Abstract
In total knee arthroplasty (TKA) and total hip replacement (THR) the restoration of the normal joint function represents a fundamental feature. A prosthetic joint must be able to provide motions and to transmit functional loads.
As reported in the literature, the stress distribution may be altered in bones after the implantation of a total joint prosthesis. Some scientific works have also correlated uncemented TKA to a progressive decrease of bone density below the tibial component. Antibiotic-loaded bone cements are commonly employed in conjunction with systemic antibiotics to treat infections. Furthermore, nanoparticles with antimicrobial activity have been widely analysed. Accordingly, the current research was focused on a preliminary analysis of the mechanical and antibacterial activity of a PMMA-based bone cement loaded with gold nanoparticles. The obtained results demonstrated that nanocomposite cements with a specific concentration of gold nanoparticles improved the punching performance and antibacterial activity. However, critical aspects were found in the optimization of the nanocomposite bone cement.
Keywords: Nanocomposite, Bone cement, Gold nanoparticles, Antibacterial activity, Mechanical properties
Graphical abstract
Highlights
-
•
Evaluation of the in vitro effects of bacterial adhesion and proliferation on modified bone cement samples.
-
•
Assessment of anti-bacterial and anti-biofilm activities of the nanocomposite bone cement.
-
•
Analysis of the effect of the inclusion of gold nanoparticles on mechanical performances of a PMMA-based bone cement.
1. Introduction
The restoration of the normal joint function represents a crucial aspect in total knee arthroplasty (TKA) and total hip replacement (THR). A prosthetic joint must be able to provide motions and to transmit functional loads, thus long life resistance should be ensured. It needs to avoid disruption of the interface between implant and bone as well as to minimize damage in articulating surfaces. In the last few years, research attention has been focused on the possibility to make total joint prostheses more efficacious in relieving pain due to arthritis and also in restoring the stability and the mobility of a damaged joint [1], [2]. With regard to a total joint prosthesis, stress distribution may be altered and surrounding bone tissue normally adapts its density and structure to the current loads according to “Wolff's Law”. Using finite element analysis, many studies have demonstrated the potential alteration of the stress distribution in bones after the implantation of a total joint prosthesis [1], [3]. Some scientific works have also correlated uncemented TKA to a significant and progressive decrease of bone density below the tibial component, reaching 22% at 3 years follow-up.
Fixation of total joint components with bone cement is considered the current “gold standard” [4]. Excellent long-term results were found for cemented total arthroplasty and loosening of cemented components has been considered a rare complication in most large clinical series [1], [5], [6]. Cements based on self-polymerizing poly(methyl methacrylate) (PMMA) are the most used commercial synthetic biomaterials for anchoring a prosthetic component to bone.
They consist of a solid powder phase made of PMMA and/or copolymers and a liquid monomer phase. Additionally, the powder contains dibenzoyl peroxide (BPO) as initiator for radical polymerization, a radiopaque medium and sometimes an antibiotic. The main components of the liquid phase are MMA and, in some bone cements, other esters of acrylic acid or methacrylic acid, one or more amines (i.e. activators/co-initiators for the formation of radicals), a stabilizer and, possibly, a colorant [7], [8]. With regard to hip joints, antibiotic-loaded bone cements are commonly used in conjunction with systemic antibiotics to treat infections. Recent studies have shown that the kinetics of the antibiotic elution from the cement is characterized by a maximum concentration in the first post-operative hours followed by a long tail characterized by lower concentrations. These concentrations may be sub-inhibitory, thus allowing for both the formation of a biofilm on the surface of the spacer and the antibiotic-resistance phenomena, with a consequent increase of treatment failure rates.
Different nanoparticles with antimicrobial activity are emerging as a new class of biomedical materials for their pronounced effect in reducing bacterial proliferation as a direct consequence of leakage leading to tissue and synthetic material degradation, causing the failure of the restoration or the implant [9].
