Abstract
Why do girls and women differ from boys and men? Gender development is typically considered to result from socialization, but sex hormones present during sensitive periods of development, particularly prenatal androgens, play an important role. Data from natural experiments, especially from females with congenital adrenal hyperplasia, show the complexity of the effects of androgens on behavior: Prenatal androgens apparently have large effects on interests and engagement in gendered activities; moderate effects on spatial abilities; and relatively small or no effects on gender identity, gender cognitions, and gendered peer involvement. These differential effects provide an opportunity to move beyond identifying sources of variation in behavior to understanding developmental processes. These processes include links among gendered characteristics, psychological and neural mechanisms underlying development, and the joint effects of biological predispositions and social experiences.
Keywords: gender development, prenatal androgens, peers
How and why do a person's sex and gender matter?1 Few questions generate as much interest and controversy as the nature, origins, and consequences of psychological differences between the sexes, and of identity as female or male. Sex and gender figure into topics as diverse as classroom composition (the benefits versus drawbacks of sex-segregated classes), career choices (women's underrepresentation in the fields of science, technology, engineering, and math, or STEM), and psychopathology (sex differences in the incidence of psychiatric illnesses). Gender identity has entered the public arena and personal stories make clear that we have much to learn about how people identify or do not identify with their biological sex, as well as about the plasticity of that identity.
The sexes differ in significant ways and are similar in other ways; accordingly, the extent to which differences and similarities are emphasized varies (1-3). The size of the difference varies across psychological domains, with very large differences in social partners (playmates and romantic and sexual partners); large differences in activities and interests, and in incidences of some forms of psychopathology; moderate differences in spatial abilities and some social behaviors, and small differences in aspects of personality (1).
Social and social-cognitive perspectives guide most psychological work on sex and gender. Sex-differential socialization by parents, peers, teachers, and other social agents, including the media, is understood to operate through modeling, rewards, and opportunities to produce different outcomes and values for boys and girls (1). Children also contribute to their own gendered socialization through schemas, that is, ideas that people have about gender that guide their perception, thinking, and behavior (e.g., 4, 5).
However, social forces do not act on blank slates. Genes and physiology play a role in gender development, as they do in other aspects of psychological development. Sex steroid hormones contribute to sex-related characteristics, particularly during early development. Research on nonhuman species shows clearly and convincingly that androgens and estrogens present early in development affect sexual differentiation of many aspects of behavior, as well as reproductive anatomy and function (6, 7).
Is early development a sensitive period for hormones to organize the human brain and behavior? Researchers cannot experimentally manipulate hormones in people like they can in other species, and human behavior is influenced strongly by social context. However, natural experiments have helped us understand whether and how prenatal hormones influence gender development. Females with classical congenital adrenal hyperplasia (C-CAH) provide the best opportunity to address this issue because they are exposed to high levels of androgens in early life (because of a genetic mutation) but are raised as girls. They are usually diagnosed and treated as newborns, so they generally have low levels of postnatal androgens.2
Females with C-CAH are generally compared to unaffected females (optimally sisters without C-CAH) and occasionally to males with and without C-CAH; sometimes, subgroups of females with C-CAH with varying degrees of exposure to androgens (indexed by genotype or severity of disease) are compared to each other. Another valuable comparison is provided by females with a mild form of the disorder—nonclassical (NC) CAH; these females are exposed to excess androgens postnatally, usually starting in midchildhood or adolescence (8), and provide a control for disease and treatment.
Girls and women with C-CAH provide researchers an opportunity to examine the relative influences on gender development of exposure to sex hormones (masculinized) and childrearing (as a girl). Studies (discussed later and reviewed in 9, 10) show that behavior in females with C-CAH is masculinized in some but not all ways, and the extent of masculinization varies across behaviors (consistent with the multidimensionality of gender; 3). These studies also show that as a group, females with C-CAH are not as masculinized as typical males, and the degree of behavioral masculinization in females with C-CAH correlates modestly with the degree of prenatal exposure to androgens inferred from genotype or severity of the disease. Furthermore, the behavior of females with C-CAH is more masculinized than that of females with NC-CAH, who are generally like typical females, as expected from their female-typical prenatal exposure to androgens (see also 11, 12).
The question is no longer whether sex hormones influence gendered behavior—they do—but how they do so. In this article, I focus on the differential effects of androgens, suggesting that they can be linked to the extent to which characteristics reflect individual preferences and skills, gender cognitions, or social relationships. I also consider how hormonal influences are mediated and moderated by the social environment, and the psychological mechanisms that mediate the behavioral effects of androgens. The findings I discuss come primarily from two sources: a long-term study of females and males with C-CAH and their unaffected siblings from childhood through adolescence into adulthood, and a multimethod study of 10- to 13-year-old girls with C-CAH and NC-CAH. I also discuss other relevant studies.
