Abstract
Objectives
The citrullinating enzyme peptidylarginine deminase type 4 (PAD4) is the target of a polyclonal group of autoantibodies in patients with rheumatoid arthritis (RA). A subgroup of such antibodies, initially identified by cross-reactivity with PAD3, is strongly associated with progression of radiographic joint damage and interstitial lung disease, and has the unique ability to activate PAD4. The features of these antibodies in terms of their T cell-dependent origin, genetic characteristics, and effect of individual antibody specificities on PAD4 function remain to be defined.
Methods
We used PAD4-tagged with the monomeric fluorescent protein mWasabi to isolate PAD4-specific memory B cells from anti-PAD4 positive patients with RA and applied single cell cloning technologies to obtain monoclonal antibodies.
Results
Among 44 single B cells, we cloned five antibodies with PAD4-activating properties. Sequence analysis, germline reversion experiments, and antigen specificity assays suggested that autoantibodies to PAD4 are not polyreactive, and arise from PAD4-reactive precursors. Somatic mutations increase the agonistic activity of these antibodies at low calcium concentrations by facilitating their interaction with structural epitopes that modulate calcium-binding site 5 in PAD4.
Conclusions
PAD4-activating antibodies directly amplify a key process in disease pathogenesis, making them unique among other autoantibodies in RA. Understanding the molecular basis for their functionality may inform the design of future PAD4 inhibitors.
Keywords: Rheumatoid Arthritis, peptidylarginine deiminase 4, autoantibody, monoclonal antibody, citrullination
Introduction
The study of monoclonal antibodies derived from patients with autoimmune diseases has provided important insights to understanding the origin of the humoral response against self-antigens1–5 and mechanisms of autoantibody pathogenicity.6–8 While autoantibodies in rheumatic diseases were first described in rheumatoid arthritis (RA),9 the molecular characterization of RA-specific antibodies lagged significantly behind other diseases. The discovery that citrullinated proteins are major targets of the humoral response in RA renewed interest on further characterizing the autoantibody repertoire in this disease.3,10–14
Citrullinated proteins are generated post-translationally by the peptidylarginine deiminases (PADs), a family of five calcium-dependent enzymes (termed PAD1–4 and 6) that convert arginine residues to citrulline residues.15 At least two major groups of autoantibodies have been linked to the pathogenic role of citrullination in RA: 1) anti-citrullinated protein antibodies (ACPAs), which likely drive effector functions by forming immune complexes16 and 2) anti-PAD4 antibodies that assist the production of citrullinated antigens by enhancing the activity of PAD4.17 Antibody-mediated PAD4 activation is driven by a subgroup of anti-PAD4 antibodies initially recognized by their cross-reactivity with PAD3.17 These PAD3/PAD4 cross-reactive antibodies (anti-PAD3/4XR) enhance PAD4 activity by increasing the sensitivity of the enzyme to calcium. Together, ACPAs and antibodies to PAD4 may create a feed-forward loop that promotes effector functions via immune complex formation and autoantigen production.
Initial studies on disease-specific monoclonal antibodies from patients with RA have been centered on ACPAs.3,11–14,18 A major conclusion from these studies is that ACPAs may originate from polyreactive precursors with their specificities generated as result of antigen-driven affinity maturation.3,11,18 To gain further mechanistic insights into the development and function of the anti-PAD4 autoantibody repertoire, we determined the molecular features and antigen binding properties of monoclonal antibodies to PAD4 cloned from memory B cells of patients with RA.
Materials and Methods
Patients
All samples were collected after obtaining signed informed consent in accordance with the Johns Hopkins Institutional Review Board. Patients with RA were recruited from the Johns Hopkins Arthritis Center and screened for anti-PAD4 and anti-PAD3/4XR antibodies in serum by immunoprecipitation (IP) as previously described. 17 Peripheral blood anti-PAD4 single memory B cells were purified from three patients who had with high titer anti-PAD4 and anti-PAD3/4XR antibodies, and used to generate monoclonal antibodies. Detailed descriptions are available in the online supplementary methods.
Results
Sequence analysis of anti-PAD4 antibodies cloned from single memory B cells
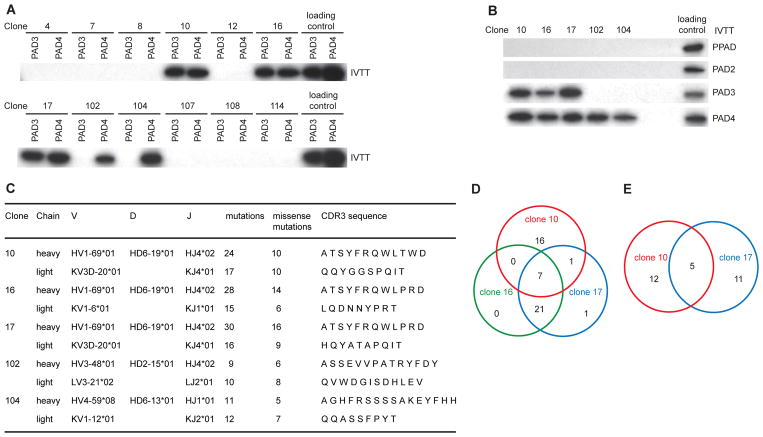
We isolated single anti-PAD4 memory B cells by flow cytometry using recombinant PAD4 tagged with the monomeric fluorescent protein mWasabi (Fig. S1). In total, 44 single memory B cells with high binding to mWasabi-PAD4 were collected from three patients with RA. Heavy chain (IgH) and corresponding light chain (IgL) variable gene sequences were amplified from 32 cells and used to generate IgG1 monoclonal antibodies. The antibodies were screened by immunoprecipitation (IP) (Fig. 1A), identifying five monoclonal antibodies with specificity to PAD4. Clones 10, 16 and 17 derived from patient #1 were cross-reactive with PAD3, while clones 102 and 104 from patient #3 only recognized PAD4 (anti-PAD4-only antibodies). No antibodies to PAD4 were isolated from patient #2. None of the anti-PAD4 antibodies recognized PAD2 or the PAD from Porphyromonas gingivalis (PPAD) (Fig. 1B).
