Abstract
Over the past few years, the influence of static or dynamic magnetic fields on biological systems has become a topic of considerable interest. Magnetism has recently been implicated to play significant roles in the regulation of cell responses and, for this reason, it is revolutionizing many aspects of healthcare, also suggesting new opportunities in tissue engineering.
The aim of the present study was to analyze the effect of the application mode of a time-dependent magnetic field on the behavior of human mesenchymal stem cells (hMSCs) seeded on 3D additive-manufactured poly(ɛ-caprolactone)/iron-doped hydroxyapatite (PCL/FeHA) nanocomposite scaffolds.
Keywords: Additive manufacturing, Scaffold, Magnetic field, Cell-material interaction
Graphical abstract
Highlights
-
•
Additive Manufacturing for bone tissue engineering.
-
•
Analysis of the effect of the application mode of a time-dependent magnetic field on the cell-behavior.
-
•
3D nanocomposite magnetic scaffolds.
1. Introduction
In the field of tissue engineering, one of the most promising challenges is to provide living constructs that are able to integrate with the surrounding tissues. To overcome the limitations related to the static culture systems, such as limited diffusion and inhomogeneous cell-matrix distribution, over the past years different kinds of scaffolds and bioreactors have been designed. As already recognized, scaffolds should possess a set of chemical, biochemical and morphological cues in order to promote and to control specific events at the cellular and tissue levels. On the contrary, the ideal feature of a bioreactor is that it should supply suitable levels of oxygen, nutrients, cytokines, growth factors, and mechanical stimulation for cell migration and scaffold colonization. A valuable candidate for tissue engineering applications should be a 3D additive-manufactured magnetic scaffold, which could satisfy the above reported requirements. It will provide a morphologically controlled and tailored structure with interconnected pores of different sizes [1], [2], [3], [4]. Furthermore, a magnetic scaffold could be used also as its own bioreactor. The possibility to magnetically switch-on/switch-off the scaffold could be used at the same time for delivering biomolecules, such as angiogenic factors, and stem cells, as well as for the stimulation of cell adhesion, proliferation and differentiation [1], [2], [3], [4].
In order to better understand the possible range of biomedical applications for magnetic devices and their potentials, the effects of magnetic fields on human tissues need to be well investigated.
The influence of static or variable magnetic fields on biological systems has become a topic of considerable interest. By this point of view, magnetism has been recently implicated to play significant roles in the regulation of cell responses. As reported in the literature, many works aimed to investigate the influence of a static magnetic field on the biological systems. It has also been demonstrated that Static Moderate-intensity Field (SMFs) (1 mT–1 T) are capable of affecting a number of biological phenomena such as cell proliferation [5], [6], migration [5], [7] and orientation [5], [8].
It has been found that SMFs have no lethal effect on cell growth and the cells have the ability to survive under normal growing conditions [9], [10], [11]. SMFs can prevent decrease in bone mineral density [5], [12], and promote the healing of bone fractures [5], [13], [14]. Using an electromagnetic bioreactor (magnetic field intensity, 2 mT; frequency, 75 Hz), Fassina L. et al. (2006) investigated the effect of the electromagnetic stimulation on proliferation and calcified matrix production of a human osteogenic sarcoma cell line (SAOS-2); cell proliferation was twice as high, the expression of decorin, osteocalcin, osteopontin, type I collagen, and type III collagen was greater and calcium deposition under magnetic stimulation was fivefold higher than that without electromagnetic stimulation [15].
Chiu K.H. et al. (2007) studied the potential differentiation of osteoblasts after treatment with a static magnetic field (0.4 T for 6 h) [16]. The authors highlighted that during SMF stimulation, the cell membrane could be assumed to be the target. Phospholipids can be oriented by SMF, resulting in an over-deformation of the cell membrane. SMF affects osteoblastic maturation by increasing the membrane rigidity, reducing its fluidity as well as the decreasing proliferation-promoting effects of growth factors at the membrane domain. Consequently, they observed an increase in the Alkaline Phosphatase (ALP) activity and a change in cell morphology [16]. A similar effect was observed by Feng S.W. et al. (2010). In particular, they studied the influence of a static magnetic field on osteoblast cells grown on poly(L-lactide) (PLLA) scaffolds. The results of their study suggested that human osteosarcoma cells (MG63) seeded on PLLA scaffolds and treated with SMF had a more differentiated phenotype. With regard to cell morphology, it was evidenced that the effect of SMFs depends on cell type and field strength [17].
Along these lines, the study from Sato K. et al. (1992) showed how the application of a static magnetic field of 1.5 T for 96 h on “immortal” human cervical cancer cells (HeLa) did not produce significant changes in cell shape [18]. Such results are in contrast with those obtained from Pacini S. et al. (1999), who reported dramatic changes in the morphology of human neural cells (FNC-B4) derived from human olfactory epithelium after exposure to a magnetic resonance tomography [19].
