Abstract
Surgical aortic valve replacement is the gold standard procedure to treat patients with severe, symptomatic aortic valve stenosis or insufficiency. Bioprosthetic valves are used for surgical aortic valve replacement with a much greater prevalence than mechanical valves. However, bioprosthetic valves may fail over time because of structural valve deterioration; this often requires intervention due to severe bioprosthetic valve stenosis or regurgitation or a combination of both. In select patients, transcatheter aortic valve replacement is an alternative to surgical aortic valve replacement. Transcatheter valve-in-valve (ViV) replacement is performed by implanting a transcatheter heart valve within a failing bioprosthetic valve. The transcatheter ViV operation is a less invasive procedure compared with reoperative surgical aortic valve replacement, but it has been associated with specific complications and requires extensive preoperative work-up and planning by the heart team. Data from experimental studies and analyses of results from clinical procedures have led to strategies to improve outcomes of these procedures. The type, size, and implant position of the transcatheter valve can be optimized for individual patients with knowledge of detailed dimensions of the surgical valve and radiographic and echocardiographic measurements of the patient's anatomy. Understanding the complexities of the ViV procedure can lead surgeons to make choices during the original surgical valve implantation that can make a future ViV operation more technically feasible years before it is required.
Keywords: surgical aortic valve replacement, transcatheter aortic valve replacement, valve-in-valve repair, bioprosthetic valves, SAVR, TAVR
INTRODUCTION
The treatment of choice for severe aortic valve stenosis (AS) has been surgical aortic valve replacement (SAVR) for most patients with acceptable surgical risks. Transcatheter aortic valve replacement (TAVR) has become an accepted, less-invasive alternative for selected patients with severe aortic stenosis, and the indications are expanding.1 Patients undergoing SAVR usually have their valve replaced with either a bioprosthetic or mechanical valve. The trend toward greater prevalence of bioprosthetic valve use has been dramatic over the past 2 decades (Figure 1).1 This preference for bioprosthetic valves is due to multiple factors, including an aging population presenting for SAVR, an increased understanding of the thromboembolic and anticoagulant-related hemorrhage complications associated with mechanical valves,2 improved durability of newer-generation tissue valves,3–5 increased safety of reoperative AVR procedures,6 and the desire of younger patients to avoid long-term anticoagulation.
Figure 1.
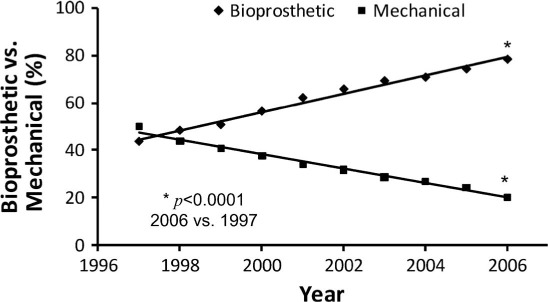
Percentage use of bioprosthetic valves vs mechanical valves from 1997 through 2006. Bioprosthetic valve use increased progressively during 10 years. Asterisk indicatesP < .0001. Reprinted with permission.1
THE PROBLEM: BIOPROSTHETIC STRUCTURAL VALVE DETERIORATION
Several randomized trials and retrospective studies have shown similar long-term survival even in patients younger than 70 years old undergoing SAVR with bioprosthetic or mechanical valves.7–10 Compared with mechanical valves, SAVR using bioprosthetic valves has been associated with fewer bleeding complications but a higher risk of reoperation for structural valve deterioration (SVD).7,10 Advances in bioprosthetic valve designs, physiologic fixation, and anticalcification treatment of the leaflets have reduced the risk of reoperation from SVD in newer-generation tissue valves compared with earlier tissue valves.3,4,11 Long-term freedom from bioprosthetic SVD is decreased in patients who are younger at the time of SAVR and in patients with higher postprocedural transvalvular gradients.3,12 The younger a patient is at the time of bioprosthetic valve implantation, the greater the likelihood of reoperation for SVD in that patient's lifetime (Figure 2).13 In severe aortic stenosis, the primary goal of SAVR is to decrease the transvalvular gradient by improving the aortic valve area (AVA).
Figure 2.

Lifetime risk of reoperation as a function of age at surgical aortic valve replacement. Reprinted with permission.13
The effective orifice area (EOA) of the surgically implanted valve is determined by the patient's aortic annulus size and the anatomically and functionally obstructive elements of the surgical valve as well as surgical implantation techniques. If the native aortic annulus is small, the surgeon may choose to enlarge it to accommodate a larger valve size, implant the valve in a supra-annular position, or choose a valve with a larger effective orifice area (homograft, stentless bioprostheic valve, or externally mounted stented bioprosthetic valve) to achieve a better hemodynamic result. High transvalvular gradients from a lower EOA indexed to the patient have been shown to negatively impact left ventricular mass regression, survival, quality of life, and bioprosthetic valve durability following SAVR.14–18
While reoperative SAVR can often be performed safely,19,20 many patients are elderly and have medical comorbidities that can significantly increase the risks of mortality or major morbidity following redo SAVR.21,22 Age greater than 80 years, coronary artery disease, prior bypass surgery with patent left internal mammary artery near the sternum, low ejection fraction, congestive heart failure, frailty, poor conditioning, calcified ascending aorta, and renal failure can severely affect the early and long-term survival and recovery from redo SAVR.
TRANSCATHETER AORTIC VALVE REPLACEMENT
TAVR for native aortic stenosis has been shown to improve survival compared with best medical practice in patients deemed inoperable for SAVR.23,24 Randomized trials have also proven noninferiority of TAVR compared with SAVR for patients at high risk25,26 and, more recently, at intermediate risk for SAVR.27,28 Randomized trials comparing TAVR with SAVR in low-risk patients are currently enrolling patients. At present, TAVR is performed using self-expanding or balloon-expandable valves. Commercially available self-expanding valves use a nitinol frame and include the Medtronic CoreValve (Medtronic, Inc.) and the newer-generation Medtronic Evolut™ R and Pro valves, which have the ability to be recaptured and redeployed to optimize positioning. The commercially available balloon-expandable valves include the Edwards SAPIEN valves (Edwards Lifesciences Corporation). The early-generation Edwards SAPIEN valve was mounted on a larger catheter and sheath system that often required transapical access.
