Summary
Background
Estimates of influenza-associated mortality are important for national and international decision making on public health priorities. Previous estimates of 250 000–500 000 annual influenza deaths are outdated. We updated the estimated number of global annual influenza-associated respiratory deaths using country-specific influenza-associated excess respiratory mortality estimates from 1999–2015.
Methods
We estimated country-specific influenza-associated respiratory excess mortality rates (EMR) for 33 countries using time series log-linear regression models with vital death records and influenza surveillance data. To extrapolate estimates to countries without data, we divided countries into three analytic divisions for three age groups (<65 years, 65–74 years, and ≥75 years) using WHO Global Health Estimate (GHE) respiratory infection mortality rates. We calculated mortality rate ratios (MRR) to account for differences in risk of influenza death across countries by comparing GHE respiratory infection mortality rates from countries without EMR estimates with those with estimates. To calculate death estimates for individual countries within each age-specific analytic division, we multiplied randomly selected mean annual EMRs by the country’s MRR and population. Global 95% credible interval (CrI) estimates were obtained from the posterior distribution of the sum of country-specific estimates to represent the range of possible influenza-associated deaths in a season or year. We calculated influenza-associated deaths for children younger than 5 years for 92 countries with high rates of mortality due to respiratory infection using the same methods.
Findings
EMR-contributing countries represented 57% of the global population. The estimated mean annual influenza-associated respiratory EMR ranged from 0·1 to 6·4 per 100 000 individuals for people younger than 65 years, 2·9 to 44·0 per 100 000 individuals for people aged between 65 and 74 years, and 17·9 to 223·5 per 100 000 for people older than 75 years. We estimated that 291 243–645 832 seasonal influenza-associated respiratory deaths (4·0–8·8 per 100 000 individuals) occur annually. The highest mortality rates were estimated in sub-Saharan Africa (2·8–16·5 per 100 000 individuals), southeast Asia (3·5–9·2 per 100 000 individuals), and among people aged 75 years or older (51·3–99·4 per 100 000 individuals). For 92 countries, we estimated that among children younger than 5 years, 9243–105 690 influenza-associated respiratory deaths occur annually.
Interpretation
These global influenza-associated respiratory mortality estimates are higher than previously reported, suggesting that previous estimates might have underestimated disease burden. The contribution of non-respiratory causes of death to global influenza-associated mortality should be investigated.
Introduction
Annual influenza epidemics result in substantial mortality, especially among adults aged 65 years and older. Previous estimates attributed to WHO1 indicated that 250 000–500 000 influenza-associated deaths occur annually, corresponding to estimates of 3·8–7·7 deaths per 100 000 individuals calculated using 2005 UN Department of Economic and Social Affairs World Population Prospects.2 The methods used to calculate this WHO estimate have not been published and might not have accounted for annual variability in the incidence of influenza virus infection, the age and health status of populations, or risk of influenza death across countries. A 2013 study,3 which used data from 2005 to 2009, suggested that 148 000–249 000 annual influenza respiratory deaths might occur each year.3 Current, reliable global and country-specific influenza-associated mortality estimates are needed to inform decisions about the value of influenza prevention and control and to inform global public health priorities.
Estimating the burden of annual influenza epidemics is challenging for many countries because of the requirement for high-quality systematic vital records and local viral surveillance data. As a result, most influenza-associated mortality estimates have been obtained from high-income countries with a temperate climate.4–26 Many developing or recently industrialised countries have leveraged improvements in influenza surveillance data27 to develop country-specific influenza-associated mortality estimates, which are generally higher (4·3–31·6 per 100 000 individuals) than WHO-attributed estimates,28–31 implying that the current global influenza death estimates might underestimate the global mortality burden of influenza. The wide variation in influenza-associated excess mortality estimates between countries and between seasons and years highlights the effect of specific circulating influenza viruses on mortality and the need to include more country-specific estimates in global mortality estimation to better quantify global disease burden. Furthermore, global and country-specific estimates should be updated periodically to account for changes in population demographics, improvements in health care, and viral evolution.
Improved methods to estimate baseline and influenza-associated mortality have been developed.32–37 However, influenza virus infections are rarely confirmed systematically by laboratory diagnosis, and thus influenza deaths might be attributed to other comorbid conditions or secondary infections. Ecological models are commonly used to estimate influenza-associated mortality. Vital records death data, which have varying levels of quality, completeness, and population coverage, are systematically coded for cause of death using the International Classification of Diseases (ICD),38,39 and categories of death commonly associated with influenza, including respiratory or circulatory causes,40 are modelled with virological data to identify periods of influenza virus circulation and to estimate influenza-associated excess deaths. The inclusion of virological data is important for the quantification of influenza-associated deaths because the circulating strains of the virus subtypes vary from year to year, which can affect annual mortality.40,41 The application of these methods is challenging in many low-income and middle-income countries because they often have year-round or multiple peaks in influenza virus circulation, vital records data that are not of sufficient quality, or they have too few years of reliable surveillance data for robust estimates despite recent efforts to improve and expand surveillance.27,35,42,43
We aimed to estimate the number of country-specific influenza-associated respiratory deaths and to update global and regional influenza-associated respiratory death estimates among individuals younger than 65 years, 65–74 years, and 75 years and older, using excess death estimates from contributing countries extrapolated to countries without such data.
Methods
Study design and data sources
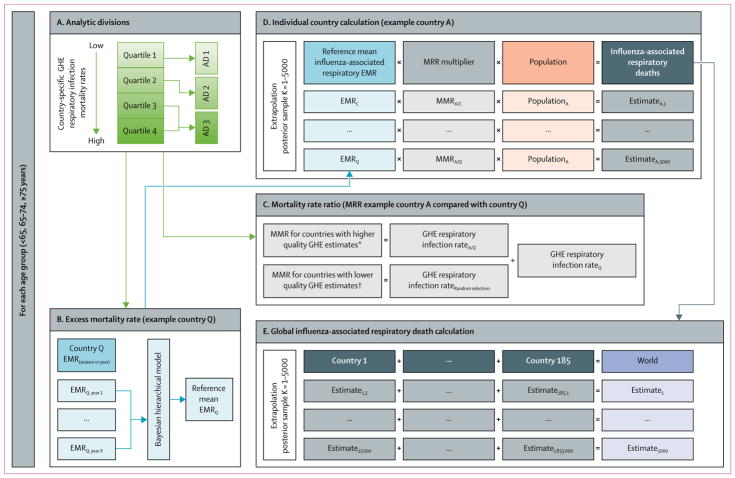
We developed a statistical modelling approach to generate country-specific estimates of influenza-associated death for countries with no available estimates accounting for uncertainty in estimated risk of influenza death and variability between countries (figure 1). This analysis complied with the WHO Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER),44,45 and a GATHER checklist for this study is included in the appendix.
Figure 1. Extrapolation model approach for individual country and global estimates of influenza-associated respiratory deaths.
Country refers to WHO member states, Hong Kong, Taiwan, or regions within a country. WHO GHE were used to categorise countries into ADs and in the extrapolation model. Usability was defined using the following equation: usability (%)=completeness of death registration data (%) × (1–proportion of ill-defined death registration data). AD=analytic division. EMR=excess mortality rate. MMR=mortality rate ratio. GHE=Global Health Estimates. *Countries with at least 5 years of death registration data available (starting from 2005) with a mean usability of all available years (from 2000 onwards) of 60% or more if International Classification of Diseases-coded registration data for specific causes of death were reported, or with a mean usability of 80% or more if only summarised causes of death data were reported. †Countries with no annual death registration data available or countries with less than 5 years of death registration available (starting from 2005) with a mean usability of all available years (from 2000 forward) of less than 60% if International Classification of Diseases-coded registration data for specific causes of death were reported, or a mean usability of 80% or less if only summarised causes of death data were reported.
In this study, the terms country or countries referred to WHO member states, Hong Kong, Taiwan, or regions within a country. We contacted potential collaborators in 62 countries who were identified from literature published in the past 20 years or through the solicitation of data from surveillance or research contacts. Of the 62 countries, 47 contributed data: 33 countries provided model estimates of age-specific influenza-associated respiratory excess mortality rates (EMRs), 13 countries contributed age-specific annual respiratory deaths, and one country contributed annual estimates of influenza-associated respiratory death using a multiplicative model (table 1). Of the 15 remaining countries contacted, eight did not respond to collaboration requests, five were unable to share data by September, 2017, and two had vital records data of insufficient quality.
Table 1.