As an example, the in vitro antimicrobial effect of dental restorative materials loaded with nanoparticles has been highlighted by do Amaral et al. (2016), even if to date the in vivo performance of such nanocomposites is still missing [10]. The efficacy of silver ions on a variety of bacterial strains of oral streptococci has been widely studied [11], [12], [13]. Stickler et al. (2000) suggested that 0.5% by weight of silver nanoparticles (AgNPs, 38 nm mean size) in an acrylic dental resin exhibited a remarkable antibacterial effect against the escherichia coli, while increasing mechanical properties [14]. Similar results have been found by Ahn et al. (2009) for an orthodontic adhesive containing 500 ppm of AgNPs (5 nm mean size) [15].
On the other hand, Melo et al. (2013) demonstrated that a remineralization effect was well evident for adhesive nanocomposites obtained including up to 40% by weight of amorphous calcium phosphate nanoparticles (mean size of 116 nm) and 0.08% of AgNPs (mean size of 2.7 nm), as a consequence of the release of calcium and phosphorus ions [16]. An antimicrobial effect due to the release of silver ions was also found [16].
A variety of nanoparticles are currently under investigation in order to improve antibacterial properties of non-degradable dental materials [17] or bone cements, i.e. titanium dioxide [18] and gold [19].
Prokopovich et al. showed that oleic acid capped AgNPs encapsulated into PMMA-based bone cement samples exhibited a potent antimicrobial activity against Methicillin Resistant Staphylococcus aureus, Staphylococcus epidermidis, Acinetobacter baumannii at nanoparticle concentrations as low as 0.05% (w/w). Furthermore, the mechanical properties and cytotoxicity of the bone cement containing these NPs were assessed to guarantee that such material is safe to be used in orthopaedic surgical practice [20].
Silver and gold nanoparticles (20–27 nm average size) were incorporated in PU (Polyurethane), PCLm (Polycaprolactam), PC (polycarbonate) and PMMA (Sawant et al., 2013) by swelling and casting methods under ambient conditions. The formation of E. coli biofilm has been highlighted on these nanocomposites, also providing a positive correlation between the contact angle of the neat surface and the number of attached colonies, carbohydrates and proteins. Bacterial attachment was highest on PC and least on PU nanocomposite. The inclusion of Ag and/or Au nanoparticles into the polymeric matrix seems to reduce growth of organisms, as well as proteins and carbohydrates. This study indicated that these nanocomposites could represent a valuable candidate for implant applications [21].
In this scenario, because of their pronounced activity in inhibiting the proliferation and multiplication of gram negative and gram positive bacteria, gold nanoparticles (AuNPS) are widely used in the experimental field for preventing contamination of the medical device in turn linked to the formation of a biofilm where different fractions of microorganism play specific roles.
In biofilm forming, bacteria are preserved from the unfavourable influence of the environment conditions [22]. They are protected from immune system response of the host (hinders phagocytosis, chemotaxis, opsonisation), thus allowing a decrease in antibiotics and antibodies penetration [23].
Initial bacterial adhesion, irreversible attachment, micro-colony formation, maturation and detachment represent the different stages in biofilm formation [22], [24]. Biofilm-submerged bacteria possess 1000-fold increased resistance to a majority of the antimicrobials [25].
In a challenging scenario of a continuous optimization of prosthetic implants and new diagnostic techniques, the knowledge of the structure-mechanical property-function relationships represents a crucial aspect.
In this context, the aim of the current research was to evaluate the in vitro effects of bacterial adhesion and proliferation on modified bone cement samples obtained by adding gold nanoparticles (10–20 nm in diameter). Using the confocal laser scanning microscopy (CLSM), the antibacterial and antibiofilm activities of the nanocomposite bone cement were evaluated by visualizing and quantifying the distribution of bacterial cells and the extracellular matrix (biofilm) deposition on the bone cement surface. Small punch tests and compression tests were employed to assess the effect of the inclusion of gold nanoparticles on the mechanical performances of a PMMA-based bone cement.
2. Materials and methods
High-viscosity Smartset Hv Bone Cement was kindly provided by DePuy International Ltd. Gold nanoparticles 10–20 nm in diameter (99.95% purity) were purchased from NanoAmor (Nanostructured and Amorphous Materials, Inc.).
Bacterial strains isolated from patients with peri-prosthetic joint infection were used: Methicillin Resistant Staphilococcus Aureus (MRSA) and Pseudomonas Aeruginosa. Bacterial strains were identified on the basis of their biochemical profile using automatic systems (Vitek, bio Merieux; Phoenix Becton Dickinson) and the proteomic profile by MALDI-TOF MS (Microflex LT; Bruker Daltonics).
