Abstract
Importance
Immune dysfunction underlies the pathogenesis of rheumatoid arthritis (RA) and inflammatory bowel disease (IBD). Immunosuppressive therapy is the standard of care for these diseases. Both immune dysfunction and therapy-related immunosuppression can inhibit cancer-related immune surveillance in this population. Drug-induced immunosuppression is a risk factor for non-melanoma skin cancer (NMSC), particularly squamous cell tumors. For patients with a history of NMSC, data are limited on the impact of these drugs on the risk of additional NMSCs.
Objective
To determine the relative hazard of a second NMSC in RA or IBD patients who use methotrexate, anti-tumor necrosis factor (anti-TNF) therapy, or thiopurines after an initial NMSC.
Design
Retrospective cohort study.
Setting
Individuals enrolled in Medicare.
Participants
Individuals with RA or IBD from 2006–2012.
Exposure
Exposure to methotrexate, thiopurines, anti-TNFs, sulfasalazine, hydroxychloroquine, abatacept, or rituximab after the incident NMSC surgery.
Outcome
A second NMSC occurring ≥1 year after the incident NMSC, using Cox regression models.
Results
Among 9,460 individuals (6,841 with RA, 2,788 with IBD), the incidence rate of second NMSC per 1,000 person-years was 58.2 (95% CI, 54.5–62.1) and 58.9 (53.2–65.2) in RA and IBD, respectively. Among RA patients, methotrexate used in conjunction with other medications was associated with an increased risk of second NMSC (HR 1.60, 95% CI 1.08–2.37). Adjusted for other medications, the risk of NMSC increased with >1 year of methotrexate use (HR 1.24, 95% CI 1.04–1.48). Compared to methotrexate alone, the addition of anti-TNF drugs was significantly associated with risk of NMSC (HR 1.49, 95% CI 1.03–2.16). Abatacept and rituximab were not associated with increased NMSC risk. The HRs for >1 year of thiopurine and anti-TNF use for IBD were 1.49 (95% CI 0.98–2.27) and 1.36 (95% CI 0.76–2.44), respectively.
Conclusion
Methotrexate use is associated with an increased risk of second NMSC. Anti-TNF use may increase the risk of second NMSC when used with methotrexate for RA. Whether thiopurine and/or anti-TNF use in IBD increases the risk of second NMSC is uncertain; further long term studies are required before one can conclude that these immunosuppressive therapies do not increase the risk of second NMSC.
Introduction
The incidence of non-melanoma skin cancer (NMSC) is increasing in Caucasian populations worldwide1. Dermatologists, rheumatologists, gastroenterologists, and primary care physicians are capable of recognizing NMSC lesions, with most being treated by dermatologists2. Although initial surgical treatment is usually curative, the risk of second primary NMSC is high3. Major risk factors for NMSC are skin pigmentation and solar damage to the skin4. Medications that accelerate the phototoxic process have been associated with an increased incidence of NMSC5. Several medications are considered, “photosensitizers”, including methotrexate and thiopurines 6, 7. This effect has not been reported with biologic therapies. Immunosuppression is also believed to be a risk factor for NMSC, particularly squamous cell carcinoma; this has prompted frequent screening of individuals after solid organ transplantation8.
Immune dysfunction underlies the pathogenesis of rheumatoid arthritis (RA) and inflammatory bowel disease (IBD). Immunosuppressive therapies have become the standard for treating these diseases9, 10. Methotrexate was one of the first therapies with demonstrated benefit in RA, and has remained a cornerstone therapy11. Similarly, in IBD, thiopurine has increased dramatically in recent decades12. These therapies have been further augmented by medications targeting tumor necrosis factor–alpha (anti-TNFs) and other targets, collectively referred to as biologic therapies13, 14. These agents are used alone or in combination with methotrexate or thiopurines15–20.
Methotrexate, thiopurines, and anti-TNFs have been associated with hematologic and dermatologic malignancies21–23. Thiopurines appear to increase the risk of NMSC both during active use of the drug and possibly after the medication is discontinued24, 25. Similar effects were appreciated with methotrexate in a systematic review assessing the risk of NMSC in patients with psoriasis26, 27. The association between anti-TNFs and NMSC is less clear, with conflicting results among several studies 22, 27, 28.
For patients with a prior malignancy, there are limited data regarding the impact of these medications on the risk of cancer recurrence or a second primary. The available data are generally in small cohorts and have combined different cancer types29–31. In this study, we assessed the risk of a second NMSC in Medicare beneficiaries with RA or IBD exposed to methotrexate, thiopurines, or biologics. We hypothesized an increased risk for a second NMSC among patients with exposure to photosensitizers such as methotrexate or thiopurines relative to those who have received agents without these effects, such as anti-TNFs or non-immunosuppressive therapies.
Patients and Methods
We performed a retrospective cohort study among subjects with RA or IBD using Medicare data from 2006–2012. This cohort has been used previously to evaluate the comparative effectiveness and safety of medications used to treat IBD and RA28, 32–34.
Inclusion criteria
Individuals aged ≥18 years with a diagnosis of RA or IBD based upon ICD-9 diagnosis codes, and an incident NMSC diagnosis after enrollment within Medicare from 2006–2010, were considered eligible for this study. Follow-up time continued for this cohort until 12/31/2012. Incident diagnoses of NMSC were identified using an adapted claims-based algorithm35, combining diagnostic codes for NMSC and dermatologic procedures (See supplemental materials).
