Abstract
Substantial evidence suggests that chronic human cytomegalovirus (hCMV) infection contributes significantly to T-cell immunosenescence and adverse health outcomes in older adults. As such, it is important to search for compounds with anti-hCMV properties. Studies have shown that resveratrol, a sirtuin activator, suppresses hCMV infection. Here we report suppressive effects of sirtinol, a sirtuin antagonist, on hCMV infection and its cellular and molecular consequences. Human diploid fibroblast WI-38 cells were infected by hCMV Towne strain in the absence or presence of sirtinol. hCMV replication was measured using qPCR. Senescent phenotype was determined by senescence-associated β galactosidase (SA-β-Gal) activity. Expression of hCMV immediate early (IE) and early (E) proteins and senescence-associated proteins (pRb and Rb, p16INK4, and p53) and production of reactive oxygen species (ROS) were assessed using standard laboratory assays. The results demonstrated that sirtinol suppressed hCMV infection as well as hCMV-induced activation of molecular mechanisms of senescence and ROS production. While underlying molecular mechanisms remain to be elucidated, these findings indicate sirtinol as a novel and potent anti-hCMV agent with the potential to be developed as an effective treatment for chronic hCMV infection and its cellular and molecular consequences that are important to ageing and health of older adults.
Keywords: sirtinol, hCMV infection, hCMV-induced cellular senescence, reactive oxygen species
1. Introduction
Human cytomegalovirus (hCMV) is a large DNA virus that can persist in cells of the myeloid lineage, such as monocytes and macrophages, establishing chronic or persistent infection in some immunocompetent individuals (Smith et al., 2004; Wreghitt et al., 2003). Chronic CMV infection is highly prevalent in older adults based on anti-CMV IgG serology. Substantial evidence suggests that chronic CMV infection contributes significantly to age-related T-cell immunosenescence and adverse health outcomes (Leng et al., 2011b; Pawelec et al., 2005). For example, a large number of studies have shown clonal expansion of CD4+ and CD8+ T cells specific to CMV pp65 or immediate early (IE)-1 epitopes in seropositive older persons (Hadrup et al., 2006; Khan et al., 2004; Koch et al., 2007; Ouyang et al., 2003; Pourgheysari et al., 2007; Vescovini et al., 2007). Several studies including our own have demonstrated broad impact of chronic CMV infection on T-cell immunity beyond those to pp65 or IE-1 epitopes including significant alterations of commonly identified T-cell phenotypes (Di Benedetto et al., 2015; Li et al., 2014a; Olsson et al., 2000; Sylwester et al., 2005). In addition to its impact on T-cell immunity, hCMV has been reported to induce cellular senescence (Noris et al., 2002; Wolf et al., 2012). At the population level, anti-CMV seropositivity has been proposed as a key component of “immune risk profile” which predicted mortality in the Swedish OCTO and NONA immune studies (Strindhall et al., 2007; Wikby et al., 2006), and others have shown significant associations between positive anti-CMV IgG serology and frailty, disability, and mortality (Aiello et al., 2008; Roberts et al., 2010; Schmaltz et al., 2005; Wang et al., 2010). More recently, we have observed that presence of hCMV viral DNA in periperhal blood monocytes as detected by a nested PCR-based assay is likely a better indicator of chronic CMV infection than positive anti-CMV IgG serology in terms of its associations with pp65-specific CD8+ T-cell expansion and immune activation in older adults (Leng et al., 2011a; Leng et al., 2011b), and our longitudinal study, albert at only two time points with a small sample size, has confirmed this obersavation and linked chronic CMV infection to elevated inflammation (Li et al., 2014b). Because of the significant adverse health impact of this viral infection, searching for effective interventional strategies has benn identified by the Institute of Medicine as a top priority (Arvin et al., 2004; Stratton K, 2000).
Sirtuins (SIRT), particularly SIRT-1, are key regulators of metabolism that promote cell survival and extend lifespan (Cohen et al., 2004). Over the years, a number of sirtuin activators and inhibitors have been identified and evaluated for their biological activities (Villalba and Alcain 2012). Resveratrol, probably the most extensively studied SIRT-1 activator, is known to promote mitochondrial biogenesis, suppress apoptosis, and reduce signs of aging (Smith, et al., 2009; Pearson, et al., 2008). Resveratrol was also reported to have suppressive effect against hCMV infection (Evers et al., 2004). Sirtinol and its analogues are sirtuin antagonists targeting to SIRT-1 and SIRT-2 (Grozinger et al., 2001; Mai et al., 2005). At a high concentration (50μM or higher), sirtinol has been shown to induce senescence-like growth arrest in human breast cancer MCF-7 and lung cancer H1299 cells (Ota et al., 2006; Li et al., 2008). Blockage of SIRT-1 activity by sirtinol and other inhibitors led to endothelial dysfunction in the rat aorta (Zarzuelo et al., 2013). In our study of hCMV infection in human diploid fibroblasts, sirtinol was employed to block SIRT-1 activity in an attempt to antagonize suppressiion of reveratrol on hCMV infection. To our supprise, sirtinol inhibited hCMV infection as well. Here, we report such supprising effect of sirtinol. We also describe the suppressive effect of sirtinol on hCMV-induced activation of molecular mechanisms of senescence and production of reactive oxygen species (ROS), likely secondary to its supprssion of hCMV infection.
2. Materials and Methods
2.1. Sirtinol and antibodies
Sirtinol (2-[(2-hydroxynaphthalen-1-ylmethylene)- amino]-N-(1-phenyl-ethyl)-benzamide) was purchased from Sigma-Aldrich (St. Louis, MO) and prepared in 20 mM stocks in dimethylsulfoxide (DMSO) stored at −20 °C until use.
Primary antibodies including anti-p53, anti-p16INK4, and anti-β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-retinoblastoma (Rb) and anti- phosphorylated Rb (pRbSer780) were purchased from Cell Signaling Technology (Danvers, MA). Anti-hCMV immediate-early (IE) proteins at apparent molecular weights of 86, 68–72, and 38kD primary antibody was obtained from Millipore Corporation (Billerica, MA) and Anti-hCMV early protein (UL44) was from Virusys Corporation (Randallstown, MD).