2.1. Preparation of miniature disk-shaped specimens
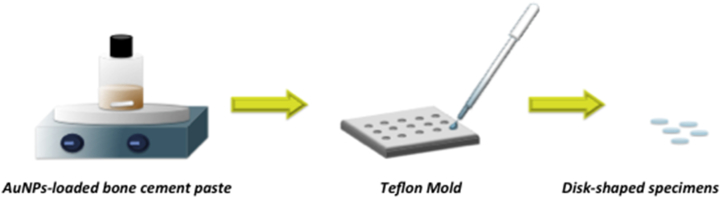
Miniature disk-shaped specimens with a diameter of 6.4 mm and a thickness of 0.5 mm were prepared using molding technique (Fig. 1). Briefly, different concentration of gold nanoparticles (0.25% w/w, 0.5% w/w and 1% w/w) were added to the solid phase of the Smartset Hv Bone cement and gently mixed with the liquid phase, in order to improve the antibacterial activity of the neat cement without negatively altering the mechanical performances. The obtained paste was poured into the mould to obtain miniature disk-shaped samples of nanocomposite cement. Miniature disk-shaped specimens of Smartset Hv bone cement were also used as control.
Fig. 1.
Schematic representation of the technique used to obtain disk-shaped specimens.
Using the same approach, cylindrical specimens (5.0 mm in diameter, 10.0 mm in height) were also prepared for further mechanical compression tests.
2.2. Small punch tests
Small punch tests were carried out on the miniature disk-shaped specimens with a diameter of 6.4 mm and a thickness of 0.5 mm according to the ASTM F2183 standard, in order to evaluate maximum load and displacement at maximum load. All the tests were performed using an INSTRON 5566 testing machine.
The experimental set-up consisted of a die, a hemispherical head punch and a guide for the punch. The specimen was loaded axisymmetrically in bending by the hemispherical head punch at a constant displacement rate of 0.5 mm/min until failure occurred. The values of load and displacement of the punch were recorded continuously while performing the test.
2.3. Compression tests
Compression tests were performed on the cylindrical specimens in order to highlight the effect of the inclusion of gold nanoparticles on the mechanical properties of bone cement samples.
Each cylindrical specimen was characterized by a diameter of 5.0 mm and a height (h0) of 10.0 mm. All the tests were carried out at a rate of 1 mm/min up to a strain value of 0.3 mm/mm, using an INSTRON 5566 testing system.
The engineering stress (σ) was evaluated as follows:
| σ = F/A |
where F is the load and A is the total area of the cross section of the specimen.
The engineering strain (ε) was evaluated as the ratio between the height variation (Δh) and initial height (h0) of the specimen:
| ε = Δh/h0 |
Mechanical results were reported as mean ± standard deviation.
2.4. Scanning electron microscopy
Scanning electron microscopy (SEM) was used to evaluate the morphology of the bone cement samples obtained by adding different amounts of AuNPs (0.25%, 0.5%, 1% by weight), after mechanical tests. SEM images were collected through a scanning electron microscope (FEI Quanta FEG 200, The Netherlands).
2.5. Antibacterial activity
For each bacterial strain, miniature disk-shaped AuNPs-loaded bone cement samples (0.25%, 0.5%, 1% by weight) and controls (disk-shaped sample bone cement) were placed in Petri flasks containing 3 ml of Brain-Heart Infusion (BHI) medium with 10% (w/v) glucose. The medium was inoculated with 200 μl (0.5 McFarland) of bacterial strain and incubated in aerobic conditions. After 24 h at 37 °C, each specimen was removed from the flask and placed in another petri flask containing 3 ml of fresh BHI with 10% (w/v) glucose and incubated in aerobic conditions at 37 °C for 24 h. Specimens of AuNPs-loaded bone cement and negative controls were washed twice with sterile phosphate buffered saline (PBS) to remove non-adherent bacteria. The adherent biofilm was fixed and observed under Confocal Laser Scanning Microscopy (Leica Microsystem) after staining with the live/dead BacLight viability stain (Life Technologies, Monza, Italy), containing SYTO 9 dye and propidium iodide (PI).
