Abstract
Temporal order memory is important in daily life and is adversely affected by healthy aging. However, there is a lack of standardized neuropsychological tests designed to directly measure this construct. We developed a new test to examine incidental temporal order memory for a self-generated sequence of tasks one might complete in everyday life. Young and older adults completed the test, which assessed immediate free recall, delayed free recall, and delayed cued recall for the order of events in a sequence. Participants were given 10 cards, each listing a task one might accomplish in a typical day. Participants were asked to self-generate a “to do” list by placing the 10 cards in a sequence representing the order in which they would accomplish the tasks, but were not informed of a subsequent memory test. Immediate recall was assessed by asking participants to verbally list the tasks in the same order as the sequence that was generated. Following a 20 min delay, delayed recall was assessed using the same procedure. Delayed cued recall was assessed by giving participants the cards and asking them to place them in the correct sequence. Standardized neuropsychological measures also were administered. Older adults were significantly impaired relative to young adults on immediate recall, delayed free recall, and delayed cued recall of the sequence, controlling for item memory. Correlation analyses with standardized neuropsychological tests provide preliminary evidence for construct validity for our test, which is portable and can be rapidly administered in clinical or laboratory settings.
Keywords: aging, temporal order memory, sequence memory, construct validity, older adults
Temporal order memory involves the ability to remember the order in which events are experienced across time. Memory for the order of items or events in a sequence is thought to depend on frontal and temporal lobe function (Devito & Eichenbaum, 2011; Ekstrom, Copara, Isham, Wang, & Yonelinas, 2011). Functional neuroimaging studies have recorded activation in the frontal lobes (Cabeza, Anderson, Houle, Mangels, & Nyberg, 2000; Hayes, Ryan, Schnyer, & Nadel, 2004; Knutson, Wood, & Grafman, 2004; Rowe & Passingham, 2001) and temporal lobes (Ekstrom & Bookheimer, 2007; Lehn et al., 2009) while humans perform tasks involving memory for sequences of stimuli. Deficits on temporal order memory tasks also have been reported in humans with damage to the frontal (Daum & Mayes, 2000; Milner, Petrides, & Smith, 1985; Shimamura, Janowsky, & Squire, 1990) or temporal lobes (Downes, Mayes, MacDonald, & Hunkin, 2002; Hopkins, Kesner, & Goldstein, 1995; Mayes et al., 2001; Spiers et al., 2001).
Age-related deficits in memory for the temporal order of items or events in a sequence also have been well documented in healthy older adults (Blachstein, Greenstein, & Vakil, 2012; Kessels, Hobbel, & Postma, 2007; Newman, Allen, & Kaszniak, 2001; Old & Naveh-Benjamin, 2008; Roberts, Ly, Murray, & Yassa, 2014; Rotblatt et al., 2015; Sumida et al., 2015; Tolentino, Pirogovsky, Luu, Toner, & Gilbert, 2012; Ulbrich, Churan, Fink, & Wittmann, 2009). These deficits are presumed to stem from age-related changes in the frontal and temporal lobes. Temporal order memory deficits in older adults have been associated with age-related episodic memory impairment (Sumida et al., 2015) and also may have important implications for daily functioning. For example, temporal order memory in older adults, including those with mild cognitive impairment, was shown to be related to various activities of daily living such as meal preparation and medication management (Schmitter-Edgecombe, Woo, & Greeley, 2009). In addition, impaired temporal order memory might be a selective behavioral marker of Alzheimer’s disease (Bellassen, Iglói, de Souza, Dubois, & Rondi-Reig, 2012). Similarly, temporal order memory deficits have been reported in older adults diagnosed with mild cognitive impairment (Gillis, Quinn, Phillips, & Hampstead, 2013), which has been described as a transitional stage between normal aging and Alzheimer’s disease. Therefore, the development of tests to assess temporal order memory in older adults might have important clinical implications and be useful for detecting early changes in memory function that can be associated with neurodegenerative disease.