Subjects were required to have a baseline observation period of 6 months before the first NMSC diagnosis to assess medication exposures prior to or at the time of NMSC diagnosis36. Additionally, subjects were required to have ≥12 months of follow-up time after the first NMSC diagnosis or procedure, without an additional NMSC diagnostic or procedure code from 6–12 months to maximize the likelihood that subsequent NMSC codes represented incident events instead of follow-up for a prevalent NMSC(Supplemental Figure 1). This allowed 1 year for the completion of therapy for the incident NMSC event before diagnosis of a second NMSC; similar methods have been employed using administrative data to capture recurrent and second malignancies37.
Exclusion Criteria
Individuals were excluded if they had an NMSC diagnosis within the first 6 months of enrollment or diagnosis with any of the following conditions prior to the first NMSC diagnosis: any malignancy, psoriasis, organ transplant, HIV, xeroderma pigmentosa, or albinism.38, 39 We excluded patients with any recorded use prior to the first NMSC diagnosis of medications thought to affect the risk of NMSC, such as tacrolimus, cyclosporin, imiquimod, or fluorouracil. Individuals enrolled in Medicare Part C (Managed Medicare) were excluded, as they may have incomplete drug and outcome data. Follow-up was censored if patients met any exclusion criteria after first NMSC diagnosis.
Exposure definition
The following medications were considered exposures of interest: 1) methotrexate, 2) thiopurines (azathioprine/ 6-mercaptopurine), 3) anti-TNFs (infliximab, adalimumab, certolizumab, golimumab, or etanercept), 4) leflunomide, 5) tocilizumab, 6) abatacept, 7) rituximab, and 8) sulfasalazine (SSA) or hydroxychloroquine (HCQ). Exposure was defined as ≥2 dispensings or infusions within 4 months of each other, with at least one dispensing or infusion after the incident NMSC surgery. Exposure was further divided into current and recent exposure, where recent exposure began 90 days after the expected end of each prescription or infusion dosing interval if the medication was not continued. In addition, time updating variables describing cumulative exposure were generated for each medication. We assessed the duration of exposure for methotrexate, anti-TNFs, and thiopurines, categorized as never exposed, <1 year, 1–2 years, 2–3 years, and >3 years.
Outcome
Follow-up began 1 year after the first NMSC surgery. Follow-up ended with the earliest of the following: 1) subsequent new NMSC diagnosis using the same criteria as described above, 2) death, 3) loss of medical or prescription benefits, or 4) end of data-collection.
Potential confounders
Potential confounders were assessed at the start of follow-up, including age (in deciles), sex, race/ethnicity, urban versus rural residence, Charlson comorbidity scores, and nursing home inhabitance. Latitude was assessed as a dichotomous covariate, above or below the median for this cohort. A history of actinic keratosis and the number of dermatologic visits in the 12-month period between NMSC diagnosis and start of follow-up were assessed. Exposure to the medications of interest prior to the initial NMSC was evaluated as a potential confounder using all available data prior to the start of follow-up. Cumulative corticosteroid exposure was assessed as a confounder as a time-varying covariate measured in prednisone-equivalent dosing and categorized as no exposure, <1.5g, and >1.5g.
Statistical Analysis
Analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC) and Stata version 13.0 (StataCorp, College Station, TX). Analyses were performed separately for RA and IBD. Our data use agreement with the Center for Medicare and Medicaid Services precluded reporting results where <11 patients were exposed to a therapy or experienced the outcome. Baseline covariates were assessed using χ2 and Fisher’s exact tests as appropriate. We computed separate disease-specific Cox regression models for each exposure contrast of interest, adjusted for covariates. Covariates were selected for final multivariable models if inclusion changed the HR for the primary exposure by ≥10%. We computed time-updating HRs of each exposure of interest, adjusting for potential confounders. Test for interaction between our exposures of interest and follow-up time was used to ensure that proportional hazard assumptions were not violated (data not shown).
Because methotrexate is commonly used in combination with other therapies (HCQ, SSA or anti-TNF), we assessed the risk of NMSC with methotrexate plus HCQ or SSA versus HCQ or SSA alone and methotrexate plus anti-TNF versus anti-TNF alone. These analyses were limited to patients with documented methotrexate use prior to the first NMSC diagnosis. We computed the pooled association of methotrexate versus no methotrexate with second NMSC using fixed effects meta-analysis40, 41. Similar analyses were conducted for thiopurines and anti-TNFs in the IBD cohort. The study protocol was approved by University of Alabama at Birmingham and University of Pennsylvania institutional review boards.
Results
There were 9,460 individuals within the cohort: 6,841 had RA and 2,788 had IBD (Figure 1). 1,291 individuals developed a second NMSC. The incidence rate per 1,000 person-years of second NMSC was 58.2 (95%CI, 54.5–62.1) and 58.9 (53.2–65.2) among patients with RA and IBD, respectively. For both IBD and RA, males were more likely to have a second NMSC compared to females (p=0.01 and p<0.001, respectively) (Table 1). The median latitude for the cohort was 37.5 degrees; This was not associated with increased risk of second NMSC in IBD or RA. There was a greater prevalence of a history of actinic keratosis in those with a second NMSC in both RA and IBD (p<0.001).
