Abstract
Over the past 30 years, antiretroviral drug regimens for treating HIV infection have become more effective, safer, and more convenient. Despite 31 currently approved drugs, the pipeline of investigational HIV drugs remains full. Investigational antiretroviral drugs include the nucleoside analogue reverse transcriptase translocation inhibitor (NRTTI) MK-8591, a long-acting compound that could be dosed once weekly. Investigational nonnucleoside analogue reverse transcriptase inhibitors (NNRTIs) include doravirine, which is active in vitro against NNRTI-resistant HIV and was potent and well–tolerated when used in combination with a dual–nucleoside analogue RTI (nRTI) backbone in treatment-naive individuals. New integrase strand transfer inhibitors (InSTIs) include recently approved bictegravir, which is active against InSTI-resistant viral strains in vitro and was potent and well–tolerated in combination regimens in treatment-naive individuals, and investigational cabotegravir, which is being studied with monthly parenteral dosing for HIV maintenance treatment and with bimonthly dosing for HIV preexposure prophylaxis (PrEP). Investigational HIV entry inhibitors include the new CD4 attachment inhibitor fostemsavir, which targets HIV envelope glycoprotein 120, and recently approved ibalizumab, which binds the CD4 receptor. This article summarizes presentations by Roy M. Gulick, MD, MPH, at the IAS–USA continuing education program, Improving the Management of HIV Disease, held in Los Angeles, California, in April 2017, and at the 2017 Ryan White HIV/AIDS Program Clinical Conference, held in San Antonio, Texas, in August 2017.
Keywords: HIV, antiretroviral drugs, antiretroviral therapy, MK-8591, doravirine, bictegravir, cabotegravir, fostemsavir, ibalizumab, investigational, nucleoside, nonnucleoside, integrase, entry inhibitor
Although currently recommended antiretroviral regimens are more effective, safer, and more convenient than ever before,1,2 the pipeline of investigational antiretroviral drugs for treating HIV infection remains full. New agents include compounds in existing classes—nucleoside analogue reverse transcriptase inhibitors (nRTIs), nonnucleoside analogue RTIs (NNRTIs), protease inhibitors (PIs), and integrase strand transfer inhibitors (InSTIs)—as well as new classes of HIV entry inhibitors. Among a number of investigational drugs in the pipeline, 6 of those furthest along in development or that offer distinct advantages over currently available other drugs are discussed.
Nucleoside Analogue Reverse Transcriptase Inhibitors
For nRTIs, drugs with more convenient dosing than current once-daily schedules could offer benefits. MK-8591 (or 4’-ethynyl-2-fluoro-2’-deoxyadenosine [EFdA]) is an investigational long-acting adenosine analogue currently in phase II studies.3 MK-8591 exhibits potent activity against HIV-1 in vitro, as well as activity against HIV-2 and multidrug-resistant HIV strains,4 and has a prolonged half-life of 150 to 160 hours. It is a nucleoside nonobligate chain terminator that inhibits HIV reverse transcriptase by preventing translocation of the enzyme, and hence has been termed a nucleoside reverse transcriptase translocation inhibitor (NRTTI).5 In a study of 30 HIV-infected treatment-naive individuals, single oral doses of 0.5 to 30 mg of MK-8591 reduced plasma HIV RNA levels by up to 1.7 log10 copies/mL at 10 days, with no emergence of viral resistance, indicating that convenient weekly dosing may be possible.6
Other formulations of MK-8591 may be administered parenterally and have the potential to be combined with other antiretroviral drugs. In animal studies, a single parenteral dose of MK-8591 produced persistent target drug levels for more than 180 days7 and the compound accumulated in target sites, including lymph nodes and vaginal and rectal tissues.8 MK-8591 also prevented simian-human immunodeficieny virus (SHIV) infection when used as in PrEP macaques.9
Nonnucleoside Analogue Reverse Transcriptase Inhibitors
NNRTIs with less toxicity, better tolerability, fewer drug interactions, and activity against NNRTI-resistant viruses are needed. The investigational NNRTI furthest along in development is doravirine (MK-1439), which has been evaluated in phase III trials. Preclinical studies showed that doravirine is potent at low doses against HIV and active against viral strains with common NNRTI-resistance–associated substitutions such as K103N, Y181C, G190A, E101K, E138K, and K103N/Y181C.10 Doravirine is metabolized by cytochrome P4503A4 (CYP3A4) but is not a CYP450 inhibitor nor inducer, and thus has less potential for drug interactions than other NNRTIs.