Pacini S. et al. (2003) reported an alteration of cell morphology of human skin fibroblasts associated with a decrease in the expression of glycoconjugate sugar residues when cells were exposed to a magnetic field of 0.2 T [20].
Other studies have suggested that static magnetic fields have a detrimental effect on cell proliferation [21]. Specifically, Cunha C. et al. (2012) analyzed for the first time the effect of a static moderate intensity magnetic field (320 mT) on MG-63 human osteosarcoma cells seeded in vitro on magnetic scaffolds. The application of a SMF, either continuously or applied for 1 h per day, resulted in a negative effect on cell proliferation and osteocalcin secretion.
However, the effect was not correlated with an increase in cell apoptosis, stress or disruption of membrane integrity and morphological features, and gene expression resulted unaltered [21].
Yun H. M. et al. (2016) investigated the combined effects of the external SMF with magnetic nanocomposite scaffolds made of polycaprolactone/magnetic nanoparticles on the osteoblastic functions and bone formation. The SMF synergized with the magnetic scaffolds in the osteoblastic differentiation of primary mouse calvarial osteoblasts, including the expression of bone-associated genes (Runx2 and Osterix) and ALP activity. Current findings suggested that the combined application of external (SMF) and internal (scaffold) magnetism can be a promising tool for bone regeneration [22].
Regarding the application of variable magnetic fields, several studies have shown that continuous and prolonged exposure of cells to magnetic fields modify cell physiological activities such as proliferation, synthesis and secretion of growth factors [9], [23], [24], [25]. These physiological changes largely depend on the physical properties of electromagnetic fields such as waveform and frequency, while the applied electromagnetic field dose is a function of field strength and exposure time [9], [26].
Some studies have also underlined how Extremely Low Frequency (ELF) magnetic fields may provide advantages for in vitro tissue generation [27], [28], stimulating angiogenesis and promoting bone formation [28].
Among the first published studies, Liboff A.R. et al. (1984) assessed the influence of a sinusoidal magnetic field with frequency of 76 Hz and intensity of 0.16 μT on the fibroblast proliferation. As a consequence of the application of the magnetic field, the authors found a positive effect in terms of cell proliferation [29].
In this context, De Mattei M. et al. (1999) evaluated the effect of a pulsed electromagnetic field with frequency of 75 Hz and 1.3 ms pulses on the proliferation process of different cell lines, showing promising results [30].
The effect of a magnetic field (intensity of 7 mT, frequency of 15 Hz) on the proliferation of mouse osteoblastic cell line (MC3T3-E1) was analyzed by Diniz P. et al. (2002), observing an increase in cell proliferation after constant exposure [31].
Furthermore, Chang W. H. S. et al. (2004) reported the effect of a pulsed electromagnetic field with frequency of 15 Hz and magnetic field strength of 0.1 mT on primary mouse calvarial osteoblasts in terms of proliferation and differentiation, reporting that a constant exposure of 8 h per day for 14 days accelerated the proliferation without altering cell differentiation [32].
Recently, Martino C. F. et al. (2008) evaluated the effect of a pulsed magnetic field of 20 Gauss and 15 Hz on the growth of SAOS-2 cells. The results of their research suggest that a constant exposure does not alter cell growth [33].
Accordingly, the aim of the present study was to preliminary investigate the effect of a time-dependent magnetic field on the metabolic activity of Human Mesenchymal Stem Cells (hMSCs) seeded on 3D additive-manufactured poly(ɛ-caprolactone)/iron-doped hydroxyapatite (PCL/FeHA) scaffolds.
In particular, as it was demonstrated [21], [34], [35] that a frequency of 70 Hz and an intensity in the range of 1 mT to 1 T already provided interesting results in terms of biological phenomena and bone regeneration, the effect of a sinusoidal magnetic field with frequency of 70 Hz and intensity of 25–30 mT on cell-laden scaffolds was analyzed.
2. Materials and methods
2.1. Design and preparation of 3D nanocomposite magnetic scaffolds
PCL/FeHA nanocomposite pellets were prepared and then properly processed to fabricate scaffolds using additive manufacturing, as described in a previous work [2].
Briefly, FeHA nanoparticles were obtained through an acid-base neutralization process performed by stirring and heating a water suspension of calcium hydroxide, iron (II) chloride and iron (III) chloride at 40 °C with the drop-wise addition of an aqueous phosphoric acid solution. The reactive compounds in the solution were set at a Fe/Ca ratio of 20 mol% and a 1.67 molar ratio of (Fe + Ca)/P. After aging, the precipitate was separated from the solution by centrifugation, freeze-drying and then sieving at 150 μm [36].
