Summary
Circulating factors released from tissues during exercise have been hypothesized to mediate some of the health benefits of regular physical activity. Lipokines are circulating lipid species that have recently been reported to affect metabolism in response to cold. Here, lipidomics analysis revealed that a bout of moderate intensity exercise causes a pronounced increase in the circulating lipid 12,13-dihydroxy-9Z-octadecenoic acid (12,13-diHOME) in in male, female, young, old, sedentary, and active human subjects. In mice, both a single bout of exercise and exercise training increased circulating 12,13-diHOME and surgical removal of brown adipose tissue (BAT) negated the increase in 12,13-diHOME, suggesting that BAT is the tissue source for exercise-stimulated 12,13-diHOME. Acute 12,13-diHOME treatment of mice in vivo increased skeletal muscle fatty acid uptake and oxidation, but not glucose uptake. These data reveal that lipokines are novel exercise-stimulated circulating factors that may contribute to the metabolic changes that occur with physical exercise.
In Brief
Using an MS/MSALL lipidomics platform, Stanford et al identify 12,13-diHOME as an exercised-induced lipokine in male, female, young and old human subjects. Murine experiments show that BAT is the tissue source of exercise-induced increases in circulating 12,13-diHOME, and that this lipokine increases fatty acid uptake in skeletal muscle in vivo.
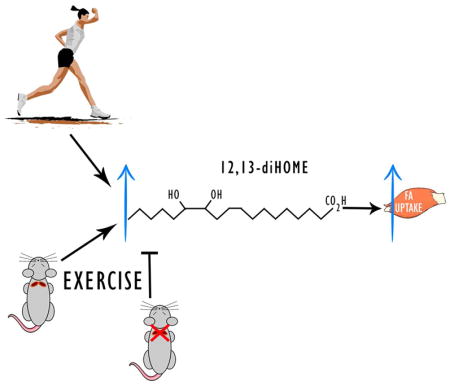
Introduction
Exercise results in adaptations to almost all tissues in the body, and these changes contribute to the beneficial effects of exercise to improve metabolic health. Even a single bout of moderate intensity exercise can have dramatic effects on glucose metabolism, lowering circulating insulin concentrations and making skeletal muscles more sensitive to insulin (Goodyear and Kahn, 1998). Exercise training, defined as repeated bouts of exercise over a period of weeks, months, or years can also result in lowering of insulin concentrations and improve glucose tolerance (Egan and Zierath, 2013; Goodyear and Kahn, 1998). Recently there has been great interest in identifying novel circulating factors that mediate the beneficial effects of exercise on health. Most of this focus has been on the investigation of muscle derived factors, known as myokines (Pedersen and Febbraio, 2008), and we and others have also hypothesized that there may be exercise-stimulated adipokines that mediate some of the benefits of exercise on health (Stanford and Goodyear, 2016; Stanford et al., 2015a).
There has been a recently identified class of lipids called “lipokines” that act as signaling molecules and can influence systemic metabolism (Cao et al., 2008; Liu et al., 2013; Lynes et al., 2017; Yore et al., 2014). Our recent study demonstrated that both acute and chronic cold exposure increase the circulating lipokine 12,13-diHOME, and an increase in this lipid is associated with improved metabolic health (Lynes et al., 2017). Lipokines can be released from adipose tissue (Cao et al., 2008; Lynes et al., 2017; Yore et al., 2014) and liver (Burhans et al., 2015; Liu et al., 2013) and have been reported to both improve skeletal muscle insulin sensitivity (Cao et al., 2008; Liu et al., 2013; Yore et al., 2014) or impair metabolic homeostasis (Burhans et al., 2015). Whether exercise can regulate circulating signaling lipokines has not been investigated. Here, we tested the hypothesis that exercise regulates the production of lipokines. We identified 12,13-diHOME as a lipokine increased in response to a single bout of exercise in humans and rodents, the tissue source of 12,13-diHOME in the mouse is BAT, and 12,13-diHOME can increase skeletal muscle fatty acid oxidation and uptake.
Results and Discussion
12,13-diHOME is an exercise-induced lipokine in humans
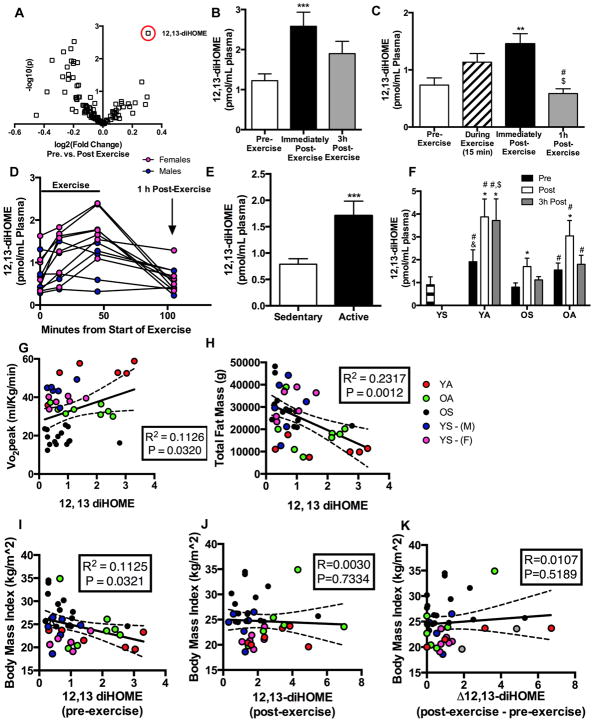
We studied two separate cohorts of human subjects to test the hypothesis that an acute bout of exercise alters the concentration of circulating lipokines. Cohort 1 (n=27) were from the greater Orlando, FL area and were healthy, young and older male subjects with a range of activity levels. Cohort 1 performed 40 min of cycle ergometer exercise at 70% heart rate reserve and blood samples were obtained before, immediately post-, and 3 h post-exercise. Plasma was analyzed by liquid chromatography tandem mass spectrometry (LC-MS/MS) to measure the concentrations of a panel of 88 mediator lipids with annotated signaling properties. These lipids were categorized by several attributes including the precursor fatty acids, pro- or anti-inflammatory effects, or the enzymes that catalyze the first step in the formation of the lipid (Figure S1A). Only 1 lipid was significantly increased and 13 lipids were significantly decreased immediately post-exercise (Table S1). The lipid significantly increased in circulation after an acute bout of exercise was the linoleic acid metabolite 12,13-diHOME (Figure 1A,B). To confirm and extend these findings, we studied a second cohort of subjects from the greater Boston, MA area. Cohort 2 subjects were healthy young (29.4 ±0.6 yr) males (n=6) and females (n=6) that were not engaged in consistent exercise training regimens. Cohort 2 performed 45 min of treadmill running at 75% VO2peak with blood sampling before, 15 min into, immediately post- and 1 h post-exercise. 12,13-diHOME tended to increase 15 min into exercise, was significantly increased immediately post-exercise, and returned to baseline by 1 h post-exercise (Figure 1C). There were no differences in baseline 12,13-diHOME between male and female subjects, although interestingly exercise increased 12,13-diHOME in all female subjects but only 4 of 6 male subjects (Figure 1D).
Figure 1. Identification of 12,13-diHOME, an exercise-induced lipokine.