Images from five randomly selected areas were acquired for each disk-shaped sample. Sequential optical sections of 2 μm were collected in sequence along the z-axis over the complete thickness of the sample. The resulting stacks of images were analysed and quantified, thus obtaining information related to the biofilm thickness on AuNPs-loaded bone cement specimens, compared with the controls.
The experiments were repeated at least three times in triplicate.
2.6. Statistical analysis
The results were analysed using ANOVA followed by Tukey post-hoc test and statistical differences were set at p < 0.05.
3. Results and discussion
3.1. Small punch tests
As a first step towards the evaluation of the mechanical properties of nanocomposite and neat bone cement, small punch test was properly selected. Small punch test is a reproducible miniature specimen test method, which was already considered to evaluate the mechanical properties of retrieved acrylic bone cement, and PCL reinforced with sol–gel synthesized organic–inorganic hybrid fillers [26], [27].
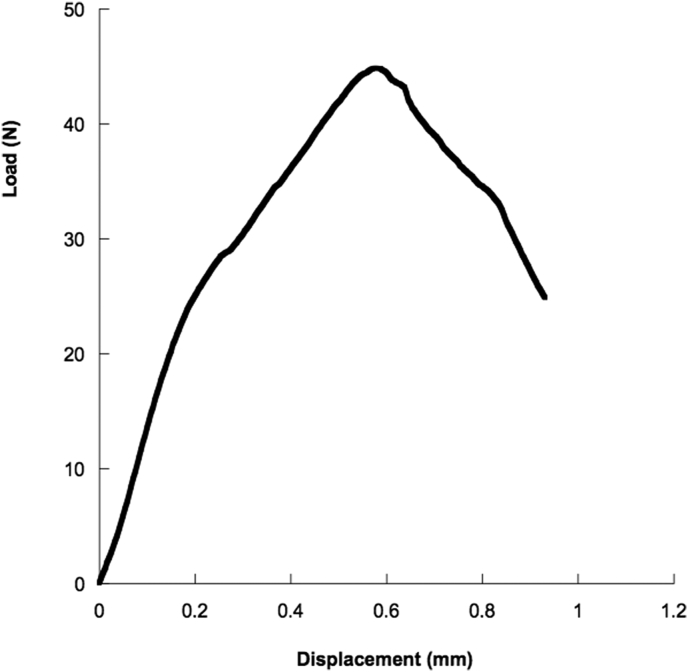
Load–displacement curves obtained from small punch tests on disk–shaped bone-cement specimens were generally characterized by an initial linear region, followed by a decrease in the curve slope until a maximum load is reached. Finally, a decrease in the load was evident until failure occurred (Fig. 2).
Fig. 2.
Typical load-displacement curve obtained from small punch tests performed on bone cement miniature disk-shaped samples.
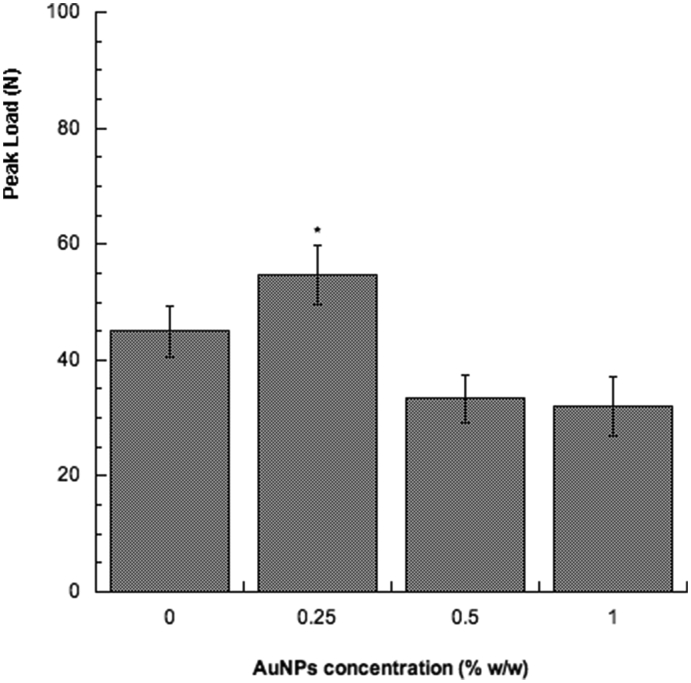
The inclusion of 0.25% by weight of AuNPs would seem to represent an effective reinforcement in terms of mechanical properties (Fig. 3), providing values of peak load which were higher than those obtained for the neat bone cement and for the other compositions of the nanocomposite bone cement.
