Abstract
Objectives
(1) Estimate the proportion of mechanically ventilated (MV) intensive care unit (ICU) patients meeting basic communication criteria who could potentially be served by assistive communication tools and speech-language consultation. (2) Compare characteristics of patients who met communication criteria with those who did not.
Design
Observational cohort study in which computerized billing and medical records were screened over a 2-year period.
Setting
Six specialty ICUs across two hospitals in an academic health system.
Participants
Eligible patients were awake, alert, and responsive to verbal communication from clinicians for at least one 12-h nursing shift while receiving MV ≥ 2 consecutive days.
Main results
Of the 2671 MV patients screened, 1440 (53.9%) met basic communication criteria. The Neurological ICU had the lowest proportion of MV patients meeting communication criteria (40.82%); Trauma ICU had the highest proportion (69.97%). MV patients who did not meet basic communication criteria (n = 1231) were younger, had shorter lengths of stay and lower costs, and were more likely to die during the hospitalization.
Conclusions
We estimate that half of MV patients in the ICU could potentially be served by assistive communication tools and speech-language consultation.
Keywords: Intensive care unit, Communication, Nursing, Artificial respiration, Patient communication
Introduction
Communication impairment presents a common, distressing problem for patients who receive mechanical ventilation (MV) during critical illness and for the clinicians who care for them.1–6 New hospital accreditation standards for patient communication include the communication disability acquired as a result of endotracheal or tracheal intubation during critical illness as a condition requiring provider assessment and accommodation.7 Augmentative and Alternative Communication (AAC) tools can be used successfully by clinicians and ICU patients to transmit or receive messages.8–13 Our previous work showed significant improvements in nurse-patient communication with training and the use of AAC.14 Although measures of sedation, coma, and severity of illness are commonly reported in critical care research, few studies have documented the proportion of mechanically ventilated ICU patients who are awake, aware and responsive to verbal communication and who therefore could be served by these simple assistive communication tools. This information is necessary to (1) appropriately plan communication supplies and support programs, (2) prepare clinicians, and (3) provide benchmarking data from which to evaluate communication support initiatives in the ICU.
The purpose of this paper is to estimate the proportion of mechanically ventilated ICU patients who meet basic communication criteria and thus could potentially benefit from the use of assistive communication tools or referral for evaluation and intervention by a speech-language pathologist. Specifically, we used communication eligibility screening data from a quality improvement study to estimate the proportion of mechanically ventilated patients who are awake, alert and responsive to verbal communication across six different specialty ICUs in two University of Pittsburgh Medical Center hospitals.
Methods
This is a descriptive analysis of the eligibility screening data from a stepped wedge crossover cluster randomized trial of nurse training in the use of assistive communication tools. The study was approved by the University of Pittsburgh Institutional Review Board. The implementation was staggered over 8 quarters in 6 ICUs (neurological, neurotrauma, trauma, transplant, cardiovascular, general medical) across two University of Pittsburgh Medical Center (UPMC) hospitals in Pittsburgh, PA. Details of the communication intervention are available online at http://go.osu.edu/speacs2 and description of the parent study design are published separately.15 In brief, the intervention consists of a 1-h web-based communication skills training program for nurses with content on assessment of communication function with nonvocal patients and augmentative and alternative communication (AAC) techniques and tools to facilitate communication with ICU patients who may have multiple impairments. “Communication carts” with low tech communication tools (e.g., communication boards, hearing aid batteries, notebooks, clipboards and felt-tip pens) were supplied to each ICU and restocked weekly during intervention phases. Table 1 describes each study ICU.
Table 1.
Study intensive care units.
| Unit | Beds | Specialty population focus |
|---|---|---|
| Transplant | 28 | Abdominal transplant pre/post-surgery; surgical oncology and, head-neck surgery |
| NeuroTrauma | 10 | Traumatic brain and spine injuries, |
| Neurological | 20 | Stroke, subarachnoid hemorrhage, brain surgery |
| Trauma | 22 | Traumatic injury, some neurological overflow |
| Cardiovascular | 24 | Cardiovascular surgery/medical cardiology |
| General medical | 20 | Mixed medical illness, respiratory failure, sepsis |
| Total | 124 |
Data collection
We identified all mechanically ventilated patients before, during, and after the intervention implementation whose first ICU admission during their hospital stay was to a study ICU during the study period and involved two consecutive days of billing for mechanical ventilation using billing records maintained by UPMC’s Medical Archival System (MARS).16 We then randomly sampled these potentially eligible patients by ICU, by study quarter, for detailed eligibility screening using a random number generator. We abstracted charts from the electronic medical record (EMR) sequentially until we had identified 30 eligible patients per unit per quarter, yielding the prespecified sample of 1440 after 24 months. We report here results from 24 months of eligibility screening from August 1, 2009 to July 31, 2011.