For Many Personal Characteristics, Androgens' Effects Track Sex Differences
Studies of females with CAH typically aim to identify the contributions of androgens to sex differences, so they focus on sex-related characteristics. For individual preferences and skills, the magnitude of androgens' effects generally parallels the magnitude of sex differences, with large effects on interests and engagement in activities, moderate effects on spatial abilities, and small to moderate effects on social and personal attributes. In general, the difference between females with and without C-CAH is smaller than the sex difference in that characteristic, so early androgens do not fully account for sex differences. This is not surprising, given social and nonhormonal biological influences on gender development (1).
Interests and Participation in Activities
The largest difference between females with and without C-CAH concerns interests and engagement in gendered activities (9).3 Across development, from childhood to adulthood, girls and women with C-CAH are more interested in and participate more in male-typed activities and are less interested in and participate less in female-typed activities than are girls and women without C-CAH or with NC-CAH (seen in observations, daily reports of time use, and global reports). Differences are large (see Figure 1, panel A for a composite measure of activities in 10- to 13-year-old girls with C-CAH and NC-CAH).
Figure 1. Scores on gendered characteristics in girls with C-CAH and NC-CAH.
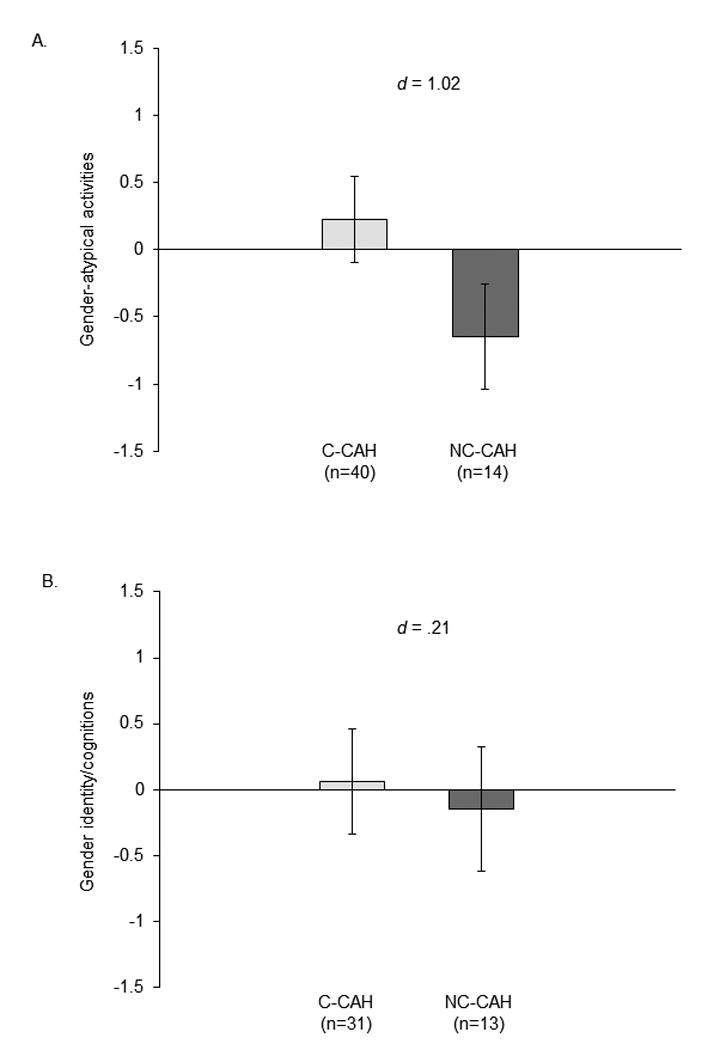
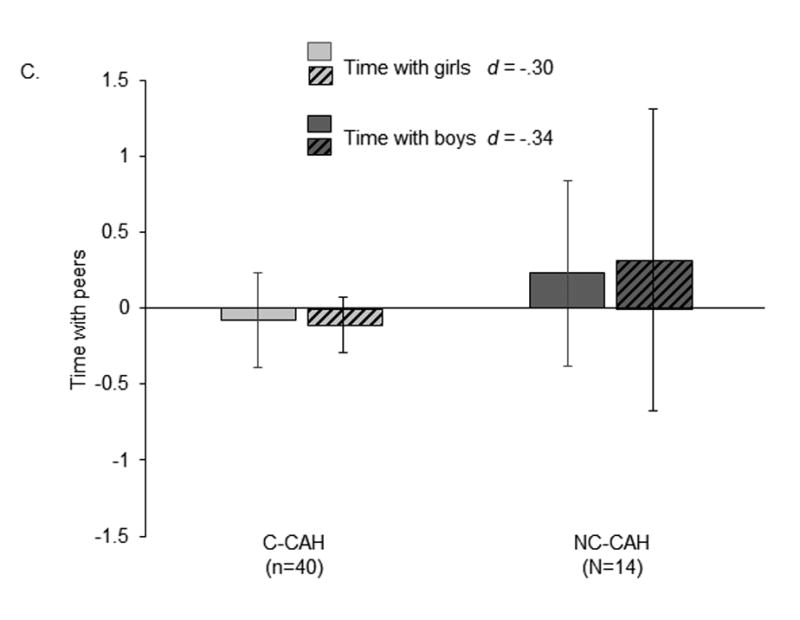
Scores are standardized (z-scores) to facilitate comparison across characteristics. Bars represent mean scores; lines are 95% confidence intervals; d = mean of CAH - mean of NC-CAH/average standard deviation. Data from (18). Panel A: Gender-atypical activities. Composite measure of male-typed minus female-typed activities (percentage of time, interest, and participation in those activities); coefficient alpha = .73. High scores reflect gender-atypical activities (more male-typed relative to female-typed). Panel B: Gender identity/cognitions. Composite measure of core gender identity, gender typicality, gender contentedness, intergroup bias, felt pressure for gender conformity, gender role attitudes, gender centrality, and gender evaluation; coefficient alpha = .70. High scores reflect female-typical/traditional cognitions. Panel C: Time spent with girls and with boys (proportion: time with peers/time with all people). High scores reflect proportionately more time spent with female and male peers.
Most studies of interests and participation in activities involve global reports from females with C-CAH and their parents. Participation in gender-atypical activities by girls with C-CAH has been confirmed with direct measures. In two studies using free-play sessions, girls with C-CAH played more with boys' toys (especially toy vehicles) and less with girls' toys (including dolls) than did their sisters without CAH (16, 17). In another study using daily phone diaries, girls with C-CAH reported spending more time in male-typical activities and less time in female-typical activities than did those with NC-CAH (18).
