Abstract
Background
The higher risk of heart failure (HF) in African-American and Hispanic women compared to White women is related to the higher burden of risk factors (RF) in minorities. However, it is unclear if there are differences in the association between the number of RF for HF and the risk of development of HF and death within racial/ethnic groups.
Methods and Results
In the Women’s Health Initiative (1993–2010), African-American (n=11,996), White (n=18,479), and Hispanic (n=5,096) women with 1, 2, or 3+ baseline RF were compared to women with 0 RF within their respective racial/ethnic groups to assess risk of developing HF or all-cause mortality before and after HF, using survival analyses. After adjusting for age, socioeconomic status, and hormone therapy, the subdistribution hazard ratio (95% confidence interval) of developing HF increased as number of RF increased (p<0.0001, interaction of race/ethnicity and RF number p=0.18): African-Americans 1 RF: 1.80(1.01–3.20), 2 RF: 3.19(1.84–5.54), 3+ RF: 7.31(4.26–12.56); Whites 1 RF: 1.27(1.04–1.54), 2 RF: 1.95(1.60–2.36), 3+ RF: 4.07(3.36–4.93); Hispanics 1 RF: 1.72(0.68–4.34), 2 RF: 3.87(1.60–9.37), 3+ RF: 8.80(3.62–21.42). Risk of death before developing HF increased with subsequent RF (p<0.0001), but differed by racial/ethnic group (interaction p=0.001). The number of RF was not associated with the risk of death after developing HF in any group (p=0.25, interaction p=0.48).
Conclusions
Among diverse racial/ethnic groups, an increase in the number of baseline RF was associated with higher risk of HF and death before HF, but was not associated with death after HF. Early RF prevention may reduce the burden of HF across multiple racial/ethnic groups.
INTRODUCTION
Heart failure (HF) is one of the leading causes of morbidity and mortality in older women and is expected to rise in prevalence by 25% over the next two decades due to an aging United States (U.S.) population.1–4 Despite advances in therapy, HF continues to disproportionately affect African-American women compared to White women.2,5–7 In general, socioeconomic factors and predisposing risk factors (RF) including hypertension and diabetes have been identified as important contributors to this excess risk.2,6,8 Despite data on the incidence and prevalence of HF in women, which demonstrate higher risk of HF in African-American and Hispanic women, data examining the differential development of HF and subsequent risk of death in these groups are lacking.2,9
African-American and Hispanic women have higher prevalence of established RF for HF, including atherosclerosis, hypertension, diabetes, obesity, and sedentary activity.2,3 The population attributable risk for HF based upon individual RF has been widely described in Whites, small populations of African-Americans, and to a lesser extent in Hispanics.2,10–12 In most studies, individual RF have been associated with greater risk of developing HF in African-Americans and Hispanics.2 Yet, the impact of these RF may be much greater in African-American and Hispanic women who often have multiple RF.2 A strong target for reducing racial/ethnic disparities in HF may include identifying the risk of HF and death before and after developing HF, dependent upon the number of RF.
To address these questions, we examined HF development among African-American, White, and Hispanic women, using data from the Women’s Health Initiative (WHI) study, a large population-based study of postmenopausal women. Within each racial/ethnic group, we tested the hypothesis that compared to women with 0 baseline RF, women with subsequently higher number of baseline RF would have increased risk of developing HF and dying before or after developing HF.
METHODS
The data, analytic methods, and study materials are available to other researchers for purposes of reproducing the results or replicating the procedure upon request and approval of the Women’s Health Initiative Publications and Presentations Committee.
Data Source
The WHI is a National Heart, Lung, and Blood Institute supported study of U.S. postmenopausal women followed for over 20 years for evaluation of cardiovascular disease, cancer, and osteoporosis.13 The original WHI population is one of the largest female-only population studies, including 161,808 diverse racial/ethnic women who were enrolled for either randomized clinical trials or an observational study.14 The study includes self-reported medical information collected through interviews and surveys, anthropometric measurements by WHI personnel, and review of medical records for outcome determination.13
Study Cohort
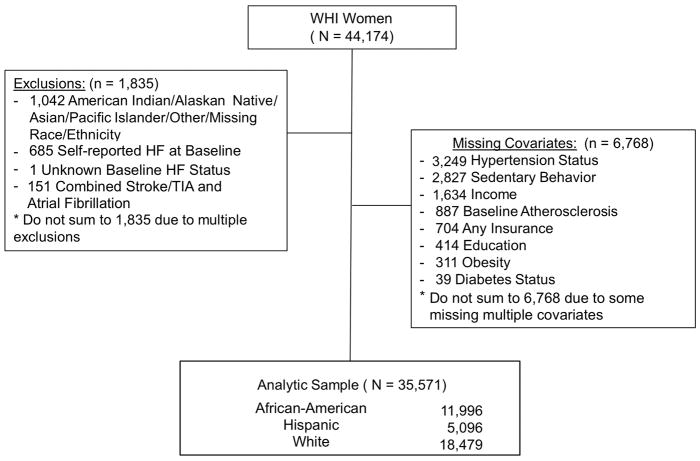
A sub-cohort of the original WHI population was selected to study the epidemiology of HF in postmenopausal women.5 This sample included 44,174 postmenopausal women, who underwent annual assessment for HF adjudication from baseline enrollment (1993–1998) through 2010. Race and ethnicity were self-identified as Non-Hispanic African-American (African-American), Hispanic, and Non-Hispanic White (White). This population included all participants who were randomized to the WHI hormone therapy trial (n=27,347) and an over-sampling of minorities to include all non-hormone trial African-American (n=11,880) and Hispanic (n=4,947) women. Adjudicated HF and death were as of data release of December 2014. Overlapping exclusions included: participants of other races/ethnicities due to their small number of events (n=1,042), self-report of HF or unknown HF at baseline (n=686), participants with stroke attributed to atrial fibrillation since the covariates could not be separated (n=151). Participants with missing covariates (n=6,768) were also excluded since the objective of this study was to assess risk based upon number of RF upon study enrollment. Patients with missing covariates had no difference in incident HF or death than the final analysis sample (Supplemental Table 1). The final sample included 35,571 participants (Figure 1). The study was approved by the human subjects review committee at each WHI participating institution, and all participants provided written informed consent.