2.2. Cell Culture and hCMV infection
Primary human embryonic lung diploid fibroblasts (WI-38) were purchased from the American Tissue Culture Collection (ATCC, Manassas, VA) and were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin. The cells were young at population doubling (PD) of 32 or less and became replicative senescent at PD50 or higher. The cultured cells were split in ratios of 1:2 or 1:4 when the confluence of the culture was over 80%. The cumulative population doublings were calculated as log2 (D/D0), where D and D0 are defined as the density of cells at the time of harvesting and seeding, respectively. All experiments were performed using cells that were between 27–32 PD for hCMV infections unless indicated otherwise.
Stocks of the Towne strain of hCMV (ATCC, VR977) were routinely prepared and viral titers were measured in MRC-5 cells as previously described(Li et al., 2015). Supernatants collected from uninfected MRC-5 culture were processed the same way as viral stock and used as mock control medium. WI-38 cells seeded at 2×104cells/cm2 in DMEM–10% FBS were cultured for 24h after which the cells were serum starved for 48–72 h in DMEM–0.2% FBS to synchronize cells in G0 phase of cell cycle before infection. Virus inoculum was added for 2 h at 37°C and then discarded and replaced with fresh DMEM–0.2% FBS. Mock-infected controls were exposed to an equal volume of mock control medium described above. Cells were harvested at indicated time points post infection for further testing.
For cultures in the presence of sirtinol, sirtinol was added 2h before (−2h) or after (+2h) virus inoculation (−2h) with its presence until cells were harvested.
2.3. MTT assay
MTT assay was performed as previously described (Mao et al., 2010). Briefly, cells were seeded in flat-bottomed 96-well microplates at the density of 3×103 cells in 0.2 ml per well. After 24 h, cells were incubated in the culture medium containing sirtinol at different concentrations for 48 h. Then 20 μL MTT [3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-diphenlytetrazoliumbromide] of 5 mg/mL was added to each well. After incubation for 4 h, the supernatant was discarded and 0.2mL DMSO was added to stop reactions. The absorbance values of each well were determined spectrophotometrically at 490 nm using BIO-RAD microplate reader (Hercules, CA).
2.4. qPCR
DNA was extracted from harvested cells using Qiagen Mini-DNA Kit (Valencia, CA). qPCR was performed to determine hCMV viral DNA copy numbers using iQ SYBR Green Supermix kit (Bio-Rad) in a total volume of 20 μl containing 10 ng of total DNA and CFX96 C1000 Touch Real-Time PCR System Detector (Bio-Rad). Primers targeted to hCMV UL123 region were employed as previously described (Leng et al., 2011b): forward 5′-TCTGCCAGGACATCTTTCTC-3′and reverse 5′GTGACCAAGGCCACGACGTT-3′. Amplification cycles were performed according to the manufacturer’s instructions: 3 min at 95°C, followed by 40 cycles of 5 sec at 95°C, and 30 sec at 60°C. hCMV viral copy numbers were calculated using a standard curve generated with series of dilutions of Quantitated Viral DNA PCR control of hCMV AD169 strain purchased from Advanced Biotechnologies (Columbia, MD).
2.5. Western blot analysis
Harvested cells were washed with ice-cold PBS and then lysed with cell lysis buffer containing protease inhibitors cocktail (Cell Signaling Technology). Protein concentrations were determined by BCA protein assay kit purchased from Pierce Companies (Dallas, TX). Total 50 μg of protein extracts were loaded and electrophoresed on 12% SDS polyacrylamide gel and transferred to a polyvinylidene fluoride (PVDF) membrane (Bio-Rad). The membranes were subsequently probed with specific primary antibodies as listed above, respectively. Secondary antibody used for detection was linked with horseradish peroxidase. The enhanced chemiluminescence (ECL) method was used to detect the conjugated horseradish peroxidase.
2.6. SA-β-Gal staining
Levels of SA-β-Gal were determined essentially the same as previously described (Dimri et al., 1995; Mao et al., 2010). Briefly, cells were washed in PBS and fixed for about 5 min in 0.25% glutaraldehyde. After extensive washing in PBS, cells were incubated at 37°C in the staining solution without CO2. Staining was evident in 2 to 4 h and was maximal in 12 to 16 h. The percentage of SA-β-gal positive cells out of the total number of cells was counted. Average percentages were obtained from three independent experiments.
2.7. Measurement of intracellular reactive oxygen species (ROS)
The intracellular ROS level was measured as described previously (Mao et al., 2010). Briefly, on the day for measurement, cells were trypsinized and collected for further staining. After 30 min incubation with the ROS probe 2′, 7′-dichlorodihydrofluorecein diacetate (H2DCFDA, 10 μM) at 37 °C in the dark, the intracellular fluorescence intensity was measured by flow cytometer. The value of fluorescence intensity was positively correlated to the intracellular ROS production.
2.8. Statistical analysis
Data were expressed as means ± SD. Data was analyzed using two-way ANOVA with SPSS 15.0 software, P<0.05 was considered significant.
3. Results
3.1. Sirtinol has potent suppressive effect on hCMV infection
Pilot experiments of hCMV Towne strain infection in WI-38 human diploid fibroblasts at doubling time (PD) 30 with series of multiplicity of infection (MOI) indicated that infection became rapidly lytic at MOI of 0.1 or above with significant cell lysis within 72 hours after virus inoculation (data not shown). At MOI of 0.01, sporadic plaques started to occur 5 days after virus inoculation and viral copies were still readily detected at 7 days post infection (dpi). Since rapid lytic infection at high MOI was not optimal for in-depth analysis of hCMV replication and protein expression or hCMV-induced cellular senescence and ROS production, this lower MOI was chosen for all subsequent experiments.