Eligibility criteria confirmed by the EMR included: (1) first ICU admission during the hospital stay in a study unit; and (2) invasive mechanical ventilation via endotracheal tube (ET tube) or tracheostomy for 2 or more calendar days (e.g., non-invasive mechanical ventilation or invasive mechanical ventilation for < 2 days excluded). Once these criteria were confirmed, we screened the EMR for a maximum of 28 ICU days for basic communication criteria, reflecting the patient’s potential to have been served by the assistive communication tools taught as part of the intervention study.
Basic communication criteria consisted of the patient being awake, alert, and responsive to verbal communication from clinicians. We operationalized this criteria as being awake for at least one 12-h nursing shift while receiving MV. Evidence of wakefulness included any of the following: (1) the patient responding to and/or following commands, (2) nursing note description of patient as alert, arousable, anxious, or awake, (3) a score of 6 (obeys verbal commands) for the Best Motor Response on the Glasgow Coma Scale,17 (4) a score of ≥4 on the Riker Sedation Agitation Scale,18 (5) a score of 1–3 on the Modified Ramsay Sedation Scale,19 and/or (6) responsive to verbal communication from clinicians via head nods, gestures, or other nonvocal method.
Statistical analysis
Data analysis was conducted using IBM SPSS Statistics (version 20.0, IBM Corp., Armonk, NY). We descriptively summarized the number of patients identified using billing records, those further screened for detailed eligibility criteria using the EMR, the frequency of eligibility, and the frequency and reason for ineligibility. The data were screened for accuracy, missing values, outliers, and underlying statistical assumptions. The distribution of the continuous variables age, ICU length of stay, hospital length of stay, and cost-adjusted charges were not normally distributed therefore medians and interquartile ranges were reported. Frequency count and percentages were calculated for categorical variables.
We calculated the proportion of MV patients who were awake, alert, and responsive to verbal communication from clinicians overall and by unit by subtracting those confirmed ineligible (who were not actually mechanically ventilated for 2 days, were admitted first to a non-study ICU or time period, were children or prisoners) from the denominator, then dividing the number of patients who met basic communication criteria by the total number screened. We used Pearson chi-square and Mann–Whitney U tests to compare demographic and clinical characteristics of MV patients who were awake and, alert, or responsive to verbal communication from clinicians with those who were not.
Results
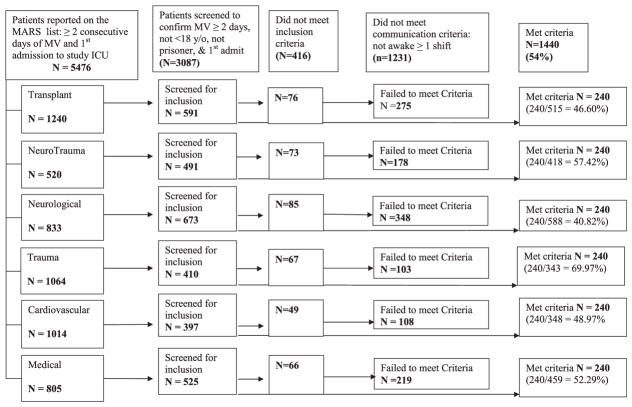
Billing records identified 5476 potentially eligible patients over a period of 24 months; 3087 were screened to achieve the pre-specified sample size of 1440. Reasons for study ineligibility included less than 2 days of mechanical ventilation (n = 274), a previous ICU admission during the hospital stay (n = 92), non-study ICU (n = 30), age < 18 years or prisoner (n = 20) and not awake and alert or responsive to verbal communication from clinicians (n = 1231) (Fig. 1).
Fig. 1.
Quarters 1–8: SPEACS-2 eligibility screening by unit.
Among 2671 MV patients in 6 study ICUs in 2 hospitals, 53.9% met basic communication criteria (Table 2). The neurological ICU had the lowest proportion of MV patients meeting communication criteria (40.82%) and the Trauma ICU had the highest proportion (69.97%). Patients who met communication criteria were more likely to have diagnoses of septicemia, and pneumonia; while patients who did not meet criteria were more likely to have an intracerebral hemorrhage, cerebral occlusion with infarct, and alcoholic cirrhosis of the liver. Those MV patients who did not meet basic communication criteria (n = 1231) were younger, had shorter lengths of stay and lower costs, and were more likely to die during the hospitalization. Patients who met communication criteria were more often discharged to skilled nursing facility or long term acute care hospitals (Table 3).
