Abstract
Transcatheter mitral valve replacement (TMVR) is a novel approach for treatment of severe mitral regurgitation. A number of TMVR devices are currently undergoing feasibility trials using both transseptal and transapical routes for device delivery. Overall experience worldwide is limited to fewer than 200 cases. At present, the 30-day mortality exceeds 30% and is attributable to both patient- and device-related factors.
TMVR has been successfully used to treat patients with degenerative mitral stenosis (DMS) as well as failed mitral bioprosthesis and mitral repair using transcatheter mitral valve-in-valve (TMViV)/valve-in-ring (ViR) repair. These patients are currently treated with devices designed for transcatheter aortic valve replacement. Multicenter registries have been initiated to collect outcomes data on patients currently undergoing TMViV/ViR and TMVR for DMS and have confirmed the feasibility of TMVR in these patients. However, the high periprocedural and 30-day event rates underscore the need for further improvements in device design and multicenter randomized studies to delineate the role of these technologies in patients with mitral valve disease.
Keywords: Transcatheter mitral valve replacement, mitral regurgitation, degenerative mitral stenosis, valve-in-valve repair, valve-in-ring repair
INTRODUCTION AND BACKGROUND
The prevalence of moderate or severe mitral regurgitation (MR) in the United States is between 2 and 2.5 million and is expected to reach 5 million by 2030.1 The 2013 American College of Cardiology/American Heart Association and 2012 European Society of Cardiology guidelines recommend mitral valve (MV) surgery for severe symptomatic MR or asymptomatic MR with left ventricular (LV) dysfunction.2,3 However, large surveys in western populations4 have documented that only a small percentage of patients undergo potentially curative mitral surgery, whereas patients with advanced age, severe LV dysfunction, and comorbidities are typically denied surgery.5
The swift metamorphosis of transcatheter aortic valve replacement (TAVR) from an investigational technique for inoperable patients6,7 to an established therapy for severe aortic stenosis (AS)8,9 in intermediate- and high-risk subgroups has stimulated efforts to develop transcatheter approaches for treating MV disease. In addition, patients with degenerative mitral stenosis (DMS),10 a failed surgical mitral bioprosthesis,11 and prior mitral repair also represent cohorts that may benefit from less-invasive alternatives to high-risk redo MV surgery. As a result, transcatheter MV replacement (TMVR) is the latest iteration of catheter-based strategies that attempts to fulfill the unmet need for percutaneous treatment of MV disease.
This review highlights the current nascent state of TMVR and summarizes relevant insights from the limited contemporary experience with this procedure in three patient cohorts: those with severe degenerative or functional MR, failed mitral bioprostheses or repair, and DMS.
TMVR FOR MITRAL REGURGITATION
An ideal transcatheter treatment for MR should accomplish the following objectives: (1) expand the patient population that can be offered a viable treatment, including patients with unacceptable risk for open surgery; (2) successfully treat the pathophysiology, including both primary and secondary MR; (3) provide lasting reduction and elimination of MR with no recurrence; (4) ideally be performed percutaneously without needing protracted general anesthesia or extracorporeal circulatory support (ECC); and (5) deliver the device with relatively simple access and closure to minimize the risk of major cardiac or vascular complications.
The fact that MV disease is undertreated worldwide along with the potential broad applicability of this new technology (in contrast to transcatheter mitral repair, which is feasible in only a limited number of patients) is driving interest in TMVR. However, a number of formidable challenges must be overcome in designing a device that targets the MV complex. Figure 1 enumerates some of the constraints in developing a successful TMVR device. At the time of this writing, at least six devices have been implanted in human patients.12 These devices are based on nitinol alloy platforms enclosing bovine or porcine leaflets, and they employ diverse mechanisms to anchor and stabilize them in the mitral annulus (Table 1). All TMVR devices are implanted using transapical (TA) access except the CardiAQ device (Edwards Lifesciences Corporation), which has been successfully implanted using antegrade transseptal (TS) as well as TA access.13 The current clinical experience and status of the clinical program is summarized in Table 2.14
Figure 1.