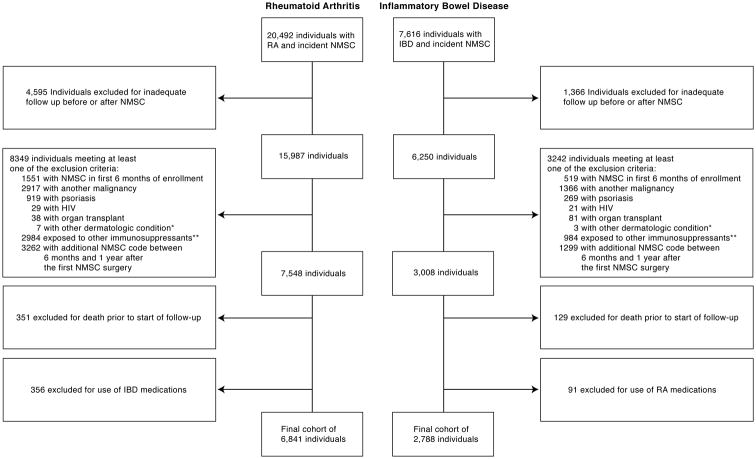
Figure 1.
Identification of cohort meeting inclusion and exclusion criteria.
Flowchart depicting identification of individuals meeting inclusion and exclusion criteria for study in both RA and IBD.
*Other dermatologic conditions consisted of xeroderma pigmentosa and albinism
** Other immunosuppressive therapies included tacrolimus, cyclosporin, imiquimod, or fluorouracil.
*** Cumulative exposure time is reported in Median (IQR), and is among those who had received the drug.
Table 1.
Baseline Characteristics of individuals at cohort entry
| Characteristic | Group | RA | IBD | ||
|---|---|---|---|---|---|
| + NMSC (n=932) | − NMSC (n=5909) | + NMSC (n=381) | − NMSC (n=2407) | ||
| Age | < 50 | <10 | 63 (1.1%) | 12 (3.1%) | 48 (2.0%) |
| 51–60 | 23 (2.5%) | 216 (3.7%) | <10 | 89 (3.7%) | |
| 61–70 | 268 (28.8%) | 1633 (27.6%) | 113 (29.7%) | 674 (28.0%) | |
| 71–80 | 453 (48.6%) | 2635 (44.6%) | 179 (47.0%) | 1035 (43.0%) | |
| 81+ | 183 (19.6%) | 1362 (23.0%) | 73 (19.2%) | 561 (23.3%) | |
| Criteria for initial Medicare eligibility | Old Age and Survivors Insurance | 757 (81.2%) | 4609 (78.0%) | 329 (86.4%) | 2031 (84.4%) |
| Disability and/or ESRD | 175 (18.8%) | 1300 (22.0%) | 52 (13.6%) | 376 (15.6%) | |
| Sex | Female | 582 (62.4%) | 4310 (72.9%) | 200 (52.5%) | 1432 (59.5%) |
| Male | 350 (37.6%) | 1599 (27.1%) | 181 (47.5%) | 975 (40.5%) | |
| Race | White | 925 (99.2%) | 5802 (98.2%) | 376 (98.7%) | 2375 (98.7%) |
| Black | <10 | <10 | <10 | <10 | |
| Other | <10 | 98 (1.7%) | <10 | 27 (1.1%) | |
| Hispanic Ethnicity | <10 | 133 (2.3%) | <10 | 28 (1.2%) | |
| Residence | Rural | 280 (30.0%) | 1861 (31.9%) | 86 (22.6%) | 584 (24.8%) |
| Urban | 652 (70.0%) | 3981 (68.1%) | 294 (77.4%) | 1774 (75.2%) | |
| Latitude (at or above median) | At or above median | 445 (47.7%) | 2948 (49.9%) | 173 (45.5%) | 1196 (49.7%) |
| Below median | 487 (52.3%) | 2961(50.1%) | 208(54.6%) | 1211(50.3%) | |
| Skilled nursing facility | Yes | 43 (4.6%) | 457 (7.7%) | 18 (4.7%) | 196 (8.1%) |
| Charlson co-morbidity score | 0 | 413 (44.3%) | 2527 (42.8%) | 182 (47.8%) | 1092 (45.4%) |
| 1 | 137 (14.7%) | 930 (15.7%) | 54 (14.2%) | 345 (14.3%) | |
| 2+ | 382 (41.0%) | 2452 (41.5%) | 145 (38.1%) | 970 (40.3%) | |
| Prior Anti-TNF | 314 (33.7%) | 2184 (37.0%) | 43 (11.3%) | 264 (11.0%) | |
| Prior Methotrexate | 815 (87.4%) | 5005 (84.7%) | 10 (2.6%) | 96 (4.0%) | |
| Prior Thiopurines | -- | -- | 99 (26.0%) | 529 (22.0%) | |
| Prior Abatacept | 35 (3.8%) | 298 (5.0%) | -- | -- | |
| Prior Rituximab | 17 (1.8%) | 152 (2.6%) | -- | -- | |
| History of Actinic Keratotsis | 615 (66.0%) | 3128 (52.9%) | 249 (65.4%) | 1330 (55.3%) | |
| Dermatology encounters in past year | Mean (SD) | 1.7 (1.7) | 1.4 (1.5) | 1.8 (1.6) | 1.6 (1.8) |
Risk of second NMSC with methotrexate use in RA
The median methotrexate exposure time after an initial NMSC in RA was 1.64 years (IQR:1.15–2.31) and 2.61 years (IQR:1.80–3.80) among those with and without a second NMSC, respectively. Among SSA/HCQ users, there was an increased risk of second NMSC with methotrexate exposure, though this was not statistically significant (HR 1.81, 95%CI 0.94–3.52) (Table 2). Similarly, there was an increased but not statistically significant risk of second NMSC with methotrexate use in patients also treated with anti-TNFs (HR 1.50, 95%CI 0.92–2.44). Using meta-analytic methods to pool these groups, methotrexate exposure was associated with an increased risk of second NMSC (HR 1.60, 95%CI 1.08–2.37).