(A)Volcano plot of 88 lipids comparing the fold induction after acute exercise to the p value; 12,13-diHOME is circled in red (Cohort 1, n=27). See: Table S1. (B) Plasma concentration of 12,13-diHOME before, immediately post, and 3h post acute exercise (Cohort 1, n=27). Symbols are differences vs. pre exercise (**P<0.01). (C) Plasma concentration of 12,13-diHOME before, during and 1h after exercise (Cohort 2, n=12). Symbols are differences compared to: pre exercise (**P<0.01); immediately post exercise (#P<0.05); and 15 min into exercise ($P<0.05). (D) Plasma concentrations of 12,13-diHOME before, during and after exercise for each individual subject (Cohort 2, n=12; n=6 male and n=6 female). (E) Plasma concentrations of baseline (pre-exercise) 12,13-diHOME in sedentary (n=30) vs. active (n=13) (Cohort 1 and males from Cohort 2). Symbols are differences compared to pre-exercise values (***P<0.001). Plasma concentration of 12,13-diHOME before, immediately post, and 3h post an acute bout of exercise in subjects from Cohort 1 (F) YS (n=4; historical data), YA (n=6), OS (n=14), and OA (n=7). Asterisks represent differences compared to pre-exercise values (*P<0.05; **P<0.01); compared to OS at the same time point (#P<0.05); compared to YS at the same time point (&P<0.05); or compared to OA at the same time point ($P<0.05). Correlations of baseline (pre-exercise) 12,13-diHOME (pmol/mL plasma) and (G) VO2peak (n=39), (H) total fat mass (n=39), and (I) BMI (n=39). See: Figure S1 and Table S2. Correlations of (J) post-exercise 12,13-diHOME (pmol/mL plasma) and BMI (n=39) and (K) the difference in post-exercise to pre-exercise 12,13-diHOME (pmol/mL plasma) (Δ12,13-diHOME) and BMI (n=39). Correlations include data from Cohorts 1 and 2; Red (Young Active-YA, n=6), green (Older Active-OA, n=7), and black (Older Sedentary-OS, n=14) circles represent Cohort 1, while the blue (Young Sedentary Males - YS-M, n=6) and pink (Young Sedentary Females - YS-F, n=6) represent Cohort 2. For B-K, Data are mean ± SEM.
Effects of activity level on exercise-stimulated 12,13-diHOME
To determine if chronic levels of physical activity affect baseline concentrations of 12,13-diHOME, we compared sedentary (n=30) and active (n=13) subjects from Cohorts 1 and 2. Active subjects had significantly higher 12,13-diHOME concentrations at baseline (Figure 1E), indicating that activity status could be a factor in determining 12,13-diHOME concentrations. Because Cohort 1 had male subjects with a range of ages and activity levels, we analyzed the effects of exercise on 12,13-diHOME based on both factors. These male subjects were categorized as: young sedentary (YS) (age 24–42 yr; n=4); young active (age 24–40 yr; n=6); older sedentary (age 65–90 yr; n=14); and older active (age 65–90 yr; n=7). Historical data from our recent study was used provide comparison to the cohort of young sedentary (YS) male subjects (age 24–42 yr; n=4) (Lynes et al., 2017). Active subjects performed aerobic exercise at least 3 days/week during the previous 6 months, and sedentary performed < 1 exercise session per week. Young active (YA), older active (OA), and older sedentary (OS) subjects all had a significant increase in 12,13-diHOME immediately post-exercise, although the effect was greater in the active subjects (Figure 1F). At 3 h post-exercise, only YA subjects had an increase in circulating 12,13-diHOME. Taken together these data show that a single bout of exercise increases circulating 12,13-diHOME in humans regardless of gender, age, or activity level.
Resting 12,13-diHOME concentrations are correlated with fat mass
We next examined if there was a relationship with 12,13-diHOME and cardiorespiratory fitness, measured by VO2peak. 12,13-diHOME was positively correlated with VO2peak in both cohorts (Figure 1G). The direct correlation between cardiovascular fitness and 12,13-diHOME was specific for 12,13-diHOME; VO2peak was not significantly correlated with other linoleic acid metabolites including 12,13-epOME, 9,10-epOME, and 9,10-diHOME (Figure S1B–D). 12,13-diHOME also correlated with total fat mass (Figure 1H), body mass index (BMI) (Figure 1I), body weight (Figure S1E), and triglycerides (Figure S1F). There was no correlation between 12,13-diHOME and fasting glucose concentrations (Figure S1G), and none of these factors were correlated with 12,13-epOME, 9,10-epOME, and 9,10-diHOME (Figure S1H–J; data not shown).
Since 12,13-diHOME was positively correlated with VO2peak, and negatively correlated with fat mass and BMI, we performed co-variate analyses to determine if the increase in 12,13-diHOME was driven primarily by fat mass. Co-variate analyses revealed that when % fat mass is accounted for, 12,13-diHOME is only significantly correlated with circulating triglycerides (Table S2).
Given the relationship between BMI and baseline 12,13-diHOME concentrations, we also determined if BMI or fat mass were important factors in the increase in 12,13-diHOME in response to exercise. There was no correlation between BMI and Immediate Post-Exercise 12,13-diHOME concentrations (Figure 1J) or between BMI and the increase (delta) in 12,13-diHOME with acute exercise (Figure 1K). Thus, the post-exercise increase in 12,13-diHOME is independent of BMI and indicates a specific effect of acute exercise to increase 12,13-diHOME.
12,13-diHOME is increased with exercise in mice
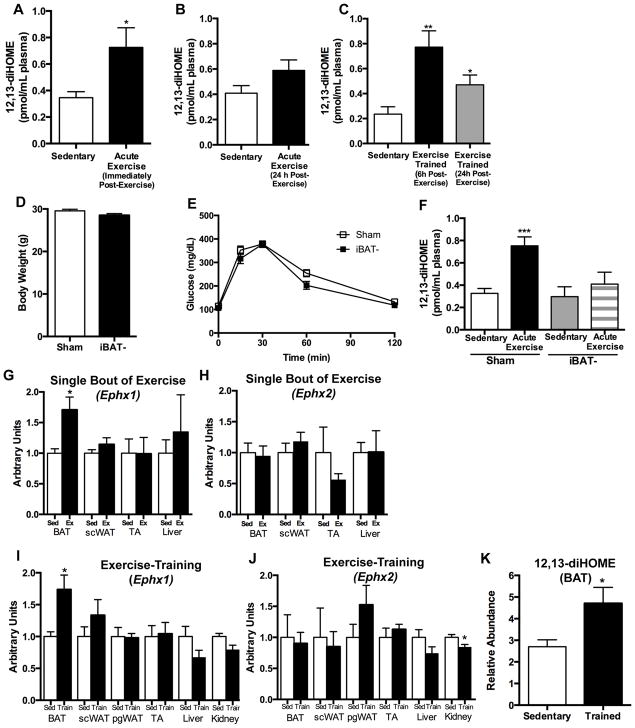
A mouse model was used to investigate mechanisms of exercise regulation of 12,13-diHOME. Interestingly, and consistent with our previous study (Lynes et al., 2017), 12,13-diHOME concentrations were lower in mice compared to humans, which may be due to differences in fatty acid precursors, enzyme activity, or general animal physiology. To study exercise in mice, 10 wk old C57BL/6 male mice were either: housed singularly in static cages for 3 wks (Sedentary); housed singularly in static cages for 3 wks followed by a single bout of moderate intensity treadmill exercise (Acute Exercise); or housed singularly in a cage containing an exercise wheel for 3 wks (Exercise Trained; 7.4±0.6 km/day). Consistent with the human studies, a single bout of exercise significantly increased circulating 12,13-diHOME in mice (Figure 2A). At 24 h post-exercise, there was no longer a significant increase in 12,13-diHOME (Figure 2B). Chronic exercise training by wheel running also significantly increased circulating 12,13-diHOME at both 6 and 24 h after removal of mice from wheel cages (Figure 2C). Body mass and fat mass were decreased after 3 wks of wheel running (Figure S2A) and thus, similar to the human data, it is likely that a decrease in fat mass contributes to the increase in 12,13-diHOME in the trained mice.
