Abstract
We report a case of 56-year-old man presented to us with chief complaints of frothy urine and leg swelling. A urinalysis revealed nephrotic-range proteinuria. Haematological investigations revealed thrombocytosis, leucocytosis and peripheral blood smear showed a leucoerythroblastic picture. JAK 2 mutation was positive. To confirm the diagnosis of myeloproliferative neoplasm, bone marrow biopsy was done, which was suggestive of primary myelofibrosis. The patient underwent kidney biopsy due to rapidly declining renal function and persistent proteinuria, which was suggestive of focal segmental glomerulosclerosis. Early glomerulopathy is rare in myeloproliferative neoplasm, and aggressive follow-up is required to prevent progression of kidney disease.
Keywords: malignant and benign haematology, renal system, proteinurea
Background
Primary myelofibrosis is a myeloproliferative neoplasm characterised by clonal proliferation of myeloid cells. Prefibrotic primary myelofibrosis (PMF) is the least common among chronic myeloproliferative disorders with a reported incidence of 1.5 per 100 000 population.1 Renal involvement in myeloproliferative neoplasms is uncommon but extramedullary haematopoiesis can at times lead to obstructive uropathy and renal failure. Glomerulopathy is even more unusual in myeloproliferative neoplasm (MPN). According to literature, there are only a few reported cases of glomerular disease with myelofibrosis, hence we present an interesting case of focal segmental glomerulosclerosis with prefibrotic myelofibrosis.
Case presentation
A 56-year-old man presented to us with a history of frothy urine and leg oedema for the last 6 months. There was no history of fever, cough, shortness of breath and decrease urine output. There is no significant past medical history. No family member was suffering from similar illness. On examination, bilateral pedal oedema was present, the spleen was palpable 2 cm below left costal margin, rest examination was unremarkable. On further biochemical and haematological investigations, urine microscopy revealed significant proteinuria, a 24-hour urinary protein was 3.1 g and serum creatinine was 2.1 mg/dL. Complete blood count revealed haemoglobin (Hb)—13.0 g/dL, white cell count—28×109/L and platelet count of 842×109/L (table 1). The peripheral blood smear was suggestive of the leucoerythroblastic picture with few tear drop cells. Serum lactate dehydrogenase (LDH) was raised (1142 IU/L). To confirm the diagnosis of myeloproliferative disorders, bone marrow biopsy was done which was suggestive of PMF(figures 1 and 2). In addition, the JAK 2 V617F mutation was also positive. Breakpoint cluster region-Abelson (BCR-ABL) translocation was absent. Our patient was classified in intermediate risk-1 according to Dynamic International Prognostic Scoring System. The patient was put on cytoreductive therapy with hydroxyurea (1000 mg/day). Subsequently, the patient was referred to nephrology department to determine the cause of progressive renal dysfunction. After 2 weeks, the patient presented in nephrology department with repeat urinalysis showing persistent nephrotic range proteinuria and serum creatinine of 2.3 mg/dL. Ultrasound abdomen was suggestive of the mild renal parenchymal disease. Viral markers (HbsAg, antihepatitis C virus, HIV) were negative. Serum electrophoresis study and antinuclear antibodies were negative (table 1). Renal biopsy was performed to know the underlying cause of rapidly declining renal dysfunction and proteinuria. Light microscopy section of kidney biopsy shows total 22 glomeruli out of which, 10 are globally sclerosed, 1 of the glomeruli shows ischaemic wrinkling and 2 of the glomeruli show segmental sclerosis (figure 3). All the medium-sized vessels and arterioles show medial thickening and luminal narrowing with hyaline arteriosclerosis (figure 4). The patient was prescribed corticosteroid in a tapering dose. After 1 month of follow-up, the patient was symptomatically better, urine microscopy revealed proteinuria in traces with creatinine 1.3 mg/dL. Complete blood count showed Hb 11.3 g/dL, total leucocyte—6.9×109/L, platelet count—346×109/L.
Table 1.
Biochemical and haematological investigations
| White cell count | Hb—13.0 gm/dl, white cell count 28×109/L Platelets— 842×109/L, MCV—79.7 fL |
| Peripheral Blood film | Leucoerythroblastic picture, few tear drop cells |
| LDH | 1142 IU/L |
| JAK2 V617F | Positive |
| RT-PCR for BCR-ABLtranslocation | Negative |
| Urine microscopy | Protein ++++ |
| Creatinine | 2.1 mg/dL |
| 24-hour urine protein | 3.1 g |
| Liver function test | Total protein 5.0 g/dL, albumin 2.90 g/dL SGOT 52 U/L, SGPT 32 U/L |
| Viral markers | HbsAg—negative, anti-HCV—negative, HIV—negative |
Hb, haemoglobin; HbsAg, hepatitis B surface antigen; HCV, hepatitis C virus; LDH, lactate dehydrogenase; MCV, mean cell volume; SGOT, Serum Glutamic Oxaloacetic Transaminase; SGPT, Serum Glutamic Pyruvic Transaminase.
