Abstract
Over time, risk assessment has shifted from establishing relationships between exposure to a single chemical and a resulting adverse health outcome, to evaluation of multiple chemicals and disease outcomes simultaneously. As a result, there is an increasing need to better understand the complex mechanisms that influence risk of chemical and non-chemical stressors, beginning at their source and ending at a biological endpoint relevant to human or ecosystem health risk assessment. Just as the Adverse Outcome Pathway (AOP) framework has emerged as a means of providing insight into mechanism-based toxicity, the exposure science community has seen the recent introduction of the Aggregate Exposure Pathway (AEP) framework. AEPs aid in making exposure data applicable to the FAIR (i.e., findable, accessible, interoperable, and reusable) principle, especially by (1) organizing continuous flow of disjointed exposure information;(2) identifying data gaps, to focus resources on acquiring the most relevant data; (3) optimizing use and repurposing of existing exposure data; and (4) facilitating interoperability among predictive models. Herein, we discuss integration of the AOP and AEP frameworks and how such integration can improve confidence in both traditional and cumulative risk assessment approaches.
Keywords: Aggregate Exposure Pathway, risk assessment, Adverse Outcome Pathway
A New Direction and New Challenges for Risk Assessment
Advances in analytical methods and predictive models have contributed to a rapid evolution in the fields of exposure science, toxicology, and epidemiology. The resulting increase in the rate, scope, and magnitude of data generation within these fields has shifted the focus of risk assessment from observing apical responses to understanding how chemical or non-chemical stressors interact with biological receptors that, when perturbed, lead to apical responses [1]. The risk assessment community is provided with the opportunity to not only investigate causal relationships between a single stressor and a single disease, but also to systematically identify multiple stressors and biological mechanisms that contribute to a common disease or that impact various species. This new opportunity also presents new challenges, such as the organization of large, disjointed streams of information from various disciplines into coherent knowledge able to support risk assessment. Frameworks are essential in addressing this particular challenge, as they provide a generic scaffold to aid in the acquisition, organization, integration, harmonization, and application of data.
Frameworks for Toxicological and Exposure Sciences
The field of toxicity has seen numerous benefits arising from the widespread acceptance and broad applicability of science-based organizing frameworks, such as the Mode of Action (MOA) and the Adverse Outcome Pathway (AOP). The widely accepted MOA framework uses chemical-specific toxicity information to describe a biologically plausible series of key events leading to an effect, for supporting human health risk assessment [2,3]. More recently, the AOP framework has emerged as a mechanism for organizing chemical-independent knowledge and data through a series of causally connected biological key events, thereby allowing for a mechanism-based evaluation of human and ecological health risks arising from chemical exposure [4–6]. Organizing frameworks such as AOPs have led to advances in experimental design and identification of data gaps and research needs, in addition to benefiting such applications as high-throughput toxicity testing and chemical-specific MOA analyses.
The field of exposure science has also evolved to a point at which it is poised to reap similar benefits from a complementary framework capable of more efficient acquisition, organization, and interpretation of exposure knowledge, data, and predictions. To meet these growing needs, the Aggregate Exposure Pathway (AEP) framework was proposed as a means to assemble existing knowledge of exposure, spanning from a stressor’s origin to its concentration at a site of action [7]. AEPs share several key features with AOPs. Specifically, (1) development of AOPs and AEPs proceeds in an incremental manner, thus allowing both frameworks to continuously evolve as new data become available or new information is discovered; (2) both frameworks are general constructs intended to support qualitative and quantitative uses of their contained information; and (3) individual AOPs and AEPs are themselves modular constructs, and can be incorporated into larger networks to model complex systems.