In a phase I study of antiretroviral therapy–naive individuals, 7 days of treatment with doravirine produced a rapid decrease of 1.5 log10 copies/mL in plasma HIV RNA levels.11 In a double-blind phase II trial, 216 antiretroviral therapy–naive participants with HIV RNA levels of 1000 copies/mL or higher and CD4+ cell counts of 100/µL or higher were randomly assigned to receive coformulated (indicated with a /) tenofovir disoproxil fumarate (TDF)/emtricitabine plus either doravirine (n = 108) or efavirenz (n = 108).12 At 48 weeks, 78% of the doravirine group and 79% of the efavirenz group had HIV RNA levels below 40 copies/mL.
Two phase III registrational trials were recently completed and presented.13,14 In the first trial, 769 treatment-naive participants with HIV RNA levels of 1000 copies/mL or higher and no resistance to study drugs by genotypic testing were randomly assigned to receive 2 nRTIs (mostly TDF-based combinations) plus either double-blinded doravirine 100 mg or the PI darunavir 800 mg boosted with ritonavir 100 mg.13 At 48 weeks, 84% of the doravirine group and 80% of the darunavir/ritonavir group had HIV RNA levels below 50 copies/mL. Protocol-defined virologic failure was uncommon, and no resistance mutations were identified in cases of virologic failure. Treatment was discontinued due to adverse events in 2% and 3% of participants, respectively.
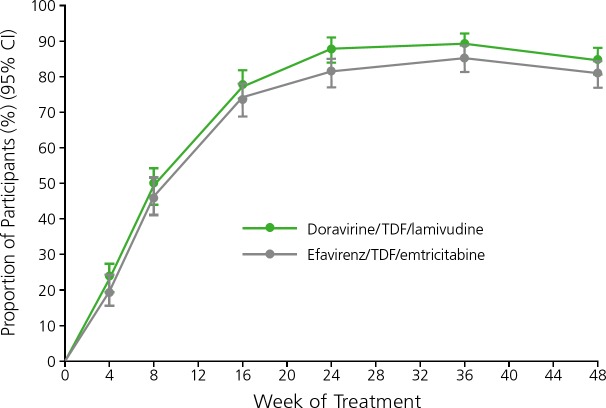
The second trial randomly assigned 728 treatment-naive participants to receive double-blinded doravirine/TDF/lamivudine or efavirenz/TDF/emtricitabine.14 At 48 weeks, 84% of the doravirine group and 81% of the efavirenz group achieved virologic suppression (HIV RNA level <50 copies/mL) and fulfilled prespecified noninferiority criteria (Figure 1). There were differences in the proportions of participants who reported dizziness (9% in the doravirine group vs 37% in the efavirenz group) and sleep disorders (12% in the doravirine group vs 26% in the efavirenz group), although rates of study drug discontinuation were similar.
Figure 1.
Rates of virologic suppression among treatment-naive HIV-infected individuals treated with coformulated (indicated with a /) doravirine/tenofovir disoproxil fumarate (TDF)/lamivudine or efavirenz/TDF/emtricitabine in a phase III trial. CI indicates confidence internal. Adapted from Squires et al.14
These studies are under review by the US Food and Drug Administration (FDA) with a target action date of October 2018 and could support approval of doravirine. To date, there are no clinical data on the effects of a doravirine-based regimen in individuals with NNRTI-resistant virus.