EMR-contributing countries with modelled influenza-associated excess respiratory mortality or annual estimates of respiratory mortality by age group for validation
| Income level* | Global population (%)† | Seasons or years with estimates available | Time series | Type of influenza virus data used in statistical model | Analytic division
|
|||
|---|---|---|---|---|---|---|---|---|
| Age<65 years | Age65–74 years | Age≥75 years | ||||||
| Countries or territories with modelled influenza-associated respiratory EMRs (EMR-contributing countries) | ||||||||
| Sub-Saharan Africa | ||||||||
| Kenya | Low | 0·63% | 2007–13 | Monthly | Influenza virus | 3 | 3 | 3 |
| South Africa | Upper middle | 0·74% | 1999–2013 | Monthly (1999–2008) and weekly (2009–13) | Influenza virus | 3 | 3 | 3 |
| Europe | ||||||||
| Austria | High | 0·12% | 1999/2000–2009/10 | Weekly | Influenza virus | 1 | 1 | 1 |
| Czech Republic | High | 0·14% | 1999/2000–2010/11 | Weekly | Virus type | 1 | 2 | 2 |
| Denmark | High | 0·08% | 2002/03–2013/14 | Weekly | Virus subtype | 1 | 1 | 2 |
| Germany | High | 1·10% | 2001/02–2013/14 | Weekly | NA | 1 | 1 | 1 |
| Israel | High | 0·11% | 2004/05–2013/14 | Weekly | Virus subtype | 1 | 2 | 2 |
| Netherlands | High | 0·23% | 1999/2000–2010/11 | Weekly | Virus type | 1 | 1 | 2 |
| Norway | High | 0·07% | 1999/2000–2014/15 | Weekly | Virus type | 1 | 1 | 2 |
| Portugal | High | 0·14% | 1999/2000–2013/14 | Weekly | NA | 2 | 2 | 3 |
| Romania | Upper middle | 0·27% | 2000/01–2014/15 | Monthly | Virus subtype | 3 | 2 | 1 |
| Serbia | Upper middle | 0·12% | 2000/01–2010/11 | Weekly | NA | 1 | 1 | 1 |
| Spain | High | 0·63% | 2000/01–2012/13 | Weekly | Virus subtype | 1 | 1 | 1 |
| Switzerland | High | 0·11% | 1999/2000–2013/14 | Weekly | Virus subtype | 1 | 1 | 1 |
| UK‡ | High | 0·88% | 2006/07–2011/12 | Weekly | Virus type | 1 | 1 | 2 |
| The Americas | ||||||||
| Argentina | Upper middle | 0·59% | 2002–09 | Monthly | NA | 3 | 3 | 3 |
| Southern Brazil | Upper middle | 2·83% | 2002–09 | Monthly | NA | 2 | 3 | 3 |
| Canada | High | 0·49% | 1999/2000–2007/08 | Weekly | Virus type | 1 | 1 | 1 |
| Chile | High | 0·24% | 2002–09 | Weekly | NA | 1 | 1 | 2 |
| Mexico | Upper middle | 1·73% | 2002/03–2009/10 | Weekly | NA | 2 | 2 | 2 |
| Paraguay | Lower middle | 0·09% | 2002–09 | Weekly | NA | 2 | 2 | 2 |
| USA | High | 4·39% | 1999/2000–2014/15 | Weekly | Virus subtype | 2 | 2 | 1 |
| Uruguay | High | 0·05% | 2004, 2007–2009 | Weekly | NA | 2 | 2 | 2 |
| Southeast Asia | ||||||||
| India | Lower middle | 17·87% | 2010–13 | Weekly | Virus subtype | 3 | 3 | 3 |
| Thailand | Upper middle | 0·93% | 2006–11 | Weekly | Virus subtype | 3 | 3 | 3 |
| Western Pacific | ||||||||
| Australia | High | 0·33% | 2003–09 | Weekly | Virus subtype | 1 | 1 | 1 |
| China | Upper middle | 18·76% | 2004/05–2009/10 | Weekly | Virus subtype | 1 | 2 | 1 |
| Hong Kong§ | High | 0·10% | 2000–13 | Weekly | Virus subtype | 2 | 3 | 3 |
| Japan | High | 1·73% | 1999/2000–2010/11 | Weekly | Virus subtype | 1 | 2 | 3 |
| New Zealand | High | 0·06% | 2002–13 | Weekly | Virus subtype | 1 | 1 | 1 |
| Singapore | High | 0·08% | 2004–11 | Weekly | Influenza virus | 2 | 3 | 3 |
| South Korea | High | 0·69% | 2003/04–2013/14 | Weekly | Virus type | 1 | 2 | 2 |
| Taiwan§ | High | 0·32% | 2000/01–2013/14 | Weekly | Virus subtype | 2 | 2 | 3 |
| Countries with annual estimates of respiratory deaths by age group (validation countries) | ||||||||
| Eastern Mediterranean | ||||||||
| Kuwait | High | 0·05% | 2000–11 | ·· | ·· | 1 | 3 | 3 |
| Morocco | Lower middle | 0·47% | 2010–13 | ·· | ·· | 3 | 3 | 2 |
| The Americas | ||||||||
| Colombia | Upper middle | 0·66% | 2002–08 | ·· | ·· | 2 | 2 | 2 |
| Costa Rica | Upper middle | 0·07% | 2002–09 | ·· | ·· | 1 | 1 | 1 |
| Cuba | Upper middle | 0·16% | 2005, 2007, and 2009 | ·· | ·· | 2 | 3 | 3 |
| Ecuador | Upper middle | 0·22% | 2002–09 | ·· | ·· | 3 | 2 | 3 |
| El Salvador | Lower middle | 0·08% | 2002–09 | ·· | ·· | 3 | 3 | 3 |
| Guatemala | Lower middle | 0·22% | 2005–09 | ·· | ·· | 3 | 3 | 3 |
| Panama | Upper middle | 0·05% | 2004, 2006–09 | ·· | ·· | 2 | 2 | 2 |
| Peru | Upper middle | 0·43% | 2005–07 | ·· | ·· | 3 | 3 | 3 |
| Venezuela | Upper middle | 0·42% | 2002, 2006–07 | ·· | ·· | 2 | 2 | 2 |
| Southeast Asia | ||||||||
| Indonesia | Lower middle | 3·51% | 2014 | ·· | ·· | 3 | 2 | 2 |
| Western Pacific | ||||||||
| Mongolia | Lower middle | 0·04% | 2002–10 | ·· | ·· | 3 | 1 | 1 |
| Countries with estimates of influenza-associated excess respiratory mortality obtained using multiplier approach | ||||||||
| Bangladesh | Low | 2·19% | 2010/11–2011/12 | ·· | ·· | 3 | 2 | 1 |
EMR=excess mortality rate. NA=not available.
Income level categorisation based on World Bank classification.46
Influenza-associated EMR estimates for England and Wales only.
Analytic divisions for Hong Kong and Taiwan were based on vital records data from the Census and Statistics Department of the Hong Kong Government and the Ministry of Health and Welfare in Taiwan, because WHO Global Health Estimates did not include Hong Kong or Taiwan.
The 33 countries with annual influenza-associated excess mortality estimates are referred to as EMR-contributing countries. For these countries, we requested at least 4 years of weekly or monthly vital records mortality data collected between 1999 and 2015 for three age groups (<65 years, 65–74 years, and ≥75 years) for all respiratory causes of death (ICD ninth revision48 codes 460–519; ICD tenth revision49 codes, J00–J99). We also requested viral surveillance data, including the total number of specimens tested for influenza and the number of positive specimens for each influenza type and subtype, for the same time period. For EMR-contributing countries with these data that were not able to share with external partners, we requested influenza-associated excess respiratory mortality estimates for the three age groups for at least four separate seasons or years (appendix).
13 countries provided the annual number of all ICD-coded respiratory deaths for the three age groups, which were used to validate the extrapolated estimates. One country provided estimates of influenza-associated acute respiratory infection deaths obtained using a multiplicative model with verbal autopsy and viral surveillance data, which were used as a comparison for our extrapolated estimates. The type and quality of data obtained from these 14 validation countries is described in the appendix.
WHO Global Health Estimates
We used 2015 WHO Global Health Estimates (GHE) respiratory infection mortality rates,47,50 including both lower respiratory infection (ICD tenth revision49 codes J09–J22, P23, and U04) and upper respiratory infection (ICD codes49 J00–J06) mortality estimates to assign countries to age-specific analytic divisions. We also used the 2015 GHE mortality rates to calculate respiratory infection mortality rate ratios (MRRs) to account for differences in risk of influenza-associated or respiratory death between EMR-contributing countries and countries without excess mortality estimates. GHE respiratory infection mortality rate estimates were obtained for three age groups (<65 years, 65–74 years, and ≥75 years). Hong Kong and Taiwan were not included in 2015 GHE mortality estimates and thus respiratory infection mortality rates were obtained from their 2015 ICD-coded vital records. Estimation methods for GHE differed by country depending on quality and availability of cause-specific death information (appendix).50
Population estimates
2015 population estimates were obtained from the UN Department of Economic and Social Affairs World Population Prospects2 for 184 countries and the US Census Bureau46 mid-year population estimates for one country.
Calculation of mortality estimates
We used a step-wise approach to extrapolate influenza-associated excess mortality rates from countries with estimates to countries without estimates (figure 1). First, we used the 2015 GHE respiratory infection mortality rates47 to group countries into analytic divisions, based on the assumption that variability in these rates reflected variability in circulation of influenza viral strains or case fatality ratio between countries. To establish analytic divisions for each age group, we categorised countries into quartiles according to GHE respiratory infection mortality rate (figure 1A). Because respiratory infection mortality rates varied widely by age, countries were sometimes categorised into different quartiles according to age group. Few EMR-contributing countries were grouped into the fourth quartile, therefore the third and fourth quartiles were combined resulting in three analytic divisions for each age group (nine analytic divisions in total; appendix). For each age-specific analytic division, the number of EMR-contributing countries ranged between six and 18 (table 2). We estimated global influenza-associated respiratory deaths for 185 countries. For this global analysis, the world was defined as WHO member states, Hong Kong, and Taiwan. 11 member states (Andorra, Cook Islands, Dominica, Saint Kitts and Nevis, Monaco, Marshall Islands, Niue, Nauru, Palau, San Marino, and Tuvalu) were excluded, which comprised 0·006% of the global population. Influenza-associated death estimates were not calculated for these 11 member states because 2015 GHE estimates for respiratory infection mortality rates for these countries were not available.
Table 2.
