Abstract
Three-dimensional aggregation of human mesenchymal stem cells (hMSCs) has been used to enhance their therapeutic properties but current fabrication protocols depend on laboratory methods and are not scalable. In this study, we developed thermal responsive poly(N-isopropylacrylamide) grafted microcarriers (PNIPAM-MCs), which supported expansion and thermal detachment of hMSCs at reduced temperature (23.0 °C). hMSCs were cultured on the PNIPAM-MCs in both spinner flask (SF) and PBS Vertical-Wheel (PBS-VW) bioreactors for expansion. At room temperature, hMSCs were detached as small cell sheets, which subsequently self-assembled into 3D hMSC aggregates in PBS-VW bioreactor and remain as single cells in SF bioreactor owing to different hydrodynamic conditions. hMSC aggregates generated from the bioreactor maintained comparable immunomodulation and cytokine secretion properties compared to the ones made from the AggreWell®. The results of the current study demonstrate the feasibility of scale-up production of hMSC aggregates in the suspension bioreactor using thermal responsive microcarriers for integrated cell expansion and 3D aggregation in a close bioreactor system and highlight the critical role of hydrodynamics in self-assembly of detached hMSC in suspension.
Keywords: Thermal Responsive Microcarriers, hMSCs Expansion, Spinner Flask, PBS Vertical-Wheel Bioreactor, 3D Aggregation Culture
1. Introduction
Human mesenchymal stem cells (hMSCs) isolated from multiple adult tissues are the most commonly-tested adult stem cells in cell therapy in a wide range of diseases. However, it is well-documented that in vitro expansion increases hMSC population heterogeneity and reduces therapeutic potency, contributing to inconsistent clinical outcome. Preserving the therapeutic potency of culture-expanded hMSCs requires novel technology that restores the innate hMSC properties.
Recent studies show that hMSC from various sources have an innate ability to spontaneously assemble into 3D aggregates that restore and improve a wide range of therapeutic properties including multi-potentiality, secretion of trophic factors, and immunomodulatory properties [1, 2]. Several studies using 3D aggregate-derived hMSCs have shown better therapeutic outcomes in disease models, including stroke, myocardial infarction, hindlimb ischemia, and Parkinson’s disease when compared to 2D cultured hMSCs [1, 3]. Studies have shown that even short-term aggregation significantly enhanced hMSC properties and therapeutic potential. For examples, one-day preconditioning of hMSCs as 3D aggregates enhanced angiogenesis and infarct size reduction in rat myocardial infraction (MI) model [4], and three-day culture of hMSCs as aggregates improved the endogenous neurogenesis and survival after transplantation in rat Parkinson disease model [5]. Therefore, there is an increasing interest in the scalable production of hMSC aggregates.
The current protocols for the production of hMSC 3D aggregates are based on laboratory and non-scalable methods, including hanging drop, centrifugation, and ultra-low attachment plates [3]. These laboratory methods require separate steps in cell expansion and aggregation and are not scalable. The limitation of the laboratory techniques has motivated the development of advanced aggregate production system such as the robot-assisted hanging drop culture that has the ability for high-throughput production of cellular spheroids [6]. Recently, our laboratory has reported the production of hMSC aggregates with controlled size distribution in the Wave Bioreactor [7]. While these methods have the potential for large scale production of 3D cell aggregates, they still require separate steps in cell expansion and formation of aggregates.
Microcarrier-based bioreactors have been widely used for the large scale production of adherent mammalian cells because they provide a closed culture environment that facilitates automation and real-time sampling and monitoring [8]. Microcarriers in spinner flask (SF) and the PBS vertical wheel (PBS-VW) bioreactors have been used in clinical-scale expansion of hMSCs [9–11]. To date, however, no studies have reported the production of 3D hMSC aggregates in a microcarrier bioreactor system. In microcarrier-based bioreactors, proteolytic enzyme treatment is frequently used to detach the adherent cells from microcarreirs but the presence of the proteolytic enzyme prevents cell aggregation. Alternatively, surface coating of multi-well plates and microcarriers with thermal responsive polymers such as poly(N-isopropylacrylamide) (PNIPAM) induce cell detachment at a reduced temperature in the absence of proteolytic enzyme. Tamura et al. reported the synthesis of PNIPAM functional groups onto microcarriers for the large-scale culture of Chinese hamster ovary (CHO) cells with high proliferation and cell detachment efficiency of [12]. In a subsequent study, Yang et al. reported hMSC expansion on PNIPAM modified Cytodex-3 microcarrier in a spinner flask bioreactor, in which hMSCs were harvested as single cells after 5 days of expansion [13]. Our prior study showed that hMSCs detached from a thermal responsive culture surface formed 3D hMSC aggregates with enhanced clonogenic properties compared to the aggregates formed by enzymatically detached hMSCs owing to the retention of endogenous extracellular matrix (ECM) following thermal detachment [14].