PCL pellets (average molecular weight of 65,000, Sigma-Aldrich, St. Louis, MO) were dissolved in tetrahydrofuran (THF, Sigma-Aldrich, St. Louis, MO) with stirring at room temperature. FeHA nanoparticles and, subsequently, ethanol were added to the PCL/THF solution during stirring. A PCL/filler weight ratio (w/w) of 80/20 was employed. To optimize the dispersion of FeHA nanoparticles in the polymer solution, an ultrasonic bath (Branson 1510 MT, Danbury, CT) was also used.
The obtained nanocomposite pellets consisting of PCL loaded with FeHA fillers were processed using 3D fiber deposition technique to manufacture 3D cylindrical scaffolds with a 0°/0°/90°/90° lay-down pattern (Fig. 1).
Fig. 1.

Representative image of a 3D nanocomposite magnetic scaffold with a 0°/0°/90°/90° lay-down pattern, a fiber diameter of 500 μm, a layer thickness of 390–400 μm and a strand distance of 1800 μm.
In particular, 3D PCL/FeHA 80/20 scaffolds were built by extruding and depositing the fibers along specific directions according to the properly selected lay-down pattern. The PCL/FeHA pellets were placed in a stainless steel syringe and heated to a temperature of about 120 °C using a cartridge unit placed on the mobile arm of a 3D plotter dispensing machine (Envisiontec GmbH, Gladbeck, Germany).
A nitrogen pressure of 8.5–8.9 bar was applied to the syringe through a cap. The material was extruded through a nozzle with an inner diameter of 600 μm, and the continuous filament was deposited at a speed of 30 mm/min.
The polymeric and nanocomposite scaffolds were characterized by a fiber diameter of 500 μm, a layer thickness of 390–400 μm and a strand distance (i.e., center-to-center fiber distance) of approximately 1800 μm.
2.2. Morphological analysis
In order to evaluate morphological features as well as cell adhesion and shape, scanning electron microscopy (SEM) was performed through a FEI Quanta FEG 200 scanning electron microscope (The Netherlands).
In the case of cell-laden scaffolds, the culture media was removed and the samples were rinsed three times with PBS. Then, cell-constructs were fixed in 2.5% glutaraldehyde (pH 7.4) (Sigma Aldrich, Italy) for 20 min at room temperature. The samples were washed and dehydrated in an ethanol series (70, 80, 90, 95, and 100% v/v), dried air, gold sputtered and analyzed by SEM.
2.3. Cell culture
Human mesenchymal stem cells (hMSCs; Clonetics, Italy), at the fourth passage, were cultured in α-modified Eagle's medium (α-MEM) (Bio-Whittaker, Belgium) containing 10% (v/v) fetal bovine serum (FBS, Gibco™, Thermo Fisher Scientific), 100 U/mL penicillin and 0.1 mg/mL streptomycin (HyClone, UK), in a humidified atmosphere at 37 °C and 5% CO2. Scaffolds for cell cultures (3 mm in thickness and 6 mm in diameter) were prepared soaking the structures in 70% ethanol 3 times (30 min/cycle), then in phosphate-buffered saline (PBS, Sigma Aldrich, Italy) with 1% antibiotic/antimycotic 3 times (2 h) and, finally, in cell culture medium for pre-wetting (2 h). Cells (density 1.0 × 104 cells/sample) suspended in FBS, were statically seeded onto the scaffolds. After 30 min of incubation, culture medium was added to each well containing one cell-laden scaffold.
2.3.1. Cell metabolic activity
Cell metabolic activity was evaluated using the Alamar Blue Assay (AbD Serotec Ltd,UK). This is based on a redox reaction that occurs in the mitochondria of the cells; the colored product is transported out of the cell and can be spectrophotometrically measured.
After 1, 4, 7, 14, 21, 28, 35 days from cell seeding, the cell-constructs were rinsed with PBS, and for each sample, 200 μl of Dulbecco's modified Eagle's medium. (DMEM) without Phenol Red (HyClone, UK) containing 10% (v/v) Alamar Blue was added, followed by incubation in 5% CO2 diluted atmosphere for 4 h at 37 °C.
One hundred microliters of the solution was subsequently removed from the wells and transferred to a 96-well plate. The optical density was immediately measured with a spectrophotometer (Sunrise; Tecan, Männedorf, Zurich, Switzerland) at wavelengths of 570 and 595 nm. The number of viable cells is correlated with the magnitude of dye reduction and is expressed as a percentage of Alamar Blue reduction, according to the manufacturer's protocol. Each experiment was repeated at least 3 times in triplicate.
The cellular morphology was observed through confocal laser scanning microscopy (CLSM, Zeiss LSM 510/Confocor 2, Oberkochen, Germany) and ER-Tracker™ green for live-cell endoplasmic reticulum labeling. The images of cell constructs were acquired by using a Ar excitation laser at the wavelength of 488 nm and a 10× objective.
