Abstract
The white-backed planthopper, Sogatella furcifera, is a phloem sap feeder that secretes watery and gelling saliva during feeding. In this study, we identified the major proteins in watery saliva of S. furcifera by shotgun LC-MS/MS analysis combined with transcriptomic analysis. A total of 161 proteins were identified, which were divided into 8 function categories, including enzymes, transporter, calcium ion binding protein, salivary sheath protein, cytoskeleton protein, DNA-, RNA-, and protein-binding or regulating proteins, other non-enzyme proteins and unknown proteins. Gene expression pattern of 11 secretory proteins were analyzed by real time quantitative-PCR. We detected the mucin-like protein, which had a unique expression level in salivary gland, most likely to be a candidate effector involved in regulation of plant defense. This study identified the watery saliva component of S. furcifera and it provided a list of proteins which may play a role in interaction between S. furcifera and rice.
Introduction
Saliva is an important biochemical interface determining the compatibility between sap-sucking insects and their host plants [1, 2]. The saliva includes bioactive compounds that have a range of functions from degrading plant cell and digesting nutrients to eliciting or inhibiting plant defense [3]. Hemipterans secrete two types of saliva: gelling saliva and watery saliva. Gelling saliva is used to form salivary sheath in order to facilitate stylet penetration, while watery saliva involves in the regulation of plant defense [4].
White-backed planthopper, Sogatella furcifera, a critical phloem sap feeder, causes considerable damage by sucking plant sap and transmitting plant viral diseases [5]. It also secretes gelling and watery saliva during feeding process like other hemipterans. Previous studies have detected the saliva composition in many species of aphids [1, 6–9], Nephotettix cincticeps [10], Lygus Hesperus [11] and Nilaparvata lugens [4, 12]. Huang et al. [13] compared the secreted saliva composition of three planthopper species and revealed the ubiquitous and specific saliva compounds in different insects. Li et al. [14] sequenced and assembled the transcriptome of S. furcifera salivary gland, laying the foundation of research on saliva of S. furcifera.
In this study, shotgun liquid chromatography-tandem mass spectrometry (LC-MS/MS) was carried out to identify the watery saliva component of S. furcifera, identification of salivary protein was combined with transcriptome of S. furcifera and N. lugens salivary gland. We quantified the relative expression of predicted secretory proteins and detected many salivary proteins that may modulate plant defense. This study exhibits an overview of S. furcifera salivary proteins and makes it possible to understand the mechanism of interaction between white-backed planthopper and rice. Moreover, we make an attempt to exploit the candidate effectors in S. furcifera, which may provide a new target for pest management.
Materials and methods
Insects
The S. furcifera populations were originally collected from Xing'an County, Guilin, Guangxi Zhuang Autonomous Region, China, in 2013, which is the Scientific Observing and Experimental Station of Crop Pests of Guilin/Guilin Branch, Institute of Plant Protection, Chinese Academy of Agricultural Sciences. The field studied did not involve endangered or protected species, and no specific permissions were required for these activities in this station. The insects were reared on susceptible rice TN1. The colony was maintained in insect cages in a thermostat at 27 ± 1°C and 80 ± 10% RH, under a 16:8 L: D photoperiod.
Collection and concentration of watery saliva
Each saliva collection container was prepared by stretching two layers of Parafilm over one side of a sterile glass tube (4 cm × 8 cm) with 200 μL 15% sucrose diet. 50 S. furcifera adults were held in each tube and we collected 8 000 adults in total. To attract the insects and keep humid, another side of the tube was wrapped up with a piece of wet black cloth. The collection tubes were placed in a thermostat at 27 ± 1°C and 80 ± 10% RH, under a 16:8 L: D photoperiod. After 24 h of stylet-probing and salivary discharging by the insects, collection tubes were removed from the thermostat. Diet was collected under sterile conditions by making a small incision in the Parafilm with an injector needle and pouring the stylet-probed diet into sterile centrifuge tubes with pipette. Saliva collection was conserved under -80°C.
The collection of saliva was centrifuged at 7 000 g at 4°C for 30 min to remove the impurity and deposit. The supernatant was ultrafiltered with 3 kDa molecular weight cut offs (Amicon-4 Ultra; Millipore, MA, USA), and then concentrated by adding fivefold volume of trichloroacetic acid/acetone (1/9) solution. After incubating at -20°C for 4 h and centrifuging at 6 000 g at 4°C for 40 min, the supernatant was removed and the precipitation was washed 3 times with chilled acetone. Sample was dissolved in SDT buffer (4% sodium dodecylsulfate (SDS, Bio-Rad, Hercules, CA, USA), 100 mM Tris-HCl, 1 mM dithiothreitol (DTT, Bio-Rad), pH 7.6) and incubated in hot water for 15 min and centrifuged at 14 000 g for 40 min.