Mean annual influenza-associated EMR per 100 000 population by age group for EMR-contributing countries
| Age <65 years | Age 65–74 years | Age ≥75 years | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Mean annual EMR* (SE) | Analytic division† | Mean annual EMR* (SE) | Analytic division† | Mean annual EMR* (SE) | Analytic division† | |
| Argentina | 3·0 (0·6) | 3 | 44·0 (7·8) | 3 | 223·5 (44·4) | 3 |
|
| ||||||
| Australia | 0·5 (0·2) | 1 | 3·5 (1·7) | 1 | 20·8 (9·8) | 1 |
|
| ||||||
| Austria | 1·0 (0·4) | 1 | 11·9 (3·9) | 1 | 63·8 (9·5) | 1 |
|
| ||||||
| Southern Brazil | 1·0 (0·2) | 2 | 19·8 (5·6) | 3 | 111·1 (40·5) | 3 |
|
| ||||||
| Canada | 0·4 (0·1) | 1 | 6·1 (2·2) | 1 | 44·5 (12·5) | 1 |
|
| ||||||
| Chile | 1·4 (0·2) | 1 | 30·4 (6·3) | 1 | 187·4 (39·9) | 2 |
|
| ||||||
| China | 0·7 (0·3) | 1 | 19·1 (7·0) | 2 | 112·7 (34·3) | 1 |
|
| ||||||
| Czech Republic | 0·5 (0·1) | 1 | 5·5 (0·9) | 2 | 20·4 (3·5) | 2 |
|
| ||||||
| Denmark | 0·6 (0·2) | 1 | 4·2 (1·6) | 1 | 51·0 (14·3) | 2 |
|
| ||||||
| Germany | 0·4 (0·1) | 1 | 2·9 (1·3) | 1 | 21·0 (7·6) | 1 |
|
| ||||||
| Hong Kong‡ | 0·4 (0·05) | 2 | 12·0 (1·3) | 3 | 84·6 (9·3) | 3 |
|
| ||||||
| India | 2·2 (1·2) | 3 | 35·5 (12·3) | 3 | 88·1 (30·4) | 3 |
|
| ||||||
| Israel | 0·2 (0·05) | 1 | 3·2 (0·8) | 2 | 29·3 (4·9) | 2 |
|
| ||||||
| Japan | 0·2 (0·03) | 1 | 3·5 (0·4) | 2 | 27·5 (2·9) | 3 |
|
| ||||||
| Kenya§ | 6·4 (2·5) | 3 | ·· | 3 | ·· | 3 |
|
| ||||||
| Mexico | 1·4 (0·3) | 2 | 19·3 (4·5) | 2 | 68·9 (22·1) | 2 |
|
| ||||||
| Netherlands | 0·4 (0·1) | 1 | 12·6 (2·5) | 1 | 91·1 (19·9) | 2 |
|
| ||||||
| New Zealand | 0·3 (0·1) | 1 | 4·8 (1·2) | 1 | 36·7 (7·7) | 1 |
|
| ||||||
| Norway | 0·9 (0·2) | 1 | 9·2 (2·2) | 1 | 75·1 (8·1) | 2 |
|
| ||||||
| Portugal | 2·2 (0·7) | 2 | 25·3 (7·2) | 2 | 118·1 (41·2) | 3 |
|
| ||||||
| Paraguay | 0·6 (0·1) | 2 | 10·3 (3·2) | 2 | 92·0 (22·2) | 2 |
|
| ||||||
| Romania | 0·6 (0·1) | 3 | 6·7 (1·1) | 2 | 20·2 (3·6) | 1 |
|
| ||||||
| Serbia | 0·7 (0·2) | 1 | 6·6 (2·2) | 1 | 17·9 (5·6) | 1 |
|
| ||||||
| Singapore | 1·5 (0·2) | 2 | 39·5 (4·2) | 3 | 187·9 (16·1) | 3 |
|
| ||||||
| South Africa | 5·2 (0·4) | 3 | 37·4 (4·0) | 3 | 123·3 (7·5) | 3 |
|
| ||||||
| South Korea | 0·1 (0·03) | 1 | 3·8 (1·0) | 2 | 24·9 (6·6) | 2 |
|
| ||||||
| Spain | 0·3 (0·1) | 1 | 6·8 (1·6) | 1 | 54·7 (15·0) | 1 |
|
| ||||||
| Switzerland | 0·3 (0·03) | 1 | 4·8 (0·6) | 1 | 33·4 (6·2) | 1 |
|
| ||||||
| Taiwan‡ | 0·2 (0·05) | 2 | 3·4 (1·0) | 2 | 33·4 (6·7) | 3 |
|
| ||||||
| Thailand | 1·4 (0·9) | 3 | 25·0 (16·0) | 3 | 118·9 (76·0) | 3 |
|
| ||||||
| UK¶ | 2·4 (1·9) | 1 | 17·3 (13·2) | 1 | 66·6 (39·9) | 2 |
|
| ||||||
| Uruguay | 2·4 (3·0) | 2 | 40·6 (42·6) | 2 | 199·5 (148·2) | 2 |
|
| ||||||
| USA | 0·6 (0·1) | 2 | 8·6 (1·0) | 2 | 49·4 (6·2) | 1 |
EMR=excess mortality rate.
Mean annual respiratory EMRs per 100 000 population for EMR-contributing countries were calculated using an age-specific and country-specific Bayesian hierarchical model for seasonal or annual estimates and their standard errors.
Analytic divisions were calculated using WHO Global Health Estimates of respiratory infection mortality.
The analytic divisions for Hong Kong and Taiwan were based on vital records data from the Census and Statistics Department of the Hong Kong Government and the Ministry of Health and Welfare in Taiwan because WHO Global Health Estimates did not generate estimates for Hong Kong or Taiwan.
Influenza-associated respiratory EMR estimates were only available for the youngest age group (<65 years).
Influenza-associated respiratory EMR estimates include deaths in England and Wales only.
We estimated country-specific influenza-associated respiratory EMRs per 100 000 population for the 33 EMR-contributing countries using time series log-linear regression models. Different models were selected for each country on the basis of data availability and quality of information (appendix). 26 countries included data on influenza-like illness consultations or the percentage of people who tested positive for each influenza virus type or subtype, and five countries accounted for respiratory syncytial virus circulation by including the percentage of people who tested positive for respiratory syncytial virus. Seven countries did not have sufficient viral surveillance data for modelling. For these countries, linear regression with a Serfling33 approach was used in addition to either pneumonia and influenza mortality data, influenza-like illness activity, or viral surveillance information to define the influenza virus circulation season for each of the countries.29 To account for temporal correlation in the residuals, we used bootstrapping to calculate individual year or season standard errors around these estimates.32
To account for variability between countries and annual variability in excess mortality estimates within each analytic division, we used a Bayesian hierarchical model incorporating individual season or year estimates and standard errors to calculate age-specific and country-specific mean annual EMRs per 100 000 population to represent the collection of annual estimates available from each EMR-contributing country (figure 1B).
Posterior inference was done using the Markov chain Monte Carlo method (appendix). We applied a constraint to ensure that the estimated mean annual EMRs were not less than 0 (table 2). We excluded estimates from the 2009 influenza A(H1N1)pdm09 period, specifically estimates for the 2009–10 season for temperate countries in the northern hemisphere and 2009 annual estimates for countries with a tropical climate and temperate countries in the southern hemisphere, because the effect of the influenza A(H1N1)pdm09 virus on mortality was different from that of typical seasonal epidemics.3,51
To account for differences in the risk of influenza death between countries, we calculated MRRs using two formulas (figure 1C) on the basis of the quality of GHE source information and cause-of-death estimates (appendix).50 The MRRs for countries with higher quality GHE cause-of-death estimates50 were calculated by dividing that country’s GHE respiratory infection mortality rate by the GHE respiratory infection mortality rate for a randomly selected reference EMR-country for each extrapolation simulation. The MRRs for countries with lower quality GHE cause-of-death estimates50 were calculated by replacing the individual country’s GHE respiratory infection mortality rate with a randomly selected GHE respiratory infection mortality rate from the distribution of GHE respiratory infection mortality rates from all countries within that age-specific analytic division and dividing by the GHE respiratory infection mortality rate for the randomly selected reference EMR-country. We used this approach to account for unknown error in GHE respiratory infection mortality rates for countries with poor quality or no vital records data. To verify our MRR approach, we plotted GHE respiratory infection mortality rates by mean annual EMR rates from the 33 countries stratified by age group.
We estimated the number of influenza-associated respiratory deaths within each age-specific analytic division for each country by extrapolating randomly selected mean annual influenza-associated EMRs from the 33 EMR-contributing countries (figure 1D). For each posterior sample within an analytic division, we multiplied a randomly selected mean annual EMR from a contributing country, or reference EMR, by the age-specific and country-specific MRR and population to generate a distribution of possible influenza-associated respiratory death estimates for each country. This process was repeated for each posterior sample (n=5000) by randomly selecting a different reference EMR and associated MRR to account for variability caused by the choice of reference countries. To obtain individual country estimates, we used interval estimates corresponding to the 95% credible interval (95% CrI) of the Bayesian posterior distributions to represent the range of possible influenza-associated respiratory deaths in a specific year or season (appendix).
Individual country posterior distributions were summed within each age-specific analytic division to calculate regional and global estimates (figure 1E). We used the 95% CrI of the posterior distribution to represent the range of possible global annual influenza-associated deaths in a specific season or year because of expected annual variability in circulating virus strains. We also summed estimates by WHO region and World Bank classification.52 After evaluating and validating our extrapolation approach, we replaced extrapolated estimates for each EMR-contributing country with the mean annual influenza-associated respiratory EMR and death estimate from the Bayesian hierarchical model, with the exception of Brazil and Kenya because their provided estimates were not nationally representative. The proportion of the median influenza-associated respiratory death estimates that were derived from either EMR-contributing country estimates or from our extrapolation model was calculated to compare the proportion of the total estimate that was derived from countries with EMR data with the proportion that were extrapolated.