Fig. 1.
Characterization of anti-PAD4 monoclonal antibodies derived from RA memory B cells. (A) Monoclonal antibodies with reactivity to PAD3/4 or PAD4 were screened by IP using [35S]methionine-labeled PAD3 and PAD4 as antigens. Representative data from 12 of 32 monoclonal antibodies are shown. (B) IP of radiolabelled P. gingivalis PAD (PPAD), PAD2, PAD3 and PAD4 using anti-PAD3/4XR and anti-PAD4-only monoclonal antibodies (3.4 nM antibody per IP assay). Radiolabelled products directly resolved by SDS–PAGE are shown to demonstrate loading. (C) Ig gene usage, mutation number and CDR3 amino acid sequences of monoclonal antibodies to PAD3/4 (clones 10, 16 and 17) and antibodies with reactivity only to PAD4 (clones 102 and 104). (D and E) Venn diagrams show the number of SHM shared by clones 10, 16 and 17 in the heavy chain (D) and by clones 10 and 17 in the light chain (E). IPs were performed on 2 different occasions with similar results.
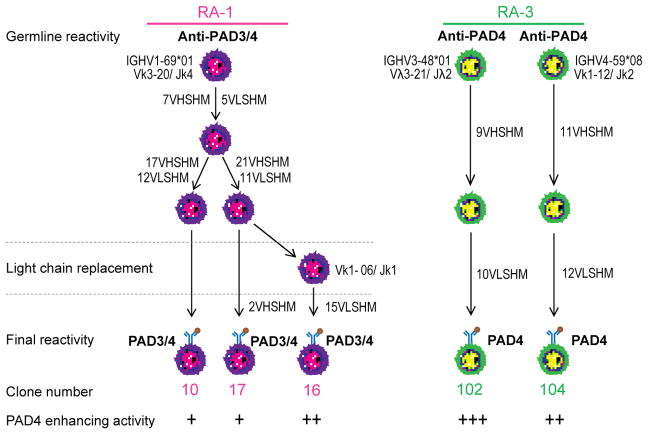
Based on the IgH V-D-J usage, complementarity-determining region 3 (CDR3) sequence, CDR3 length and the presence of common mutations, monoclonals 10, 16 and 17 were determined to be clonally related (Fig. 1C). The number of somatic hypermutations (SHM) shared by the IgH of antibodies 10, 16 and 17 are shown in Figure 1D. Clones 16 and 17 had the highest similarity with 28 common mutations. Antibody 10 showed 24 mutations, 7 of which were shared by clones 16 and 17. Clones 10 and 17 also shared a related Vκ gene rearrangement (IGKV3D-20/IGKJ4), similar CDR3 sequence, and 5 mutations (Fig. 1C and 1E), confirming that these were derived from the same B cell precursor. However, clone 16 used a different Vκ gene rearrangement (IGKV1-06/IGKJ1) and had a different CDR3 sequence, suggesting the possibility of light chain replacement.19,20 Antibodies 102 and 104 were not clonally related (Fig. 1C). The enrichment of SHM in all antibodies suggests that they originated from antigen experienced cells.
Anti-PAD4 autoantibodies arise from autoreactive precursors
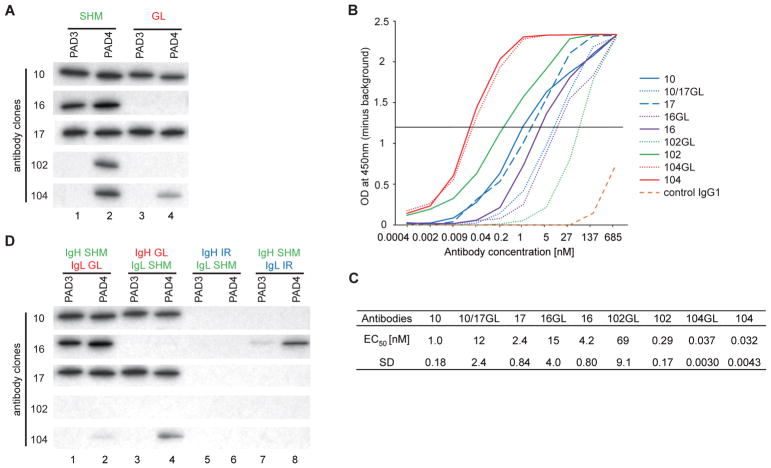
To determine whether anti-PAD3 and PAD4 reactivity was due to SHM, we reverted the IgH and IgL chain genes of the antibodies to their germline form and antigen binding was addressed by IP. Binding to PAD3 and PAD4 by clones 10 and 17 was not affected when reverted to germline (Fig. 2A). Similarly, clone 104 retained PAD4 reactivity, albeit less, in its germline form (Fig. 2A). In contrast, reversion of mutations in clone 16 and 102 led to a complete loss of PAD binding (Fig. 2A). Since IP is not sensitive enough to detect low affinity antibodies or quantitatively compare antibody binding, the binding efficiency of mutated and germline antibodies were additionally compared by ELISA (Fig. 2B and 2C). In contrast to IP, all mutated and germline antibodies showed reactivity to PAD4 when compared with control IgG1, suggesting that these antibodies were derived from B cell precursors for which reactivity to PAD4 resulted from V(D)J rearrangements. Except for mutated and germline clone 104, which have similar antigen binding, reversion to germline importantly decreased the binding of the other antibodies to PAD4, supporting that their reactivity was enhanced through affinity maturation.