Integrase Strand Transfer Inhibitors
For InSTIs, agents with more convenient dosing or with activity against InSTI-resistant viral strains are needed. The recently approved InSTI bictegravir is active against InSTI-resistant virus in vitro15 and has a half-life of approximately 18 hours, with no pharmacokinetic boosting required for once-daily dosing. Bictegravir does not inhibit or induce CYP3A4 or uridine 5’-diphospho (UDP)-glucuronosyltransferase, indicating a low potential for drug interactions.16
In a phase I study, 20 participants who were treatment-naive or had been off antiretroviral therapy for at least 12 weeks and had not previously taken an InSTI, with HIV RNA levels of 10,000 to 400,000 copies/mL and CD4+ cell counts above 200/µL, received 10 days of bictegravir once daily at 5 mg, 25 mg, 50 mg, or 100 mg and experienced dose-related decreases in HIV RNA levels of 1.45 log10 to 2.43 log10 copies/mL.17
In a phase II study, 98 treatment-naive participants who had HIV RNA levels above 1000 copies/mL and CD4+ cell counts of 200/µL or higher were randomly assigned to receive tenofovir alafenamide (TAF)/emtricitabine plus either bictegravir (n = 65) or dolutegravir (n = 33).18 At 48 weeks, 97% of the bictegravir group and 91% of the dolutegravir group had HIV RNA levels below 50 copies/mL. Virologic failure occurred in 2% and 6%, respectively, with no drug resistance observed in these individuals. Adverse events and abnormal laboratory test results were similar between the 2 groups.
Phase III registrational studies of bictegravir were recently completed and presented (Figure 2.)19,20 In one study, 645 treatment-naive participants with HIV RNA levels of 500 copies/mL or higher and glomerular filtration rates of 50 mL/min or higher who were negative for the HLA-B∗5701 allele were randomly assigned to receive double-blinded bictegravir/TAF/emtricitbine or dolutegravir/abacavir/lamivudine.19 At 48 weeks, 92% of the bictegravir group and 93% of the dolutegravir group had achieved virologic suppression (HIV RNA levels <50 copies/mL), fulfilling prespecified noninferiority criteria. Adverse events (all grades) were similar between the 2 regimens, with the exception of nausea (10% in the bictegravir group vs 23% in the dolutegravir group).
Figure 2.
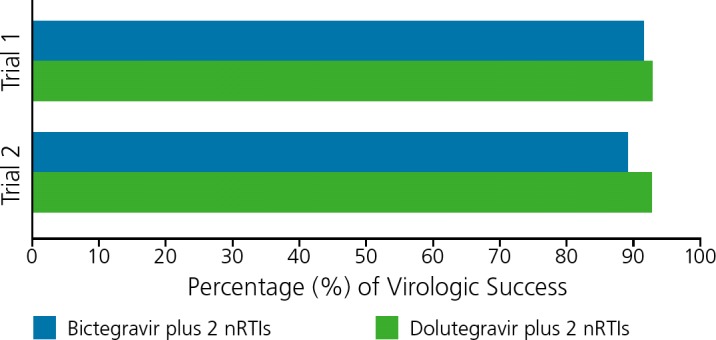
Rates of virologic suppression at 48 weeks in 2 phase III trials of HIV-infected individuals treated with coformulated nucleoside analogue transcriptase inhibitors (nRTIs) tenofovir alafenamide (TAF)/emtricitabine plus either the investigational drug bictegravir or plus dolutegravir.19,20
In a second study, 645 treatment-naive participants with HIV RNA levels of 500 copies/mL or higher and glomerular filtration rates of 30 mL/min were randomly assigned to receive a double-blinded regimen of bictegravir/TAF/emtricitabine or of dolutegravir plus TAF/emtricitabine.20 At 48 weeks, 89% of the bictegravir group and 93% of the dolutegravir group had achieved virologic suppression (HIV RNA levels <50 copies/mL), fulfilling prespecified noninferiority criteria, and adverse events were similar between the 2 regimens. These studies supported FDA approval of coformulated BIC/TAF/FTC for the treatment of HIV infection in early February 2018.
