Abstract
OBJECTIVE
To quantify the association between having a prior bed occupant or roommate with a positive blood, respiratory, urine, or wound culture and subsequent infection with the same organism.
DESIGN
Case-control study.
SETTING
The study included 4 hospitals within an academically affiliated network in New York City, including a community hospital (221 beds), a pediatric acute-care hospital (283 beds), an adult tertiary-/quaternary-care hospital (647 beds), and a pediatric and adult tertiary-/quaternary-care hospital (914 beds).
PATIENTS
All 761,426 inpatients discharged from 2006 to 2012 were eligible. Cases included all patients who developed a healthcare-associated infection (HAI) with Staphylococcus aureus, Acinetobacter baumannii, Streptococcus pneumoniae, Pseudomonas aeruginosa, Klebsiella pneumoniae, Enterococcus faecalis, or Enterococcus faecium. Controls were uninfected patients matched by fiscal quarter, hospital, and length of stay. For each bed occupied during the 3–5-day period prior to infection, microbiology results for assigned roommates and the patient who occupied the bed immediately prior to the case were collected. For controls, the day of infection of the matched case served as the reference point.
RESULTS
In total, 10,289 HAIs were identified. In a multivariable analysis controlling for both exposures and patient characteristics, the odds of cases having been exposed to a prior bed occupant with the same organism were 5.83 times that of controls (95% confidence interval [CI], 3.62–9.39), and the odds of cases having been exposed to a roommate with the same organism were 4.82 times that of controls (95% CI, 3.67–6.34).
CONCLUSION
Infected or colonized roommates and prior occupants do pose a risk, which may warrant enhanced terminal and intermittent cleaning measures.
More than 700,000 healthcare-associated infections (HAIs) occur in US hospitals each year.1 These infections, considered to be largely preventable, accrue $28–45 billion annually in excess healthcare costs and are fatal in nearly 6% of cases.2–4 Efforts to improve quality of care while reducing costs have made HAI prevention a national priority and have sparked a surge of innovative measures aimed at curtailing their spread.5,6 Many of these interventions have specifically targeted high-risk patients with protocols for the care and maintenance of indwelling devices, with measurable but varied success.7
Meanwhile, a growing body of evidence demonstrating widespread contamination of hospital rooms and equipment has led to increasing concern about the risks posed to all patients by current cleanliness standards and practices.8 Numerous products are being developed and marketed to hospitals for the purpose of improving environmental disinfection, with particular attention given to routine cleaning for patients with multidrug-resistant organisms and terminal cleaning after discharge.9 However, very few studies have actually examined whether there is a link between contamination in patient rooms and risk of infection. Given the constraints of financial and human resources for infection prevention and control, it is important to quantify the potential impact of enhanced environmental cleanliness. Our study addressed this question by evaluating whether there is an association between HAIs and exposure to infected or colonized hospital roommates or prior room occupants using 7 years of data from 4 inpatient acute-care hospitals.
METHODS
Sample and Setting
This study was conducted in 4 inpatient hospitals in New York City. The hospitals, all part of the same healthcare network, included a community hospital (221 beds), a pediatric acute-care hospital (283 beds), an adult tertiary-/quaternary-care hospital (647 beds), and a pediatric and adult tertiary-/quaternary-care hospital (914 beds). All patients discharged between January 1, 2006, and December 31, 2012, were eligible for inclusion. This study was reviewed and approved by the institutional review boards of the study facilities, and waivers of informed consent were granted.
Data Collection
All study data were collected retrospectively. Data were sourced from multiple electronic systems used for clinical documentation and administrative purposes throughout the hospital network and were linked using unique medical record numbers and dates of admission and discharge.10 Demographic information and patient characteristics were sourced from administrative data and included age, sex, risk of mortality as measured by the Charlson comorbidity index, and specific comorbid conditions including malignancies, renal failure, and diabetes. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes were used to create the Charlson comorbidity index and to identify comorbid conditions. Patient room and bed assignments for each day of hospitalization were collected from the admission–discharge–transfer system. Culture results and antibiogram data including date and site of culture collection were obtained from clinical microbiology records.