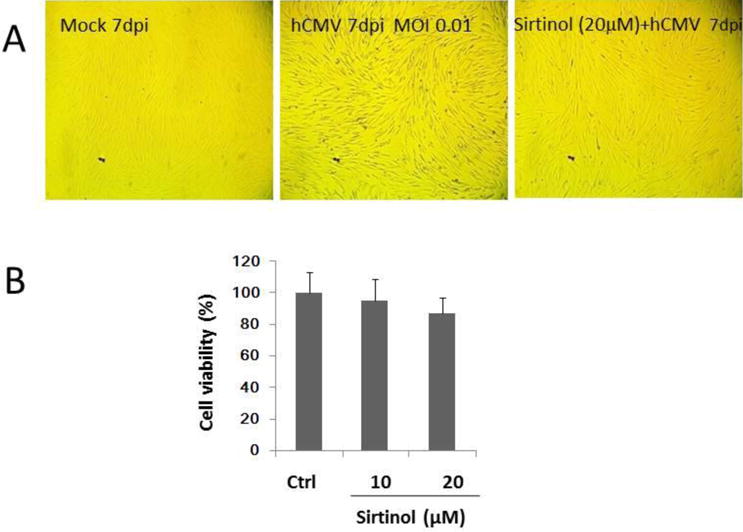
Previous studies in human breast cancer MCF-7 cells and MCF-7 and MCF-7 doxirubicin-resistant cells showed significant cytotoxicity of sirtinol at the concentration of 50μM or higher (Li et al., 2008). Here, we examined potential toxicity of sirtinol treatment to WI-38 fibroblasts. As shown in Fig. 1A, no significant sirtinol toxicity was detectable at the concentration of 10μM or 20μM by the MTT assay. In addition, cells treated with sirtinol (at 20μM) showed no significant morphological changes or evidence of cell damage compared to the mock infected controls (Fig. 1B, right versus left panels).
Fig. 1.
Effect of sirtinol treatment on cell viability of low passage WI-38 cells (30PD) (panel A) and effects of hCMV infection alone or hCMV infection with pretreatment of sirtinol on morphological changes of low passage WI-38 cells (panel B). For panel A, WI-38 cells (3×104) were cultured in DEME-0.2% FBS for 48–72 h and then treated with sirtinol at indicated concentration for 48 h and then with MTT for 4 h. The reaction was stopped by DMSO and cell viability was determined spectrophotometrically at 490 nm. For panel B, under the same culture condition, WI-38 cells were pretreated with sirtinol (20μM) for 2h followed by hCMV inoculation at MOI of 0.01 and cultured for additional 7 days. Representative photographs shown are from 4 repeated experiments taken at day 7 post infection (dpi) using a digital camera with experimental conditions stated on each photograph.
We then investigated the effect of sirtinol treatment on hCMV infection. As shown in Fig. 1B, cells infected by hCMV at MOI of 0.01 demonstrated significant morphological changes and plaque formation at 7 dpi (middle panel), while cells treated with sirtinol (at 20μM) had similar morphology as the mock control with no plaque formation.
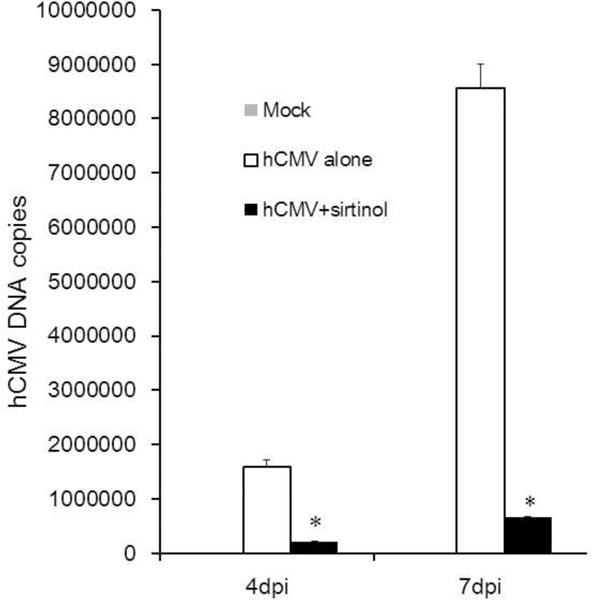
Next, we evaluated hCMV viral replication by qPCR in the presence or absence of sirtinol at 4 and 7 dpi. As shown in Fig. 2, sirtinol treatment dramatically reduced hCMV viral copies with over 95% suppression of viral replication at both 4 dpi and 7 dpi, demonstrating potent anti-hCMV activity of sirtinol.
Fig. 2.
Effects of sirtinol on hCMV viral replication in WI-38 cells. Low passage WI-38 cells (30PD) (3×104) were cultured in DEME-0.2% FBS for 48–72 h and then treated with sirtinol (20μM) for 2h before hCMV inoculation (MOI of 0.01). Cells were harvested at indicated time points for viral DNA copies determined using qPCR. Data were obtained from three independent experiments. *P<0.001 vs hCMV alone.