Table 2.
Risk of a second NMSC with immunosuppressive therapy in patients with Rheumatoid Arthritis and Inflammatory Bowel Disease
| Combination of interest | Events | Person-years | Incidence rate (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|
| RA | ||||
|
| ||||
| Methotrexate | ||||
| MTX with SSA/HCQ vs | 72 | 913 | 78.9 (61.7–99.3) | 1.81 (0.94–3.52)a,b |
| SSA/HCQ monotherapy | 10 | 223 | 44.9 (21.5–82.6) | Ref |
|
| ||||
| MTX with Anti-TNF vs | 122 | 1,715 | 71.1 (59.08–84.94) | 1.50(0.92–2.44)a,b |
| Anti-TNF monotherapy | 19 | 367 | 51.8 (31.2–80.8) | Ref |
|
| ||||
| Anti-TNF | ||||
| Anti-TNF with MTX vs | 109 | 1,465 | 74.4 (61.1–89.8) | 1.49 (1.03–2.16)c |
| MTX alone (ref) | 335 | 4,631 | 72.3 (64.8–80.5) | Ref |
|
| ||||
| Abatacept | ||||
| Abatacept with MTX vs | 66 | 76.0 (24.7–177.4) | 1.40 (0.48–4.03)d | |
| MTX alone (ref) | 319 | 4,311 | 74.0 (66.1–82.6) | Ref |
|
| ||||
| Rituximab | ||||
| Rituximab with MTX vs | 19 | 103.2 (12.5–373.0) | 1.44 (0.26–8.08)e | |
| MTX alone (ref) | 320 | 4,301 | 74.4 (66.5–83.0) | Ref |
|
| ||||
| IBD | ||||
|
| ||||
| Thiopurine vs | 63 | 717 | 87.9 (67.5–112.4) | 1.23 (0.78–1.94)f |
| Anti-TNF (ref) | 26 | 375 | 69.4 (45.3–101.6) | Ref |
|
| ||||
| Anti-TNF with thiopurine vs | 85 | 71.0 (26.0–154.5) | 0.79 (0.30–2.08)g | |
| anti-TNF monotherapy (ref) | 26 | 351 | 74.0 (48.3–108.4) | Ref |
No covariates modified the HR by > 10%; covariates assessed included: anti-TNFs, SSA/HCQ, leflunomide, abatacept, rituximab, age, sex, median latitude, cumulative steroid exposure, and number of dermatology encounters in the year following surgery for the incident NMSC.
Using meta-analytic methods to pool these two groups, methotrexate exposure was associated with an increased risk of second NMSC (HR 1.60, 95% CI 1.08–2.37)
Adjusted for Anti-TNF exposure prior to the incident NMSC diagnosis, no other covariates modified the HR by > 10%;.
Adjusted for abatacept exposure prior to the incident NMSC diagnosis, no other covariates modified the HR by > 10%;
Adjusted for rituximab exposure prior to the incident NMSC diagnosis, no other covariates met the 10% change criteria for confounding.
No covariates modified the HR by > 10%;.
Adjusted for thiopurine exposure prior to the incident NMSC diagnosis, no other covariates modified the HR by > 10%.
We evaluated the impact of duration of methotrexate therapy after incident NMSC, categorizing methotrexate exposure as never exposed, short-term exposure (<1 year), and exposure >1 year. We observed an increased risk of NMSC with increasing duration of exposure, particularly among those exposed for >1 year (HR 1.24, 95%CI 1.04–1.48) (Table 3). After adjusting for age, sex, other immunosuppressive therapies, and latitude, the risk of a second NMSC increased with longer methotrexate exposure (<1 year HR 1.10, 95%CI 0.84–1.44; 1–2 years HR 1.16, 95%CI 0.95–1.41; 2–3 years HR 1.36, 95%CI1.05–1.77; >3 years HR 1.59, 95%CI 1.09–2.32).
Table 3.
Cumulative duration of medication exposure on risk of second NMSC in Rheumatoid Arthritis
| Drug | Never exposed | Recent exposure | Short-Term exposure (<1year) | Long-term exposure (>1year) |
|---|---|---|---|---|
| Methotrexate | ||||
| Events | 271 | 54 | 85 | 499 |
| Person-years | 5,041 | 1,647 | 1,089 | 7,538 |
| Incidence Rate per 1000 person-years | 53.8 (47.5–60.5) | 32.8 (24.6–42.8) | 78.1 (62.4–96.5) | 66.2 (60.5–72.3) |
| Adjusted HRa | Ref | 0.81 (0.60–1.11) | 1.12 (0.86–1.47) | 1.24 (1.04–1.48) |
| Anti-TNF | ||||
| Events | 662 | 32 | 39 | 176 |
| Person-years | 10,875 | 926 | 440 | 3,073 |
| Incident Rate | 60.9 (56.3–65.7) | 34.6 (23.6–48.8) | 88.5 (63.0–121.0) | 57.3 (49.1–66.4) |
| Adjusted HRa | Ref | 0.87 (0.59–1.29) | 1.43 (1.01–2.04) | 1.11 (0.87–1.42) |
| Abatacept | ||||
| Events | 858 | 5 | 15 | 31 |
| Person-time | 14,397 | 212 | 205 | 500 |
| Incident Rate | 59.6 (55.7–63.7) | 23.6 (7.6–55.0) | 73.3 (41.0–120.9) | 62.0 (42.1–88.0) |
| Adjusted HRa | Ref | 0.72 (0.29–1.79) | 1.48 (0.87–2.51) | 1.51 (0.94–2.41) |
| Rituximab | ||||
| Events | 890 | 6 | 5 | 8 |
| Person-time | 14,837 | 179 | 119 | 180 |
| Incident Rate | 60.0 (56.1–64.1) | 33.6 (12.3–73.1) | 41.8 (13.6–97.6) | 44.6 (19.2–87.8) |
| Adjusted HRa | Ref | 1.01 (0.44–2.35) | 0.78 (0.32–1.92) | 1.20 (0.55–2.61) |
Adjusted for use of anti-TNFs, SSA/HCQ, methotrexate, leflunomide, abatacept, rituximab, age, sex, median latitude, cumulative steroid exposure, and number of dermatology encounters in the year following surgery for the incident NMSC.