The newer-generation SAPIEN XT is constructed of bovine pericardial leaflets with a cobalt chromium frame that allows a smaller-diameter catheter. The third-generation SAPIEN 3 valve is designed for more stable deployment and has a skirt around its base to decrease the incidence of paravalvular leak (PVL). Increased operator experience and technical advancements in transcatheter valve and delivery system designs have rapidly improved outcomes of TAVR procedures.29,30
THE TRANSCATHETER VALVE-IN-VALVE CONCEPT
The idea of deploying a TAVR valve within a failing bioprosthetic valve, referred to as TAVR “Valve-in-Valve” (ViV), was inevitable given the clinical success of TAVR in native aortic valve stenosis and the high risk for reoperation in a growing population of patients with bioprosthetic SVD. In 2007, the technical feasibility of ViV was studied in pigs using transapical access to deploy 23-mm Cribier-Edwards transcatheter valves into Carpentier-Edwards valves in the aortic and mitral positions.31 In 2010, the hemodynamic function of TAVR was studied in vitro in a series of experiments implanting 23-mm SAPIEN valves in variously sized Carpentier-Edwards PERIMOUNT valves32,33 and, later, 23- and 26-mm Evolut R valves in variously sized Hancock II valves.34 The data revealed important information to help determine the optimum valve size and depth of implantation for clinical TAVR ViV procedures. The first clinical transcatheter ViV case was reported by Wenaweser et al. in 2007 using a self-expanding Medtronic CoreValve,35 and individual case reports followed.36–42 Several case series and registries of ViV operations have been reported in the past 7 years.43–48
Increased use of the off-label ViV technique has presented more lessons and a greater understanding of the procedure's complexity. The heterogeneity of each bioprosthetic valve type and size, modes of failure (calcification vs leaflet tear, tissue valve stenosis vs regurgitation), valve position (aortic, mitral, tricuspid, pulmonary), type of transcatheter heart valve (THV) implanted, delivery system access, and depth of implant can all dramatically alter the procedural success and outcomes. As of 2015, transcatheter ViV has been approved by the U.S. Food and Drug Administration (FDA) for use in the aortic position for both the CoreValve and SAPIEN XT valves.49
STENTED BIOPROSTHETIC VALVES
A thorough understanding of the design and structure of bioprosthetic valves is critical to the procedural success of implanting the best available valve into an optimum position within the failing surgical valve. Bioprosthetic valves are either stented or stentless. Stented bioprosthetic valves are constructed of a sewing ring around a base, with three struts that act as a scaffold to support the tissue leaflets. The xenograft leaflets are fashioned from bovine pericardium or porcine valve leaflets that often have an anticalcification treatment and are fixed internally or externally to the struts. The sewing ring is made of fabric of varying thickness and internal support. The struts are composed of metal alloy or plastic material of varying thickness, rigidity, and heights to support the leaflets throughout the cardiac cycle (Figure 3).13 The labelled size of the bioprosthetic valve refers to the external diameter of the sewing ring that corresponds to the landing zone of the native valve annulus measured by the surgeon at the time of initial implant. More important for ViV procedures is the bioprosthetic valve's actual internal diameter, which takes into account the internal diameter of the sewing ring, the struts, and the leaflets. The internal diameters of many commercially available bioprosthetic valves can be found from various industry sources and must always be measured meticulously by computerized tomography (CT) imaging and echocardiography. Different valve types have various radiopaque elements that can help align the THV for accurate depth and positioning during ViV procedures.
Figure 3.
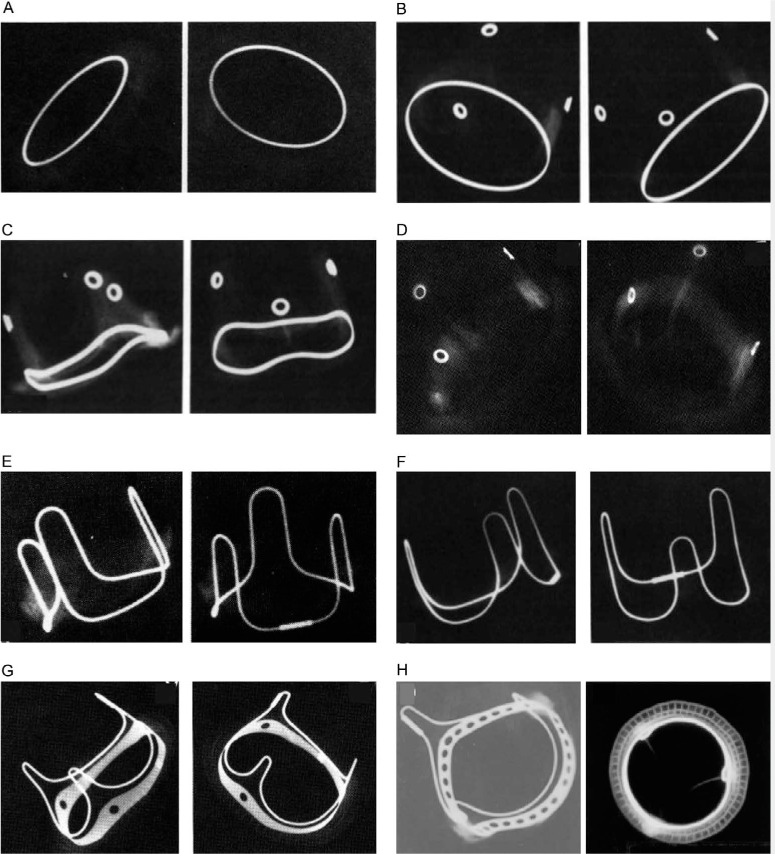
Radiographic appearances of various stented bioprosthetic valves. (A) The Hancock standard valve has a radiopaque Haynes alloy flat base ring. (B) The Hancock Modified Orifice valve has a radiopaque flat base ring (Haynes alloy) and metal eyelets (Haynes alloy) located at the apices of each stent post. (C) The Medtronic Hancock II valve has a radiopaque saddle-shaped base ring (Haynes alloy) and metal eyelets (Haynes alloy) located at the apices of each stent post. (D) The Medtronic Mosaic valve has radiopaque metal eyelets only. (E) The Carpentier-Edwards (CE) Porcine Standard valve has a radiopaque continuous wire form (Elgiloy Specialty Metals) that outlines the stent posts (U-shaped loops) and the base ring between the stent posts. The base ring is otherwise radiolucent. (F) The CE Porcine Supra-Annular Valve (SAV) is similar to the CE Porcine Standard valve (E) except that the CE porcine SAV has “less sharp” transition angles between base ring and stent posts. (G) The CE Pericardial valve has a flattened radiopaque base ring with three holes. A narrow wire form outlines the three stent posts and the base ring in between. (H) The CE Perimount standard has a radiopaque base ring that contains multiple holes and a separate narrow wire form that outlines the stent posts and the base ring in between. Reprinted with permission.13
STENTLESS BIOPROSTHETIC VALVES
Stentless valves are used in the aortic and pulmonary valve positions either in a subcoronary position or as a full root replacement. The lack of struts to support the leaflets allows for a greater effective orifice area. Stentless valves are often used in a small aortic annulus to avoid patient-prosthesis mismatch or in root replacement to treat aortic root aneurysm, dissection, or infection. The subcoronary technique usually involves attaching the sewing ring to the annulus and sewing the valve directly into the sinuses of Valsalva. The full root replacement requires reattaching the coronary arteries as buttons into the root above the valve leaflets. The lack of radiopaque markers in stentless valves makes it difficult to image for positioning when performing ViV. The coronary arteries can be in close proximity to the annulus, and the space between the THV stent and the sinuses of Valsalva is not protected by the struts (as it is in stented valves), making the coronary arteries more vulnerable to potential obstruction by the stentless valve leaflets or the THV itself.