Study Design
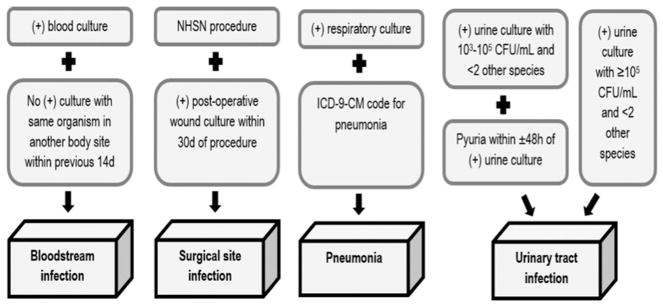
A matched case-control design was used to evaluate the association between having a prior bed occupant or roommate with a positive blood, respiratory, urine, or wound culture and subsequent infection with the same organism and comparable antibiotic sensitivity profile. These infections were chosen because they are some of the most common HAIs occurring in inpatient settings, because they are reliably identifiable in electronic records, and because patients can develop such infections through contact with contaminated hands or surfaces. Cases included all patients who developed a hospital-acquired bloodstream infection, urinary tract infection, surgical site infection, or pneumonia with 1 of the following organisms: oxacillin-sensitive Staphylococcus aureus, oxacillin-resistant S. aureus, ampicillin-sulbactam-sensitive Acinetobacter baumannii, ampicillin-sulbactam-resistant A. baumannii, penicillin-sensitive Streptococcus pneumonia, penicillin-resistant S. pneumonia, levofloxacin-sensitive Pseudomonas aeruginosa, levofloxacin-resistant P. aeruginosa, imipenem-sensitive Klebsiella pneumoniae, imipenem-resistant Klebsiella pneumoniae, vancomycin-sensitive Enterococcus faecalis and E. faecium and vancomycin-resistant E. faecalis and E. faecium. These organisms were chosen to represent a broad range of pathogens commonly seen in inpatient settings with various modes of transmission, preferential body sites of infection, and differing viability on healthcare surfaces. HAIs were detected via electronic algorithms analogous to the Centers for Disease Control and Prevention National Healthcare Safety Network surveillance definitions (Figure 1).10,11
FIGURE 1.
Electronic algorithms used to identify healthcare-associated infections based on the Centers for Disease Control and Prevention National Healthcare Safety Network surveillance guidelines.10,11 Data from the institutions’ clinical microbiology laboratories were used in conjunction with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes.
Controls were matched to cases in a 1:1 ratio and were randomly selected from all patients who (1) never had a positive culture with the organism under investigation during their hospitalization, (2) were admitted during the same fiscal quarter as the case, (3) were admitted to the same hospital as the case, and (4) had a length of stay at least as long as the case’s length of stay prior to infection.
Exposures
Exposure to prior bed occupant
All beds that each case occupied during the 3–5-day period prior to infection were identified using a computerized algorithm. For each of these beds, a second algorithm was applied to identify the patient who occupied the bed immediately prior to the case. A third algorithm searched the clinical microbiology data to determine whether any of the previous occupants had a positive culture with the organism of interest at any point prior to being discharged from the bed they occupied prior to the case. The same process was applied for controls, with the matched case’s day of infection serving as the reference point. For example, if the matched case had an infection on day 10, we looked back 3–5 days from day 10 of the control’s hospital stay.
Exposure to hospital roommate
All rooms that each case occupied during the 3–5-day period prior to infection were identified using a computerized algorithm. For each of these rooms, a second algorithm was applied to identify any other patients assigned on the same date(s) as the case. A third algorithm searched the clinical microbiology data to determine whether any of the roommates had a positive culture with the organism of interest at any point prior to sharing a room with the case. The same process was applied for controls, with the matched case’s day of infection serving as the reference point.
Data Analysis
Bivariate comparisons between cases and controls with respect to exposure to infected or colonized prior room occupants, exposure to infected or colonized roommates, age, Charlson comorbidity index, sex, presence of malignancies, renal failure, and diabetes mellitus were conducted within each organism category using the χ2 test for independence, the Fisher exact test, or the 2-sample t test, as appropriate. The overall numbers and proportions of cases and controls exposed to infected or colonized prior room occupants and roommates were tabulated to determine crude odds ratios (OR) and 95% confidence intervals (CI). Multivariable logistic regression analysis was performed to calculate adjusted odds ratios mutually controlling for both exposures and all patient characteristics and comorbidities assessed in the bivariate comparisons.