For countries with high respiratory infection mortality in children, we also obtained estimates of influenza-associated excess mortality for children younger than 5 years for a subanalysis. Of the 185 countries that we had estimated global influenza-associated respiratory deaths for, two (1%; Hong Kong and Taiwan) did not have GHE respiratory infection mortality estimates for children younger than 5 years and therefore could not be categorised into an analytic division. Once grouped into analytic divisions, only countries in analytic division 3 (n=92) were selected for subanalysis. We used the same analytic and extrapolation approach as previously described to estimate respiratory influenza-associated deaths for the 92 countries with the highest child respiratory infection mortality, which were primarily located in sub-Saharan Africa, southeast Asia, and the Eastern Mediterranean region. The number of deaths in children younger than 5 years for these 92 countries represents 95% of all respiratory infection deaths globally in this age group (appendix).47,50 Influenza-associated respiratory EMRs from 1999 to 2015 for Kenya, India, Romania, and South Africa were used as reference EMRs. These EMR-contributing countries had sufficient vital records death data for children younger than 5 years for modelling. EMR estimates were not available for this age group in countries with lower overall respiratory infection mortality rates or countries in analytic divisions 1 or 2 because too few deaths were recorded each week in these countries to estimate excess mortality using time series log-linear models.
Evaluation of extrapolation approach
To evaluate each country’s effect on the global estimate, we did the extrapolation by removing one EMR-contributing country from the model at a time. We also compared each EMR-contributing country’s mean annual influenza-associated respiratory death 95% CrIs to the extrapolated respiratory death estimate calculated when that specific country was removed from the analysis and with the extrapolated annual death interval estimate for that country using all EMRs within the analytic division. To assess differences between our extrapolation and the country-specific EMR, we considered non-noticeable differences to be if the 95% CrIs overlapped or if the difference in medians was 10% or more when all countries were included in the extrapolation.
For another validation comparison, we calculated the proportion of all respiratory deaths that were influenza-associated for 32 of the 33 EMR-contributing countries that provided the total age-specific number of respiratory deaths by year or season. Within each age-specific analytic division, we calculated the range of the proportion of influenza-associated deaths of all respiratory deaths across all annual estimates for each EMR-contributing country. We applied the range within each analytic division to the annual age-specific ICD-coded respiratory deaths provided by 13 validation countries. We evaluated our extrapolation estimates by comparing the range of influenza-associated respiratory deaths estimated using the proportion of influenza-associated respiratory deaths out of all respiratory deaths with the 95% CrI estimates from the posterior distribution for the 13 validation countries. We considered our extrapolation model to have reasonably estimated influenza-associated deaths for these countries if the intervals overlapped. For the other validation country—Bangladesh—which provided annual estimates of influenza-associated mortality calculated using a multiplier approach, we compared our extrapolated estimate with the mean annual estimate for that country. We considered non-noticeable differences to be if the 95% CrIs overlapped. We considered that an overlap in these ranges implied that our extrapolation model reasonably estimated influenza-associated deaths for these countries compared with alternative methods of estimating influenza-associated deaths using a proportion.
Role of the funding source
There was no funding source for this study. The corresponding author had full access to all the data and had final responsibility for the decision to submit for publication.
Results
The 33 EMR-contributing countries accounted for 57% of the global population (4 153 487 759 of 7 335 457 097 individuals; appendix). Of these 33 countries, two (6%) were in sub-Saharan Africa, 13 (39%) were in Europe, eight (24%) were in the Americas (three [9%] in North America and five [15%] in central or South America), two (6%) in southeast Asia, and eight (24%) in the western Pacific. EMR-contributing countries provided between four and 16 annual influenza-associated respiratory EMR estimates calculated between 1999 and 2015 for the three age groups (>65 years, 65–74 years, and ≥75 years).
The estimated mean annual influenza-associated respiratory EMR ranged from 0·1 to 6·4 per 100 000 individuals for people younger than 65 years, 2·9 to 44·0 per 100 000 individuals for people aged between 65 and 74 years, and 17·9 to 223·5 per 100 000 for people older than 75 years (table 2; appendix).
We found a positive correlation (r2=0·29 in the ≥75 years age group to r2=0·74 in the <65 years age group; appendix) between GHE respiratory infection mortality rates and the mean annual influenza-associated excess mortality rates for the EMR-contributing countries (appendix). MRRs were calculated using 2015 GHE respiratory infection mortality estimates. For example, the country-specific and age-specific MRR for analytic division 2 among people aged 65–74 years was calculated as follows: for countries with higher quality GHE cause-of-death estimates, the numerator was their country-specific respiratory infection mortality rate estimate (44·2–93·5 per 100 000 individuals) and the denominator was the GHE respiratory infection rate for the randomly selected reference EMR-contributing country within that analytic division (45·9–87·6 per 100 000 individuals; table 3). For countries with lower quality GHE cause-of-death estimates, the MRR numerator represented the distribution of estimates for all countries within the analytic divisions. The range of country-specific and age-specific MRRs are shown in the appendix.
Table 3.
WHO Global Health Estimates of respiratory infection mortality rate (per 100 000 individuals) by age group and analytic division
| Analytic division 1(n=46) | Analytic division 2(n=46) | Analytic division 3(n=93) | |
|---|---|---|---|
| Number of EMR-contributing countries | |||
|
| |||
| Age group | |||
| <65 years | 18 | 9 | 6 |
| 65–74 years | 13 | 12 | 7 |
| ≥75 years | 11 | 11 | 10 |
|
|
|||
| WHO Global Health Estimates of respiratory infection mortality rate (per 100 000) | |||
|
|
|||
| All countries | |||
| <65 years | 0·8–5·3 | 5·3–12·8 | 13·0–181·6 |
| 65–74 years | 5·4–43·0 | 44·2–93·5 | 96·2–897·1 |
| ≥75 years | 9·3–277·7 | 279·1–553·8 | 571·6–2996·2 |
| EMR-contributing countries | |||
| <65 years | 0·9–5·2 | 5·3–12·8 | 13·1–42·8 |
| 65–74 years | 8·5–41·7 | 45·9–87·6 | 108·5–301·5 |
| ≥75 years | 94·6–275·8 | 302·7–545·7 | 677·2–1650·3 |
Data are n, or range. Analytic divisions were calculated using WHO Global Health Estimates of respiratory infection mortality. EMR=excess mortality rate.
Using data from 1999–2015, we estimated that between 291 243 and 645 832 influenza-associated respiratory deaths (4·0–8·8 per 100 000 individuals) occur annually (table 4). The median estimated number of global influenza-associated respiratory deaths (409 111) represent 13% of all GHE respiratory infection deaths (3 200 874 [appendix]). Country-specific influenza-associated respiratory death estimates are shown in the appendix. The annual number of influenza-associated respiratory deaths was highest among people younger than 65 years (175 303 deaths, 95% CrI 67 255–342 576)—accounting for 42% of all global influenza-associated respiratory deaths—and people older than 75 years (172 420, 122 876–237 933)—accounting for 41% of all global influenza-associated respiratory deaths (appendix). The highest rates of influenza-associated respiratory deaths were among people older than 75 years (51·3–99·4 per 100 000 individuals; table 4, figure 2). Across all age groups, the widest range of deaths was in sub-Saharan Africa (27 813–163 074; 17%) and the highest proportion of deaths occurred in southeast Asia (68 258–178 049; 25% of total deaths) and the western Pacific (67 728–141 436; 25%; table 4; appendix). The widest range of influenza-associated respiratory death rates occurred in countries in sub-Saharan Africa, the Eastern Mediterranean, and southeast Asia (appendix). Lower-middle-income and low-income countries had a wider range of influenza-associated deaths (112881–420841 annual deaths) compared with high-income and upper middle-income countries (table 4). The highest number of annual influenza-associated respiratory deaths and mortality rates were in countries with the highest respiratory infection mortality (ie, analytic division 3) for all age groups (appendix). For our global estimates of median influenza-associated respiratory deaths, 73% (128 749) of estimates were derived from the extrapolation model for people younger than 65 years, 39% (27 456) for individuals aged 65–74 years, and 32% (53 911) for people aged 75 years and older (appendix).
Table 4.