2.4. Magnetic stimulation
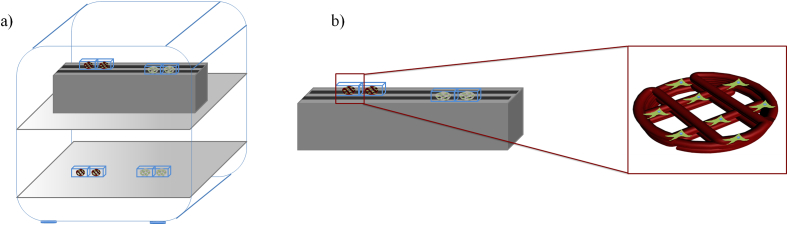
At 1 day after cell seeding, two different conditions (continuous and discontinuous stimulation) were considered for the exposure to an external sinusoidal magnetic field. In particular, some cell-laden scaffolds (Group 1) were exposed to a magnetic field which was continuously applied for 6 h per day, whereas some others (Group 2) were stimulated using a discontinuous application of a magnetic field for 6 h per day (20 intervals - 18 min each). Further cell-laden scaffolds, which were not magnetically stimulated, were used as control and placed in the same incubator. Fig. 2 reports a schematic representation of the experimental setup. The electromagnet was placed below the wells to expose the cell-laden scaffolds to the time-dependent magnetic field. Two adjacent wells used for cell culture were set apart at a distance of more than 10 mm as the edge-to-edge distance, trying to avoid the mutual influence. The experiment was repeated three times in triplicate. PCL/Mag. and PCL/FeHA/Mag. indicate polymeric and nanocomposite scaffolds of both groups, stimulated by a sinusoidal magnetic field. “Mag.” has been introduced to denote the application of a magnetic field.
Fig. 2.
Schematic representation of the experimental setup used to perform the analyses on 3D cell-laden scaffolds: a) magnet-equipped incubator; b) details related to the magnetic stimulation system.
2.5. Results and discussion
Unlike the previously reported study [1], [2], [3], the current research may be considered as a preliminary systematic approach for analyzing the effects of the application of an external time-dependent magnetic field in conjunction with 3D nanocomposite magnetic scaffolds on the metabolic activity of hMSCs. The performances of fully biodegradable and 3D PCL/FeHA porous scaffolds with magnetic properties were previously evaluated by experimental/theoretical in vitro studies and in vivo tests on rabbit [3]. FeHA nanoparticles were uniformly and randomly distributed in the polymer matrix [3]. An improvement in mechanical performances was shown for the nanocomposite magnetic structures if compared to the neat PCL ones [3]. The results were also confirmed by finite element analysis [3]. Finally, magnetic measurements, which were performed by a superconducting quantum interference device (SQUID) magnetometer, confirmed the superparamagnetic character of the nanocomposite materials, showing a very low coercive field, as well as a sigmoidal shape of the magnetization curve at 37 °C [1]. The capability of a magnetic scaffold to absorb magnetic nanoparticles in water solution, in presence of a neodymium magnet was also demonstrated [2].
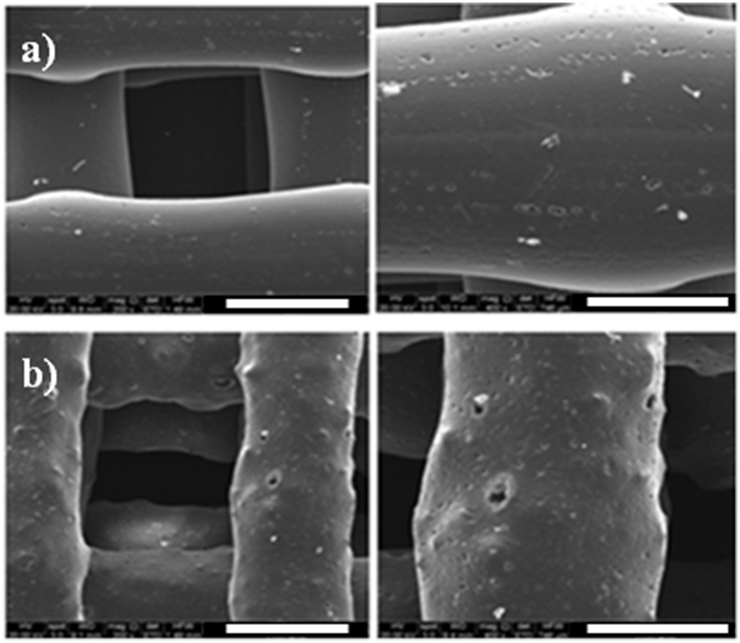
Results from SEM analyses on the obtained 3D additive-manufactured scaffolds highlighted a well-organized structure (i.e. architecture, fiber spacing, effective fiber diameter) allowing the evaluation of the morphological features of the polymeric and nanocomposite scaffolds. In particular, PCL and PCL/FeHA fibers showed a mean diameter of 500 μm (Fig. 3). It is worth noting that the nanocomposite magnetic surface was characterized by a greater roughness, due to the presence of FeHA nanoparticles, which has been shown to strongly affect cell behaviors [1]. As widely reported, surface topography and chemistry play an important synergistic role in the cell-material interaction [1].