The mode of failure of stentless valves is often aortic regurgitation from malcoaptation of the leaflets or leaflet tears. Calcification may occur more in the aortic root than in the leaflets. The sewing ring can anchor the THV but often not as reliably as the more rigid stented valves. Careful measurements and preoperative planning are required to avoid malpositioning, valve embolism or migration, and coronary obstruction. The implant position usually requires expert echocardiographic imaging intraoperatively since fluoroscopy may not be as reliable due to a lack of calcification and radiopaque markers, and aortic regurgitation can make angiographic imaging of the leaflets difficult.50 Studies have reported successful ViV implantation with stentless valves using either balloon-expandable42,51 or self-expandable THV.52,53 Due to the concern for adequate anchoring, oversizing the THV for ViV in stentless valves has been suggested as long as the leaflets are not too bulky and the coronary artery heights are acceptable for the oversized THV.51
ACCESS FOR THE DEPLOYMENT SYSTEM
Vascular and access site complications during or after TAVR have decreased dramatically over the past decade. Operator experience and anticipating complications have both played a major role in improving procedural safety. Careful examination of the preoperative CT and angiography has allowed operators to predict potential complications and adjust the transfemoral strategy for alternative access. Newer valve innovations allowing smaller-diameter delivery systems have greatly decreased the reliance on the transapical approach for balloon-expandable THV. While transapical access allows a more direct approach to the aortic and mitral valves–with the stability of the “valve-on-a-stick” as opposed to the relative instability of the long, flexible wires–it requires a minithoracotomy and direct ventriculotomy.
Alternative access options have also decreased the prevalence of direct iliac artery or “chimney” iliac artery graft access that requires retroperitoneal or transabdominal surgery in patients with severe iliofemoral disease. Transfemoral artery with percutaneous access is currently the most common approach for TAVR and ViV. TAVR has been performed successfully using direct aortic, subclavian artery (with or without a Dacron graft), or carotid artery access. ViV in the aortic position also most commonly employs percutaneous transfemoral access. Alternative access for aortic ViV has been via transapical, direct aortic,54 subclavian, or carotid artery approaches.53 ViV for bioprosthetic valves in the mitral position can be done directly with transapical access or via the femoral55 or internal jugular vein crossing the interatrial septum. Access for ViV in the tricuspid or pulmonary valve positions is usually via the femoral vein, but the internal jugular vein can be useful depending on the angle of the orientation of the tricuspid valve.
TRANSCATHETER HEART VALVES FOR AORTIC VALVE-IN-VALVE PROCEDURES
Aortic ViV procedures are most often performed with the balloon-expandable Edwards SAPIEN XT or S3 valves or the self-expandable Medtronic CoreValve or Evolut R valves. The Medtronic CoreValve and Edwards SAPIEN XT valve have been approved by the FDA for aortic ViV procedures in patients who are at high or extreme risk for SAVR. Edwards SAPIEN XT has also been FDA approved for use in intermediate-risk patients. Each THV has unique properties that can be advantageous or detrimental to outcomes. For instance, the Edwards SAPIEN XT or S3 valves can be deployed via a transapical approach if required. The SAPIEN XT or S3 valve, the CoreValve, or the Evolut R valve can be deployed via any of the previously mentioned arterial or direct aortic access options. The design of the SAPIEN valves causes the THV leaflets to be positioned intra-annularly, whereas the CoreValve and Evolut R valves allow for a supra-annular leaflet position. The taller height of the nitinol stent in the CoreValve and Evolut R valves may make coronary ostium access more difficult after deployment. The higher rates of permanent pacemaker implantation after self-expandable vs balloon-expandable THV reported after TAVR is not as significant after ViV, and the overall pacemaker requirement is much lower following ViV compared with TAVR.56 The self-expandable stents do not mandate rapid pacing for deployment, although it is often used for a more stable deployment. The Medtronic Evolut R can be recaptured, repositioned, and redeployed prior to release from the delivery system, which can aid in optimal positioning within the bioprosthetic valve.
The results of ViV have been improving with clinical experience and critical analysis of outcomes data from individual cases, case series, large registries, and in vitro experiments. Preoperative evaluation and surgical strategies have evolved as specific beneficial and detrimental outcomes are recognized, reported, and scrutinized. Patients undergoing ViV are at risk for the same complications that can occur with TAVR. However, the rigid structure and round geometry of the stented bioprosthetic valves may allow more consistent anchoring and fit of the expanded THV, resulting in less PVL when the valve is positioned appropriately. Moreover, the risk of annular disruption is less likely unless balloon expansion is aggressively performed. The rigid stented valves also protect the conduction system from compression with valve expansion, which lowers, but does not eliminate, the incidence of permanent pacemaker requirement compared with TAVR.56,57 The complications that have been specifically related to ViV procedures are high postprocedural gradients, coronary obstruction, and malposition and possible migration of the valve.
CORONARY OBSTRUCTION
Coronary artery ostial obstruction may occur during TAVR and cause immediate or delayed myocardial ischemia. Acute obstruction usually results in rapid hemodynamic compromise and cardiogenic shock requiring immediate recognition and intervention. Even with rapid response, coronary obstruction by THV may have poor outcomes. Preoperative evaluation to recognize patients at high risk for coronary obstruction has decreased the risk of this complication during TAVR in native valves to < 1%.58 However, the risk of coronary obstruction is higher in ViV, especially in patients presenting with bioprosthetic aortic stenosis.59 The orientation of most stented bioprosthetic valves places the coronary ostia above the lowest point of the stented frame, and the struts are purposely placed away from the coronary ostia whenever possible. Patients with bicuspid aortic valves or anomalous positioning of the coronary arteries may have the coronary ostia closer to the struts of the implanted valve. Stentless bioprosthetic valves or prior aortic root replacement may result in a coronary artery location close to the surgical valve leaflets. Supra-annular positioning of the stented bioprosthetic valve decreases the distance of the sewing ring from the coronary ostia.