To determine with greater certainty whether a prior occupant or roommate was the source of exposure, we compared isolates for a sample of exposed case-roommate and case-prior-occupant pairs. Because molecular typing of isolates was not available, we compared antimicrobial susceptibilities based on available antibiogram data. Klebsiella pneumoniae was selected for this subanalysis due to the range of antibiotics tested for this organism in the study institutions. The tested antibiotics included cefepime, ceftriaxone, gentamicin, imipenem, levofloxacin, meropenem, piperacillin-tazobactam, tobramycin, and trimethoprim.
RESULTS
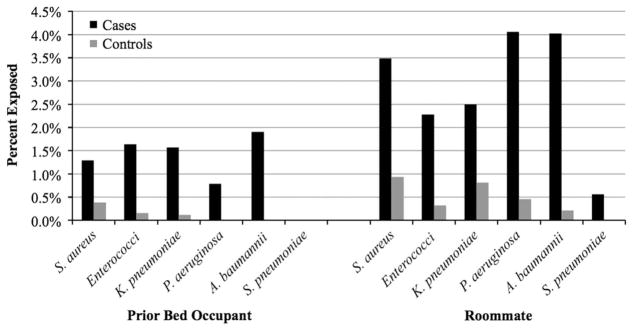
Patient admissions across the 4 facilities totaled 761,426 during the study period. Overall, 10,289 HAI cases were identified, and eligible controls were available for 10,033 cases (97.5%). The median length of stay for cases was 25 days (interquartile range, 14–46). Table 1 displays bivariate comparisons between cases and controls with respect to demographic characteristics, comorbid conditions, and exposure to infected or colonized roommates and prior bed occupants by organism. In total, 136 cases were exposed to a prior bed occupant with the same organism compared with 20 controls (crude odds ratio [OR], 6.88; 95% confidence interval [CI], 4.30–11.01). Furthermore, 309 cases were exposed to a roommate with the same organism compared with 64 controls (crude OR, 4.95; 95% CI, 3.78–6.49). Less than 2% of cases were exposed to a previous bed occupant with the same organism and <4% of cases were exposed to roommates with the same organism (Figure 2). In the multivariable analysis controlling for patient characteristics and mutually controlling for each exposure, the odds of cases having been exposed to a prior bed occupant with the same organism were 5.83 times that of controls (95% CI, 3.62–9.39), and the odds of cases having been exposed to a roommate with the same organism were 4.82 times that of controls (95% CI, 3.67–6.34) (Table 2).
TABLE 1.
Bivariate Comparisons Between Cases and Controls With Respect to Demographic Characteristics, Comorbid Conditions, and Exposure to Infected or Colonized Roommates and Prior Bed Occupantsa
| Variable | Sensitive Isolates | Resistant Isolates | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Cases | Controls | P Value | Cases | Controls | P Value | |
| Acinetobacter baumannii | N =258 | N =258 | N = 214 | N =214 | ||
| Infected or colonized prior occupant | 3 (1.1) | 0 (0) | .25 | 6 (2.8) | 0 (0) | .01 |
| Infected or colonized roommate | 3 (1.1) | 0 (0) | .25 | 16 (7.5) | 1 (0.5) | <.001 |
| Age, y | 54.6 (25.6) | 54.2 (25.8) | .84 | 46.6 (30.3) | 47.5 (30.8) | .74 |
| Charlson comorbidity index | 5.9 (5.2) | 4.9 (4.8) | .02 | 3.7 (5.5) | 3.7 (4.1) | .97 |
| Female | 113 (43.8) | 126 (48.8) | .25 | 116 (54.2) | 116 (54.2) | 1.00 |
| Malignancies | 51 (19.8) | 30 (11.6) | .01 | 20 (9.4) | 27 (12.6) | .28 |