Cabotegravir, an investigational InSTI in advanced development, has a similar structure and resistance profile to dolutegravir. Cabotegravir demonstrates potent activity against HIV in oral formulation at a range of doses and is also available as a nanoformulation for parenteral administration. In a phase I study, cabotegravir administered parenterally exhibited a half-life of 21 to 50 days, which supports monthly or bimonthly dosing.21 In the phase II ECLAIR study of HIV-seronegative men,22 14 (16%) of 86 participants had detectable levels of study drug 1 year after their last injection.23
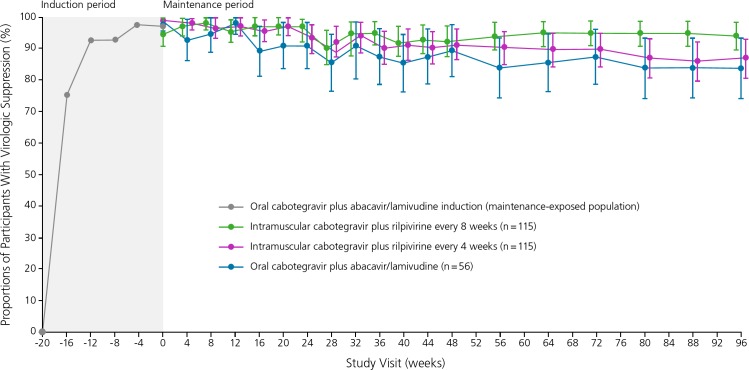
In the phase IIb LATTE-2 (Long-Acting Antiretroviral Treatment Enabling–2) study, 309 treatment-naive HIV-infected participants received run-in treatment with oral cabotegravir 30 mg plus abacavir/lamivudine for 20 weeks. Participants with virologic suppression were then randomly assigned to receive either intramuscular cabotegravir 400 mg plus intramuscular rilpivirine 600 mg every 4 weeks (n = 115), intramuscular cabotegravir 600 mg plus intramuscular rilpivirine 900 mg every 8 weeks (n = 115), or continue oral cabotegravir 30 mg plus abacavir/lamivudine daily (n = 56).24 At 96 weeks, HIV RNA levels were suppressed below 50 copies/mL in 87% in the group that received the injectable regimen every 4 weeks, 94% in the group that received the injectable regimen every 8 weeks, and 84% in the group that received the oral regimen daily (Figure 3). Adverse events were limited to injection site reactions, which occurred in most participants but were generally mild or moderate in intensity (lasting a median of 3 days), and led to treatment discontinuation in only 2 (1%) participants. Subsequent analysis revealed a few cases of virologic breakthrough in the group that received intramuscular treatment every 8 weeks. Thus, cabotegravir with monthly intramuscular dosing is currently being examined as maintenance treatment in phase III studies.25
Figure 3.
Rates of virologic suppression among HIV-infected individuals treated with the investigational drug cabotegravir 30 mg plus coformulated (indicated with a /) abacavir/lamivudine orally for 20 weeks (induction phase), followed by continued cabotegravir 400 mg plus rilpivirine 600 mg intramuscularly every 4 weeks, cabotegravir 600 mg plus rilpivirine 900 mg intramuscularly every 8 weeks, or cabotegravir 30 mg plus abacavir/lamivudine orally daily (maintenance therapy) in the phase II LATTE-2 (Long-Acting Antiretroviral Treatment Enabling-2) trial. Adapted from Margolis et al.24
An injectable formulation of cabotegravir is also being investigated for HIV preexposure prophylaxis (PrEP). The phase II HIV Prevention Trials Group (HPTN) 077 study enrolled 199 low-risk, HIV-uninfected participants (66% women, 34% men) and randomly assigned them (3:1) to receive oral cabotegravir for 4 weeks followed by itnramuscular cabotegravir 800 mg every 12 weeks or 600 mg every 8 weeks (or matching placebos).26 There were no differences in safety or tolerability except for injection site reactions occurring in 34% of those who received cabotegravir and in 2% of those who received a placebo injection. Drug concentrations were lower with cabotegravir 800 mg given every 12 weeks, and investigators concluded that cabotegravir 600 every 8 weeks was optimal. These results support the design and implementation of the HPTN 083 study,27 with a target population of 4500 high-risk men who have sex with men and transgender women who have sex with men who are randomly assigned to receive double-blinded daily oral TDF/emtricitabine or intramuscular cabotegravir every 8 weeks. The primary endpoint of HPTN 083 is incident HIV infections over an expected duration of 4.5 years, and the study is fully powered for noninferiority with a margin of 23%. The first study participant was enrolled in December 2016. A parallel study will enroll at-risk, HIV-uninfected African women.28
Entry Inhibitors
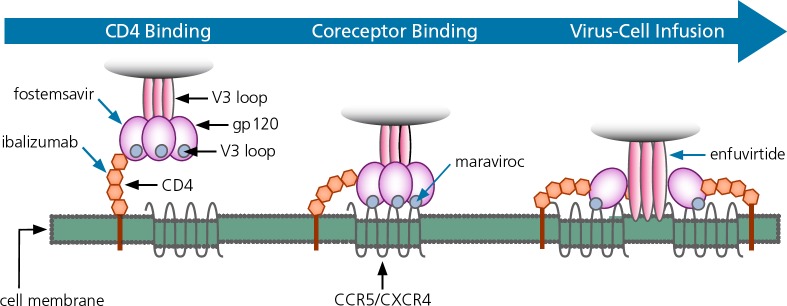
Entry inhibitors with novel mechanisms of action and greater convenience in dosing would be useful. The 3 steps of HIV entry are: 1) HIV binding to the CD4 receptor; 2) subsequent binding to the chemokine receptor (either CC chemokine receptor 5 [CCR5] or C-X-C chemokine receptor type 4 [CXCR4]); and 3) fusion of the viral and host cell membranes (Figure 4).29 Currently available entry inhibitors are the CCR5 receptor antagonist maraviroc and the fusion inhibitor enfuvirtide.