Figure 1. Study Profile.
The final study sample excluded participants with missing covariates and races/ethnicities that did not include African-American, White, and Hispanic women. HF indicates heart failure; TIA, transient ischemic attack; WHI, Women’s Health Initiative.
Outcomes of interest
Participants were followed until development of the following outcomes: incident HF requiring hospitalization and all-cause mortality before or after developing HF. Data on incident HF was abstracted annually from medical records after self-report of hospitalizations. A trained committee adjudicated these hospitalizations as definite or probable HF based upon symptoms, physical exam, clinical data, and therapy during a hospitalization, which has been described elsewhere.3,15 All-cause mortality was collected during routine WHI follow-up by family report and death certificate.15
Explanatory Variables-RF for HF
Established RF for HF were defined per 2013 American College of Cardiology/American Heart Association Stage A classifications.3 RF for HF included having 1+ of the following risks in the absence of symptomatic HF: atherosclerosis (includes Stage B classifications of prior hospitalization for myocardial infarction since this is one of the largest etiologies of HF, prior percutaneous coronary intervention, prior coronary artery bypass graft surgery, history of carotid artery disease, stroke/transient ischemic attack or peripheral vascular disease), diabetes mellitus (self-report of physician diagnosis and taking hypoglycemic medications), hypertension (self-report and taking antihypertensive medications, systolic blood pressure ≥ 140 mmHg, or diastolic blood pressure ≥ 90 mmHg by measurement upon enrollment), obesity (body mass index ≥ 30 kg/m2 by measurement upon enrollment), and a recognized RF for HF not included as Stage A: sedentary activity (<500 Metabolic equivalent of task minute/week)16. Metabolic syndrome was not included but can be inferred from the component RF captured. Other established RF for HF were excluded since they were unavailable in the WHI dataset (cardiotoxin exposure and family history of cardiomyopathy). No baseline cardiac imaging was available for the population with RF for HF.
Statistical Analysis
Baseline patient characteristics were compared between race and ethnicities, using chi-square tests for categorical variables and analysis of variance for continuous variables. We consider three outcomes: (1) time to HF from baseline, (2) time to death before HF, and (3) time to death after HF. For (1), time to HF from baseline was defined as the number of days from enrollment to the first occurrence of HF. A participant was censored at last contact. Death was modelled as a competing risk for HF using Fine and Gray method, where subdistribution hazard was estimated 17. For (2), time to death before HF was defined as the number of days from enrollment to death. Follow-up for death was censored at last contact and we restrict participants to those who did not have incident HF during the follow-up. For (3) time to death after HF was defined as time from first incidence of HF to death, and participants were censored at last contact. Cox proportional hazard regression models were used for (2) and (3). Separate models were built for each outcome and three sets of models were fitted: (a) menopausal hormone therapy “control”: where menopausal hormone therapy was adjusted given increased risk of cardiovascular disease with menopausal hormone therapy irrespective of race/ethnicity.18; (b) age + menopausal hormone therapy: where age was added to the model (a); and (c) socioeconomic status + age + menopausal hormone therapy: where socioeconomic status (education, income, and insurance) was added to the model (b), given known racial/ethnic differences in HF presentation 3. First, models were fitted for all the participants with race/ethnicity as a covariate to estimate subdistribution hazard ratios (SHR) or hazard ratios (HR) and 95% confidence interval (CI) for the number of baseline RF (1, 2, or 3+) with 0 RF as reference for the outcomes of interest. Then interactions between race/ethnicity and number of baseline RF were tested to determine if risks varied by race/ethnicity. Finally, we built models within each race/ethnicity group to estimate SHR, HR and 95% CI for the number of baseline RF. Hispanic women were not assessed for all-cause mortality after HF since the event rate of incident HF was too low to yield precise estimates.
In the secondary analysis, the association between an individual RF and HF, death before HF, and death after HF were performed using model (c) described above plus adjustment for all other RF. Statistical analyses were performed using SAS 9.4 (Cary, NC), and the significance level was set at 0.05 for all tests.