The objective of our current study is to test the feasibility of hMSC aggregate production using thermal responsive PNIPAM grafted-microcarriers (PNIPAM-MCs) in suspension bioreactors (as illustrated in Figure 1). The results demonstrate proof of concept for the large-scale formation of hMSC aggregates in a PBS Vertical Wheel (PBS-VW) bioreactor by inducing thermal release of hMSC following a 3-fold expansion on the PNIPAM-MCs. The bioreactor-derived hMSC aggregates maintain immunomodulatory properties comparable to the ones produced via commercial AggreWell®.
Figure 1. Thermal Responsive Microcarrier Bioreactor System Diagram and Timeline.
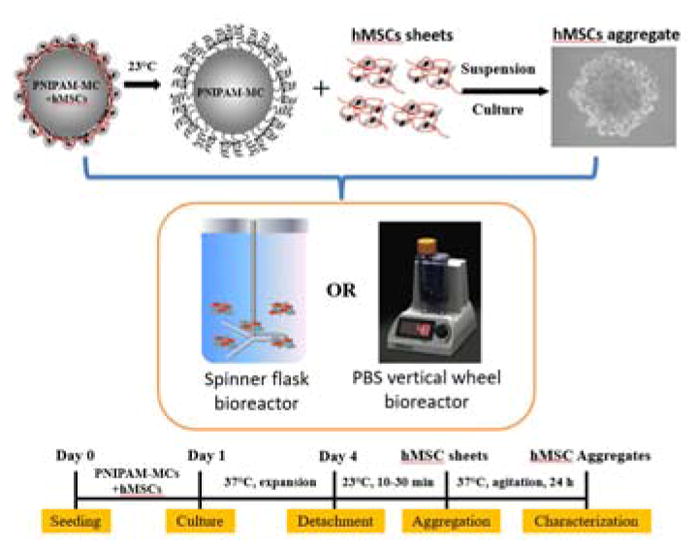
hMSCs were seeded expanded on thermal responsive PNIPAM grafted microcarriers (PNIPAM-MCs) in bioreactors. After expansion, hMSCs were released from the PNIPAM-MCs and subsequently self-assembled into 3D aggregates in suspension culture. Experimental timeline shows the non-invasive expansion and aggregation of hMSCs. Figure was partially adapted from https://www.pbsbiotech.com/.
2. Material and Methods
2.1. PNIPAM grafted microcarriers synthesis
PNIPAM grafted microcarriers were fabricated following the methods detailed in Tamura et al. [12]. In brief, N-isopropylacrylaminde monomer (NIPAM, 2.4 g, 22.0 mmol) was purified and completely dissolved in 60.0 mL 2-propanol (Mixture 1). Nitrogen gas was inserted in the flask for deoxygenation of the mixture. The following procedures were carried out in a nitrogen environment. Copper(I) chloride (79.0 mg, 0.8 mmol), copper(II) dichloride (11.0 mg, 80.0 μmol) and tris ((2-dimenthylamino)ethyl) amine (Me6TREN, 203 mg, 0.86 mmol) were well mixed with 20.0 mL 2-propanol with agitation (Mixture 2). One gram of chloromethylated poly(styrene) (CMPS) microcarriers (50–100 mesh) was added to a 3-neck round bottom flask. Then Mixture 1 and Mixture 2 were successively added to the 3-neck flask, and agitated under room temperature for 24 hours or until reaction completed. Me6TREN was prepared according to the reference [15]. For purification, PNIPAM-MCs were collected by centrifugation, and the catalyst and solvent were removed via 3 times of methanol washes under ultrasonic agitation. Further purification of the microcarriers was carried out by adding 50.0 mmol/L EDTA solution and shaking for 1 day to remove the copper catalysts. The PNIPAM-MCs were extensively washed with Milli-Q water and air-dried. All chemicals were purchased from Sigma Aldrich (St. Louis, MO) unless otherwise noted.
To verify the grafting reaction on the PNIPAM-MCs, the attenuated total reflection-Fourier transform infrared (ATR-FTIR) spectra of PNIPAM-MCs were determined using an Alpha FTIR spectrometer with a Platinum ATR Quick Snap sampling module (single reflection diamond crystal) (BRUKER, Billerica, MA). A small portion (~10 mg) of microcarrier sample was placed on the sample station, and the spectra from 500–4000 cm−1 were collected at the resolution of 4 cm−1. CMPS microcarriers (50–100 mesh) from Sigma Aldrich without any treatment, referred to as Control-MCs, were used as controls in all bioreactor experiment.
2.2. hMSCs expansion on PNIPAM grafted microcarriers
hMSCs were acquired from Tulane Center for Stem Cell and Research and Regenerative Medicine. Standardized frozen hMSCs harvested from healthy human bone marrow were thawed and cultured following the protocol from our prior publication [16]. Briefly, hMSCs were cultured in complete culture medium (CCM), which was formulated as minimum essential medium-α (Life Technologies, Carlsbad, CA) with 10% v/v fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA) and 1% v/v penicillin/streptomycin (Life Technolgies, Carlsbad, CA). All cell culture was carried out in a standard incubator with 5% CO2 under 37°C. hMSCs from passage 5–6 were used in all experiments.