Figure 4.
Mechanisms by which HIV entry inhibitors block virus from entering a cell. Fostemsavir and ibalizumab are investigational drugs. Enfuvirtide and maraviroc have been approved by the US Food and Drug Administration. CCR5 indicates CC chemokine receptor 5; CXCR4, C-X-C chemokine receptor type 4; gp, glycoprotein. Adapted from Moore et al.29
A new class of HIV entry inhibitors, CD4 attachment inhibitors, targets the first step of entry. Fostemsavir is a small molecule inhibitor that binds HIV envelope glycoprotein 120 (gp120) and prevents virus from binding to the CD4 receptor. Fostemsavir is an oral prodrug of temsavir, the active compound, with potent activity in vitro against HIV,30 and pharmacokinetics that support once-daily dosing with no need for pharmacologic boosting. In a phase I dose-escalation study in 48 participants who were treatment-naive or had been off antiretroviral treatment for 8 weeks or longer, higher doses of fostemsavir led to a reduction in HIV RNA level of 1.5 log10 copies/mL.31 Twelve percent of participants had baseline envelope polymorphisms that rendered fostemsavir inactive, raising the issue of the need for patient screening for polymorphisms.
In a phase II study, treatment-experienced participants who previously received at least 1 antiretroviral drug for at least 1 week and who had virus susceptible to temsavir (IC50 < 100nM) were randomly assigned to receive TDF and raltegravir plus fostemsavir 400 mg twice daily (n = 50), 800 mg twice daily (n = 50), 600 mg once daily (n = 50), or 1200 mg once daily (n = 50), or plus atazanavir/ritonavir (n = 50).32 At week 48, 61% to 82% of the fostemsavir groups and 71% of the atazanavir/ritonavir group had HIV RNA levels below 50 copies/mL in a modified intent-to-treat analysis. Fostemsavir 1200 mg daily was selected as the dose for the open-label continuation study after week 48 for all participants who received fostemsavir.33 At week 96, virologic suppression was observed in 61% of the fostemsavir group and 53% of the atazanavir/ritonavir group.34 Treatment-related grade 2 to 4 adverse events were observed in 9% of the fostemsavir group and 37% of the atazanavir/ritonavir group, with hyperbilirubinemia observed in 12% of the latter group. Adverse events led to treatment discontinuation in 2.5% and 10.0%, respectively. In recognition of its novel mechanism of action, fostemsavir was granted “breakthrough status” by the FDA in July 2015. A phase III trial of fostemsavir enrolled 272 heavily-experienced participants who continued their regimens and randomly added 3:1 fostemsavir or placebo.35 At 8 days, the primary endpoint of the study, HIV RNA decreased 0.8 log10 copies/mL (fostemsavir) vs. 0.2 (placebo, P < .0001). Participants then optimized their background regimens and continued or added fostemsavir 600 mg bid. At 24 weeks, 54% had HIV RNA levels below 40 copies/mL and 12 (4%) had discontinued study medications due to adverse events.