The mechanism of coronary obstruction during ViV is usually displacement of the bioprosthetic valve leaflet towards the sinus of Valsalva, thereby obstructing the diastolic perfusion of the coronary ostium. The covered portion of the THV stent can also obstruct the coronary artery. Most often, the left coronary artery is involved, whereas obstruction of the right coronary ostium is less common. Preoperative characteristics that increase the risk of coronary artery obstruction include lower height of the coronary ostium above the valve annulus, narrow sinuses, narrow or low sinotubular junction, tall or bulky leaflets, and externally fixed leaflets or stentless valves.60 These risk factors must be carefully analyzed with preoperative imaging and review of the valve type and technical details from the original SAVR, when available. Patients at high risk for coronary obstruction should be considered for redo SAVR if they are reasonable surgical candidates.
The risk of coronary obstruction can be further assessed during the ViV procedure by careful aortic root and direct coronary angiography. In patients at high risk for coronary obstruction and for redo SAVR, placing a guidewire with or without an undeployed coronary stent in the coronary artery can offer potentially lifesaving access to the coronary artery at risk should coronary obstruction occur. Stented bioprosthetic valves with tissue wrapped externally around the struts, such as the MITROFLOW (Sorin Group USA, Inc., Arvada, CO) and Trifecta™ (St. Jude Medical, St. Paul, MN) valves, may have an increased risk of coronary artery obstruction by the bioprosthetic valve leaflets after ViV. The THV type and size are also important considerations. A lower-profile balloon-expandable valve may be chosen if the coronary artery at risk is above the top of the bioprosthetic valve struts. A smaller-diameter valve or underinflation of the valve may result in less outward excursion of the leaflets,60 but the resulting postprocedural gradients must be considered with this option. While the Valve-in-Valve International Data Registry (VIVID) reported a 3.5% incidence of coronary artery obstruction with a mortality rate of 57.1%,61 the incidence may be decreasing due to earlier recognition of the risk factors and honing of procedural strategies during ViV.57,60,62,63
POSTPROCEDURAL GRADIENTS AFTER AORTIC VALVE-IN-VALVE
TAVR involves expanding the THV within the native valve, most often anchoring the THV within a calcified native valve annulus and variably mobile calcified valve leaflets. The radial forces of the balloon-expandable and self-expanding THV can expand the native valve structures. The relative size match of the THV to the native valve and the degree of calcification determines the completeness of expansion possible. An underexpanded valve can be postdilated with a balloon, often resulting in less PVL or immediate improvement of transvalvular gradients. Mean transvalvular gradients following TAVR in native valves usually range from 0 to 10 mm Hg.59 The average mean gradients following ViV are higher, from 12.4 to 16 mm Hg.56,57,59 While overexpansion of THV within a stentless bioprosthesis is often done to improve anchoring, opening a THV within a stented bioprosthetic valve offers fewer options.
The size and type of valve and the etiology of structural valve deterioration, as well as aortic root anatomy and valve implantation techniques, greatly influence the postprocedural gradients. The choice of THV type and size and the implant depth within the surgical valve are critical to optimize early and late outcomes of ViV. Smaller valves at the time of SAVR with increased gradients can result in earlier structural valve deterioration due to leaflet strain resulting in early calcification or leaflet tear.3 It is necessary yet often difficult to determine whether high transvalvular gradients occurring years after SAVR are due to SVD or patient-prosthesis mismatch at the time of the initial surgery. Serial echocardiography may help but may not be available for review. In vitro experiments have examined the effects of valve type, size, and implant depth on postimplant gradients.34,64–66 The supra-annular configuration of the Medtronic CoreValve and Evolut R valve had lower gradients than size-matched intra-annular Edwards SAPIEN XT valves within stented valves in vitro. The internally attached leaflet valves tended to have lower gradients than the externally attached valves. Implant depth was critical, with higher implant depth conferring an advantage with lower gradients. Larger-size THV had better gradients except when placed into valves with a smaller internal diameter; this is likely due to underexpansion of the THV.34
In an in-depth analysis of the VIVID registry, Simonato et al.67 reported the postprocedural gradients of ViV patients with various stented valves according to the THV implanted and the depth of implant. They defined high or low depth of impact post hoc based on the relative likelihood of increased gradients post-ViV. For Medtronic CoreValve Evolut R valves (157 patients), high implantation was defined as implant depth of ≤ 5 mm and low implantation defined as > 5 mm below the bioprosthetic sewing ring. For Edwards SAPIEN XT valves (135 patients), high implantation was defined as implantation depth less than or equal to 10% of the height of the valve after expansion. High postprocedural gradients were defined as transvalvular mean gradient > 20 mm Hg after ViV. In the CoreValve group, the incidence of high gradient was 15% if the valve was implanted with a depth ≤ 5 mm and 34.2% if implanted > 5 mm in depth (P = .03). In patients undergoing ViV with a SAPIEN XT valve, the high-implantation group had elevated gradients in 18.5% vs 43.5% in the low-implantation group (P = .03). The SAPIEN XT high-implantation group had more patients with small bioprosthetic valves and more patients with aortic stenosis as a result of SVD, which should put that group at higher risk for high gradients post-ViV. A valve implanted too high increases the risk of THV malposition with potential migration or embolization. Multivariate analysis from the study showed that higher implantation depth and use of CoreValve Evolut R for ViV decreased the likelihood of high postprocedural gradients, whereas SVD with aortic stenosis or mixed stenosis and regurgitation increased the risk of higher gradients compared with SVD due to aortic regurgitation. Patients with SVD and small bioprosthetic valves with an internal diameter < 20 mm are more likely to have high gradients (mean gradient > 20 mm Hg) after ViV when performed with SAPIEN THV than with CoreValve THV.
With the constraints of the prior surgical valve, the ViV procedure must be carefully planned based on (1) angiographic, CT, and echocardiographic imaging; (2) previous operative reports and information about the bioprosthetic valve type and size; and (3) commercially available information about the true internal diameter, radiopaque markings, leaflet configuration, strut height, and rigidity. Useful downloadable applications such as the ViV Aortic app and the ViV Mitral app (UBQO, London, UK) have been developed to help physicians access information about specifications of various bioprosthetic valves in clinical use. If the valve type and labelled size are known and selected, the app will show examples of the in situ and fluoroscopic appearance, stent internal diameter and height, true internal diameter including the leaflets, suggestions for types of THV to be used, in situ photographs and fluoroscopic appearance of the suggested valve deployed within the given bioprosthetic valve, and optimal depth of deployment for each suggested THV. While these apps are a helpful guide, the strategy of the operation is always determined by the physician with meticulous preoperative planning and intraoperative decision making.