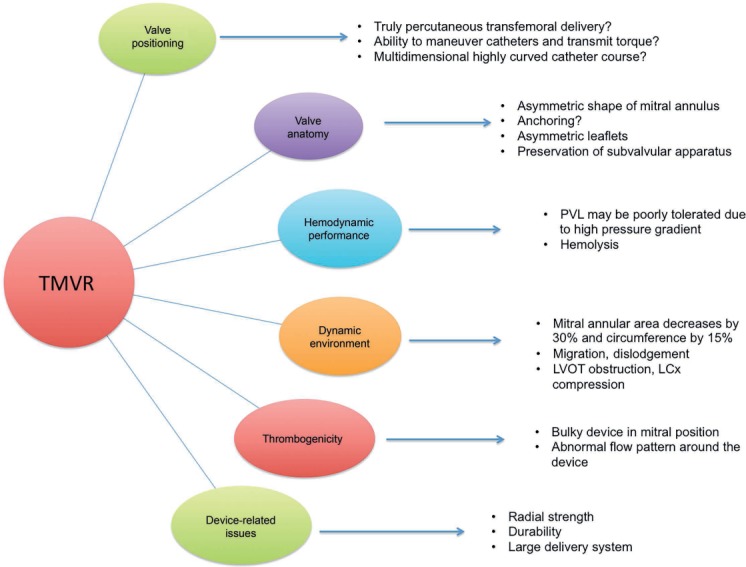
Challenges in the successful development of a transcatheter mitral valve replacement (TMVR) device. Multiple interrelated factors influence the optimal positioning and performance of a TMVR device. PVL: paravalvular leak; LVOT: left ventricular outflow tract; LCx: left circumflex artery. Adapted from De Backer et al.12
Table 1.
Salient features of current transcatheter mitral valve replacement (TMVR) platforms. Comparison of relevant features pertaining to device design, anchoring mechanism, delivery approach, and sheath size.

Table 2.
Current experience and status of clinical programs with transcatheter mitral valve replacement platforms. Adapted from Krishnaswamy et al.14
Procedure
The preparation for TMVR requires a detailed multimodality evaluation with both 2- and 3-dimensional (2D/3D) transesophageal echocardiography (TEE) and multiplanar computed tomography imaging with overlay and/or cardiac magnetic resonance imaging. These imaging technologies help ascertain (1) the mechanism and severity of mitral dysfunction, (2) accurate measurements of the left ventricle, mitral annulus, interatrial septum, left ventricular outflow tract (LVOT) and predicted neo-LVOT diameter after TMVR, and (3) the location and course of the left anterior descending and diagonal arteries relevant to TA access and myocardial thickness at the apex.
Transapical TMVR
In transapical TMVR, the patient is under general anesthesia, and the procedure is performed in a hybrid operating suite under 3D TEE and fluoroscopy guidance. A TA approach typically involves a minithoracotomy with purse-string sutures for access site management. The LV apex is accessed with a standard puncture needle, and a 6F sheath is introduced inside the left ventricle. A J-tip guidewire is then advanced into a pulmonary vein and exchanged for a stiff guidewire. The access site is predilated, and the TMVR device delivery system is positioned and deployed under fluoroscopic and TEE guidance during rapid ventricular pacing (Figure 2).
Figure 2.
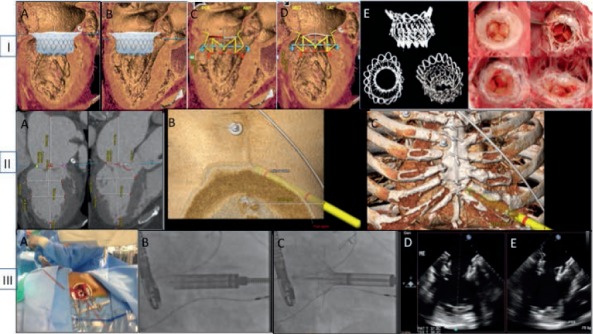
Transapical (TA) transcatheter mitral valve replacement preprocedural planning and device deployment. Panel I (A–F) illustrates preprocedural planning with emphasis on computed tomography (CT) overlay and predicting device anchoring and effect on left ventricular outflow tract (LVOT) dimensions. Panel II (A–C) illustrates use of CT techniques to predict TA access site and sheath trajectory. Panel III (A–E) demonstrates TA access, device deployment, and postdeployment echo assessment.