For the SF bioreactor experiments, 2.0 million hMSCs and 1.0 gram of PNIPAM grafted microcarriers were added in a 125.0 mL Wheaton Celstir® Suspension Culture spinner flask (VWR international, Radnor, PA) with Wheaton Biostir® Stirrers. The final volume of culture medium was adjusted to 100.0 mL. Initial seeding conditions are as follows: the stirrer is programmed to 85 rpm agitation for 5 minutes and then stopped for 30 minutes, and was repeated for a total of 20 cycles. The SF bioreactor was placed in a standard culture incubator with 5% CO2 under 37°C for up to 7 days at 85 rpm. Half of the culture medium was replaced every other day with medium sampling at the same time. To determine the time required for hMSC detachment from PNIPAM-MCs, hMSCs were seeded with PNIPAM-MCs in the SF bioreactor for 2 days culture, and 2.0 mL sample was re-plated on the ultra-low attachment surface (Corning, Corning, NY) and incubated at room temperature (23.0 °C). Majority of cell detachment occurs during the initial 30 minutes of incubation based on DAPI (Sigma Aldrich St. Louis, MO) staining of the microcarreirs.
To determine the kinetics of hMSCs growth on the microcarriers, an MTT assay was used according to vender’s instruction. Briefly, cell and microcarriers were mixed with 100 μL MTT reagent and incubated at 37°C for 2 hours. The purple color of the mixture was extracted by DMSO and the absorbance was read at 590 nm on a iMark™ microplate reader (BioRad, Hercules, CA). For metabolic analysis, supernatants were collected from SF bioreactor at days 1, 3, 5 and 7, and glucose and lactate concentrations measured using an YSI 2500 Biochemistry Select Analyzer (Yellow Spring, OH). The specific metabolic consumption and production rates as well as the yield of lactate from glucose were calculated using the equations detailed in previous studies [17, 18].
For the PBS-VW bioreactor experiments, 2.0 million hMSCs were seeded with 0.5 g PNIPAM grafted microcarriers in 60.0 mL CCM in a 0.1-MAG PBS-VW bioreactor (PBS Biotech, Camarillo, CA). The vertical-wheel speed was set at 60 rpm for 2 min and then stopped for 28 min, and this cycling was repeated for the first 4 hours, followed by 6 days of culture at 60.0 rpm in a standard CO2 incubator with a media change at day 3. To initiate cell detachment, the PBS-VW bioreactor was moved to room temperature (23 °C) for 10 min at 60.0 rpm. Microcarriers were removed by settling down and aspiration several times. Final volume of medium was adjusted to 60.0 mL. Then the bioreactor was moved back to standard incubator and detached hMSCs sheets were continuously cultured up to 24 hours to form aggregates. As controls, hMSC aggregates were formed in AggreWell® (STEMCELL Technologies, Cambridge, MA) following the commercial product manual. Cell viability was determined by ReadyProbes™ Cell Viability Imaging Kit (ThermoFisher, Waltham, MA). IDO activity was determined by following the methods outlined in our previous publication [19]. Levels of cytokines were determined by enzyme-linked immunosorbent assay (ELISA) (R&D System, Minneapolis, MN) following the vender’s protocol.
2.3. Statistics and data analysis
All experiments were performed at least in triplicate (n = 3), and representative data are presented in the manuscript. Experimental results are expressed as means ± standard deviation (SD) of the samples. Statistical analysis was performed by one-way ANOVA and Tukey’s post hoc test for multiple comparisons, and significance at p < 0.05 was included.
3. Results and Discussion
3.1. Synthesis and characterization of PNIPAM grafted microcarriers
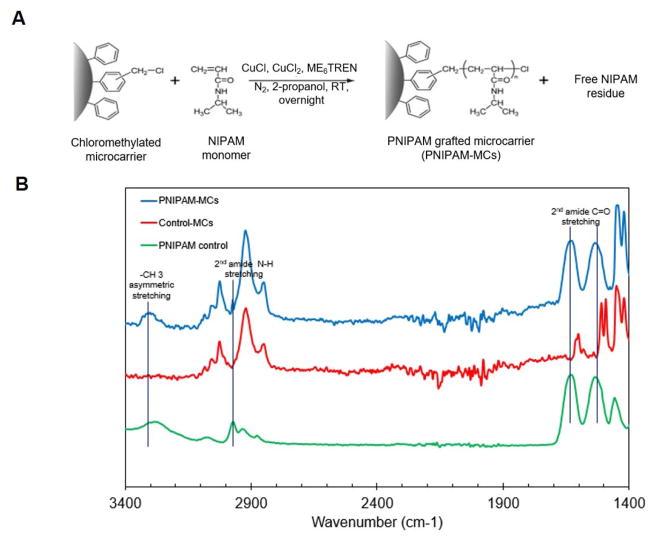
The PNIPAM-MCs were fabricated by initiating ATRP reaction on the commercial CMPS microcarriers as illustrated in Figure 2A. The polymerization reaction was initiated at the chloromethyl functional groups located at the surface of CMPS microcarrier, which allows for the direct quantification of grafted PNIPAM polymer chains. After reaction, ATR-FTIR was used to characterize the PNIPAM polymer chains grafted on the microcarriers. As shown in Figure 2B, PNIPAM-MCs have characteristic peaks at the following positions: 3,292 cm−1 for secondary amide N-H stretching, 2,970 cm−1 for the -CH3 asymmetric stretching, 1650 cm−1 and 1,550 cm−1 for the secondary amide C=O stretching [13, 20], which are not presented on the Control-MCs, confirming the presence of the PNIPAM polymer chains on the PNIPAM-MCs.
