Abstract
HIV-infected persons are more likely to have chronic pain, receive opioid analgesic treatment, receive higher doses of opioids, and to have substance use disorders and mental illness compared with the general population, putting them at increased risk for opioid use disorder. Management of opioid use in HIV-infected individuals can be complex, and the limited data on opioid treatment in this population are conflicting with regard to its effect on HIV outcomes. Buprenorphine treatment for opioid use disorder improves HIV outcomes and other outcomes. This article summarizes a presentation by Chinazo O. Cunningham, MD, MS at the IAS–USA continuing education program, Improving the Management of HIV Disease, held in Atlanta, Georgia, in March 2017.
Keywords: HIV, opioid analgesia, opioids, chronic pain, opioid use disorder, buprenorphine
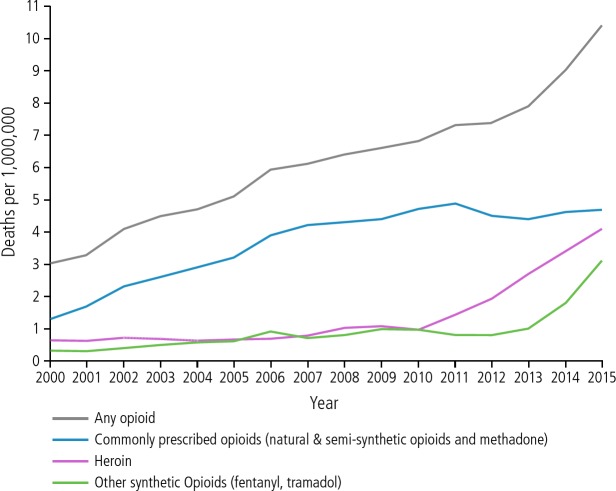
The opioid epidemic in the United States is reflected in the increasing number of drug overdose deaths since 2000, with more recent dramatic increases in overdose deaths attributable to heroin and other synthetic opioids (Figure 1). Any illness with a high mortality rate should prompt decisive action. There is an enormous gap in treatment for opioid use disorder. National data from 2013 indicate that of an estimated 22.5 million people with substance use disorder, only 2.6 million (12%) have received any type of treatment.1
Figure 1.
Overdose deaths involving opioids in the United States, 2000 to 2015. Adapted from Centers for Disease Control and Prevention.24
Pain, Opioid Analgesics, and HIV
Chronic pain is more common among HIV-infected versus -uninfected individuals, with estimates of its prevalence ranging from 25% to 90%.2–9 Pain among HIV-infected individuals has diverse etiologies, including HIV infection itself, aging, and the adverse effects of medications. Opioids are more commonly prescribed to HIV-infected individuals, and estimates of the proportion of infected individuals prescribed opioids range from 21% to 53%.4,10–12 HIV-infected persons also receive higher doses of opioids and are more likely to have substance use disorder and mental illness.4,9,10 Thus, risk of opioid misuse is elevated in the HIV-infected population.
Current Guidelines for Opioid Treatment for Chronic Pain
The Centers for Disease Control and Prevention (CDC) guidelines for prescribing opioids for non–cancer-related chronic pain are summarized in the Table.13 The guidelines cover 3 major areas: 1) when to initiate or continue opioid treatment for chronic pain; 2) which opioid to choose, dose and duration of treatment, and follow-up and discontinuation; and 3) assessing risks and harms. Practitioners should prescribe nonpharmacologic or nonopioid therapies when possible, establish goals of treatment, and discuss risks and benefits with patients. If an opioid is prescribed, it should be an immediate-release, short-acting agent at the lowest effective dose. The currently recommended dose for an opioid is below the range of 50 to 90 morphine milligram equivalents (MMEs). Risk of overdose increases as MMEs increase.
Table.
Centers for Disease Control and Prevention Guideline for Prescribing Opioids for Non-Cancer-Related Chronic Pain
| Initiating Treatment With Opioids | Determining Opioid Selection, Dose, Duration, Follow-up, and Discontinuation | Assessing Risks and Harms of Treatment With Opioids |
|---|---|---|
|
|
|
Data compiled from Dowell et al.13
The CDC guidelines also specify that opioids should be prescribed in no greater quantity than needed (enough for ≤3–7 days). Concurrent use of opioids and benzodiazepines should be avoided. Naloxone should be considered for high-risk patients. The New York State Department of Health permits free naloxone kits to be dispensed, and practitioners have a standing order that patients may obtain naloxone kits from pharmacies. A prescription drug monitoring program (eg, Internet System for Tracking Over-Prescribing [I-Stop] in New York State) and urine drug tests should be used to monitor persons receiving opioid treatment.