The influence of androgens on activity preferences extends to occupational interests and choices. Adolescent and adult women with C-CAH reported more interest than their sisters without C-CAH in male-dominated occupations, preferring jobs involving things (e.g., chemist, carpenter) to jobs involving people (e.g., teacher, interior designer; 19). Furthermore, these interests have translated into career selection: Women with C-CAH were more likely than women without CAH to be employed in male-dominant occupations (those with no more than a quarter of females in the profession; 20) and to be in the top 20th percentile of income (unsurprising given pay disparities in male- versus female-dominant careers; 21).
Many studies with similar findings across methods, labs, and nations provide compelling evidence that gendered interests and associated behavioral choices are influenced by the amount of androgens present during prenatal development. But studies of females with C-CAH are not perfect experiments and alternative explanations must be considered. The main concern is that behavioral masculinization reflects parents' responses to the masculinized genitalia of girls with C-CAH, particularly their encouragement of male-typed behavior. We lack evidence that this happens; if anything, parents tend to encourage female-typed behavior (12, 17). An additional concern is that behavioral changes result from other hormones atypical in CAH (e.g., cortisol) or from having a serious chronic illness requiring lifelong medication. Other hormones and factors related to illness are unlikely to have selective effects on gendered characteristics; they would also affect males with C-CAH and some would also affect females with NC-CAH, which is not the case (see Figure 1A, and 1, 10).
Additional evidence confirms the effects of androgens on gendered activities. The degree of prenatal exposure to androgens (inferred from type of mutation in the CYP21A2 gene that causes CAH and the severity of the disease) is related linearly and moderately to the extent of interest in male-typed activities in females with CAH (e.g., 12, 20, 22). Other studies involving natural experiments (conditions in which exposure to hormones is dissociated from the sex children are raised) confirm a link between interest in activities and prenatal exposure to androgens (9). Evidence also indicates a link between typical variations in prenatal testosterone levels (e.g., measured in amniotic fluid) and childhood play, although this work requires replication (23).
A fundamental question for developmentalists concerns the psychological mechanisms underlying the effects of androgens on activities. How does exposure to moderate levels of androgens during prenatal development facilitate play with toy trucks and interest in STEM careers? The gendered toy preferences of children are similar to sex-related object preferences in rhesus monkeys (24). These differences may be driven in part by sex differences in the predisposition to imitate propulsive movement (hitting versus cradling an object; 25), and by preferences for engaging with people over objects (19). This topic is worth further investigation because it helps us understand how biological predispositions play out over development.