Currently, there is only one neuropsychological test, with known psychometric properties, that was specifically developed to assess temporal order memory (Bauer, Leventon, & Varga, 2012; Bauer, Dikmen, Heaton, Mungas, Slotkin, & Beaumont, 2013). Originally devised for use in children, Bauer and colleagues developed the Picture Sequence Memory Test (PSMT) for the National Institutes of Health Toolbox. The PMST involves the presentation of a series of four pictured activities in a fixed sequence on a computer screen, which are simultaneously described verbally by the experimenter/clinician. After viewing the sequence and hearing the description, the participant is asked to place the four pictures of the activities in the correct sequence. Dikmen et al. (2014) published validation data for the PMST for ages 20 to 85 and the data indicate good test-retest reliability and construct validity for the test. The remaining aforementioned studies have been conducted using a variety of experimental tests developed to assess temporal order memory in older adults and other populations. These tests often require participants to remember the order in which stimuli are presented on a two-dimensional grid or whether a word was presented on one list versus a different list presented at different points in time. For example, Kessels et al. (2007) developed a task that required young and older adults to remember a sequence of seven pictures of everyday objects (e.g. a camera) presented one at a time in seven different positions on a 5 × 5 grid on a computer screen. In a study by Parkin, Hunkin, & Walter (1995), young and older adults were required to remember two lists of declarative sentences and then were asked whether each sentence was from List 1 or List 2. Roberts et al. (2014) showed young and older adults a series of pictures of common objects on a computer screen. Then participants were shown two pictures from the series and were asked to indicate which one came earlier in the sequence. These laboratory-based tests have provided considerable and highly valuable insight into the effects of aging on temporal order memory and the neural substrates that might support this construct. However, few of these tests have been developed to resemble daily living tasks that involve temporal order memory. Our goal was develop new test of temporal order memory involving a “to-do” list, which is commonly used in daily life. The majority of existing temporal order memory tests provide the participant with explicit instructions to remember the order in which the stimuli are presented and the sequences are generated for (not by) the participants. In daily life, an individual rarely knows at encoding whether or not he/she will need to remember a specific event or its relationship in time to other events. Furthermore, in daily life, individuals often structure their own sequences of events, which is not reflected in current tests where the sequences are typically generated for the participant. Therefore, we designed a new test to examine age-related differences in incidental temporal order memory for a self-generated sequence of tasks one might complete in everyday life. The test assesses immediate free recall, delayed free recall, and delayed cued recall for the temporal order of events in a sequence. We also assessed preliminary evidence for construct validity of our test in a sample of healthy older adults. This test differs from existing tests because the sequences were self generated, the participants were not specifically told to remember the stimuli, and the test was designed to resemble everyday memory by using a “to-do” list.
Methods
The study participants (n=75) included 45 young adults between the ages of 18 and 25 (M = 19.71 years, SD = 1.73), and 30 healthy older adults ages 65 and older (M = 72.37 years, SD = 6.23). Young adults were recruited from a pool of Introductory Psychology undergraduate students enrolled at San Diego State University. Older adults were recruited from multiple community centers in the area. The proportion of males and females was nearly identical in the young adult (55.56% female) and older adult (56.67% female) groups. On average, older adults completed significantly more years of education (M = 15.4, SD = 1.08) compared to young adults (M = 12.91, SD = 2.31), F(1,73) = 39.352, p < .001, d = 1.20. In addition, older adults (M = 63.23, SD = 3.39) had significantly higher raw scores than young adults (M = 62.13, SD = 3.32) on the Wide Range Achievement Test-4 (WRAT; Wilkinson & Robertson, 2006), F(1, 68) = 5.18 p < .05, a measure of word reading ability and a proxy measure of education level. Raw scores on the WRAT-4 were moderately correlated with years of education (r = .42, p < .001). Participants who endorsed higher than moderate depression were excluded from the study. This included young adults with a Beck Depression Inventory-II (BDI-II; Beck, Steer, Ball, & Ranieri, 1996) score higher than 19 and older adults with a Geriatric Depression Scale-Short Form (GDS; Yesavage, Brind, Rose, & Lum, 1983) score higher than 10. The average BDI score for younger adults was 7.38 (SD = 4.05) and the average GDS score for older adults was 1.82 (SD = 2.26). Additional exclusionary criteria for older adults included scores lower than 130 on the Dementia Rating Scale-2, a test of global cognitive functioning (DRS-2; Jurica, Leitten, & Mattis, 2001). The average DRS-2 score for older adults was 139.40 (SD = 3.70). After applying the exclusionary criteria, the final sample included the aforementioned 75 participants.
Participants completed the Temporal Order Memory for Everyday Events (TOMEE) test, a new memory test assessing immediate and delayed free recall, recognition, and delayed cued recall for the temporal order of events in a sequence. Participants were given 10 cards, each listing a task one might accomplish in a typical day. The tasks included going to the ATM, shopping for groceries, taking a picture, returning a phone call, reading a book, checking the mailbox, taking out the trash, doing the laundry, browsing the internet, and visiting the post office. These tasks were selected because they are very common to everyday life for both young and older adults and the events do not have a clear temporal relationship or order to them. Participants were asked to self-generate a “to do” list by placing the 10 cards in a temporal sequence representing the order in which they would accomplish the tasks. Participants were not informed of any subsequent memory tests during generation of the sequence (encoding). A metal board was provided that had numbered positions from 1–10 (representing the order of the events) vertically down the left side of the board. The participant could then place each event card (which had a magnetic backing) in the desired numbered position in the sequence for ease of recording the participants’ self generated sequence. Fifteen seconds were allotted for review of the event cards following completion of the list. The board and cards were removed from view, followed by a 30 s stimulus-free delay period. Next, immediate recall was assessed by asking participants to verbally list the events in the same order as the sequence generated during encoding. The responses were recorded by the examiner in a test booklet. Then a 20 min delay period was implemented before assessing delayed recall. During the delay period, participants completed a brief nonverbal neuropsychological test, the Trail Making Test from the Delis-Kaplan Executive Function System (D-KEFS; Delis, Kaplan, & Kramer, 2001), followed by a short break. Following the delay, delayed free recall was assessed using the same procedure implemented during immediate recall. Finally, delayed cued recall for the sequence was assessed immediately after delayed free recall. The stack of 10 initial event cards was shuffled and presented to the participant who was asked to place the cards in the order representing the original sequence using the previously described metal board. The number of events recalled on each index was scored using two methods: ordinal positioning and sequential clustering. In ordinal positioning, one point was granted each time the event was in the same ordinal position in the recalled sequence as it was on the participant’s original “to do” list (1 through 10). In sequential clustering, one point was granted for each event recalled that was sequentially adjacent to another event, based on the participant’s original “to do” list. The TOMEE also assesses recall of the events, independent of the sequence. The total number of events recalled during immediate and delayed free recall, regardless of whether the event was placed in the correct sequence, also was recorded. This index provides a measure of item memory.
