) in the high-dosage cohort showed slightly better effectiveness of NOACs compared with the main analysis. However, effectiveness in both groups was still lower than in the respective PSM-VKA cohorts (Table S4).
Discussion
We found a superiority of VKA therapy over NOAC therapy regarding most observed effectiveness/safety outcomes such as all-cause death or acute hospitalizations due to IS as well as severe bleedings. This result could be confirmed in different statistical analyses, for different follow-up periods as well as for different sample definitions. Only the analysis of hemorrhagic stroke did not show any significant differences between the compared therapies. In NOAC agent-specific analyses, we could show that the composite efficacy outcome analysis showed superiority of VKAs over all 3 observed NOAC agents, whereas in terms of the safety outcome CO2, only Rivaroxaban was inferior to VKAs.
Our numbers are not in line with known RCTs and the majority of previous observational studies that compared NOAC versus VKA therapy in AF patients.3–6 This may have been caused by different factors. First, patient characteristics in clinical trial AF populations differed markedly from our real-world AF population. We applied a minimum of exclusion criteria to describe real-world OAC therapy in AF patients with a maximum of external validity. In contrast, most trials excluded a substantial number of AF patients. So, 21.05% of patients in the NOAC cohort and 23.57% of patients in the VKA cohort would have been excluded in most RCTs8,9,15 because of endocarditis, valve disorders, heart valve replacements, pulmonary embolism, thyrotoxicosis, or any previous cardiac surgery. Consequently, our analyzed AF population was older and more comorbid than clinical trial populations (mean age for PSM-groups 78 years in our study versus 71/72 years (RE-LY), 73/73 years (Rocket-AF) and 70/70 years (Aristotle).8,9,15 This may have influenced our results as, for example, safety of NOACs has been reported to depend on the number and type of co-medications patients receive.21,22
Second, we observed an early non-persistence to NOAC/VKA treatment, defined as only 1 prescription of the respective agent followed by a follow-up prescription of another agent or no follow-up prescription of the same agent within 180 days, in 19.4% and 45.9% of NOAC and VKA populations, respectively. This non-persistence rate is in line with rates reported in previous observational studies,16,17,23 but, with regard to VKA therapy, is much higher than non-persistence in known clinical trials, which was reported to be between 10.2% and 27.5% for a 12-month period.8,9,15 So, VKA therapy seems to be associated with a higher early therapy discontinuation risk than NOAC therapy. In a separate study based on a similar dataset, it could be observed that older, more comorbid female patients who face surgeries and do not visit a cardiologist regularly, face an exceptionally high VKA discontinuation risk.24
Third, non-adherence to NOAC treatment might be common. So, for example, a recent US claims data analysis that concluded that Dabigatran is more effective than Warfarin, limited observation of patients until a gap of only 3 days, leading to a follow-up period of only 98 days per patient.14 Our approach requiring 1 OAC prescription in 180 days includes potential non-adherence of patients which describes the real-world OAC treatment of German AF patients with much higher external validity. Consequently, our follow-up period was almost 4 times as long as in the above-mentioned study. In line with this, one of our sensitivity analyses confirmed that event rates associated with a shorter follow-up period (end of follow-up in case of a treatment gap of >30 days) were lower in terms of the effectiveness outcome CO1 and higher for the safety outcome CO2 than in the main analysis (Table S4). Nevertheless, NOAC therapy was still associated with higher event rates in comparison to VKA therapy with respect to most of the observed outcomes.
Fourth, in line with previous observational studies13,14 but in contrast to recent RCTs, a substantial percentage of older AF patients treated with NOACs received low-dosage therapy (Rivaroxaban 15 or 10 mg, Dabigatran 75 or 110 mg, Apixaban 2.5 mg) in our study. In 49.4% of these patients, no diagnosis of renal insufficiency/failure could be found. Our sensitivity analysis confirmed that there was indeed a poorer effectiveness of NOACs in patients who received the low-dosage therapy. It can therefore be assumed that, as this was also found in France13 and the USA,25 German NOAC prescription practice is strongly guided by bleeding risk. But, as in RCTs, low dosages of both Dabigatran and Rivaroxaban have been shown to be associated with lower efficacy compared with well-managed VKA therapy;5 this prescription practice waives effectiveness.