Fig. 3.
Different images of 3D additive-manufactured PCL (a) and PCL/FeHA (b) scaffolds obtained through 3D fiber deposition technique: (left) Scale Bar - 500 μm, (right) Scale Bar - 300 μm.
However, over the past few years, magnetism and magnetic materials have revolutionized different aspects of healthcare suggesting new opportunities in the diagnostics and in bone tissue engineering [1], [2], [3], [4]. In particular, magnetic field is considered as a potent stimulator of cell behaviors, including cell proliferation, migration and differentiation. Furthermore, with regard to the healing of damaged tissues and further diseases, its contribution has been widely recognized. The current research aimed to investigate the effect of a time-dependent field on the hMSCs functions. Previous works have already demonstrated that the magnetic nanocomposite scaffolds could stimulate the proliferation and osteogenic differentiation of hMSCs [1], [2], [3], [4]. On the other hand, the application of external magnetic fields, either static or dynamic, has shown to beneficially affect bone regeneration. Therefore, in the present work it was hypothesized that the specific combination of time-dependent magnetic fields with magnetic scaffolds might generate synergistic microenvironments favorable for cell activity.
Results obtained from Alamar Blue assay performed on Group 1 (frequency 70 Hz, intensity 30 mT for 6 h per day) and control samples have shown that prolonged exposure time to a sinusoidal magnetic field seems to negatively affect cell viability (Fig. 4). In particular, unstimulated PCL and PCL/FeHA scaffolds provided values of the percentage of Alamar Blue reduction and a peak of viability (at 14 days after cell seeding) which were generally higher than those obtained from magnetically stimulated ones. On the contrary, magnetically stimulated PCL and PCL/FeHA structures provided lower values of percentage of Alamar Blue reduction and a peak of viability at 4 days after cell seeding.
Fig. 4.
Results obtained from Alamar Blue Assay on Group 1 reported as mean value ± standard deviation. n = 5.
At 35 days after cell seeding, SEM images (Fig. 5) have shown that an increase in the number of hMSCs, which adhered to unstimulated PCL and PCL/FeHA scaffolds, is quite evident if compared to magnetized cell-constructs. In particular, hMSCs were well spread and better adhered to PCL/FeHA scaffolds in comparison to those seeded on the PCL structures. On the other hand, these results should seem to be consistent with those obtained from the Alamar Blue assay.
Fig. 5.
SEM images of different cell-constructs: a) PCL, b) PCL/FeHA, c) PCL/Mag., d) PCL/FeHA/Mag. Scale Bar - 500 μm.
Fig. 6 reports a higher magnification of a central region of the PCL/FeHA scaffold at 35 days after cell seeding.
Fig. 6.

SEM image: higher magnification of a central region of a PCL/FeHA scaffold, Scale Bar - 200 μm.
The Alamar Blue assay performed on Group 2 (frequency 70 Hz, intensity 30 mT for 6 h per day, 20 intervals of 18 min) showed interesting results in terms of cell viability/proliferation (Fig. 7).
Fig. 7.
Results obtained from Alamar Blue assay on Group 2 reported as mean value ± standard deviation. n = 5.
hMSCs were viable on both PCL and PCL/FeHA scaffolds. In particular, higher values of the percentage of Alamar Blue reduction were achieved for PCL/FeHA scaffolds, if compared to PCL ones. These results should be probably ascribed to the greater roughness of nanocomposites that should improve cell adhesion. On the other hand, the nanoparticle inclusion also enhances the material hydrophilicity, as already evidenced by the lower values of water contact angle obtained for the nanocomposite structures in comparison with those of neat PCL [1].
It is worth noting that at 14 days after cell seeding unstimulated PCL and PCL/FeHA scaffolds showed a peak of viability, which was obtained for the stimulated structures at 4 days after cell seeding. This would seem to suggest that the application of the magnetic field may induce an earlier stop of cell proliferation.
At 4 and 14 days after cell seeding, SEM analyses (Fig. 8) provided interesting results in terms of cell adhesion.
Fig. 8.
SEM images of different cell-constructs at 4 and 14 days after cell seeding: a) PCL, b) PCL/FeHA, c) PCL/Mag., d) PCL/FeHA/Mag. Scale Bar - 500 μm.
In particular, at 4 days the effect of magnetic stimulation resulted in higher number of cells which adhered to the scaffolds and in better spreading on both polymeric and nanocomposite structures, in comparison to the unstimulated scaffolds. Furthermore, at 14 days after cell seeding, cells were well spread on the different kinds of additive-manufactured scaffolds.