| Renal failure | 93 (36.1) | 59 (22.9) | .001 | 24 (11.2) | 18 (8.4) | .33 |
| Diabetes mellitus | 61 (23.6) | 53 (20.5) | .40 | 36 (16.8) | 37 (17.3) | .90 |
| Enterococci | N =1,259 | N =1,259 | N =1,238 | N = 1,238 | ||
| Infected or colonized prior occupant | 13 (1.0) | 2 (0.2) | .004 | 28 (2.3) | 2 (0.2) | <.001 |
| Infected or colonized roommate | 32 (2.5) | 3 (0.2) | <.001 | 25 (2.0) | 5 (0.4) | <.001 |
| Age, y | 54.5 (25.3) | 52.9 (26.3) | .10 | 60.0 (19.1) | 56.6 (24.1) | <.001 |
| Charlson comorbidity index | 5.7 (6.0) | 4.9 (5.2) | <.001 | 6.7 (4.4) | 5.3 (5.6) | <.001 |
| Female | 559 (44.4) | 523 (41.5) | .15 | 579 (46.8) | 570 (46.0) | .72 |
| Malignancies | 264 (20.1) | 210 (16.7) | .006 | 417 (33.7) | 191 (15.4) | <.001 |
| Renal failure | 343 (27.2) | 258 (20.5) | <.001 | 457 (36.9) | 310 (25.0) | <.001 |
| Diabetes mellitus | 313 (24.9) | 259 (20.6) | .01 | 296 (23.9) | 285 (23.0) | .60 |
| Klebsiella pneumoniae | N =1,091 | N =1,091 | N = 629 | N =629 | ||
| Infected or colonized prior occupant | 20 (1.8) | 1 (0.1) | <.001 | 7 (1.1) | 1 (0.2) | .07 |
| Infected or colonized roommate | 36 (3.3) | 11 (1.0) | <.001 | 7 (1.1) | 3 (0.5) | .34 |
| Age, y | 55.8 (25.4) | 52.1 (27.1) | .001 | 57.4 (22.9) | 55.1 (25.2) | .09 |
| Charlson comorbidity index | 6.4 (5.4) | 4.4 (4.0) | .001 | 6.6 (5.0) | 5.0 (5.2) | <.001 |
| Female | 508 (46.6) | 501 (45.9) | .76 | 292 (46.4) | 283 (45.0) | .61 |
| Malignancies | 340 (31.2) | 143 (13.1) | <.001 | 151 (24.0) | 88 (14.0) | <.001 |
| Renal failure | 324 (29.7) | 214 (19.6) | <.001 | 228 (36.3) | 136 (21.6) | <.001 |
| Diabetes mellitus | 234 (21.5) | 216 (19.8) | .34 | 165 (26.2) | 135 (21.5) | .047 |
| Pseudomonas aeruginosa | N =1,027 | N =1,027 | N = 500 | N =500 | ||
| Infected or colonized prior occupant | 11 (1.1) | 0 (0) | <.001 | 1 (0.2) | 0 (0) | .999 |
| Infected or colonized roommate | 51 (5.0) | 6 (0.6) | <.001 | 11 (2.2) | 1 (0.2) | .006 |
| Age, y | 57.6 (25.1) | 53.7 (25.4) | <.001 | 58.9 (22.8) | 57.4 (23.2) | .33 |
| Charlson comorbidity index | 6.1 (4.7) | 4.8 (4.7) | <.001 | 5.6 (4.1) | 5.2 (4.5) | .10 |
| Female | 474 (46.2) | 462 (54.0) | .60 | 236 (47.2) | 232 (46.4) | .80 |
| Malignancies | 219 (21.3) | 144 (14.0) | <.001 | 76 (15.2) | 62 (12.4) | .20 |
| Renal failure | 277 (27.0) | 221 (21.5) | .004 | 157 (31.4) | 109 (21.8) | <.001 |
| Diabetes mellitus | 244 (23.8) | 200 (19.5) | .02 | 138 (27.6) | 123 (24.6) | .28 |
| Staphylococcus aureus | N =2,008 | N =2,008 | N =1,632 | N = 1,632 | ||
| Infected or colonized prior occupant | 21 (1.1) | 9 (0.5) | .03 | 26 (1.6) | 5 (3.5) | <.001 |
| Infected or colonized roommate | 81 (4.0) | 25 (1.3) | <.001 | 46 (2.8) | 9 (0.6) | <.001 |
| Age, y | 50.8 (25.8) | 52.0 (26.6) | .15 | 60.5 (22.4) | 56.1 (24.3) | <.001 |
| Charlson comorbidity index | 5.3 (4.9) | 4.5 (4.4) | <.001 | 6.6 (4.5) | 4.9 (4.5) | <.001 |
| Female | 824 (41.0) | 873 (43.5) | .12 | 678 (41.5) | 731 (44.8) | .06 |
| Malignancies | 335 (16.7) | 223 (11.1) | <.001 | 309 (18.9) | 207 (12.7) | <.001 |