All participants also completed the following standardized neuropsychological tests: the Hopkins Verbal Learning Test-Revised (HVLT-R; Brandt & Benedict, 2001); the D-KEFS Trail Making Test (Delis et al., 2001); the D-KEFS phonemic fluency test (Delis et al., 2001); the Benton Judgment of Line Orientation Test (Benton, Sivan, Hamsher, Verney, & Spreen, 1983); and the WRAT-4 (Wilkinson & Robertson, 2006). A test battery was designed that could be relatively brief to administer in one test session along with the TOMEE (considering fatigue effects in older adults), which would provide a measure in the primary domains of memory, language, executive functioning, attention, and visuospatial perception. The WRAT-4 was included as a proxy measure of education (given that the college students in our young adult group have not yet achieved their peak education) and as a measure of word reading to assess this ability in our older adults and its relationship to performance on the TOMEE. The test battery required approximately 2–2.5 hours to administer in one session at San Diego State University. The tests were administered in the following order: TOMEE immediate recall, D-KEFS Trail Making Test, TOMEE delayed recall, BDI-II/GDS, HVLT-R immediate recall, Benton Judgment of Line Orientation Test, HVLT-R delayed recall, WRAT-4, D-KEFS phonemic fluency test, and DRS-2 (older adults only). All procedures were approved by the Institutional Review Board at San Diego State University and all participants provided informed consent prior to participation in this study.
Results
Temporal Order Memory of Everyday Events Test
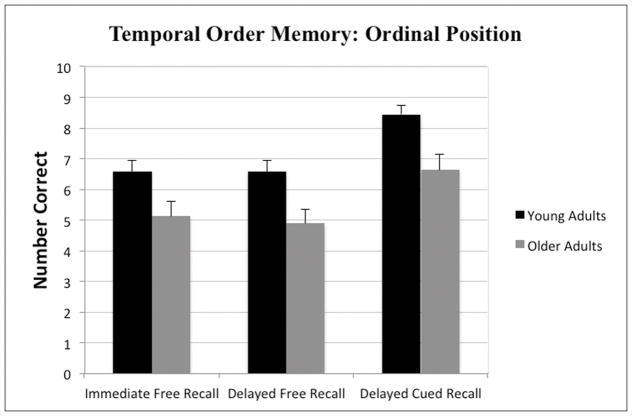
Mixed model 2 × 3 Analysis of Variance (ANOVA) tests with age-group as a between group variable (young adults, older adults) and TOMEE memory index (immediate free recall, delayed free recall, delayed cued recall) as within group variables were used to assess performance on the TOMEE using the two aforementioned scoring methods. Using the ordinal position scoring method, the analyses revealed a significant main effects of age-group F(1, 73) = 13.80, p < .001 and memory index F(2, 146) = 21.87, p < .001; however, the interaction was not significant F(2, 146) = .15, p = .86. As shown in Figure 1, young adults outperformed older adults on all three memory indices, which also is evidenced by the significant main effect of age-group. A Newman-Keuls posthoc comparison test of the main effect of memory index revealed that participants performed significantly better (p < .05) on the delayed cued recall index compared to both the immediate and delayed free recall indices, which did not differ significantly from one another.
Figure 1.
Mean (+SE) number of events placed in the correct ordinal position by young and older adults on the TOMEE for Immediate Free Recall, Delayed Free Recall, and Delayed Cued-Recall indices.
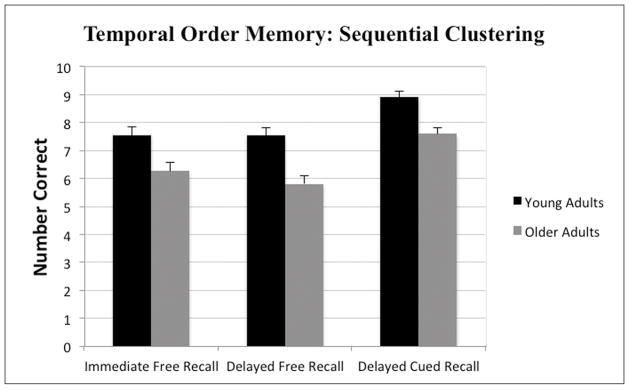
Using the sequential clustering scoring method, the analyses revealed a significant main effects of age-group F(1, 73) = 13.41, p < .001 and memory index F (2, 146) = 26.69, p < .001; however, the interaction was not significant F(2, 146) = .57, p = .57. As shown in Figure 2, young adults outperformed older adults on all three memory indices, which also is evidenced by the significant main effect of age-group. A Newman-Keuls posthoc comparison test of the main effect of memory index revealed that participants performed significantly better (p < .05) on the delayed cued recall index compared to both the immediate and delayed free recall indices, which did not differ significantly from one another. Since both scoring methods yielded highly similar results, the ordinal position scoring method was used in subsequent analyses.