Risk of second NMSC with anti-TNF, rituximab, and abatacept use for RA
Among patients treated with methotrexate for RA, there was no significant increased risk with rituximab or abatacept use, although anti-TNF use was statistically significant when adjusted for anti-TNF use prior to the first NMSC (HR 1.49, 95%CI1.03–2.16) (Table 2). When stratified by exposure duration, short-term anti-TNF use was significantly associated with increased risk (<1 year HR 1.43, 95%CI 1.01–2.04), but longer use was not (Table 3, Supplemental Table 3). Risks attributable to leflunomide were not calculated due to limited numbers of events. Cumulative steroid exposure was not significantly associated with risk of second NMSC in RA (p=0.53).
Risk of second NMSC with thiopurine and anti-TNF use for IBD
Among patients with IBD, thiopurine use was not associated with increased risk of second NMSC when used in combination with anti-TNF versus anti-TNF monotherapy (HR 0.79, 95%CI 0.30–2.08) (Table 2). In comparisons of anti-TNF versus thiopurine monotherapy, thiopurines appeared to have a higher incidence rate of second NMSC, although this was not statistically significant. Longer duration of anti-TNF use was not associated with an increased risk of a second NMSC (Supplemental Table 3). The risk of a second NMSC was increased with short-term (HR 1.53, 95%CI 0.87–2.70) thiopurine therapy and was nearly statistically significantly increased with >1 year of thiopurine therapy (HR 1.49, 95%CI 0.98–2.27) (Table 4). When further stratified by duration of exposure, the degree of risk remained similar, though was not significant (2–3 years: HR 1.57, 95%CI 0.83–2.97; >3 years HR 1.49, 95%CI 0.60–3.73). Cumulative steroid exposure was not significantly associated with second NMSC in IBD (p=0.89).
Table 4.
Cumulative impact of medication exposure on risk of second NMSC in Inflammatory Bowel Disease
| Drug | Never exposed | Recent exposure | Short-Term exposure (<1year) | Long-term exposure (>1year) |
|---|---|---|---|---|
| Anti-TNF | ||||
| Events | 332 | 4 | 8 | 27 |
| Person-time | 5,635 | 116 | 84 | 424 |
| Incidence rate per 1,000 person years | 58.9 (52.7–65.6) | 34.4 (9.4–88.2) | 95.0 (41.0–187.9) | 63.7 (42.0–92.8) |
| Adjusted HRa | Ref | 0.95 (0.33–2.66) | 1.34 (0.64–2.81) | 1.36 (0.76–2.44) |
| Thiopurine | ||||
| Events | 295 | 6 | 16 | 54 |
| Person-time | 5,240 | 189 | 132 | 697 |
| Incidence rate per 1,000 person years | 56.3 (50.1–63.1) | 31.7 (11.6–69.0) | 121.0 (69.2–196.5) | 77.5 (58.2–101.1) |
| Adjusted HRa | Ref | 0.70 (0.30–1.65) | 1.53 (0.87–2.70) | 1.49 (0.98–2.27) |
Adjusted for use of anti-TNFs and thiopurines, age, sex, median latitude, cumulative steroid exposure, and number of dermatology encounters in the year following surgery for the incident NMSC.
Discussion
In this retrospective cohort study, we examined the impact of immunosuppressant therapies on the risk of a second NMSC in patients with RA and IBD. We hypothesized that immunosuppressant medications used to treat RA and IBD, particularly methotrexate and thiopurines which are photosensitizing, may increase the risk of a second NMSC 5. Among individuals with RA, methotrexate and anti-TNF drugs were both associated with an increased risk of second NMSC diagnosis. A similar strength of association was seen for anti-TNFs among the IBD cohort although this was not statistically significant. Abatacept or rituximab in conjunction with methotrexate were not associated with a significantly increased risk for a second NMSC compared to methotrexate monotherapy, although the point estimates were similar to anti-TNF agents and sample sizes were small. Therefore, one cannot interpret these results as demonstrating that these agents are safer alternatives. Among individuals with IBD, thiopurine use approached statistical significance for the association with an increased risk of second NMSC and the estimated relative risk was similar to that seen for methotrexate in RA.
For patients with RA, the incidence of second NMSC was increased by 19 per 1000 person-years when methotrexate was used with anti-TNFs and by 34 per 1000 person-years when methotrexate was used in addition to non-immunosuppressive therapies. This translates to a number needed to treat to cause one additional NMSC per year of 52.6 and 29.4 when used with anti-TNFs or without concomitant immunosuppressant medications, respectively. Given that methotrexate is generally the first line therapy for RA, with other drugs typically added to methotrexate when needed, these data emphasize the need for intensive NMSC surveillance protocols.