Fig. 2.
The importance of SHM in antigen recognition by human monoclonal antibodies to PAD4. (A) Antibody binding to PAD3/PAD4 was addressed by IP of radiolabeled substrates using mutated (SHM) and germline (GL) reverted anti-PAD4 antibodies (3.4 nM antibody/assay). Antibodies 10GL and 17GL are the same antibody (10/17GL). (B) Anti-PAD4 antibodies and their germline variants were titrated from 0.4 pM to 685 nM against purified PAD4 by ELISA. (C) Half maximal effective concentration (EC50) for anti-PAD4 binding were calculated from B (SD, standard deviation). (D) The heavy and light chain (IgH and IgL, respectively) of SHM and GL reverted anti-PAD4 antibodies were combined to generate monoclonal antibodies (lanes 1–4). Irrelevant (IR) IgH and IgL from an antibody with no reactivity to PADs were used to produce anti-PAD4 hybrid antibodies (lanes 5–8). Antibody binding to PAD3/PAD4 was addressed by IP (3.4 nM antibody/assay) using radiolabeled substrates. The experiments in A and D were performed on two separate occasions with similar results.
Heavy and light chain requirements for PAD binding
While the IgH of antibody 16 is clonally related to antibody 10 and 17, the use of a different light chain suggests that this antibody underwent IgL replacement.19,20 Nevertheless, it is interesting that this antibody retained binding to PAD4, suggesting that the light chain replacement was insufficient to change its autoreactivity. The study of autoantibodies to DNA has shown that antigen binding can be mediated primarily by the IgH regardless of the IgL.21 To better understand the independent roles of the mutated heavy and light chains in antigen binding, monoclonals were generated that combined mutated IgH or IgL with either their corresponding germline light and heavy chains or irrelevant chains from a monoclonal with no reactivity to PADs.
Similar to the germline forms of antibodies 10, 17 and 104, reversion to germline of either IgL or IgH retained antigen binding (Fig. 2D). However, hybrid antibodies 10, 17 and 104 that used an irrelevant IgH or IgL completely lost efficient antigen binding (Fig. 2D), suggesting that their reactivity was entirely dependent on the combination of their heavy and light chains either as germline or mutated forms. Antibody 102 lost efficient PAD4 binding when either the heavy or light chain was reverted to germline (Fig. 2D) or replaced with an irrelevant IgH or IgL (Fig. 2D), demonstrating that this antibody required both mutated IgH and IgL for efficient binding to PAD4.
While clone 16 containing mutated IgL and germline igH showed no binding to PADs (Fig. 2D), mutated IgH retained reactivity to PAD3 and PAD4 when combined with any light chain (Fig. 2D), suggesting that antigen binding by this clone is largely dependent on mutations in the IgH. This may explain why replacement of the IgL in clone 16 was insufficient to silence antibody reactivity to PADs.
Mutated and germline reverted anti-PAD4 antibodies are not polyreactive and are not cross-reactive to citrullinated antigens
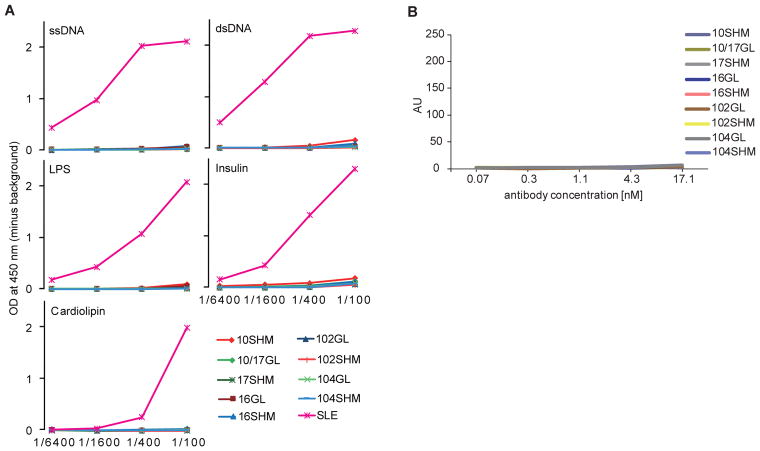
V(D)J recombination can generate harmful B cells expressing autoreactive antibodies. It is estimated that 55–75% of early immature B cells from healthy donors are polyreactive to self-antigens and lipopolysaccharide (LPS).22 While most of these cells are removed at discrete checkpoints during B cell development, their abnormal survival has been linked to autoimmune diseases.4 Since anti-PAD4 antibodies reverted to their germline form retained antigen binding, we addressed whether their reactivity to PAD4 may result from polyreactive recognition. In contrast to antibodies present in the serum from a patient with systemic lupus erythematosus (SLE) that were polyreactive, both mutated and reverted anti-PAD4 antibodies showed no polyreactivity to self-antigens (ssDNA, dsDNA, insulin and cardiolipin) or LPS (Fig. 3A). Similarly, the monoclonal antibodies showed no reactivity to cyclic citrullinated peptides (CCPs) (Fig. 3B), confirming that they were developed independently of ACPAs.