Social and Personal Attributes
Social and personal characteristics are difficult to study in rare clinical samples because the sex differences are moderate in size (requiring larger samples than typically available) and influenced by context. The effects of androgens on these characteristics are small to moderate, again tracking sex differences. For example, compared to their unaffected sisters, girls and women with C-CAH reported themselves or were reported by their parents to be less interested in babies, more likely to be aggressive, and less likely to feel empathy (9, 10).
Cognitive Abilities
Females with C-CAH appear to have better spatial abilities than females without C-CAH, including their unaffected sisters (26-28). Androgens apparently affect spatial ability directly and through interest in male-typed activities (26). Differences are not always found, a likely reflection of limited power and of aspects of the disease that uniquely affect cognition countering the effects of androgens (see 9, 10). Other cognitive abilities show small sex differences, so they are not often examined in relation to early androgens.
Exposure to Moderate Levels of Androgens Affects Gender Identity and Gender Cognition Minimally
In contrast to the findings just noted, early androgens—at least at the levels to which females with C-CAH are exposed—appear to have a small effect on gender identity and gender cognitions (see Figure 1, Panel B, for a composite measure of identity/cognitions in 10- to 13-year-old girls with C-CAH and NC-CAH).
Gender Identity
Discussions by researchers, clinicians, lay people, and others often focus on the innateness and early stability of gender identity, but gender identity develops over time and is not related simply to genes or early exposure to androgens (see 9). Most girls and women with C-CAH have a female binary identity, although they are more likely to have gender dysphoria or to transition to males, and also to identify less as female than unaffected girls and women (e.g., 29-31). In other natural experiments, findings are similar to those studies of females with C-CAH: Gender identity tends to agree more with the sex the child was raised than with early exposure to androgens, except in cases of additional exposure to androgens at puberty (9).
Gender Cognitions
Cognitive perspectives on gender development focus on the ways children actively construct gender by using socially provided gender cues to organize their world, with an array of gender cognitions influencing their behavior (4, 5, 32, 33). Gender cognitions encompass knowledge and application of gender-related stereotypes, beliefs and values, and self-concepts or self-perceptions (e.g., see Table 1 in 3), and I use the term broadly.
Studies of gender cognitions in girls with CAH go beyond examining the effects of androgens to apply constructivist perspectives to determine how gender self-concepts and stereotypes contribute to other gendered characteristics. If gender cognitions drive or result from gendered behavior, then girls with C-CAH should be more flexible in applying stereotypes (whether because of knowledge or personal attitudes), more egalitarian, and more untraditional in their thoughts and feelings about gender than girls without C-CAH. Alternatively, if gender cognitions reflect gender identity, then girls with C-CAH should have stereotypical attitudes and feelings about gender (similar to girls without C-CAH).
In our study of young adolescent girls with C-CAH and NC-CAH (13, 18), both groups had stereotypical gender cognitions, in line with their female-typical gender identity; the groups did not differ significantly in gender cognitions, including gender centrality, gender evaluation, ingroup bias, and gender attitudes. Girls with CAH had gender cognitions that were female-typical, although they were interested in and engaged in male-typed activities. Gender attitudes were linked to activities in both groups, with gender identity mediating the links: Typical gender attitudes and positive attitudes about being a girl were associated with feminine gender identity, which in turn was associated with gender-typical activities. These findings support aspects of gender constructivist theories: Cognitions are associated more closely with gender identity than with prenatal androgens.
But other apparently inconsistent findings indicate that additional work is needed to delineate cognitions in girls with CAH. In one study, girls with CAH had fewer verbal and behavioral preferences for objects labeled for girls (34). Differences across studies may reflect differences in measures; for example, focusing on objects (even gender-neutral ones) might remind girls with C-CAH that their object preferences are not typically female, whereas focusing on global attitudes might allow them to consider the ways they are like girls.
The Effects of Androgens on Gendered Peer Interactions Reflect Combined Effects of Interests, Identity, and Cognitions
The limited evidence regarding the effects of androgens on social relationships reflects the challenges of studying behavior in rare samples; gendered peer interactions in girls with C-CAH have mostly been studied with global self-reports or experimental tasks of preferences. Girls with C-CAH are more likely than typical girls to say they prefer boys as playmates (16, 35-37). The difference in playmate preference between girls with and without C-CAH is moderate in size, in contrast to the large sex difference in choice of playmates in typical children (38); it reflects that some girls with C-CAH prefer boy playmates, but many prefer girl playmates. Thus, androgens affect reported peer preferences less than activities, but more than identity and cognitions.