Fig. 3.
Peak load (N) obtained from small punch tests performed on the proposed bone cement miniature disk-shaped samples. Bars represent standard deviation. *p < 0.05 indicates statistically significant differences between neat bone cement and nanocomposites (ANOVA followed by Tukey post hoc test).
Thus, small punch tests evidenced that beyond a specific limit of nanoparticle amount, the mechanical strength of the nanocomposite bone cement decreased.
The obtained results are consistent with those reported in the literature and may be ascribed to the formation of NP clusters by further increasing the nanoparticle amount, which may act as weak points for the structure, and/or to the different ductility between the polymeric matrix and the nanoparticles. Accordingly, a different ductility between the polymeric matrix and the nanofillers can provide a weakness in the polymer-based composite structures, generating stress concentration and discontinuities in the stress transfer mechanism at the matrix/filler interface [26], [28].
3.2. Compression tests
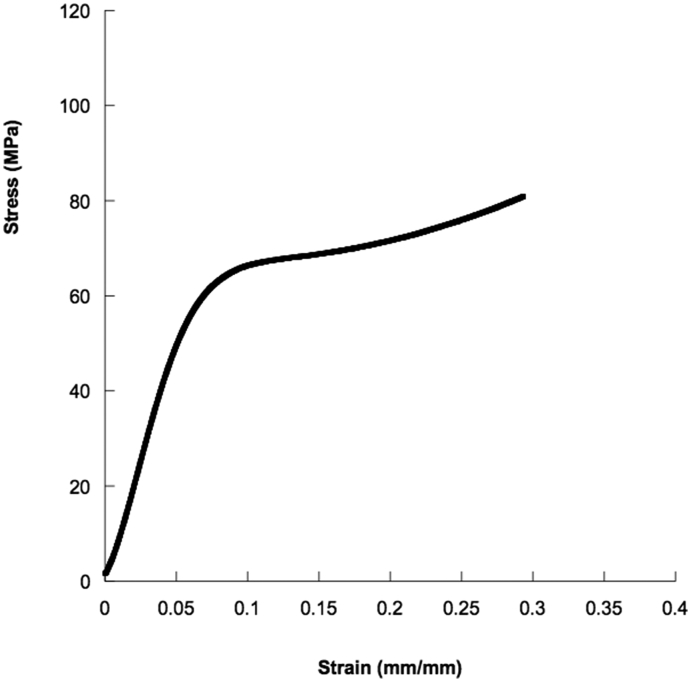
Results from compression tests on cylindrical specimens showed stress–strain curves characterized by an initial linear region (i.e., initial stiff mechanical response), followed by a zone with lower stiffness and a final stiff region (Fig. 4).
Fig. 4.
Typical stress-strain curve obtained from compression tests performed on bone cement samples.
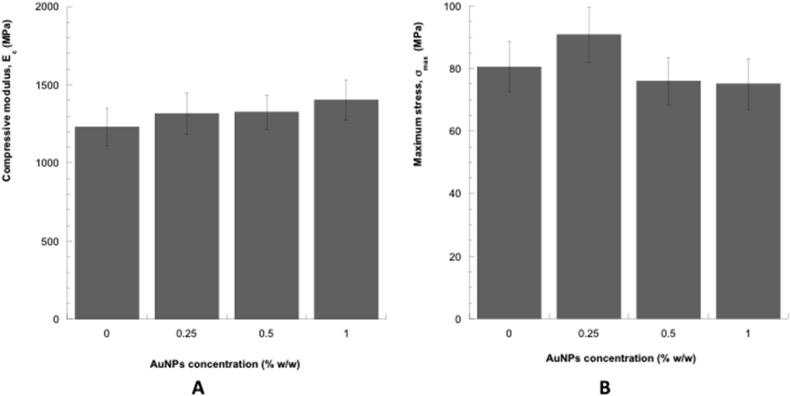
Anyway, the inclusion of AuNPs slightly improved the compressive modulus of the bone cement. In particular, the mean value of the compressive modulus for the nanocomposite bone cement obtained by adding 1% by weight of AuNPs was greater than that obtained for the neat bone cement and for the other compositions (Fig. 5).