Transseptal TMVR
The transseptal approach to TMVR is more nuanced due to the large profile of the CardiAQ device.13 After gaining transfemoral arterial and venous accesses, a transseptal puncture (TSP) is performed under TEE guidance. A second TSP is performed caudal to the first one to introduce a snare catheter that helps navigate the device across the mitral annulus. An arteriovenous loop is established between the ipsilateral femoral artery and vein by using a customized nitinol guide wire in the descending aorta that is positioned from the venous side and snared out of the ipsilateral femoral artery (Figure 3, panel I).13
Figure 3.
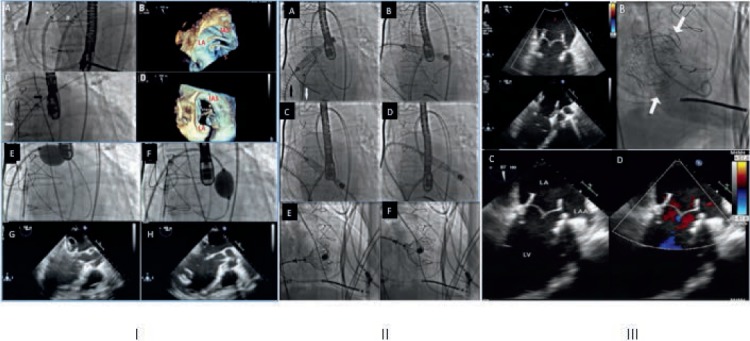
Transseptal (TS) transcatheter mitral valve replacement procedural steps. Panel I (A–H) shows dual transseptal puncture and balloon tracking. Panel II (A–F) shows device tracking and deployment. Panel III (A–D) shows device evaluation postdeployment.13
Under TEE guidance, the device capsule is inserted into the femoral vein over the nitinol wire, and the entire catheter delivery system (CDS) is tracked through the interatrial septum into the left atrium and across the mitral annulus. The capsule containing the loaded valve is directed toward the LV with the help of the snare system. Once in position across the mitral valve, the capsule is retracted and the LV anchors deployed to capture the native mitral leaflets. The position of the LV anchors is confirmed with TEE before the left atrial anchors are exposed and deployed. After documenting optimal bioprosthesis performance under fluoroscopy and TEE, the valve is released from the CDS (Figure 3, Panel II).13 Finally, the CDS is removed via the femoral vein. Three-dimensional echocardiographic reconstruction is used to confirm the optimal position and valve function. The residual interatrial communication is closed with a patent foramen ovale occluder (Figure 3, Panel III).13
TMVR Outcomes
Worldwide experience with TMVR is limited to fewer than 200 cases. A number of devices are undergoing phase I trials at present. While detailed reports are still awaited, preliminary experience indicates a high 30-day mortality ranging from 25% to 40% that has been attributed to sepsis, multiorgan failure, device thrombosis, and the inability to recover from access site complications.15 This truly reflects the formidable challenges that exist in terms of patient selection, access management, device-related factors, and extensive comorbidities of patients currently undergoing this procedure. Incremental advances in all of these intertwined variables and data from evolving clinical experience are critical for defining the role of TMVR in the realm of transcatheter mitral therapy.