RESULTS
Baseline Characteristics
During the enrollment period of 1993–1998, 35,571 women (34% African-American, 52% White, 14% Hispanic) were followed for the development of HF (Table 1). Racial/ethnic minority women were younger than White women (African-American mean age 61.4, White 64.0, Hispanic 60.2, p<0.0001). The majority of patients had 1+ RF at baseline across race and ethnicity, but African-Americans had the most RF at baseline. College education levels were higher among African-Americans (63.2%) and Whites (61.9%) than Hispanics (46.9%). Approximately half of women from all race/ethnicities had an annual income of <$35,000, and over 80% of women from all race/ethnicities had some form of health insurance.
Table 1.
Baseline Characteristics
| Characteristics RF for HF N |
African-American N (%) 11,996 |
White N (%) 18,479 |
Hispanic N (%) 5,096 |
p-value |
|---|---|---|---|---|
| Age years, Mean(SD) | 61.4 (7.1) | 64.0 (7.1) | 60.2 (6.8) | <.0001 |
| Number of RF | <.0001 | |||
| 0 | 1,315 (11.0) | 4,443 (24.0) | 1,062 (20.8) | |
| 1 | 3,301 (27.5) | 6,578 (35.6) | 1,872 (36.7) | |
| 2 | 3,859 (32.2) | 4,809 (26.0) | 1,387 (27.2) | |
| 3+ | 3,521 (29.4) | 2,649 (14.3) | 775 (15.2) | |
| Atherosclerosis | 1,128 (9.4) | 1,102 (6.0) | 316 (6.2) | <.0001 |
| Carotid Disease | 34 (0.3) | 47 (0.3) | 11 (0.2) | 0.72 |
| MI | 341 (2.8) | 358 (1.9) | 48 (0.9) | <.0001 |
| PTCA/CABG | 187 (1.6) | 292 (1.6) | 44 (0.9) | 0.0005 |
| PVD | 383 (3.2) | 273 (1.5) | 121 (2.4) | <.0001 |
| Stroke/TIA | 446 (3.7) | 408 (2.2) | 135 (2.6) | <.0001 |
| Treated Diabetes | 1,364 (11.4) | 820 (4.4) | 356 (7.0) | <.0001 |
| Hypertension | <.0001 | |||
| Never Hypertensive | 5,470 (45.6) | 12,735 (68.9) | 3,598 (70.6) | |
| Current/Untreated | 1,118 (9.3) | 1,591 (8.6) | 464 (9.1) | |
| Current/Treated | 5,408 (45.1) | 4,153 (22.5) | 1,034 (20.3) | |
| Obesity | 6,040 (50.4) | 6,729 (36.4) | 1,879 (36.9) | <.0001 |
| Sedentary Activity | 7,473 (62.3) | 10,259 (55.5) | 3,070 (60.2) | <.0001 |
| HT Study Arm | <.0001 | |||
| Not in HT | 9,769 (81.4) | 0 (0.0) | 3,914 (76.8) | |
| HT | 2,227 (18.6) | 18,479 (100.0) | 1,182 (23.2) | |
| Socioeconomic Variables | ||||
| Education | <.0001 | |||
| Less Than HS | 1,289 (10.7) | 911 (4.9) | 1,203 (23.6) | |
| HS/Vocational | 3,121 (26.0) | 6,118 (33.1) | 1,502 (29.5) | |
| Some College | 3,251 (27.1) | 5,423 (29.3) | 1,248 (24.5) | |
| College Graduate | 4,335 (36.1) | 6,027 (32.6) | 1,143 (22.4) | |
| Income | <.0001 | |||
| <$35,000 | 6,168 (51.3) | 8,799 (47.6) | 2,899 (56.9) | |
| $35,000–<$50,000 | 2,145(17.9) | 3,930 (21.3) | 817 (16.0) | |
| $50,000–<$75,000 | 1,994 (16.6) | 3,109 (16.8) | 657 (12.9) | |
| ≥$75,000 | 1,689 (14.1) | 2,641 (14.3) | 723 (14.2) | |
| Any Insurance | 11,009 (91.8) | 17,125 (92.7) | 4,137 (81.2) | <.0001 |
CABG indicates coronary artery bypass graft surgery; HF, heart failure; HS, high school; HT, hormone therapy; MI, myocardial infarction; PTCA, percutaneous transluminal coronary angioplasty; PVD, peripheral vascular disease; RF, risk factors; SD, standard deviation; TIA, transient ischemic attack.