How use of opioids may affect HIV outcomes remains unclear. It may seem that when practitioners prescribe opioid analgesics to HIV-infected patients, HIV outcomes are better, perhaps because patients are more adherent to office visits and HIV medications. However, data from the few studies in this area are conflicting. In studies of any versus no opioid analgesic use, utilization of antiretroviral therapy was higher in 1 study,4 no difference in utilization of antiretroviral therapy was observed in 3 studies,10,12,14 and no difference in adherence to antiretroviral therapy was observed in another study.11 Viral loads were higher among individuals taking opioids in 2 studies4,14 and were no different in 2 other studies.10,12 In studies comparing misuse versus no misuse of opioids, adherence to antiretroviral therapy was worse among participants who misused opioids.11,15
Interpretation of urine toxicology test results is more complex than is generally thought.16 There are many causes of false-positive or -negative findings. Among the most common mistakes in interpretation of urine toxicology testing are those involving oxycodone—a frequently prescribed controlled substance—and fentanyl. A urine screening assay that specifically includes oxycodone should be used. If oxycodone is taken in high enough doses, “spillover” may occur resulting in positive findings for opiates. Similarly, a urine test result positive for opiates may be associated with use of morphine, codeine, hydrocodone, or heroin, as there is no way to distinguish among these in a screening assay. Metabolites of fentanyl are not detected in typical screening tests. With regard to use of benzodiazepines, individuals taking clonazepam typically have test results negative for benzodiazepines. Thus, to accurately interpret the results of urine testing, metabolic pathways and which metabolites are being tested in screening assays must be understood. If there is doubt about the results of a urine screening test, confirmatory testing using gas chromatography mass spectrometry should be ordered, as it provides levels of all metabolites and accurate identification of the cause of a positive test result for opiates.
Treatment of Opioid Use Disorder
Pharmacologic treatment of opioid use disorder consists of the opioid antagonist naltrexone and the opioid agonists buprenorphine and methadone. Buprenorphine, which can be prescribed by HIV practitioners, has positively affected HIV treatment outcomes and other outcomes among HIV-infected individuals.
Methadone is a full opioid agonist (as are most opioids including morphine, hydrocodone, codeine, and oxycodone). Buprenorphine is a partial opioid agonist, a crucial difference with regard to its safety profile and some of the clinical challenges. Buprenorphine and methadone each target the opioid µ receptor. Buprenorphine has a higher affinity for the µ receptor than any full opioid agonist—a desirable characteristic in that the drug acts to block other opioids from the µ-receptor. Buprenorphine is typically administered sublingually in a tablet or film formulation, and for the treatment of opioid addiction, methadone is administered orally in a liquid formulation. They have similar half-lives (24–36 hours) and each is metabolized by cytochrome P450enzymes.
The opioid effect of a full opioid agonist, such as morphine, continues to increase as the dose increases (Figure 2), also increasing the risk of respiratory depression and fatal overdose. The opioid effect of buprenorphine has a ceiling, plateauing at increasing doses and greatly reducing the risk of respiratory depression when used alone. Risk of fatal overdose is increased when other central nervous system depressants such as alcohol or benzodiazepines are used concomitantly with buprenorphine.
Figure 2.
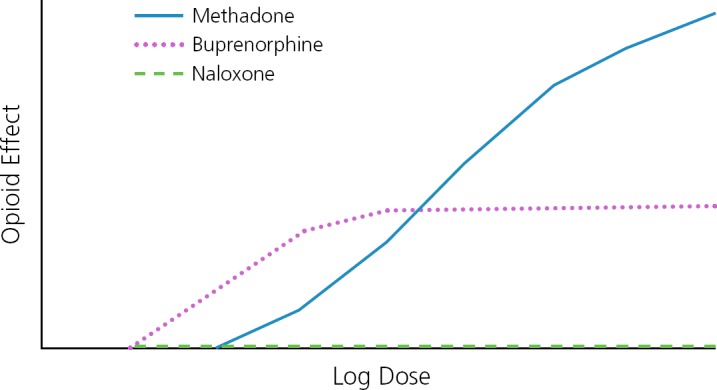
Conceptual representation of the opioid effects and log doses of the full opioid agonist methadone, the partial opioid agonist buprenorphine, and the opioid antagonist naloxone. Adapted from Substance Abuse and Mental Health Services Administration.25
With regard to treatment delivery, methadone is highly regulated and buprenorphine is minimally regulated. For persons receiving treatment from methadone maintenance treatment programs, methadone must be delivered by a physician at that program and counseling visits, urine toxicology tests, dosing, dispensing, prescribing, and availability are regulated at the state and federal levels. Buprenorphine treatment can be administered in any clinic setting, including syringe-exchange programs. Physicians, nurse practitioners, and physician assistants may prescribe buprenorphine, which is particularly important in the context of HIV care, given the crucial role of nurse practitioners and physician assistants in this setting. To prescribe buprenorphine, physicians must complete 8 hours of training and nurse practitioners and physician assistants must complete 24 hours of training to receive a waiver and Drug Enforcement Administration “X” number. Buprenorphine is dispensed in community pharmacies and can be prescribed in a 30-day supply with refills. Practitioners may only prescribe buprenorphine for 30 patients during the first year but can apply to treat up to 100 and, in some situations, up to 275 patients thereafter.