Table 2.
Potentially eligible patients identified through billing data and further screened for being awake, alert, and attempting to communicate for at least one nursing shift, SPEACS-2 study 2009–2011.
| Unit | Billing dataa | EMR screened | Inclusion criteria not met | Assessed for “awake” criteria | Met “awake” criteria | Proportion |
|---|---|---|---|---|---|---|
| Transplant | 1240 | 591 | 76 | 515 | 240 | 46.60% |
| NeuroTrauma | 520 | 491 | 73 | 418 | 240 | 57.42% |
| Neurological | 833 | 673 | 85 | 588 | 240 | 40.82% |
| Trauma | 1064 | 410 | 67 | 343 | 240 | 69.97% |
| Cardiovascular | 1014 | 397 | 49 | 348 | 240 | 68.97% |
| Medical | 805 | 525 | 66 | 459 | 240 | 52.29% |
| Total | 5476 | 3087 | 416 | 2671 | 1440 | 53.91% |
mechanical ventilation for >2 days, and first ICU admission during incident hospital stay; EMR – electronic medical record.
Table 3.
Characteristics of patients mechanically ventilated for 2 or more days who met and did not meet communication criteria in 6 study units in 2 hospitals, 2009–2011.
| Variable | Awake and alert or responsive to verbal communication by clinicians at least one nursing shift | ||
|---|---|---|---|
| Yes (n = 1440) | No (n = 1231) | p-value | |
| Age, median (IQR) (N = 1211a) | 62 (23) | 60 (23) | <0.001c |
| Female, n (%) (N = 1211a) | 686 (47.6%) | 525 (42.6%) | 0.027 |
| Race, n (%) (N = 1435b, 1076a) | <0.001 | ||
| White | 1291 (89.7%) | 922 (75.6%) | |
| Black | 132 (9.2%) | 135 (11.1%) | |
| Other | 12 (0.8%) | 19 (1.6%) | |
| Unknown/missing | 5 (0.3%) | 144 (11.8%) | |
| Top 8 principal diagnosis, n (%) (N = 1439b, 1208a) | |||
| Septicemia NOS | 121 (8.4%) | 76 (6.2%) | 0.028 |
| Intracerebral hemorrhage | 49 (3.4%) | 90 (7.3%) | <0.001 |
| Acute respiratory failure | 66 (4.6%) | 49 (4%) | 0.444 |
| Subarachnoid hemorrhage | 45 (3.1%) | 49 (4%) | 0.232 |
| Cerebrovascular accident | 29 (2%) | 46 (3.7%) | 0.007 |
| Alcoholic cirrhosis of the liver | 17 (1.2%) | 34 (2.8%) | 0.003 |
| Pneumonia, organism NOS | 29 (2%) | 8 (0.6%) | 0.003 |
| Cirrhosis of liver NOS | 23 (1.6%) | 15 (1.2%) | 0.410 |
| Hospital type – unit type, n (%) | <0.001 | ||
| Tertiary referral – transplant | 240 (16.7%) | 272 (22.1%) | |
| Tertiary referral – neurotrauma | 240 (16.7%) | 171 (13.9%) | |
| Tertiary referral – Neurology | 240 (16.7%) | 355 (28.8%) | |
| Tertiary referral – trauma | 240 (16.7%) | 106 (8.6%) | |
| Community – cardiovascular | 240 (16.7%) | 110 (8.9%) | |
| Community – Mixed med-surg | 240 (16.7%) | 217 (17.6%) | |
| ICU length of stay in days, median (IQR) (N = 1222a) | 9 (11) | 5 (6) | <0.001c |
| Hospital length of stay in days, median (IQR) (N = 1211a) | 15 (14) | 9 (11) | <0.001c |
| Cost-adjusted charges in dollars, median (IQR) (N = 1069b, N = 847a) | 42,432 (42,141) | 28,779 (33,451) | <0.001c |
| Discharge disposition, n (%) (N = 1220a) | <0.001 | ||
| Dead | 234 (16.3%) | 370 (30.3%) | <0.001 |
| Home | 330 (22.9%) | 318 (26.1%) | 0.080 |
| Hospice | 34 (2.4%) | 42 (3.4%) | 0.104 |
| Skilled nursing facility | 334 (23.2%) | 209 (17.1%) | <0.001 |
| Long term acute care hospital | 205 (14.3%) | 41 (3.4%) | <0.001 |
| Rehabilitation | 271 (18.8%) | 205 (16.8) | 0.145 |
| Transfer to other facility | 30 (2.1%) | 35 (2.9%) | 0.204 |
ICU – intensive care unit; NOS – not otherwise specified.