Fig. 3.
Mutated and germline human anti-PAD4 antibodies are not polyreactive and have no cross-reactivity to citrullinated antigens. (A) The specificity of mutated and GL anti-PAD4 monoclonal antibodies against ssDNA, dsDNA, LPS, insulin or cardiolipin was addressed by ELISA. The assays were performed in duplicate using serial dilutions of monoclonal antibody (stock 6.8 μM) or human SLE serum as positive control. (B) Reactivity of SHM and GL anti-PAD4 monoclonal antibodies to cyclic citrullinated peptide 3 (CCP3) was addressed by ELISA. The assays were performed in duplicate.
Functional characterization of human anti-PAD4 monoclonal antibodies
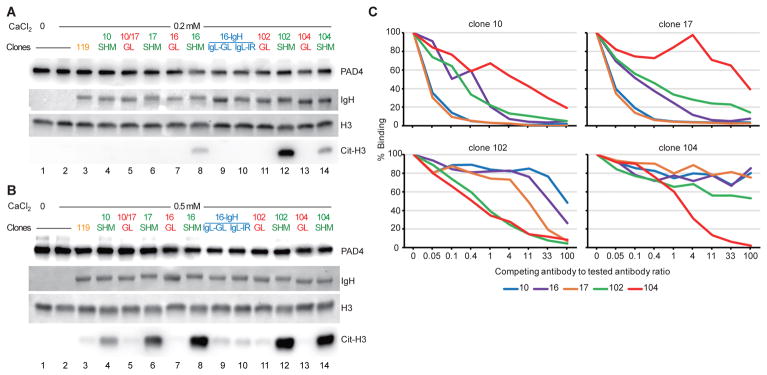
Autoantibodies that enhance PAD4 activity are strongly associated with more erosive arthritis and interstitial lung disease (ILD) in patients with RA.17,23,24 These antibodies were studied as polyclonal IgG purified from the serum of patients with RA, so the functional effects of individual antibody specificities could not be determined. The monoclonal anti-PAD4 antibodies developed here were studied for their individual capacity to activate PAD4 over a physiologic range of calcium concentrations in synovial fluid (0.2 to 1.0 mM), which are suboptimal to activate the enzyme in vitro.17 Using 0.2 mM calcium and enzymatic reactions in the linear range to citrullinate histone H3, a physiologic target of PAD4,25 clone 102 demonstrated the highest agonistic activity (Fig. 4A, lanes 12), followed by clones 16 and 104 (Fig. 4A, lanes 8 and 14, respectively).
Fig. 4.
Functional characterization of mutated (SHM) and germline reverted (GL) anti-PAD4 monoclonal antibodies. (A and B) Histone H3 (H3) was citrullinated with PAD4 at 0.2 mM (A) or 0.5 mM (B) calcium in presence of mutated or GL anti-PAD4 antibodies (0.14 μM) (lanes 4–14). Clone 16 also included the analysis of antibodies containing mutated heavy chain (IgH) combined with GL light chain (IgL-GL) (lane 9) or an irrelevant IgL (IgL-IR) (lane 10), both with reactivity to PAD3 and PAD4. As controls, H3 and PAD4 were incubated without (lane 1) or with calcium (lanes 2 and 3) in the absence (lane 1 and 2) or presence (lane 3) of an irrelevant antibody with no reactivity to PADs (clone 119). IgH and H3 were detected by Ponceau S staining. PAD4 and citrullinated H3 (cit-H3) were detected by immunoblotting. The experiments were performed on three separate occasions with similar results. (C) Competition assays among different anti-PAD4 monoclonal antibodies were performed by measuring murinized antibody binding to PAD4 following incubation with increasing amounts of competing (human) antibodies (murinized:human antibody ratio from 1:0 to 1:100). The “% Binding” represents the amount of murinized antibody bound to PAD4 in relation to the amount of antibody binding observed in the absence of competing antibody. The data are from the average of 2 independent assays.
At 0.5 and 1.0 mM calcium, the enhancing PAD4 activity by clones 16, 102 and 104 became more prominent, with clones 10 and 17 also showing some functional activity (Fig. 4B and S2). Importantly, while the germline reversions conserved antigen recognition (Fig. 2B), these monoclonal antibodies failed to enhance PAD4 activity at 0.2, 0.5 or 1.0 mM calcium (Fig. 4A and B, and S2). Similarly, antibodies containing mutated IgH from clone 16 combined with its corresponding germline IgL or an irrelevant IgL (Fig. 4A and B), both of which retained PAD reactivity (Fig. 2A), were also unable to activate the enzyme. At non-physiologic saturating calcium concentrations (i.e. 5 mM), PAD4 was fully active independently of any antibody (Fig. S3).