Figure 2. Synthesis and Characterization of PNIPAM-MCs.
(A) Scheme of synthesizing PNIPAM-MC via atom transfer radical polymerization (ATRP). (B) Attenuated total reflection (ATR) characterization of PNIPAM (green line), control microcarrier (Control-MCs, red line) and PNIPAM-MCs (blue line).
A variety of methods have been used to coat or chemically synthesize thermal responsive PNIPAM polymer on microcarriers to achieve non-enzymatic cell detachment at a reduced temperature. Yang et al reported synthesizing PNIPAM polymer onto Cytodex-3 microcarriers via conjugation of carboxylic acid group of the PNIPAM-COOH to the amine groups pre-synthesized on the Cytodex-3 surface [13]. However, this method of direct conjugation requires multiple reaction steps and has low specificity. Tamura et al. reported grafting of PNIPAM polymer chains on CMPS microcarriers via surface initiated atom transfer radical polymerization (ATRP) [12]. This method provides a simple route of direct NIPAM polymerization onto the microcarrier without pre-treatment and is adopted in the current study.
3.2. hMSCs growth and detachment on PNIPAM grafted microcarriers in SF bioreactor
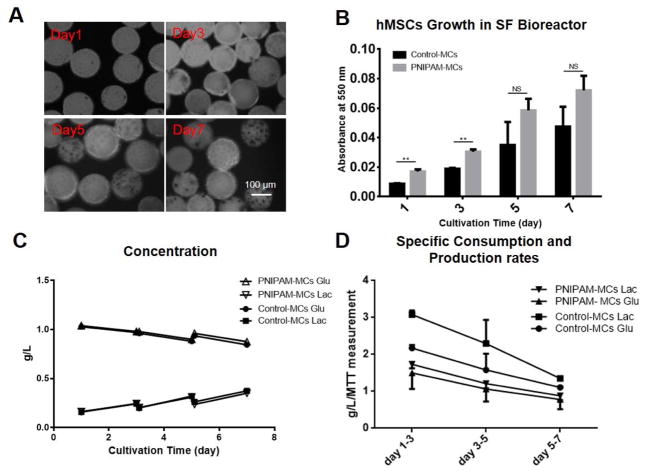
To investigate the ability of PNIPAM-MCs in supporting hMSCs expansion, hMSCs were seeded on PNIPAM-MCs or Control-MCs in the SF bioreactor for the analysis of growth kinetics and metabolic activity. As shown in Figure 3A and B, hMSC exhibited higher seeding efficiency (shown at day 1) but comparable proliferation rates on PNIPAM-MCs compared to the control over a 7-day culture in the SF bioreactor. At day 7, hMSCs on the PNIPAM-MCs exhibited a 4.7-fold expansion comparable to the 4.9-fold on Control-MCs withno significant difference. These results are also comparable to previous hMSC culture studies using commercial Cytodex-3 and CultiSpher-S microcarriers in a SF bioreactor [13, 21]. Our results also showed that PNIPAM-MCs have better cell adhesion compared to Control-MCs, presumably owing to increased hydrophilicity as reported previously [12].
Figure 3. hMSCs Cultured on PNIPAM-MCs in Spinner Flask Bioreactor.
(A) MTT staining of hMSCs on PNIPAM grafted microcarriers in spinner flask at day 1, 3, 5 and 7. (B) MTT measurement of hMSCs growth in spinner flask bioreactor using PNIPAM-MCs and Control-MCs. (C) Glucose and lactate concentration for hMSCs on Control-MCs and PNIPAM-MCs. (D) Specific glucose consumption and lactate production rates for hMSCs cultured with Control-MCs and PNIPAM-MCs. Data represent the mean of at least 3 independent determinations; errors represent the standard error in the mean. NS: p > 0.05, *: p < 0.05, **: p < 0.01.
Glucose and lactate concentrations were determined from the culture medium samples before and after partial medium changes every other day. As shown in Figure 3C and D, the specific glucose consumption/lactate production rates are higher at the beginning and decrease over time for the Control-MCs and the PNIPAM-MCs with a consistent lactate/glucose yield of approximately 1.4 [22].. Comparable trends were also reported in the expansion of umbilical cord-derived MSCs using CultiSpher-S microcarriers in SF bioreactor [18]. PNIPAM-MCs were also effective in supporting expansion and detachment of other types of adherent mammalian cells such as CHO and primary bovine aortic endothelial cells [12, 23], suggesting the potential use in a broad range of cell types for other applications.