The recently approved monoclonal antibody ibalizumab binds not to HIV but to the host cell at the second domain of the CD4 receptor, thereby preventing conformational changes and HIV entry. Ibalizumab has been in development for more than 10 years, with parenteral formulations given every 1 to 4 weeks in different study populations. In a phase II study of 113 treatment-experienced individuals with resistance to 3 antiretroviral drug classes, approximately 40% achieved virologic suppression (HIV RNA level <50 copies/mL) with an ibalizumab-containing optimized background regimen.36
Ibalizumab was evaluated in 40 individuals who had resistance to antiretroviral drugs from 3 classes, with HIV RNA levels above 1000 copies/mL on stable antiretroviral therapy for at least 6 months, and had sensitivity to at least 1 antiretroviral drug.37,38 Participants continued their current antiretroviral regimens, and then received ibalizumab 800 mg intravenously on day 7 and were assessed for virologic responses at day 14, the primary endpoint of the study. At that time, 60% of participants experienced at least a 1 log10 copies/mL decrease in HIV RNA level. Subsequently, participants optimized their antiretroviral regimen based on treatment history and drug resistance testing and received ibalizumab 800 mg intravenously on day 21 and then every 2 weeks thereafter. With this strategy, at week 24, participants experienced a mean decrease in HIV RNA level of 1.6 log10 copies/mL and 43% experienced suppressed HIV RNA levels below 50 copies/mL. Twenty-seven of these participants continued ibalizumab and their optimized background regimen in an open-label extension study and 59% had HIV RNA levels below 50 copies/mL at 48 weeks.39 These data supported FDA approval of ibalizumab for heavily treatment-experienced adults with mulitdrug resistant HIV-1 infection in whom their current regimen had failed.
Footnotes
Presented by Dr Gulick in April and August 2017. First draft prepared from transcripts by Matthew Stenger. Reviewed and edited by Dr Gulick in March 2018.
Financial affiliations in the past 12 months: Dr Gulick has no relevant financial affiliations to disclose.
References
- 1. Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA panel. JAMA. 2016;316(2):191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed on February 20, 2018.
- 3. ClinicalTrials.gov. MK-8591 with doravirine and lamivudine in participants infected with human immunodeficiency virus type 1 (MK-8591-011) (DRIVE2Simplify). http://clinicaltrials.gov/ct2/show/NCT03272347. Accessed on February 20, 2018.
- 4. Nakata H, Amano M, Koh Y, et al. Activity against human immunodeficiency virus type 1, intracellular metabolism, and effects on human DNA polymerases of 4’-ethynyl-2-fluoro-2’-deoxyadenosine. Antimicrob Agents Chemother. 2007;51(8):2701–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Michailidis E, Marchand B, Kodama EN, et al. Mechanism of inhibition of HIV-1 reverse transcriptase by 4’-Ethynyl-2-fluoro-2’-deoxyadenosine triphosphate, a translocation-defective reverse transcriptase inhibitor. J Biol Chem. 2009;284(51):35681–35691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matthews RP, Schurmann D, Rudd DJ, et al. Single doses as low as 0.5 mg of the novel NRTTI MK-8591 suppress HIV for at least seven days. [Abstract TUPDB0202LB] 9th International AIDS Society Conference on HIV Science. July 23–26, 2017; Paris, France.
- 7. Grobler J, Friedman E, Barrett SE, et al. Long-acting oral and prenteral dosing of MK-8591 for HIV treatment or prophylaxis. [Abstract 98.] Conference on Retroviruses and Opportunistic Infections (CROI). February 22–25, 2016; Boston, MA.
- 8. Grobler J, McHale C, Freddo C, et al. MK-8591 concentrations at sites of HIV transmission and replication. [Abstract 435.] Conference on Retroviruses and Opportunistic Infections (CROI). February 13–16, 2017; Seattle, Washington.