SVD in stented bioprosthetic valves resulting in aortic stenosis or mixed aortic stenosis and regurgitation is a risk factor for increased gradients after ViV due to thickened, fibrotic, or calcified leaflets decreasing the inner area within the relatively fixed valve structure. Pre-ViV balloon dilatation of bioprosthetic valves has been performed with caution, but most ViV procedures do not use predilatation due to the concern over leaflet or calcium fragmentation and embolism or acute aortic regurgitation with rapid hemodynamic compromise. High-pressure balloon dilatation of very small (labelled sized 19 or 21) bioprosthetic valves has been performed experimentally and clinically to fracture the sewing ring of valves in the aortic and pulmonary position, enabling implantation of a THV in patients at high risk for redo valve replacement.69,70 While this has been performed successfully, the risk of embolization, acute aortic regurgitation, or rupture of the aortic annulus must be factored into the risk/benefit calculation, which most often would favor redo SAVR. In addition, the physical characteristics of the valve sewing ring must be understood prior to attempting this maneuver. Post-ViV balloon dilation can be performed, especially in valves placed in the supra-annular position in bioprosthetic valves with more flexible struts. Fluoroscopic imaging can often show underexpansion of the THV, and in the setting of PVL or higher-than-expected gradients, this can improve outcomes.
VALVE-IN-VALVE COMPARED WITH REOPERATION SAVR
Several retrospective studies have compared the results of ViV versus redo SAVR.57,62,71 Despite higher predicted risk, operative mortality (0–6.4% for ViV vs 0–6.5% for redo SAVR) and 1-year survival rates were similar. In a case-matched comparison between patients undergoing ViV or redo SAVR, Ejiofor et al.57 showed no statistical difference in operative mortality, strokes, permanent pacemakers, coronary obstruction, or mean postprocedural gradients. The ViV group had a higher incidence of mild PVL with no moderate or severe PVL, but there was no difference in 3-year survival (76.3% in the ViV group and 78.7% in the redo SAVR group). The redo SAVR patients had longer ICU and hospital stays and a significantly higher incidence of new onset atrial fibrillation (18.6% for ViV and 63.6% for redo SAVR).
Phan and colleagues56 reported the results of a meta-analysis showing no difference in operative mortality despite older age (mean age 77.5 years in the ViV group and 66.7 years in the redo SAVR group) and despite more comorbidities and higher EuroSCORES in the ViV group. The rates of stroke and bleeding were higher after redo SAVR than ViV, but there was no significant difference in the need for a permanent pacemaker, the incidence of PVL, or the pooled postprocedural mean transvalvular gradients (15.2 mm Hg for ViV vs 13.5 mm Hg for redo SAVR; P = .55). In another meta-analysis, Villablanca et al.72 reported no difference in long-term all-cause mortality, but ViV patients had a lower incidence of stroke, atrial fibrillation, acute kidney injury, and major bleeding. Redo SAVR had a lower incidence of vascular complications, aortic regurgitation, and pacemaker implantation.
CONCLUSION
While redo SAVR is still considered the treatment of choice for younger patients at good surgical risk with severe SVD, ViV can be considered as an alternative, especially in older patients and those with comorbidities who are at higher risk for redo SAVR. The differential diagnosis of SVD must be considered, and it is critical to rule out high gradients due to patient-prosthesis mismatch and endocarditis in patients with bioprosthetic aortic valve stenosis. It is also important to differentiate between PVL and intravalvular aortic regurgitation, which can be difficult due to shadowing from the bioprosthetic valve structure. Understanding the risks and technical limitations of the ViV procedure is necessary in order to offer patients a valid informed consent that includes an accurate assessment of the risks and benefits of ViV vs redo SAVR vs medical management.
When ViV is considered to be the appropriate procedure, the surgeon must thoroughly analyze the type and size of valve used, how it was implanted, and the anatomy of the aortic root, coronary arteries, and vascular access options. Choosing the appropriate THV type, size, and implant depth during preoperative planning requires diligent analysis of preoperative imaging as well as the use of adjuncts such as published images and apps to anticipate the fit of the ViV, the fluoroscopic appearance, and visible landmarks. The experience with ViV should also influence the strategies of SAVR in anticipation of a potential ViV procedure that may be required in the future. With the trend toward more frequent use of bioprosthetic valves in younger patients, surgeons should consider the valve type and size at the time of the SAVR. For instance, a 78-year-old patient weighing more than 280 pounds recently presented with SVD; the surgeon had implanted a 27-mm Medtronic Mosaic valve 13 years prior with an aortic root enlargement to avoid using a 23-mm bioprosthetic valve at the time of initial SAVR. The ViV procedure was technically simplified by the larger valve, and the postprocedural gradient was 5 mm Hg. The heart team was “virtually” thanking the original surgeon throughout the procedure for making the ViV procedure more technically favorable.
KEY POINTS
Transcatheter aortic valve-in-valve procedures can be performed safely in selected patients as a less-invasive option to avoid reoperative surgical aortic valve replacement.
Thorough and detailed preoperative planning by the heart team is critical to optimize the outcomes of the operation.
Knowing the results of experimental and clinical valve-in-valve procedures helps physicians evaluate the risks and benefits for individual patients to determine whether valve-in-valve or redo surgical aortic valve replacement is preferable.
Choices of valve types, valve sizes, and implantation techniques at the time of the original surgical aortic valve replacement can affect the technical success of a potential transcatheter valve-in-valve procedure years later.
Footnotes
Conflict of Interest Disclosure
Dr. Reardon conducts research on behalf of Medtronic, Boston Scientific Corp., and St. Jude Medical, Inc./Abbott.