The structure and terminology of AEPs were proposed to mirror those same aspects of AOPs, in order to provide a foundation for seamless integration and interoperability between the two frameworks. The two basic components in an AOP are the key event (KE), representing a measureable change in a biological state, and the key event relationship (KER), representing the causal relationship between an upstream and a downstream KE [8]. Both components are supported by a weight of evidence approach to increase consistency and confidence in potential applications of AOPs. In an AEP, these are mirrored by a key exposure state (KES), defined as the state of a stressor in space and time, and the key transitional relationship (KTR), which is either the transport of a stressor across media within one AEP or the transformation of one stressor to another involving two AEPs [7]. An AOP has two specialized KEs, a MIE and an adverse outcome (AO). Similarly, an AEP also has two specialized KESs; these are a source and a target site exposure (TSE), defined as the state of a stressor at its origin and at a site of action, respectively.
Terminating an AEP at the TSE allows for an intuitive integration of the AEP and the AOP frameworks. A TSE describes the state of a stressor at a target site that corresponds to a potential MIE, and an MIE represents the initial interaction between a stressor and a molecular target that can be causally linked to an AO [9]. Placing this integration in a risk assessment context, (1) the AOP framework organizes key biological processes underlying toxicity and can guide selection of experimental testing (e.g., in vitro or in vivo) and non-testing (e.g., in silico) approaches for hazard identification; (2) the AEP framework organizes available exposure information to facilitate predictions of exposures at relevant sites of action; and (3) dose-response analysis provides the effective concentration at which a change in a biological response becomes significant. Thus, integrating the AEP and the AOP frameworks with dose-response analyses allows various pieces of information to be pieced together based on a more holistic understanding of the fate, transport, and pharmacokinetic processes that move a stressor from its source to potential TSE(s), and subsequently, the potential to assess health impacts of real-world exposure scenarios that result in specific AOs.
Integrating the AEP and the AOP Frameworks for Risk Assessment
As risk assessment moves towards an effort to better understand how multiple chemical and non-chemical stressors interact with multiple biological pathways that lead to or modify a common apical outcome [10], information organized in the AEP and the AOP frameworks can be integrated to address risk queries in a comprehensive, efficient, and effective manner. Coupling the AEP and AOP frameworks can provide a better context for interpreting dose-response data from high-throughput toxicity testing for individual chemicals. In addition, the AEP framework provides a consistent mechanism for integrating exposure information to more accurately consider multiple chemicals in a cumulative risk assessment.
Toxicity testing is increasingly conducted via high throughput (HT) in vitro tests that monitor molecular or cellular perturbations that eventually lead to a designated risk-based endpoint (e.g., general liver toxicity or, specifically, steatosis) [11,12]. The AOP framework was developed to connect the MIE(s) used for HT toxicity testing to a respective endpoint [13]. In an analogous manner, specific TSEs can connect the original source of a chemical or non-chemical stressor, or measures other than the source indicating the presence of that stressor in the environment, to its concentration at the site corresponding to the MIE. For stressors that do not, or are unable to, have their concentrations measured at the target site, other exposure information in their AEPs may be used to predict the TSEs using appropriate models. If TSEs cannot be estimated/measured or if an AEP does not exist for a stressor, data gaps can be identified, and exposure assessment strategies can then be developed for these stressors.
Recent efforts have been made to translate biologically-effective in vitro concentrations into in vivo exposures [14–16], and in vitro concentrations might be better represented in an intracellular manner [17–20]. AEPs organize exposure information within different media and across different testing systems, and thus they can support the development of modeling tools to convert these in vitro concentrations to relevant TSEs [14,21–25]. For example, in vitro dose-response data can be used to predict in vivo responses using physiologically based pharmacokinetic (PBPK) and pharmacodynamic models [26]. These predictive models, together with read-across approaches [27], can be used to estimate in vivo effects based on in vitro perturbations or in silico predictions for a large number of data-poor chemicals.