Outcomes
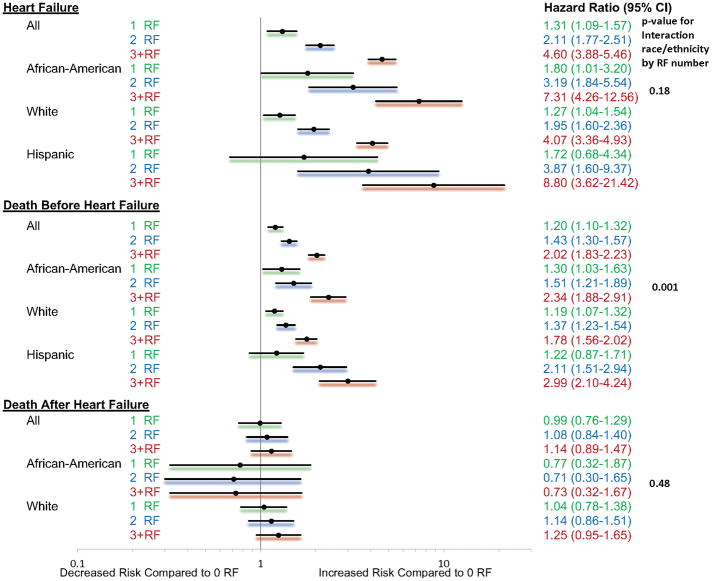
Over an average 13 years of follow-up, 4.4% (n=524) African-American, 6.3% (n=1,166) White women, and 1.8% (n=89) Hispanic women developed symptomatic HF requiring hospitalization. In the “control” which was adjusted for menopausal hormone therapy and fully adjusted models for age and socioeconomic status, having an additional RF was associated with a significantly higher risk of developing HF, compared to 0 RF [Table 2, Figure 2; SHR (95% CI): 1 RF: 1.31 (1.09–1.57); 2 RF: 2.11 (1.77–2.51); 3+ RF: 4.60 (3.88–5.46); p<0.0001]. An increasing number of RF increased the risk of HF irrespective of race/ethnicity (p=0.17 for interaction of race/ethnicity and number of RF). Within each racial/ethnicity group, in the fully adjusted model, compared to 0 RF, SHR (95%CI) of developing HF in African-Americans for 1, 2 and 3+ RF were 1.80 (1.01–3.20), 3.19 (1.84–5.54), and 7.31 (4.26–12.56), respectively. Among Whites, 1 RF [1.27 (1.04–1.54)], 2 RF [1.95 (1.60–2.36)] and 3+ RF [4.07 (3.36–4.93)] demonstrated a higher hazard of developing HF compared to 0 RF. Among Hispanics, 2 RF [3.87 (1.60–9.37)] and 3+ RF [8.80 (3.62–21.42)] were associated with a higher risk of developing HF but 1 RF was not associated with risk of HF [1.72 (0.68–4.34)].
Table 2.
Risk of Outcomes Based Upon Number of RF Compared to Reference of 0 RF
| HF† Hazard Ratio (95% CI) |
Subdistribution | All-cause Mortality before HF Hazard Ratio (95% CI) |
All-cause Mortality after HF Hazard Ratio (95% CI) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 RF | 2 RF | 3+ RF | 1 RF | 2 RF | 3+ RF | 1 RF | 2 RF | 3+ RF | |
| All | |||||||||
| MHT “control” | 1.38 (1.16–1.66)* | 2.26 (1.89–2.68)* | 5.23 (4.42–6.19)* | 1.25 (1.14–1.37)* | 1.49 (1.36–1.64)* | 2.23 (2.02–2.46)* | 1.25 (1.14–1.37)* | 1.49 (1.36–1.64)* | 2.23 (2.02–2.46)* |
| Age + MHT | 1.36 (1.14–1.63)* | 2.24 (1.88–2.66)* | 5.01 (4.23–5.94)* | 1.24 (1.13–1.35)* | 1.51 (1.37–1.66)* | 2.18 (1.98–2.41)* | 1.02 (0.78–1.33) | 1.12 (0.86–1.44) | 1.21 (0.94–1.55) |
| SES + Age + MHT | 1.31 (1.09–1.57)* | 2.11 (1.77–2.51)* | 4.60 (3.88–5.46)* | 1.20 (1.10–1.32)* | 1.43 (1.30–1.57)* | 2.02 (1.83–2.23)* | 0.99 (0.76–1.29) | 1.08 (0.84–1.40) | 1.14 (0.89–1.47) |
| African-Americans | |||||||||
| MHT “control” | 1.96 (1.10–3.48)* | 3.72 (2.15–6.43)* | 8.92 (5.22–15.24)* | 1.41 (1.12–1.77)* | 1.73 (1.39–2.16)* | 2.77 (2.23–3.43)* | 1.41 (1.12–1.77)* | 1.73 (1.39–2.16)* | 2.77 (2.23–3.43)* |
| Age + MHT | 1.87 (1.05–3.32)* | 3.46 (2.00–5.99)* | 8.33 (4.87–14.25)* | 1.34 (1.07–1.69)* | 1.63 (1.31–2.04)* | 2.61 (2.10–3.24)* | 0.82 (0.34–1.98) | 0.78 (0.34–1.81) | 0.83 (0.37–1.89) |
| SES + Age + MHT | 1.80 (1.01–3.20)* | 3.19 (1.84–5.54)* | 7.31 (4.26–12.56)* | 1.30 (1.03–1.63)* | 1.51 (1.21–1.89)* | 2.34 (1.88–2.91)* | 0.77 (0.32–1.87) | 0.71 (0.30–1.65) | 0.73 (0.32–1.67) |
| Whites | |||||||||
| MHT “control” | 1.33 (1.10–1.62)* | 2.03 (1.67–2.46)* | 4.56 (3.78–5.52)* | 1.23 (1.11–1.37)* | 1.39 (1.24–1.55)* | 1.92 (1.69–2.17)* | 1.23 (1.11–1.37)* | 1.39 (1.24–1.55)* | 1.92 (1.69–2.17)* |
| Age + MHT | 1.32 (1.08–1.60)* | 2.06 (1.70–2.49)* | 4.37 (3.61–5.28)* | 1.22 (1.10–1.36)* | 1.44 (1.28–1.61)* | 1.89 (1.67–2.14)* | 1.06 (0.80–1.42) | 1.17 (0.89–1.55) | 1.31 (1.00–1.72) |
| SES + Age + MHT | 1.27 (1.04–1.54)* | 1.95 (1.60–2.36)* | 4.07 (3.36–4.93)* | 1.19 (1.07–1.32)* | 1.37 (1.23–1.54)* | 1.78 (1.56–2.02)* | 1.04 (0.78–1.38) | 1.14 (0.86–1.51) | 1.25 (0.95–1.65) |
| Hispanics | |||||||||
| MHT “control” | 1.75 (0.70–4.39)* | 4.10(1.72–9.77)* | 9.52 (4.03–22.50)* | 1.24 (0.89–1.74)* | 2.19 (1.57–3.04)* | 3.24 (2.29–4.59)* | NA | NA | NA |
| Age + MHT | 1.75 (0.70–4.40)* | 4.08(1.71–9.71)* | 9.27 (3.91–21.95)* | 1.24 (0.88–1.73)* | 2.18 (1.57–3.03)* | 3.09 (2.19–4.38)* | NA | NA | NA |
| SES + Age + MHT | 1.72 (0.68–4.34) | 3.87(1.60–9.37)* | 8.80 (3.62–21.42)* | 1.22 (0.87–1.71) | 2.11 (1.51–2.94)* | 2.99 (2.10–4.24)* | NA | NA | NA |
indicates significant p-value <0.0001.