Buprenorphine is primarily used in a coformulation with the opioid antagonist naloxone (buprenorphine/naloxone). When taken sublingually as directed, buprenorphine is absorbed and naloxone is not. When buprenorphine/naloxone is crushed and injected, the naloxone will cause immediate opioid withdrawal, making misuse of this coformulation unlikely.
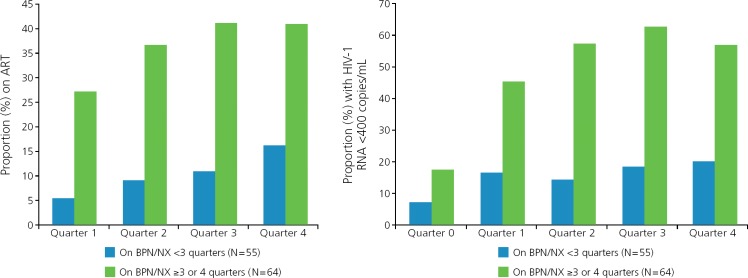
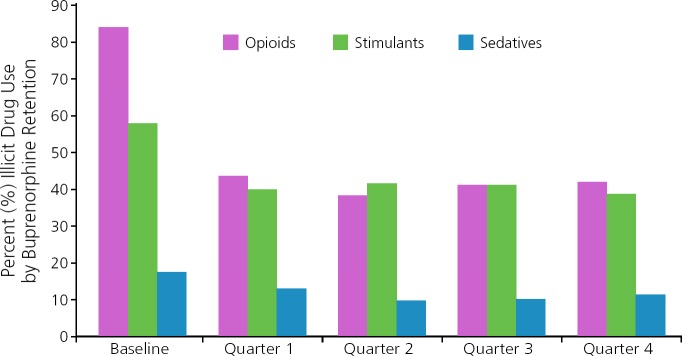
Buprenorphine integrates well into HIV treatment. The multisite BHIVES (Buprenorphine HIV Evaluation and Support) Initiative was primarily a prospective cohort design that included 386 HIV-infected participants with opioid dependence who were eligible for buprenorphine treatment.17 Participants were followed up for 12 months, with outcomes obtained from interviews every 3 months and medical record review. Proportions of participants who initiated antiretroviral therapy and achieved viral suppression (HIV RNA level <400 copies/mL) were substantially higher among those taking buprenorphine/naloxone for at least 3 of 4 quarters than those taking it for less than 3 quarters during a year period (Figure 3).18 Retention in treatment with buprenorphine/naloxone was associated with reduced use of opioids and stimulants and no change in use of sedatives compared with baseline (Figure 4).19 Retention in treatment with buprenorphine/naloxone also led to improvements in physical and mental quality of life.20 Individuals with cocaine use had no difference in buprenorphine treatment outcomes than those without cocaine use. No adverse effect on liver enzymes has been observed with buprenorphine/naloxone despite the fact that many HIV-infected individuals studied also had HCV infection.21–23
Figure 3.
HIV outcomes are improved with retention on buprenorphine treatment (P ≤ .05 for all comparisons with baseline). Adapted from Altice et al.18
Figure 4.
Drug treatment outcomes are improved with retention on buprenorphine treatment. Adapted from Fiellin et al.19
Summary
The opioid epidemic in the United States continues to grow. Although there has been a plateau in opioid-analgesic–related deaths, overall, opioid-related deaths continue to increase. A large gap remains between the number of persons eligible for treatment for opioid use disorder and the number of those receiving treatment. There are many challenges to managing pain with opioids, including interpretation of urine toxicology test results. How use of opioid analgesics affects HIV outcomes remains unclear. However, available data indicate that integration of buprenorphine with HIV treatment is associated with many positive outcomes.
Footnotes
Presented by Dr Cunningham in February 2017. First draft prepared from transcripts by Matthew Stenger. Reviewed and edited by Dr Cunningham in October, 2017.
Financial affiliations in the past 12 months: Dr Cunningham holds stock and stock options for Quest Diagnostics. Her spouse is employed by and holds stock options for Quest Diagnostics.