REFERENCES
- 1. Brown JM, O'Brien SM, Wu C, Sikora JA, Griffith BP, Gammie JS.. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg. 2009; 137: 82– 90. [DOI] [PubMed] [Google Scholar]
- 2. Zellner JL, Kratz JM, Crumbley AJ 3rd, Stroud MR, Bradley SM, Sade RM, Crawford FA Jr.. Long-term experience with the St. Jude Medical valve prosthesis. Ann Thorac Surg 1999; 68: 1210– 8. [DOI] [PubMed] [Google Scholar]
- 3. Johnston DR, Soltesz EG, Vakil N, . et al. Long-term durability of bioprosthetic aortic valves: implications from 12,569 implants. Ann Thorac Surg. 2015. April; 99 4: 1239– 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bourguignon T, Lhommet P, El Khoury R, . et al. Very long-term outcomes of the Carpentier-Edwards Perimount aortic valve in patients aged 50–65 years. Eur J Cardiothorac Surg. 2016. May; 49 5: 1462– 8. [DOI] [PubMed] [Google Scholar]
- 5. McClure RS, Narayanasamy N, Wiegerinck E, . et al. Late outcomes for aortic valve replacement with the Carpentier-Edwards pericardial bioprosthesis: up to 17-year follow-up in 1,000 patients. Ann Thorac Surg. 2010. May; 89 5: 1410– 6. [DOI] [PubMed] [Google Scholar]
- 6. Leontyev S, Borger MA, Davierwala P, . et al. Redo aortic valve surgery: early and late outcomes. Ann Thorac Surg. 2011. April; 91 4: 1120– 6. [DOI] [PubMed] [Google Scholar]
- 7. Hammermeister KE, Sethi GK, Henderson WG, Oprian C, Kim T, Rahimtoola S.. A comparison of outcomes in men 11 years after heart-valve replacement with a mechanical valve or bioprosthesis. Veterans Affairs Cooperative Study on Valvular Heart Disease. N Engl J Med. 1993. May 6; 328 18: 1289– 96. [DOI] [PubMed] [Google Scholar]
- 8. Chan V, Jamieson WR, Germann E, . et al. Performance of bioprostheses and mechanical prostheses assessed by composites of valve-related complications to 15 years after aortic valve replacement. J Thorac Cardiovasc Surg. 2006. June; 131 6: 1267– 73. [DOI] [PubMed] [Google Scholar]
- 9. Ruel M, Chan V, Bédard P, . et al. Very Long-Term Survival Implications of Heart Valve Replacement With Tissue Versus Mechanical Prostheses in Adults <60 Years of Age. Circulation. 2007; 116 11 Suppl: 1294– 300. [DOI] [PubMed] [Google Scholar]
- 10. Chiang YP, Chikwe J, Moskowitz AJ, Itagaki S, Adams DH, Egorova NN.. Survival and long-term outcomes following bioprosthetic vs mechanical aortic valve replacement in patients aged 50 to 69 years. JAMA. 2014. October 1; 312 13: 1323– 9. [DOI] [PubMed] [Google Scholar]
- 11. Reul GJ Jr, Cooley DA, Duncan JM, . et al. Valve failure with the Ionescu-Shiley bovine pericardial bioprosthesis: analysis of 2680 patients. J Vasc Surg. 1985. January; 2 1: 192– 204. [PubMed] [Google Scholar]
- 12. Urso S, Calderón P, Sadaba R, . et al. Patient-prosthesis mismatch in patients undergoing bioprosthetic aortic valve implantation increases risk of reoperation for structural valve deterioration. J Card Surg. 2014. July; 29 4: 439– 44. [DOI] [PubMed] [Google Scholar]
- 13. Piazza N, Bleiziffer S, Brockmann G, . et al. Transcatheter aortic valve implantation for failing surgical aortic bioprosthetic valve: from concept to clinical application and evaluation (part 1). JACC Cardiovasc Interv. 2011. July; 4 7: 721– 32. [DOI] [PubMed] [Google Scholar]
- 14. Mohty D, Dumesnil JG, Echahidi N, . et al. Impact of prosthesis-patient mismatch on longterm survival after aortic valve replacement: influence of age, obesity, and left ventricular dysfunction. J Am Coll Cardiol. 2009. January 6; 53 1: 39– 47. [DOI] [PubMed] [Google Scholar]
- 15. Hong S, Yi G, Youn YN, Lee S, Yoo KJ, Chang BC.. Effect of the prosthesis-patient mismatch on long-term clinical outcomes after isolated aortic valve replacement for aortic stenosis: a prospective observational study. J Thorac Cardiovasc Surg. 2013. November; 146 5: 1098– 104. [DOI] [PubMed] [Google Scholar]
- 16. Pibarot P, Weissman NJ, Stewart WJ, . et al. Incidence and sequelae of prosthesis-patient mismatch in transcatheter versus surgical valve replacement in high-risk patients with severe aortic stenosis: a PARTNER trial cohort--a analysis. J Am Coll Cardiol. 2014. September 30; 64 13: 1323– 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mihaljevic T, Nowicki ER, Rajeswaran J, . et al. Survival after valve replacement for aortic stenosis: implications for decision making. J Thorac Cardiovasc Surg. 2008. June; 135 6: 1270– 8; discussion 1278–9. [DOI] [PubMed] [Google Scholar]
- 18. Flameng W, Herregods MC, Vercalsteren M, Herijgers P, Bogaerts K, Meuris B.. Prosthesis-patient mismatch predicts structural valve degeneration in bioprosthetic heart valves. Circulation. 2010. May 18; 121 19: 2123– 9. [DOI] [PubMed] [Google Scholar]
- 19. Cohn LH, Aranki SF, Rizzo RJ, . et al. Decrease in operative risk of reoperative valve surgery. Ann Thorac Surg. 1993. July; 56 1: 15– 20; discussion 20–1. [DOI] [PubMed] [Google Scholar]
- 20. Chan V, Lam BK, Rubens FD, . et al. Long-term evaluation of biological versus mechanical prosthesis use at reoperative aortic valve replacement. J Thorac Cardiovasc Surg. 2012. July; 144 1: 146– 51. [DOI] [PubMed] [Google Scholar]
- 21. Kirsch M, Nakashima K, Kubota S, Houël R, Hillion ML, Loisance D.. The risk of reoperative heart valve procedures in Octogenarian patients. J Heart Valve Dis. 2004. November; 13 6: 991– 6; discussion 996. [PubMed] [Google Scholar]
- 22. Jaussaud N, Gariboldi V, Giorgi R, . et al. Risk of reoperation for aortic bioprosthesis dysfunction. J Heart Valve Dis. 2009. May; 18 3: 256– 61. [PubMed] [Google Scholar]
- 23. Leon MB, Smith CR, Mack M, . et al .; PARTNER Trial Investigators Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010. October 21; 363 17: 1597– 607. [DOI] [PubMed] [Google Scholar]
- 24. Popma JJ, Adams DH, Reardon MJ, . et al .; CoreValve United States Clinical Investigators Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014. May 20; 63 19: 1972– 81. [DOI] [PubMed] [Google Scholar]