TMVR FOR FAILED SURGICAL MITRAL REPAIR AND REPLACEMENT: VALVE-IN-VALVE AND VALVE-IN-RING
Mitral valve repair and replacement with bioprosthetic valves is favored over mechanical MVR as they obviate the need for life-long anticoagulation, particularly in elderly patients with bleeding risk factors.16 Bioprosthetic valves can often last between 10 and 20 years depending on the patient's age and comorbidities, with a risk for reoperation due to valve dysfunction of 4.1%, 13.6%, 18.8%, and 23.5%, at 5, 10, 15, and 20 years, respectively.17
Redo mitral surgery in patients older than 75 years of age is associated with high perioperative morbidity and mortality that often exceeds 15%.18 For patients who are at an acceptable surgical risk for redo surgery, transcatheter mitral valve-in-valve/valve-in-ring (TMViV/ViR) replacement has emerged as a feasible alternative. Current clinical experience with this procedure is based on TAVR/transcatheter pulmonary valves implanted in patients on a compassionate off-label basis.19
TMViV/ViR Procedure
Prior to performing any TMViV/ViR procedure, it is routine to secure both a multimodality evaluation that pinpoints the type of prosthetic valve dysfunction as well as an accurate evaluation of the size and type of prosthetic mitral valve (or ring). In particular, the native D-shaped annuloplasty ring may predispose to paravalvular regurgitation (PVR) following valve implantation. Since a circular-shaped device is critical to ensure efficient sealing and prevent significant postprocedural PVR, it is prudent to select deformable complete and rigid semilunar annuloplasty rings for this procedure. It is also important to achieve at least 10% oversizing of the transcatheter valve compared to the true internal diameter of the surgical device.20 However, extreme oversizing can result in an underexpanded valve with impaired leaflet coaptation, elevated transvalvular gradient, and possibly limited durability. The general procedural principles involving a TA and TS approach are similar to those described for TMVR for native mitral valves, with a few technical details specific to TMViV/ViR: (1) Balloon predilation prior to implantation is generally avoided unless the crimped valve cannot be tracked across the bioprosthesis; (2) the transcatheter valve is positioned 3 to 5 mm atrially in relation to the sewing cuff of the surgical valve and deployed with a slow balloon inflation technique under rapid ventricular pacing21; and (3) for TMViR, the transcatheter valve is centered in the ring with equal portions in the left atrium and LV (Figure 4).19
Figure 4.
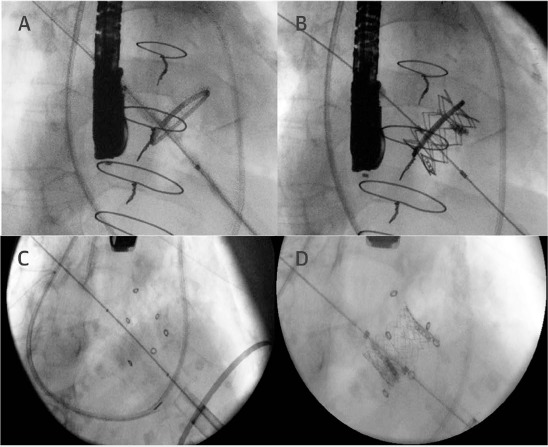
(A) Transcatheter mitral valve-in-valve/valve-in-ring (ViV/ViR) fluoroscopic image of a 28-mm Edwards Physio 1 ring (Edwards Lifesciences Corporation). (B) Final fluoroscopic image after the implantation of a 23-mm Edwards SAPIEN XT transcatheter heart valve inside the ring (ViR procedure) via a transapical approach. (C) Fluoroscopic image of a failing 23-mm Mosaic valve (Medtronic) in the mitral position. (D) Postprocedural fluoroscopic image showing a 23-mm Edwards SAPIEN XT transcatheter heart valve implanted within the surgical heart valve through a transapical route.19
TMVIV/VIR OUTCOMES
The largest data pool of TMViV procedures has been presented from the multicenter Valve-in-Valve International Data (VIVID) registry that included 349 patients who had TMViV and 88 patients who had TMViR.14 The following are key observations pertaining to outcomes after TMViV/ViR procedures from the VIVID registry:
These procedures have been performed in elderly patients with significant comorbidities. (Mean age 74 years and Society of Thoracic Surgeons' [STS] Online Risk Calculator score of 12.9%).