Figure 1.
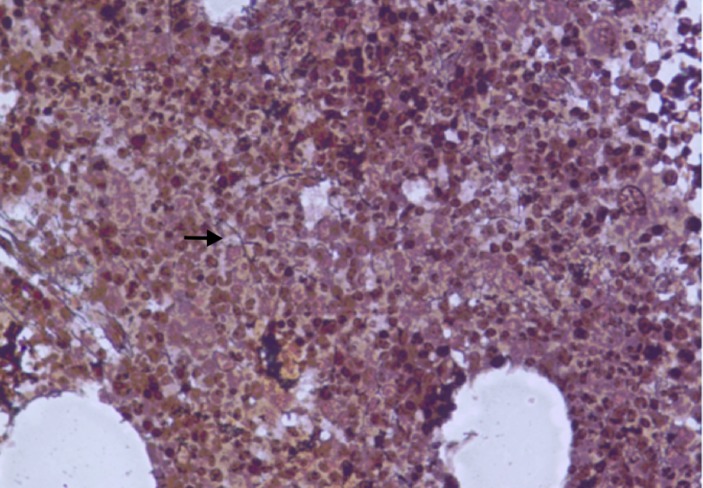
Bone marrow trephine biopsy showing megakaryocyte proliferation and atypia with hypercellularity (H&E).
Figure 2.
Reticulin stain of the bone marrow biopsy showing scattered linear reticulin with no intersection (black arrow).
Figure 3.
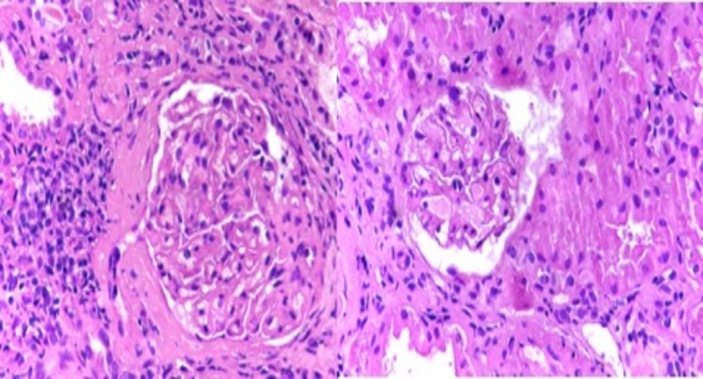
Light microscopic image showing glomerular tuft with segmental sclerosis, hypercellularity with loss of capillary lumen (focal segmental glomerulosclerosis) (H&E stain, 40×).
Figure 4.
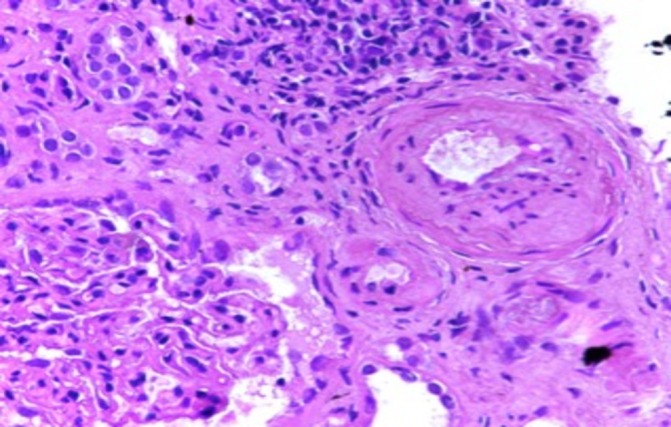
Light microscopic image of kidney biopsy with medium and small arteries showing medial thickening, lumen narrowing and tubules show regenerative changes (periodic acid–Schiff stain 40×).
Treatment
Hydroxyurea 1000 mg/day.
Prednisolone 1 mg/kg to be tapered in 1 month.
Outcome and follow-up
The patient was doing well at 3 months of follow-up with renal functions returning to normal. The patient developed anaemia probably due to the adverse effect of hydroxyurea, subsequently, the dose was reduced.