After 7 days of expansion in a standard CO2 incubator at 37 °C, the SF bioreactor was removed to room temperature to induce hMSC detachment from the PNIPAM-MCs. After 30 min at room temperature at 60.0 rpm, hMSCs detached from microcarriers mainly as single cells. Compared to cell detachment in static culture, agitation in the SF bioreactor accelerated cell detachment with majority of the cells detached. However, the detached hMSCs remained floating and were unable to aggregate into spheroids even after extended agitation for up to 24 hours at 37 °C, suggesting the unfavorable hydrodynamics conditions in the SF bioreactor for hMSC aggregation.
3.3. hMSCs detachment and aggregation in the PBS-VW bioreactor
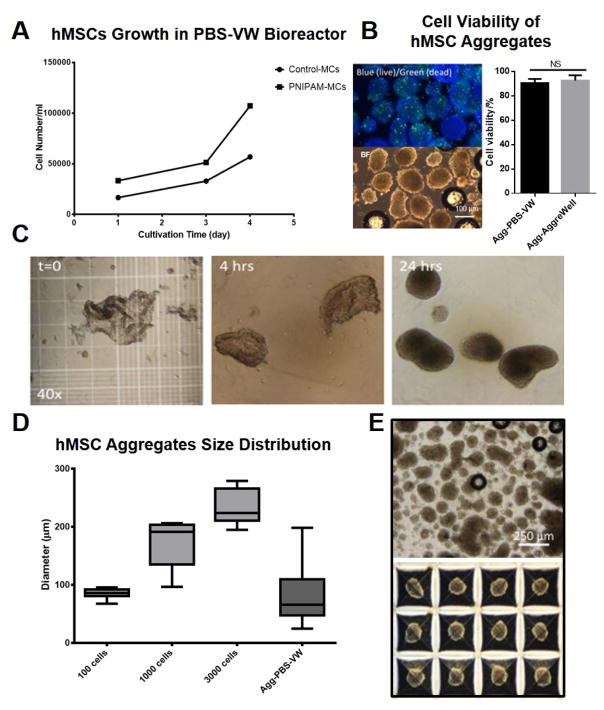
To determine the influence of bioreactor hydrodynamic conditions on hMSC aggregation, a PBS-VW bioreactor was used to expand hMSCs on the PNIPAM-MCs followed by thermal release at room temperature (Agg-PBS-VW). For comparison, aggregates generated via AggreWell® (Agg-AggreWell), a commercial plate that supports hMSCs aggregation by centrifugation, were used as controls. As shown in Figure 4A, hMSCs have higher seeding efficiency and comparable expansion on the PNIPAM-MCs in the PBS-VW bioreactor as compared to the results from the SF bioreactor. Cell number increase 3.4 folds for PNIPAM-MCs and 3.2 folds for Control-MCs at day 4. The results are also comparable with the growth results recently reported using Synthemax II microcarriers in the PBS-VW bioreactor [9]. To induce hMSC aggregation following detachment, the PBS-VW bioreactor was moved to room temperature for 10 min under agitation to initiate cell release from the PNIPAM-MCs. As shown in Figure 4C, hMSCs were detached from the PNIPAM-MCs as small cell sheets, and subsequently formed aggregates after 24 hours of agitation at 60 rpm in a standard CO2 incubator. No significant difference in cell viability between the Agg-PBS-VW (90.6%) and the Agg-AggreWell (92.6%) as shown in Figure 4B. The Agg-PBS-VW have comparable diameter as the Agg-AggreWell of 100 cells with a broader size distribution (Figure 4D and E).
Figure 4. hMSCs cultured on PNIPAM grafted microcarriers and aggregation in PBS-VW bioreactor.
(A) Growth curve of hMSCs cultured on Control-MCs and PNIPAM-MCs in PBS-VW bioreactor. (B) Cell viability in aggregates determined by LIVE/DEAD staining after 24h aggregation from PBS-VW bioreactor and AggreWell®. (C) hMSCs sheet detached from PNIPAM-MCs was cultured in PBS-VW bioreactor. After 24h, hMSCs sheets self-assembled into 3D aggregates. (D) and (E) Size and distribution of hMSC aggregates generated via PBS-VW bioreactor and AggreWell®, respectively. Data represent the mean of at least 3 independent determinations; errors represent the standard error in the mean. NS: p > 0.05.
Effective cell detachment from microcarrier using proteolytic enzyme treatment following expansion has been a major barrier because of low detachment and harvest efficiency [24]. Nienow et al. reported a significant increase of cell detachment efficiency by increasing the impeller speed (about 5-fold increase in impeller speed) in the SF bioreactor following enzymatic treatment [25]. Under these conditions, however, hMSCs are detached as single cells and cannot form 3D aggregates in SF bioreactor. In our previous study using thermal responsive 2D culture plates, hMSCs detached as small cell sheets from the thermal responsive culture surface readily formed 3D aggregates [14]. The thermally released hMSC clusters have a higher quantity of ECM proteins that not only preserves cell-cell contacts and their biological properties but also enhance 3D aggregation by reinforcing cell-cell and cell-ECM interactions, promoting hMSC aggregation [14].