Death was treated as a competing risk for the time to HF.
CI indicates confidence interval; MHT, menopausal hormone therapy; NA, not applicable; RF, risk factors; SES, socioeconomic status. MHT “control” represents the unadjusted value since many patients were exposed to MHT, which has a known cardiovascular disease risk. Age + MHT and SES + Age + MHT represent the adjusted models. Time to all-cause mortality after HF was not analyzed in Hispanics who had too few HF events.
Figure 2. Risk of Outcomes Based Upon Number of RF.
The model is fully adjusted for menopausal hormone therapy status, age, and socioeconomic status. The reference is zero RF. CI indicates confidence interval.
Prior to developing HF, death occurred in 12.0% (n=1376) African-American, 7.2% (n=358) Hispanic, and 15.3% (n=2656) White women. In the fully adjusted model, an additional RF modestly increased the risk of all-cause mortality prior to developing HF [Table 2, Figure 2; HR (95% CI): 1 RF: 1.20 (1.10–1.32); 2 RF: 1.43 (1.30–1.57); 3+ RF: 2.02 (1.83–2.23); p<0.0001]. An increasing number of RF increased the risk of death before HF differently based upon race/ethnicity (p=0.001 for interaction of race/ethnicity and number of RF). In the fully adjusted model, compared to 0 RF, 1RF [1.30 (1.03–1.63)], 2 RF [1.51 (1.21–1.89)] and 3+ RF [2.34 (1.88–2.91)] were associated with all-cause mortality prior to developing HF in African-Americans. Among Whites, 1 RF [1.19 (1.07–1.32)], 2 RF [1.37 (1.23–1.54)] and 3+ RF [1.78 (1.56–2.02)] were associated with all-cause mortality prior to developing HF. Similarly among Hispanics, 2 RF [2.11 (1.51–2.94)] and 3+ RF [2.99 (2.10–4.24)] demonstrated an increased risk of HF compared with 0 RF, but 1 RF was not associated with mortality [1.22 (0.87–1.71)].
After developing HF, 49.4% (n=259) African-American, 37.1% (n=33) Hispanic, and 51.1% (n=596) White women died. An increasing number of RF was not associated with risk of death after HF [Table 2, Figure 2; HR (95% CI): 1 RF: 0.99 (0.76–1.29); 2 RF: 1.08 (0.84–1.40); 3+ RF: 1.14 (0.89–1.47)]. This pattern occurred irrespective of race/ethnicity (p=0.48 for interaction of race/ethnicity and number of RF).
In the secondary analysis, adjusted for menopausal hormone therapy, age, socioeconomic status, and other RF, each individual RF was associated with increased risk of developing HF (Table 3). Diabetes was associated with significantly different risk of developing HF by race/ethnicity [African-American 2.11 (1.71–2.61), White 2.78 (2.33–3.32), Hispanic 4.21 (2.52–7.02), p=0.04 for interaction of diabetes with race/ethnicity, Supplemental Table 2]. Each of the individual RF was associated with death before HF with the exception of obesity, which was associated with different risk of death before HF across race/ethnicity [African-American 1.08 (0.97–1.20), White 0.93 (0.85–1.01), Hispanic 1.67 (1.34–2.07), p<0.0001 for interaction of obesity with race/ethnicity]. After HF, only atherosclerosis and diabetes were associated with increased risk of death. Obesity was associated with reduced risk of death after HF [0.85 (0.74–0.99)]. There were no significant differences in risk of death after HF with individual RF across race/ethnicity (Supplemental Table 2).
Table 3.