Recently, the Integrated Approaches to Testing and Assessment (IATA) was proposed to interpret results from novel test methods and models, to facilitate their application in public health decision making [13]. IATA can serve as a flexible and suitable tool for decision making, by integrating information needed to support the decision in a systematic manner [28]. IATA includes newly developed in vitro systems and measurement technologies (e.g., HT screening and high content imaging), as well as computational approaches, such as quantitative structure activity relationships and PBPK models [29]. The AOP framework has been adopted as the preferred mechanism for integrating toxicity data needed for hazard characterization as part of IATA [28], and AEPs could serve a similar role in organizing exposure information. IATA approaches also can provide insight into a more reliable and comprehensive exposure-based risk assessment.
The paradigm shift in risk assessment towards mechanism-based IATA approaches for observation of apical responses, and away from a full battery of animal tests, fundamentally compels an increased emphasis on exposure. The authors of the National Academy of Sciences’ report regarding toxicity testing in the 21st Century called for the consideration of exposure information at each step of the toxicity testing process [12]. The type and degree of necessary toxicological information can be resolved by beginning risk assessment with exposure characterization, thus preventing a waste of resources and collection of nonessential toxicological information that provides no improvement in the ability to manage risk [30,31]. Realization of such an exposure-driven approach, of course, requires access to the more comprehensive exposure information emerging from new tools and approaches in exposure science [32].
Cumulative risk assessment (CRA) involves determination of potential additive effects arising from exposure to multiple stressors that have a common mechanism of action. CRA is often conducted in a hazard-based manner using the highest estimated exposure concentrations, in order to be health protective. However, the likelihood of real-world exposure scenarios in which all chemicals are present at such high concentrations drops precipitously as more chemicals are included in the risk assessment. AEPs could help in determining likely co-exposure scenarios, both by making existing data simple to find and by enabling more sophisticated modeling approaches that incorporate extra available exposure information.
Networks of AEPs can be formed as chemical mixtures are sampled within the same environmental or biological media. While compiling such co-exposure information in a wide variety of media is certainly a herculean task for any individual investigator, the AEP framework can allow for incremental development of AEPs such a network through crowdsourcing efforts by the exposure community. As the co-exposure network continues to evolve by including data obtained from more media types containing similar chemicals, the TSEs of these stressors are less likely to be estimated in isolation, and confidence in co-exposure of chemical candidates can be greatly improved.
In addition to a network that connects KESs from individual AEPs via shared media (e.g., multiple endocrine disrupting compounds measured within the same riverine water sample), another type of network uses KTRs to connect the AEP of a parent chemical with AEPs of its degradation and biotransformation products. Such a network of AEPs can be used to investigate potential health risks related to the introduction of a parent chemical triggering a single MIE, to multiple MIEs linked to multiple AOPs with similar AOs, or to multiple MIEs linked to multiple AOPs with different AOs (Fig. 1). A similar type of network connects an AEP for a shared degradate/metabolite to several AEPs for its parent precursors (Fig. 2). One such an example is acetaldehyde, which exists on its own in the environment and as an industrial product, is produced endogenously, and is generated through metabolism of ethyl acetate, vinyl acetate, and ethanol [33]. In this type of AEP network, exposure information organized for the shared metabolite and multiple parent precursors not only allows for health risks to be evaluated by accounting for co-exposure scenarios for these parents, but also can be used to prioritize source management or mitigation efforts. For example, if all possible parents of the shared metabolite acetaldehyde can be measured in air, and it is found that vinyl acetate exists at a higher concentration than all other parents, then it can be assumed that reducing levels of vinyl acetate may lower acetaldehyde air concentrations.
Figure 1.