Discussion
Primary myelofibrosis is a myeloproliferative neoplasm arising from the neoplastic transformation of early haematopoietic stem cells.2 Bone marrow fibrosis (myelofibrosis) and extramedullary haematopoiesis are important features of PMF, which can involve any organ. The renal involvement is rare in MPN. Oesterling JE et al reported a case of obstructive uropathy caused by extramedullary haematopoiesis (EMH) in renal pelvis, ureter and bladder.3 Renal failure associated with hyperuricaemia and secondary amyloidosis with idiopathic myelofibrosis were also reported in some cases.4 Kwak and Lee reported a case of renal EMH in a patient of myelofibrosis.5 In our patient, kidney biopsy revealed focal segmental glomerulosclerosis (FSGS) without EMH and amyloidosis. FSGS is a histological lesion, characterised by the presence of sclerosis in parts (segmental) of some (focal) glomeruli on light microscopic examination. Secondary FSGS can occur due to drugs, toxins, viral infections (particularly HIV), vasculitis and diabetes. In our patient, none of these risk factors were present. Kaygusuz et al described a case of FSGS with myelofibrosis.6 Early renal involvement is uncommon in myelofibrosis. Said et al reported a case series of 11 patients with MPN-related glomerulopathy, which included 8 patients of PMF. The mean time from diagnosis of the neoplasm to kidney biopsy was 7.2 years in all cases.7
In our case, glomerulopathy was presented very early with prefibrotic myelofibrosis which was unusual. A similar case was earlier described by Rajasekaran et al with early MPN-related glomerulopathy in a 60-year-old man.8 The pathogenesis of glomerulopathy in MPN is still unclear. However, increased expression of platelet-derived growth factor and transforming growth factor-beta (TGF-beta) may have a role in glomerulosclerosis by enhancing the synthesis of collagen and fibronectin. TGF-beta also has a proapoptotic effect on podocytes which may promote podocyte depletion and FSGS lesions.9 The prognosis of MPN-related FSGS remains poor despite immunosuppressive therapy and treatment of underlying neoplasm. In our case, the patient improved after 1 month of steroid therapy. Long-term follow-up is required to know the exact course of glomerulopathy in MPN. It remains to be seen whether FSGS is a part of the wider spectrum of MPN-associated renal injury. We conclude that aggressive screening for proteinuria is required in PMF even at an early stage with long-term follow-up.
Learning points.
Myeloproliferative neoplasm (MPN)-related glomerulopathy can present as a part of the wider spectrum of renal disease in myeloproliferative disorders.
Though glomerulopathy usually appears late in course of MPN, it can present at an early stage.
Aggressive approach with proteinuria screening is needed to identify renal disease in myelofibrosis.
Long-term follow-up is required to know the nature of the progression of renal disease in MPN.
Footnotes
Contributors: DSM and GKB for concept, design and drafting of manuscript. AP and NB for analysis and critical revision. All authors approved the final version of manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Mesa RA, Silverstein MN, Jacobsen SJ, et al. Population-based incidence and survival figures in essential thrombocythemia and agnogenic myeloid metaplasia: an olmsted county study, 1976-1995. Am J Hematol 1999;61:10–15. [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A. Primary myelofibrosis: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol 2016;91:1262–71. 10.1002/ajh.24592 [DOI] [PubMed] [Google Scholar]
- 3.Oesterling JE, Keating JP, Leroy AJ, et al. Idiopathic myelofibrosis with myeloid metaplasia involving the renal pelves, ureters and bladder. J Urol 1992;147:1360–2. 10.1016/S0022-5347(17)37566-3 [DOI] [PubMed] [Google Scholar]
- 4.Ferhanoğlu B, Erzin Y, Başlar Z, et al. Secondary amyloidosis in the course of idiopathic myelofibrosis. Leuk Res 1997;21:897–8. 10.1016/S0145-2126(97)00049-0 [DOI] [PubMed] [Google Scholar]
- 5.Kwak HS, Lee JM. CT findings of extramedullary hematopoiesis in the thorax, liver and kidneys, in a patient with idiopathic myelofibrosis. J Korean Med Sci 2000;15:460–2. 10.3346/jkms.2000.15.4.460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaygusuz I, Koc M, Arikan H, et al. Focal segmental glomerulosclerosis associated with idiopathic myelofibrosis. Ren Fail 2010;32:273–6. 10.3109/08860220903573286 [DOI] [PubMed] [Google Scholar]
- 7.Said SM, Leung N, Sethi S, et al. Myeloproliferative neoplasms cause glomerulopathy. Kidney Int 2011;80:753–9. 10.1038/ki.2011.147 [DOI] [PubMed] [Google Scholar]
- 8.Rajasekaran A, Ngo TT, Abdelrahim M, et al. Primary myelofibrosis associated glomerulopathy: significant improvement after therapy with ruxolitinib. BMC Nephrol 2015;16:121 10.1186/s12882-015-0121-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wada T, Pippin JW, Terada Y, et al. The cyclin-dependent kinase inhibitor p21 is required for TGF-beta1-induced podocyte apoptosis. Kidney Int 2005;68:1618–29. 10.1111/j.1523-1755.2005.00574.x [DOI] [PubMed] [Google Scholar]