Risk of Outcomes Based Upon Individual RF
| HF | All-cause Mortality Before HF | All-cause Mortality After HF | |
|---|---|---|---|
| Subdistribution Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | |
| All | |||
| Atherosclerosis | 2.05(1.80–2.34)* | 1.56(1.42–1.72)* | 1.22(1.03–1.45)* |
| Treated Diabetes | 2.54(2.23–2.90)* | 2.03(1.84–2.24)* | 1.22(1.03–1.45)* |
| Hypertension | 1.72 (1.56–1.91)* | 1.30(1.23–1.38)* | 0.98(0.84–1.14) |
| Obesity | 1.44(1.30–1.59)* | 1.03(0.96–1.24) | 0.85(0.74,0.99)* |
| Sedentary Activity | 1.24(1.12–1.37)* | 1.17(1.10–1.24)* | 1.15(0.99–1.34) |
| African-Americans | |||
| Atherosclerosis | 1.94(1.55–2.43)* | 1.58(1.36–1.84)* | 1.16(0.87–1.55) |
| Treated Diabetes | 2.11(1.71–2.61)* | 1.97(1.71–2.26)* | 1.20(0.92–1.57) |
| Hypertension | 2.10(1.69–2.60)* | 1.30(1.16–1.45)* | 0.96(0.70–1.30) |
| Obesity | 1.58(1.31–1.90)* | 1.08(0.97–1.20) | 0.81(0.62–1.06) |
| Sedentary Activity | 1.30(1.07–1.58)* | 1.21(1.08–1.35)* | 0.95(0.72–1.25) |
| Whites | |||
| Atherosclerosis | 2.13(1.81–2.52)* | 1.48(1.30–1.70)* | 1.26(1.02–1.55)* |
| Treated Diabetes | 2.78(2.33–3.32)* | 1.96(1.68–2.30)* | 1.23(0.98–1.54) |
| Hypertension | 1.58(1.39–1.78)* | 1.35(1.24–1.46)* | 0.99(0.84–1.17) |
| Obesity | 1.38(1.22–1.56)* | 0.93(0.85–1.01) | 0.87(0.73–1.04) |
| Sedentary Activity | 1.20(1.06–1.36)* | 1.14(1.05–1.23)* | 1.22(1.02–1.46)* |
| Hispanics | |||
| Atherosclerosis | 1.87(0.99–3.52) | 1.99(1.45–2.74)* | NA |
| Treated Diabetes | 4.21(2.52–7.02)* | 2.56(1.89–3.46)* | NA |
| Hypertension | 2.13(1.36–3.34)* | 1.08(0.86–1.35) | NA |
| Obesity | 1.52(0.98–2.37) | 1.67(1.34–2.07)* | NA |
| Sedentary Activity | 1.46(0.91–2.33) | 1.35(1.08–1.68)* | NA |
Results are fully adjusted for menopausal hormone therapy, age, socioeconomic status and other risk factors.
indicates p-value <0.05. CI indicates confidence interval; NA, not applicable. All-cause mortality after HF was not applicable in Hispanics who had too few HF events to detect death after HF.
DISCUSSION
In one of the largest studies of a diverse racial and ethnic population of women, we found that an additional established RF for HF was associated with significantly increased risk of HF and death prior to developing HF among African-American, Hispanic, and White women. Conversely, additional RF for HF were not associated with increased risk of death after HF in either African-American or White women.
Our study supports findings from smaller studies that address racial/ethnic differences in risk of HF based upon the number of ideal RF.11,19 In the Jackson Heart Study of African-Americans (n=4,195), a progressively reduced risk of incident HF was demonstrated with an increased number of “Life’s Simple 7” ideal RF, including: ideal blood pressure, glucose, lipids, weight, daily exercise, balanced meals, and tobacco free lifestyle.11 Similarly in the Multi-Ethnic Study of Atherosclerosis (n=6,506), an increased number of ideal RF was associated with a trend towards reduced incidence of HF in African-Americans, Whites, and Hispanics.19 African-Americans and Hispanics demonstrated the greatest benefit with each additional ideal RF.19
The decreased risk of death with more ideal RF has been demonstrated in a survey study that does not address racial/ethnic differences.20 In the National Health and Nutrition Examination Survey (n=44,959), a higher number of “Life’s Simple 7” ideal RF was associated with reduced risk of all-cause death.20 However, associations between the number of RF and risk of death after HF are less described in diverse racial/ethnic populations. Numerous studies have developed calculators to predict the risk of death after developing HF, but most assess risk based upon specific types of RF, not the number of RF.3
The WHI study is unique by demonstrating the risk of HF and death before or after HF based upon the number of baseline RF. Most studies have not addressed this combination of progressive events within the same population or within diverse racial/ethnic groups. Our study and the “Life’s Simple 7” literature suggest that preventing established RF and pursuing ideal management of RF may reduce racial/ethnic disparities in HF incidence and death prior to HF. 11,19,20 However, there is a noteworthy distinction. The lack of an association between the number of RF and risk of death after HF in African-Americans and Whites implies that the preventative RF target should occur before HF develops. Additional study is warranted in a larger population of Hispanic women to determine if the same conclusion applies.
Multiple observational studies have demonstrated the obesity paradox in HF, as demonstrated in this study.21,22 Obesity was associated with increased risk of HF irrespective of race/ethnicity and increased risk of death before HF in Hispanics. However, obesity was protective for death after HF. Exercise is recommended in obese patients with HF since exercise is associated with improved exercise capacity and quality of life.3,23 The definitive trial has not been performed to determine if purposeful weight loss causes increased mortality among obese HF patients.