Most ViV/ViR procedures have been performed using TA access, while transseptal and left atrial accesses have been used for the remaining 20%. General anesthesia was used in almost all cases (98.9%). Most procedures have been performed with a balloon expandable device (Edwards SAPIEN, SAPIEN XT, Melody).
The mechanism of bioprosthetic valve failure included pure regurgitation (45%), pure stenosis (23%), and combined stenosis and regurgitation (32%).
Balloon predilation was performed in 24% of cases. Valve malpositioning was reported in 6.6%.
Postprocedure mean gradient was 6.3 mm Hg and significant (moderate or more) paravalvular leak (PVL) was reported in 3.5% of cases. LVOT obstruction was noted in 7% cases with a significantly higher incidence in ViR cases (8%) than in ViV (2.5%). A small valve size (< 25 mm) was a key predictor of significantly elevated gradient (> 10 mm Hg). Significant residual MR (> moderate) was more likely in ViR than in ViV cases.
Overall 30-day mortality was 8.5% (7.7% and 11.4% in ViV and ViR procedures, respectively; P = .15) and the occurrence of stroke was 2.5% (2.9% and 1.1% in ViV and ViR procedures, respectively). Late mortality (beyond 12 months) was 20.5%.
TMVR FOR DEGENERATIVE MITRAL STENOSIS
The underlying pathological lesion in DMS is mitral annular calcification (MAC),22 which reduces anterior leaflet mobility and impairs diastolic dilatation of the annulus. Surgical treatment of DMS is technically challenging and associated with significant complications including intractable bleeding from the LV wall, posterior ventricular rupture, separation of the left atrium and LV, circumflex artery injury, and significant PVL post MVR.23,24
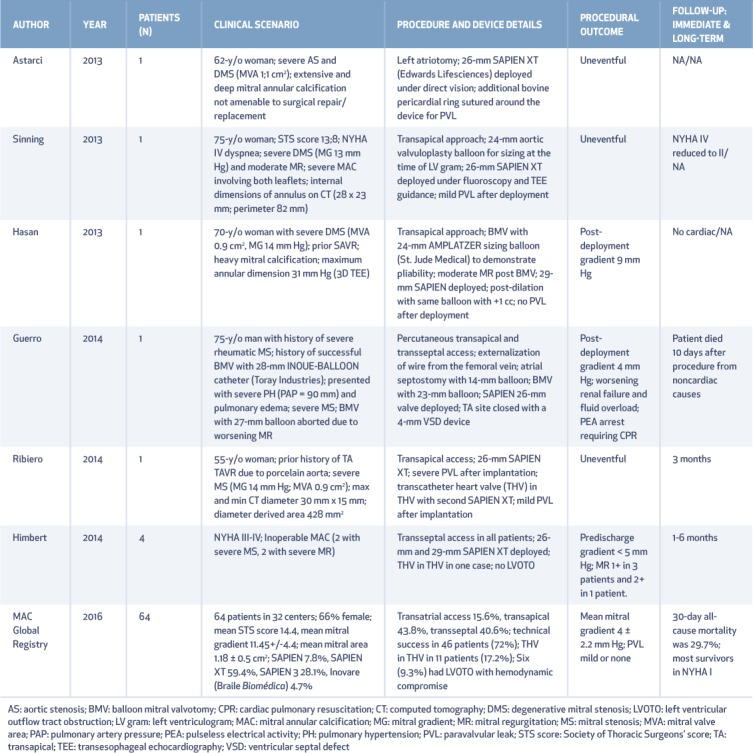
TMVR has been performed on an off-label basis at multiple centers for DMS, with most cases using balloon expandable Edwards SAPIEN/SAPIEN XT valves (Edwards Lifesciences Corporation) (Figure 5).25 Table 3 details relevant patient and procedural details from reported case series.26 The largest accrued experience of TMVR for DMS has been documented in the Mitral Annular Calcification (MAC) Global registry26 that enrolled 64 patients who underwent TMVR on a compassionate basis. Mean age of patients was 73 ± 13 years, 66% were female, and mean STS score was 14.4 ± 9.5%. The mean mitral gradient was 11.45 ± 4.4 mm Hg and the mean mitral area was 1.18 ± 0.5 cm.
