Abstract
Achieving cure of HIV infection requires eliminating all replication-competent virus from the reservoir of latently infected cells or completely inhibiting infected cells from emerging from latency. Strategies include very early use of antiretroviral therapy; hematopoietic stem cell transplantation; “shock-and-kill” approaches; immune therapy with immune checkpoint inhibitors; gene therapy, including use of CC chemokine receptor 5-modified CD4+ T cells; and broadly neutralizing antibody therapy. Success is likely to require a combination of approaches. This article summarizes a presentation by Daniel C. Douek, MD, PhD, at the IAS–USA continuing education program held in Berkeley, California, in May 2017.
Keywords: HIV, cure, hematopoietic stem cell transplant, antiretroviral therapy, shock and kill, gene therapy, immune checkpoint inhibitors, broadly neutralizing antibody therapy, latent infection
People on suppressive antiretroviral therapy acquire a reservoir of quiescent HIV-infected T cells that persists for life. These cells can undergo clonal expansion and maintain or increase the size of the reservoir without producing virus. If antiretroviral therapy is interrupted, production of HIV by these cells is observed within 2 to 4 weeks. Thus in the absence of antiretroviral therapy, cells that harbor quiescent replication-competent virus can rekindle HIV replication and transmission. The task in achieving cure of HIV infection is to eliminate all replication-competent virus in the reservoir or to attain lifelong remission, that is, sustained aviremia in the absence of antiretroviral therapy over an individual's lifetime.
How can we cure HIV-infected people? Numerous mechanisms account for HIV persistence. However, a unifying theme in cure strategies is to find and diminish the size of the HIV reservoir. Potential strategies include using early antiretroviral therapy to reduce seeding of the latent pool; reversing latency (“shock-and-kill” approach); increasing HIV-specific immune function (eg, with vaccines); reducing immune activation; using gene therapy to target the virus and the host; and using allogeneic hematopoietic stem cell transplantation. Combinations of these or other approaches may be necessary.
Hematopoietic Stem Cell Transplantation
Cure has only been achieved in 1 person, Timothy R. Brown, also referred to as the Berlin patient. He received a hematopoietic stem cell transplant from a donor whose cells were resistant to HIV infection (CC chemokine receptor 5 [CCR5] delta32/delta32). Brown, who has not received antiretroviral therapy for more than 10 years, has been doing well and has no evidence of replication-competent HIV. No viral DNA has been found in his peripheral blood mononuclear cells, and there is no convincing evidence for a nonartefactual signal in any assay for HIV nucleic acids,1 along with waning HIV antibodies and the absence of HIV-specific T cells. Although the transplantation approach is considered an important proof of concept in achieving cure, the risk associated with transplantation makes it unlikely that it will ever translate into an accessible method for all HIV-infected people.
In the case of 2 other individuals, known as the Boston patients, who received hematopoietic stem cell transplants from donors with cells susceptible to HIV infection, viral recrudescence was observed despite the 1000- to 10,000-fold reductions in viral reservoir size achieved.2 In one patient, viral rebound occurred after approximately 9 months off antiretroviral therapy and was attributed to a single virus. Thus, although kinetic modeling has indicated that a reduction of 100,000-fold in the reservoir is needed to achieve cure, the finding that a single virus may cause recrudescence suggests that cure is dependent on eliminating all latent replication-competent viruses or completely inhibiting their ability to emerge from latency.
Very Early Treatment
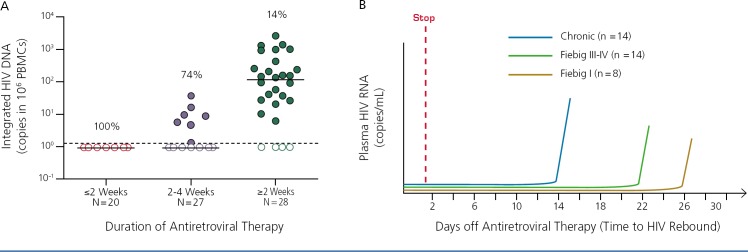
Can very early antiretroviral therapy reduce the size of the latent reservoir and play a role in cure? Studies of early reservoir dynamics in the absence of treatment indicate that about the time HIV RNA becomes detectable, the reservoir size begins to increase dramatically, with an apparent 100-fold increase over the next 2 weeks.3 The reservoir is largely established by week 4 of infection. However, very early antiretroviral treatment can substantially reduce the size of the reservoir. As shown in Figure 1A, initiation of treatment within 2 weeks of infection results in nearly undetectable reservoir size compared with initiation after 2 to 4 weeks of infection or during chronic infection. However, there is no clinically significant delay in time to viral rebound after stopping treatment. In the data shown in Figure 1B, median time to viral rebound was 14 days in chronic infection, 22 days in Fiebig stage III or IV infection, and 26 days in Fiebig stage I infection.4–6 Thus, it appears that there is a limit to the potential effect of even very early treatment in preventing recrudescence from a diminished reservoir.