| Renal failure | 475 (23.7) | 394 (19.6) | .002 | 564 (34.6) | 351 (21.5) | <.001 |
| Diabetes mellitus | 443 (22.1) | 445 (22.2) | .94 | 459 (28.1) | 361 (22.1) | <.001 |
| Streptococcus pneumoniae | N =107 | N =107 | N =70 | N =70 | ||
| Infected or colonized prior occupant | 0 (0) | 0 (0) | N/A | 0 (0) | 0 (0) | N/A |
| Infected or colonized roommate | 0 (0) | 0 (0) | N/A | 1 (1.4) | 0 (0) | .999 |
| Age, y | 55.6 (23.4) | 56.7 (26.6) | .75 | 49.9 (23.2) | 50.2 (28.4) | .94 |
| Charlson comorbidity index | 4.8 (4.1) | 5.6 (4.5) | .27 | 5.8 (5.6) | 3.9 (3.6) | .02 |
| Female | 44 (41.1) | 44 (44.1) | 1.00 | 20 (28.6) | 29 (41.4) | .11 |
| Malignancies | 17 (15.9) | 13 (12.2) | .43 | 13 (18.6) | 0 (0) | <.001 |
| Renal failure | 26 (24.3) | 26 (24.3) | 1.00 | 14 (20.0) | 12 (17.1) | .66 |
| Diabetes mellitus | 19 (17.8) | 18 (16.8) | .86 | 15 (21.4) | 12 (17.1) | .52 |
Categorical variables are number (%), with bivariate comparisons conducted using the χ2 test for independence or the Fisher exact test. Continuous variables are mean (standard deviation) with bivariate comparisons conducted using a 2-sample t test.
FIGURE 2.
Percent of patients exposed to infected prior bed occupants and roommates in controls versus cases having healthcare-associated infections with Staphylococcus aureus, Acinetobacter baumannii, Streptococcus pneumoniae, Pseudomonas aeruginosa, Klebsiella pneumoniae, Enterococcus faecalis, and Enterococcus faecium exposed to prior bed occupants and roommates infected or colonized with the same organism.
TABLE 2.
Association Between Healthcare-Associated Infection and Exposure to Infected or Colonized Prior Bed Occupants and Roommates
| Exposure | Odds Ratio (95% CI)a |
|---|---|
| Exposure to infected or colonized prior occupant | 5.83 (3.62–9.39) |
| Exposure to infected or colonized roommate | 4.82 (3.67–6.34) |
| Age, y | 1.00 (0.999–1.001) |
| Charlson comorbidity index | 1.04 (1.03–1.05) |
| Female | 1.00 (0.95–1.06) |
| Malignancies | 1.61 (1.48–1.76) |
| Renal failure | 1.50 (1.40–1.60) |
| Diabetes mellitus | 1.03 (0.96–1.11) |
NOTE. CI, confidence interval.
Results of multivariable logistic regression analysis.
In the K. pneumoniae subanalysis comparing antibiotic sensitivity of case isolates with roommate isolates, antibiogram data were available for 38 of 43 exposed case-roommate pairs. Among them, 22 pairs (58%) had identical susceptibility profiles. Notably, among the remaining 16 pairs, most (n =11, 69%) displayed additional antibiotic resistance in the case isolate, leaving open the possibility that resistance was acquired during the roommate’s course of treatment and that the more resistant isolate was passed on to the case. For prior room-occupant pairs, susceptibility data were available for 20 of 27 pairs and 11 of those (55%) had identical susceptibility profiles. Among the remaining 9 pairs, 6 (67%) displayed additional antibiotic resistance in the case isolate.