Our recent study has shown that the hydrodynamic conditions in the Wave bioreactor influenced hMSC aggregation and aggregate size distribution by controlling the frequency and stability of cell-cell contacts during aggregation [7]. In contrast to the SF bioreactor, the thermally detached hMSC sheets readily aggregated in the PBS-VW bioreactor due to the favorable hydrodynamic environment in the PBS-VW bioreactor. In our recent study, computational fluid dynamics (CFD) simulation showed that average shear stress of 0.04 Pa in the bulk fluid in the Wave bioreactor prevented hMSC aggregation [7]. Using similar simulation approach, others have shown that SF bioreactor of similar dimensions and operation conditions as used in the current study have significantly higher shear stress with broader distribution (0.04 – 0.14 Pa at 30 rpm and 0.2 – 0.42 Pa at 80 rpm), suggesting the hydrodynamic environment of high fluid shear stress in the SF bioreactor is the major factor preventing spontaneous aggregation of detached hMSC cells [26, 27]. Together, the results demonstrate the feasibility of 3D aggregation of the thermally released hMSC in the PBS-VW bioreactor and highlight the key role of hydrodynamic environment in spontaneous aggregation of detached hMSC sheets.
3.4. Immuno-response and secretory profile of bioreactor-generated hMSCs aggregates
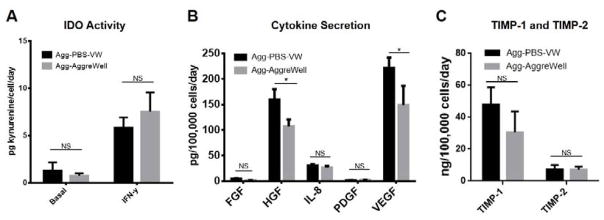
To examine the biological properties of hMSC aggregates from the PBS-VW bioreactor, Agg-PBS-VW and Agg-AggreWell (100 cells/aggregate) were treated with IFN-γ to determine their immunosuppressive properties. The levels of IDO activities for both Agg-PBS-VW and Agg-AggreWell are comparable before and after IFN-γ treatment, as shown in Figure 5A. As the secretion of trophic factors is an important property in hMSC’s clinical application, the levels of FGF, HGF, IL-8, PDGF, TIMP-1, TIMP-2, and VEGF by Agg-PBS-VW and Agg-AggreWell were analyzed. As shown in Figure 5B and C, the Agg-PBS-VW has higher levels of HGF, and VEGF secretion and comparable levels of FGF, IL-8, PDGF, TIMP-1 and TIMP-2. One of our previous studies has shown that hMSC aggregates formed from thermally released hMSCs exhibit high survival rate and lower apoptosis under an in vitro ischemic condition compared to the aggregates formed from enzymatic harvested hMSCs [14]. Together, these results demonstrate the enhancement of biological properties in hMSC aggregates formed by thermal-released hMSCs.
Figure 5. hMSCs immunomodulation and cytokine secretion.
(A) IDO activity of hMSCs aggregates generated via PBS-VW bioreactor and AggreWell®. (B) and (C) Cytokine secretion of hMSCs aggregates generated via PBS-VW bioreactor and AggreWell®. 100 cells/aggregate from AggreWell® was tested. Data represent the mean of at least 3 independent determinations; errors represent the standard error in the mean. NS: p> 0.05, *: p < 0.05.
Aggregation-mediated hMSCs functional enhancement is increasingly recognized with highly therapeutic potentials. [1, 3]. Several groups have reported upregulation of properties via 3D aggregation of hMSCs with commercial device. Baraniak et al. has demonstrated 3D hMSCs aggregates made via AggreWell® preserved the multilinage-differentiation potential of hMSCs regardless of size difference and culture time [28]. hMSCs aggregates made via AggreWell® also exhibited enhanced IDO activity and cytokine secretion [29–31]. In this study, Agg-PBS-VW and Agg-AggreWell are comparable for their immunomodulation and cytokine secretion profile, demonstrated the enhancement of hMSCs therapeutic potential via bioreactor culture of hMSCs aggregates.
To date, large scale production of hMSC aggregates in bioreactor remain a major barrier in clinical application of 3D hMSCs culture. The results of the current study demonstrated feasibility of production of hMSC aggregates in the PBS-VW bioreactor using thermal responsive microcarriers for sequential cell expansion and 3D aggregation. This method integrates cell expansion and aggregation in a single, closed bioreactor system, facilitating scaling up and regulatory approval. While the effects of bioreactor hydrodynamic conditions on aggregate size distribution remains to be investigated, the PNIPAAM-MCs have the potential to significantly advance the technology for the scalable production of hMSC aggregates.
Highlights.
PNIPAM was grafted onto microcarriers to induce cell release at room temperature.
PNIPAM microcarriers supported cell expansion in both PBS-VW and SF bioreactors.
hMSCs detached as cell sheets in PBS-VW and formed aggregates.
High shear stress prevented hMSC aggregation in SF bioreactor.
hMSCs aggregates from PBS-VW have comparable enhancement in secretory function.