References
- 1. Socias ME, Volkow N, Wood E. Adopting the ‘cascade of care’ frame-work: an opportunity to close the implementation gap in addiction care? Addiction. 2016;111(12):2079–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dobalian A, Tsao JC, Duncan RP. Pain and the use of outpatient services among persons with HIV: results from a nationally representative survey. Med Care. 2004;42(2):129–138. [DOI] [PubMed] [Google Scholar]
- 3. Frich LM, Borgbjerg FM. Pain and pain treatment in AIDS patients: a longitudinal study. J Pain Symptom Manage. 2000;19(5):339–347. [DOI] [PubMed] [Google Scholar]
- 4. Edelman EJ, Gordon K, Becker WC, et al. Receipt of opioid analgesics by HIV-infected and uninfected patients. J Gen Intern Med. 2013;28(1):82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tsao JC, Stein JA, Dobalian A. Pain, problem drug use history, and aberrant analgesic use behaviors in persons living with HIV. Pain. 2007;133(1–3):128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Breitbart W, Dibiase L. Current perspectives on pain in AIDS. Oncology (Williston Park). 2002;16(7):964–982. [PubMed] [Google Scholar]
- 7. Breitbart W, Dibiase L. Current perspectives on pain in AIDS. Oncology (Williston Park). 2002;16(6):818–835. [PubMed] [Google Scholar]
- 8. Tsao JC, Dobalian A, Naliboff BD. Panic disorder and pain in a national sample of persons living with HIV. Pain. 2004;109(1–2):172–180. [DOI] [PubMed] [Google Scholar]
- 9. Tsao JC, Plankey MW, Young MA. Pain, psychological symptoms and prescription drug misuse in HIV: A literature review. J Pain Manag. 2012;5(2):111–118. [PMC free article] [PubMed] [Google Scholar]
- 10. Silverberg MJ, Ray GT, Saunders K, et al. Prescription long-term opioid use in HIV-infected patients. Clin J Pain. 2012;28(1):39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jeevanjee S, Penko J, Guzman D, Miaskowski C, Bangsberg DR, Kushel MB. Opioid analgesic misuse is associated with incomplete antiretroviral adherence in a cohort of HIV-infected indigent adults in San Francisco. AIDS Behav. 2014;18(7):1352–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koeppe J, Lyda K, Armon C. Association between opioid use and health care utilization as measured by emergency room visits and hospitalizations among persons living with HIV. Clin J Pain. 2013;29(11):957–961. [DOI] [PubMed] [Google Scholar]
- 13. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49. [DOI] [PubMed] [Google Scholar]
- 14. Onen NF, Barrette EP, Shacham E, Taniguchi T, Donovan M, Overton ET. A review of opioid prescribing practices and associations with repeat opioid prescriptions in a contemporary outpatient HIV clinic. Pain Pract. 2012;12(6):440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robinson-Papp J, Elliott K, Simpson DM, Morgello S. Problematic prescription opioid use in an HIV-infected cohort: the importance of universal toxicology testing. J Acquir Immune Defic Syndr. 2012;61(2):187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Starrels JL, Becker WC, Alford DP, Kapoor A, Williams AR, Turner BJ. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med. 2010;152(11):712–720. [DOI] [PubMed] [Google Scholar]
- 17. Weiss L, Egan JE, Botsko M, Netherland J, Fiellin DA, Finkelstein R. The BHIVES collaborative: organization and evaluation of a multisite demonstration of integrated buprenorphine/naloxone and HIV treatment. J Acquir Immune Defic Syndr. 2011;56 Suppl 1:S7–13. [DOI] [PubMed] [Google Scholar]
- 18. Altice FL, Bruce RD, Lucas GM, et al. HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. JAIDS. 2011;56(Suppl 1):S22–S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fiellin DA, Weiss L, Botsko M, et al. Drug treatment outcomes among HIV-infected opioid-dependent patients receiving buprenorphine/naloxone. JAIDS. 2011;56(Suppl 1):S33–S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396(6712):690–695. [DOI] [PubMed] [Google Scholar]
- 21. Vergara-Rodriguez P, Tozzi MJ, Botsko M, et al. Hepatic safety and lack of antiretroviral interactions with buprenorphine/naloxone in HIV-infected opioid-dependent patients. JAIDS. 2011;56(Suppl 1):S62–S67. [DOI] [PubMed] [Google Scholar]
- 22. Sullivan LE, Botsko M, Cunningham CO, et al. The impact of cocaine use on outcomes in HIV-infected patients receiving buprenorphine/naloxone. J Acquir Immune Defic Syndr. 2011;56 Suppl 1:S54–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cunningham CO, Giovanniello A, Kunins HV, Roose RJ, Fox AD, Sohler NL. Buprenorphine treatment outcomes among opioid-dependent cocaine users and non-users. Am J Addict. 2013;22(4):352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Centers for Disease Control and Prevention. Opioid data analysis. http://www.cdc.gov/drugoverdose/data/analysis.html. Accessed on September 6, 2017.
- 25. Substance Abuse and Mental Health Services Administration. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction. http://www.naabt.org/documents/TIP40.pdf. Accessed on September 6, 2017. [PubMed]