Figure 5.
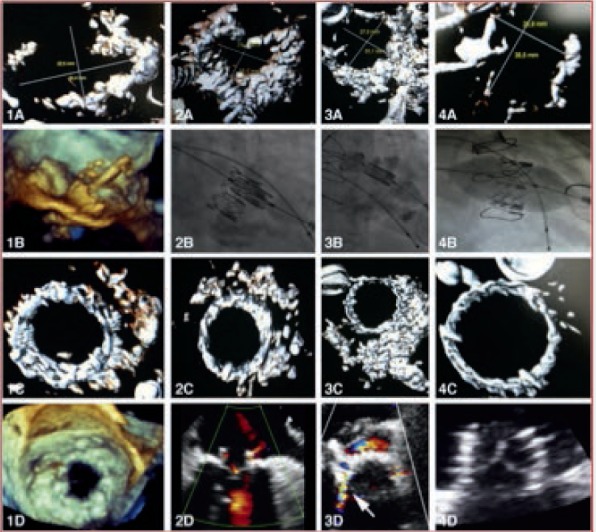
Transcatheter mitral valve replacement for degenerative mitral stenosis. (A) Images are for patients 1 to 4 in respective columns (1A, 2A, 3A, 4A). Computed tomography of mitral annular calcifications. (1B) Three-dimensional transesophageal echocardiography of valve implantation within the mitral annulus. (2B-4B) Fluoroscopy of valve implantation within the mitral annulus. (1C-4C) Computed tomography postimplantation. (1D) Three-dimensional echocardiography postimplantation. (2D-4D) Two-dimensional echocardiography postimplantation.25
Table 3.
Published reports of transcatheter mitral valve replacement in degenerative mitral stenosis, including clinical presentation, device details, and follow-up. Adapted from Guerrero et al.26
Access was transatrial in 15.6% of patients, transapical in 43.8%, and transseptal in 40.6%. Technical success was achieved in 46 (72%) patients, primarily limited by the need for a second valve in 11 (17.2%). Six (9.3%) had LV tract obstruction with hemodynamic compromise. Mean mitral gradient postprocedure was 4 ± 2.2 mm Hg, and PVL was mild or absent in all. Thirty-day all-cause mortality was 29.7% (cardiac: 12.5%, noncardiac: 17.2%); 84% of the survivors with available follow-up data were in New York Heart Association functional class I or II at 30 days (n = 25). These results highlight the feasibility of a transcatheter approach in this patient cohort. The high mortality on follow-up highlights the significant comorbidities of the patient population, which underscores the need for a more deliberate evaluation of the role of TMVR for DMS. Recently, the Mitral Implantation of Transcatheter Valves in Native Mitral Stenosis (MITRAL) trial has been approved as a multicenter investigational device exemption pilot study to evaluate outcomes for mitral implantation of the Edwards SAPIEN XT transcatheter valve in patients with severe DMS who are not candidates for surgery.12 The inclusion criteria specify patients with severe DMS (mitral valve area ≤ 1.5 cm2) who are symptomatic during a stress test or in New York Heart Association class II or greater. Exclusion criteria include patients with a history of recent myocardial infarction, complex coronary artery disease/hypertrophic obstructive cardiomyopathy, or those who received a permanent cardiac implant within the last 30 days. This study is expected to provide valuable insights into the role of TMVR for patients with DMS and the device improvements necessary to achieve optimal outcomes in these patients.