Estimated annual influenza-associated respiratory deaths and mortality rate per 100 000 population by age group, WHO region, and World Bank income classification
| Age <65 years | Age 65–74 years | Age ≥75 years | All ages | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| Median annual influenza- associated respiratory mortality* (95% CrI) |
Annual influenza- associated respiratory mortality 95% CrI, per 100 000 individuals* |
Proportion of total influenza- associated respiratory deaths† |
Median annual influenza- associated respiratory mortality* (95% CrI) |
Annual influenza- associated respiratory mortality 95% CrI, per 100 000 individuals* |
Proportion of total influenza- associated respiratory deaths† |
Median annual influenza- associated respiratory mortality* (95% CrI) |
Annual influenza- associated respiratory mortality 95% CrI, per 100 000 individuals* |
Proportion of total influenza- associated respiratory deaths† |
Median annual influenza- associated respiratory mortality* (95% CrI) |
Annual influenza- associated respiratory mortality 95% CrI, per 100 000 individuals* |
Proportion of total influenza- associated respiratory deaths† |
|
| Worldwide | 175 303 | 1·0–5·1 | 42% | 72 720 | 13·3–27·8 | 17% | 172 420 | 51·3–99·4 | 41% | 409 111 | 4·0–8·8 | .. |
| (67 255–342 576) | ( 48 810–102 187) | (122 876–237 933) | (291 243–645 832) | |||||||||
| WHO Regions | ||||||||||||
| Sub-Saharan Africa | 51 767 | 1·0–13·3 | 29% | 8841 | 12·0–80·4 | 13% | 11 874 | 36·3–286·9 | 7% | 69 359 | 2·8–16·5 | 17% |
| (9607–127 569) | (2648–17 744) | (3477–27 502) | (27 813–163 074) | |||||||||
| The Americas | 12 778 | 0·8–2·3 | 8% | 9160 | 10·5–21·5 | 13% | 29 908 | 52·2–105·7 | 17% | 51 674 | 4·2–7·3 | 12% |
| (7027–20 203) | (6236–12 776) | (21 624–43 777) | (41 007–71 710) | |||||||||
| Europe | 10 476 | 0·5–2·5 | 6% | 6566 | 5·0–17·2 | 9% | 27 509 | 23·4–70·7 | 16% | 44 774 | 3·1–8·0 | 11% |
| (4179–19 518) | (3732–12 753) | (15 679–47 438) | (28 457–72 627) | |||||||||
| Eastern Mediterranean | 23 648 | 0·7–11·6 | 13% | 4753 | 7·4–54·1 | 7% | 5777 | 21·8–129·9 | 3% | 33 528 | 2·1–13·4 | 8% |
| (4468–71 208) | (1349–9905) | (2035–12 114) | (13 350–86 490) | |||||||||
| Southeast Asia | 51 412 | 1·2–6·6 | 32% | 21 782 | 14·2–47·5 | 30% | 30 936 | 44·6–130·5 | 18% | 105 167 | 3·5–9·2 | 25% |
| (21 044–119 561) | (10 499–35 014) | (16 602–48 618) | (68 258–178 049) | |||||||||
| Western Pacific | 16 951 | 0·4–1·7 | 10% | 20 425 | 7·4–27·7 | 28% | 66 529 | 45·3–133·2 | 38% | 103 728 | 3·6–7·5 | 25% |
| (7197–28 946) | (8903–33 078) | (33 858–99 617) | (67 728–141 436) | |||||||||
| World Bank income classifications | ||||||||||||
| High income | 9531 | 0·5–1·5 | 6% | 9639 | 6·1–12·0 | 13% | 45 372 | 33·9–58·6 | 26% | 64 680 | 3·9–6·4 | 16% |
| (5312–16 208) | (7161–14 078) | (34 309–59 355) | (51 083–83 623) | |||||||||
| Upper middle income | 30 848 | 0·8–2·2 | 18% | 26 328 | 10·6–29·8 | 36% | 78 997 | 56·8–143·7 | 45% | 136 017 | 4·0–7·3 | 32% |
| (17 221–48 991) | (14 242–39 974) | (44 854–113 418) | (97 776–181 157) | |||||||||
| Lower middle income | 83 604 | 1·2–7·3 | 49% | 28 442 | 16·7–47·3 | 39% | 38 418 | 44·3–133·9 | 23% | 148 105 | 3·4–10·2 | 36% |
| (30 191–184 009) | (15 467–43 723) | (21 438–64 775) | (90 738–270 831) | |||||||||
| Low income | 45 848 | 0·8–13·9 | 26% | 7793 | 8·9–67·0 | 11% | 10 099 | 27·5–209·4 | 6% | 60 979 | 2·5–16·7 | 15% |
| (6731–119 752) | (2047–15 378) | (2980–22 685) | (22 143–150 010) | |||||||||
CrI=credible interval.
After applying our extrapolation approach to all countries, excess mortality rate (EMR)-contributing country extrapolated estimates were replaced with mean annual influenza-associated respiratory EMRs and death estimates, with the exception of Brazil and Kenya.
Estimates do not add up across all age groups because age-specific models were run separately and were not additive. Estimated proportions were calculated for each of 5000 extrapolation simulations and the median proportion from the 5000 simulations is presented.
Figure 2. Estimated country-specific and age-specific influenza-associated respiratory mortality rates.
Median influenza-associated respiratory mortality rates (per 100 000 individuals) in people aged (A) 65 years or younger, (B) 65–74 years, and (C) 75 years and older.
We estimated 9243–105 690 annual influenza-associated respiratory deaths occur among children younger than 5 years for 92 countries (appendix). The overall rate of influenza-associated respiratory deaths among children younger than 5 years ranged from 2·1 to 23·8 per 100 000 population (appendix). Of the 47 countries in sub-Saharan Africa, we categorised 45 (96%) into analytic division 3 and estimated that between 721 and 46 336 (0·5–29·0 per 100 000) influenza-associated deaths occur annually among children younger than 5 years in this region (appendix). For southeast Asia, we extrapolated deaths for eight (73%) of 11 countries and estimated that 5565–42 536 (3·2–24·5 per 100 000) influenza-associated deaths occur annually among children younger than 5 years in the region. For children younger than 5 years, 71% (31 622) of median influenza-associated respiratory death estimates were derived from the extrapolation model (appendix).
When we compared global seasonal influenza-associated death estimates using all EMR-contributing countries with estimates when one EMR-contributing country was removed, we observed few differences by age group, analytic division, or WHO region (appendix). In the youngest age group (<65 years), when Argentina was removed, the upper 95% CrI of the death estimates decreased from 552 727 to 410 635 deaths and when Romania was removed the width of the interval estimates increased by 22 468 deaths (appendix). Additionally, when Thailand was removed from the extrapolation, the median influenza-associated respiratory deaths in the youngest age group (<65 years) increased by 24 528 deaths. In the 65–74 year age group, removing any EMR-contributing country had little effect on the overall estimates. In the oldest age group (≥75 years), when Australia was removed, the width of the interval estimates increased by 120 801 deaths; however, when Argentina was removed the width of the interval estimate decreased by 67 832 deaths (appendix). When we compared our extrapolated estimates across analytic divisions and WHO region, we found that removing Argentina from estimates for the youngest age group (<65 years) decreased the death interval by 140 164 deaths, but had a smaller effect on analytic divisions and regional comparisons for the older age groups compared with the youngest age group (appendix). We did not observe any other noticeable differences in the analytic division death intervals for the older age groups (65–74 and ≥75 years) when other EMR-contributing countries were removed. We observed no noticeable differences in WHO regional interval estimates in the older age groups (65–74 and ≥75 years) when we removed an EMR-contributing country (appendix). We also compared interval estimates for each EMR-contributing country using only their mean annual influenza-associated respiratory death estimates with those estimated from the full extrapolation model and an extrapolation model in which the EMR-contributing country was removed (appendix). All EMR-contributing countries had intervals that overlapped considerably with those derived from extrapolations, including the comparison between the mortality estimate derived using the multiplier approach (for Bangladesh) and the extrapolated estimates. All interval influenza-associated respiratory death estimates from our extrapolation model were more likely to have wider interval widths than those countries where the interval of influenza-associated respiratory death estimates were replaced with their own mean annual estimates of influenza-associated excess mortality. Additionally, we applied the proportion of influenza-associated respiratory deaths out of the total number of respiratory deaths (appendix) to the annual number of respiratory deaths from the 13 validation countries and found that the extrapolated 95% CrIs and the range of proportions overlapped for all validation countries (appendix).
Discussion
We estimated that more influenza-associated respiratory deaths occur annually worldwide than reported in the WHO-attributed estimate, which included both respiratory and circulatory deaths. Among the six WHO regions, the highest burden of annual influenza-associated deaths was in sub-Saharan Africa, the western Pacific, and southeast Asia. The widest range of influenza-associated mortality rates was observed among people aged 75 years and older or in countries in the Eastern Mediterranean, sub-Saharan Africa, and southeast Asia. These results suggest considerable temporal variability in influenza-associated deaths that are likely to be due to differences in circulation of virus strains and their severity from year to year. Furthermore, our results underscore the effect influenza viral infections have on population health and provide burden estimates to inform local decision making and global policies for influenza prevention and control measures.
Our estimates of influenza-associated respiratory mortality (291 243–645 832 deaths annually; 4·0–8·8 per 100 000 individuals) are higher than previously published estimates,3 which estimated 148 000–249 000 annual influenza-associated respiratory deaths, and the WHO-attributed estimate1 of 250 000–500 000 respiratory and circulatory deaths (3·8–7·7 per 100 000 individuals). One explanation for the difference is that our extrapolation uses an adjustment factor that accounts for differences in influenza death risk between countries and possible differences in underlying health status and access to care, whereas Simonsen and colleagues3 imputed estimates for countries without influenza EMRs using selected non-influenza health indicators to predict influenza mortality. Previous estimates might not have accounted for global variability in influenza mortality risk. Furthermore, our estimates included rates from a higher quantity of resource-limited countries with higher observed influenza-associated EMRs. However, it was not possible to fully evaluate the discrepancies between the attributed estimate1 and our estimate because the methods used for the WHO-attributed estimate were not available.
Another model53 from the Institute of Health Metrics and Evaluation (IHME) estimated that 2 500 300–2 860 800 global lower respiratory infection deaths and 55 700–122 100 global influenza-attributed lower respiratory infection deaths occurred in 2015. IHME attributed approximately 3% of lower respiratory infection deaths to influenza as the primary cause of death, compared with 13% in our model. IHME estimates attribute deaths to influenza by calculating an aetiological fraction of influenza virus detections, and their estimates assume that these influenza-attributed deaths were the primary cause of death. Our estimates might be higher because we did not require influenza virus detection recognising that many people with an influenza virus infection who subsequently die are more likely to have another cause of death listed on their death certificate. Estimating the number of deaths directly attributable to influenza is difficult because influenza virus infections are rarely confirmed virologically or specified as the cause of death on death certificates.40,54,55 Additionally, many influenza-associated deaths occur as a result of secondary complications when influenza viruses are no longer detectable.40,54,55 We recognised the scarcity of high-quality mortality data in lower-income countries and accounted for this by including uncertainty in the extrapolation, which might also explain differences between IHME and our estimates. Additional comparisons between these three methods are described in the appendix.