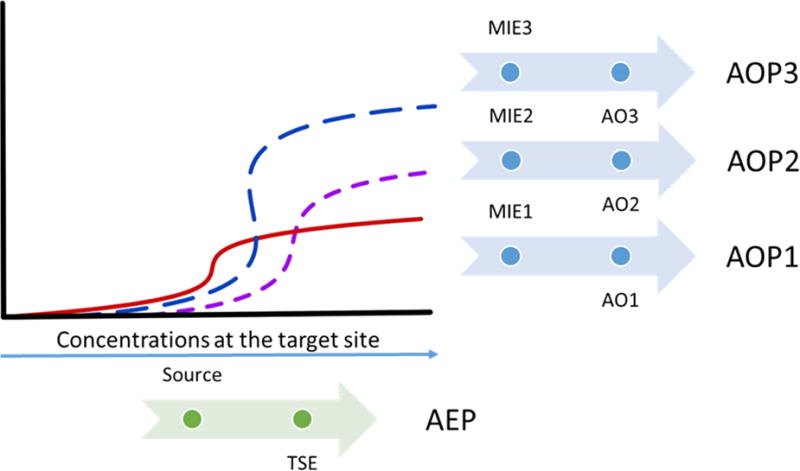
Integration of dose-response data with multiple Adverse Outcome Pathways (AOPs) and one single Aggregate Exposure Pathway (AEP). The concentration of one stressor at its target site of action (i.e., target site exposure; TSE) can potentially link to several molecular initiating events (MIEs) that lead to different adverse outcomes (AOs) upon perturbation of the molecular targets, provided the TSE is sufficient to eliciting a specific MIE.
Figure 2.
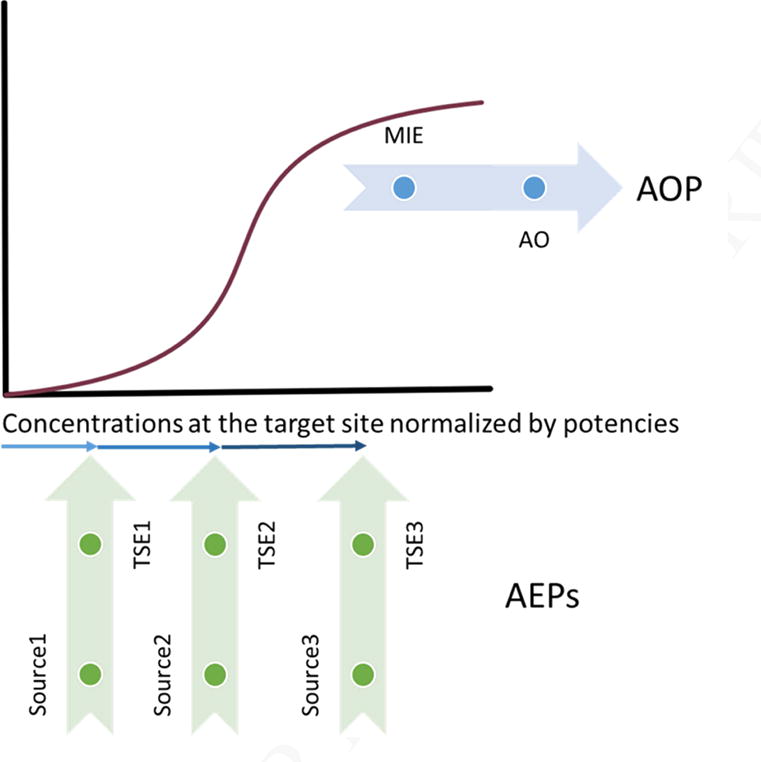
Integration of dose-response data with one Adverse Outcome Pathway (AOP) and multiple Aggregate Exposure Pathways (AEPs). The concentrations of multiple stressors at the same target site (i.e., target site exposures; TSEs) can lead to the activation of the same molecular initiating event (MIE) and result in one specific adverse outcome (AO). This feature is especially of interest in cumulative risk assessment because the concentration of one stressor alone may be insufficient to trigger the MIE, but addition of TSEs from multiple stressors may be sufficient.