In addition, at 4 days after cell seeding CLSM analyses performed on all the cell-constructs provided interesting results in terms of hMSCs adhesion and spreading. ER-Tracker™ green was used to visualize the cell cytoskeleton. In particular, better cell adhesion and spreading were well evident for the stimulated nanocomposite scaffolds in comparison with cells seeded on the neat PCL ones. As an example, CLSM images of the stimulated cell constructs are reported in Fig. 9.
Fig. 9.
CLSM images of different cell-constructs at 4 days after cell seeding: a) PCL/Mag., b) PCL/FeHA/Mag. Scale Bar - 100 μm.
In conclusion, Group 1 and Group 2 constructs showed a similar behavior. However, unlike Group 1, Group 2 did not exhibit lower values of percentage of Alamar Blue reduction. This effect should be ascribed to an overheating effect of the environment, probably due to the application of a continuous magnetic field. Fogolìn et al. (2004) demonstrated that a lower cultivation temperature resulted in reduced metabolic rates and prolonged viability. Temperature shift from 37 °C to 33° C in batch cell cultures leads to a decreased cell growth and a maintenance of high cell viability [37].
2.6. Conclusions
Benefiting from previous results, nanocomposite scaffolds were firstly designed by embedding FeHA nanoparticles into a PCL matrix. A PCL/FeHA weight ratio (w/w) of 80/20 was used.
Cell-material interaction and morphological features were assessed through scanning electron microscopy. The possibility to enhance cell proliferation employing a sinusoidal magnetic field was demonstrated.
The obtained results could open new perspectives for the application of magnetic fields and cell-laden scaffolds for bone tissue engineering. Thus, this study may be considered as a first step of a future complex work with the aim of studying the effect of a sinusoidal magnetic field on cell differentiation, taking into account also the possibility to suitably modify the intensity and the frequency of the applied field in order to optimize the magnetic stimulation process.
Acknowledgments
The authors would like to thank Dr. Anna Tampieri, Dr. Monica Sandri from Institute of Science and Technology for Ceramics, National Research Council, for providing the FeHA nanoparticles.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Gloria A., Russo T., D'Amora U., Zeppetelli S., D'Alessandro T., Sandri M., Bañobre-Lopez M., Piñeiro-Redondo Y., Uhlarz M., Tampieri A., Rivas J., Herrmannsdörfer T., Dediu V.A., Ambrosio L., De Santis R. Magnetic poly(ɛ-caprolactone)/iron-doped hydroxyapatite nanocomposite substrates for advanced bone tissue engineering. J. R. Soc. Interface. 2013;10:1–11. doi: 10.1098/rsif.2012.0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Santis R., D'Amora U., Russo T., Ronca A., Gloria A., Ambrosio L. 3D fibre deposition and stereolithography techniques for the design of multifunctional nanocomposite magnetic scaffolds. J. Mater. Sci. Mater. Med. 2015;26:250. doi: 10.1007/s10856-015-5582-4. [DOI] [PubMed] [Google Scholar]
- 3.De Santis R., Russo A., Gloria A., D'Amora U., Russo T., Panseri S., Sandri M., Tampieri A., Marcacci M., Dediu V.A., Wilde C.J., Ambrosio L. Towards the design of 3D fiber-deposited poly(ɛ-caprolactone)/iron-doped hydroxyapatite nanocomposite magnetic scaffolds for bone regeneration. J. Biomed. Nanotechnol. 2015;11:1236–1246. doi: 10.1166/jbn.2015.2065. [DOI] [PubMed] [Google Scholar]
- 4.D'Amora U., Russo T., De Santis R., Gloria A., Ambrosio L. Hybrid nanocomposites with magnetic activation for advanced bone tissue engineering. In: Tampieri A., Sprio S., editors. Bio-inspired Regenerative Medicine: Materials, Processes and Clinical Applications. Pan Stenford Publishing; 2016. p. 179. ISBN: 9789814669146. [Google Scholar]
- 5.Xiaolan B., Hadjiargyrou M., Di Masi E., Meng Y., Simon M., Tan Z., Rafailovich M.H. The role of moderate static magnetic fields on biomineralization of osteoblasts on sulfonated polystyrene films. Biomaterials. 2011;32:7831–7838. doi: 10.1016/j.biomaterials.2011.06.053. [DOI] [PubMed] [Google Scholar]