Our estimated range of 9243–105 690 annual influenza-associated deaths among children younger than 5 years in countries with high child respiratory infection mortality estimated using EMRs from four countries was similar to the influenza-associated acute lower respiratory infection death estimate by Nair and colleagues56 (28 000–111 500 annual deaths), which used severe acute lower respiratory infection case fatality proportions from 17 countries. We chose to only estimate deaths for countries with a higher burden of GHE respiratory infection mortality because EMRs for countries with a lower burden of respiratory infection mortality were not available since few deaths occur each week among children younger than 5 years.57 Furthermore, we were concerned about overestimating burden by extrapolating estimates from countries with higher burden of influenza-associated respiratory deaths to countries with a lower burden. However, we do not believe that excluding these countries from our estimation substantially affected our estimates since the countries included in our extrapolation represent 95% of all GHE respiratory infection deaths in this age group. Future studies could explore the availability of influenza-associated EMRs or alternative methods to estimate influenza-associated deaths in children younger than 5 years from countries with lower respiratory infection mortality burden for children younger than 5 years to inform a model that could estimate deaths for this age group for all countries.
Our approach to estimate global seasonal influenza-associated mortality used a multistep approach to generate estimates. The use of analytic divisions allowed us to group countries with similar respiratory infection mortality rates and extrapolate estimates of influenza-associated mortality from countries with similar mortality risk. We evaluated different approaches for categorising countries into analytic divisions52,58–60 to account for variability in the influenza attack rate or case fatality ratio between countries. Nevertheless, quantifying differences in seasonal influenza attack rate between countries was challenging because such information is scarce. Thus, we assumed a constant attack rate between countries and explored various sources to account for differences in the case fatality ratio such as poverty levels, health indicators, health-seeking behaviours, or respiratory infection rates. We assumed that the GHE respiratory infection estimates address differences in case fatality ratio, and categorised countries into analytic divisions using GHE respiratory infection mortality rates.
GHE respiratory infection mortality rates show that risk of respiratory infection mortality varies by country and these differences might be influenced by multiple factors such as quality and access to health care and differences in the underlying health status of the population. Because some of these influencing factors are known and others are not, we developed MRRs from the GHE respiratory infection estimates as a proxy for overall differences in mortality risk between countries and used these ratios to extrapolate influenza-associated EMRs from countries with available estimates to those without. Furthermore, to address the annual variability in influenza-associated deaths related to changes in circulating virus strains from year to year, we included all annual EMRs from contributing countries and presented a possible range of estimated annual influenza-associated deaths for country, region, and global estimates.
Our methods and estimates did have limitations. Our estimates are limited by the availability of vital records and viral surveillance data, especially in sub-Saharan Africa, the Eastern Mediterranean, southeast Asia, other low-income countries, and countries with a tropical climate. Specifically, estimates derived for countries in analytic division 3 might have less precision because this division contained only a small number of EMR-contributing countries to inform the extrapolation and lower-quality GHE respiratory infection mortality rates to adjust for differences in risk. This limitation was observed for the youngest age group in analytic division 3, which contained only a small number of EMR-contributing countries, whereby the inclusion of a mean annual EMR in conjunction with a lower GHE respiratory infection rate for Argentina led to higher MMRs when Argentina served as the reference country, resulting in wider death intervals. Additionally, the highest rates of influenza-associated mortality were observed in sub-Saharan Africa, the Eastern Mediterranean, and southeast Asia where vital records data are scarce. This highlights the need for more low-income countries and countries with high respiratory mortality to establish or maintain vital records systems and contribute to global estimates. Nevertheless, our model used EMR estimates from 33 countries (21 high-income countries), which represented 57% of the global population.
Additionally, available vital records data might be limited by poor reliability of cause-of-death coding despite an internationally standardised coding system, and differences might exist in death coding practices both within and between countries, which might bias national level coding efforts. Furthermore, the regression models used to estimate excess mortality have inherent uncertainty and error, which could affect the accuracy of death estimates, and model-fit information might not provide reliable guidance for interpretation or certainty of EMR estimates. Although we used Bayesian inference to generate probabilistic distributions for mortality estimates and accounted for various sources of uncertainty, the modelling framework relies on assumptions that might affect the accuracy and precision of the estimates. For example, the Bayesian approach for combining estimates from different countries incorporates uncertainties including standard errors, but does not account for systematic bias without additional information (eg, the degree of overestimation using a Serfling model because of the scarcity of available viral data). Additionally, we assumed that any of the EMR-contributing country estimates were equally likely to serve as a reference and a more informative choice of reference country might result in smaller interval widths. Extrapolation simulations were done using mean annual EMRs and represent the collection of estimates provided by countries and thus do not represent any specific year. Because of the paucity of data, our hierarchical model also assumed the EMRs to be independent between countries and seasons; however, if spatial and temporal dependence were present, that might have resulted in narrower interval estimates. Also, the GHE respiratory infection mortality estimates used to develop the MRR for our analysis were limited by variability in source information quality and availability between countries. Furthermore, 2015 GHE respiratory infection mortality rates for 11 countries (representing 0·006% of the world population) were not available. Since respiratory infection rates are used throughout the extrapolation, we could not estimate influenza-associated deaths for these countries. The exclusion of these countries is unlikely to have affected overall influenza-associated death estimates. Finally, in the MRR calculation, we assumed a constant influenza attack rate between countries, which might not be true and could result in overestimation or underestimation of the number of deaths. We were not able to account for differences in influenza-virus circulation between countries in our extrapolation, indicating a need for additional studies to evaluate how seasonal influenza attack rates might vary by geographical region.
Our estimates reflect only influenza-associated respiratory mortality, which is likely to underestimate the true burden of influenza on deaths. Influenza virus infection is also associated with hospital admission for circulatory problems and deaths, especially among older adults.40,61–66 Some national studies14,22 that estimated both respiratory and non-respiratory (eg, circulatory) deaths found that non-respiratory deaths accounted for more than half of all influenza-associated deaths. Other studies7,18,30 found that non-respiratory deaths contributed equally or accounted for fewer than half of all influenza-associated deaths. These studies suggest that the effect of influenza on non-respiratory causes of death might be more variable between countries and our estimates therefore only represent a portion of all annual influenza-associated deaths globally. Additional efforts to quantify the burden of both respiratory and non-respiratory causes of death are needed to provide a more comprehensive estimate of the global influenza-associated mortality burden.
Our 95% CrI estimates are intended to represent influenza-associated respiratory deaths between 1999 and 2015 and might reflect the variability in circulating influenza virus strains between seasons. Although our methods to estimate influenza-associated excess mortality included influenza virus type and subtype when possible, future studies should consider quantifying the effect of influenza virus type and subtypes on global deaths. Although regression models to estimate influenza-associated EMRs adjusted for respiratory syncytial virus when possible, few data were available to account for respiratory syncytial virus or other respiratory pathogens in the extrapolation. Additional studies to estimate global burden should also attempt to quantify the burden of respiratory syncytial virus and other respiratory pathogens and develop methods to account for other infectious respiratory pathogens. Estimates should be revisited periodically as new country data become available, to improve methods of estimation for both excess mortality and extrapolation, and to improve methods to account for differences in risk of influenza-associated death between countries.
This study shows that influenza contributes to a substantial annual burden of deaths globally, with the greatest effect among low-income countries in sub-Saharan Africa and southeast Asia. These estimates imply a greater burden of influenza deaths than previously recognised. Country and global policy makers should consider these data to inform influenza prevention and control programmes, especially the possible introduction or expansion of vaccination programmes. Although countries with influenza vaccination programmes have estimated reductions in severe disease and deaths,67 relatively few countries have robust seasonal influenza vaccination programmes despite WHO recommendations68 for use in key target groups. The considerable annual burden of influenza presented here could inform the efforts of global partners, such as Gavi, the Vaccine Alliance, and the Partnership for Influenza Vaccine Introduction, to increase accessibility to influenza vaccines in low-income and middle-income countries. Further work to refine the estimates by including non-respiratory causes of death are ongoing as part of a multilateral consortium of scientists and public health officials.
Supplementary Material
Research in context.
Evidence before this study
Previous estimates commonly attributed to WHO indicate that 250 000–500 000 deaths occur annually worldwide due to seasonal influenza viruses. However, no information has been published about the methods or data sources used to calculate these global estimates. These estimates began to be cited in publications and on WHO’s website around 2004, suggesting that the estimates might have been generated using data from the 1990s. Since the 1990s, improvements in influenza virus surveillance and vital records systems in many countries have led to more estimates of influenza-associated mortality for countries across the world, including some estimates from middle-income and low-income countries. Global estimates of pandemic influenza deaths are available; however, these estimates might not meet the requirements for global seasonal influenza estimates because deaths during pandemic periods are likely to differ from seasonal epidemics. We searched PubMed for articles that estimated influenza-associated excess deaths or excess mortality published between Jan 1, 1960 and Dec 31, 2012, using the search terms “influenza”, “death”, “mortality”, “excess death”, “excess mortality”, “Serfling”, “negative binomial”, “time series”, “respiratory”, “circulatory”, “pneumonia”, and “influenza” with no language restrictions. Previously published data were not suitable for our extrapolation model to estimate global influenza deaths because of heterogeneity between the age groups studied and death outcomes (eg, pneumonia and influenza, respiratory, all-cause, or circulatory) investigated. Thus, we initiated an effort to directly collect data from partners around the world using common age groups and death outcomes to update and improve global seasonal influenza-associated mortality estimates.