KEY POINTS
Transcatheter mitral valve replacement (TMVR) is a novel approach in the treatment of severe mitral regurgitation (MR). It offers a potentially durable solution to the problem of severe MR in a wide spectrum of patients who are unsuitable for mitral valve surgery
TMVR is presently being studied in patients with severe native MR, degenerated mitral bioprosthesis, or degenerative mitral stenosis. A number of devices are being evaluated in feasibility studies. Both transapical and transseptal routes have been used for delivering TMVR devices.
Preliminary results with TMVR have demonstrated a high 30-day mortality rate. This is indicative of the prohibitive risk profile of patients currently being treated with TMVR and procedural challenges surrounding very bulky high profile devices.
CONCLUSION
TMVR is currently in the early stages of clinical evaluation. The role of this technology in the treatment of MVD will be influenced by the concurrent progress in three interrelated spheres, which include (1) technological developments in device design to address problems of truly percutaneous TA access and closure, TS deliverability, anchoring, thrombosis, and LVOT obstruction (this includes imaging algorithms to accurately predict the impact of a TMVR device in a dynamic environment such as the mitral annulus); (2) the development of novel transcatheter mitral repair technologies that replicate surgical annuloplasty (clinical results with follow-up data will reveal if the role of TMVR and TMV replacement will be competitive or complimentary); and most importantly, (3) the ability to identify patients who stand to benefit the most from these approaches, which is critical to the success of these procedures.
Footnotes
Conflict of Interest Disclosure:
The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
REFERENCES
- 1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M.. Burden of valvular heart diseases: a population-based study. Lancet. 2006. September 16; 368 9540: 1005– 11. [DOI] [PubMed] [Google Scholar]
- 2. Vahanian A, Alfieri O, Andreotti F, . et al. Guidelines on the management of valvular heart disease (version 2012). The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2012. October; 33 19: 2451– 96. [DOI] [PubMed] [Google Scholar]
- 3. Nishimura RA, Otto CM, Bonow RO, . et al .; American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014. June 10; 63 22: e57– 185. [DOI] [PubMed] [Google Scholar]
- 4. Iung B, Baron G, Butchart EG, . et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003. July; 24 13: 1231– 43. [DOI] [PubMed] [Google Scholar]
- 5. Mirabel M, Iung B, Baron G, . et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J. 2007. June; 28 11: 1358– 65. [DOI] [PubMed] [Google Scholar]
- 6. Cribier A, Eltchaninoff H, Bash A, . et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002. December 10; 106 24: 3006– 8. [DOI] [PubMed] [Google Scholar]
- 7. Makkar RR, Fontana GP, Jilaihawi H, . et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012. May 3; 366 18: 1696– 704. [DOI] [PubMed] [Google Scholar]
- 8. Popma JJ, Adams DH, Reardon MJ, . et al .; CoreValve United States Clinical Investigators Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014. May 20; 63 19: 1972– 81. [DOI] [PubMed] [Google Scholar]
- 9. Mack MJ, Leon MB, Smith CR, . et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015. June 20; 385 9986: 2477– 84. [DOI] [PubMed] [Google Scholar]
- 10. Labovitz AJ, Nelson JG, Windhorst DM, Kennedy HL, Williams GA.. Frequency of mitral valve dysfunction from mitral anular calcium as detected by Doppler echocardiography. Am J Cardiol. 1985. January 1; 55 1: 133– 7. [DOI] [PubMed] [Google Scholar]