Figure 2.
Mean (+SE) number of events placed in correct sequential clusters by young and older adults on the TOMEE for Immediate Free Recall, Delayed Free Recall, and Delayed Cued-Recall indices.
The TOMEE also assesses recall of events, independent of sequence. The number of events recalled during immediate and delayed free recall was analyzed to provide a measure of item memory for the events. A 2 × 2 mixed model ANOVA with age-group as a between group variable (young adults, older adults) and TOMEE memory index (immediate free recall, delayed free recall) as within group variables revealed a significant main effects of age-group F(1, 73) = 9.07, p < .01 and memory index F(1, 73) = 4.78, p < .05; however, the interaction was not significant F(1, 73) = .58, p = .45. The results show that young adults recalled significantly more events, independent of sequence, than older adults during immediate free recall (young adults M = 8.84, SD = 1.02; older adults M = 8.26, SD = 1.34) and delayed free recall (young adults M = 8.67, SD = 1.31; older adults M = 8.03, SD = 1.50).
Although item memory was qualitatively similar between young and older adults (difference was less than one point), analyses revealed that the difference reached statistical significance. Thus, to account for item memory when assessing recall of events in the sequence, the total number of events recalled in the correct sequence (i.e., temporal order memory, based on the ordinal position scoring method) was divided by the total number of events recalled irrespective of sequence (i.e., item memory). ANOVA tests revealed that the ratio scores were significantly larger for young adults (M = 0.73, SD = 0.22) compared to older adults (M = 0.65, SD = 0.25) for immediate free recall, F(1,73) = 4.18, p < .05, d = 0.34. Similarly, the ratio scores were significantly larger for young adults (M = 0.75, SD = 0.22) compared to older adults (M = 0.63, SD = 0.23) for delayed free recall, F(1,73) = 6.00, p < .05, d = 0.53.
Standardized Neuropsychological Measures
One-way ANOVA tests were conducted to assess age group differences on all five standardized neuropsychological tests. The data from young and older adults, along with the results of the statistical analyses and associated Cohen’s d effect size estimates are reported in Table 1. Young adults significantly outperformed older adults on measures of memory, executive functioning, attention, motor speed, and visuospatial perception. No significant age differences were found on fluency measures of language and older adults outperformed young adults on word reading. To facilitate comparisons with standardized neuropsychological tests, data from young and older adults on the immediate free recall, delayed free recall, and delayed cued recall memory indices from the TOMEE were analyzed using one-way ANOVA tests and associated Cohen’s d effect size estimates were calculated. A Bonferroni correction was applied to control for multiple comparisons and the alpha level was set at .017. The data and results also are shown in Table 1.
Table 1.
Mean (SD) raw scores of young and older adults on the temporal order memory test and standardized neuropsychological tests, along with the results of one-way analysis of variance tests comparing age group differences and associated Cohen’s d effects size estimates.
| YA (n = 45) | OA (n = 30) | F | p | d | |
|---|---|---|---|---|---|
| Temporal Order Memory | |||||
| Immediate Free Recall | 6.58 (2.37) | 5.13 (2.56) | 6.28 | <.01** | 0.59 |
| Delayed Free Recall | 6.57 (2.37) | 4.90 (2.44) | 8.81 | <.01** | 0.70 |
| Cued Recall | 8.44 (1.92) | 6.63 (2.72) | 10.86 | <.01** | 0.75 |
| Verbal Memory | |||||
| HVLT-R Immediate Free Recall | 28.91 (2.42) | 27.52 (4.51) | 6.25 | <.05* | 0.39 |
| HVLT-R Delayed Free Recall | 10.56 (1.21) | 9.94 (2.20) | 7.67 | <.01** | 0.35 |
| Language | |||||
| Phonemic Fluency (Total) | 40.38 (7.13) | 42.53 (13.63) | 0.80 | .37 | -- |
| Attention | |||||
| DKEFS Trails: Trial 1 | 17.38 (5.07) | 22.65 (6.98) | 51.35 | <.001** | 0.86 |
| Executive Functioning | |||||
| DKEFS Trails: Trial 4 | 63.51 (18.38) | 94.48 (48.22) | 40.76 | <.001** | 0.85 |
| Visuospatial Perception | |||||
| Benton JoLO | 25.44 (3.92) | 23.96 (4.30) | 8.72 | <.01** | 0.36 |
| Word Reading | |||||
| WRAT-4 | 62.13 (3.31 | 64.20 (5.01) | 4.64 | <.05* | 0.49 |
p < .05,
p < .01 or lower.