Fifth, all major trials comparing NOACs with VKAs used Warfarin as VKA agent of choice. In contrast, 99.4% of the AF patients in our sample received Phenprocoumon, which is known to differ from Warfarin, at least in terms of its pharmacokinetic characteristics.26
Sixth, in contrast to most recent studies, the mean age and CCI of AF patients receiving NOAC therapy in Germany are higher than that of patients receiving VKA therapy.27 This might mean that even if a PSM procedure, including several variables describing the comorbidity and stroke risk profile of AF patients, was implemented, remaining undetected patient characteristics may have influenced the results of our analysis.
Seventh, the real-world quality of anticoagulation with VKA therapy in the German setting might be better than in many other countries. This was not only confirmed by previous clinical trials,18,28,29 but also by previous German observational studies30 and might partly explain the good effectiveness and safety of VKA therapy in our dataset.
Limitations
Due to our retrospective observational design, residual bias is the main potential weakness of our study. To address this weakness, we observed multiple outcomes based on PSM cohorts, analyzed time-to-first event in both univariate and multivariate Cox regression analyses, and ran 3 sensitivity analyses.
As is the case for all retrospective database analyses, diagnosis or outcome misclassification, although non-differential, constitutes a limitation. To minimize the risk resulting from this limitation, we only observed confirmed events requiring an acute hospital admission.
Moreover, we observed minor differences in prescribed DDDs for Heparins and Clopidogrel between the cohorts (0.5 DDDs per patient month in favor of VKA) and in the frequency of other previous cardiovascular events (in favor of NOAC), which may also have influenced event rates.
Finally, market entry date for NOACs differed between the 3 observed agents. This led to a systematic difference in follow-up time of patients who received these agents (Table S3). However, due to our methodology that was mainly based on time-to-event analyses, a systematic bias in that respect is not expected. Furthermore, follow-up times of the main cohorts were very similar.
Conclusion and implications for practice
When a NOAC therapy is compared with a VKA therapy, the latter seems to be more effective and safe in the real-world treatment of German AF patients.
Acknowledgments
This work was financially supported by AOK PLUS. Since UM is employed by AOK PLUS, AOK PLUS as the study sponsor was involved in this study in the following way: conception/design of the study, data collection, validation of database, and interpretation of results in discussion section.
Footnotes
Author contributions
SM, AG: project lead, participated in writing all parts of the paper, statistical analysis; UM, AP, AS, SM: conception/design of the study, data collection, validation of database, and interpretation of results in discussion section; SGS: conception/design of the study and interpretation of results in discussion section with regard to clinical practice. All authors made substantial contributions to all the following: 1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, 2) drafting the article or revising it critically for important intellectual content, 3) final approval of the version to be submitted.
Disclosure
UM received modest honoraria from several pharmaceutical companies. SGS received modest honoraria from BMS/Pfizer and Bayer Vital, and modest research grant support from BMS/Pfizer and Daiichi Sankyo. SM and AG participated in this study as staff members of IPAM. AP and AS do not have any conflicts of interest except those potentially related to their employer, AOK Baden-Württemberg and AOK Bayern, respectively. The authors report no other conflicts of interest in this work.