Since the monoclonal antibodies reverted to germline retained some PAD4 reactivity at high concentrations (Fig. 2B), we tested their ability to activate PAD4 using increasing amounts of antibody (Fig. S2). Unlike mutated anti-PAD4 antibodies, the germline versions required at least 10 times more antibody to induce even minimal PAD4 activity. Together, these data demonstrate that, while antigen experience is not necessary to produce anti-PAD4 autoantibodies, affinity maturation is required for the generation of efficient agonistic antibodies to PAD4.
Functional antibodies target different epitopes in PAD4
While agonistic anti-PAD4 antibodies were initially recognized by their cross-reactivity with PAD3,17 it is noteworthy that we identified functional autoantibodies derived from anti-PAD3/4XR positive patients that only have reactivity to PAD4. This indicates that more than one epitope may be targeted to activate the enzyme. To address whether agonistic anti-PAD4 antibodies target distinct or overlapping epitopes, the antibodies were murinized and their binding to PAD4 was addressed by ELISA in the presence of increasing amounts of competing human anti-PAD4 antibodies (Fig. 4C). With the exception of antibody 16, the rest of the clones were efficiently generated as murine antibodies, which retained similar PAD4 reactivity as their human counterparts (Fig. S4). Antigen binding by the clonally related antibodies 10 and 17 was completely blocked by one another, supporting that they target the same epitope (Fig. 4C). Analysis of the other antibodies, however, is more complex because their considerable differences in binding efficiency (Fig, 2C). Human clone 16, for which binding efficiency is lower than its clonally related antibodies 10 and 17, required 4–10 times more antibody to efficiently block these clones (Fig. 4C). Antibody 104, which has the highest relative affinity, completely blocked clone 102 and partially blocked clones 10 and 17 at high concentrations, but was not significantly blocked by any of other anti-PAD4 antibodies (Fig. 4C). Clone 102 partially blocked antibodies 10 and 17, but was only significantly blocked by clone 104 (Fig. 4C). Based on the distinct recognition of PAD3 (Fig. 1A), at least 2 epitopes are likely to be targeted by antibodies that enhance PAD4 activity. Nevertheless, it is possible that antibody 102 may recognized a third epitope that partially overlaps with clones 10, 16 and 17 (PAD3/4XR antibodies) and clone 104 (anti-PAD4-only antibody).
To gain further insights into the structural basis of the interaction between the monoclonal antibodies and PAD4, we attempted to map the regions targeted by the antibodies using PAD4 deletion mutants. PAD4 consists of a C-terminal domain and an N-terminal domain that is divided into subdomains 1 and 2 (Fig. S5A).26 Under standard conditions used to IP PAD4, none of the antibodies showed binding to any domain (Fig. S5B). When the antibody concentration and the incubation time were increased in the IP assays (Fig. S5C), clone 104 showed reactivity to the N-terminal domain and subdomain 1. However, the other antibodies still failed to react with any subdomain of PAD4 (Fig. S5C). These findings strongly suggest that the epitope targeted by the majority of functional anti-PAD4 antibodies is structural in nature, requiring folding of the complete molecule.
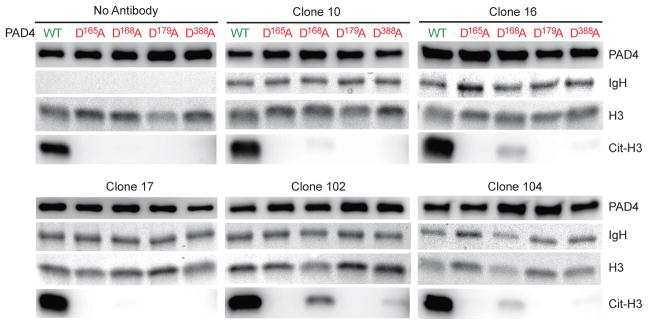
Agonistic autoantibodies enhance PAD4 activity by regulating the enzyme at calcium-binding site 5
Since agonistic anti-PAD4 antibodies work by lowering the calcium requirement for PAD4 activity, we explored potential sites of interaction using PAD4 mutants that lack the ability to bind calcium at specific sites. PAD4 has 5 calcium-binding sites designated Ca1-Ca5. Ca1 and Ca2 in the C-terminal domain are required for catalysis, while Ca3-Ca5 are likely relevant to regulate enzyme activity.26 Aspartic acid residues that coordinate calcium-binding at Ca3 and Ca5 (D165), Ca3 and Ca4 (D179), only Ca4 (D388) and only Ca5 (D168) were changed to alanine.27 Then, the PAD4 mutants were used to test the functional activity of the antibodies. None of the PAD4 mutants were active in the absence of anti-PAD4 antibodies (Fig. 5). However, the activity of the D168A mutant (Ca5) was partially rescued by antibody clones 10, 16, 102 and 104 (Fig. 5). Interestingly, the enhancing effect of the monoclonal antibodies on the mutant D168A was proportional to their activity on wild type PAD4 (i.e. 102 > 104 = 16 > 10) (Fig. 4B and 5). Together, these data suggest that functional anti-PAD4 antibodies decrease the calcium requirement of PAD4 by regulating the enzyme at Ca5. Although clones 16, 102 and 104 showed some minimal effect on the activity of the mutant D388A, Ca5 appears to be the most important site of interaction.