Fig. 5.
(A) Compressive modulus (Ec,) and (B) maximum stress at a strain of 0.3 mm/mm (σmax,) obtained from compression tests performed on the different cylindrical samples. Bars represent standard deviation.
On the other hand, the inclusion of 0.25% by weight of AuNPs seemed to improve the maximum stress of the bone cement, whereas a reduction was observed by further increasing the AuNPs concentration. However, no statistically significant differences were detected between neat bone cement and nanocomposites.
3.3. Scanning electron microscopy
SEM analyses performed on the proposed samples allowed to visualize the presence of defects and/or empty spaces mainly generated during mechanical tests. Furthermore, morphological features related to the PMMA-based bone cement/filler interface and also the presence of clusters were observed (Fig. 6).
Fig. 6.
Typical SEM images of different sections of bone cement samples (left) and AuNPs-loaded bone cement samples (middle and right), after mechanical tests.
3.4. Antibacterial activity
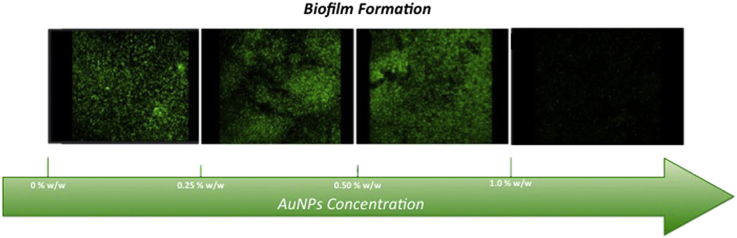
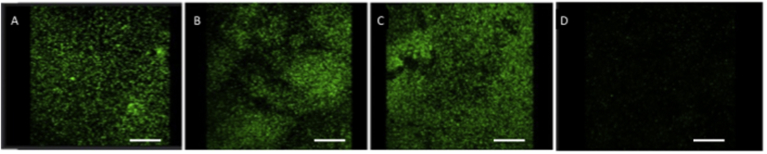
Fig. 7 shows CLSM images obtained through live/dead BacLight viability stain performed on the surface of all AuNPs-loaded bone cement disks or control samples. It is worth noting that living bacteria and biofilm appear greater for bone cement loaded with 0.25% and 0.50% by weight of gold nanoparticles, if compared to the data obtained for samples loaded with 1% by weight of gold nanoparticles. A reduction in thickness of the biofilm and also a decreasing in the ratio between live and dead bacteria per unit area have been observed. Live bacterial cells reduced up to 54% and 56% for MRSA and Pseudomonas, respectively, on bone cement samples obtained by adding 1% by weight of AuNPs, with respect to the control samples (without AuNPs). Furthermore, 3D reconstruction of the biofilm has allowed to observe a decrease of the biofilm thickness by approximately 74%.
Fig. 7.
Confocal laser scanning microscopy shows the formation of MRSA biofilm on cement specimens. Cement without AuNPs (control), (A); Cement-AuNPs (0.25% w/w), (B); Cement-AuNPs (0.50% w/w), (C); Cement-AuNPs (1% w/w), (D). Biofilms were stained with live/dead BacLight viability stain (Life Technologies, Monza, Italy): SYTO9 (green) represents viable cells; propidium iodide (red) represents dead cells. Cement-AuNPs 1% by weight represents the most effective in reducing biofilm formation. In all images the scale bar = 30 μm.
4. Conclusion
Mechanical analyses provided interesting information on the effect of the inclusion of AuNPs into a PMMA-based bone cement. Even if the addition of nanoparticles generally improves the mechanical properties of a polymeric matrix, it is also worth noting that the nanoparticles may act as “weak points” beyond a threshold concentration and the performance of the nanocomposites decreases.
In particular, it was found that the inclusion of 0.25% by weight of AuNPs significantly improved the punching performances, without negatively altering the compressive properties of the bone cement.