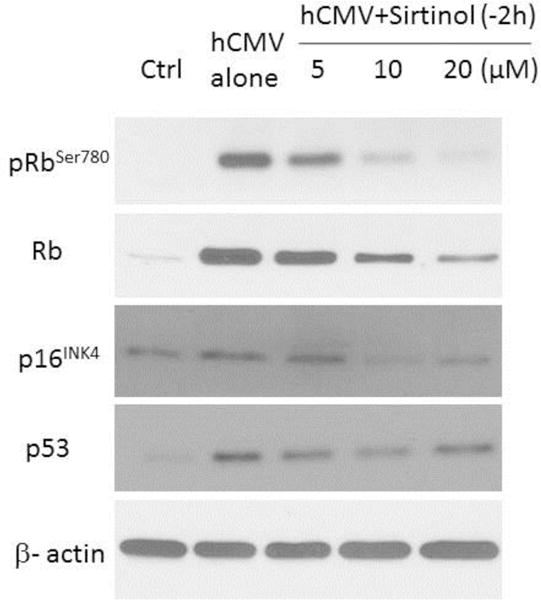
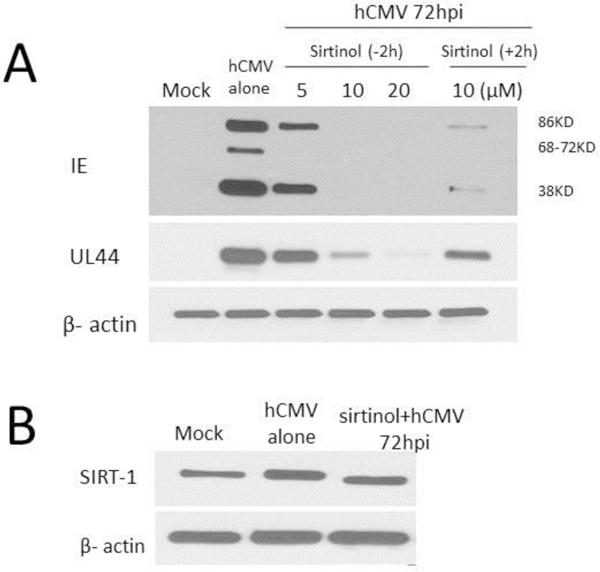
We further tested effects of sirtinol on protein expression of hCMV IE and early (UL44) genes through Western blot analysis. As shown in Fig. 3, sirtinol suppressed hCMV protein expression in a dose-dependent fashion (complete suppression of the 68–72-kD components of the IE at the concentration of 5μM and all three components at 10μM and 20μM) at 72 hpi when sirtinol was added 2 hours before hCMV inoculation (−2 h). While complete suppression of the 68–72-kD component expression at 72 hpi was still achieved when sirtinol (at 10μM) was added 2 hours after hCMV inoculation (+2 h), such delay led to weaker suppressive effects of sirtinol on the expression of the 86 and 38-kD component of the IE and early (UL44) protein.
Fig. 3.
Effects of sirtinol on expression of hCMV viral immediate early (IE) and early UL44 protein expression in young WI-38 fibroblasts (30 PD). Serum starved low passage WI-38 cells (30PD) were treated with sirtinol at the indicated concentrations added 2h before (−2h) or at 10μM added 2h after (+2h) hCMV inoculation (MOI of 0.01), and then were harvest at 72 hour post infection (hpi) for protein expression using Western blot analysis. Beta-actin expression levels are shown in both panels as loading control.
3.2. Sirtinol suppresses hCMV-induced activation of molecular mechanisms of senescence
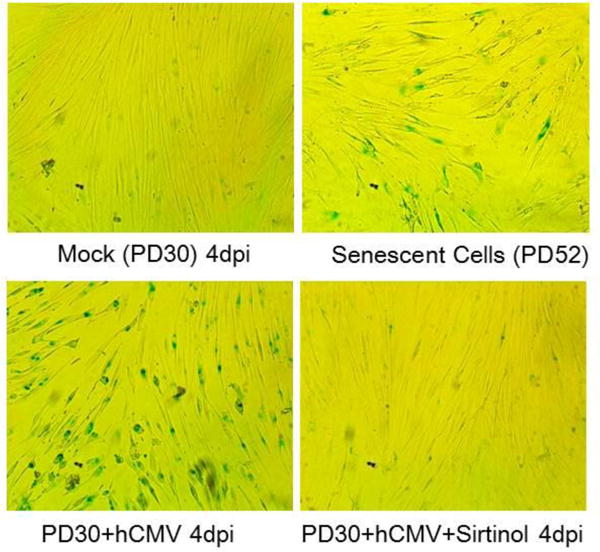
It should be noted that hCMV infection was carried out in growth arrested low-passage or young WI38 cells synchronized in G0 phase of cell cycle. To distinguish from the term cellular senescence which is defined as irreversible cell cycle arrest induced in proliferating cells, hCMV-induced phenotype in quiescent cells observed in the current study is referred as activation of molecular mechanisms of senescence. To investigate the impact of sirtinol on hCMV-induced activation of molecular mechanisms of senescence, first, we used SA-β-Gal staining to examine whether hCMV infection would lead to the development of this phenotype in low passage or young WI-38 fibroblasts at population doubling 30 (PD30). This was based on the previously observation by Noris and colleagues that hCMV infected low passage HELF and HDF cells manifested premature senescent phenotype by positive SA-β-Gal staining (Noris et al., 2002). GLB1 gene encodes β-galactosidase and hCMV Towne strain does not contain GLB1 gene sequence, excluding the possibility that hCMV infection itself causes spurious positive SA-β-Gal staining. As shown in Fig. 4, low passage WI-38 fibroblasts at PD30 with hCMV infection at MOI of 0.01 showed over 90% SA-β-Gal positive staining at 4 dpi (lower left panel), similar to or higher than that of high passage, replicative senescent cells (PD52, upper right panel), while mock infected low passage cells showed only sporadic staining (upper left panel).
Fig. 4.
SA-β-Gal staining ofWI-38 cells under the following conditions: replicative senescent cells at 52 population doublings (52 PD, upper right panel) as a positive control; Low passage or young cells at PD30 either with Mock infection control (upper left panel), HCMV infection alone at MOI of 0.01 (lower left panel), or same hCMV infection with pretreatment of sirtinol (20μM, lower right panel), all at 4 dpi. Representative photos shown are from three repeated experiments.
We then investigated expression of retinoblastoma (Rb), phosphorylated Rb (pRb Ser780), p16INK4, and p53, the critical molecules in cell cycle and cellular senescence. In the absence of hCMV infection, these molecules were not detected or expressed at low level (“Mock” lane, Fig. 5 panel A). hCMV infection induced or upregulated expression of these senescent molecules with the induction of pRbSer780, Rb, and p53 being most profound (“hCMV alone” lane, Fig. 5 panel A). As shown in Fig. 5 panel A (“hCMV+Sirtinol” lanes), sirtinol had significant suppressive effect of hCMV-induced expression of Rb, pRb Ser780, p16INK4, and p53 with stronger suppression demonstrated at the concentration of 10μM or 20μM. Quantification of relative expression levels under these experimental conditions is shown in Fig. 5 panel B. Taken together, these experiments demonstrated potent suppressive effect of sirtinol on hCMV-induced activation of molecular mechanisms of senescence including expression of senescence-associated proteins.