References
- 1.Gomez-Outes A, Suarez-Gea ML, et al. Discovery of anticoagulant drugs: a historical perspective. Curr Drug Discov Technol. 2012;9:83–104. doi: 10.2174/1570163811209020083. [DOI] [PubMed] [Google Scholar]
- 2.Elgendy AY, Mahtta D, Barakat AF, et al. Meta-analysis of safety and efficacy of uninterrupted non-vitamin K antagonist oral anticoagulants versus vitamin K antagonists for catheter ablation of atrial fibrillation. Am J Cardiol. 2017;120:1830–1836. doi: 10.1016/j.amjcard.2017.07.096. [DOI] [PubMed] [Google Scholar]
- 3.Dentali F, Riva N, Crowther M, Turpie AGG, Lip GYH, Ageno W. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation. 2012;126:2381–2391. doi: 10.1161/CIRCULATIONAHA.112.115410. [DOI] [PubMed] [Google Scholar]
- 4.Miller CS, Grandi SM, Shimony A, Filion KB, Eisenberg MJ. Meta-analysis of efficacy and safety of new oral anticoagulants (Dabigatran, Rivaroxaban, Apixaban) versus Warfarin in patients with atrial fibrillation. Am J Cardiol. 2012;110:453–460. doi: 10.1016/j.amjcard.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 5.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with Warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 6.Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GYH. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123:638. doi: 10.1016/j.amjmed.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 7.Gomez-Outes A, Terleira-Fernandez AI, Calvo-Rojas G, Suarez-Gea ML, Vargas-Castrillon E. Dabigatran, Rivaroxaban, or Apixaban versus Warfarin in patients with nonvalvular atrial fibrillation: a systematic review and meta-analysis of subgroups. Thrombosis. 2013;2013:640723. doi: 10.1155/2013/640723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, et al. RE-LY Steering Committee and Investigators Dabigatran versus Warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 9.Patel MR, Mahaffey KW, Garg J, et al. ROCKET AF Investigators Rivaroxaban versus Warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 10.Mekaj YH, Mekaj AY, Duci SB, Miftari EI. New oral anticoagulants: their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther Clin Risk Manag. 2015;11:967–977. doi: 10.2147/TCRM.S84210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauer KA. Pros and cons of new oral anticoagulants. Hematology Am Soc Hematol Educ Program. 2013;2013:464–470. doi: 10.1182/asheducation-2013.1.464. [DOI] [PubMed] [Google Scholar]
- 12.Abraham NS, Singh S, Alexander GC, et al. Comparative risk of gastrointestinal bleeding with Dabigatran, Rivaroxaban, and Warfarin: population based cohort study. BMJ. 2015;350:h1857. doi: 10.1136/bmj.h1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maura G, Blotière P-O, Bouillon K, et al. Comparison of the short-term risk of bleeding and arterial thromboembolic events in nonvalvular atrial fibrillation patients newly treated with Dabigatran or Rivaroxaban versus vitamin K antagonists: a French nationwide propensity-matched cohort study. Circulation. 2015;132:1252–1260. doi: 10.1161/CIRCULATIONAHA.115.015710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham DJ, Reichman ME, Wernecke M, et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with Dabigatran or Warfarin for nonvalvular atrial fibrillation. Circulation. 2015;131:157–164. doi: 10.1161/CIRCULATIONAHA.114.012061. [DOI] [PubMed] [Google Scholar]
- 15.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus Warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 16.Laliberté F, Cloutier M, Nelson WW, et al. Real-world comparative effectiveness and safety of Rivaroxaban and Warfarin in nonvalvular atrial fibrillation patients. Curr Med Res Opin. 2014;30:1317–1325. doi: 10.1185/03007995.2014.907140. [DOI] [PubMed] [Google Scholar]