HVLT-R = Hopkins Verbal Learning Test-Revised
DKEFS = Delis Kaplan Executive Function System
JoLO = Benton Judgment of Line Orientation Test
WRAT-4 = Word Reading subtest of the Wide Range Achievement Test-Fourth Edition
Correlations between the Temporal Order Memory and Standardized Measures
As shown in Table 2, Pearson r partial correlation analyses (controlling for age) were used to examine the relationship between the TOMEE indices using the ordinal position scoring method and standardized neuropsychological tests in older adults. These analyses were conducted to assess preliminary evidence for construct validity of our test in a sample of healthy older adults. All three TOMEE indices were significantly correlated with measures of verbal memory. However, the indices were not correlated with measures of phonemic fluency, attention, executive functioning, visuospatial perception, or word reading. In addition, DRS-2 scores in older adults did not correlate with TOMEE indices of immediate free recall (r = .03, p = .87), delayed free recall (r = .14, p = .45), or delayed cued recall (r = .35, p = .06). GDS scores in older adults also did not correlate with TOMEE indices of immediate free recall (r = .13, p = .50), delayed free recall (r = −.07, p = .71), or delayed cued recall (r = −.02, p = .99). Similarly, BDI-II scores in young adults did not correlate with TOMEE indices of immediate free recall (r = −.12, p = .43), delayed free recall (r = −.16, p = .30) or delayed cued-recall (r = −.05, p = .74).
Table 2.
Pearson r partial correlation coefficients (controlling for age) and associated p-values for relationships between the TOMEE ordinal position scoring method for the Immediate, Delayed, and Delayed Cued-Recall indices and raw scores from standardized neuropsychological measures in older adults.
| Temporal Order Memory
|
||||||
|---|---|---|---|---|---|---|
| Immediate Recall | Delayed Recall | Cued Recall | ||||
| r | p | r | p | r | p | |
| Verbal Memory | ||||||
| HVLT-R Immediate Recall | .42 | .05* | .40 | .05* | .44 | .05* |
| HVLT-R Delayed Recall | .46 | .05* | .47 | .01* | .54 | .01* |
| Language | ||||||
| Phonemic Fluency | .26 | .18 | .26 | .18 | .10 | .59 |
| Attention | ||||||
| D-KEFS Trails: Trial 1 | .11 | .57 | .12 | .52 | −.003 | .99 |
| Executive Function | ||||||
| D-KEFS Trails: Trial 4 | −.26 | .18 | −.23 | .23 | −.34 | .07 |
| Visuospatial Perception | ||||||
| Benton JoLO | −.06 | .75 | −.14 | .49 | .06 | .74 |
| Word Reading | ||||||
| WRAT-4 | .07 | .74 | .18 | .34 | .34 | .07 |
Indicates statistical significance
HVLT-R = Hopkins Verbal Learning Test-Revised
DKEFS = Delis Kaplan Executive Function System
JoLO = Benton Judgment of Line Orientation Test
WRAT-4 = Word Reading subtest of the Wide Range Achievement Test-Fourth Edition
Discussion
In the present study, older adults performed worse than young adults on immediate free recall, delayed free recall, and delayed cued recall for the temporal order of everyday events as assessed by our new test. Moderate to large Cohen’s d effect sizes associated with age group differences were observed, suggesting that our new test may provide a sensitive measure of temporal order memory impairment in older adults. Given that aging has been shown to adversely affect verbal memory (e.g. Kramer, Yaffe, Lengenfelder, & Delis, 2003), we conducted subsequent analyses to account for item memory for the events, while assessing recall of the events in the sequence. We found that the age-related differences in temporal order memory remained significant when item memory was accounted for in the analyses. Therefore, these findings provide evidence that poorer performance in older adults relative to young adults on the free recall indices on our test reflects impairment in the ability to remember the temporal sequence of the events rather than solely impaired item memory for the events.
Overall, the present findings are consistent with previous studies reporting that temporal order memory is impaired in older adults compared to young adults (Kessels et al., 2007; Tolentino et al., 2012). However, this is one of the first studies to show age-related impairment on a test involving incidental encoding for a self-generated sequence that resembles temporal order memory as it is relied upon in daily life. Several tests have been designed to assess temporal order memory in both healthy and impaired individuals (Daum & Mayes, 2000; Dikmen et al., 2014; Hayes et al., 2004; Hopkins et al., 1995; Kessels et al., 2007; Knutson et al., 2004; Lehn et al., 2009; Parkin et al., 1995). Although studies using these measures have offered insight into sequential memory, the measures were not designed to be representative of everyday experiences and only one to our knowledge has validation data across the lifespan and has evidence for test-retest reliability and construct validity. Our utilization of a “to do” list in our test may be representative of the process by which individuals recall information that is necessary for completing daily activities in a particular sequence and therefore provides novel insight into temporal order memory function as it is pertains to daily life. Additionally, our test assesses multiple indices of temporal order memory and can dissociate deficits in temporal order memory from deficits in item memory for the events irrespective of their temporal sequence.