Figure 1.
Effect of early antiretroviral therapy on reservoir size (A) and time to rebound after therapy interruption by infection stage (B). Chronic HIV infection has a range of 5–29 days; median, 14 days; Feibig stage III or IV infection has a range of 14–77 days; median, 22 days; and Feibig stage I infection has a range of 13–48 days; median, 26 days. PBMC indicates peripheral blood mononuclear cells. Adapted from Rothenberger et al. 2015,4 Kroon et al. 2016,5 and Colby et al. 20176
Shock and Kill
The strategy of “shock and kill,” relies on a latency reversing agent (LRA) to reactivate HIV transcription in latently infected cells. The immune system then recognizes and kills the infected cells. Administration of antiretroviral therapy throughout the shock-and-kill process protects against the propagation of new infection.
Many LRAs currently are being investigated, including epigenetic modifiers such as histone deacetylase (HDAC) inhibitors, toll-like receptor agonists, activators of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-KB), disulfiram, immune checkpoint inhibitors, and agents that affect the STAT5 signaling pathway and mTOR signaling. However, studies to date indicate that compared with maximal T-cell activation, few LRAs work well ex vivo with cells from HIV-infected patients. In clinical trials of LRAs, increases in cell-associated and plasma HIV RNA have been observed, with the reservoir size increasing and no detection of infected cells being eliminated.7
As to the “kill” part of the strategy, various studies have shown that neither the virus nor the immune system is effective in clearing infected cells after latency reversal; in one in vitro model, infected resting CD4+ T cells survived despite viral cytopathic effects.8 Further, because most of the virus has mutated to escape immune responses, escape variants dominate in the latent reservoir of people with chronic infection.9 Therapeutic vaccines to augment immune responses have resulted in transient expansion of T cells that do not recognize escaped HIV epitopes.10 At least 40 clinical trials of vaccines to increase the magnitude of HIV-specific immune response have been completed in the past 2 decades, and overall results show that vaccination is safe and immunogenic, but ineffective in eliminating virus.
A number of shock-and-kill studies have combined LRAs with approaches such as therapeutic vaccines, interferon, and broadly neutralizing antibodies to enhance immune response. In one study of 20 individuals on antiretroviral therapy who had a viral load below 50 HIV RNA copies/mL for more than 3 years, the combination of the HDAC inhibitor romidepsin and the HIV peptide vaccine resulted in no change in integrated DNA or infectious virus. A statistically significant decline in total HIV DNA was observed; however, the effect was clinically meaningless, because viral rebound after cessation of anti-retroviral therapy was always observed within 2 to 4 weeks.11
In a recent study, use of a different HIV vaccine in combination with romidepsin was associated with viral rebound within 4 weeks of interruption of antiretroviral drugs in 8 participants; 5 other participants exhibited sustained lower level viremia during the interruption.12
Immune Therapy
More promising are strategies that reverse the exhaustion of HIV-specific CD8+ T cells with the use of immune checkpoint inhibitors, which have become effective in treating a variety of malignancies. T-cell exhaustion may arise from the interaction of the cell surface marker PD-1 (programmed death-1) with PD-L1 (programmed death-ligand 1). This interaction serves to shut down the T-cell response to such infected cells. Immune checkpoint inhibitors such as monoclonal antibodies that target PD-1, PD-L1, and cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) may be used to block this interaction. These inhibitors have been shown to enhance HIV-specific T-cell responses in ex vivo studies. A number of small clinical trials are underway to evaluate the effects of the immune checkpoint inhibitors pembrolizumab, nivolumab, ipilimumab, and atezolizumab in HIV-infected individuals with malignancies. This strategy stands a good chance of reinvigorating HIV-specific immune response, perhaps when used in combination with LRA; however, the safety of these approaches is an ever present consideration and remains the subject of much discussion.
Gene Therapy
The aim of gene therapy is to deliver a therapeutic agent to a cell using a gene; to inhibit or kill the HIV genome in the cell, such as with anti-HIV antisense RNA, targeted DNA nucleases, and transdominant Rev; or to remove something that HIV needs, such as CCR5, by using antisense RNA, intrabodies, or targeted DNA nucleases. As shown in the cases of the Berlin patient and the Boston patients, cure requires removing the virus and the target cells (eg, by inhibiting or eliminating CCR5).