Protein digestion
Filter-aided sample preparation (FASP) method [15] was used for protein digestion. 30 μL protein sample was added to 100 mM DTT and incubated in hot water for 5 min. After adding 200 μL UA buffer (8 M urea (Bio-Rad), 150 mM Tris-HCl, pH 8.0), the mixture was transferred to 10 kDa ultrafiltration centrifuge tube (Sartorius, Gottingen, Germany) and centrifuged twice at 14 000 g for 15 min. 100 μL IAA buffer (100 mM IAA in UA) was added to the tube and incubated in dark for 30 min at room temperature after vortexing for 1 min at 600 rpm. Then centrifuge at 14 000 g for 15 min. Sample was added 100 μL UA buffer and centrifuged at 14 000 g for 15 min. Perform this procedure twice. Then sample was digested with 1 μg trypsin (Promega, Madison, USA) in 40 μL 100 mM NH4HCO3 (Sigma, St. Louis, MO, USA) buffer. After vortexing for 1 min at 600 rpm, the mixture was reacted for 16–18 h at 37°C. Sample was centrifuged at 14 000 g for 15 min and transferred to a new collection tube. 40 μL 25 mM NH4HCO3 was added to the tube and then centrifuged at 14 000 g for 15 min to collect filtrate. C18 Cartridge (Sigma) was used to desalinate the peptides. 15 μL 0.1% formic acid (FA, Sigma) was added after lyophilizing the peptides. Peptides were qualified at OD280.
LC/MS-MS analysis
LC/MS-MS was performed on an Easy nLC (Thermo Fisher Scientific, MA, USA) coupled with Q Exactive mass spectrometer (Thermo Fisher Scientific). 6 μL digested peptides were used for LC/MS-MS analysis. The mobile phases A was 0.1% FA/H2O and mobile phases B was 0.1% FA/H2O and 84% acetonitrile (ACN, Merck, Darmstadt, Germany)/H2O. The peptides were loaded in C18-reversed phase column (Thermo Scientific Acclaim PepMap100, 100 μm × 2 cm, nanoViper C18) and separated in analytical column (Thermo scientific EASY column, 10 cm, ID 75 μm, 3 μm, C18-A2) at a flow rate of 300 nL/min. The liquid phase gradient was as follows: 0% B to 55% B at 0–110 min, 55% B to 100% B at 110–115 min and 100% B at 115–120 min.
Peptide separations were analyzed using a Q Exactive mass spectrometer by dynamically choosing the 20 most abundant ions from one full mass scan (300–1800 m/z) for high-energy collisional dissociation (HCD) fragmentation. Normalized collision energy was 27 eV and dynamic exclusion was 60 s. Under fill ratio was defined as 0.1%. First level of mass spectrum resolution was 70 000 at m/z 200 and second level was 17 500 at m/z 200. Automatic gain control (AGC) target was 3e6.
Protein identification
MS/MS spectra were searched using Maxquant software (Max Planck Institute of Biochemistry in Martinsried, Germany, version 1.3.0.5) against three databases: (1) the public Uniprot database with parameters set for Auchenorrhyncha (http://www.uniprot.org, 16 168 coding protein sequences); (2) transcriptomic database of S. furcifera salivary gland (http://www.ncbi.nlm.nih.gov/sra, accession number SRR3211109); and (3) transcriptomic database of N. lugens salivary gland (http://www.ncbi.nlm.nih.gov/sra, accession number SRR5149721). For protein identification, search parameters were as follows: (1) enzyme, trypsin; (2) max missed cleavages, 2; (3) MS/MS tolerance, 20 ppm; (4) fixed modifications, carbamidomethyl (C); (5) variable modifications, oxidation (M), acetyl (protein N-term); (6) database pattern: reverse; (7) protein and peptide false discovery rate (FDR), ≤0.01.