Fig. 5.
(A) Effects of sirtinol on hCMV-induced expression of Rb, pRb Ser780, p16INK4, and p53 in WI-38 fibroblasts at 72 hours post infection (hpi). Serum starved low passage WI-38 cells (PD30) were treated by sirtinol at indicated concentration added 2h before hCMV inoculation (MOI 0.01) (−2h) and then were harvested at 72 hpi for expression of the above molecules using Western blot analysis. Beta-actin expression level is shown as loading control. Representative images were acquired from three different experiments. (B) Quantitative analysis of the protein levels of Rb, pRbSer780, p16INK4, and p53. Bars represent relative protein levels counted as D1/D0 (the value for Mock was set as 1.0), where D0 and D1 stand for the optical density of beta-actin ladder and sample ladder, respectively. The optical density for each ladder was calculated by Image J soft ware. Data were obtained from three independent experiments. *P < 0.05 versus Mock group; **P < 0.01 versus Mock group; #P < 0.05 versus hCMV alone group; ##P < 0.01 versus hCMV alone group.
3.3. Sirtinol suppresses hCMV-induced ROS production
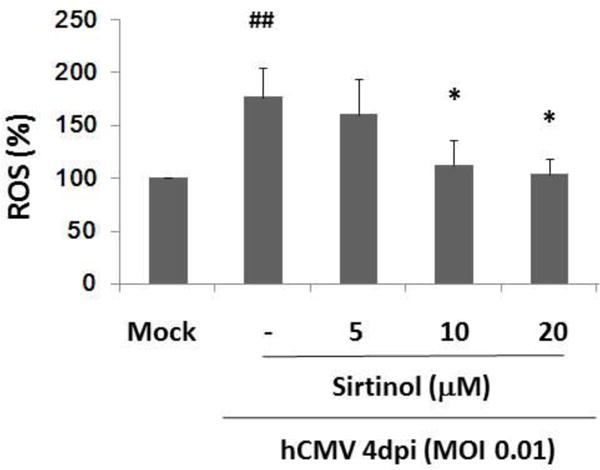
Oxidative stress has been a major theory of ageing. It postulates that detrimental molecules such as reactive oxygen species (ROS) produced and accumulated over time lead to oxidative damages to cellular macromolecules and contribute to the decline of cellular function and progressive organism ageing (Harman, 1956; Loeb et al., 2005). We and others have demonstrated that oxidative damage induces cellular senescence and reduction of ROS production can protect cells from developing senescence (Mao et al., 2010; Toussaint et al., 2000). In rat smooth muscle cells, hCMV infection was demonstrated to induce production of reactive oxygen intermediates that might be important for CMV immediate early (IE) gene expression and viral replication (Speir et al., 1996). Here, we investigated whether hCMV infection induced ROS production in young WI-38 fibroblasts. As shown in Fig. 6, hCMV infection induced significantly increased ROS production in the absence of sirtinol. Sirtinol at the concentration of 10μM or 20μM suppressed hCMV-induced ROS production almost down to the production level observed under mock control.
Fig. 6.
Effects of sirtinol on hCMV-induced ROS production in low passage WI-38 cells (30PD). Serum starved young WI-38 cells (30PD) were mock infection treated, hCMV infection alone (MOI 0.01), or pretreated with sirtinol at indicated concentrations added 2h before hCMV inoculation (MOI 0.01), and then were harvested at 4 dpi for ROS measurement by Flow cytometry analysis using probe H2DCFDA (% of ROS production shown on the Y axis). Data were obtained from three independent experiments. ##P<0.01, vs Mock infection group;*P<0.05, vs hCMV infection alone group.
4. Discussion
This study, for the first time, reports surprising inhibitory effect of sirtinol on hCMV infection in human diploid fibroblasts. This potent suppression is further supported by the impact of sirtinol of the cellular and molecular consequences of hCMV infection, namely, hCMV-induced activation of molecular mechanisms of senescence and ROS production.
As hCMV infection was conducted in arrested cells, the observed phenotype induced by hCMV infection is referred as activation of molecular mechanisms of senescence. Data on hCMV-induced activation of molecular mechanisms of senescence and ROS production described in this study, while confirmatory to previous studies (Noris et al., 2002; Wolf et al., 2012; Speir et al., 1996) support the notion that hCMV infection has broad impact on cellular and molecular processes of ageing beyond T-cell immunosenescence. Such impact, in addition to its direct negative consequences to ageing, may be linked to immune dysregluation and poor health in older adults. For example, senescent cells have been identified as a major source of chornic inflammation (Freund et al., 2010) and chronic inflammation has been recognized as a major contributor to frailty and other age-relate chronic conditions (Franceschi and Campisi, 2014; Leng et al., 2007). Consistent with this, in the longitudinal study cited above, older women with hCMV viral DNA in their peripheral blood monocytes had elevated circulating IL-6 levels at two time points over 12 years apart (Li et al., 2014b).
Perhaps the most striking and novel aspect of this study is the anti-hCMV activity of sirtinol. Evers and colleagues (Evers et al., 2004) have shown anti-CMV activity of resveratrol, a prototype of sirtuin activator that primarily stimulates Sirt-1 (Villalba and Alcain, 2012). The anti-hCMV properties of human sirtuins have further demonstrated by recent observation that knockdown of each of the 7 sirtuins with siRNA (siSIRT1 to −7) enhanced hCMV viral replication (Koyuncu et al., 2014). Sirtinol, a well-characterized sirtuin inhibitor (Grozinger et al., 2001; Mai et al., 2005; Villalba and Alcain, 2012), surprisingly displayed potent anti-hCMV activity. While resveratrol is known to inhibit replicative senescence and have anti-oxidative property (Giovannelli et al., 2011; Liu et al., 2015), here we have further shown, also for the first time, the suppressive effects of sirtinol on hCMV-induced activation of molecular mechanisms of senescence and ROS production. These suppressive effects are likely mediated by molecular mechanisms other than SIRT-1 pathway as sirtinol at the tested concentration showed minimal or no suppression on SIRT-1 expression (data not shown). This possibility is further suggested by previous studies in which IC50 value of sirtinol was 131μM for its inhibition of SIRT-1 (Grozinger et al., 2001; Mai et al., 2005) and sirtinol concentration in the current study was far below these IC50 values. One such non-SIRT-1-mediated mechanism could be the suppression of hCMV-induced p16INK4 expression by sirtinol as hCMV-induced p16 NK4 expression has been shown to be required for optimal hCMV replication (Zannetti et al., 2006). Whether SIRT-2 inhibition is involved in sirtinol’s anti-hCMV effect deserves further investigation. Potential effects of sirtinol on a number of newly defined molecular pathways that regulate hCMV replication (Roy and Arav-Boger, 2014) will also need to be investigated.