Acknowledgments
This work is supported by NIH R01 NS102395-01A1 and R43 HL128698-01. We are thankful for Dr Kenneth Hanson from Department of Chemistry in FSU for kindly providing the ATR-FTIR instrument.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sart S, Tsai AC, Li Y, Ma T. Three-dimensional aggregates of mesenchymal stem cells: cellular mechanisms, biological properties, and applications. Tissue engineering Part B, Reviews. 2014;20:365–380. doi: 10.1089/ten.teb.2013.0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Follin B, Juhl M, Cohen S, Perdersen AE, Kastrup J, Ekblond A. Increased Paracrine Immunomodulatory Potential of Mesenchymal Stromal Cells in Three-Dimensional Culture. Tissue engineering Part B, Reviews. 2016;22:322–329. doi: 10.1089/ten.teb.2015.0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Tsai A-C, Yuan X, Ma T. The Biology and Therapeutic Application of Mesenchymal Cells. John Wiley & Sons, Inc; 2016. The effect of three-dimensional aggregates on the biology of mesenchymal stromal cells; pp. 75–90. [Google Scholar]
- 4.Kim JH, Park IS, Park Y, Jung Y, Kim SH, Kim SH. Therapeutic angiogenesis of three-dimensionally cultured adipose-derived stem cells in rat infarcted hearts. Cytotherapy. 2013;15:542–556. doi: 10.1016/j.jcyt.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Schwerk A, Altschuler J, Roch M, Gossen M, Winter C, Berg J, Kurtz A, Steiner B. Human adipose-derived mesenchymal stromal cells increase endogenous neurogenesis in the rat subventricular zone acutely after 6-hydroxydopamine lesioning. Cytotherapy. 2015;17:199–214. doi: 10.1016/j.jcyt.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Tung YC, Hsiao AY, Allen SG, Torisawa YS, Ho M, Takayama S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. The Analyst. 2011;136:473–478. doi: 10.1039/c0an00609b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai AC, Liu Y, Yuan X, Chella R, Ma T. Aggregation kinetics of human mesenchymal stem cells under wave motion. Biotechnology journal. 2017;12 doi: 10.1002/biot.201600448. [DOI] [PubMed] [Google Scholar]
- 8.Sart S, Agathos SN. Large-Scale Expansion and Differentiation of Mesenchymal Stem Cells in Microcarrier-Based Stirred Bioreactors. Methods in molecular biology. 2016;1502:87–102. doi: 10.1007/7651_2015_314. [DOI] [PubMed] [Google Scholar]
- 9.Sousa MF, Silva MM, Giroux D, Hashimura Y, Wesselschmidt R, Lee B, Roldao A, Carrondo MJ, Alves PM, Serra M. Production of oncolytic adenovirus and human mesenchymal stem cells in a single-use, Vertical-Wheel bioreactor system: Impact of bioreactor design on performance of microcarrier-based cell culture processes. Biotechnology progress. 2015;31:1600–1612. doi: 10.1002/btpr.2158. [DOI] [PubMed] [Google Scholar]
- 10.Gross RE, Watts RL, Hauser RA, Bakay RA, Reichmann H, von Kummer R, Ondo WG, Reissig E, Eisner W, Steiner-Schulze H, Siedentop H, Fichte K, Hong W, Cornfeldt M, Beebe K, Sandbrink R, Spheramine G. Investigational, Intrastriatal transplantation of microcarrier-bound human retinal pigment epithelial cells versus sham surgery in patients with advanced Parkinson’s disease: a double-blind, randomised, controlled trial. The Lancet Neurology. 2011;10:509–519. doi: 10.1016/S1474-4422(11)70097-7. [DOI] [PubMed] [Google Scholar]
- 11.Dos Santos F, Campbell A, Fernandes-Platzgummer A, Andrade PZ, Gimble JM, Wen Y, Boucher S, Vemuri MC, da Silva CL, Cabral JM. A xenogeneic-free bioreactor system for the clinical-scale expansion of human mesenchymal stem/stromal cells. Biotechnology and bioengineering. 2014;111:1116–1127. doi: 10.1002/bit.25187. [DOI] [PubMed] [Google Scholar]
- 12.Tamura A, Kobayashi J, Yamato M, Okano T. Temperature-responsive poly(N-isopropylacrylamide)-grafted microcarriers for large-scale non-invasive harvest of anchorage-dependent cells. Biomaterials. 2012;33:3803–3812. doi: 10.1016/j.biomaterials.2012.01.060. [DOI] [PubMed] [Google Scholar]
- 13.Yang HS, Jeon O, Bhang SH, Lee SH, Kim BS. Suspension culture of mammalian cells using thermosensitive microcarrier that allows cell detachment without proteolytic enzyme treatment. Cell transplantation. 2010;19:1123–1132. doi: 10.3727/096368910X516664. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Ma T. Endogenous extracellular matrices enhance human mesenchymal stem cell aggregate formation and survival. Biotechnology progress. 2013;29:441–451. doi: 10.1002/btpr.1686. [DOI] [PubMed] [Google Scholar]