The inherent nature of an AEP, in organizing exposure information for a stressor in environmental, biological, and manufactured media, also allows for a natural integration among humans and ecosystems. Such integration offers the opportunities to investigate how human activities (e.g., land management and development) impact the natural functioning of ecosystems, as well as how both humans and wildlife affect each other in a reciprocal manner. Combined with the AOP framework, it is possible to further explore how the effects caused by one stressor may exacerbate effects of other stressors or on another species. For example, larval settlement and growth of a benthic polychaete was shown to be significantly reduced in hypoxic waters when compared to normoxic conditions with similar concentrations of polybrominated diphenyl ethers, despite the ability to adapt to heavily polluted environments [34]. Perhaps most difficult to capture in the AEP and the AOP frameworks are “indirect effects”, such as loss of food resources or habitat structure, since these effects are only implicitly embedded in stressor concentrations or related to AOs. In this context, integrating AEPs and AOPs to fully encompass ecological risk assessment is not trivial, but can have substantial influences on ecological health.
Finally, temporal variation embedded into the KESs of specific AEPs could allow the exposome and epidemiological analyses to meet their critical need for investigating changes in exposure and resulting impacts (e.g., epigenetic alterations, disease development and progression). The exposome was proposed as a means for identifying risk factors that could lead to disease in humans, by considering all possible exposures (e.g., endogenous hormones, gut microbiota, lifestyle, psychological stress, chemicals) for an individual from conception to death through exposomics applications [35]. This definition of the exposome reflects the concept of integrating the AEP and the AOP frameworks, which elucidates the linkage between exposure, affected biological processes, and resulting diseases so that confidence might be improved in the exposure-disease relationships informed by exposome research.
Conclusions
The examples presented in this work only just begin to touch upon the many applications potentially offered by the AEP framework. The AEP framework is not meant to act as a new direction for exposure science or a new modeling tool for risk assessment, but rather provides a standard mechanism for organizing exposure data. Such organization is critical for exposure science, where data is derived from separate sub-disciplines, so that information may be more easily incorporated into existing risk assessment methods. Exposure information organized within AEPs provides a wide range of exposure metrics that are able to link to available toxicity information or to identify data gaps, for use in various risk assessment applications. For example, although emphasis is often placed on TSEs at a molecular or cellular level, TSEs can also be defined in the context of epidemiology findings that consider exposure at the level of individuals. Assembly of AEPs into AEP networks naturally integrates information across multiple chemicals and exposure media, thereby promoting more realistic estimates of exposures in cumulative risk assessment. The unique aspects that AEPs provide in support of various resources stand to aid in advancing of 21st century exposure science and risk assessment strategies.
Acknowledgments
The authors would like to thank several internal U.S. EPA reviewers for their comments on a draft of this manuscript. Special thanks to the 2016 AEP workshop attendees. Funding for Dr. Leonard was provided by the Oak Ridge Institute for Science and Education Research Participation Program at the US EPA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Disclaimer: The U.S. Environmental Protection Agency has provided administrative review and has approved this paper for publication. The views expressed in this paper are those of the authors and do not necessarily reflect the views of the U.S. Environmental Protection Agency.