DISCUSSION
The long campaign toward improving patient safety and reducing preventable deaths in hospitals has had many successes.12,13 Still, too many Americans continue to die unnecessarily from infections they contract while in the hospital.4 The need to focus on prevention is ever more acute with the proliferation of multidrug resistant organisms and increasingly limited options for successful treatment.14
We now have strong evidence that interventions designed to improve environmental cleanliness do make a difference. Indeed, the first multicenter randomized controlled trial to determine the efficacy of enhanced terminal cleaning procedures for patients with multidrug-resistant organisms was recently published. This study of 9 hospitals showed a statistically significant decrease in organism acquisition when targeted cleaning methods—particularly ultraviolet light technology—were incorporated into the standard cleaning protocol, adding only 4 extra minutes to the total cleaning time.15 Although some roommate-to-roommate transmission may occur outside of the physical environment (eg, with healthcare workers serving as vectors), it is likely that at least some roommate transmission and all prior occupant transmission involves environmental reservoirs.
As the largest study to quantify the association between HAIs and exposure to infected or colonized previous bed occupants and roommates, encompassing data from all inpatient units in 4 acute-care hospitals and surveying exposure to 6 different organisms, our analysis serves to illustrate how many infections might be prevented by implementing enhanced cleaning measures. Previous studies showed mixed findings due to wide variations in sample size, study quality, design, patient population, and definitions of exposures and outcomes, though the majority did find statistically significant relationships between at least 1 of their exposures and outcomes of interest.16 Our findings reveal robust and statistically significant associations, with exposure to an infected or colonized prior bed occupant conferring a nearly 6-fold increase in the odds of infection, and exposure to an infected or colonized roommate conferring a nearly 5-fold increase. These results might actually underestimate the true association because, by limiting the retrospective period to the most likely period of exposure (3–5 days prior to infection), we captured only a portion of roommates and prior room occupants who could have been sources of exposure.11 Still, the prevalence of exposure as operationalized in this analysis was low, suggesting that the overall contribution of prior bed occupants and roommates defined in this way to the overall incidence of HAIs is relatively small.
The primary limitation of this study was the unavailability of molecular typing, which made it impossible to determine with certainty whether a case acquired a pathogen genetically identical to that of the roommate or prior occupant presumed to be the source of exposure. Nonetheless, our conclusion that there is a true chain of transmission from prior bed occupants and roommates remains plausible for 2 reasons. First, we performed a subanalysis to assess whether isolates were phenotypically similar regarding their susceptibility to a variety of antibiotic agents and still found statistically significant associations between prior bed occupant or roommate exposure and the development of HAIs. Furthermore, in most cases where antibiotic sensitivity did differ, resistance was more prevalent among the cases than among the prior occupants or roommates presumed sources of exposure. This finding supports the possibility that resistance could have been acquired during the roommate or prior occupant’s antibiotic therapy, with the resistant organism then passed to the case.17 Second, the epidemiological association we identified remained sizeable and highly significant even after controlling for several potential confounders. However, the association could be due to an unknown confounder that we were unable to identify or measure in this retrospective study. For example, certain rooms may be reserved for the highest-risk patients, meaning that patients assigned to such rooms could have a both a higher risk of exposure due to their room placement as well as a higher risk of infection due to their condition upon admission to the unit. Indeed, some statistically significant differences between cases and controls were observed at baseline regarding the comorbid conditions that affect infection risk. However, the associations remained robust in the multivariable model controlling for these variables, suggesting that confounding by factors related to patient severity was minimal if at all present. Finally, with the exception of unit-specific protocols in response to outbreaks, universal surveillance was not conducted at the study institutions, meaning that some colonization may have gone undetected.
The human and financial costs associated with HAIs are unacceptably high and may continue to grow along with antimicrobial resistance and the shortage of novel therapies on the immediate horizon.14 In light of mounting evidence that patients harboring pathogens do contaminate their hospital rooms,18 that current standards for cleaning and disinfection are not sufficient for decontamination,19 and that exposure to contaminated rooms confers a 5- to 6-fold increase in odds of infection, hospitals must take action by adopting proven methods for reducing environmental contamination.