- 15.Ciampolini M, Nardi N. Five-Coordinated High-Spin Complexes of Bivalent Cobalt, Nickel, andCopper with Tris(2-dimethylaminoethyl)amine. Inorg Chem. 1966;5:41–44. [Google Scholar]
- 16.Tsai AC, Liu Y, Yuan X, Ma T. Compaction, fusion, and functional activation of three-dimensional human mesenchymal stem cell aggregate. Tissue engineering Part A. 2015;21:1705–1719. doi: 10.1089/ten.tea.2014.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dos Santos F, Andrade PZ, Boura JS, Abecasis MM, da Silva CL, Cabral JM. Ex vivo expansion of human mesenchymal stem cells: a more effective cell proliferation kinetics and metabolism under hypoxia. Journal of cellular physiology. 2010;223:27–35. doi: 10.1002/jcp.21987. [DOI] [PubMed] [Google Scholar]
- 18.Mizukami A, Fernandes-Platzgummer A, Carmelo JG, Swiech K, Covas DT, Cabral JM, da Silva CL. Stirred tank bioreactor culture combined with serum-/xenogeneic-free culture medium enables an efficient expansion of umbilical cord-derived mesenchymal stem/stromal cells. Biotechnology journal. 2016;11:1048–1059. doi: 10.1002/biot.201500532. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Munoz N, Tsai AC, Logan TM, Ma T. Metabolic Reconfiguration Supports Reacquisition of Primitive Phenotype in Human Mesenchymal Stem Cell Aggregates. Stem cells. 2017;35:398–410. doi: 10.1002/stem.2510. [DOI] [PubMed] [Google Scholar]
- 20.Li D, Zhang XY, Yao JF, Simon GP, Wang HT. Stimuli-responsive polymer hydrogels as a new class of draw agent for forward osmosis desalination. Chem Commun. 2011;47:1710–1712. doi: 10.1039/c0cc04701e. [DOI] [PubMed] [Google Scholar]
- 21.Yuan Y, Kallos MS, Hunter C, Sen A. Improved expansion of human bone marrow-derived mesenchymal stem cells in microcarrier-based suspension culture. Journal of tissue engineering and regenerative medicine. 2014;8:210–225. doi: 10.1002/term.1515. [DOI] [PubMed] [Google Scholar]
- 22.Tsai AC, Liu YJ, Ma T. Expansion of human mesenchymal stem cells in fibrous bed bioreactor. Biochem Eng J. 2016;108:51–57. [Google Scholar]
- 23.Matsuzaka N, Nakayama M, Takahashi H, Yamato M, Kikuchi A, Okano T. Terminal-functionality effect of poly(N-isopropylacrylamide) brush surfaces on temperature-controlled cell adhesion/detachment. Biomacromolecules. 2013;14:3164–3171. doi: 10.1021/bm400788p. [DOI] [PubMed] [Google Scholar]
- 24.de Soure AM, Fernandes-Platzgummer A, da Silva CL, Cabral JM. Scalable microcarrier-based manufacturing of mesenchymal stem/stromal cells. J Biotechnol. 2016;236:88–109. doi: 10.1016/j.jbiotec.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Nienow AW, Rafiq QA, Coopman K, Hewitt CJ. A potentially scalable method for the harvesting of hMSCs from microcarriers. Biochem Eng J. 2014;85:79–88. [Google Scholar]
- 26.Ismadi MZ, Hourigan K, Fouras A. Experimental Characterisation of Fluid Mechanics in a Spinner Flask Bioreactor. Processes. 2014;2:753. [Google Scholar]
- 27.Liovic P, Sutalo ID, Stewart R, Glattauer V, Meagher L. Fluid Flow and Stresses on Microcarriers in Spinner Flask Bioreactors. Ninth International Conference on CFD in the Minerals and Process Industries; Melbourne, Australia. 2012. [Google Scholar]
- 28.Baraniak PR, McDevitt TC. Scaffold-free culture of mesenchymal stem cell spheroids in suspension preserves multilineage potential. Cell and tissue research. 2012;347:701–711. doi: 10.1007/s00441-011-1215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimmermann JA, McDevitt TC. Pre-conditioning mesenchymal stromal cell spheroids for immunomodulatory paracrine factor secretion. Cytotherapy. 2014;16:331–345. doi: 10.1016/j.jcyt.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Rettinger CL, Fourcaudot AB, Hong SJ, Mustoe TA, Hale RG, Leung KP. In vitro characterization of scaffold-free three-dimensional mesenchymal stem cell aggregates. Cell and tissue research. 2014;358:395–405. doi: 10.1007/s00441-014-1939-0. [DOI] [PubMed] [Google Scholar]
- 31.Cook MM, Futrega K, Osiecki M, Kabiri M, Kul B, Rice A, Atkinson K, Brooke G, Doran M. Micromarrows--three-dimensional coculture of hematopoietic stem cells and mesenchymal stromal cells. Tissue engineering Part C, Methods. 2012;18:319–328. doi: 10.1089/ten.TEC.2011.0159. [DOI] [PubMed] [Google Scholar]