We also examined the relationship between performance on our test and the aforementioned standardized tests to provide preliminary evidence for construct validity in older adults. One type of construct validity, convergent validity, is established when scores on a test are correlated with variables that are theoretically related to the construct of interest. In contrast, discriminant validity is supported when scores on the measure demonstrate no relationships with variables that are theoretically distinct from the construct of interest (Groth-Marnat, 2009). Performance on our test was significantly correlated with performance on other measures of memory, providing preliminary evidence for convergent validity of our test. Immediate and delayed recall indices on our test were significantly correlated with immediate and delayed recall indices on the HVLT-R, a well validated, standardized measure of verbal memory thought to be sensitive to temporal lobe function. However, performance on our test was not found to be correlated with performance on tests assessing cognitive constructs not theoretically related to the construct of memory, including language, attention, visuospatial perception and word reading. These findings provide preliminary evidence for divergent validity of our test. These findings also illustrate that any age-related deficits on our test were not solely due to impairments in language or general attention in older adults. A trend level association (p = .07) was found between performance on the delayed cued recall index of our test and Trial 4 of the D-KEFS Trail Making Test, which assesses set-shifting aspects of executive function thought to be dependent on frontal lobe function. Given the relationship between temporal order memory and frontal lobe function, this association might be of interest in the context of a large sample. Our findings offer preliminary evidence for construct validity and possible insight into the neural correlates of test performance; however, future studies involving larger samples clearly are needed.
Scores on a measure of global cognitive functioning (DRS-2) in older adults did not correlate with performance on any indices from our test, indicating that age-related deficits on our test were not likely due to global cognitive impairment in the older adults. Similarly, scores on standardized measures of depression did not correlate with performance on our test in young or older adults. All participants were screened for depression and none of the participants endorsed more than moderate depressive symptoms (based on BDI-II and GDS scores). In addition, older adults completed significantly more years of education than young adults and scored significantly higher on the WRAT-4, which is a proxy measure for education level and a measure of word reading. Therefore, worse performance on our test in older adults relative to young adults was not due to lower education levels or impaired word reading. In addition, the young and older adult groups did not differ in sex, which is another important demographic variable. Taken together, these data provide evidence that demographic variables, mood, and global cognitive functioning were assessed and controlled for in the present study.
In the present study, older adults performed worse than young adults on standardized measures of memory, attention, executive functioning, and visuospatial perception. These findings are consistent with well-documented evidence of age-related declines in these domains (Bartrés-Faz et al., 2001). However, older adults were comparable to young adults on a standardized measure of phonemic fluency and even outperformed young adults on a measure of word reading, which is consistent with prior studies indicating that language functioning is generally well preserved in healthy older adults (Van Gorp, Satz, Kiersch, & Henry, 1986).
Moreover, the effect sizes for age-related differences on the immediate recall and delayed free recall indices from our test were 44% and 50% larger, respectively, than the effect sizes for age-related differences on the HVLT-R. This finding provides preliminary evidence that our test may have incremental value above and beyond standardized tests of verbal memory in assessing age-related memory impairment. For example, Blachstein et al. (2012) examined the sensitivity of direct and indirect measures of temporal order memory to the effects of aging using a Hebrew version of the Rey Auditory Verbal Learning Test (AVLT) and found that a more direct measure of temporal order memory (explicit reordering of words into original order of presentation) was more vulnerable to the effects of aging relative to an indirect measure (spontaneous order in which list is recalled). In addition, the direct measure was more significantly correlated than the indirect measure with other verbal memory measures derived from the Rey AVLT. Coupled with the findings from Blachstein et al. (2012), the present findings provide evidence that temporal order memory tests might be useful in the assessment of age-related memory impairment.
The present study is not without limitations. The relatively small sample size lessens the generalizability of the findings. However, age-related differences on the TOMEE were statistically significant and associated with moderate to large effect sizes, providing evidence that the findings are robust in the context of a relatively small sample. Additionally, non-significant correlations should be interpreted with caution given the relatively small sample size. Another limitation was the use of a relatively small battery of neuropsychological tests, which limits our ability to thoroughly assess the psychometric properties of our test. Future studies involving larger samples are clearly needed to extend the current findings. Finally, everyday experiences occur across much longer periods of time compared to the present test, which does limit the generalizability of the present findings to everyday temporal order memory.
In conclusion, we developed a new test to assess memory for an incidentally encoded, self-generated sequence of events and found significant age-related impairments on the test, which were associated with moderate to large effect sizes. We also found preliminary evidence for construct validity of the test in older adults. Age-related temporal order memory deficits have been well documented using experimental laboratory tests (Blachstein et al., 2012; Dikmen et al., 2014; Kessels et al., 2007; Newman et al., 2001; Old & Naveh-Benjamin, 2008; Roberts et al., 2014; Rotblatt et al., 2015; Sumida et al., 2015; Tolentino et al., 2012; Ulbrich et al., 2009) and may be related to various activities of daily living (Schmitter-Edgecombe et al., 2009); however, there is only one standardized test of temporal order memory currently available. Although future studies are clearly needed, the present study represents the first to our knowledge to develop a test of temporal order memory that relates to everyday life with evidence for construct validity in older adults. Given recent findings that impaired temporal order memory might be a selective behavioral marker of Alzheimer’s disease (Bellassen et al., 2012) and evidence this deficit may be detectable in older adults diagnosed with mild cognitive impairment who are at risk for AD (Gillis et al., 2013), temporal order memory tests might have important clinical implications. Our test is a highly portable, rapidly administered measure that can be used in research and clinical settings.