- 11. Jamieson WRE, Burr LH, Miyagishima RT, . et al. Reoperation for bioprosthetic mitral structural failure: risk assessment. Circulation. 2003. September 9; 108 Suppl 1: II98– 102. [DOI] [PubMed] [Google Scholar]
- 12. De Backer O, Piazza N, Banai S, . et al. Percutaneous transcatheter mitral valve replacement: an overview of devices in preclinical and early clinical evaluation. Circ Cardiovasc Interven. 2014. June; 7 3: 400– 9. [DOI] [PubMed] [Google Scholar]
- 13. Ussia GP, Quadri A, Cammalleri V, . et al. Percutaneous transfemoral-transseptal implantation of a second-generation CardiAQ mitral valve bioprosthesis: first procedure description and 30-day follow-up. EuroIntervention. 2016. February; 11 10: 1126– 31. [DOI] [PubMed] [Google Scholar]
- 14. Krishnaswamy A, Mick S, Navia J, Gillinov AM, Tuzcu EM, Kapadia SR.. Transcatheter mitral valve replacement: A frontier in cardiac intervention. Cleve Clin J Med. 2016. November; 83 11 Suppl 2: S10– S17. [DOI] [PubMed] [Google Scholar]
- 15. Bapat V, Buellesfeld L, Peterson MD, . et al. Transcatheter mitral valve implantation (TMVI) using the Edwards FORTIS device. EuroIntervention. 2014. September; 10 Suppl U: U120– 8. [DOI] [PubMed] [Google Scholar]
- 16. Gammie JS, Sheng S, Griffith BP, . et al. Trends in mitral valve surgery in the United States: results from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg. 2009. May; 87 5: 1431– 7; discussion 1437–9. [DOI] [PubMed] [Google Scholar]
- 17. Chan V, Malas T, Lapierre H, . et al. Reoperation of left heart valve bioprostheses according to age at implantation. Circulation. 2011. September 13; 124 11 Suppl: S75– 80. [DOI] [PubMed] [Google Scholar]
- 18. Balsam LB, Grossi EA, Greenhouse DG, . et al. Reoperative valve surgery in the elderly: predictors of risk and long-term survival. Ann Thorac Surg. 2010. October; 90 4: 1195– 200; discussion 1201. [DOI] [PubMed] [Google Scholar]
- 19. Paradis JM, Del Trigo M, Puri R, Rodés-Cabau J.. Transcatheter Valve-in-Valve and Valve-in-Ring for Treating Aortic and Mitral Surgical Prosthetic Dysfunction. J Am Coll Cardiol. 2015. November 3; 66 18: 2019– 37. [DOI] [PubMed] [Google Scholar]
- 20. Cheung A, Webb JG, Barbanti M, . et al. 5-year experience with transcatheter transapical mitral valve-in-valve implantation for bioprosthetic valve dysfunction. J Am Coll Cardiol. 2013. April 30; 61 17: 1759– 66. [DOI] [PubMed] [Google Scholar]
- 21. Cheung A, Al-Lawati A. Transcatheter mitral valve-in-valve implantation: current experience and review of literature. Curr Opin Cardiol. 2013. March; 28 2: 181– 6. [DOI] [PubMed] [Google Scholar]
- 22. Abramowitz Y, Jilaihawi H, Chakravarty T, Mack MJ, Makkar RR.. Mitral Annulus Calcification. J Am Coll Cardiol. 2015. October 27; 66 17: 1934– 41. [DOI] [PubMed] [Google Scholar]
- 23. Spencer FC, Galloway AC, Colvin SB.. A clinical evaluation of the hypothesis that rupture of the left ventricle following mitral valve replacement can be prevented by preservation of the chordae of the mural leaflet. Ann Surg. 1985. December; 202 6: 673– 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sud K, Agarwal S, Parashar A, . et al. Degenerative Mitral Stenosis: Unmet Need for Percutaneous Interventions. Circulation. 2016. April 19; 133 16: 1594– 604. [DOI] [PubMed] [Google Scholar]
- 25. Himbert D, Bouleti C, Iung B, . et al. Transcatheter valve replacement in patients with severe mitral valve disease and annular calcification. J Am Coll Cardiol. 2014. December 16; 64 23: 2557– 8. [DOI] [PubMed] [Google Scholar]
- 26. Guerrero M, Dvir D, Himbert D, . et al. Transcatheter Mitral Valve Replacement in Native Mitral Valve Disease With Severe Mitral Annular Calcification: Results From the First Multicenter Global Registry. JACC Cardiovasc Interv. 2016. July 11; 9 13: 1361– 71. [DOI] [PubMed] [Google Scholar]