On the other hand, the most effective solution in the formation of a biofilm on the cement was achieved by a concentration of 1.0% by weight. This should prevent failures and consequent further surgery.
This preliminary research has evidenced some critical features in the optimization of AuNPs-loaded bone cement, which should enhance the mechanical performances of the neat cement, while reducing the thickness of the biofilm and the ratio live/dead bacterial cells. For this reason, the optimization of nanocomposites and the prediction of the mechanical behavior may be achieved by integrating experimental tests and mathematical models [29], [30].
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Ronca D., Gloria A., De Santis R., Russo T., D'Amora U., Chierchia M., Nicolais L., Ambrosio L. Critical analysis on dynamic-mechanical performance of spongy bone: the effect of an acrylic cement. Hard Tissue. 2014;3:1–7. [Google Scholar]
- 2.Font-Rodriguez D.E., Scuderi G.R., Insall J.N. Survivorship of cemented total knee arthroplasty. Clin. Orthop. Relat. Res. 1997;345:79–86. [PubMed] [Google Scholar]
- 3.Huiskes R., Weinans H., Grootenboer H.J., Dalstra M., Fudala B., Slooff T.J. Adaptive bone-remodeling theory applied to prosthetic–design analysis. J. Biomech. 1987;20:1135–1150. doi: 10.1016/0021-9290(87)90030-3. [DOI] [PubMed] [Google Scholar]
- 4.Vaishya R., Chauhan M., Vaish A. Bone cement. J. Clin. Orthop. Trauma. 2013;4:157–163. doi: 10.1016/j.jcot.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranawat C.S., Flynn W.F., Deshmukh R.G. Impact of modern technique on long-term results of total condylar knee arthroplasty. Clin. Orthop. Relat. Res. 1994;309:131–135. [PubMed] [Google Scholar]
- 6.Ritter M.A., Herbst S.A., Keating E.M., Faris P.M., Meding J.B. Long term survival analysis of a posterior cruciate retaining total condylar total knee arthroplasty. Clin. Orthop. Relat. Res. 1994;309:136–145. [PubMed] [Google Scholar]
- 7.Kuhn K.D. Spinger-Verlag; Heidelberg: 2000. Bone Cements. [Google Scholar]
- 8.De Santis R., Mollica F., Ronca D., Ambrosio L., Nicolais L. Dynamic mechanical behaviour of PMMA based bone cements in wet environment. J. Mater Sci. Mater Med. 2003;14:583–594. doi: 10.1023/a:1024014822277. [DOI] [PubMed] [Google Scholar]
- 9.Espelid I., Tveit A.B., Erickson R.L., Keck S.C., Glasspoole E.A. Radiopacity of restorations and detection of secondary caries. Dent. Mater. 1991;7:114–117. doi: 10.1016/0109-5641(91)90056-5. [DOI] [PubMed] [Google Scholar]
- 10.do Amaral G.S., Cássia Negrini T., Maltz M., Arthur R.A. Restorative materials containing antimicrobial agents: is there evidence for their antimicrobial and anticaries effects? A systematic review. Aust. Dent. J. 2016;61:6–15. doi: 10.1111/adj.12338. [DOI] [PubMed] [Google Scholar]
- 11.Rogers L. The bactericidal action of organic silver salts and other antiseptics on the dysentery Bacillus. Indian J. Med. Res. 1913;1:263–269. [Google Scholar]
- 12.Morones J.R., Elechiguerra J.L., Camacho A., Holt K., Kouri J.B., Ramírez J.T., Yacaman M.J. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto K., Ohashi S., Aono M., Kokubo T., Yamada I., Yamauchi J. Antibacterial activity of silver ions implanted in SiO2 filler on oral streptococci. Dent. Mater. 1996;12:227–229. doi: 10.1016/s0109-5641(96)80027-3. [DOI] [PubMed] [Google Scholar]