This study is subject to several limitations. First, RF for HF did not include family history of cardiomyopathy and metabolic syndrome. However, metabolic syndrome should be represented with the capture of diabetes, hypertension, and obesity. Also, an interdependent relationship of the individual RF may influence HF outcomes. For this reason, we also assessed the risk of each outcome for each individual RF. Second, some of the patients may have structural heart disease without symptoms of HF since WHI participants had no imaging at baseline, and patients with prior myocardial infarction were not excluded. Systolic dysfunction at baseline was likely low given small rates of myocardial infarction at baseline, but the presence of left ventricular hypertrophy was likely missed given high baseline prevalence of hypertension.24 Third, incident HF only included hospitalized events and may underreport development of symptomatic HF. However, the population that is hospitalized is at highest risk for death25 and thus a key group for study. Fourth, the socioeconomic status of participants demonstrate a non-representative sample of the U.S. racial/ethnic minorities, but this is one of the largest population studies of diverse racial/ethnic women. Finally, the exact time of development of baseline RF prior to WHI enrollment is not well captured for all RF, and new development of some RF during follow-up were not collected. However, the risk of long-term events after baseline provides important data that may inform public health targets.
CONCLUSIONS
Among the WHI population of postmenopausal women, compared to 0 RF, each additional RF was associated with an increased risk of HF and death before HF among African-American, Hispanic, and White women. However, additional RF for HF were not associated with increased risk of death after HF diagnosis among either African-American or White women. Racial/ethnic disparities in HF may be reduced by seeking RF prevention. Further study of RF prevention interventions in longitudinal populations is warranted.
Supplementary Material
CLINICAL IMPLICATIONS.
What is new?
Across multiple racial/ethnic groups, the number of risk factors for heart failure is associated with risk of developing new onset heart failure and death before heart failure.
The number of risk factors for heart failure is not associated with risk of death after heart failure.
What are the clinical implications?
Public efforts should target primary prevention of risk factors for heart failure, especially among African-Americans who have the highest rates of heart failure.
Acknowledgments
We have gratitude for the WHI participants, clinical sites, investigators, and staff of which otherwise this study would not be possible.
Funding Sources
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. Dr. Breathett received support from the National Institute of Health (NIH) L60 MD010857 and the University of Arizona Health Sciences, Strategic Priorities Faculty Initiative Grant.
Appendix: A Short List of Women’s Health Initiative Investigators
Program Office: Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller (National Heart, Lung, and Blood Institute, Bethesda, Maryland).
Clinical Coordinating Center: Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg (Fred Hutchinson Cancer Research Center, Seattle, WA).
Investigators and Academic Centers: JoAnn E. Manson (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA); Barbara V. Howard (MedStar Health Research Institute/Howard University, Washington, DC); Marcia L. Stefanick (Stanford Prevention Research Center, Stanford, CA); Rebecca Jackson (The Ohio State University, Columbus, OH); Cynthia A. Thomson (University of Arizona, Tucson/Phoenix, AZ); Jean Wactawski-Wende (University at Buffalo, Buffalo, NY); Marian Limacher (University of Florida, Gainesville/Jacksonville, FL); Robert Wallace (University of Iowa, Iowa City/Davenport, IA); Lewis Kuller (University of Pittsburgh, Pittsburgh, PA); Sally Shumaker (Wake Forest University School of Medicine, Winston-Salem, NC).
Women’s Health Initiative Memory Study: Sally Shumaker (Wake Forest University School of Medicine, Winston-Salem, NC).
Footnotes
Conflict of Interest Disclosures
There are no disclosures.
References
- 1.Heart Failure Fact Sheet. Data & Statistics. DHDSP|CDC; [Accessed October 10, 2013]. http://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_heart_failure.htm. [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P Subcommittee O behalf of the AHASC and SS. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA Guideline for the Management of Heart Failure A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 4.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PWF, Woo YJ. Forecasting the Future of Cardiovascular Disease in the United States A Policy Statement From the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 5.Eaton CB, Abdulbaki AM, Margolis KL, Manson JE, Limacher M, Klein L, Allison MA, Robinson JG, Curb JD, Martin LA, Liu S, Howard BV. Racial and ethnic differences in incident hospitalized heart failure in postmenopausal women: the Women’s Health Initiative. Circulation. 2012;126:688–696. doi: 10.1161/CIRCULATIONAHA.111.066688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eaton CB, Pettinger M, Rossouw J, Martin LW, Foraker R, Quddus A, Liu S, Wampler NS, Hank Wu W-C, Manson JE, Margolis K, Johnson KC, Allison M, Corbie-Smith G, Rosamond W, Breathett K, Klein L. Risk Factors for Incident Hospitalized Heart Failure With Preserved Versus Reduced Ejection Fraction in a Multiracial Cohort of Postmenopausal Women. Circ Heart Fail. 2016;9:e002883. doi: 10.1161/CIRCHEARTFAILURE.115.002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breathett K, Baliga RR, Capers Q. Review of Heart Failure Management in African-Americans. In: Baliga RR, Haas GJ, editors. Management of Heart Failure. Springer; London: 2015. pp. 277–286. [Google Scholar]