Added value of this study
Previous global influenza mortality estimates were calculated more than 10 years ago and detailed information about the methods used are not available. Since these estimates were made available by WHO the number of countries with capacity to calculate national estimates for seasonal influenza-associated excess mortality has increased. We initiated a project to use this additional information to update and improve global estimates of influenza-associated respiratory mortality. We worked with collaborators from 47 countries to develop an innovative statistical model to calculate global estimates of influenza-associated mortality using vital records and viral surveillance data, including estimates for 1999–2015 from countries with data, which were extrapolated to countries without such data. Our study presents a comprehensive analysis of influenza-associated mortality, in which we provide extensive details about methods used and account for differences between countries. In this study, we calculated country-specific estimates for influenza-associated respiratory deaths in three age groups (<65 years, 65–74 years, and ≥75 years) and did a subanalysis for children younger than 5 years, which might help country-level policy makers to understand the impact of influenza virus infection on their populations. Additionally, our analysis complies with the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) recommendations.
Implications of all the available evidence
Our estimates of influenza-associated respiratory mortality are higher than previous estimates. The results of this study help to improve understanding about the burden of influenza viral infections and emphasise the need for continued support to detect, prevent, and control influenza viruses. Furthermore, this study provides age-specific and country-specific influenza-associated respiratory death estimates that could be used by countries to inform prevention and control measures for influenza virus infection in their population. This study improves on earlier estimates by including primary data from 47 countries to estimate global influenza-associated deaths and by validating our extrapolation models.
Acknowledgments
We thank Juan Yang (China Center for Disease Control and Prevention), Christina Bancej and Myriam Saboui (Public Health Agency of Canada), Anne Mazick (Statens Serum Institut), Silke Buda, and Walter Haaz (Robert Koch Institut), Catharina Yekti Praptiningsih (Centers for Disease Control and Prevention, Jakarta, Indonesia Office), Rita Dichtiar (Israel Center for Disease Control), Jacco Wallinga (Dutch National Institute for Public Health), Jennifer Haubrock and Ben Waite (Institute of Environmental Science and Research, New Zealand), Birgitte Freiesleben de Blasio (Norwegian Institute of Public Health, Norway), Leticia Coppola and Viviana Ramas (National Influenza Reference Center, Department of Public Health Laboratory, Ministry of Health, Uruguay), Jeremy Reich and Zachary Owens (Rollins School of Public Health, Emory University, Atlanta, GA, USA), Daniel Hogan, Colin Mathers, and Julia Fitzner (WHO, Geneva, Switzerland). We would also like to thank Jim Walters and Graphics Services (Centers for Disease Control and Prevention) for their assistance with the production of graphics, and Jessica Kolling and the Geospatial Research, Analysis, and Services Program (GRASP; Centers for Disease Control and Prevention). The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the US Centers for Disease Control and Prevention.
Footnotes
Contributors
ADI, M-AW, and JSB conceived the study. ADI contacted and engaged potential partners for collaboration in the global network, communicated progress of the global estimation, and requested feedback. ADI and KMR compiled, managed, cleaned, evaluated, and analysed country-specific data. ADI and KMR developed statistical models for country-specific data for some contributing countries and communicated and discussed findings with country-specific partners before finalising estimates to use in global models. HHC conceptualised and developed the global statistical model. ADI, KMR, HHC, and JSB drafted the manuscript, created the tables and figures, compiled feedback, and addressed all comments from coauthors. DJM, RP, ST, CC, JMG, DS, BJC, PW, JK, LWA, MP, MR-F, HY, LE, AK, GE, LvA, SPdS, SA, and UB developed country-specific statistical models to estimate influenza-associated mortality, analysed country-specific data, provided statistical feedback on global models, and provided feedback on manuscript drafts. All other authors listed as network collaborators provided data, reviewed country-specific results, or reviewed, contributed to, and approved final results, manuscript drafts, and this publication.
Declaration of interests
CC reports grants from the US Centers for Disease Control and Prevention during the conduct of the study, and grants from Sanofi Pasteur, and travel expenses paid for by Parexel, outside the submitted work. BJC reports grants from the National Institute of Allergy and Infectious Diseases, the Health and Medical Research Fund of Hong Kong, Harvard Center for Communicable Disease Dynamics, the National Institute of General Medical Sciences, and the University Grants Committee of Hong Kong, during the conduct of the study; and grants from Sanofi Pasteur, outside the submitted work. JK reports grants from the Foundation for Influenza Epidemiology and travel expenses paid for by the Global Influenza Initiative, outside the submitted work. All other authors declare no competing interests.
This online publication has been corrected. The corrected version first appeared at thelancet.com on January 19, 2018
Funding
None.
References
- 1.WHO. [accessed May 10, 2016];Influenza (seasonal) fact sheet. 2016 http://www.who.int/mediacentre/factsheets/fs211/en/
- 2.UN Population Division. [accessed May 12, 2016];World population prospects. 2017 http://esa.un.org/unpd/wpp/Download/Standard/Population/
- 3.Simonsen L, Spreeuwenberg P, Lustig R, et al. Global mortality estimates for the 2009 influenza pandemic from the GLaMOR project: a modeling Study. PLoS Med. 2013;10:e1001558. doi: 10.1371/journal.pmed.1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow A, Ma S, Ling AE, Chew SK. Influenza-associated deaths in tropical Singapore. Emerg Infect Dis. 2006;12:114–21. doi: 10.3201/eid1201.050826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foppa IM, Hossain MM. Revised estimates of influenza-associated excess mortality, United States, 1995 through 2005. Emerg Themes Epidemiol. 2008;5:26. doi: 10.1186/1742-7622-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gran JM, Kacelnik O, Grjibovski AM, Aavitsland P, Iversen BG. Counting pandemic deaths: comparing reported numbers of deaths from influenza A(H1N1)pdm09 with estimated excess mortality. Influenza Other Respir Viruses. 2013;7:1370–79. doi: 10.1111/irv.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green HK, Andrews N, Fleming D, Zambon M, Pebody R. Mortality attributable to influenza in England and Wales prior to, during and after the 2009 pandemic. PLoS One. 2013;8:e79360. doi: 10.1371/journal.pone.0079360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo HW, Schmid D, Liu YL, Lachner P, Allerberger F. Influenza-related excess mortality, Austria 2001 till 2009. Wein Klin Wochenschr. 2011;123:593–98. doi: 10.1007/s00508-011-0019-7. [DOI] [PubMed] [Google Scholar]
- 9.Kyncl J, Prochazka B, Goddard NL, et al. A study of excess mortality during influenza epidemics in the Czech Republic, 1982–2000. Eur J Epidemiol. 2005;20:365–71. doi: 10.1007/s10654-005-1067-y. [DOI] [PubMed] [Google Scholar]
- 10.Lee VJ, Yap J, Ong JB, et al. Influenza excess mortality from 1950–2000 in tropical Singapore. PLoS One. 2009;4:e8096. doi: 10.1371/journal.pone.0008096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemaitre M, Carrat F, Rey G, Miller M, Simonsen L, Viboud C. Mortality burden of the 2009 A/H1N1 influenza pandemic in France: comparison to seasonal influenza and the A/H3N2 pandemic. PLoS One. 2012;7:e45051. doi: 10.1371/journal.pone.0045051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linhart Y, Shohat T, Bromberg M, Mendelson E, Dictiar R, Green MS. Excess mortality from seasonal influenza is negligible below the age of 50 in Israel: implications for vaccine policy. Infection. 2011;39:399–404. doi: 10.1007/s15010-011-0153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Cuadrado T, de Mateo S, Jimenez-Jorge S, Savulescu C, Larrauri A. Influenza-related mortality in Spain, 1999–2005. Gac Sanit. 2012;26:325–29. doi: 10.1016/j.gaceta.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 14.Muscatello DJ, Newall AT, Dwyer DE, Macintyre CR. Mortality attributable to seasonal and pandemic influenza, Australia, 2003 to 2009, using a novel time series smoothing approach. PLoS One. 2014;8:e64734. doi: 10.1371/journal.pone.0064734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nunes B, Viboud C, Machado A, et al. Excess mortality associated with influenza epidemics in Portugal, 1980 to 2004. PLoS One. 2011;6:e20661. doi: 10.1371/journal.pone.0020661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rizzo C, Bella A, Viboud C, et al. Trends for influenza-related deaths during pandemic and epidemic seasons, Italy, 1969–2001. Emerg Infect Dis. 2007;13:694–99. doi: 10.3201/eid1305.061309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schanzer DL, Sevenhuysen C, Winchester B, Mersereau T. Estimating influenza deaths in Canada, 1992–2009. PLoS One. 2013;8:e80481. doi: 10.1371/journal.pone.0080481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu P, Goldstein E, Ho LM, et al. Excess mortality associated with influenza A and B virus in Hong Kong, 1998–2009. J Infect Dis. 2012;206:1862–71. doi: 10.1093/infdis/jis628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zucs P, Buchholz U, Haas W, Uphoff H. Influenza associated excess mortality in Germany, 1985–2001. Emerg Themes Epidemiol. 2005;2:6. doi: 10.1186/1742-7622-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Estimates of deaths associated with seasonal influenza—United States, 1976–2007. MMWR Morb Mortal Wkly Rep. 2010;59:1057–62. [PubMed] [Google Scholar]