Figure 2. 12,13-diHOME is increased in mice following exercise, and BAT is required for exercise-induced increase in 12,13-diHOME.
Circulating 12,13-diHOME (A) immediately post an acute bout of exercise (n=7 sedentary, n=6 acute exercise), (B) 24 h post an acute bout of exercise (n=4/group), and (C) 6 h or 24 h removed from 3 wks of exercise training (n=8 sedentary, n=6 6 h post-exercise, n=6 24 h post-exercise). See: Figure S2. Asterisks represent differences compared to sedentary mice (*P<0.05; **P<0.01). (D) Body weight, (E) glucose tolerance test, and (F) circulating 12,13-diHOME in Sham mice and iBAT- at rest (Sham, n=4; iBAT-, n=7) and after an acute bout of exercise (Sham, n=6; iBAT-, n=6). Asterisks represent differences compared to sedentary, Sham mice (***P<0.001). Gene expression of (G) Ephx1 and (H) Ephx2 in sedentary mice (Sed) and after acute exercise (Ex) in mice, and (I) Ephx1 and (J) Ephx2 after 3 wks of exercise training (n=6/group). (K) 12,13-diHOME concentrations measured by LC-MS/MS in BAT after 3 wks of exercise in mice. See: Table S3. Asterisks represent differences compared to sedentary (*P<0.05). For A-K, data are means ± s.e.m
BAT is the tissue source for the exercise-induced increase in circulating 12,13-diHOME
Our recent study determined that BAT is the source of the increase in circulating 12,13-diHOME in cold exposed mice (Lynes et al., 2017). To investigate whether BAT was the source of 12,13-diHOME with acute exercise, mice underwent sham surgery or had BAT surgically removed from the intrascapular region (iBAT-), the depot which accounts for ~60% of BAT in a mouse. Eight wks later, body weights and glucose tolerance were not different between Sham and iBAT- mice (Figure 2D,E). There was no difference in basal 12,13-diHOME concentrations between Sham and iBAT- mice, indicating that there are other tissues that contribute to circulating 12,13-diHOME levels (Figure 2F). Exercise significantly increased 12,13-diHOME in the Sham mice, but the effects of exercise were fully blunted in the iBAT- mice (Figure 2F). Thus, although liver, kidney, and white adipose tissue have all been shown to express 12,13-diHOME, this experiment identifies iBAT as the tissue responsible for the exercise-induced increase in circulating 12,13-diHOME.
We next assessed the effects of exercise training on the expression of genes responsible for the production of 12,13-diHOME. Biosynthesis of 12,13-diHOME is regulated by soluble epoxide hydrolases (sEH) of which Ephx1 and Ephx2 are the major isoforms expressed in adipose tissue (Lynes et al., 2017). A single bout of exercise increased Ephx1 in BAT, but did not affect Ephx1 expression in subcutaneous white adipose tissue (scWAT), tibialis anterior muscle (TA), or liver (Figure 2G). Ephx2 was not changed in any tissue after exercise (Figure 2H).
After 3 wks of exercise training, gene expression of Ephx1 and Ephx2 was measured in BAT, scWAT, perigonadal white adipose tissue (pgWAT), tibialis anterior muscle, liver, and kidney. Training increased Ephx1 in BAT (Figure 2I), but not in any other tissue. Exercise training had no effect on Ephx2 expression in BAT, scWAT, pgWAT, muscle, or liver, but significantly decreased Ephx2 expression in the kidney (Figure 2J). The function of the decrease in Ephx2 in the kidney with exercise is unknown. These data indicate that both a single bout of exercise and exercise training increase Ephx1 expression only in BAT.
To determine if exercise training increases 12,13-diHOME in BAT, mice underwent 3 wks of exercise training by wheel running and BAT was analyzed by LC-MS/MS lipidomics. Of the 88 lipid species measured (Table S3), only 12,13-diHOME was significantly increased in BAT from exercise-trained mice (Figure 2K). Together, these data indicate that BAT is the tissue source for the exercise-induced increase in circulating 12,13-diHOME.
12,13-diHOME increases skeletal muscle fatty acid uptake in vivo
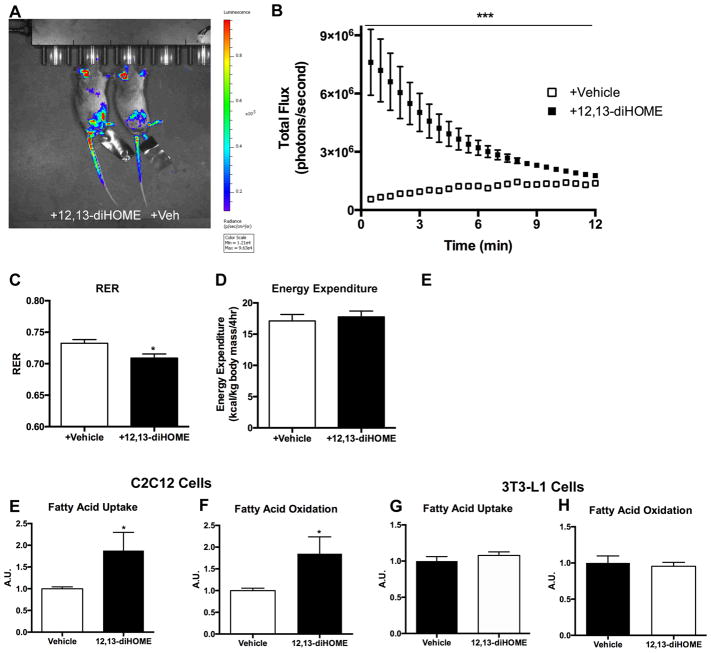
A major source of energy for the working skeletal muscles during exercise comes from the uptake and oxidation of fatty acids. To test the hypothesis that 12,13-diHOME increases fatty acid uptake and oxidation in skeletal muscle in vivo, we generated ACTA1Cre+/− Rosa(stop)Luc+/− mice that constitutively express a bioluminescent reporter in skeletal muscle. Mice were injected intravenously with FFA-SS-Luc, a fatty acid conjugated to luciferin (Henkin et al., 2012; Liao et al., 2005), in the presence of 12,13-diHOME or a vehicle control. Injection of 12,13-diHOME increased fatty acid uptake in skeletal muscle (Figure 3A,B, Video S1). This increase was most prominent immediately after injection and remained significantly elevated above vehicle for 10 min. These intriguing in vivo data provide a potential physiological function for the exercise-induced increase in 12,13-diHOME. Consistent with these findings, acute injection of wild-type mice with 12,13-diHOME resulted in a decreased respiratory exchange ratio (RER), indicating increased lipid oxidation and supporting the hypothesis that 12,13-diHOME increases fatty acid oxidation in vivo (Figure 3C). The decrease in RER was independent of any change in energy expenditure (Figure 3D).
Figure 3. 12,13-diHOME enhances fatty acid uptake into skeletal muscle.