- 9. Markowitz M, Gettie A, St. Bernard L, et al. Low dose MK-8591 Protects Rhesus Macaques Against Rectal SHIV Infection. In Special Issue: Abstracts From the 2018 Conference on Retroviruses and Opportunistic Infections. Top Antivir Med. 2018;26(e-1):32–33. [Google Scholar]
- 10. Lai MT, Feng M, Falgueyret JP, et al. In vitro characterization of MK-1439, a novel HIV-1 nonnucleoside reverse transcriptase inhibitor. Antimicrob Agents Chemother. 2014;58(3):1652–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schurmann D, Sobotha C, Gilmartin J, et al. A randomized, double-blind, placebo-controlled, short-term monotherapy study of doravirine in treatment-naive HIV-infected patients. AIDS. 2016;30:57–63. [DOI] [PubMed] [Google Scholar]
- 12. Gatell JM, Morales-Ramirez JO, Hagins DP, et al. Forty-eight-week efficacy and safety and early CNS tolerability of doravirine (MK-1439), a novel NNRTI, with TDF/FTC in ART-naive HIV-positive patients. J Int AIDS Soc. 2014;17(4):Suppl 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Molina JM, Squires K, Sax PE, et al. Doravirine is non-inferior to darunavir/r in phase 3 treatment-naive trial at week 48. Lancet HIV. In press.
- 14. Squires KE, Molina J-M, Sax PE, et al. Fixed dose combination of doravirine/lamivudine/TDF is non-inferior to efavirenz/emtricitabine/TDF in treatment-naïve adults with HIV-1 infection: week 48 results of the Phase 3 DRIVE-AHEAD study. [Abstract TUAB0104LB.] 9th International AIDS Society Conference on HIV Science. July 23–26, 2017; Paris, France.
- 15. Tsiang M, Jones GS, Goldsmith J, et al. Antiviral activity of bictegravir (GS-9883), a novel potent HIV-1 integrase strand transfer inhibitor with an improved resistance profile. Antimicrob Agents Chemother. 2016;60(12):7086–7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang H, Custodio JM, Wei X, et al. Clinical pharmacology of the HIV integrase strand transfer inhibitor bictegravir. [Abstract 40.] Conference on Retroviruses and Opportunistic Infections (CROI). February 13–16, 2017; Seattle, Washington.
- 17. Gallant JE, Thompson M, DeJesus E, et al. Antiviral activity, safety, and pharmacokinetics of bictegravir as 10-day monotherapy in HIV-1-infected adults. JAIDS. 2017;75(1):61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sax P.E., DeJesus E., Crofoot G., et al. Bictegravir versus dolutegravir, each with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection: a randomised, double-blind, phase 2 trial. Lancet HIV. 2017;14(4):e154–e160. [DOI] [PubMed] [Google Scholar]
- 19. Gallant J, Lazzarin A, Mills A, et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet. 2017;390:2063–2072. [DOI] [PubMed] [Google Scholar]
- 20. Sax PE, Pozniak A, Montes ML, et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet. 2017;390:2073–2082. [DOI] [PubMed] [Google Scholar]
- 21. Spreen W, Ford SL, Chen S, et al. GSK1265744 pharmacokinetics in plasma and tissue after single-dose long-acting injectable administration in healthy subjects. JAIDS. 2014;67(5):481–486. [DOI] [PubMed] [Google Scholar]
- 22. Markowitz M, Frank I, Grant RM, et al. Safety and tolerability of long-acting cabotegravir injections in HIV-uninfected men (ECLAIR): a multicentre, double-blind, randomised, placebo-controlled, phase 2a trial. Lancet HIV. 2017;4(8):e331–e340. [DOI] [PubMed] [Google Scholar]
- 23. Ford S, Stancil B, Markowitz M, et al. ECLAIR study of cabotegravir LA injections: characterization of safety and PK during the ‘PK Tail’ phase. [Abstract OA12.06LB.] HIV Research for Prevention Conference. October 17–21, 2016; Chicago, Illinois.
- 24. Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017;390(10101):1499–1510. [DOI] [PubMed] [Google Scholar]
- 25. ClinicalTrials.gov. Study to evaluate the efficacy, safety, and tolerability of long-acting intramuscular cabotegravir and rilpivirine for maintenance of virologic suppression following switch from an integrase inhibitor in HIV-1 infected therapy naive participants. http://clinicaltrials.gov/ct2/show/NCT02938520. Accessed on February 20, 2018.