A promising approach is nuclease-based gene therapy targeting CCR5. In this approach, CD4+ T cells or CD34+ hematopoietic stem cells are removed from HIV-infected individuals and treated with a zinc-finger nuclease that recognizes and cleaves the CCR5 gene. This results in cells that no longer express the CCR5 coreceptor. After these cells expand, they are infused back into the individual. The approach is minimally invasive, with low risk of severe adverse effects. It is also more accessible than hematopoietic stem cell transplantation, with no need for donors and no risk of graft-versus-host disease.
A number of gene therapy studies in HIV-infected individuals who are aviremic and on antiretroviral therapy are under way. Initial findings include the long-term persistence in vivo of CCR5-modified CD4+ T cells after a single infusion and durable increases in CD4+ T memory stem cells enriched for modified CCR5.13 A reduction in the size of the HIV reservoir was observed in all patients over 3 years, and viral kinetics suggest replacement of infected cells over time.
A reduction in the HIV set point was observed when anti-retroviral therapy was interrupted at 6 weeks after infusion; this set point correlated with the amount of CCR5-modified CD4+ T cells in the infusion received by the individuals. At last reporting, 4 of 16 patients receiving treatment have remained off antiretroviral drugs for more than 22 weeks.13 These findings indicate that administering cells resistant to HIV infection when antiretroviral therapy is stopped reduces the size of the reservoir and the HIV set point. Although these early findings are extremely encouraging, a primary question is how scalable an approach this will prove to be.
Broadly Neutralizing Antibodies Against HIV Envelope
Broadly neutralizing antibodies that target the HIV envelope (Env) may be an approach to a cure because they can block viral entry into cells and mediate the killing of infected cells. Numerous monoclonal antibodies (mAbs) with varying inhibitory potency and breadth of coverage of diverse viruses have been discovered in recent years.
A phase I study evaluating VRC01, a mAb that targets the CD4 binding site of HIV Env, showed that a single infusion in viremic patients was associated with responses consisting of sustained suppression, transient suppression, or no suppression with the degree of suppression dependent upon the sensitivity of the virus to VRC01 (Figure 2).14 Additional phase I trials of VRC01 in individuals who interrupt antiretroviral therapy have shown that the majority of participants had viral rebound within 5 weeks, even with high plasma levels of VRC01, and rebound was associated with emergence of resistant virus. However, a modest but statistically significant delay in viral rebound was observed compared with historical controls, lending some optimism to the findings (Figure 3).15 Similar findings were made in a study of the 3BNC117 antibody.16
Figure 2.
Log10 change in viral load from individual baseline for 8 study participants longitudinally for 90 days after infusion of VRC01, a monoclonal antibody targeting the CD4 binding site of HIV Env (day 0). Adapted from Lynch et al. 2015.14
Figure 3.
Viral load after cessation of antiretroviral therapy in patients receiving VRC01, a monoclonal antibody targeting the CD4 binding site of HIV Env, in the AIDS Clinical Trials Group (ACTG) A5340 trial (A) and National Institutes of Health (NIH) (B) phase I trials. Percentage of viral suppression in the phase I trial participants compared with participants in previous ACTG trials (C) after treatment discontinuation. Adapted from Bar et al. 2016.15
To overcome the pitfalls of single-agent mAbs, desirable characteristics of second-generation mAb products include a 10-fold greater potency than current agents, coverage of 98% to 99% of virus envelope diversity to prevent escape, administration via subcutaneous injection once every 4 to 6 months instead of by intravenous infusion every 2 months with current products, and a cost comparable with antiretroviral drugs.
Greater potency and breadth of coverage can be engineered as well into mAbs. The 10E8 antibody, for instance, exhibits excellent breadth of coverage but has limited potency. A few amino acid mutations engineered into the antibody creates a product with the same breadth of coverage but at 1000 times greater potency. In addition, antibodies can be formulated into combined products that exhibit greater breadth of coverage and potency than that offered by a single antibody (Figure 4).17
Figure 4.
An HIV cross-monoclonal antibody (mAb) and the parental antibodies from which each cross-mAb was derived (A). The percentage of viruses neutralized by 10E8/P140 and 10E8/iMab and their parental mAbs that target Env or CD4/CCR5 indicate greater breadth of coverage and potency (B). IC50 indicates 50% inhibitory concentration. Adapted from Huang et al. 2016.17
In addition, engineered mutations to the Fc portion of an antibody are capable of dramatically prolonging the product's half-life, for example, by protecting it from endosomal degradation. In one approach, the addition of 2 amino acid mutations to VRC01 may extend the half-life of the antibody in healthy participants by at least 4-fold, with therapeutic levels appearing to be maintained for 6 months.