(A) Representative images of luciferase activity in ACTAcre+/−Rosa(stop)Luc+/− injected intravenously with luciferin conjugated fatty acid and 12,13-diHOME (mouse on left) or vehicle (mouse on right). Data are representative images at 8 min. (B) Quantification of luciferase activity after injection; luminescence was measured every 30s for 15 min. (n=6/group). (C) Average respiratory exchange ratio (RER) and (D) energy expenditure as measured by CLAMS for 4 h at room temperature in mice acutely treated with 12,13-diHOME (n=5) or vehicle (n=4). Fatty acid uptake and oxidation measured by 14C radiolabeled palmitic acid in differentiated (E,F) C2C12 cells and (G,H) 3T3-L1 cells incubated with either 12,13-diHOME or vehicle. The data was normalized by protein content (n=5/group). Asterisks represent differences compared to vehicle (*P<0.05; (***P<0.001). For A–H, data are means ± s.e.m.
To determine if the effects on fatty acid uptake and oxidation were cell autonomous and specific to skeletal muscle cells, we measured the effects of 12,13-diHOME incubation on the uptake of radiolabeled palmitate in differentiated C2C12 myotubes and 3T3-L1 white adipocytes. Consistent with the in vivo data, there was a significant increase in fatty acid uptake (Figure 3E) and oxidation (Figure 3F) in C2C12 cells. 12,13-diHOME did not increase fatty acid uptake or oxidation in 3T3-L1 cells, indicating that 12,13-diHOME does not upregulate lipid metabolism in white adipocytes (Figure 3G,H). The mechanism for the increase in fatty acid uptake and oxidation in skeletal muscle and in cells is not known, but it is likely that 12,13-diHOME activates signaling pathways leading to translocation of fatty acid transporters.
12,13-diHOME increases mitochondrial respiration in muscle cells
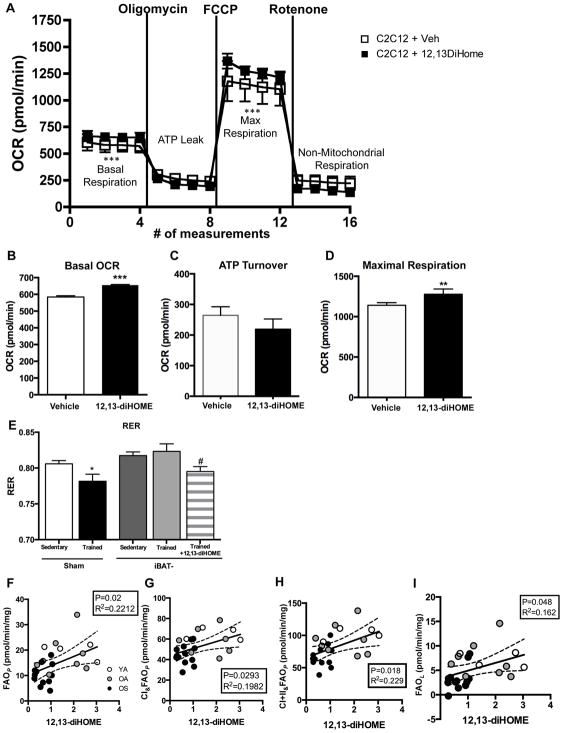
To determine if 12,13-diHOME regulates mitochondrial function in muscle, differentiated C2C12 myotubes were incubated with 12,13-diHOME and then analyzed for mitochondrial respiration. 12,13-diHOME incubation increased basal oxygen consumption rate (OCR) and respiratory capacity in C2C12 cells (Figure 4A,B). 12,13-diHOME did not affect ATP turnover (Figure 4A,C), but did increase maximal uncoupled respiration (Figure 4A,D). In 3T3-L1 cells, 12,13-diHOME did not increase OCR under any condition (data not shown).
Figure 4. 12,13-diHOME increases respiration in skeletal muscle.
(A) Bioenergetic profile of differentiated C2C12 cells treated with 12,13-diHOME or vehicle. (B) Basal OCR, (C) ATP turnover, and (D) maximal respiration were measured (n=5/group). Asterisks represent differences compared to sedentary (**P<0.01, ***P<0.001). (E) Average RER measured by CLAMS for 4 h at room temperature in Sham-sedentary (n=4), Sham-trained (n=4), iBAT- sedentary (n=4), iBAT- trained (n=5), or iBAT- trained acutely treated with 12,13-diHOME (n=5). Asterisks represent differences compared to Sham-sedentary mice (*P<0.05); or compared to iBAT- sedentary mice (#P<0.05). Respiration was measured in permeabilized fiber bundles by high resolution respirometry in subjects from Cohort 1. Pre-exercise 12,13-diHOME correlated with ADP stimulated respiration; (F) FAO supported OXPHOS (FAOP), (G) complex I and FAOP (CI&FAOP) mitochondrial, (H) complex I, II and FAO supported coupled respiration (CI+II&FAOP), and (I) non-ADP stimulated (FAOL) respiration (n=27). For A–I, data are means ± s.e.m.
Function and mechanism of increased 12,13-diHOME with exercise training
Our data support the concept that the increase in 12,13-diHOME after acute exercise functions to regulate fatty acid uptake and metabolism. Therefore, we utilized sham and iBAT- mice to determine if increased 12,13-diHOME with training regulates fat utilization in vivo. Sham and iBAT- mice were sedentary or exercise trained for 3 wks and RER was measured in metabolic cages. RER was significantly reduced in the sham-trained mice compared to the sham-sedentary mice. In contrast, there was no effect of exercise training on RER in the iBAT- mice. However, when the trained iBAT- mice were treated with 12,13-diHOME, RER was significantly decreased (Figure 4E). These data indicate an essential role for 12,13-diHOME in the regulation of fatty acid metabolism with exercise training.
To identify a mechanism for increased fat utilization with 12,13-diHOME in skeletal muscle, we determined the effects of injecting mice with 12,13-diHOME in vivo on the expression of mitochondrial and fatty acid oxidation genes in tibialis anterior muscle. 12,13-diHOME increased expression of several genes involved in mitochondrial activity and biogenesis (citrate synthase, Nrf1, Nrf2), and fatty acid uptake (Cd36, Fatp4) (Figure S2B). 12,13-diHOME also increased expression of Nrf1 and Nrf2 in the heart (Figure S2C). There was no effect of 12,13-diHOME on mitochondrial or fatty acid oxidation genes in scWAT, pgWAT, liver, or BAT (Figure S2D–G). These findings are consistent with the data showing that 12,13-diHOME regulates fatty acid metabolism in skeletal muscle.
12,13-diHOME correlates to greater skeletal muscle respiration in humans
We next determined if 12,13-diHOME was correlated with mitochondrial respiration in permeabilized fiber bundles from vastus lateralis biopsy specimens from human subjects (Cohort 1). ADP stimulated respiration (FAOP) in the presence of palmitoylcarnitine/malate (PCM), maximal complex I and FAOP respiration (CI&FAOP), and maximal complex I, II and FAOP respiration (CI+II&FAOP) were significantly correlated to circulating 12,13-diHOME (Figure 4F–I). Non-ADP stimulated respiration (FAOL), was also significantly correlated to 12,13-diHOME (Figure 4H). These data indicate that circulating 12,13-diHOME is correlated to increased capacity for mitochondrial respiration in skeletal muscle. Together with the finding that 12,13-diHOME increases maximal respiratory capacity of the C2C12 myotubes, these data raise the possibility that increases in circulating 12,13-diHOME with exercise functions to help increase the respiratory capacity of a working skeletal muscle and may enhance exercise capacity. This will be an interesting question for future investigation.