Suppressive effects of sirtinol on hCMV-induced activation of molecular mechanisms of senescence and ROS production could be secondary to its suppression on hCMV infection itself. One potential mechanism in particularly could be the suppression of the expression of hCMV 86-kD IE protein by sirtinol as this hCMV IE protein is known to induce premature senescence (Noris et al., 2002; Wolf et al., 2012). Suppression of hCMV-induced expression of p16INK4 and p53 could be another potential mechanism as p16INK4a and p53/p21WAF1pathways play important roles in initiation of cellular senescence. The atypical response of increase in pRb phosphorylation to hCMV infection was also observed by Noris and colleagues (Noris et al., 2002). One possible explanation is that hCMV can cause cell cycle arrest and senescence, at the same time, induces S phase-promoting activities required for DNA synthesis to the advantage of viral replication. Phosphorylation of pRb causes it to lose its growth-inhibitory properties and converts it into perhaps an inert state. Important questions including why increased p53 expression did not interfere with hCMV stimulation of pRb phosphorylation deserve further investigation. Of note, sirtinol itself has been shown to induce senescence in human cancer cells (Ota et al., 2006) and is considered, among other sirtuin inhibitors, for its potential as anticancer agent (Hu et al., 2014). However, the concentration of sirtinol required for such effect was 100μM, 5 times higher than the concentration tested in this study.
The observation of sirtinol as a novel and potent anti-hCMV agent provides a basis for potential development of sirtinol or its derivatives as effective anti-hCMV therapies. The need for the development of novel interventional strategies is urgent both for the treatment of clinically overt hCMV infection as well as for the alleviation of adverse impact of chronic CMV infection on immunity and health of older adults. This is because currently available anti-hCMV therapies primarily rely on viral DNA polymerase inhibitors. They have several important drawbacks including dose-limiting renal (foscarnet and cidoforvir) or bone marrow (ganciclovir) toxicities as well as the clinical prevalence of drug-resistance and cross-resistant hCMV strains (Baldanti and Gerna, 2003; Griffiths, 2002).
This study has limitations. For example, the study described here was conducted in WI-38, a commonly used human diploid fibroblast cell line. Findings from this study may not be generalizable to other cell types that hCMV is also known to infect. However, results from our experiments in THP-1 derived macrophages were consistent with findings obtained in WI-38 cells (data not shown). Another limitation is that we conducted the experiments only with hCMV Towne strain. As hCMV is well known for the variations between laboratory and clinical strains as well as among clinical varaints (Wilkinson et al., 2015), the results of this study will need to be validated with other hCMV strains. Despite of these limitations, our findings suggest that sirtinol has potent suppressive effect of hCMV infection and hCMV-induced activation of molecular mechanisms of senescence and ROS production. They also support broad impact of hCMV infection on cellular and molecular processes of ageing beyond T-cell immunosenescence This study and subsequent studies may pave the way for potential development of sirtinol or its derivatives as effective anti-hCMV therapies.
Highlights.
Sirtinol suppresses hCMV replication and protein expression in human fibroblasts
Sirtinol suppresses hCMV-induced cellular senescence and protein expression
Sirtinol suppresses hCMV-induced production of reactive oxygen species
Acknowledgments
Dr. Genxiang Mao is an Irma and Paul Milstein Program for Senior Health fellow supported by the MMAAP Foundation (http://www.mmaapf.org).
Funding:
This work was supported in part by NIH grants R21-AG-043874 and R01AI108907 as well as funding from the Milstein Medical Asian American Partnership (MMAAP) Foundation (http://www.mmaapf.org) to Dr. Sean X. Leng.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiello AE, Haan MN, Pierce CM, Simanek AM, Liang J. Persistent infection, inflammation, and functional impairment in older Latinos. J Gerontol A Biol Sci Med Sci. 2008;63:610–618. doi: 10.1093/gerona/63.6.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R. Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin Infect Dis. 2004;39:233–239. doi: 10.1086/421999. [DOI] [PubMed] [Google Scholar]
- Baldanti F, Gerna G. Human cytomegalovirus resistance to antiviral drugs: diagnosis, monitoring and clinical impact. J Antimicrob Chemother. 2003;52:324–330. doi: 10.1093/jac/dkg354. [DOI] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Di Benedetto S, Derhovanessian E, Steinhagen-Thiessen E, Goldeck D, Muller L, Pawelec G. Impact of age, sex and CMV-infection on peripheral T cell phenotypes: results from the Berlin BASE-II Study. Biogerontology. 2015;16:631–643. doi: 10.1007/s10522-015-9563-2. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers DL, Wang X, Huong SM, Huang DY, Huang ES. 3,4′,5-Trihydroxy-trans-stilbene (resveratrol) inhibits human cytomegalovirus replication and virus-induced cellular signaling. Antiviral Res. 2004;63:85–95. doi: 10.1016/j.antiviral.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S4–9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannelli L, Pitozzi V, Jacomelli M, Mulinacci N, Laurenzana A, Dolara P, Mocali A. Protective effects of resveratrol against senescence-associated changes in cultured human fibroblasts. J Gerontol A Biol Sci Med Sci. 2011;66:9–18. doi: 10.1093/gerona/glq161. [DOI] [PubMed] [Google Scholar]