- 26. Landovitz R, Li S, Grinsztejn B, et al. Safety, tolerability and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected women and men: HPTN 077. [Abstract TUAC0106LB.] 9th International AIDS Society Conference on HIV Science. July 23–26, 2017; Paris, France.
- 27. ClinicalTrials.gov. Safety and efficacy study of injectable cabotegravir compared to daily oral tenofovir disoproxil fumarate/emtricitabine (TDF/FTC), for pre-exposure prophylaxis in HIV-uninfected cisgender men and transgender women who have sex with men. http://clinicaltrials.gov/ct2/show/NCT02720094. Accessed on February 20, 2018.
- 28. ClinicalTrials.gov. Evaluating the safety and efficacy of long-acting injectable cabotegravir compared to daily Oral TDF/FTC for pre-exposure prophylaxis in HIV-uninfected women. http://clinicaltrials.gov/ct2/show/NCT03164564. Accessed on February 20, 2018.
- 29. Moore JP, Doms RW. The entry of entry inhibitors: a fusion of science and medicine. Proc Natl Acad Sci USA. 2003;100:10598–10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nowicka-Sans B, Gong YF, McAuliffe B, et al. In vitro antiviral characteristics of HIV-1 attachment inhibitor BMS-626529, the active component of the prodrug BMS-663068. Antimicrob Agents Chemother. 2012;56(7):3498–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nettles RE, Schurmann D, Zhu L, et al. Pharmacodynamics, safety, and pharmacokinetics of BMS-663068, an oral HIV-1 attachment inhibitor in HIV-1-infected subjects. J Infect Dis. 2012;206(7):1002–1011. [DOI] [PubMed] [Google Scholar]
- 32. Lalezari JP, Latiff GH, Brinson C, et al. Safety and efficacy of the HIV-1 attachment inhibitor prodrug BMS-663068 in treatment-experienced individuals: 24 week results of AI438011, a phase 2b, randomised controlled trial. Lancet HIV. 2015;2(10):e427–e437. [DOI] [PubMed] [Google Scholar]
- 33. Thompson M, Lalezari JP, Kaplan R, et al. Safety and efficacy of the HIV-1 attachment inhibitor prodrug fostemsavir in antiretroviral-experienced subjects: week 48 analysis of AI438011, a Phase IIb, randomized controlled trial. Antivir Ther. 2017;22:215–223. [DOI] [PubMed] [Google Scholar]
- 34. DeJesus E, Martins M, Stoehr A, et al. Attachment inhibitor prodrug BMS-663068 in ARV-experienced subjects: weeks 96 analysis. [Abstract 472.] Conference on Retroviruses and Opportunistic Infections (CROI). February 22–25, 2016; Boston, Massachusetts.
- 35. Kozal M, Aberg J, Pialoux G, et al. Phase 3 study of fostemsavir in heavily treatment-experienced HIV-1–infected participants: Day 8 and week 24 primary efficacy and safety results (BRIGHTE Study, formerly 205888/AI438-047). 16th European AIDS Conference; October 25–27, 2017; Milan, Italy.
- 36. Khanlou H, Devente J, Fessel J, et al. Durable efficacy and continued safety of ibalizumab in treatment-experienced patients. [Abstract LB9.] 49th Annual Meeting of the Infectious Diseases Society of America (IDSA). October 20–23, 2011; Boston, Massachusetts.
- 37. Lalezari J, Fessel WJ, Schrader S, et al. Primary efficacy endpoint and safety results of ibalizumab (IBA) in a phase 3 study of heavily treatment-experienced patients with multi-drug resistant (MDR) HIV-1 infection. [Abstract LB-6.] ID Week. October 26–30, 2016; New Orleans, Louisiana.
- 38. Lewis S, Fessel J, Emu B, et al. Long-acting ibalizumab in patients with multi-drug resistant HIV-1: a 24-week study. [Abstract 449LB.] Conference on Retroviruses and Opportunistic Infections (CROI). February 13–16, 2017; Seattle, Washington.
- 39. Emu B, Fessel WJ, Schrader S, et al. 48-Week Safety and Efficiency On-Treatment Analysis of Ibalizumab in Patients with Multi-Drug Resistant HIV-1. ID Week 10-6-2017; San Diego, California, USA.