12,13-diHOME does not affect skeletal muscle glucose uptake
Glucose is another important fuel source during exercise, thus we also tested the hypothesis that 12,13-diHOME would increase glucose uptake in skeletal muscle. In contrast to the effects of 12,13-diHOME to increase fatty acid uptake and oxidation in C2C12 myotubes, 12,13-diHOME did not affect glucose uptake in these cells (Figure S3A). To confirm these findings, we isolated mouse soleus and extensor digitorum longus (EDL) skeletal muscles and incubated them with either vehicle or 12,13-diHOME for 1 h. There was no effect of 12,13-diHOME on rates of glucose uptake in either muscle (Figure S3B,C). The finding that 12,13-diHOME regulates fatty acid uptake and oxidation, but not glucose uptake, is interesting in light of our data showing that 12,13-diHOME is correlated with circulating triglycerides but not circulating glucose in human subjects (Figure S1F,G).
Collectively, these data identify 12,13-diHOME as a circulating lipokine released during moderate intensity exercise in humans and mice. In human subjects, the increase in 12,13-diHOME after acute exercise was present regardless of age, gender, activity levels, BMI or fat mass. Baseline 12,13-diHOME was higher in physically active compared to sedentary subjects, although this comparison was made in male subjects and cannot be generalized to females. Our correlation analyses suggest that the relationship between 12,13-diHOME and both activity and fitness level is driven by the fat mass of the subjects. It will be important in future studies to determine the direct effects of an exercise training program, independent of changes in fat mass, on 12,13-diHOME in both in male and female subjects.
The mouse experiments show that the tissue source for an increase in 12,13-diHOME with a single bout of exercise is iBAT, and that an increase in 12,13-diHOME increases fatty acid uptake in skeletal muscle in vivo. Importantly, a direct role of 12,13-diHOME to mediate exercise-induced changes in RER and fatty acid oxidation was determined in a mouse model. In the absence of BAT (iBAT-), there was no training effect of RER determined. However, when iBAT- mice were treated with 12,13-diHOME their RER decreased to the level of exercise-trained mice, indicating an essential role for 12,13-diHOME to contribute, at least in part, to the metabolic response to exercise.
A previous study aimed at identifying biomarkers for oxidative stress showed that extremely high intensity, prolonged (75 km) cycling exercise in elite male cyclists increased several linoleic acid metabolites including 13 HODE + 9-HODE, 9,10-diHOME, and 12,13-diHOME (Nieman et al., 2014). In the current study, the only linoleic acid metabolite that was increased with moderate intensity exercise was 12,13-diHOME. Whether the severity and duration of the exercise in the previous study increased a greater number of linoleic acid metabolites is not known, nor is it known how these metabolites may function in response to severe exercise. Although we could not directly test the hypothesis, our data suggests that 12,13-diHOME may function specifically in skeletal muscle to increase fatty acid uptake and oxidation and mitochondrial activity.
Our recent study investigated the effects of cold exposure on BAT and identified 12,13-diHOME as a metabolite elevated in response to both short term (1 h) and chronic (7–11 days) cold exposure in rodents and humans (Lynes et al., 2017). We demonstrated that 12,13-diHOME is a cold-induced lipokine released from BAT and functions to decrease circulating triglycerides and promote fatty acid uptake specifically in BAT. The parallels between the effects of exercise and cold exposure on 12,13-diHOME are striking and somewhat unexpected. For one, both short term and chronic treatments with these stimuli increase circulating 12,13-diHOME to similar concentrations. In addition, both cold and exercise increase 12,13-diHOME in BAT, which is thought to be the tissue source of circulating 12,13-diHOME. This is surprising because while cold exposure is a well-known and potent stimulator of BAT activity, most investigations have shown that exercise training decreases BAT activity in humans and rodents (Motiani et al., 2017; Vosselman et al., 2015; Wu et al., 2014). We propose that cold causes the release of 12,13-diHOME from BAT to function in an autocrine manner to provide fuel for the BAT, whereas exercise causes the release of 12,13-diHOME from BAT to function in an endocrine manner, resulting in stimulation of fatty acids into the working skeletal muscle.
While the current investigation has focused on the identification of 12,13-diHOME as the first BAT-derived molecule regulated by exercise, in future studies it will be important to investigate the physiological consequences of the signaling lipids that are significantly decreased by exercise, as these factors may play important roles in regulating the metabolic effects of exercise. In sum, these data provide a previously unidentified role for BAT in the metabolic response to exercise and the first evidence that BAT-derived molecules, including signaling lipids, can increase fatty acid oxidation and uptake in skeletal muscle.
Limitations of Study
While our human cohort consisted of male subjects of various ages and activity levels, our female subjects were all young and sedentary. Although the male and female young sedentary subjects had a similar increase in 12,13-diHOME after an acute bout of exercise, it is not clear if this would be the same in female subjects of varying ages or activity levels. In addition, only male C57BL/6 mice were used, and it is possible that female mice or mice of a different strain would have a different response to exercise. A further limitation of our study is that these experiments were all performed in healthy human subjects and we identified fat mass is an important driver of 12,13-diHOME. Thus, obese subjects may not have the same response to acute exercise.
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological Samples | ||
| Human Tissue Samples | This Paper | N/A |
| Human Tissue Samples | (Lynes et al., 2017) | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| FFA-SS-Luc | Intrace Medical | N/A |
| 12,13-diHOME | Cayman Chemical | Cat#10009832 |
| Critical Commercial Assays | ||
| Triglycerides Assay Kit | Roche | COBAS INTEGRA TRIGL, test ID 0-010 |
| Glucose Assay Kit | Roche | COBAS INTEGRA Glucose HK Gen.3 |
| Experimental Models: Cell Lines | ||
| C2C12 Cells | ATCC | Cat # CRL-1772 |
| 3T3-L1 Cells | ATCC | Cat # CL-173 |
| Experimental Models: Organisms/Strains | ||
| Mouse: C57BL/6 | Charles River Laboratories | Strain code: 027 |
| Mouse: ACTA1cre+/− | The Jackson Laboratory | Stock no. 006149 |
| Mouse: Rosa(stop)Luc+/+ | The Jackson Laboratory | Stock no. 005125 |
| Mouse: CD-1 IGS | Charles River Laboratories | Strain code: 022 |
| Oligonucleotides | ||
| A full list of Primers is in Table S4 | This paper | N/A |
| Software and Algorithms | ||
| GraphPad Prism 7 | GraphPad Software | https://www.graphpad.com/scientific-software/prism/ |
| Image J Java 1.6.0_2.4 | NIH | https://imagej.nih.gov/ij/ |
| Living Image Software (IVIS Imaging Systems) | Perkin Elmer | http://www.perkinelmer.com/product/li-software-for-spectrum-1-seat-add-on-128113 |
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Kristin Stanford (kristin.stanford@osumc.edu).