- Griffiths PD. The 2001 Garrod lecture. The treatment of cytomegalovirus infection. J Antimicrob Chemother. 2002;49:243–253. doi: 10.1093/jac/49.2.243. [DOI] [PubMed] [Google Scholar]
- Grozinger CM, Chao ED, Blackwell HE, Moazed D, Schreiber SL. Identification of a class of small molecule inhibitors of the sirtuin family of NAD-dependent deacetylases by phenotypic screening. J Biol Chem. 2001;276:38837–38843. doi: 10.1074/jbc.M106779200. [DOI] [PubMed] [Google Scholar]
- Hadrup SR, Strindhall J, Kollgaard T, Seremet T, Johansson B, Pawelec G, thor Straten P, Wikby A. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol. 2006;176:2645–2653. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hu J, Jing H, Lin H. Sirtuin inhibitors as anticancer agents. Future Med Chem. 2014;6:945–966. doi: 10.4155/fmc.14.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N, Hislop A, Gudgeon N, Cobbold M, Khanna R, Nayak L, Rickinson AB, Moss PA. Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J Immunol. 2004;173:7481–7489. doi: 10.4049/jimmunol.173.12.7481. [DOI] [PubMed] [Google Scholar]
- Koch S, Larbi A, Ozcelik D, Solana R, Gouttefangeas C, Attig S, Wikby A, Strindhall J, Franceschi C, Pawelec G. Cytomegalovirus infection: a driving force in human T cell immunosenescence. Ann N Y Acad Sci. 2007;1114:23–35. doi: 10.1196/annals.1396.043. [DOI] [PubMed] [Google Scholar]
- Koyuncu E, Budayeva HG, Miteva YV, Ricci DP, Silhavy TJ, Shenk T, Cristea IM. Sirtuins are evolutionarily conserved viral restriction factors. MBio. 2014;5:e02249. doi: 10.1128/mBio.02249-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BY, Han JA, Im JS, Morrone A, Johung K, Goodwin EC, Kleijer WJ, DiMaio D, Hwang ES. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 2006;5:187–195. doi: 10.1111/j.1474-9726.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- Leng SX, Li H, Xue QL, Tian J, Yang X, Ferrucci L, Fedarko N, Fried LP, Semba RD. Association of detectable cytomegalovirus (CMV) DNA in monocytes rather than positive CMV IgG serology with elevated neopterin levels in community-dwelling older adults. Exp Gerontol. 2011a;46:679–684. doi: 10.1016/j.exger.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng SX, Qu T, Semba RD, Li H, Yao X, Nilles T, Yang X, Manwani B, Walston JD, Ferrucci L, Fried LP, Margolick JB, Bream JH. Relationship between cytomegalovirus (CMV) IgG serology, detectable CMV DNA in peripheral monocytes, and CMV pp65(495–503)-specific CD8+ T cells in older adults. Age (Dordr) 2011b;33:607–614. doi: 10.1007/s11357-011-9205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55:864–871. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- Li H, Mao G, Carlson J, Leng SX. A novel flow cytometry-based tool for determining the efficiency of human cytomegalovirus infection in THP-1 derived macrophages. J Virol Methods. 2015;221:127–130. doi: 10.1016/j.jviromet.2015.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Margolick JB, Bream JH, Nilles TL, Langan S, Bui HT, Sylwester AW, Picker LJ, Leng SX. Heterogeneity of CD4+ and CD8+ T-cell responses to cytomegalovirus in HIV-infected and HIV-uninfected men who have sex with men. J Infect Dis. 2014a;210:400–404. doi: 10.1093/infdis/jiu093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Weng P, Najarro K, Xue QL, Semba RD, Margolick JB, Leng SX. Chronic CMV infection in older women: longitudinal comparisons of CMV DNA in peripheral monocytes, anti-CMV IgG titers, serum IL-6 levels, and CMV pp65 (NLV)-specific CD8(+) T-cell frequencies with twelve year follow-up. Exp Gerontol. 2014b;54:84–89. doi: 10.1016/j.exger.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu R, Zhang XM, Li DD, He QY. Mechanism of apoptosis induced by SIRT1 deacetylase inhibitors in human breast cancer MCF-7 drug-resistant cells. Acta Pharmaceutica Sinica. 2008;43:1003–1010. [PubMed] [Google Scholar]
- Liu T, Qi H, Ma L, Liu Z, Fu H, Zhu W, Song T, Yang B, Li G. Resveratrol Attenuates Oxidative Stress and Extends Life Span in the Annual Fish Nothobranchius guentheri. Rejuvenation Res. 2015;18:225–233. doi: 10.1089/rej.2014.1618. [DOI] [PubMed] [Google Scholar]
- Loeb LA, Wallace DC, Martin GM. The mitochondrial theory of aging and its relationship to reactive oxygen species damage and somatic mtDNA mutations. Proc Natl Acad Sci U S A. 2005;102:18769–18770. doi: 10.1073/pnas.0509776102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai A, Massa S, Lavu S, Pezzi R, Simeoni S, Ragno R, Mariotti FR, Chiani F, Camilloni G, Sinclair DA. Design, synthesis, and biological evaluation of sirtinol analogues as class III histone/protein deacetylase (Sirtuin) inhibitors. J Med Chem. 2005;48:7789–7795. doi: 10.1021/jm050100l. [DOI] [PubMed] [Google Scholar]
- Mao GX, Wang Y, Qiu Q, Deng HB, Yuan LG, Li RG, Song DQ, Li YY, Li DD, Wang Z. Salidroside protects human fibroblast cells from premature senescence induced by H(2)O(2) partly through modulating oxidative status. Mech Ageing Dev. 2010;131:723–731. doi: 10.1016/j.mad.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Noris E, Zannetti C, Demurtas A, Sinclair J, De Andrea M, Gariglio M, Landolfo S. Cell cycle arrest by human cytomegalovirus 86-kDa IE2 protein resembles premature senescence. J Virol. 2002;76:12135–12148. doi: 10.1128/JVI.76.23.12135-12148.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson J, Wikby A, Johansson B, Lofgren S, Nilsson BO, Ferguson FG. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech Ageing Dev. 2000;121:187–201. doi: 10.1016/s0047-6374(00)00210-4. [DOI] [PubMed] [Google Scholar]