Fig. 5.
Agonistic antibodies partially restore the activity of calcium-binding site 5 (Ca5) mutant PAD4. Histone H3 (H3) was incubated with wild type (WT) or PAD4 mutants at calcium-binding sites Ca3 and Ca5 (D165A), Ca5 (D168A), Ca3 and Ca4 (D179A) or Ca4 (D388A) in the presence of 10 mM CaCl2, with or without anti-PAD4 monoclonal antibodies. After 2 hrs at 37°C, PAD4 and citrullinated H3 (Cit-H3) were detected by immunoblotting. IgH and H3 were detected by Ponceau S staining as loading control. The experiments were performed on two separate occasions with similar results.
Discussion
Analysis of recombinant antibodies cloned from single new emigrant and mature naïve RA B cells provided initial evidence of defective central and peripheral checkpoints in B cell tolerance in patients with RA.4,18 In a prior study, these antibodies were shown to be polyreactive to self-antigens and LPS, with a subgroup showing low reactivity to IgG and cyclic citrullinated peptides.18 Further studies have shown that ~25% of synovial IgG-expressing B cells are specific to citrullinated antigens in ACPA+ RA patients.3
Despite the considerable understanding of ACPA generation gained through the study of recombinant antibodies cloned from single RA B cells,3,11 monoclonal antibodies to PAD4 have not previously been identified. The antibodies identified in our current study have unique features that provide novel insights into the understanding of the humoral autoimmune response in RA. In contrast to ACPAs and other antibodies cloned from patients with RA,3,11,13,14 the antibodies identified in our current study are not polyreactive and antigen recognition was independent of SHM. All clones appear to have emerged from anti-PAD4 B cell precursors (Fig. 6), which likely bypassed early tolerance checkpoints. Nevertheless, our results demonstrate that affinity maturation is essential for the antibodies to gain efficient agonistic effect against PAD4.
Fig. 6.
Genetic characteristics and functional features of RA-derived monoclonal antibodies to PAD4. Antibodies lineage trees were generated by comparison of Ig VH sequences. Monoclonal antibodies were derived from 2 patients with RA (RA-1 and RA-3). Clonally related antibodies were identified based on sequences derived from a single germline rearrangement characterized by the same V, D and J gene usage, CDR3 length and sequence. The numbers beside the arrows represent the number of mutations acquired in the heavy (VHSHM) and light (VLSHM) chain. The germline and mutated antibody reactivities are specified, as well as their capacity to enhance PAD4 activity.
While polyclonal RA sera containing agonistic antibodies to PAD4 were initially identified by their cross-reactivity with PAD3, the cloning of individual antibodies from these patients demonstrated that the functional effect on PAD4 is not driven by antibodies binding to a single epitope. Instead, antibodies targeting different sites on the enzyme, including at least one epitope with structural similarity to PAD3, was able to enhance PAD4 activity.
Although we have cloned human anti-PAD4 agonistic antibodies, the study has some limitations. First, only a few antibodies were obtained, which may not represent the complete repertoire of agonistic anti-PAD4 antibodies found in RA. Secondly, the exact mechanism by which the antibodies enhance PAD4 activity will require the structural definition of the antibody-PAD4 complex. Nevertheless, functional analysis of PAD4 mutants identified Ca5 as a potential site that is commonly regulated by four of the five antibodies. The increase in agonistic activity of antibodies to PAD4 as a result of SHM likely reflects the ability of the antibody to maintain a stable transition state of the enzyme resembling binding of calcium to Ca5. Considering that this transition state is likely favored when the enzyme is active, it is possible that a conformational state during PAD4 activation may be the primary antigen that drives affinity maturation of functional antibodies. As such, conditions that promote abnormal hyperactivation of PAD4, such as leukotoxic hypercitrullination in neutrophils,28,29 may lead to both the production of high affinity ACPAs and functional anti-PAD4 antibodies in RA. The finding that ACPAs and antibodies to PAD4 co-exist in pre-clinical RA30–32 further supports the hypothesis that conditions of PAD4 hyperactivation may be prevalent in the initiation phase of the disease. Since the generation of agonistic PAD4 antibodies is dependent on the accumulation of somatic mutations and is likely driven by chronic exposure to epitopes of enzymatically active PAD4, this may explain why functional antibodies are largely found in patients with advanced disease.17
In PAD4, Ca5 is positioned near the surface of subdomain 2 at the interface between the N- and C-terminal domains in a region shown to be important for full activation of the related enzyme PAD2.26,33 This location may allow Ca5 to be accessible to functional autoantibody binding and potentially to other regulatory molecules. Although pharmacologic PAD inhibition has been focused on blocking the catalytic site in the enzyme, the finding that autoantibodies can modulate PAD4 activity by regulating Ca5 offers an additional target for inhibitor development. Molecules designed to interact with Ca5 could potentially block PAD4 hyperactivation with a limited effect on physiologic functions that require lower enzyme activity. Similarly, such molecules may be useful to counteract PAD4 interactions with agonistic autoantibodies in patients with RA. In addition to mechanistic insights, the analysis of patient-derived monoclonal antibodies, as we have employed here, offers the opportunity to address pathogenic pathways in erosive RA and to define approaches to offset the consequences of such damage.