p-values from Pearson Chi Square.
reflects the variations in the sample size that did not meet awake criteria due to missingness.
reflects the variations in the awake sample size due to missingness.
denotes p-values from Mann–Whitney U.
Discussion
In this retrospective longitudinal observational study of a mixture of medical and subspecialty ICUs in one tertiary referral and one community academic-affiliated hospitals, we found that half (53.9%) of the mechanically ventilated ICU patients met minimum criteria for communication during sustained periods of wakefulness. This demonstrates a very large population that could be served by simple assistive communication tools. If use of these tools provides even small improvements in patients’ frustration20 and agitation, the impact could be clinically significant.
Our findings that slightly more than half of MV patients are awake and alert, or attempting to communicate at some point during their period of MV is higher than the point prevalence of 18.4% reported by Thomas and Rodriguez.21 This difference could be explained by fluctuation in the patients’ communication ability over the course of an ICU stay. In addition, we reviewed records for up to 28 days of MV for incidence of communication ability rather than a single randomly selected day. Moreover, Thomas and Rodriguez used a different denominator, all patients in the ICU, as compared to our sample of patients with 2 or more days on MV. They employed additional exclusion criteria such as history of speechlessness, and pre-existing use or the inability to use adaptive communication devices.21 In contrast, our sample inclusion criteria were intentionally liberal and likely captured some patients with minimal communication ability and cognitive impairments, such as delirium and/or mild sedation. We chose to include these patients because our previous work14 showed that some basic communication could be facilitated with ICU patients who have multiple communication impairments, including delirium, and because the training intervention and communication tools specifically address these deficits.
Zubow and Hurtig recently reviewed the electronic medical records of all patients 3 years old or older in ICUs at University of Iowa Hospitals and Clinics over a 7-day period to determine the number of patients meeting candidacy requirements for AAC or Assistive Technology services.22 The criteria were Sedation Agitation Scale18 scores >4 (calm or agitated) and the patient’s inability to independently access the nurse call system. Exclusion criteria included: pre-existing communication impairments, deaf or hard of hearing, non-English speaking, English as a second language, and communication disorders resulting from brain injury or stroke. Of all ICU patients reviewed, 33% met candidacy for AAC or Assistive Technology services.22 This proportion is lower than our estimate due to the exclusion criteria and shorter observation period. Despite methodological differences across studies, all show that a clinically significant proportion of ICU patients who are unable to speak have communication ability and could potentially benefit from assistive communication tools and techniques and/or a consultation from a speech-language pathologist. Our method of daily evaluation for a prolonged period (i.e., up to 28 days on mechanical ventilation) underscores the importance of daily assessment and accommodation for communication ability.
Using national estimates of 790,257 MV hospitalizations annually in the US,23 we estimate that 425,079 MV patients annually may have communicative ability at some time during their period of intubation and mechanical ventilation. As critical care clinical practice moves toward less sedation, promoting wakefulness and early mobilization during MV,24–26 the proportion of awake and potentially communicative patients is likely to increase thus increasing the need for communication support. Communication ability assessments for intubated, nonvocal patients should include evaluation of consciousness and attention, oral motor function, upper motor function and consistent YES-NO signal.27,28
The Neurological ICU had the lowest proportion of patients meeting communication criteria. There are several clinical explanations for this difference. Neurological insults often involve the brain centers that control communication comprehension, expression or both. Moreover, neurologically-injured ICU patients are more likely to experience decreased level of consciousness or coma than patients with other diagnoses. Further, care of the patient with stroke, subarachnoid hemorrhage or brain surgery often involves pharmacologically-induced deep sedation. In these cases, communication may be challenging, impossible or contraindicated. Interestingly, we found a relatively high (57.4%) incidence of patients in the NeuroTrauma ICU who were awake and showed at least minimal ability to communicate which indicates a different case mix (e.g., traumatic brain and spinal injuries) than the Neurological ICU (see Table 1) and care protocols that avoid pharmacological sedation/coma.