- 21.Freitas FT, Souza LR, Azziz-Baumgartner E, et al. Influenza-associated excess mortality in southern Brazil, 1980–2008. Epidemiol Infect. 2013;141:1731–40. doi: 10.1017/S0950268812002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redlberger-Fritz M, Aberle JH, Popow-Kraupp T, Kundi M. Attributable deaths due to influenza: a comparative study of seasonal and pandemic influenza. Eur J Epidemiol. 2012;27:567–75. doi: 10.1007/s10654-012-9701-y. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen J, Mazick A, Glismann S, Molbak K. Excess mortality related to seasonal influenza and extreme temperatures in Denmark, 1994–2010. BMC Infect Dis. 2011;11:350. doi: 10.1186/1471-2334-11-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wijngaard CC, Asten L, Koopmans MP, et al. Comparing pandemic to seasonal influenza mortality: moderate impact overall but high mortality in young children. PLoS One. 2012;7:e31197. doi: 10.1371/journal.pone.0031197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park M, Wu P, Goldstein E, Joo Kim W, Cowling BJ. Influenza-associated excess mortality in South Korea. Am J Prev Med. 2016;50:e111–19. doi: 10.1016/j.amepre.2015.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen C, Walaza S, Treurnicht FK, et al. In- and out-of-hospital mortality associated with seasonal and pandemic influenza and respiratory syncytial virus in South Africa, 2009–2013. Clin Infect Dis. 2017 doi: 10.1093/cid/cix740. published online Oct 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polansky LS, Outin-Blenman S, Moen AC. Improved global capacity for influenza surveillance. Emerg Infect Dis. 2016;22:993–1001. doi: 10.3201/eid2206.151521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aungkulanon S, Cheng P-Y, Kusreesakul K, et al. Influenza-associated mortality in Thailand, 2006–2011. Influenza Other Respir Viruses. 2015;9:298–304. doi: 10.1111/irv.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng PY, Palekar R, Azziz-Baumgartner E, et al. Burden of influenza-associated deaths in the Americas, 2002–2008. Influenza Other Respir Viruses. 2015;9(suppl 1):13–21. doi: 10.1111/irv.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tempia S, Walaza S, Viboud C, et al. Deaths associated with respiratory syncytial and influenza viruses among persons ≥5 years of age in HIV-prevalent area, South Africa, 1998–2009. Emerg Infect Dis. 2015;21:600–08. doi: 10.3201/eid2104.141033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng L, Shay DK, Jiang Y, et al. Influenza-associated mortality in temperate and subtropical Chinese cities, 2003–2008. Bull World Health Organ. 2012;90:279–88b. doi: 10.2471/BLT.11.096958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldstein E, Viboud C, Charu V, Lipsitch M. Improving the estimation of influenza-related mortality over a seasonal baseline. Epidemiology. 2012;23:829–38. doi: 10.1097/EDE.0b013e31826c2dda. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serfling RE. Methods for current statistical analysis of excess pneumonia-influenza deaths. Public Health Rep. 1963;78:494–506. [PMC free article] [PubMed] [Google Scholar]
- 34.Simonsen L, Clarke MJ, Stroup DF, Williamson GD, Arden NH, Cox NJ. A method for timely assessment of influenza-associated mortality in the United States. Epidemiology. 1997;8:390–95. doi: 10.1097/00001648-199707000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Thompson WW, Weintraub E, Dhankhar P, et al. Estimates of US influenza-associated deaths made using four different methods. Influenza Other Respir Viruses. 2009;3:37–49. doi: 10.1111/j.1750-2659.2009.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicoll A, Ciancio BC, Lopez Chavarrias V, et al. Influenza-related deaths—available methods for estimating numbers and detecting patterns for seasonal and pandemic influenza in Europe. Euro Surveill. 2012;17:20162. doi: 10.2807/ese.17.18.20162-en. [DOI] [PubMed] [Google Scholar]
- 37.Nicholson KG, Wood JM, Zambon M. Influenza. Lancet. 2003;362:1733–45. doi: 10.1016/S0140-6736(03)14854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathers CD, Fat DM, Inoue M, Rao C, Lopez AD. Counting the dead and what they died from: an assessment of the global status of cause of death data. Bull World Health Organ. 2005;83:171–77. [PMC free article] [PubMed] [Google Scholar]
- 39.Muscatello DJ, Amin J, MacIntyre CR, et al. Inaccurate ascertainment of morbidity and mortality due to influenza in administrative databases: a population-based record linkage study. PLoS One. 2014;9:e98446. doi: 10.1371/journal.pone.0098446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 41.Simonsen L, Clarke MJ, Williamson GD, Stroup DF, Arden NH, Schonberger LB. The impact of influenza epidemics on mortality: introducing a severity index. Am J Public Health. 1997;87:1944–50. doi: 10.2105/ajph.87.12.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katz MA, Schoub BD, Heraud JM, Breiman RF, Njenga MK, Widdowson M-A. Influenza in Africa: uncovering the epidemiology of a long-overlooked disease. J Infect Dis. 2012;206(suppl 1):S1–4. doi: 10.1093/infdis/jis548. [DOI] [PubMed] [Google Scholar]
- 43.Johnson LEA, Muir-Paulik SA, Kennedy P, et al. Capacity building in national influenza laboratories—use of laboratory assessments to drive progress. BMC Infect Dis. 2015;15:501. doi: 10.1186/s12879-015-1232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.The GATHER Working Group. [accessed April 10, 2017];Guidelines for accurate and transparent health estimates reporting. 2016 http://gather-statement.org/
- 45.Stevens GA, Alkema L, Black RE, et al. Guidelines for accurate and transparent health estimates reporting: the GATHER statement. Lancet. 2016;388:e19–23. doi: 10.1016/S0140-6736(16)30388-9. [DOI] [PubMed] [Google Scholar]
- 46.United States Census Bureau. International programs. [accessed May 12, 2016];International data base. http://www.census.gov/population/international/data/idb/informationGateway.php.
- 47.WHO. Estimates for 2000–2015. [accessed Feb 22, 2017];Cause specific mortality. 2017 http://www.who.int/healthinfo/global_burden_disease/estimates/en/index1.html.
- 48.Centers for Disease Control and Prevention. [accessed March 3, 2012];Classification of Diseases and Injuries. (9). ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Publications/ICD-9/ucod.txt.
- 49.WHO. [accessed March 3, 2012];International statistical classification of diseases and related health problems. 2011 10th revision. http://www.who.int/classifications/icd/ICD10Volume2_en_2010.pdf.
- 50.WHO. [accessed Feb 22, 2017];WHO methods and data sources for country-level causes of death 2000–2015. 2017 http://www.who.int/healthinfo/global_burden_disease/GlobalCOD_method_2000_2015.pdf?ua=1.
- 51.Dawood FS, Iuliano AD, Reed C, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis. 2012;12:687–95. doi: 10.1016/S1473-3099(12)70121-4. [DOI] [PubMed] [Google Scholar]
- 52.The World Bank. [accessed April 3, 2015];World Bank country and lending Groups. 2015 https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
- 53.GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Douglas RG., Jr Influenza: the disease and its complications. Hosp Pract. 1976;11:43–50. doi: 10.1080/21548331.1976.11707045. [DOI] [PubMed] [Google Scholar]
- 55.Thompson WW, Comanor L, Shay DK. Epidemiology of seasonal influenza: use of surveillance data and statistical models to estimate the burden of disease. J Infect Dis. 2006;194(suppl 2):S82–91. doi: 10.1086/507558. [DOI] [PubMed] [Google Scholar]
- 56.Nair H, Brooks WA, Katz M, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet. 2011;378:1917–30. doi: 10.1016/S0140-6736(11)61051-9. [DOI] [PubMed] [Google Scholar]
- 57.Wong KK, Jain S, Blanton L, et al. Influenza-associated pediatric deaths in the United States, 2004–2012. Pediatrics. 2013;132:796–804. doi: 10.1542/peds.2013-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.WHO. [accessed April 1, 2015];Influenza transmission zones. 2011 http://www.who.int/csr/disease/swineflu/Influenza_transmission_zones.pdf?ua=1.
- 59.UN Statistics Division. [accessed April 1, 2015];Millennium development indicators: world and regional groupings. 2014 http://mdgs.un.org/unsd/mdg/Host.aspx?Content=Data/RegionalGroupings.htm.
- 60.UNICEF. [accessed April 1, 2015];Where we work. https://www.unicef.org/where-we-work.
- 61.Fleming DM. The contribution of influenza to combined acute respiratory infections, hospital admissions, and deaths in winter. Commun Dis Public Health. 2000;3:32–38. [PubMed] [Google Scholar]
- 62.Fleming DM, Cross KW, Pannell RS. Influenza and its relationship to circulatory disorders. Epidemiol Infect. 2005;133:255–62. doi: 10.1017/s0950268804003231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reichert TA, Simonsen L, Sharma A, Pardo SA, Fedson DS, Miller MA. Influenza and the winter increase in mortality in the United States, 1959–1999. Am J Epidemiol. 2004;160:492–502. doi: 10.1093/aje/kwh227. [DOI] [PubMed] [Google Scholar]
- 64.Simonsen L, Clarke MJ, Williamson GD, Stroup DF, Arden NH, Schonberger LB. The impact of influenza epidemics on mortality: introducing a severity index. Am J Public Health. 1997;87:1944–50. doi: 10.2105/ajph.87.12.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Collins SD. Excess mortality from causes other than influenza and pneumonia during influenza epidemics. Public Health Rep. 1932;47:2159–79. [Google Scholar]
- 66.Warren-Gash C, Bhaskaran K, Hayward A, et al. Circulating influenza virus, climatic factors, and acute myocardial infarction: a time series study in England and Wales and Hong Kong. J Infect Dis. 2011;203:1710–18. doi: 10.1093/infdis/jir171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kostova D, Reed C, Finelli L, et al. Influenza illness and hospitalizations averted by influenza vaccination in the United States, 2005–2011. PLoS One. 2013;8:e66312. doi: 10.1371/journal.pone.0066312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vaccines against influenza WHO position paper—November 2012. Wkly Epidemiol Rec. 2012;87:461–76. No authors listed. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.