Supplementary Material
Acknowledgments
The authors would like to thank Eric Meffre, Ph.D from Yale University for kindly providing the plasmids used to clone the monoclonal antibodies; Michelle K. Jones from the Johns Hopkins Arthritis Center for recruiting patients for the study; and Raffaello Cimbro (current) and Francis J. Chrest (former) from the Bayview Immunomics Core for their technical contribution to this work.
Funding information.
This study was sponsored by MedImmune, the global biologics R&D arm of AstraZeneca, The Jerome L. Greene Foundation, and grant number P30AR053503 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIAMS or the National Institutes of Health.
Footnotes
Competing interests
FA, ED and AR received a grant from Medimmune and are authors on issued Patent No. 8,975,033 entitled “Human Autoantibodies Specific for PAD3 which are Cross-reactive with PAD4 and their Use in the Diagnosis and Treatment of Rheumatoid Arthritis and Related Diseases”. ED previously served on the scientific advisory board for Padlock Therapeutics, Inc. FA serves as consultant for Bristol-Myers Squibb Company. GPS is employed by MedImmune LLC, and owns stocks in Astra Zeneca. TM is employed by Gilead Science and is a former employee of MedImmune LLC. JS, ED, AR and FA submitted an invention disclosure by the Johns Hopkins University that covers the sequences and the use of the human anti-PAD4 monoclonal antibodies. The remaining authors declare that they have no competing interests.
Contributorship
All authors contributed to the preparation of this manuscript and approved the final version for publication. JS planned the study, cloned, expressed, purified and characterized the monoclonal antibodies, analyzed/interpreted the data, and wrote the manuscript. ED and AR conceptualized the study and analyzed data. KS performed immunoprecipitation assays. GPS and TM provide expertise in the analysis of monoclonal antibodies. COB III provided RA samples. MFK generated recombinant proteins. FA conceptualized and designed the study, participated in data analysis, directed the project, and wrote the manuscript.
Ethical approval information
This study was conducted with the approval of the Johns Hopkins Institutional Review Board.
Data sharing statement
All data relevant for this study have been included in the manuscript. Reagents derived from this project will be available from FA under a material transfer agreement with the Johns Hopkins University.
References
- 1.Rahman A, Giles I, Haley J, Isenberg D. Systematic analysis of sequences of anti-DNA antibodies--relevance to theories of origin and pathogenicity. Lupus. 2002;11:807–23. doi: 10.1191/0961203302lu302rr. [DOI] [PubMed] [Google Scholar]
- 2.Deftos M, Olee T, Carson DA, Chen PP. Defining the genetic origins of three rheumatoid synovium-derived IgG rheumatoid factors. J Clin Invest. 1994;93:2545–53. doi: 10.1172/JCI117265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amara K, Steen J, Murray F, et al. Monoclonal IgG antibodies generated from joint-derived B cells of RA patients have a strong bias toward citrullinated autoantigen recognition. J Exp Med. 2013;210:445–55. doi: 10.1084/jem.20121486. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Curr Opin Immunol. 2008;20:632–8. doi: 10.1016/j.coi.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Yurasov S, Wardemann H, Hammersen J, et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201:703–11. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J, Jacobi AM, Wang T, et al. Polyreactive autoantibodies in systemic lupus erythematosus have pathogenic potential. J Autoimmun. 2009;33:270–4. doi: 10.1016/j.jaut.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrenstein MR, Katz DR, Griffiths MH, et al. Human IgG anti-DNA antibodies deposit in kidneys and induce proteinuria in SCID mice. Kidney Int. 1995;48:705–11. doi: 10.1038/ki.1995.341. [DOI] [PubMed] [Google Scholar]
- 8.Im SR, Im SW, Chung HY, Pravinsagar P, Jang YJ. Cell- and nuclear-penetrating anti-dsDNA autoantibodies have multiple arginines in CDR3 of VH and increase cellular level of pERK and Bcl-2 in mesangial cells. Mol Immunol. 2015;67:377–87. doi: 10.1016/j.molimm.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 9.Waaler E. On the occurrence of a factor in human serum activating the specific agglutintion of sheep blood corpuscles. Acta Path Microbiol Scan. 1940;17:172–88. doi: 10.1111/j.1600-0463.2007.apm_682a.x. [DOI] [PubMed] [Google Scholar]
- 10.Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101:273–81. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corsiero E, Bombardieri M, Carlotti E, et al. Single cell cloning and recombinant monoclonal antibodies generation from RA synovial B cells reveal frequent targeting of citrullinated histones of NETs. Ann Rheum Dis. 2016;75:1866–75. doi: 10.1136/annrheumdis-2015-208356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li S, Yu Y, Yue Y, et al. Autoantibodies From Single Circulating Plasmablasts React With Citrullinated Antigens and Porphyromonas gingivalis in Rheumatoid Arthritis. Arthritis Rheumatol. 2016;68:614–26. doi: 10.1002/art.39455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuda R, Ozawa T, Kobayashi E, et al. Monoclonal antibody against citrullinated peptides obtained from rheumatoid arthritis patients reacts with numerous citrullinated microbial and food proteins. Arthritis Rheumatol. 2015;67:2020–31. doi: 10.1002/art.39161. [DOI] [PubMed] [Google Scholar]