Actual differences in demographic characteristics (age, race, gender) between patients who met basic communication criteria and those who did not are small and statistical significance is likely a result of the large sample size. Higher cost, longer lengths of stay, diagnostic, and discharge disposition (long-term acute care) differences in the group meeting communication criteria do indicate a constellation of prolonged critical illness, whereas shorter stays and higher mortality among the group not meeting communication criteria may indicate greater acute illness severity. We intentionally chose to include patients who died in the comparison because communication at end-of-life in the ICU may be profoundly important for patient comfort, family members, and clinicians. Indeed, despite differences between groups, 13% of patients who met communication criteria died in hospital. Thus, patients at high risk of dying in the ICU should be considered for assistive communication services if they meet communication criteria.
This study has several limitations. Generalizability may be limited given a regional sample in academic-affiliated hospitals. Additionally, we limit analyses to patients with MV of at least 2 days’ duration. Using billing records to identify MV patients is subject to some misspecification, typically due to billing across midnight (and therefore < 2 full days duration) or for non-invasive MV. However, unless billing varies systematically with the patient’s ability to communicate, there is no reason to believe that this would introduce bias into our estimates. Finally while there was a significant difference noted in age between the two groups, the difference in the means is only two years and thus may not be clinically significant.
Conclusion
In conclusion, half of MV patients in the ICU could be served by augmentative and alternative communication. This supports Patient-Centered Communication Standards recently promulgated by The Joint Commission.7,29 The variability between specialty ICUs suggest a need for unit-based programs and services targeted to the unique communication needs of specialty populations.
Acknowledgments
Acknowledgment for research support: Robert Wood Johnson Foundation Interdisciplinary Nursing Quality Research Initiative grant (#66633).
Drs. Nilsen and Tate received post-doctoral research training support from the National Institute of Nursing (2T32 NR 8857-6 A1; Erlen) and the National Institute of Mental Health (T32-MH19986; Reynolds) respectively.
References
- 1.Karlsson V, Bergbom I, Forsberg A. The lived experiences of adult intensive care patients who were conscious during mechanical ventilation: a phenomenological-hermeneutic study. Intensive Crit Care Nurs. 2012;28:6–15. doi: 10.1016/j.iccn.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Khalaila R, Zbidat W, Anwar K, Bayya A, Linton DM, Sviri S. Communication difficulties and psychoemotional distress in patients receiving mechanical ventilation. Am J Crit Care. 2011;20:470–479. doi: 10.4037/ajcc2011989. [DOI] [PubMed] [Google Scholar]
- 3.Rotondi AJ, Chelluri L, Sirio C, et al. Patients’ recollections of stressful experiences while receiving prolonged mechanical ventilation in an intensive care unit. Crit Care Med. 2002;30:746–752. doi: 10.1097/00003246-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Nelson JE, Meier DE, Litke A, Natale DA, Siegel RE, Morrison RS. The symptom burden of chronic critical illness. Crit Care Med. 2004;32:1527–1534. doi: 10.1097/01.ccm.0000129485.08835.5a. [DOI] [PubMed] [Google Scholar]
- 5.Carroll SM. Nonvocal ventilated patients perceptions of being understood. West J Nurs Res. 2004;26:85–103. doi: 10.1177/0193945903259462. Discussion 4–12. [DOI] [PubMed] [Google Scholar]
- 6.Menzel LK. Factors related to the emotional responses of intubated patients to being unable to speak. Heart Lung. 1998;27:245–252. doi: 10.1016/s0147-9563(98)90036-x. [DOI] [PubMed] [Google Scholar]
- 7.The Joint Commission. [Accessed 23.07.10];New and Revised Standards and EPs for Patient-Center Communication-Hospital Accreditation Program. 2010 Available at, http://www.jointcommission.org/NR/rdonlyres/26D4ABD6-3489-4101-B397-56C9EF7CC7FB/0/Post_PatientCenteredCareStandardsEPs_20100609.pdf.