Extending work using global reports, my colleagues and I studied time spent by girls with C-CAH and NC-CAH with same- and other-sex peers (18). This work allowed us to integrate several aspects of gender by identifying the contributions to gendered peer interactions of experienced similarity (behavioral compatibility) and expected similarity (gender cognitions; 39). Girls with C-CAH allow a stronger test of these influences than is possible in typical children because of the dissociation of their male-typed activities (which would lead them to boys), and their female gender identity and cognitions (which would lead them to girls). The peer interactions of girls with C-CAH can tell us about the relative effects of behavior and gender cognition. Girls with C-CAH might have fewer interactions with both boys and girls than would typical girls: Their identity and cognitions attract them to girls, who do not return the attraction because of lack of shared interests; boys would not engage with them because they are girls. Alternatively, they might interact more with both boys and girls, seeking out boys whose interests in activities are compatible, and girls for similar gender identity and cognitions. Or they might have more interactions with boys and fewer interactions with girls, but to a lesser extent than if behavioral compatibility were the only influence on peer interactions.
In daily phone diary reports of time spent with male and female peers, few girls with C-CAH or NC-CAH reported time spent with boys, and the groups did not differ significantly in time spent with either boys or other girls (18; see Figure 1, Panel C for time spent with female and with male peers). Across all girls, time spent with other girls was significantly and moderately predicted by both gender-typed activities and gender identity/cognitions (predictors were composites, as shown in Figure 1, A and B.) Furthermore, degree of prenatal exposure to androgens, indexed by CAH type, indirectly influenced time spent with girls through gender-typed activities. These results confirm and extend findings in typical children that peer interactions are influenced by both experienced and expected similarity (39).
Lessons From CAH for Gender Developmentalists
Studies of females with CAH confirm findings from research with nonhuman animals that sex-related behavior is influenced by sex hormones during sensitive developmental periods; such evidence is increasingly being confirmed in other types of studies with humans. Findings described earlier reveal the complexity of the effects of androgens on human behavior. Data from the 10- to 13-year-old girls in our study illustrate how androgens affect gendered activities much more than identity/cognitions and time spent in interactions with peers (see Figure 1).
But studies of females with CAH go beyond partitioning variance into effects due to androgens versus socialization, and exemplify the value of natural experiments to address questions about psychological development. For example, as noted earlier, data from females with CAH tested constructivist theories of gender development (4, 33), given the increased variation in activities by girls with C-CAH and the differential effects of androgens on activities and identity/cognitions.
Females with CAH can help us explain gender development as joint effects of nature and nurture (e.g.,40, 41), testing Maccoby's proposition that “whatever differential predispositions boys and girls may have, it is likely that the way they are enacted will depend greatly on the social conditions provided by the adults and peers with whom they interact” (42, p. 404). For example, in our study of 10- to 13-year-old girls with CAH, my colleagues and I are examining how family socialization effects are correlated with and moderated by prenatal exposure to androgens; we hypothesize that gender-typed socialization will be more effective (i.e., associated with more gender typing) in girls with NC-CAH than in girls with C-CAH, and that socialization is driven partly by the girls themselves, so girls with C-CAH receive less traditional gender socialization than girls with NC-CAH.
Looking Ahead
It is heartening to move away from the nature-nurture argument toward an integrated understanding of gender development, as suggested in Figure 2. Data are needed to test the paths; most evidence addresses simple links between causes and outcomes. Figure 2 highlights several points about gender development. Biological and social processes work together, and are likely correlated with each other and interact to affect development. It is important to identify mechanisms mediating causes and outcomes; studies of neural sex differences will be most meaningful when brain structure and activation are linked to psychological mechanisms, and examined in relation to both experiences and hormones. Equifinality applies to gendered characteristics as it does to other developmental outcomes. For example, spatial ability is facilitated by prenatal exposure to androgens, but may also develop from social experiences; transgender identity results from exposure to high levels of prenatal androgens in a few girls (such as those with C-CAH), but from nonhormonal biological factors or gendered socialization experiences in most girls. The advances we have made and the challenges ahead make this an exciting time for research in gender development.
Figure 2. Simple process model of links between prenatal androgens and sex-typed behaviors.
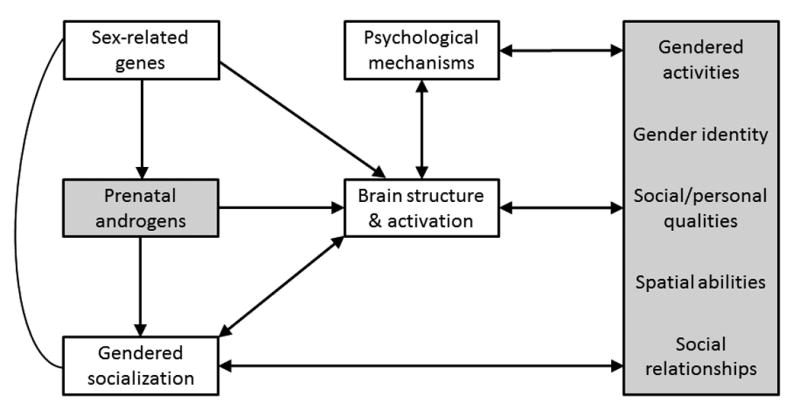
(gray boxes), considering neural and psychological mediators of links, and including effects of gendered socialization and genes (nonhormonal biological processes) (white boxes). Magnitudes of paths likely change with development and vary for different behaviors, contexts, and individuals. Adapted and modified from (10). Used by permission.