- 14.Stickler D.J. Biomaterials to prevent nosocomial infections: is silver the gold standard? Curr. Opin. Infect. Dis. 2000;13:389–393. doi: 10.1097/00001432-200008000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Ahn S.J., Lee S.J., Kook J.K., Lim B.S. Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dent. Mater. 2009;25:206–213. doi: 10.1016/j.dental.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Melo M.A., Cheng L., Zhang K., Weir M.D., Rodrigues L.K., Xu H.H. Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate. Dent. Mater. 2013;29:199–210. doi: 10.1016/j.dental.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Souza G.M. Nanoparticles in restorative materials. In: Kishen A., editor. Nanotechnology in Endodontics. Springer International Publishing; 2015. pp. 139–171. [Google Scholar]
- 18.Poosti M., Ramazanzadeh B., Zebarjad M., Javadzadeh P., Naderinasab M., Shakeri M.T. Shear bond strength and antibacterial effects of orthodontic composite containing TiO2 nanoparticles. Eur. J. Orthod. 2013;35:676–679. doi: 10.1093/ejo/cjs073. [DOI] [PubMed] [Google Scholar]
- 19.Tay F.R., Durán G., Paula A.J., Durán N. Advances in dental materials through nanotechnology: facts, perspectives and toxicological aspects. Trends Biotechnol. 2015;33:621–636. doi: 10.1016/j.tibtech.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Prokopovich P., Köbrick M., Brousseau E., Perni S. Potent antimicrobial activity of bone cement encapsulating silver nanoparticles capped with oleic acid. J. Biomed. Mater. Res. B Appl. Biomater. 2015;103:273–281. doi: 10.1002/jbm.b.33196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawant S.N., Selvaraj V., Prabhawathi V., Doble M. Antibiofilm properties of silver and gold incorporated PU, PCLm, PC and PMMA nanocomposites under two shear conditions. PLoS One. 2013;8 doi: 10.1371/journal.pone.0063311. e63311 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolwzan B. Analysis of the phenomenon of biofilm - the conditions of its formation and functioning. Ochr. Sr. 2011;33:3–14. [Google Scholar]
- 23.Bartoszewicz M., Rygiel A. Biofilm as the basic mechanism of surgical site infection-prevention methods in topical treatment. Chir. Pol. 2006;8:171–178. [Google Scholar]
- 24.O'Toole G., Kaplan H.B., Kolter R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 25.Czaczyk K., Myszka K. The mechanisms of bacterial biofilms resistance to antimicrobial agents. Biotechnologia. 2007;76:40–52. [Google Scholar]
- 26.Russo T., Gloria A., D-Antò V., D'Amora U., Ametrano G., Bollino F., De Santis R., Ausanio G., Catauro M., Rengo S., Ambrosio L. Poly(ε-caprolactone) reinforced with sol – gel synthesized organic – inorganic hybrid fillers as composite substrates for tissue engineering. J. Appl. Biomater. Biomech. 2010;8:146–152. [PubMed] [Google Scholar]
- 27.Dunne N.J., Leonard D., Daly C., Buchanan F.J., Orr J.F. Validation of the small punch test as a technique for characterizing the mechanical properties of acrylic bone cement. Proc. Inst. Mech. Eng. H. 2006;220:11–21. doi: 10.1243/095441105X68980. [DOI] [PubMed] [Google Scholar]
- 28.Gloria A., Russo T., D'Amora U., Zeppetelli S., D'Alessandro T., Sandri M., Bañobre-López M., Piñeiro-Redondo Y., Uhlarz M., Tampieri A., Rivas J., Herrmannsdörfer T., Dediu V.A., Ambrosio L., De Santis R. Magnetic poly(ε-caprolactone)/iron-doped hydroxyapatite nanocomposite substrates for advanced bone tissue engineering. J. R. Soc. Interface. 2013;10 doi: 10.1098/rsif.2012.0833. 20120833 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Domingos M., Gloria A., Coelho J., Bartolo P., Ciurana J. Three-dimensional printed bone scaffolds: the role of nano/micro-hydroxyapatite particles on the adhesion and differentiation of human mesenchymal stem cells. Proc. Inst. Mech. Eng. H. 2016:1–10. doi: 10.1177/0954411916680236. [DOI] [PubMed] [Google Scholar]
- 30.Nicolais L., Gloria A., Ambrosio L. The mechanics of biocomposites. In: Ambrosio L., editor. Biomedical Composites. CRC Press; London, UK: 2010. pp. 411–440. [Google Scholar]