Conclusion
A greater understanding of the size, location, and maintenance of the HIV reservoir has been attained and is likely to improve strategies aimed at cure of infection. Reservoir size can be reduced with early antiretroviral therapy, but the clinical significance of such a reduction remains uncertain. Latency reversing agents have shown poor reactivation of virus and no reduction in reservoir size. Therapeutic vaccines generally have not shown promising effects in human studies. Hematopoietic stem cell transplantation has been shown to work in 1 patient, but it is not a scalable approach. Gene therapy may be used to target HIV and CCR5, and clinical studies show some reservoir reduction, but scalability is an issue. Env-specific mAbs are in promising proof-of-concept studies, with more potent combinations and bispecific mAbs being developed. Combinations of approaches may need to be used to increase the chances of achieving cure, including LRAs plus mAbs, gene therapy plus mAbs plus LRAs, and others.
Footnotes
Presented by Dr Douek in April 2017. First draft prepared from transcripts by Matthew Stenger. Reviewed and edited by Dr Douek in August 2017.
Financial affiliations in the past 12 months: Dr Douek has no relevant financial affiliations to disclose.
References
- 1. Yukl SA, Boritz E, Busch M, et al. Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin patient. PLoS Pathog. 2013;9(5):e1003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hill AL, Rosenbloom DI, Goldstein E, et al. Real-time predictions of reservoir size and rebound time during antiretroviral therapy interruption trials for HIV. PLoS Pathog. 2016;12(4):e1005535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ananworanich J, Chomont N, Eller LA, et al. HIV DNA set point is rapidly established in acute HIV infection and dramatically reduced by early ART. EBioMedicine. 2016;11:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rothenberger MK, Keele BF, Wietgrefe SW, et al. Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proc Natl Acad Sci USA. 2015;112(10):E1126–E1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kroon E, Ananworanich J, Eubanks K, et al. Effect of vorinostat, hydroxychloroquine and maraviroc combination therapy on viremia following treatment interruption in individuals treated during acute HIV infection. 21st International AIDS Conference. July 18–22, 2016; Durban, South Africa.
- 6. Colby D, Chomont N, Kroon E, et al. HIV RNA rebound postinterruption in persons suppressed in Fiebig I acute HIV. 24th Conference on Retroviruses and Opportunistic Infections (CROI). February 13–16, 2017; Seattle, Washington.
- 7. Laird GM, Bullen CK, Rosenbloom DI, et al. Ex vivo analysis identifies effective HIV-1 latency-reversing drug combinations. J Clin Invest. 2015;125(5):1901–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shan L, Deng K, Shroff NS, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36(3):491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deng K, Pertea M, Rongvaux A, et al. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature. 2015;517(7534):381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Casazza JP, Bowman KA, Adzaku S, et al; VRC 101 Study Team. Therapeutic vaccination expands and improves the function of the HIV-specific memory T-cell repertoire. J Infect Dis. 2013;207(12):1829–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leth S, Schleimann MH, Nissen SK, et al. Combined effect of Vacc-4x, recombinant human granulocyte macrophage colony-stimulating factor vaccination, and romidepsin on the HIV-1 reservoir (REDUC): a single-arm, phase 1B/2A trial. Lancet HIV. 2016;3(10):e463–e472. [DOI] [PubMed] [Google Scholar]
- 12. Mothe B, Moltó J, Manzardo C, et al. Viral control induced by HIV-consv vaccines & romidepsin in early treated individuals. 24th Conference on Retroviruses and Opportunistic Infections (CROI). February 13–16, 2017; Seattle, Washington.
- 13. Zeidan J, Lee GK, Benne C, et al. T-cell homeostasis and CD8 responses predict viral control post SB-728-T treatment. 23rd Conference on Retroviruses and Opportunistic Infections (CROI). February 22–25, 2016; Boston, Massachusetts.
- 14. Lynch RM, Boritz E, Coates EE, et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med. 2015;7(319):319ra206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bar KJ, Sneller MC, Harrison LJ, et al. Effect of HIV antibody VRC01 on viral rebound after treatment interruption. N Engl J Med. 2016;375(21):2037–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scheid JF, Horwitz JA, Bar-On Y, et al. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature. 2016;535(7613):556–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang Y, Yu J, Lanzi A, et al. Engineered bispecific antibodies with exquisite HIV-1-neutralizing activity. Cell. 2016;165(7):1621–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]