Bioinformatic analysis
Gene Ontology (GO) annotations of identified proteins were assigned according to information available in the Swiss-Prot/TrEMBL (http://www.uniprot.org/) and Gene Ontology database (http://geneontology.org/). MS-identified proteins were categorized by molecular function, biological process and cellular component. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway of identified proteins was performed by BlastKOALA in KEGG database (http://www.kegg.jp/). Signal peptide was determined by SignalP 4.1 Server (http://www.cbs.dtu.dk/services/SignalP/). Subcelluar localization was predicted by TargetP 1.1 Server (http://www.cbs.dtu.dk/services/TargetP/). THMHH Server v. 2.0 (http://www.cbs.dtu.dk/services/ TMHMM/) was used to predict transmembrane helices in proteins. Protein domains were determined by Pfam version 31.0 (http://pfam.xfam.org/).
Prediction of secretory protein and amplification of candidate sequence
Probable secretory proteins were predicted by signal peptide and transmembrane domain. In eukaryotes, there are two cases for secretory proteins: (1) presence of a signal peptide and absence of transmembrane domain; (2) presence of a signal peptide and a transmembrane domain, and meanwhile, the transmembrane domain was in range of the signal peptide [9]. Proteins qualified the conditions were searched in the transcriptomic database of S. furcifera salivary gland and verified using blastx to search for similar sequences in NCBI non-redundant protein database with default parameters. The open reading frame (ORF) of each candidate gene was determined using the ExPASY Translate Tool (http://web.expasy.org/translate/). Gene-specific primers (S1 Table) designed by Primer Premier 5.0 were used to clone the complete ORF or partial sequences of each salivary protein gene. The total RNA from S. furcifera adult was extracted by using TRIzol Reagent (Invitrogen, MA, USA) according to manufacturer’s instructions. Template cDNA was synthesized using the Fast Quant RT kit (TIANGEN, Beijing, China). The PCR products were cloned into the pEASY-T1 vector (TransGen Biotech, Beijing, China) and the insert was sequenced with standard M13 primers.
Real-time quantitative PCR (qPCR) for gene expression analysis
To investigate the tissue-, developmental stage- and sex-specific expression patterns of S. furcifera secretory protein genes, real-time qRCR was conducted using an ABI 7500 Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA). The tissues including salivary gland, head (without salivary gland), gut, testis, ovary and remaining body were dissected from S. furcifera adults under an anatomical lens (Leica Microsystems GmbH, Wetzlar, Germany) with microforceps (Shanghai Medical Instruments Ltd., Corp.) in chilled 1×Phosphate Buffered Saline (1×PBS) solution (pH 7.2, Life Technologies Corporation, NY, USA). Different developmental stages of S. furcifera including egg, 1st-2nd instar nymphs, 3rd-4th instar nymphs, 5th instar nymphs, newly emerged female and male adults, and female and male adults after molting for 5 days were collected. For tissues, total RNA was isolated using the PureLink RNA Mini Kit (Life Technologies, Carlsbad, CA, USA). Total RNA of the whole insect was extracted by using TRIzol Reagent (Invitrogen).
Gene-specific primers (S2 Table) were designed using primer3 web (version 4.0.0) (http://primer3.ut.ee/) and Beacon Designer 7.90, and the cDNA was prepared according to the instruction. The S. furcifera housekeeping gene Ribosomal protein L9 (GeneBank accession number KP735523) and Ribosomal protein L10 (GeneBank accession number KP735524) were used as internal control [16]. The specificity and efficiency of each primer was validated by analyzing standard curves with a fivefold cDNA dilution series.
Each qPCR reaction was conducted in a 20 μl mixture containing 10 μl of Bester® SybrGreen qPCR mastermix (DBI® Bioscience, Germany), 0.4 μl of each primer (10 μM), 0.04 μl of 50 × ROX Reference Dye, 4 μl of sample cDNA and 5.16 μl of sterilized H2O. The first-strand cDNA and a no-reverse-transcription control were used as templates for three biological replicates under the following reaction program: an initial denaturation step at 95°C for 5 min, followed by 40 cycles of 95°C for 10 s and 60°C for 31 s, melt curves stages at 95°C for 15 s, 60°C for 1 min, and 95°C for 15 s. Relative quantification of salivary protein genes in different tissues and developmental stages was performed with the 2-ΔΔCt method [17]. Data analysis was performed using the SPSS Statistics 20.0 software (IBM SPSS Statistics Inc., Chicago, IL, USA). A one-way nested analysis of variance (ANOVA) and Duncan’s multiple range test (p < 0.05) were used to calculate the relative expression of each target gene. The values were presented as the mean ± SE when applicable.