Acknowledgments
The work reported in this article was supported by grants HD19644, HD044398, and HD057930 from the National Institutes of Health. I appreciate the many people who have contributed to the research; for especially significant help, I thank Kristina Bryk, who skillfully managed the projects and contributed to all stages from conceptualization through dissemination; collaborators, particularly Adriene Beltz and Susan McHale; pediatric endocrinologists and family support group staff who assisted with recruiting families; Diana Crom, who coordinated the intensive data collection for the study of preteen girls; and graduate and undergraduate students, and research assistants who helped coordinate studies, and collect and process data. I am especially grateful to the families who participated in the studies.
Footnotes
For a discussion of terminology regarding sex and gender, and its application to work discussed here, see (1).
The degree of exposure to androgens varies among individuals with C-CAH as a result of the specific mutation causing the condition and is reflected in the severity of the disease.
Examples of male- and female-typed activities include childhood toys (e.g., trucks versus dolls), adolescent and adult hobbies (e.g., sports, interest in building things versus dancing, crafts), household chores (e.g., home repairs versus preparing food), and academic pursuits (e.g., math versus language arts). For information on the gendered nature of these activities, see (13-15.)
References
- 1.Blakemore JEO, Berenbaum SA, Liben LS. Gender development. New York, NY: Psychology Press/Taylor & Francis; 2009. [Google Scholar]
- 2.Hyde JS. The gender similarities hypothesis. American Psychologist. 2005;60:581–592. doi: 10.1037/0003-066x.60.6.581. [DOI] [PubMed] [Google Scholar]
- 3.Ruble DN, Martin CL, Berenbaum SA. Gender development. In: Eisenberg N, editor. Handbook of child psychology Social, emotional, and personality development. 6th. Vol. 3. New York, NY: Wiley; 2006. pp. 858–932. [Google Scholar]
- 4.Martin CL, Halverson CF. A schematic processing model of sex typing and stereotyping in children. Child Development. 1981;52:1119–1134. doi: 10.2307/1129498. [DOI] [Google Scholar]
- 5.Martin CL, Ruble DN. Children's search for gender cues: Cognitive perspectives on gender development. Current Directions in Psychological Science. 2004;13:67–70. doi: 10.1111/j.0963-7214.2004.00276.x. [DOI] [Google Scholar]
- 6.de Vries GJ, Fields CT, Peters NV, Whylings J, Paul MJ. Sensitive periods for hormonal programming of the brain. In: Andersen SL, Pine DS, editors. Current Topics in the Behavioral Neurosciences: The Neurobiology of Childhood. Vol. 16. Berlin, Germany: Springer; 2014. pp. 79–108. [DOI] [PubMed] [Google Scholar]
- 7.Wallen K. The organizational hypothesis: Reflections on the 50th anniversary of the publication of Phoenix, Goy, Gerall, and Young (1959) Hormones and Behavior. 2009;55:561–565. doi: 10.1016/j.yhbeh.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Merke DP, Bornstein SR. Congenital adrenal hyperplasia. Lancet. 2005;365:2125–2136. doi: 10.1016/S0140-6736(05)66736-0. [DOI] [PubMed] [Google Scholar]
- 9.Berenbaum SA, Beltz AM. Sexual differentiation of human behavior: Effects of prenatal and pubertal organizational hormones. Frontiers in Neuroendocrinology. 2011;32:183–200. doi: 10.1016/j.yfrne.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Berenbaum SA, Beltz AM. How early hormones shape gender development. Current Opinion in Behavioral Sciences. 2016;7:53–60. doi: 10.1016/j.cobeha.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall CM, Jones JA, Meyer-Bahlburg HFL, Dolezal C, Coleman M, Foster P, Clayton PE. Behavioral and physical masculinization are related to genotype in girls with congenital adrenal hyperplasia. Journal of Clinical Endocrinology and Metabolism. 2004;89:419–424. doi: 10.1210/jc.2003-030696. [DOI] [PubMed] [Google Scholar]
- 12.Nordenström A, Servin A, Bohlin G, Larsson A, Wedell A. Sex-typed toy play behavior correlates with the degree of prenatal androgen exposure assessed by CYP21 genotype in girls with congenital adrenal hyperplasia. Journal of Clinical Endocrinology and Metabolism. 2002;87:5119–5124. doi: 10.1210/jc.2001-011531. [DOI] [PubMed] [Google Scholar]