- 25. Smith CR, Leon MB, Mack MJ, . et al .; PARTNER Trial Investigators Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011. June 9; 364 23: 2187– 98. [DOI] [PubMed] [Google Scholar]
- 26. Adams DH, Popma JJ, Reardon MJ.. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014. September 4; 371 10: 967– 8. [DOI] [PubMed] [Google Scholar]
- 27. Reardon MJ, Van Mieghem NM, Popma JJ, . et al .; SURTAVI Investigators Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017. April 6; 376 14: 1321– 31. [DOI] [PubMed] [Google Scholar]
- 28. Leon MB, Smith CR, Mack MJ, . et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016. April 28; 374 17: 1609– 20. [DOI] [PubMed] [Google Scholar]
- 29. Gurvitch R, Tay EL, Wijesinghe N, . et al. Transcatheter aortic valve implantation: lessons from the learning curve of the first 270 high-risk patients. Catheter Cardiovasc Interv. 2011. December 1; 78 7: 977– 84. [DOI] [PubMed] [Google Scholar]
- 30. Suri RM, Minha S, Alli O, . et al. Learning curves for transapical transcatheter aortic valve replacement in the PARTNER-I trial: Technical performance, success, and safety. J Thorac Cardiovasc Surg. 2016. September; 152 3: 773– 780.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Walther T, Falk V, Dewey T, . et al. Valve-in-a-valve concept for transcatheter minimally invasive repeat xenograft implantation. J Am Coll Cardiol. 2007. July 3; 50 1: 56– 60. [DOI] [PubMed] [Google Scholar]
- 32. Azadani AN, Jaussaud N, Matthews PB, Ge L, Chuter TA, Tseng EE.. Transcatheter aortic valves inadequately relieve stenosis in small degenerated bioprostheses. Interact Cardiovasc Thorac Surg. 2010. July; 11 1: 70– 7. [DOI] [PubMed] [Google Scholar]
- 33. Azadani AN, Tseng EE. Transcatheter valve-in-valve implantation for failing bioprosthetic valves. Future Cardiol. 2010. November; 6 6: 811– 31. [DOI] [PubMed] [Google Scholar]
- 34. Azadani AN, Reardon M, Simonato M, . et al. Effect of transcatheter aortic valve size and position on valve-in-valve hemodynamics: An in vitro study. J Thorac Cardiovasc Surg. 2017. June; 153 6: 1303– 15. [DOI] [PubMed] [Google Scholar]
- 35. Wenaweser P, Buellesfeld L, Gerckens U, Grube E.. Percutaneous aortic valve replacement for severe aortic regurgitation in degenerated bioprosthesis: the first valve in valve procedure using the Corevalve Revalving system. Catheter Cardiovasc Interv. 2007. November 1; 70 5: 760– 4. [DOI] [PubMed] [Google Scholar]
- 36. Walther T, Kempfert J, Borger MA, . et al. Human minimally invasive off-pump valve-in-a-valve implantation. Ann Thorac Surg. 2008. March; 85 3: 1072– 3. [DOI] [PubMed] [Google Scholar]
- 37. Ruiz CE, Laborde JC, Condado JF, Chiam PT, Condado JA.. First percutaneous transcatheter aortic valve-in-valve implant with three year follow-up. Catheter Cardiovasc Interv. 2008. August 1; 72 2: 143– 8. [DOI] [PubMed] [Google Scholar]
- 38. Cheung A, Webb JG, Wong DR, . et al. Transapical transcatheter mitral valve-in-valve implantation in a human. Ann Thorac Surg. 2009. March; 87 3: e18– 20. [DOI] [PubMed] [Google Scholar]
- 39. Ye J, Webb JG, Cheung A, . et al. Transcatheter valve-in-valve aortic valve implantation: 16-month follow-up. Ann Thorac Surg. 2009. October; 88 4: 1322– 4. [DOI] [PubMed] [Google Scholar]
- 40. Klaaborg KE, Egeblad H, Jakobsen CJ, . et al. Transapical transcatheter treatment of a stenosed aortic valve bioprosthesis using the Edwards SAPIEN Transcatheter Heart Valve. Ann Thorac Surg. 2009. June; 87 6: 1943– 6. [DOI] [PubMed] [Google Scholar]
- 41. Kelpis TG, Mezilis NE, Ninios VN, Pitsis AA.. Minimally invasive transapical aortic valve-in-a-valve implantation for severe aortic regurgitation in a degenerated stentless bioprosthesis. J Thorac Cardiovasc Surg. 2009. October; 138 4: 1018– 20. [DOI] [PubMed] [Google Scholar]
- 42. Rodés-Cabau J, Dumont E, Doyle D, Lemieux J.. Transcatheter valve-in-valve implantation for the treatment of stentless aortic valve dysfunction. J Thorac Cardiovasc Surg. 2010. July; 140 1: 246– 8. [DOI] [PubMed] [Google Scholar]
- 43. Webb JG, Wood DA, Ye J, . et al. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation. 2010. April 27; 121 16: 1848– 57. [DOI] [PubMed] [Google Scholar]
- 44. Seiffert M, Franzen O, Conradi L, . et al. Series of transcatheter valve-in-valve implantations in high-risk patients with degenerated bioprostheses in aortic and mitral position. Catheter Cardiovasc Interv. 2010. October 1; 76 4: 608– 15. [DOI] [PubMed] [Google Scholar]
- 45. Gurvitch R, Cheung A, Ye J, . et al. Transcatheter valve-in-valve implantation for failed surgical bioprosthetic valves. J Am Coll Cardiol. 2011. November 15; 58 21: 2196– 209. [DOI] [PubMed] [Google Scholar]
- 46. Bedogni F, Laudisa ML, Pizzocri S, . et al. Transcatheter valve-in-valve implantation using Corevalve Revalving System for failed surgical aortic bioprostheses. JACC Cardiovasc Interv. 2011. November; 4 11: 1228– 34. [DOI] [PubMed] [Google Scholar]
- 47. Wilbring M, Alexiou K, Tugtekin SM, . et al. Transcatheter valve-in-valve therapies: patient selection, prosthesis assessment and selection, results, and future directions. Curr Cardiol Rep. 2013. March; 15 3: 341. [DOI] [PubMed] [Google Scholar]
- 48. Mylotte D, Lange R, Martucci G, Piazza N.. Transcatheter heart valve implantation for failing surgical bioprostheses: technical considerations and evidence for valve-in-valve procedures. Heart. 2013. July; 99 13: 960– 7. [DOI] [PubMed] [Google Scholar]
- 49. Deeb GM, Chetcuti SJ, Reardon MJ, . et al. 1-year results in patients undergoing transcatheter aortic valve replacement with failed surgical bioprostheses. JACC Cardiovasc Interv. 2017. May 22; 10 10: 1034– 44. [DOI] [PubMed] [Google Scholar]
- 50. Dumont E, Lemieux J, Doyle D, Rodés-Cabau J.. Feasibility of transapical aortic valve implantation fully guided by transesophageal echocardiography. J Thorac Cardiovasc Surg. 2009. October; 138 4: 1022– 4. [DOI] [PubMed] [Google Scholar]