- Ota H, Tokunaga E, Chang K, Hikasa M, Iijima K, Eto M, Kozaki K, Akishita M, Ouchi Y, Kaneki M. Sirt1 inhibitor, Sirtinol, induces senescence-like growth arrest with attenuated Ras-MAPK signaling in human cancer cells. Oncogene. 2006;25:176–185. doi: 10.1038/sj.onc.1209049. [DOI] [PubMed] [Google Scholar]
- Ouyang Q, Wagner WM, Voehringer D, Wikby A, Klatt T, Walter S, Muller CA, Pircher H, Pawelec G. Age-associated accumulation of CMV-specific CD8+ T cells expressing the inhibitory killer cell lectin-like receptor G1 (KLRG1) Exp Gerontol. 2003;38:911–920. doi: 10.1016/s0531-5565(03)00134-7. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Akbar A, Caruso C, Solana R, Grubeck-Loebenstein B, Wikby A. Human immunosenescence: is it infectious? Immunol Rev. 2005;205:257–268. doi: 10.1111/j.0105-2896.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourgheysari B, Khan N, Best D, Bruton R, Nayak L, Moss PA. The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire. J Virol. 2007;81:7759–7765. doi: 10.1128/JVI.01262-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am J Epidemiol. 2010;172:363–371. doi: 10.1093/aje/kwq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Arav-Boger R. New cell-signaling pathways for controlling cytomegalovirus replication. Am J Transplant. 2014;14:1249–1258. doi: 10.1111/ajt.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005;53:747–754. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- Smith JJ, Kenney RD, Gagne DJ, Frushour BP, Ladd W, et al. Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Syst Biol. 2009;3:31. doi: 10.1186/1752-0509-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MS, Bentz GL, Alexander JS, Yurochko AD. Human cytomegalovirus induces monocyte differentiation and migration as a strategy for dissemination and persistence. J Virol. 2004;78:4444–4453. doi: 10.1128/JVI.78.9.4444-4453.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speir E, Shibutani T, Yu ZX, Ferrans V, Epstein SE. Role of reactive oxygen intermediates in cytomegalovirus gene expression and in the response of human smooth muscle cells to viral infection. Circ Res. 1996;79:1143–1152. doi: 10.1161/01.res.79.6.1143. [DOI] [PubMed] [Google Scholar]
- Stratton KDJ, Lawrence R. Vaccines for the 21st Century: A Tool for Decision Making. The National Academies Press; 2000. [PubMed] [Google Scholar]
- Strindhall J, Nilsson BO, Lofgren S, Ernerudh J, Pawelec G, Johansson B, Wikby A. No Immune Risk Profile among individuals who reach 100 years of age: findings from the Swedish NONA immune longitudinal study. Exp Gerontol. 2007;42:753–761. doi: 10.1016/j.exger.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint O, Medrano EE, von Zglinicki T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp Gerontol. 2000;35:927–945. doi: 10.1016/s0531-5565(00)00180-7. [DOI] [PubMed] [Google Scholar]
- Vescovini R, Biasini C, Fagnoni FF, Telera AR, Zanlari L, Pedrazzoni M, Bucci L, Monti D, Medici MC, Chezzi C, Franceschi C, Sansoni P. Massive load of functional effector CD4+ and CD8+ T cells against cytomegalovirus in very old subjects. J Immunol. 2007;179:4283–4291. doi: 10.4049/jimmunol.179.6.4283. [DOI] [PubMed] [Google Scholar]
- Villalba JM, Alcain FJ. Sirtuin activators and inhibitors. Biofactors. 2012;38:349–359. doi: 10.1002/biof.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GC, Kao WH, Murakami P, Xue QL, Chiou RB, Detrick B, McDyer JF, Semba RD, Casolaro V, Walston JD, Fried LP. Cytomegalovirus infection and the risk of mortality and frailty in older women: a prospective observational cohort study. Am J Epidemiol. 2010;171:1144–1152. doi: 10.1093/aje/kwq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikby A, Nilsson BO, Forsey R, Thompson J, Strindhall J, Lofgren S, Ernerudh J, Pawelec G, Ferguson F, Johansson B. The immune risk phenotype is associated with IL-6 in the terminal decline stage: findings from the Swedish NONA immune longitudinal study of very late life functioning. Mech Ageing Dev. 2006;127:695–704. doi: 10.1016/j.mad.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Wilkinson GW, Davison AJ, Tomasec P, Fielding CA, Aicheler R, Murrell I, Seirafian S, Wang EC, Weekes M, Lehner PJ, Wilkie GS, Stanton RJ. Human cytomegalovirus: taking the strain. Med Microbiol Immunol. 2015;204:273–284. doi: 10.1007/s00430-015-0411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J, Weinberger B, Grubeck-Loebenstein B. The immunoregulatory effects of CMV-infection in human fibroblasts and the impact on cellular senescence. Immun Ageing. 2012;9:1. doi: 10.1186/1742-4933-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wreghitt TG, Teare EL, Sule O, Devi R, Rice P. Cytomegalovirus infection in immunocompetent patients. Clin Infect Dis. 2003;37:1603–1606. doi: 10.1086/379711. [DOI] [PubMed] [Google Scholar]
- Zannetti C, Mondini M, De Andrea M, Caposio P, Hara E, Peters G, Gribaudo G, Gariglio M, Landolfo S. The expression of p16INK4a tumor suppressor is upregulated by human cytomegalovirus infection and required for optimal viral replication. Virology. 2006;349:79–86. doi: 10.1016/j.virol.2006.01.042. [DOI] [PubMed] [Google Scholar]
- Zarzuelo MJ, López-Sepúlveda R, Sánchez M, Romero M, Gómez-Guzmán M, Ungvary Z, Pérez-Vizcaíno F, Jiménez R, Duarte J. SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: implications for vascular aging. Biochem Pharmacol. 2013;85:1288–1296. doi: 10.1016/j.bcp.2013.02.015. [DOI] [PubMed] [Google Scholar]