- 13.Endendijk JJ, Beltz AM, McHale SM, Bryk K, Berenbaum SA. Linking prenatal androgens to gender-related attitudes, identity, and activities: Evidence from girls with congenital adrenal hyperplasia. Archives of Sexual Behavior. 2016;45:1807–1815. doi: 10.1007/s10508-016-0693-7. [DOI] [PubMed] [Google Scholar]
- 14.Berenbaum SA. Effects of early androgens on sex-typed activities and interests in adolescents with congenital adrenal hyperplasia. Hormones and Behavior. 1999;35:102–110. doi: 10.1006/hbeh.1998.1503. [DOI] [PubMed] [Google Scholar]
- 15.McHale SM, Kim JY, Whiteman S, Crouter AC. Links between sex-typed time use in middle-childhood and gender development in early adolescence. Developmental Psychology. 2004;40:868–881. doi: 10.1037/0012-1649.40.5.868. [DOI] [PubMed] [Google Scholar]
- 16.Berenbaum SA, Snyder E. Early hormonal influences on childhood sex-typed activity and playmate preferences: Implications for the development of sexual orientation. Developmental Psychology. 1995;31:31–42. doi: 10.1037/0012-1649.31.1.31. [DOI] [Google Scholar]
- 17.Pasterski VL, Geffner ME, Brain C, Hindmarsh P, Brook C, Hines M. Prenatal hormones and postnatal socialization by parents as determinants of male-typical toy play in girls with congenital adrenal hyperplasia. Child Development. 2005;76:264–278. doi: 10.1111/j.1467-8624.2005.00843.x. [DOI] [PubMed] [Google Scholar]
- 18.Berenbaum SA, Beltz AM, Bryk KL, McHale SM. Gendered peer involvement in girls with congenital adrenal hyperplasia: Effects of prenatal androgens, gendered activities, and gender cognitions. Archives of Sexual Behavior. doi: 10.1007/s10508-017-1112-4. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beltz AM, Swanson JL, Berenbaum SA. Gendered occupational interests: Prenatal androgen effects on psychological orientation to things versus people. Hormones and Behavior. 2011;60:313–317. doi: 10.1016/j.yhbeh.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frisén L, Nordenström A, Falhammar H, Filipsson H, Holmdahl G, Janson PO, Nordenskjöld A. Gender role behavior, sexuality, and psychosocial adaptation in women with congenital adrenal hyperplasia due to CYP21A2 deficiency. Journal of Clinical Endocrinology & Metabolism. 2009;94:3432–3439. doi: 10.1210/jc.2009-0636. [DOI] [PubMed] [Google Scholar]
- 21.Strandqvist A, Falhammar H, Lichtenstein P, Hirschberg AL, Wedell A, Norrby C, Nordenskjöld A. Suboptimal psychosocial outcomes in patients with congenital adrenal hyperplasia: Epidemiological studies in a nonbiased national cohort in Sweden. Journal of Clinical Endocrinology & Metabolism. 2014;99:1425–1432. doi: 10.1210/jc.2013-3326. [DOI] [PubMed] [Google Scholar]
- 22.Meyer-Bahlburg HFL, Dolezal C, Baker SW, Ehrhardt AA, New MI. Gender development in women with congenital adrenal hyperplasia as a function of disorder severity. Archives of Sexual Behavior. 2006;35:667–684. doi: 10.1007/s10508-006-9068-9. [DOI] [PubMed] [Google Scholar]
- 23.Hines M, Constantinescu M, Spencer D. Early androgen exposure and human gender development. Biology of Sex Differences. 2015;6:3. doi: 10.1186/s13293-015-0022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassett JM, Siebert ER, Wallen K. Sex differences in rhesus monkey toy preferences parallel those of children. Hormones and Behavior. 2008;54:359–364. doi: 10.1016/j.yhbeh.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benenson JF, Tennyson R, Wrangham RW. Male more than female infants imitate propulsive motion. Cognition. 2011;121:262–267. doi: 10.1016/j.cognition.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Berenbaum SA, Bryk KLK, Beltz AM. Early androgen effects on spatial and mechanical abilities: Evidence from congenital adrenal hyperplasia. Behavioral Neuroscience. 2012;126:86–96. doi: 10.1037/a0026652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hampson E, Rovet JF. Spatial function in adolescents and young adults with congenital adrenal hyperplasia: Clinical phenotype and implications for the androgen hypothesis. Psychoneuroendocrinology. 