- 6.Ross S.M. Combined DC and ELF magnetic fields can alter cell proliferation. Bioelectromagnetics. 1990;11:27–36. doi: 10.1002/bem.2250110105. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto Y., Kawasumi M., Saito M. Effect of static magnetic field on cell migration. Electr. Eng. Jpn. 2007;160:46–52. [Google Scholar]
- 8.Kotani H., Kawaguchi H., Shimaoka T., Iwasaka M., Ueno S., Ozawa H., Nakamura K., Hoshi K. Strong static magnetic field stimulates bone formation to a definite orientation in vitro and in vivo. J. Bone Min. Res. 2002;17:1814–1821. doi: 10.1359/jbmr.2002.17.10.1814. [DOI] [PubMed] [Google Scholar]
- 9.Noriega-Luna B., Sabanero M., Sosa M., Avila-Rodrigueza M. Influence of pulsed magnetic fields on the morphology of bone cells in early stages of growth. Micron. 2011;42:600–607. doi: 10.1016/j.micron.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Miyakoshi J. Effects of static magnetic fields at the cellular level. Prog. Biophys. Mol. Biol. 2005;87:213–223. doi: 10.1016/j.pbiomolbio.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Rosen A.D. Mechanism of action of moderate-intensity static magnetic fields on biological systems. Cell. Biochem. Biophys. 2003;39:163–173. doi: 10.1385/CBB:39:2:163. [DOI] [PubMed] [Google Scholar]
- 12.Yan Q.C., Tomita N., Ikada Y. Effects of static magnetic field on bone formation of rat femurs. Med. Eng. Phys. 1998;20:397–402. doi: 10.1016/s1350-4533(98)00051-4. [DOI] [PubMed] [Google Scholar]
- 13.Bruce J.N., Criscuolo G.R., Merrill M.J., Moquin R.R., Blacklock J.B., Oldfield E.H. Vascular permeability induced by protein product of malignant brain tumors: inhibition by dexamethasone. J. Neurosurg. 1987;67:880–884. doi: 10.3171/jns.1987.67.6.0880. [DOI] [PubMed] [Google Scholar]
- 14.Degen I.L., Stetsula V.I. Consolidation of bone fragments in a constant magnetic field. Ortop. Travmatol. Protez. 1971;32:45–48. [PubMed] [Google Scholar]
- 15.Fassina L., Visai L., Benazzo F., Benedetti L., Calligaro A., Cusella De Anegelis M.G., Farina A., Maliardi V., Magenes G. Effects of electromagnetic stimulation on calcified matrix production by SAOS-2 cells over a polyurethane porous scaffold. Tissue Eng. 2006;12:1985–1999. doi: 10.1089/ten.2006.12.1985. [DOI] [PubMed] [Google Scholar]
- 16.Chiu K.H., Ou K.L., Lee S.Y., Lin C.T., Chang W.J., Chen C.C., Huang H.M. Static magnetic fields promote osteoblast-like cells differentiation via increasing the membrane rigidity. Ann. Biomed. Eng. 2007;35:1932–1939. doi: 10.1007/s10439-007-9370-2. [DOI] [PubMed] [Google Scholar]
- 17.Feng S.W., Lo Y.J., Chang W.J., Lin C.T., Lee S.Y., Abiko Y., Huang H.M. Static magnetic field exposure promotes differentiation of osteoblastic cells grown on the surface of a poly-L-lactide substrate. Med. Biol. Eng. Comput. 2010;48:793–798. doi: 10.1007/s11517-010-0639-5. [DOI] [PubMed] [Google Scholar]
- 18.Sato K., Yamaguchi H., Miyamoto H., Kinouchi Y. Growth of human cultured cells exposed to a non-homogeneous static magnetic field generated by Sm-Co magnets. Biochem. Biophys. Acta. 1992;1136:231–238. doi: 10.1016/0167-4889(92)90111-n. [DOI] [PubMed] [Google Scholar]
- 19.Pacini S., Vannelli G.B., Barni T., Ruggiero M., Sardi I., Pacini P., Gulisano M. Effect of 0.2 T static magnetic field on human neurons: remodeling and inhibition of signal transduction without genome instability. Neurosci. Lett. 1999;267:185–188. doi: 10.1016/s0304-3940(99)00362-6. [DOI] [PubMed] [Google Scholar]
- 20.Pacini S., Gulisano M., Peruzzi B., Sgambati E., Gheri G., Bry S.G., Vannucchi S., Polli G., Ruggiero M. Effects of 0.2 mT static magnetic field on human skin fibroblasts. Cancer Detect. Prev. 2003;27:327–332. doi: 10.1016/s0361-090x(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 21.Cunha C., Panseri S., Marcacci M., Tampieri A. Evaluation of the effects of a moderate intensity static magnetic field application on human osteoblast-like cells. Am. J. Biomed. Eng. 2012;2:263–268. [Google Scholar]
- 22.Yun H.M., Ahn S.J., Park K.R., Kim M.J., Kim J.J., Jin G.Z., Kim H.W., Kim E.C. Magnetic nanocomposite scaffolds combined with static magnetic field in the stimulation of osteoblastic differentiation and bone formation. Biomaterials. 2016;85:88–98. doi: 10.1016/j.biomaterials.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 23.Chang K., Chang W.H.S., Wu M.L., Shih C. Effects of different intensities of extremely low frequency pulsed electromagnetic fields on formation of osteoclast-like cells. Bioelectromagnetics. 2003;24:431–439. doi: 10.1002/bem.10118. [DOI] [PubMed] [Google Scholar]