- 51. Bapat V, Davies W, Attia R, . et al. Use of balloon expandable transcatheter valves for valve-in-valve implantation in patients with degenerative stentless aortic bioprostheses: Technical considerations and results. J Thorac Cardiovasc Surg. 2014. September; 148 3: 917– 22; discussion 922–4. [DOI] [PubMed] [Google Scholar]
- 52. Sarkar K, Ussia GP, Tamburino C.. Transcatheter aortic valve implantation for severe aortic regurgitation in a stentless bioprosthetic valve with the Core Valve revalving system-technical tips and role of the Accutrak system. Catheter Cardiovasc Interv. 2011. September 1; 78 3: 485– 90. [DOI] [PubMed] [Google Scholar]
- 53. Huber C, Praz F, O'Sullivan CJ, . et al. Transcarotid aortic valve-in-valve implantation for degenerated stentless aortic root conduits with severe regurgitation: a case series. Interact Cardiovasc Thorac Surg. 2015. June; 20 6: 694– 700. [DOI] [PubMed] [Google Scholar]
- 54. Cockburn J, Trivedi U, Hildick-Smith D.. Transaortic transcatheter aortic valve implantation within a previous bioprosthetic aortic valve replacement. Catheter Cardiovasc Interv. 2011. September 1; 78 3: 479– 84. [DOI] [PubMed] [Google Scholar]
- 55. Himbert D1, Brochet E, Radu C, . et al. Transseptal implantation of a transcatheter heart valve in a mitral annuloplasty ring to treat mitral repair failure. Circ Cardiovasc Interv. 2011. August; 4 4: 396– 8. [DOI] [PubMed] [Google Scholar]
- 56. Phan K, Zhao DF, Wang N, Huo YR, Di Eusanio M, Yan TD.. Transcatheter valve-in-valve implantation versus reoperative conventional aortic valve replacement: a systematic review. J Thorac Dis. 2016. January; 8 1: E83– 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ejiofor JI, Yammine M, Harloff MT, . et al. Reoperative Surgical Aortic Valve Replacement Versus Transcatheter Valve-in-Valve Replacement for Degenerated Bioprosthetic Aortic Valves. Ann Thorac Surg. 2016. November; 102 5: 1452– 8. [DOI] [PubMed] [Google Scholar]
- 58. Ribeiro HB, Webb JG, Makkar RR, . et al. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. J Am Coll Cardiol. 2013. October 22; 62 17: 1552– 62. [DOI] [PubMed] [Google Scholar]
- 59. Dvir D, Webb JG, Bleiziffer S, . et al .; Valve-in-Valve International Data Registry Investigators Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA. 2014. July; 312 2: 162– 70. [DOI] [PubMed] [Google Scholar]
- 60. Dvir D, Leipsic J, Blanke P, . et al. Coronary obstruction in transcatheter aortic valve-in-valve implantation: preprocedural evaluation, device selection, protection, and treatment. Circ Cardiovasc Interv. 2015. January; 8 1. [DOI] [PubMed] [Google Scholar]
- 61. Dvir D, Webb J, Brecker S, . et al. Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: results from the global valve-in-valve registry. Circulation. 2012. November 6; 126 19: 2335– 44. [DOI] [PubMed] [Google Scholar]
- 62. Silaschi M, Wendler O, Seiffert M, . et al. Transcatheter valve-in-valve implantation versus redo surgical aortic valve replacement in patients with failed aortic bioprostheses. Interact Cardiovasc Thorac Surg. 2017. January; 24 1: 63– 70. [DOI] [PubMed] [Google Scholar]
- 63. Blanke P, Soon J, Dvir D, . et al. Computed tomography assessment for transcatheter aortic valve in valve implantation: The vancouver approach to predict anatomical risk for coronary obstruction and other considerations. J Cardiovasc Comput Tomogr. 2016. Nov-Dec; 10 6: 491– 9. [DOI] [PubMed] [Google Scholar]
- 64. Sedaghat A, Sinning JM, Utzenrath M, . et al. Hydrodynamic Performance of the Medtronic CoreValve and the Edwards SAPIEN XT Transcatheter Heart Valve in Surgical Bioprostheses: An In Vitro Valve-in-Valve Model. Ann Thorac Surg. 2016. January; 101 1: 118– 24. [DOI] [PubMed] [Google Scholar]
- 65. Midha PA, Raghav V, Condado JF, . et al. Valve Type, Size, and Deployment Location Affect Hemodynamics in an In Vitro Valve-in-Valve Model. JACC Cardiovasc Interv. 2016. August 8; 9 15: 1618– 28. [DOI] [PubMed] [Google Scholar]
- 66. Doose C, Kütting M, Egron S, . et al. Valve-in-valve outcome: design impact of a pre-existing bioprosthesis on the hydrodynamics of an Edwards Sapien XT valve. Eur J Cardiothorac Surg. 2017. March 1; 51 3: 562– 70. [DOI] [PubMed] [Google Scholar]
- 67. Simonato M, Webb J, Kornowski R, . et al. Transcatheter Replacement of Failed Bioprosthetic Valves: Large Multicenter Assessment of the Effect of Implantation Depth on Hemodynamics After Aortic Valve-in-Valve. Circ Cardiovasc Interv. 2016. June; 9 6. [DOI] [PubMed] [Google Scholar]
- 68. Dvir D, Barbanti M, Tan J, Webb JG.. Transcatheter aortic valve-in-valve implantation for patients with degenerative surgical bioprosthetic valves. Curr Probl Cardiol. 2014. January; 39 1: 7– 27. [DOI] [PubMed] [Google Scholar]
- 69. Tanase D, Grohmann J, Schubert S, Uhlemann F, Eicken A, Ewert P.. Cracking the ring of Edwards Perimount bioprosthesis with ultrahigh pressure balloons prior to transcatheter valve in valve implantation. Int J Cardiol. 2014. October 20; 176 3: 1048– 9. [DOI] [PubMed] [Google Scholar]
- 70. Nielsen-Kudsk JE, Christiansen EH, Terkelsen CJ, . et al. Fracturing the Ring of Small Mitroflow Bioprostheses by High-Pressure Balloon Predilatation in Transcatheter Aortic Valve-in-Valve Implantation. Circ Cardiovasc Interv. 2015. August; 8 8: e002667. [DOI] [PubMed] [Google Scholar]
- 71. Erlebach M, Wottke M, Deutsch MA, . et al. Redo aortic valve surgery versus transcatheter valve-in-valve implantation for failing surgical bioprosthetic valves: consecutive patients in a single-center setting. J Thorac Dis. 2015. September; 7 9: 1494– 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Villablanca PA, Mathew V, Thourani VH, . et al. A meta-analysis and meta-regression of long-term outcomes of transcatheter versus surgical aortic valve replacement for severe aortic stenosis. Int J Cardiol. 2016. December 15; 225: 234– 43. [DOI] [PubMed] [Google Scholar]