2015;54:60–70. doi: 10.1016/j.psyneuen.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Mueller SC, Temple V, Oh E, VanRyzin C, Williams A, Cornwell B, Merke DP. Early androgen exposure modulates spatial cognition in congenital adrenal hyperplasia (CAH) Psychoneuroendocrinology. 2008;33:973–980. doi: 10.1016/j.psyneuen.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berenbaum SA, Bailey JM. Effects on gender identity of prenatal androgens and genital appearance: Evidence from girls with congenital adrenal hyperplasia. Journal of Clinical Endocrinology and Metabolism. 2003;88:1102–1106. doi: 10.1210/jc.2002-020782. [DOI] [PubMed] [Google Scholar]
- 30.Dessens AB, Slijper FME, Drop SLS. Gender dysphoria and gender change in chromosomal females with congenital adrenal hyperplasia. Archives of Sexual Behavior. 2005;34:389–397. doi: 10.1007/s10508-005-4338-5. [DOI] [PubMed] [Google Scholar]
- 31.Pasterski V, Zucker KJ, Hindmarsh PC, Hughes IA, Acerini CL, Spencer D, Hines M. Increased cross-gender identification independent of gender role behavior in girls with congenital adrenal hyperplasia: Results from a standardized assessment of 4- to 11-year-old children. Archives of Sexual Behavior. 2015;44:1363–1375. doi: 10.1007/s10508-014-0385-0. [DOI] [PubMed] [Google Scholar]
- 32.Liben LS, Bigler RS. The developmental course of gender differentiation: Conceptualizing, measuring, and evaluating constructs and pathways. Monographs of the Society for Research in Child Development. 2002;67:vii–viii. 1–187. doi: 10.1111/1540-5834.t01-1-00188. Serial No. 269. [DOI] [PubMed] [Google Scholar]
- 33.Martin CL, Ruble DN, Szkrybalo J. Cognitive theories of early gender development. Psychological Bulletin. 2002;128:903–933. doi: 10.1037/0033-2909.128.6.903. [DOI] [PubMed] [Google Scholar]
- 34.Hines M, Pasterski V, Spencer D, Neufeld S, Patalay P, Hindmarsh PC, Acerini CL. Prenatal androgen exposure alters girls’ responses to information indicating gender-appropriate behaviour. Philosophical Transactions of the Royal Society of London B. 2016;371:20150125. doi: 10.1098/rstb.2015.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hines M, Kaufman F. Androgen and the development of human sex-typical behavior: Rough-and-tumble play and sex of preferred playmates in children with congenital adrenal hyperplasia (CAH) Child Development. 1994;65:1042–1053. doi: 10.1111/j.1467-8624.1994.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 36.Pasterski V, Geffner ME, Brain C, Hindmarsh P, Brook C, Hines M. Prenatal hormones and childhood sex segregation: Playmate and play style preferences in girls with congenital adrenal hyperplasia. Hormones and Behavior. 2011;59:549–555. doi: 10.1016/j.yhbeh.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Servin A, Nordenström A, Larsson A, Bohlin G. Prenatal androgens and gender-typed behavior: A study of girls with mild and severe forms of congenital adrenal hyperplasia. Developmental Psychology. 2003;39:440–450. doi: 10.1037/0012-1649.39.3.440. [DOI] [PubMed] [Google Scholar]
- 38.Martin CL, Fabes RA. The stability and consequences of young children's same-sex peer interactions. Developmental Psychology. 2001;37:431–446. doi: 10.1037/0012-1649.37.3.431. [DOI] [PubMed] [Google Scholar]
- 39.Martin CL, Fabes RA, Hanish L, Leonard S, Dinella LM. Experienced and expected similarity to same-gender peers: Moving toward a comprehensive model of gender segregation. Sex Roles. 2011;65:421–434. doi: 10.1007/s11199-011-0029-y. [DOI] [Google Scholar]
- 40.Berenbaum SA, Blakemore JEO, Beltz AM. A role for biology in gender-related behavior. Sex Roles. 2011;64:804–825. doi: 10.1007/s11199-011-9990-8. [DOI] [Google Scholar]
- 41.Eagly AH, Wood W. The nature-nurture debates: 25 years of challenges in understanding the psychology of gender. Perspectives on Psychological Science. 2013;8:340–357. doi: 10.1177/1745691613484767. [DOI] [PubMed] [Google Scholar]
- 42.Maccoby EE. Perspectives on gender development. International Journal of Behavioral Development. 2000;24:398–406. doi: 10.1080/016502500750037946. [DOI] [Google Scholar]