Acknowledgments
This research was supported by National Institutes of Health (NIH) Grant 5R01AG034202 from the National Institute on Aging awarded to Paul E. Gilbert. Shannon Y. DeJesus was supported by NIH grant 5R25GM058906 from the National Institute of General Medical Sciences (NIGMS). Charles C. Moreno was supported by NIH grant (2T34GM008303) from the NIGMS. Sarah Mattson was supported by grant U01 AA014834. We thank Petra Taylor and Lauren MacDonnell for their assistance with data collection and entry. We also thank all of the participants for their contributions to this study.
References
- Bartrés-Faz D, Junqué C, Clemente IC, Serra-Grabulosa JM, Guardia J, López-Alomar A, … Moral P. MRI and genetic correlates of cognitive function in elders with memory impairment. Neurobiology of Aging. 2001;22:449–459. doi: 10.1016/s0197-4580(01)00207-x. [DOI] [PubMed] [Google Scholar]
- Bauer PJ, Dikmen SS, Heaton RK, Mungas D, Slotkin J, Beaumont JL., III NIH Toolbox Cognition Battery (CB): measuring episodic memory. Monographs of the Society for Research in Child Development. 2013;78:34–48. doi: 10.1111/mono.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer PJ, Leventon JS, Varga NL. Neuropsychological assessment of memory in preschoolers. Neuropsychology Review. 2012;22:414–424. doi: 10.1007/s11065-012-9219-9. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment. 1996;67:588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Bellassen V, Iglói K, de Souza LC, Dubois B, Rondi-Reig L. Temporal order memory assessed during spatiotemporal navigation as a behavioral cognitive marker for differential Alzheimer’s disease diagnosis. Journal of Neuroscience. 2012;32:1942–1952. doi: 10.1523/JNEUROSCI.4556-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton A, Sivan A, Hamsher K, Varney N, Spreen O. Benton Judgment of Line Orientation. Psychological Assessment Resources; Lutz, Florida: 1983. [Google Scholar]
- Blachstein H, Greenstein Y, Vakil E. Aging and temporal order memory: A comparison of direct and indirect measures. Journal of Clinical and Experimental Neuropsychology. 2012;34:107–112. doi: 10.1080/13803395.2011.625352. [DOI] [PubMed] [Google Scholar]
- Brandt J, Benedict RHB. Hopkins verbal learning test-revised. Psychological Assessment Resources; Lutz, Florida: 2001. [Google Scholar]
- Cabeza R, Anderson ND, Houle S, Mangels JA, Nyberg L. Age-related differences in neural activity during item and temporal-order memory retrieval: A positron emission tomography study. Journal of Cognitive Neuroscience. 2000;12:197–206. doi: 10.1162/089892900561832. [DOI] [PubMed] [Google Scholar]
- Daum I, Mayes AR. Memory and executive function impairments after frontal or posterior cortex lesions. Behavioural Neurology. 2000;12:161–173. doi: 10.1155/2000/327304. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan executive function system: D-KEFS. Pearson; San Antonio, Texas: 2001. [Google Scholar]
- DeVito LM, Eichenbaum H. Memory for the order of events in specific sequences: Contributions of the hippocampus and medial prefrontal cortex. The Journal of Neuroscience. 2011;31(9):3169–3175. doi: 10.1523/JNEUROSCI.4202-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikmen SS, Bauer PJ, Weintraub S, Mungas D, Slotkin J, Beaumont JL, Gershon R, Temkin NR, Heaton RK. Measuring episodic memory across the lifespan: NIH Toolbox Picture Sequence Memory Test. Journal of the International Neuropsychological Society. 2014;20:611–619. doi: 10.1017/S1355617714000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes JJ, Mayes AR, MacDonald C, Hunkin NM. Temporal order memory in patients with Korsakoff’s syndrome and medial temporal amnesia. Neuropsychologia. 2002;40:853–861. doi: 10.1016/s0028-3932(01)00172-5. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Bookheimer SY. Spatial and temporal episodic memory retrieval recruit dissociable functional networks in the human brain. Learning & Memory. 2007;14:645–654. doi: 10.1101/lm.575107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Copara MS, Isham EA, Wang WC, Yonelinas AP. Dissociable networks involved in spatial and temporal order source retrieval. NeuroImage. 2011;56:1803–1813. doi: 10.1016/j.neuroimage.2011.02.033. [DOI] [PubMed] [Google Scholar]
- Gillis MM, Quinn KM, Phillips PA, Hampstead BM. Impaired retention is responsible for temporal order memory deficits in mild cognitive impairment. Acta Psychologica. 2013;143:88–95. doi: 10.1016/j.actpsy.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth-Marnat G. Handbook of psychological assessment. 5. John Wiley & Sons, Inc; Hoboken, N.J: 2009. [Google Scholar]
- Hayes SM, Ryan L, Schnyer DM, Nadel L. An fMRI study of episodic memory: Retrieval of object, spatial, and temporal information. Behavioral Neuroscience. 2004;118:885–896. doi: 10.1037/0735-7044.118.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins RO, Kesner RP, Goldstein M. Item and order recognition memory in subjects with hypoxic brain injury. Brain and Cognition. 1995;27:180–201. doi: 10.1006/brcg.1995.1016. [DOI] [PubMed] [Google Scholar]