- 14.van de Stadt LA, van Schouwenburg PA, Bryde S, et al. Monoclonal anti-citrullinated protein antibodies selected on citrullinated fibrinogen have distinct targets with different cross-reactivity patterns. Rheumatology (Oxford) 2013;52:631–5. doi: 10.1093/rheumatology/kes371. [DOI] [PubMed] [Google Scholar]
- 15.Bicker KL, Thompson PR. The protein arginine deiminases: Structure, function, inhibition, and disease. Biopolymers. 2013;99:155–63. doi: 10.1002/bip.22127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sokolove J, Zhao X, Chandra PE, Robinson WH. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcgamma receptor. Arthritis Rheum. 2011;63:53–62. doi: 10.1002/art.30081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darrah E, Giles JT, Ols ML, et al. Erosive Rheumatoid Arthritis Is Associated with Antibodies That Activate PAD4 by Increasing Calcium Sensitivity. Sci Transl Med. 2013;5:186ra65. doi: 10.1126/scitranslmed.3005370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samuels J, Ng YS, Coupillaud C, Paget D, Meffre E. Impaired early B cell tolerance in patients with rheumatoid arthritis. J Exp Med. 2005;201:1659–67. doi: 10.1084/jem.20042321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nemazee D, Weigert M. Revising B cell receptors. J Exp Med. 2000;191:1813–7. doi: 10.1084/jem.191.11.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalinina O, Doyle-Cooper CM, Miksanek J, et al. Alternative mechanisms of receptor editing in autoreactive B cells. Proc Natl Acad Sci U S A. 2011;108:7125–30. doi: 10.1073/pnas.1019389108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wardemann H, Yurasov S, Schaefer A, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–7. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 23.Giles JT, Darrah E, Danoff S, et al. Association of cross-reactive antibodies targeting peptidyl-arginine deiminase 3 and 4 with rheumatoid arthritis-associated interstitial lung disease. PLoS One. 2014;9:e98794. doi: 10.1371/journal.pone.0098794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navarro-Millan I, Darrah E, Westfall AO, et al. Association of anti-peptidyl arginine deiminase antibodies with radiographic severity of rheumatoid arthritis in African Americans. Arthritis Res Ther. 2016;18:241. doi: 10.1186/s13075-016-1126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakashima K, Hagiwara T, Yamada M. Nuclear localization of peptidylarginine deiminase V and histone deimination in granulocytes. J Biol Chem. 2002;277:49562–8. doi: 10.1074/jbc.M208795200. [DOI] [PubMed] [Google Scholar]
- 26.Arita K, Hashimoto H, Shimizu T, et al. Structural basis for Ca(2+)-induced activation of human PAD4. Nat Struct Mol Biol. 2004;11:777–83. doi: 10.1038/nsmb799. [DOI] [PubMed] [Google Scholar]
- 27.Liu YL, Tsai IC, Chang CW, et al. Functional roles of the non-catalytic calcium-binding sites in the N-terminal domain of human peptidylarginine deiminase 4. PLoS One. 2013;8:e51660. doi: 10.1371/journal.pone.0051660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konig MF, Andrade F. A critical reappraisal of neutrophil extracellular traps (NETs) and NETosis mimics based on differential requirements for protein citrullination. Front Immunol. 2016;7:461. doi: 10.3389/fimmu.2016.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konig MF, Abusleme L, Reinholdt J, et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci Transl Med. 2016;8:369ra176. doi: 10.1126/scitranslmed.aaj1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kokkonen H, Mullazehi M, Berglin E, et al. Antibodies of IgG, IgA and IgM isotypes against cyclic citrullinated peptide precede the development of rheumatoid arthritis. Arthritis Res Ther. 2011;13:R13. doi: 10.1186/ar3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolfenbach JR, Deane KD, Derber LA, et al. Autoimmunity to peptidyl arginine deiminase type 4 precedes clinical onset of rheumatoid arthritis. Arthritis Rheum. 2010;62:2633–9. doi: 10.1002/art.27570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sokolove J, Bromberg R, Deane KD, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One. 2012;7:e35296. doi: 10.1371/journal.pone.0035296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slade DJ, Fang P, Dreyton CJ, et al. Protein Arginine Deiminase 2 Binds Calcium in an Ordered Fashion: Implications for Inhibitor Design. ACS Chem Biol. 2015;10:1043–53. doi: 10.1021/cb500933j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darrah E, Rosen A, Giles JT, et al. Peptidylarginine deiminase 2, 3 and 4 have distinct specificities against cellular substrates: novel insights into autoantigen selection in rheumatoid arthritis. Ann Rheum Dis. 2012;71:92–8. doi: 10.1136/ard.2011.151712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konig MF, Paracha AS, Moni M, et al. Defining the role of Porphyromonas gingivalis peptidylarginine deiminase (PPAD) in rheumatoid arthritis through the study of PPAD biology. Ann Rheum Dis. 2015;74:2054–61. doi: 10.1136/annrheumdis-2014-205385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tiller T, Meffre E, Yurasov S, et al. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J Immunol Methods. 2008;329:112–24. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Souto-Carneiro MM, Longo NS, Russ DE, et al. Characterization of the human Ig heavy chain antigen binding complementarity determining region 3 using a newly developed software algorithm, JOINSOLVER. J Immunol. 2004;172:6790–802. doi: 10.4049/jimmunol.172.11.6790. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.