Experimental Model and Subject Details
Human Subjects
All participants provided written informed consent and the study protocol was approved by the Institutional Review Boards at Florida Hospital (Florida cohort) or the Joslin Diabetes Center (Massachusetts cohort). Participants from Florida cohort were all male subjects with an average age of 58.7±2.5 yr and a BMI of 24.8±0.6 kg/m2 and were categorized as: Young Active (YA) 21–40 yrs (31.1±1.5 yr), Older Active (OA) and Older Sedentary (OS) 65–90 yrs (69.8±0.7 yr), with “Active” based on engaging in aerobic exercise (running, cycling, swimming) at least 3 days/wk. Subjects were classified as “Sedentary” if they performed one or less structured exercise session/wk. Objectively measured physical activity indicated that the YA group engaged in 60 min/day of vigorous physical activity and 126 min/day of moderate physical activity. The OA group engaged in 40 min/day of vigorous physical activity and 72 min/day of moderate physical activity. The OS group engaged in 1 min/day of vigorous physical activity and 24 min/day of moderate physical activity. Inclusion criteria included stable weight, non-smoker, BMI <35 kg/m2, resting blood pressure ≤150mmHg systolic and ≤95mmHg diastolic. Exclusion criteria included inability or unwillingness to comply with the protocol, clinically significant CVD including MI within the past year, the presence of peripheral vascular disease, hepatic disease, renal disease, muscular or neuromuscular disease, hematologic/oncologic disease, peripheral neuropathy, orthopedic limitations, history of pulmonary emboli, history of alcohol or substance abuse, current use of blood thinners or any medication that can alter glucose homeostasis.
Participants from the Massachusetts cohort consisted of males (n=6) and female (n=6), all who were young (29.4 ±0.6 yr), and lean (BMI 22.5±0.8 kg/m2). There was no effect of sex or gender identity on the outcomes of the study. All subjects performed less than 150 min/week of exercise for the previous 3 months (87±16 minutes of exercise/week). Inclusion criteria included being between 18–35 yrs of age, BMI ≥20 and ≤26 and HbA1C ≤5.7%. Exclusion criteria included current dieting or weight loss efforts, heart of lung disease, acute systemic infection, currently pregnant or breast feeding, HIV/AIDS, cancer, hepatic disease, renal disease, demyelinating diseases, clinical history of hypertension (systolic >140mmHg or diastolic >90mmHg, type 1 or 2 diabetes, inability to exercise at 50% of predicted heart rate, or taking beta-blockers.
Animals
Animal procedures were approved by the Institutional Animal Use and Care Committee at The Ohio State University and Joslin Diabetes Center. Mice were housed on a 12-hour light/dark cycle (6a (ON)/6p (OFF)) at room temperature (22°C) for all experiments and fed a standard chow-diet (21% kcal from fat; PharmaServ 9F5020) and given ad libitum access to food and water. Mice were housed individually in static cages or in wheel cages (Nalgene) (Stanford et al., 2015b). For chronic exercise experiments, mice were given ad libitum access to food and water after removal from wheel cage. Any mouse that ran 10% less than the average of the trained group was excluded from analyses. For acute exercise experiments, 12 wk old male C57BL/6 mice were familiarized with the treadmill (Quinton model 42) for 2 days prior to experiment. For iBAT- mice, mice were anesthetized using isofluorane. iBAT was surgically removed and the artery immediately cauterized. Sham mice underwent the same surgical procedure, but iBAT was not removed.
For experiments measuring in vivo bioluminescent fatty acid uptake in skeletal muscle, ACTA1cre+/− mice (C57BL/6) (Stock no. 006149) were bred with Rosa(stop)Luc+/+ (FVB) (Stock no. 005125) (Jackson Laboratory). Experiments measuring skeletal muscle glucose uptake in isolated skeletal muscle glucose uptake were performed in 6 wk old, female, CD-1 IGS mice (Charles River Laboratories).
Cell Culture
Cells were housed at 37°C, 95% humidity and 5% CO2. C2C12 myoblasts (ATCC; passage 4–7) were maintained in DMEM containing 10% FBS and 1% penicillin/streptomycin. Differentiation was induced by incubating the cells in DMEM containing 2% horse serum and 1% penicillin/streptomycin for 4 days. 3T3-LI adipocytes (ATCC: passage 4–7) were maintained in DMEM containing 10% FBS and 1% penicillin/streptomycin. Differentiation was induced by incubating the cells in DMEM containing 10% FBS and 1% penicillin/streptomycin with 400ng/mL dexamethasone, 1 μg/mL insulin, and 115 μg/mL IBMX for days 0–2, 1μg/mL insulin for days 2–4, and DMEM with 10% FBS and 1% penicillin/streptomycin only for days 4–8. For both C2C12 cells (mouse skeletal muscle) and 3T3-L1 cells (mouse embryos) it is not clear if the cells were isolated from males, females, or a combination.
Method Details
Human exercise testing and monitoring
For the Florida cohort, VO2 peak was determined as peak aerobic capacity measured during a graded exercise protocol on an electronically braked cycle ergometer (Coen et al., 2013), and body composition was determined by Dual energy X-ray absorptiometry scan. The SenseWear® Pro Armband (BodyMedia) was used to monitor physical activity behaviors (Carnero et al., 2017). For the submaximal exercise test, participants were fasted overnight and were 48 h removed from the last exercise bout. Participants performed a 6-min warm-up of light cycling on the ergometer followed by 40 min at 70% of heart rate reserve (HRR). Heart rate, perceived exertion and blood pressure data were collected every 5 mins and indirect calorimetry measurements recorded (Parvo Medics). A fasting blood sample was drawn before exercise, immediately after, and 3 hrs post-exercise. Exercise was performed at room temperature (22°C). Vastus lateralis muscle biopsies were obtained under local anesthesia (Pruchnic et al., 2004). Permeabilized myofiber bundles (~1–3 mg each) were prepared immediately after the biopsy and mitochondrial respiration was evaluated by high-resolution respirometry (Coen et al., 2015).
For participants of the Massachusetts cohort, VO2peak was determined as peak aerobic capacity measured during a graded exercise modified Bruce protocol on a treadmill (Beltz et al., 2016; Bruce, 1971). For the submaximal exercise test, participants were fasted overnight and were one week removed from the determination of VO2peak. A fasting blood sample was drawn before exercise, 15 min during exercise, immediately after, and 1 h post-exercise. Exercise was performed at room temperature (22°C).
Mice and exercise
Male, 10 wk old C57BL/6 mice were fed a chow-diet (21% kcal from fat) ad libitum. Mice were housed individually in static cages or in wheel cages (Nalgene) (Stanford et al., 2015b). For chronic exercise experiments, mice were given ad libitum access to food and water after removal from wheel cage. For acute exercise experiments, 12 wk old male C57BL/6 mice were familiarized with the treadmill (Quinton model 42) for 2 days prior to experiment. Mice underwent 40 min of treadmill exercise at 0.8 mph, 10% incline and all mice were able to successfully complete the acute bout of exercise. Both acute and chronic exercise experiments were performed at room temperature (22°C).
Removal of iBAT
For iBAT- mice, mice were anesthetized using isofluorane. iBAT was surgically removed and the artery immediately cauterized. Sham mice underwent the same surgical procedure, but iBAT was not removed.
In vivo fatty acid uptake
For experiments measuring in vivo bioluminescent fatty acid uptake in skeletal muscle, ACTA1cre+/− mice (C57BL/6) (Stock no. 006149) were bred with Rosa(stop)Luc+/+ (FVB) (Stock no. 005125)(Jackson Laboratory). Male offspring carrying the cre allele were injected retro-orbitally with 1 μg/kg body weight 12,13-diHOME in 0.1% BSA PBS or methyl acetate as a vehicle and all mice were co-injected with 2μm FFA-SS-Luc (Intrace Medical). This dose is based on the circulating concentration of 12,13-diHOME in mice that are housed at room temperature, 3ng/mL (10nM). After an acute bout of exercise, 12,13-diHOME increased to 10–30ng/mL (30–100nM). This is considerably below the reported cytotoxic dose of 100mg/kg body weight (Sisemore et al., 2001). Mice at room temperature (22°C) were anesthetized and imaged (IVIS Spectrum CT) using sequential 30 second exposures for 12 min.