- Jurica PJ, Leitten CL, Mattis S. Dementia Rating Scale-2. Psychological Assessment Resources; Lutz, Florida: 2001. [Google Scholar]
- Kessels RP, Hobbel D, Postma A. Aging, context memory and binding: A comparison of “what, where and when” in young and older adults. International Journal of Neuroscience. 2007;117:795–810. doi: 10.1080/00207450600910218. [DOI] [PubMed] [Google Scholar]
- Knutson KM, Wood JN, Grafman J. Brain activation in processing temporal sequence: An fMRI study. Neuroimage. 2004;23:1299–1307. doi: 10.1016/j.neuroimage.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Yaffe K, Lengenfelder J, Delis DC. Age and gender interactions on verbal memory performance. Journal of International Neuropsychological Society. 2003;9:97–102. doi: 10.1017/s1355617703910113. [DOI] [PubMed] [Google Scholar]
- Lehn H, Steffenach HA, van Strien NM, Veltman DJ, Witter MP, Haberg AK. A specific role of the human hippocampus in recall of temporal sequences. The Journal of Neuroscience. 2009;29:3475–3484. doi: 10.1523/JNEUROSCI.5370-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes AR, Isaac CL, Holdstock JS, Hunkin NM, Montaldi D, Downes JJ, … Roberts JN. Memory for single items, word pairs, and temporal order of different kinds in a patient with selective hippocampal lesions. Cognitive neuropsychology. 2001;18:97–123. doi: 10.1080/02643290125897. [DOI] [PubMed] [Google Scholar]
- Milner B, Petrides M, Smith ML. Frontal lobes and the temporal organization of memory. Human neurobiology. 1985;4:137–142. [PubMed] [Google Scholar]
- Newman MC, Allen JJB, Kaszniak AW. Tasks for assessing memory for temporal order versus memory for items in aging. Aging, Neuropsychology, and Cognition. 2001;8:72–78. [Google Scholar]
- Old SR, Naveh-Benjamin M. Differential effects of age on item and associative measures of memory: A meta-analysis. Psychology of Aging. 2008;23:104–118. doi: 10.1037/0882-7974.23.1.104. [DOI] [PubMed] [Google Scholar]
- Parkin AJ, Hunkin NM, Walter BM. Relationships between normal aging, frontal lobe function, and memory for temporal and spatial information. Neuropsychology. 1995;9:304–312. [Google Scholar]
- Roberts JM, Ly M, Murray E, Yassa MA. Temporal discrimination deficits as a function of lag interference in older adults: Temporal Discrimination and Interference in Aging. Hippocampus. 2014;24:1189–1196. doi: 10.1002/hipo.22303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotblatt LJ, Sumida CA, Van Etten EJ, Turk EP, Tolentino JC, Gilbert PE. Differences in temporal order memory among young, middle-aged, and older adults may depend on the level of interference. Frontiers in Aging Neuroscience. 2015;7:28. doi: 10.3389/fnagi.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Passingham RE. Working memory for location and time: activity in prefrontal area 46 relates to selection rather than maintenance in memory. Neuroimage. 2001;14:77–86. doi: 10.1006/nimg.2001.0784. [DOI] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M, Woo E, Greeley DR. Characterizing multiple memory deficits and their relation to everyday functioning in individuals with mild cognitive impairment. Neuropsychology. 2009;23:168–77. doi: 10.1037/a0014186. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Janowsky JS, Squire LR. Memory for the temporal order of events in patients with frontal lobe lesions and amnesic patients. Neuropsychologia. 1990;28:803–813. doi: 10.1016/0028-3932(90)90004-8. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Burgess N, Maguire EA, Baxendale SA, Hartley T, Thompson PJ, O’Keefe J. Unilateral temporal lobectomy patients show lateralized topographical and episodic memory deficits in a virtual town. Brain: A Journal of Neurology. 2001;124:2476–2489. doi: 10.1093/brain/124.12.2476. [DOI] [PubMed] [Google Scholar]
- Sumida CA, Holden HM, Van Etten EJ, Wagner GM, Hileman JD, Gilbert PE. Who, when, and where? Age-related differences on a new memory test. Learning & Memory. 2016;23(1):38–41. doi: 10.1101/lm.039313.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolentino JC, Pirogovsky E, Luu T, Toner CK, Gilbert PE. The effect of interference on temporal order memory for random and fixed sequences in nondemented older adults. Learning & Memory. 2012;19:251–255. doi: 10.1101/lm.026062.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbrich P, Churan J, Fink M, Wittmann M. Perception of temporal order: The effects of age, sex, and cognitive factors. Aging, Neuropsychology, and Cognition. 2009;16(2):183–202. doi: 10.1080/13825580802411758. [DOI] [PubMed] [Google Scholar]
- Van Gorp WG, Satz P, Kiersch ME, Henry R. Normative data on the boston naming test for a group of normal older adults. Journal of Clinical and Experimental Neuropsychology. 1986;8(6):702–705. doi: 10.1080/01688638608405189. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ. WRAT 4: Wide Range Achievement Test; Professional Manual. Psychological Assessment Resources, Incorporated; 2006. [Google Scholar]
- Yesavage JA, Brind TL, Rose TL, Lum O. Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]