References
- 1.NRC. Using 21st Century Science to Improve Risk-Related Evaluations. National Academies Press (US); 2017. https://www.ncbi.nlm.nih.gov/books/NBK424987/ accessed April 4, 2017. [PubMed] [Google Scholar]
- 2.Boobis AR, Doe JE, Heinrich-Hirsch B, Meek MEB, Munn S, Ruchirawat M, Schlatter J, Seed J, Vickers C. IPCS framework for analyzing the relevance of a noncancer mode of action for humans. Crit Rev Toxicol. 2008;38:87–96. doi: 10.1080/10408440701749421. [DOI] [PubMed] [Google Scholar]
- 3*.Meek ME(Bette), Palermo CM, Bachman AN, North CM, Lewis R Jeffrey. Mode of action human relevance (species concordance) framework: Evolution of the Bradford Hill considerations and comparative analysis of weight of evidence. J Appl Toxicol. 2014;34:595–606. doi: 10.1002/jat.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4**.Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrrano JA, Tietge JE, Villeneuve DL. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 2010;29:730–741. doi: 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- 5.Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, Landesmann B, Lettieri T, Munn S, Nepelska M, Ottinger MA, Vergauwen L, Whelan M. Adverse outcome pathway (AOP) development I: strategies and principles. Toxicol Sci. 2014;142:312–320. doi: 10.1093/toxsci/kfu199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, Landesmann B, Lettieri T, Munn S, Nepelska M, Ottinger MA, Vergauwen L, Whelan M. Adverse Outcome Pathway Development II: Best Practices. Toxicol Sci. 2014;142:321–330. doi: 10.1093/toxsci/kfu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7**.Teeguarden JG, Tan Y-M, Edwards SW, Leonard JA, Anderson KA, Corley RA, Kile ML, Simonich SM, Stone D, Tanguay RL, Waters KM, Harper SL, Williams DE. Completing the Link between Exposure Science and Toxicology for Improved Environmental Health Decision Making: The Aggregate Exposure Pathway Framework. Environ Sci Technol. 2016;50:4579–4586. doi: 10.1021/acs.est.5b05311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.OECD. Guidance document on developing and assessing Adverse Outcome Pathways. 2013 http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2013)6&doclanguage=en.
- 9.Allen TEH, Goodman JM, Gutsell S, Russell PJ. Defining Molecular Initiating Events in the Adverse Outcome Pathway Framework for Risk Assessment. Chem Res Toxicol. 2014;27:2100–2112. doi: 10.1021/tx500345j. [DOI] [PubMed] [Google Scholar]
- 10.Moretto A, Bachman A, Boobis A, Solomon KR, Pastoor TP, Wilks MF, Embry MR. A framework for cumulative risk assessment in the 21st century. Critical Reviews in Toxicology. 2017;47:85–97. doi: 10.1080/10408444.2016.1211618. [DOI] [PubMed] [Google Scholar]
- 11.Andersen ME, Krewski D. Toxicity Testing in the 21st Century: Bringing the Vision to Life. Toxicol Sci. 2009;107:324–330. doi: 10.1093/toxsci/kfn255. [DOI] [PubMed] [Google Scholar]
- 12.Krewski *D, Acosta D, Andersen M, Anderson H, Bailar JC, Boekelheide K, Brent R, Charnley G, Cheung VG, Green S, Kelsey KT, Kerkvliet NI, Li AA, McCray L, Meyer O, Patterson RD, Pennie W, Scala RA, Solomon GM, Stephens M, Yager J, Zeise L. Toxicity testing in the 21st century: a vision and a strategy. J Toxicol Environ Health B Crit Rev. 2010;13:51–138. doi: 10.1080/10937404.2010.483176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tollefsen KE, Scholz S, Cronin MT, Edwards SW, de Knecht J, Crofton K, Garcia-Reyero N, Hartung T, Worth A, Patlewicz G. Applying Adverse Outcome Pathways (AOPs) to support Integrated Approaches to Testing and Assessment (IATA) Regul Toxicol Pharm. 2014;70:629–640. doi: 10.1016/j.yrtph.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Wetmore BA, Wambaugh JF, Ferguson SS, Sochaski MA, Rotroff DM, Freeman K, Clewell HJ, Dix DJ, Andersen ME, Houck KA, Allen B, Judson RS, Singh R, Kavlock RJ, Richard AM, Thomas RS. Integration of Dosimetry, Exposure, and High-Throughput Screening Data in Chemical Toxicity Assessment. Toxicol Sci. 2012;125:157–174. doi: 10.1093/toxsci/kfr254. [DOI] [PubMed] [Google Scholar]