Metabolic Studies
For measurements of respiratory exchange ratio (RER) and energy expenditure, mice were placed in metabolic chambers and injected intravenously with 1μg/kg body weight 12,13-diHOME in 0.1% BSA PBS or vehicle and then monitored in the Comprehensive Laboratory Animals Monitoring System (CLAMS) at room temperature (22°C) for 4h.
Skeletal muscle [3H]-2-deoxyglucose uptake
Isolated skeletal muscle glucose uptake was measured as previously described (Hayashi et al., 1998). Briefly, EDL or soleus muscles were dissected from 6wk old female CD-1 mice, mounted, and incubated in Krebs-Ringer bicarbonate buffer (KRB) pH 7.4 containing 2 mM pyruvate for 60 min. Both EDL and soleus were incubated with vehicle or 300ng/mL of 12,13-diHOME at room temperature (22°C). Following treatment, glucose transport activity was measured for an additional 10 min.
Lipidomic profiling
Lipidomic profiling was done as we have described in detail (Lynes et al., 2017). Briefly, a mixture of deuterium-labeled internal standards was added to aliquots of thawed serum, followed by cold methanol (MeOH) for Solid Phase Extraction (SPE). Following vortex, overnight storage at −20 °C, and centrifugation, the supernatant was acidified and SPE was performed (Powell, 1999). The methyl formate fractions were collected, dried under nitrogen, reconstituted in MeOH:H2O, centrifuged and the supernatant analyzed using the LC–MS/MS mediator lipidomics platform.
PCR and Cell Studies
mRNA levels of were measured by quantitative RT-PCR using primers (Table S4). Fatty acid uptake and oxidation into differentiated C2C12 muscle cells and 3T3L1 adipocytes measured using 14C-labeled palmitic acid uptake and conversion of 14C-labeled palmitic acid into CO2 as previously described (Townsend et al., 2013). Cells were treated with 1.5μM 12,13-diHOME or methyl acetate vehicle for 15 min, similar to previous studies (Lynes et al., 2017). Oxygen consumption rates (OCR) were measured in starved (1 hr) C2C12 skeletal muscle cells treated with 1.5μM 12,13-diHOME or methyl acetate vehicle for 15 min and OCR measured in 200μM palmitate (Seahorse XF24) (Vernochet et al., 2012).
Glucose uptake in C2C12 cells
2-deoxyglucose uptake in C2C12 cells was measured as previously described (Nedachi and Kanzaki, 2006). Briefly, differentiated C2C12 myotubes were serum starved for 3 h in DMEM before any treatment. Cells were incubated with 12,13-diHOME (1.5 mM; 15 min) or insulin (100 nM; 15 min). After stimulation, cells were washed with buffer containing 140 mM NaCl, 20 mM Hepes-Na (pH 7.4), 5 mM KCl, 2.5 mM MgSO4, and 1.0 mM CaCl2. Glucose transport was determined by the addition of [3H]-2-deoxyglucose for glucose for 10 min on ice. Cells were washed with ice-cold saline solution and harvested in 0.05 N NaOH to determine net accumulation of [3H]-2-deoxyglucose (Nedachi and Kanzaki, 2006).
Quantification and Statistical Analyses
Statistics
Data are mean ± SEM and significance defined as P ≤ 0.05 and determined by Student t tests or two-way ANOVA and Bonferroni post hoc analysis. All analyses were performed using features present in GraphPad Prsim (version 7; GraphPad Software, Inc. San Diego, CA). No statistical method was used to pre-determine sample size. Covariate analyses between 12,13-diHOME and body composition parameters were assessed using Spearman and Pearson correlation coefficients. The statistical parameters and the number of mice or human subjects used per experiment are found in the figure legends.
Supplementary Material
Representative imaging of FFA-SS-Luc uptake in Acta1cre+/−Rosa(stop)Luc+/− injected intravenously with luciferin-conjugated fatty acid and 12,13-diHOME or vehicle. Data from individual images using sequential, thirty second exposures over approximately 15 minutes was stacked into a movie. The animal on the right is the vehicle treated and the mouse on the left is treated with 12,13-diHOME.
Table S1, related to Figure 1. Signaling lipids in humans pre- vs. post-exercise.
Table S2, related to Figure 1. Spearman and Pearson correlation coefficient with % Fat Mass as a covariate.
Table S3, related to Figure 2. Signaling lipids in mouse BAT, Sedentary vs. Trained.
Table S4, related to Figures 2, S2. Primer sets used in this study.
Figure S1, related to Figure 1. Lipidomics in human subjects after acute exercise.
Figure S2, related to Figures 2 and 4. Body mass after exercise; gene expression after acute injection of 12,13-diHOME.
Figure S3, related to Figure 4. 12,13-diHOME does not affect glucose uptake into skeletal muscle.
Highlights.
Exercise increases circulating levels of the lipokine 12,13-diHOME in humans and mice.
Intrascapular BAT is the tissue source for the exercise-stimulated increase in 12,13-diHOME in mice.
12,13-diHOME increases fatty acid uptake and oxidation in skeletal muscle of mice.
Acknowledgments
This work was supported by National Institutes of Health Grants R01-HL138738 and K01-DK105109 (to K.I.S.); R01-DK099511 and R01-DK112283 (to L.J.G.); and 5P30 DK36836 (Joslin Diabetes Center DERC). The human studies at Florida Hospital were supported by funding from the National Institutes of Health K01-AG044437 (to PMC); correspondence for the Florida study should be directed to paul.coen@flhosp.org. The authors gratefully appreciate the contributions of our study participants and acknowledge the excellent technical assistance of the core staff at TRI-MD, Florida Hospital and the Joslin Diabetes Center.
Footnotes
Author contributions
KIS and LJG conceived and designed the study. KIS, MDL, HT, LAB, PJA, FJM, ACL, MFH, VKS, and KS performed mouse experiments. KIS and HT performed cell culture experiments. EYC, FG, NRN, and MAK performed lipidomics experiments. FJM, LAB, PJA, and ACL performed the gene expression experiments. KIS analyzed the lipidomics data with input from MAK and LJG. PMC was responsible for the human study concept and design at Florida Hospital. RJWM, JJR, and LJG were responsible for the human study concept and design at the Joslin Diabetes Center. GD conducted the human muscle respiration assays. YHT and MTZ provided oversight for the in vivo experiments. KIS, PMC and LJG interpreted the results, prepared figures, and completed statistical analysis. KIS and LJG wrote the manuscript. All authors approved the final version of the manuscript.