- 17.Bancroft T, Lim J, Wang C, Sander SD, Swindle JP. Health care resource utilization, costs, and persistence in patients newly diagnosed as having nonvalvular atrial fibrillation and newly treated with Dabigatran versus Warfarin in the United States. Clin Ther. 2016;38(3):545–56. e1–6. doi: 10.1016/j.clinthera.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Piccini JP, Hellkamp AS, Lokhnygina Y, et al. Relationship between time in therapeutic range and comparative treatment effect of Rivaroxaban and Warfarin: results from the ROCKET AF Trial. J Am Heart Assoc. 2014;3:e000521–e000521. doi: 10.1161/JAHA.113.000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fricke U, Günther J, Zawinell A. Anatomisch-therapeutisch-chemische Klassifikation mit Tagesdosen für den deutschen Arzneimittelmarkt: Methodik der ATC-Klassifikation und DDD-Festlegung; ATC-Index mit DDD-Angaben. [Anatomical Therapeutic Chemical Classification with daily doses for the German pharmaceutical market: Methodology of the ATC classification and DDD definition; ATC Index with DDD information] WIdO; 2004. German. [Google Scholar]
- 20.WHO Collaborating Centre for Drug Statistics Methodology . Guidelines for ATC classification and DDD assignment 2017. Oslo: 2016. [Accessed December 19, 2017]. [cited December 19, 2017]. Available from: https://www.whocc.no/filearchive/publications/2017_guidelines_web.pdf. [Google Scholar]
- 21.Jaspers Focks J, Brouwer MA, Wojdyla DM, et al. Polypharmacy and effects of Apixaban versus Warfarin in patients with atrial fibrillation: post hoc analysis of the ARISTOTLE trial. BMJ. 2016;353:i2868. doi: 10.1136/bmj.i2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham DJ, Reichman ME, Wernecke M, et al. Stroke, bleeding, and mortality risks in elderly Medicare beneficiaries treated with Dabigatran or Rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern Med. 2016;176:1662–1671. doi: 10.1001/jamainternmed.2016.5954. [DOI] [PubMed] [Google Scholar]
- 23.Al-Khalili F, Lindstrom C, Benson L. The safety and persistence of non-vitamin-K-antagonist oral anticoagulants in atrial fibrillation patients treated in a well structured atrial fibrillation clinic. Curr Med Res Opin. 2016;32(4):1–7. doi: 10.1185/03007995.2016.1142432. [DOI] [PubMed] [Google Scholar]
- 24.Wilke T, Groth A, Fuchs A, Pfannkuche M, Maywald U. Persistence with VKA treatment in newly treated atrial fibrillation patients: an analysis based on a large sample of 38,076 German patients. Eur J Clin Pharmacol. 2017;73(11):1437–1447. doi: 10.1007/s00228-017-2307-2. [DOI] [PubMed] [Google Scholar]
- 25.Pokorney SD, O’Brien EC, Granger CB. Assessing generalizability of trial results in general practice. Eur Heart J. 2016;37:1154–1157. doi: 10.1093/eurheartj/ehv532. [DOI] [PubMed] [Google Scholar]
- 26.Beinema M, Brouwers, Jacobus RBJ, Schalekamp T, Wilffert B. Pharmacogenetic differences between Warfarin, acenocoumarol and phenprocoumon. Thromb Haemost. 2008;100:1052–1057. [PubMed] [Google Scholar]
- 27.Michalski F, Tittl L, Werth S, et al. Selection, management, and outcome of vitamin K antagonist-treated patients with atrial fibrillation not switched to novel oral anticoagulants. Results from the Dresden NOAC registry. Thromb Haemost. 2015;114:1076–1084. doi: 10.1160/TH15-02-0116. [DOI] [PubMed] [Google Scholar]
- 28.Connolly SJ, Pogue J, Eikelboom J, et al. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008;118:2029–2037. doi: 10.1161/CIRCULATIONAHA.107.750000. [DOI] [PubMed] [Google Scholar]
- 29.Wallentin L, Lopes RD, Hanna M, et al. Efficacy and safety of Apixaban compared with Warfarin at different levels of predicted international normalized ratio control for stroke prevention in atrial fibrillation. Circulation. 2013;127:2166–2176. doi: 10.1161/CIRCULATIONAHA.112.142158. [DOI] [PubMed] [Google Scholar]
- 30.Mueller S, Pfannkuche M, Breithardt G, et al. The quality of oral anti-coagulation in general practice in patients with atrial fibrillation. Eur J Intern Med. 2014;25:247–254. doi: 10.1016/j.ejim.2013.12.013. [DOI] [PubMed] [Google Scholar]