- 15.Wetmore BA, Wambaugh JF, Ferguson SS, Li L, Clewell HJ, Judson RS, Freeman K, Bao W, Sochaski MA, Chu TM, Black MB, Healy E, Allen B, Andersen ME, Wolfinger RD, Thomas RS. Relative Impact of Incorporating Pharmacokinetics on Predicting In Vivo Hazard and Mode of Action from High-Throughput In Vitro Toxicity Assays. Toxicological Sciences. 2013;132:327–346. doi: 10.1093/toxsci/kft012. [DOI] [PubMed] [Google Scholar]
- 16.Wetmore BA. Quantitative in vitro-to-in vivo extrapolation in a high-throughput environment. Toxicology. 2015;332:94–101. doi: 10.1016/j.tox.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Armitage JM, Wania F, Arnot JA. Application of Mass Balance Models and the Chemical Activity Concept To Facilitate the Use of in Vitro Toxicity Data for Risk Assessment. Environmental Science & Technology. 2014;48:9770–9779. doi: 10.1021/es501955g. [DOI] [PubMed] [Google Scholar]
- 18.Fischer FC, Henneberger L, König M, Bittermann K, Linden L, Goss KU, Escher BI. Modeling Exposure in the Tox21 in Vitro Bioassays. Chemical Research in Toxicology. 2017;30:1197–1208. doi: 10.1021/acs.chemrestox.7b00023. [DOI] [PubMed] [Google Scholar]
- 19.Graepel R, Lamon L, Asturiol D, Berggren E, Joossens E, Paini A, Prieto P, Whelan M, Worth A. The virtual cell based assay: Current status and future perspectives. Toxicology in Vitro. 2017;45:258–267. doi: 10.1016/j.tiv.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paini A, Sala Benito JV, Bessems J, Worth AP. From in vitro to in vivo: Integration of the virtual cell based assay with physiologically based kinetic modelling. Toxicology in Vitro. 2017;45:241–248. doi: 10.1016/j.tiv.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Judson R, Houck K, Martin M, Knudsen T, Thomas RS, Sipes N, Shah I, Wambaugh J, Crofton K. In Vitro and Modelling Approaches to Risk Assessment from the U.S. Environmental Protection Agency ToxCast Programme. Basic & Clinical Pharmacology & Toxicology. 2014;115:69–76. doi: 10.1111/bcpt.12239. [DOI] [PubMed] [Google Scholar]
- 22.Leonard JA, Tan YM, Gilbert M, Isaacs K, El-Masri H. Estimating Margin of Exposure to Thyroid Peroxidase Inhibitors Using High-Throughput in vitro Data, High-Throughput Exposure Modeling, and Physiologically Based Pharmacokinetic/Pharmacodynamic Modeling. Toxicol Sci. 2016;151:57–70. doi: 10.1093/toxsci/kfw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin HM, Ernstoff A, Arnot JA, Wetmore BA, Csiszar SA, Fantke P, Zhang X, McKone TE, Jolliet O, Bennett DH. Risk-Based High-Throughput Chemical Screening and Prioritization using Exposure Models and in Vitro Bioactivity Assays. Environmental Science & Technology. 2015;49:6760–6771. doi: 10.1021/acs.est.5b00498. [DOI] [PubMed] [Google Scholar]
- 24.Wambaugh JF, Setzer RW, Reif DM, Gangwal S, Mitchell-Blackwood J, Arnot JA, Joliet O, Frame A, Rabinowitz J, Knudsen TB, Judson RS, Egeghy P, Vallero D, Cohen Hubal EA. High-Throughput Models for Exposure-Based Chemical Prioritization in the ExpoCast Project. Environmental Science & Technology. 2013 doi: 10.1021/es400482g. 130711145716006. [DOI] [PubMed] [Google Scholar]