Acknowledgments
Financial support: This project was supported by a grant from the National Institute of Nursing Research at the National Institutes of Health (grant no. R01 NR010822).
Footnotes
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.
References
- 1.Klevens RM, Edwards JR, Richards CL, et al. Estimating health care-associated infections and deaths in US hospitals, 2002. Public Health Rep. 2007;122:160–166. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott RD., II The direct medical costs of healthcare-associated infections in US hospitals and the benefits of prevention. [Accessed July 27, 2017];Centers for Disease Control and Prevention website. http://www.cdc.gov/hai/pdfs/hai/scott_costpaper.pdf. Published 2009.
- 3.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umscheid CA, Mitchell MD, Doshi JA, Agarwal R, Williams K, Brennan PJ. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol. 2011;32:101–114. doi: 10.1086/657912. [DOI] [PubMed] [Google Scholar]
- 5.Jeeva RR, Wright D. Healthcare-associated infections: a national patient safety problem and the coordinated response. Med Care. 2014;52:S4–S8. doi: 10.1097/MLR.0b013e3182a54581. [DOI] [PubMed] [Google Scholar]
- 6.Stone PW, Glied SA, McNair PD, et al. CMS changes in reimbursement for HAIs: setting a research agenda. Med Care. 2010;48:433–439. doi: 10.1097/MLR.0b013e3181d5fb3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ranji SR, Shetty K, Posley KA, et al. Technical Review, No. 9.6. Rockville, MD: Agency for Healthcare Research and Quality (US); 2007. Closing the quality gap: a critical analysis of quality improvement strategies (vol. 6, Prevention of healthcare–associated infections) [PubMed] [Google Scholar]
- 8.Dancer SJ. The role of environmental cleaning in the control of hospital-acquired infection. J Hosp Infect. 2009;73:378–385. doi: 10.1016/j.jhin.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 9.Boyce JM. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob Resist Infect Control. 2016;5:10. doi: 10.1186/s13756-016-0111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apte M, Neidell M, Furuya EY, Caplan D, Glied S, Larson E. Using electronically available inpatient hospital data for research. Clin Transl Sci. 2011;4:338–345. doi: 10.1111/j.1752-8062.2011.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. [Accessed January 24, 2018];National Academy Press website. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2001/Crossing-the-Quality-Chasm/Quality%20Chasm%202001%20%20report%20brief.pdf. Published 2001.
- 13.Wang Y, Eldridge N, Metersky ML, et al. National trends in patient safety for four common conditions, 2005–2011. N Engl J Med. 2014;370:341–351. doi: 10.1056/NEJMsa1300991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bassetti M, Merelli M, Temperoni C, Astilean A. New antibiotics for bad bugs: where are we? Ann Clin Microbiol Antimicrob. 2013;12:22. doi: 10.1186/1476-0711-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson DJ, Chen LF, Weber DJ, et al. Enhanced terminal room disinfection and acquisition and infection caused by multidrug-resistant organisms and Clostridium difficile (the Benefits of Enhanced Terminal Room Disinfection study): a cluster-randomised, multicentre, crossover study. Lancet. 2017;389:805–814. doi: 10.1016/S0140-6736(16)31588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen B, Cohen CC, Loyland B, Larson EL. Transmission of health care-associated infections from roommates and prior room occupants: a systematic review. Clin Epidemiol. 2017;9:297–310. doi: 10.2147/CLEP.S124382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freedberg DE, Salmaian H, Cohen B, Abrams JA, Larson EL. Receipt of antibiotics in hospitalized patients and risk for Clostridium difficile infection in subsequent patients who occupy the same bed. JAMA Intern Med. 2016;176:1801–1808. doi: 10.1001/jamainternmed.2016.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russotto V, Cortegiani A, Raineri SM, Giarratano A. Bacterial contamination of inanimate surfaces and equipment in the intensive care unit. J Intensive Care. 2015;3:54. doi: 10.1186/s40560-015-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siani H, Maillard JY. Best practice in healthcare environmental decontamination. Eur J Clin Microbiol Infect Dis. 2015;34:1–11. doi: 10.1007/s10096-014-2205-9. [DOI] [PubMed] [Google Scholar]