The evidence of an increased risk of a second NMSC among those treated with thiopurines for IBD was nearly statistically significant and consistent with several prior studies showing that thiopurines increase the incidence of a first NMSC. This failure to achieve statistical significance may have been due to inadequate statistical power, or may reflect a persistent effect of prior thiopurine exposure which would be expected among many of the anti-TNF treated patients in this cohort24. Likewise, if both thiopurines and anti-TNFs increase the risk of second NMSC, the association would be attenuated in direct comparisons of these two drug classes.
Anti-TNF therapy was significantly associated with the risk of second NMSC among patients with RA and the magnitude of risk was comparable in IBD. Prior studies in IBD have suggested an increased risk of melanoma with anti-TNF therapy, but there is less evidence for an increased NMSC 22, 27, 28. In RA, the association between an initial NMSC and anti-TNFs appears more firmly established, though the relationship is often complicated by concomitant methotrexate use42–46. One can hypothesize that immunosuppression from anti-TNFs might contribute to NMSC risk, particularly in patients with a prior NMSC. In contrast to thiopurines and methotrexate, anti-TNF therapy is not photosensitizing. Regardless, these data suggest that anti-TNF therapy may not be an advantageous option over thiopurines or methotrexate for patients with a prior NMSC history.
This study has several important strengths. NMSC is the most frequently diagnosed malignancy in the United States47. Therefore, the risk of recurrence is an important question commonly faced in clinical practice, and research examining this risk has been limited to case reports and series29–31. This is by far the largest study to date examining the impact of commonly used therapies in IBD and RA on the risk of cancer recurrence. Furthermore, Medicare is a geographically diverse patient population and should be generalizable to approximately 93% of older adults in the US48.
There are several potential limitations of this study. As with any retrospective study using claims-based data, there is the risk of misclassification. However, requiring both diagnostic and procedural codes to define NMSCs minimizes the probability of including individuals without a first NMSC in the cohort by maximizing the positive predictive value of a true incident event. There is also the potential for surveillance bias, as individuals with a prior NMSC who receive methotrexate or anti-TNF drugs may be more frequently surveyed than individuals not receiving these medications. To account for this, we adjusted for dermatology visits in the first year after the first NMSC. We are unable to ensure that our diagnostic codes identified exclusively second NMSC events as opposed to the initial event. However, we employed a 1-year window after the initial diagnosis to minimize this misclassification. While we were unable to specifically measure disease severity in RA or IBD, we used corticosteroid exposure as a surrogate and found no significant association with second NMSC in univariate analysis and only one model with evidence of confounding. Because we studied NMSC, we cannot generalize these results to the risk of recurrence of other cancers. Our analysis was restricted to a predominantly Caucasian population, although this is the population that is at greatest risk for NMSC. We were also unable to assess NMSC risk according to specific ethnicity or sunscreen utilization.
We had limited power to study some of the drugs of interest. There were too few patients exposed to leflunomide. Similarly, sample sizes were small for analysis of abatacept, tocilizumab, and rituximab. The estimated HR with rituximab was among the highest observed, and there was some evidence of greater risk with longer therapy, but the confidence intervals were wide. Because rituximab is recommended among the biologic therapies for use in patients with a prior history of cancer in the ACR 2012 guidelines49, this estimate may be biased by confounding by indication. Likewise, we were also unable to assess the impact of new thiopurine use in those who were thiopurine-naïve prior to their initial NMSC event due to limited numbers. This may represent reluctance on the part of providers to initiate therapy with thiopurines in individuals who have a known NMSC. Therefore, while this study demonstrated an increased risk of second NMSC with methotrexate and possibly anti-TNF drugs, we cannot conclude that the other immunosuppressive therapies represent safer alternatives. Further research examining these agents is required.
In summary, the use of immunosuppressive therapy is known to be a risk factor for NMSC, most commonly squamous cell carcinoma. Physicians treating patients with RA and IBD commonly face the decision of what medications to use in patients with a history of NMSC. In this study, methotrexate use was associated with an increased risk of a second NMSC in RA, with increasing risk with longer duration of exposure. While there appears to be a strong relationship between thiopurine use and an initial NMSC in IBD, we did not observe a statistically significant increased risk for a second NMSC, although this may have been due to reduced statistical power as the estimated relative risk with thiopurines for IBD was larger than that observed with methotrexate for RA. Lastly, anti-TNF therapy may further increase the risk of second NMSC particularly when used in conjunction with methotrexate to treat RA. These data can be used to guide therapeutic decisions in patients with prior NMSC.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported in part by the Agency for Healthcare Research & Quality (grants R01 HS018517 and U19 HS021110) and the NIH (grants K08-DK095951, K12 CA 076931, and K24-DK078228).
| Funding/Sponsor was involved? | |
|---|---|
| Design and conduct of the study | Yes___No_X_ |
| Collection, management, analysis and interpretation of data | Yes___No_X_ |
| Preparation, review, or approval of the manuscript | Yes___No_X_ |
| Decision to submit the manuscript for publication | Yes___No_X_ |
Footnotes
Author Contributions: Drs James D. Lewis and Jeffrey Curtis had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Scott, Mamtani, Lewis, Curtis. Acquisition, analysis, and interpretation of data: Scott, Mamtani, Lewis, Curtis, Brensinger, Beukelman. Drafting of the manuscript: Scott, Mamtani. Critical revision of the manuscript for important intellectual content: Scott, Mamtani, Haynes, Chiesa-Fuxench, Chen, Xie, Yun, Osterman, Margolis, Brensinger, Lewis, Curtis. Statistical analysis: Brensinger, Lewis. Obtained funding: Lewis, Curtis, Scott, Mamtani. Administrative, technical, or material support: Brensinger, Lewis, Curtis. Study supervision: Lewis, Curtis.