- 25.Sipes NS, Wambaugh JF, Pearce R, Auerbach SS, Wetmore BA, Hsieh JH, Shapiro AJ, Svoboda D, DeVito MJ, Ferguson SS. An Intuitive Approach for Predicting Potential Human Health Risk with the Tox21 10k Library. Environmental Science & Technology. 2017;51:10786–10796. doi: 10.1021/acs.est.7b00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gajewska M, Worth A, Urani C, Briesen H, Schramm KW. The acute effects of daily nicotine intake on heart rate – A toxicokinetic and toxicodynamic modelling study. Regulatory Toxicology and Pharmacology. 2014;70:312–324. doi: 10.1016/j.yrtph.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Patlewicz G. Read-across approaches - misconceptions, promises and challenges ahead. ALTEX. 2014;31:387–396. doi: 10.14573/altex.1410071. [DOI] [PubMed] [Google Scholar]
- 28.Organization for Economic Co-operation and Development. Guidance Document on the Reporting of Defined Approaches to be Used Within Integrated Approaches to Testing and Assessment. Paris, France: n.d http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2016)28&doclanguage=en (accessed December 11, 2017) [Google Scholar]
- 29.Worth AP, Patlewicz G. Integrated Approaches to Testing and Assessment. In: Eskes C, Whelan M, editors. Validation of Alternative Methods for Toxicity Testing. Springer International Publishing, Cham; 2016. pp. 317–342. http://link.springer.com/10.1007/978-3-319-33826-2_13 (accessed December 11, 2017) [DOI] [PubMed] [Google Scholar]
- 30.Doull J, Borzelleca JF, Becker R, Daston G, DeSesso J, Fan A, Fenner-Crisp P, Holsapple M, Holson J, Llewellyn GCraig, MacGregor J, Seed J, Walls I, Woo Y, Olin S. Framework for use of toxicity screening tools in context-based decision-making. Food and Chemical Toxicology. 2007;45:759–796. doi: 10.1016/j.fct.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 31*.Pastoor TP, Bachman AN, Bell DR, Cohen SM, Dellarco M, Dewhurst IC, Doe JE, Doerrer NG, Embry MR, Hines RN, Moretto A, Phillips RD, Rowlands JC, Tanir JY, Wolf DC, Boobis AR. A 21st century roadmap for human health risk assessment. Critical Reviews in Toxicology. 2014;44:1–5. doi: 10.3109/10408444.2014.931923. [DOI] [PubMed] [Google Scholar]
- 32.NAS. Using 21st Century Science to Improve Risk-Related Evaluations. National Academies of Sciences, Engineering, and Medicine; Division on Earth and Life Studies; Board on Environmental Studies and Toxicology; Committee on Incorporating 21st Century Science into Risk-Based Evaluations Using 21st Century Science to Improve Risk-Related Evaluations., National Academies Press; Washington, D.C: 2017. Advances in Exposure Science. https://www.ncbi.nlm.nih.gov/books/NBK424978/ (accessed September 11, 2017) [PubMed] [Google Scholar]
- 33.United States Environmental Protection Agency. Health Assessment Document for Acetaldehyde. Washington, DC, USA: 1987. https://nepis.epa.gov/Exe/ZyNET.exe/30001FBF.txt?ZyActionD=ZyDocument&Client=EPA&Index=1986%20Thru%201990&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&UseQField=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5CZYFILES%5CINDEX%20DATA%5C86THRU90%5CTXT%5C00000000%5C30001FBF.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1 (accessed December 11, 2017) [Google Scholar]
- 34.Shin PKS, Gopalakrishnan S, Chan AKY, Qian PY, Wu RSS. Interactive effects of hypoxia and PBDE on larval settlement of a marine benthic polychaete. Marine Pollution Bulletin. 2014;85:425–432. doi: 10.1016/j.marpolbul.2014.04.037. [DOI] [PubMed] [Google Scholar]
- 35.Wild CP. Complementing the Genome with an “Exposome”: The Outstanding Challenge of Environmental Exposure Measurement in Molecular Epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14:1847–1850. doi: 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]