- 8.Costello J. AAC intervention in the intensive care unit: the children’s hospital Boston model. AAC Augment Altern Commun. 2000;16:137–153. [Google Scholar]
- 9.Happ M, Roesch T, Garrett K. Electronic voice-output communication aids for temporarily nonspeaking patients in a medical intensive care unit: a feasibility study. Heart Lung. 2004;33:92–101. doi: 10.1016/j.hrtlng.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Radtke JV, Tate JA, Happ MB. Nurses’ perceptions of communication training in the ICU. Intensive Crit Care Nurs. 2012;28:16–25. doi: 10.1016/j.iccn.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radtke JV, Baumann BM, Garrett KL, Happ MB. Listening to the voiceless patient: case reports in assisted communication in the intensive care unit. J Palliat Med. 2011;14:791–795. doi: 10.1089/jpm.2010.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stovsky B, Rudy EB, Dragonette P. Comparison of two types of communication methods used after cardiac surgery with patients with endotracheal tubes. Heart Lung. 1988;17:281–289. [PubMed] [Google Scholar]
- 13.Beukelman D, Garrett K, Yorkston K. Augmentative Communication Strategies for Adults with Acute or Chronic Medical Conditions. Baltimore, MD: Paul H Brookes Publishing Co; 2007. [Google Scholar]
- 14.Happ MB, Garret KL, Tate JA, et al. Effect of a multi-level intervention on nurse-patient communication in the intensive care unit: results of the SPEACS trial. Heart Lung. 2014;43(2):89–98. doi: 10.1016/j.hrtlng.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Happ MB, Baumann BM, Sawicki J, Tate JA, George EL, Barnato AE. SPEACS-2: intensive care unit “communication rounds” with speech language pathology. Geriatr Nurs. 2010;31:170–177. doi: 10.1016/j.gerinurse.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Yount RJ, Vries JK, Councill CD. The medical archival system: an information retrieval system based on distributed parallel processing. Inf Process Manage. 1991;27:379–389. [Google Scholar]
- 17.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 18.Riker R, Picard J, Fraser G. Prospective evaluation of the sedation-agitation scale for adult critically ill patients. Crit Care Med. 1999;27:1325–1329. doi: 10.1097/00003246-199907000-00022. [DOI] [PubMed] [Google Scholar]
- 19.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–659. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patak L, Gawlinski A, Fung NI, Doering L, Berg J. Patients’ reports of health care practitioner interventions that are related to communication during mechanical ventilation. Heart Lung. 2004;33:308–320. doi: 10.1016/j.hrtlng.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Thomas LA, Rodriguez CS. Prevalence of sudden speechlessness in critical care units. Clin Nurs Res. 2011;20:439–447. doi: 10.1177/1054773811415259. [DOI] [PubMed] [Google Scholar]
- 22.Zubow L, Hurtig R. A demographic study of AAC/AT needs in hospitalized patients. Perspect Augment Altern Commun. 2013;22:79–90. [Google Scholar]
- 23.Wunsch H, Linde-Zwirble WT, Angus DC, Hartman ME, Milbrandt EB, Kahn JM. The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010;38:1947–1953. doi: 10.1097/CCM.0b013e3181ef4460. [DOI] [PubMed] [Google Scholar]
- 24.Vasilevskis EE, Ely EW, Speroff T, Pun BT, Boehm L, Dittus RS. Reducing iatrogenic risks: ICU-acquired delirium and weakness–crossing the quality chasm. Chest. 2010;138:1224–1233. doi: 10.1378/chest.10-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson JC, Girard TD, Gordon SM, et al. Long-term cognitive and psychological outcomes in the awakening and breathing controlled trial. Am J Respir Crit Care Med. 2010;182:183–191. doi: 10.1164/rccm.200903-0442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vasilevskis EE, Girard TD, Ely EW. The bedside diagnosis of ICU delirium: specificity is high, let’s optimize sensitivity. Am J Respir Crit Care Med. 2012;185:107–108. doi: 10.1164/ajrccm.185.1.107. Author reply 8. [DOI] [PubMed] [Google Scholar]
- 27.Garrett KL, Happ MB, Costello J, Fried-Oken M. AAC in intensive care units. In: Buekelman DR, Garrett K, Yorkston KM, editors. Augmentative Communication Strategies for Adults with Acute or Chronic Medical Conditions. Baltimore, MD: Brookes Publishing Company; 2007. [Google Scholar]
- 28.Grossbach I, Stranberg S, Chlan L. Promoting effective communication for patients receiving mechanical ventilation. Crit Care Nurse. 2011;31:46–60. doi: 10.4037/ccn2010728. [DOI] [PubMed] [Google Scholar]
- 29.The Joint Commission. Advancing effective communication, cultural competence, and patient- and family-centered Care: A roadmap for Hospitals. http://www.jointcommission.org/assets/1/6/ARoadmapforHospitalsfinalversion727.pdf2010.