Conflicts of interest/Disclosures
Dr. Lewis has served as a consultant for Takeda, Amgen, Millennium Pharmaceuticals, Prometheus, Lilly, Shire, AstraZeneca, Janssen Pharmaceuticals, Merck, and AbbVie. He has served on a Data and Safety Monitoring Board for clinical trials sponsored by Pfizer. He has received research support from Bayer, Shire, Centocor, Nestle, and Takeda. Dr. Curtis has served as a consultant for Roche/Genentech, UCB, Janssen, CORRONA, Amgen, Pfizer, BMS, Crescendo and AbbVie. He has received research support from Roche/Genentech, UCB, Janssen, CORRONA, Amgen, Pfizer, BMS, Crescendo, and AbbVie. Dr. Beukelman has served as a consultant for Genentech, Novartis, and UCB. He has received research support from Pfizer. Dr. Zhang has received research support from Amgen. Dr. Osterman has served as a consultant for Janssen, Abbott, and UCB. He has received research support from UCB. Dr. Haynes has received research support from AstraZeneca/BristolMeyersSquibb. Dr. Mamtani has served as a consultant for Takeda. Drs. Scott, Brensinger, Chiesa-Fuxench, Chen, Xie, Yun, and Margolis report no potential conflicts of interest.
References
- 1.Deady S, Sharp L, Comber H. Increasing skin cancer incidence in young, affluent, urban populations: a challenge for prevention. Br J Dermatol. 2014 doi: 10.1111/bjd.12988. [DOI] [PubMed] [Google Scholar]
- 2.Koelink CJ, Kollen BJ, Groenhof F, et al. Skin lesions suspected of malignancy: an increasing burden on general practice. BMC Fam Pract. 2014;15:29. doi: 10.1186/1471-2296-15-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol. 2000;136:1524–30. doi: 10.1001/archderm.136.12.1524. [DOI] [PubMed] [Google Scholar]
- 4.Preston DS, Stern RS. Nonmelanoma cancers of the skin. N Engl J Med. 1992;327:1649–62. doi: 10.1056/NEJM199212033272307. [DOI] [PubMed] [Google Scholar]
- 5.Robinson SN, Zens MS, Perry AE, et al. Photosensitizing agents and the risk of non-melanoma skin cancer: a population-based case-control study. J Invest Dermatol. 2013;133:1950–5. doi: 10.1038/jid.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chahidi C, Morliere P, Aubailly M, et al. Photosensitization by methotrexate photoproducts. Photochem Photobiol. 1983;38:317–22. doi: 10.1111/j.1751-1097.1983.tb02678.x. [DOI] [PubMed] [Google Scholar]
- 7.Attard NR, Karran P. UVA photosensitization of thiopurines and skin cancer in organ transplant recipients. Photochem Photobiol Sci. 2012;11:62–8. doi: 10.1039/c1pp05194f. [DOI] [PubMed] [Google Scholar]
- 8.Tessari G, Girolomoni G. Nonmelanoma skin cancer in solid organ transplant recipients: update on epidemiology, risk factors, and management. Dermatol Surg. 2012;38:1622–30. doi: 10.1111/j.1524-4725.2012.02520.x. [DOI] [PubMed] [Google Scholar]
- 9.McInnes IB, Schett G. The Pathogenesis of Rheumatoid Arthritis. New England Journal of Medicine. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 10.Abraham C, Cho JH. Inflammatory Bowel Disease. New England Journal of Medicine. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Upchurch KS, Kay J. Evolution of treatment for rheumatoid arthritis. Rheumatology (Oxford) 2012;51(Suppl 6):vi28–36. doi: 10.1093/rheumatology/kes278. [DOI] [PubMed] [Google Scholar]
- 12.Cosnes J, Nion-Larmurier I, Beaugerie L, et al. Impact of the increasing use of immunosuppressants in Crohn’s disease on the need for intestinal surgery. Gut. 2005;54:237–41. doi: 10.1136/gut.2004.045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott MJ, Maini RN, Feldmann M, et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994;344:1105–10. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- 14.Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337:1029–35. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 15.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–9. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 16.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–76. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 17.Weinblatt ME, Keystone EC, Furst DE, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- 18.Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–33. doi: 10.1053/j.gastro.2005.11.030. quiz 591. [DOI] [PubMed] [Google Scholar]
- 19.Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257–65. e1–3. doi: 10.1053/j.gastro.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 20.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–95. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 21.Kotlyar DS, Lewis JD, Beaugerie L, et al. Risk of Lymphoma in Patients with Inflammatory Bowel Disease Treated with Azathioprine and 6-Mercaptopurine: a Meta-Analysis. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Long MD, Herfarth HH, Pipkin CA, et al. Increased risk for non-melanoma skin cancer in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2010;8:268–74. doi: 10.1016/j.cgh.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long MD, Martin CF, Pipkin CA, et al. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143:390–399. e1. doi: 10.1053/j.gastro.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peyrin-Biroulet L, Khosrotehrani K, Carrat F, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology. 2011;141:1621–28. e1–5. doi: 10.1053/j.gastro.2011.06.050. [DOI] [PubMed] [Google Scholar]