- 24.Chang K., Chang W.H., Huang S., Huang S., Shih C. Pulsed electromagnetic fields stimulation affects osteoclast formation by modulation of osteoprotegerin, RANK ligand and macrophage colony-stimulating factor. J. Orthop. Res. 2005;23:1308–1314. doi: 10.1016/j.orthres.2005.03.012.1100230611. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz Z., Fisher M., Lohmann C.H., Simon B.J., Boyan B.D. Osteoprotegerin (OPG) production by cells in the osteoblast lineage is regulated by pulsed electromagnetic fields in cultures grown on calcium phosphate substrates. Ann. Biomed. Eng. 2009;37:437–444. doi: 10.1007/s10439-008-9628-3. [DOI] [PubMed] [Google Scholar]
- 26.Chang K., Chang W.H. Proceedings of the 23rd Annual Meeting of the Bioelectromagnetics Society. 2001. The influence of pulsed electromagnetic field (PEMF) on osteopenia in an ovariectomized female rat model. (St Paul, MN) [Google Scholar]
- 27.Park K.H., Soda A., Yamaguchi H., Kinouchi Y., Yoshizach K. Effects of magnetic field on collagen synthesis in osteoblasts. In: Bersani, editor. Electricity and Magnetism in Biology and Medicine. Kluwer Academic/Plenum Publishers; 1999. pp. 457–460. [Google Scholar]
- 28.Nakajima T., Ishiguro A., Wakatsuki Y. formation of super wires of clusters by self- assembly of transition metal cluster anions with metal cations. Angew. Chem. Int. Ed. 2001;40:1066–1068. doi: 10.1002/1521-3773(20010316)40:6<1066::aid-anie10660>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Liboff A.R., Williams T., Jr., Strong D.M., Wistar R., Jr. Time-varying magnetic fields: effect on DNA synthesis. Science. 1984;223:818–820. doi: 10.1126/science.6695183. [DOI] [PubMed] [Google Scholar]
- 30.De Mattei M., Caruso A., Traina G.C., Pezzetti F., Baroni T., Sollazzo V. Correlation between pulsed electromagnetic fields exposure time and cell proliferation increase in human osteosarcoma cell lines and human normal osteoblast cell in vitro. Bioelectromagnetics. 1999;20:117–182. doi: 10.1002/(sici)1521-186x(1999)20:3<177::aid-bem4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 31.Diniz P., Shomura K., Soejima K., Ito G. Effects of pulsed electromagnetic field (PEMF) stimulation on bone tissue like formation are dependent on the maturation stages of the osteoblasts. Bioelectromagnetics. 2002;23:398–405. doi: 10.1002/bem.10032. [DOI] [PubMed] [Google Scholar]
- 32.Chang W.H.S., Chen L.T., Sun J.S., Lin F.H. Effect of pulse-burst electromagnetic field stimulation on osteoblast cell activities. Bioelectromagnetics. 2004;25:457–465. doi: 10.1002/bem.20016. [DOI] [PubMed] [Google Scholar]
- 33.Martino C.F., Belchenko D., Ferguson V., Nielsen-Preiss S., Qi H.J. The effects of pulsed electromagnetic fields on the cellular activity of SaOS-2 cells. Bioelectromagnetics. 2008;29:125–132. doi: 10.1002/bem.20372. [DOI] [PubMed] [Google Scholar]
- 34.Ba X., Hadjiargyrou M., Di Masi E., Meng Y., Simon M., Tan Z., Rafailovich M.H. The role of moderate static magnetic fields on biomineralization of osteoblasts on sulfonated polystyrene films. Biomaterials. 2011;32:7831–7838. doi: 10.1016/j.biomaterials.2011.06.053. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z., Che P.L., Du J., Ha B., Yarema K.J. Static magnetic field exposure reproduces cellular effects of the Parkinson's disease drug candidate ZM241385. PLoS One. 2010;5:e13883. doi: 10.1371/journal.pone.0013883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tampieri A., D'Alessandro T., Sandri M., Sprio S., Landi E., Bertinetti L., Panseri S., Pepponi G., Goettlicher J., Bañobre-López M., Rivas J. Intrinsic magnetism and hyperthermia in bioactive Fe-doped hydroxyapatite. Acta Biomater. 2012;8:843–851. doi: 10.1016/j.actbio.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 37.Bollati Fogolın M., Wagner R., Etcheverrigaray M., Kratje R. Impact of temperature reduction and expression of yeast pyruvate carboxylase on hGM-CSF-producing CHO cells. J. Biotechnol. 2004;109:179–191. doi: 10.1016/j.jbiotec.2003.10.035. [DOI] [PubMed] [Google Scholar]