Declaration of Interests
Authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beltz NM, Gibson AL, Janot JM, Kravitz L, Mermier CM, Dalleck LC. Graded Exercise Testing Protocols for the Determination of VO2max: Historical Perspectives, Progress, and Future Considerations. Journal of sports medicine (Hindawi Publishing Corporation) 2016;2016:3968393. doi: 10.1155/2016/3968393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce RA. Exercise testing of patients with coronary heart disease. Principles and normal standards for evaluation. Annals of clinical research. 1971;3:323–332. [PubMed] [Google Scholar]
- Burhans MS, Flowers MT, Harrington KR, Bond LM, Guo CA, Anderson RM, Ntambi JM. Hepatic oleate regulates adipose tissue lipogenesis and fatty acid oxidation. Journal of lipid research. 2015;56:304–318. doi: 10.1194/jlr.M054429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnero EA, Dubis GS, Hames KC, Jakicic JM, Houmard JA, Coen PM, Goodpaster BH. Randomized trial reveals that physical activity and energy expenditure are associated with weight and body composition after RYGB. Obesity (Silver Spring) 2017 doi: 10.1002/oby.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen PM, Jubrias SA, Distefano G, Amati F, Mackey DC, Glynn NW, Manini TM, Wohlgemuth SE, Leeuwenburgh C, Cummings SR, et al. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. The journals of gerontology Series A, Biological sciences and medical sciences. 2013;68:447–455. doi: 10.1093/gerona/gls196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen PM, Menshikova EV, Distefano G, Zheng D, Tanner CJ, Standley RA, Helbling NL, Dubis GS, Ritov VB, Xie H, et al. Exercise and Weight Loss Improve Muscle Mitochondrial Respiration, Lipid Partitioning and Insulin Sensitivity Following Gastric Bypass Surgery. Diabetes. 2015 doi: 10.2337/db15-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell metabolism. 2013;17:162–184. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annual review of medicine. 1998;49:235–261. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998;47:1369–1373. doi: 10.2337/diab.47.8.1369. [DOI] [PubMed] [Google Scholar]
- Henkin AH, Cohen AS, Dubikovskaya EA, Park HM, Nikitin GF, Auzias MG, Kazantzis M, Bertozzi CR, Stahl A. Real-time noninvasive imaging of fatty acid uptake in vivo. ACS chemical biology. 2012;7:1884–1891. doi: 10.1021/cb300194b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J, Sportsman R, Harris J, Stahl A. Real-time quantification of fatty acid uptake using a novel fluorescence assay. Journal of lipid research. 2005;46:597–602. doi: 10.1194/jlr.D400023-JLR200. [DOI] [PubMed] [Google Scholar]
- Liu S, Brown JD, Stanya KJ, Homan E, Leidl M, Inouye K, Bhargava P, Gangl MR, Dai L, Hatano B, et al. A diurnal serum lipid integrates hepatic lipogenesis and peripheral fatty acid use. Nature. 2013;502:550–554. doi: 10.1038/nature12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynes MD, Leiria LO, Lundh M, Bartelt A, Shamsi F, Huang TL, Takahashi H, Hirshman MF, Schlein C, Lee A, et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nature medicine. 2017;23:631–637. doi: 10.1038/nm.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motiani P, Virtanen KA, Motiani KK, Eskelinen JJ, Middelbeek RJ, Goodyear LJ, Savolainen AM, Kemppainen J, Jensen J, Din MU, et al. Decreased insulin-stimulated brown adipose tissue glucose uptake after short-term exercise training in healthy middle-aged men. Diabetes, obesity & metabolism. 2017 doi: 10.1111/dom.12947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedachi T, Kanzaki M. Regulation of glucose transporters by insulin and extracellular glucose in C2C12 myotubes. American journal of physiology Endocrinology and metabolism. 2006;291:E817–828. doi: 10.1152/ajpendo.00194.2006. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Shanely RA, Luo B, Meaney MP, Dew DA, Pappan KL. Metabolomics approach to assessing plasma 13- and 9-hydroxy-octadecadienoic acid and linoleic acid metabolite responses to 75-km cycling. American journal of physiology Regulatory, integrative and comparative physiology. 2014;307:R68–74. doi: 10.1152/ajpregu.00092.2014. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiological reviews. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- Powell WS. Extraction of eicosanoids from biological fluids, cells, and tissues. Methods in molecular biology (Clifton, NJ) 1999;120:11–24. doi: 10.1385/1-59259-263-5:11. [DOI] [PubMed] [Google Scholar]
- Pruchnic R, Katsiaras A, He J, Kelley DE, Winters C, Goodpaster BH. Exercise training increases intramyocellular lipid and oxidative capacity in older adults. American journal of physiology Endocrinology and metabolism. 2004;287:E857–862. doi: 10.1152/ajpendo.00459.2003. [DOI] [PubMed] [Google Scholar]
- Sisemore MF, Zheng J, Yang JC, Thompson DA, Plopper CG, Cortopassi GA, Hammock BD. Cellular characterization of leukotoxin diol-induced mitochondrial dysfunction. Archives of biochemistry and biophysics. 2001;392:32–37. doi: 10.1006/abbi.2001.2434. [DOI] [PubMed] [Google Scholar]
- Stanford KI, Goodyear LJ. Exercise regulation of adipose tissue. Adipocyte. 2016;5:153–162. doi: 10.1080/21623945.2016.1191307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford KI, Middelbeek RJ, Goodyear LJ. Exercise Effects on White Adipose Tissue: Beiging and Metabolic Adaptations. Diabetes. 2015a;64:2361–2368. doi: 10.2337/db15-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford KI, Middelbeek RJ, Townsend KL, Lee MY, Takahashi H, So K, Hitchcox KM, Markan KR, Hellbach K, Hirshman MF, et al. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes. 2015b;64:2002–2014. doi: 10.2337/db14-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend KL, An D, Lynes MD, Huang TL, Zhang H, Goodyear LJ, Tseng YH. Increased mitochondrial activity in BMP7-treated brown adipocytes, due to increased CPT1- and CD36-mediated fatty acid uptake. Antioxidants & redox signaling. 2013;19:243–257. doi: 10.1089/ars.2012.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernochet C, Mourier A, Bezy O, Macotela Y, Boucher J, Rardin MJ, An D, Lee KY, Ilkayeva OR, Zingaretti CM, et al. Adipose-specific deletion of TFAM increases mitochondrial oxidation and protects mice against obesity and insulin resistance. Cell metabolism. 2012;16:765–776. doi: 10.1016/j.cmet.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosselman MJ, Hoeks J, Brans B, Pallubinsky H, Nascimento EB, van der Lans AA, Broeders EP, Mottaghy FM, Schrauwen P, van Marken Lichtenbelt WD. Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. International journal of obesity (2005) 2015 doi: 10.1038/ijo.2015.130. [DOI] [PubMed] [Google Scholar]
- Wu MV, Bikopoulos G, Hung S, Ceddia RB. Thermogenic capacity is antagonistically regulated in classical brown and white subcutaneous fat depots by high fat diet and endurance training in rats: impact on whole-body energy expenditure. The Journal of biological chemistry. 2014;289:34129–34140. doi: 10.1074/jbc.M114.591008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yore MM, Syed I, Moraes-Vieira PM, Zhang T, Herman MA, Homan EA, Patel RT, Lee J, Chen S, Peroni OD, et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell. 2014;159:318–332. doi: 10.1016/j.cell.2014.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative imaging of FFA-SS-Luc uptake in Acta1cre+/−Rosa(stop)Luc+/− injected intravenously with luciferin-conjugated fatty acid and 12,13-diHOME or vehicle. Data from individual images using sequential, thirty second exposures over approximately 15 minutes was stacked into a movie. The animal on the right is the vehicle treated and the mouse on the left is treated with 12,13-diHOME.
Table S1, related to Figure 1. Signaling lipids in humans pre- vs. post-exercise.
Table S2, related to Figure 1. Spearman and Pearson correlation coefficient with % Fat Mass as a covariate.
Table S3, related to Figure 2. Signaling lipids in mouse BAT, Sedentary vs. Trained.
Table S4, related to Figures 2, S2. Primer sets used in this study.
Figure S1, related to Figure 1. Lipidomics in human subjects after acute exercise.
Figure S2, related to Figures 2 and 4. Body mass after exercise; gene expression after acute injection of 12,13-diHOME.
Figure S3, related to Figure 4. 12,13-diHOME does not affect glucose uptake into skeletal muscle.