- 25.Abbas AM, Almukhtar RM, Loftus EV, Jr, et al. Risk of melanoma and non-melanoma skin cancer in ulcerative colitis patients treated with thiopurines: a nationwide retrospective cohort. Am J Gastroenterol. 2014;109:1781–93. doi: 10.1038/ajg.2014.298. [DOI] [PubMed] [Google Scholar]
- 26.Goldfeder KL, Levin JM, Katz KA, et al. Ultraviolet recall reaction after total body irradiation, etoposide, and methotrexate therapy. J Am Acad Dermatol. 2007;56:494–9. doi: 10.1016/j.jaad.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Krathen MS, Gottlieb AB, Mease PJ. Pharmacologic immunomodulation and cutaneous malignancy in rheumatoid arthritis, psoriasis, and psoriatic arthritis. J Rheumatol. 2010;37:2205–15. doi: 10.3899/jrheum.100041. [DOI] [PubMed] [Google Scholar]
- 28.Haynes K, Beukelman T, Curtis JR, et al. Tumor necrosis factor alpha inhibitor therapy and cancer risk in chronic immune-mediated diseases. Arthritis Rheum. 2013;65:48–58. doi: 10.1002/art.37740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poullenot F, Seksik P, Beaugerie L, et al. Risk of Incident Cancer in Patients With Inflammatory Bowel Disease Starting Anti-TNF Therapy While Having Prior Malignancy Within Past 5 Years. Gastroenterology. 2014;146:S78. doi: 10.1097/MIB.0000000000000741. [DOI] [PubMed] [Google Scholar]
- 30.Beaugerie L, Carrat F, Colombel JF, et al. Risk of new or recurrent cancer under immunosuppressive therapy in patients with IBD and previous cancer. Gut. 2013 doi: 10.1136/gutjnl-2013-305763. [DOI] [PubMed] [Google Scholar]
- 31.Strangfeld A, Hierse F, Rau R, et al. Risk of incident or recurrent malignancies among patients with rheumatoid arthritis exposed to biologic therapy in the German biologics register RABBIT. Arthritis Res Ther. 2010;12:R5. doi: 10.1186/ar2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baddley JW, Winthrop KL, Chen L, et al. Non-viral opportunistic infections in new users of tumour necrosis factor inhibitor therapy: results of the SAfety Assessment of Biologic ThERapy (SABER) Study. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-203407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solomon DH, Curtis JR, Saag KG, et al. Cardiovascular risk in rheumatoid arthritis: comparing TNF-alpha blockade with nonbiologic DMARDs. Am J Med. 2013;126:730e9–730e17. doi: 10.1016/j.amjmed.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osterman MT, Haynes K, Delzell E, et al. Comparative effectiveness of infliximab and adalimumab for Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:811–817. e3. doi: 10.1016/j.cgh.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Setoguchi S, Solomon DH, Glynn RJ, et al. Agreement of diagnosis and its date for hematologic malignancies and solid tumors between medicare claims and cancer registry data. Cancer Causes Control. 2007;18:561–9. doi: 10.1007/s10552-007-0131-1. [DOI] [PubMed] [Google Scholar]
- 36.Lewis JD, Bilker WB, Weinstein RB, et al. The relationship between time since registration and measured incidence rates in the General Practice Research Database. Pharmacoepidemiol Drug Saf. 2005;14:443–51. doi: 10.1002/pds.1115. [DOI] [PubMed] [Google Scholar]
- 37.Chubak J, Yu O, Pocobelli G, et al. Administrative data algorithms to identify second breast cancer events following early-stage invasive breast cancer. J Natl Cancer Inst. 2012;104:931–40. doi: 10.1093/jnci/djs233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frentz G, Olsen JH. Malignant tumours and psoriasis: a follow-up study. Br J Dermatol. 1999;140:237–42. doi: 10.1046/j.1365-2133.1999.02655.x. [DOI] [PubMed] [Google Scholar]
- 39.Stern RS Study PFu. The risk of melanoma in association with long-term exposure to PUVA. J Am Acad Dermatol. 2001;44:755–61. doi: 10.1067/mjd.2001.114576. [DOI] [PubMed] [Google Scholar]
- 40.Bradburn M, Deeks J, Altman D. sbe24: metan - an alternative meta-analysis command. Stata Tech Bull. 1998;44:15. [Google Scholar]
- 41.Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res. 1993;2:121–45. doi: 10.1177/096228029300200202. [DOI] [PubMed] [Google Scholar]
- 42.Mariette X, Matucci-Cerinic M, Pavelka K, et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis. 2011;70:1895–904. doi: 10.1136/ard.2010.149419. [DOI] [PubMed] [Google Scholar]
- 43.Chakravarty EF, Michaud K, Wolfe F. Skin cancer, rheumatoid arthritis, and tumor necrosis factor inhibitors. J Rheumatol. 2005;32:2130–5. [PubMed] [Google Scholar]
- 44.Askling J, Fahrbach K, Nordstrom B, et al. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf. 2011;20:119–30. doi: 10.1002/pds.2046. [DOI] [PubMed] [Google Scholar]
- 45.Moulis G, Sommet A, Bene J, et al. Cancer risk of anti-TNF-alpha at recommended doses in adult rheumatoid arthritis: a meta-analysis with intention to treat and per protocol analyses. PLoS One. 2012;7:e48991. doi: 10.1371/journal.pone.0048991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56:2886–95. doi: 10.1002/art.22864. [DOI] [PubMed] [Google Scholar]
- 47.Weinstock MA. Nonmelanoma skin cancer mortality in the United States, 1969 through 1988. Arch Dermatol. 1993;129:1286–90. [PubMed] [Google Scholar]
- 48.DeNavas-Walt C, Proctor B, Smith J U.S. Census Bureau. Income, Poverty, and Health Insurance Coverage in the United States: 2012. U.S. Government Printing Office; Washington, D.C: 2013. Current Population Reports, P60–245. [Google Scholar]
- 49.Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625–39. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.