- 8.Franciosa JA, Ferdinand KC, Yancy CW Group O behalf of the CS on HF in AAW. Treatment of Heart Failure in African Americans: A Consensus Statement. Congest Heart Fail. 2010;16:27–38. doi: 10.1111/j.1751-7133.2009.00118.x. [DOI] [PubMed] [Google Scholar]
- 9.Maas AHEM, van der Schouw YT, Regitz-Zagrosek V, Swahn E, Appelman YE, Pasterkamp G, Ten Cate H, Nilsson PM, Huisman MV, Stam HCG, Eizema K, Stramba-Badiale M. Red alert for women’s heart: the urgent need for more research and knowledge on cardiovascular disease in women: proceedings of the workshop held in Brussels on gender differences in cardiovascular disease, 29 September 2010. Eur Heart J. 2011;32:1362–1368. doi: 10.1093/eurheartj/ehr048. [DOI] [PubMed] [Google Scholar]
- 10.Dunlay SM, Roger VL, Weston SA, Jiang R, Redfield MM. Longitudinal Changes in Ejection Fraction in Heart Failure Patients With Preserved and Reduced Ejection Fraction. Circ Heart Fail. 2012;5:720–726. doi: 10.1161/CIRCHEARTFAILURE.111.966366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spahillari A, Talegawkar S, Correa A, Carr JJ, Terry JG, Lima J, Freedman JE, Das S, Kociol R, de Ferranti S, Mohebali D, Mwasongwe S, Tucker KL, Murthy VL, Shah RV. Ideal Cardiovascular Health, Cardiovascular Remodeling, and Heart Failure in Blacks: The Jackson Heart Study. Circ Heart Fail. 2017;10:e003682. doi: 10.1161/CIRCHEARTFAILURE.116.003682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalogeropoulos A, Georgiopoulou V, Kritchevsky SB, Psaty BM, Smith NL, Newman AB, Rodondi N, Satterfield S, Bauer DC, Bibbins-Domingo K, Smith AL, Wilson PWF, Vasan RS, Harris TB, Butler J. Epidemiology of Incident Heart Failure in a Contemporary Elderly Population: The Health, Aging, and Body Composition Study. Arch Intern Med. 2009;169:708–715. doi: 10.1001/archinternmed.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.About WHI - About WHI. [Accessed September 1, 2017];Women’s Health Initiative. https://www.whi.org/about/SitePages/About%20WHI.aspx.
- 14.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, Shumaker S, Wang C-Y, Stein E, Prentice RL. Implementation of the women’s health initiative study design. Ann Epidemiol. 2003;13:S5–S17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 15.Heckbert SR, Kooperberg C, Safford MM, Psaty BM, Hsia J, McTiernan A, Gaziano JM, Frishman WH, Curb JD. Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the Women’s Health Initiative. Am J Epidemiol. 2004;160:1152–1158. doi: 10.1093/aje/kwh314. [DOI] [PubMed] [Google Scholar]
- 16.Pandey A, LaMonte M, Klein L, Ayers C, Psaty BM, Eaton CB, Allen NB, de Lemos JA, Carnethon M, Greenland P, Berry JD. Relationship Between Physical Activity, Body Mass Index, and Risk of Heart Failure. J Am Coll Cardiol. 2017;69:1129–1142. doi: 10.1016/j.jacc.2016.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 18.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal Hormone Therapy and Risk of Cardiovascular Disease by Age and Years Since Menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 19.Ogunmoroti O, Oni E, Michos ED, Spatz ES, Allen NB, Rana JS, Virani SS, Blankstein R, Aronis KN, Blumenthal RS, Veledar E, Szklo M, Blaha MJ, Nasir K. Life’s Simple 7 and Incident Heart Failure: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2017:6. doi: 10.1161/JAHA.116.005180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. 2012;307:1273–1283. doi: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtis JP, Selter JG, Wang Y, Rathore SS, Jovin IS, Jadbabaie F, Kosiborod M, Portnay EL, Sokol SI, Bader F, Krumholz HM. The Obesity Paradox: Body Mass Index and Outcomes in Patients With Heart Failure. Arch Intern Med. 2005;165:55–61. doi: 10.1001/archinte.165.1.55. [DOI] [PubMed] [Google Scholar]
- 22.Powell-Wiley TM, Ngwa J, Kebede S, Lu D, Schulte PJ, Bhatt DL, Yancy C, Fonarow GC, Albert MA. Impact of Body Mass Index on Heart Failure by Race/Ethnicity From the Get With The Guidelines-Heart Failure (GWTG-HF) Registry. JACC Heart Fail. 2018;6:233–242. doi: 10.1016/j.jchf.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horwich TB, Broderick S, Chen L, McCullough PA, Strzelczyk T, Kitzman DW, Fletcher G, Safford RE, Ewald G, Fine LJ, Ellis SJ, Fonarow GC. Relation Between Body Mass Index, Exercise Training, and Outcomes in Chronic Systolic Heart Failure. Am J Cardiol. 2011;108:1754–1759. doi: 10.1016/j.amjcard.2011.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuspidi C, Sala C, Negri F, Mancia G, Morganti A Italian Society of Hypertension. Prevalence of left-ventricular hypertrophy in hypertension: an updated review of echocardiographic studies. J Hum Hypertens. 2012;26:343–349. doi: 10.1038/jhh.2011.104. [DOI] [PubMed] [Google Scholar]
- 25.Shahar E, Lee S, Kim J, Duval S, Barber C, Luepker RV. Hospitalized heart failure: rates and long-term mortality. J Card Fail. 2004;10:374–379. doi: 10.1016/j.cardfail.2004.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.