Results
Identification of proteins of watery saliva in S. furcifera
A total of 161 proteins were identified from S. furcifera secreted watery saliva by shotgun LC-MS/MS analysis (Table 1). According to their functions, the identified proteins were divided into 8 categories: (1) enzymes including oxidoreductases, hydrolases, peptidases, proteases, transferases, lyases, isomerases, ligases and ATP synthases; (2) transporter including ABC transporter, endoplasmic reticulum (ER) -Golgi transporter, ion transporter, Golgi transporter, lipid transporter, lysosome transporter, protein transporter and vacuolar transporter; (3) calcium ion binding protein; (4) putative sheath protein; (5) cytoskeleton protein; (6) DNA-, RNA-, and protein-binding or regulating proteins; (7) other non-enzyme proteins such as ubiquitin, antigen, chaperone protein, ribosomal protein, heat shock protein and signal transduction protein; (8) unknown protein.
Table 1. Summarizes the proteins of watery saliva of S. furcifera identified by LC-MS/MS.
| Protein identification | No. of unique peptidesa | Protein domainb | THMHHc | TargetPd | SignalPe | Function group |
|---|---|---|---|---|---|---|
| Cytochrome c oxidase subunit 1 | 22 | Cytochrome C and Quinol oxidase polypeptide I | 12 | S | Yes | Oxidoreductases |
| NADH-ubiquinone oxidoreductase chain 6 | 2 | plastoquinone oxidoreductase chain 6 | 4 | S | Yes | Oxidoreductases |
| NADH-ubiquinone oxidoreductase chain 3 | 3 | NADH-ubiquinone/plastoquinone oxidoreductase, chain 3 | 3 | S | Yes | Oxidoreductases |
| NADH-ubiquinone oxidoreductase chain 2 | 1 | Proton-conducting membrane transporter | 5 | S | Yes | Oxidoreductases |
| Cytochrome P450 CYP419A1 | 1 | Cytochrome P450 | 0 | / | No | Oxidoreductases |
| Cytochrome b | 3 | Cytochromeb (C-terminal)/b6/petD Cytochrome b/b6/petB; Cytochrome b(N-terminal)/b6/petB | 4 | S | No | Oxidoreductases |
| Cytochrome c oxidase subunit 2 | 1 | Cytochrome C oxidase subunit II, periplasmic domain; Cytochrome C oxidase subunit II, transmembrane domain | 2 | S | No | Oxidoreductases |
| NADH dehydrogenase subunit 6 | 1 | / | 4 | S | Yes | Oxidoreductases |
| Peptidylglycine α-hydroxylating monooxygenase | 1 | Copper type II ascorbatedependent monooxygenase, C-terminal domain; Copper type II ascorbate-dependent monooxygenase, N-terminal domain | 1 | S | Yes | Oxidoreductases |
| Inosine-5'-monophosphate dehydrogenase | 1 | IMP dehydrogenase / GMP reductase domain; CBS domain | 0 | / | No | Oxidoreductases |
| Maestro heat-like repeat-containing protein family member 1 | 1 | / | 0 | S | No | Oxidoreductases |
| Aldo-keto reductase | 1 | Aldo/keto reductase family | 0 | / | No | Oxidoreductases |
| Uncharacterized protein LOC100165697 | 1 | / | 0 | / | No | Oxidoreductases |
| Isolate 9 chitinase | 1 | Glycosyl hydrolases family 18 | 0 | M | No | Hydrolases |
| Carboxylesterase | 2 | Carboxylesterase family | 0 | S | Yes | Hydrolases |
| Non-lysosomal glucosylceramidase | 1 | Glycosyl-hydrolase family 116, catalytic region; beta-glucosidase 2, glycosyl-hydrolase family 116 N-term | 1 | / | No | Hydrolases |
| Dicer 2 | 1 | PAZ domain; Ribonuclease III domain | 0 | / | No | Hydrolases |
| Ankyrin repeat domain-containing protein | 1 | Ankyrin repeats (3 copies); Ankyrin repeats (many copies) | 0 | / | No | Hydrolases |
| Uncharacterized protein LOC105391939 isoform X3 | 1 | Phospholipase A2 | 1 | / | No | Hydrolases |
| Hypothetical protein D910_09660 | 3 | Endoribonuclease XendoU | 0 | S | No | Hydrolases |
| Uncharacterized protein LOC100164352 | 1 | / | 0 | / | No | Hydrolases |
| Hypothetical protein ANCCEY_11605 | 1 | / | 0 | / | No | Hydrolases |
| Placental protein 11 | 1 | Endoribonuclease XendoU | 1 | M | No | Hydrolases |
| Plancitoxin-1 isoform X1 | 2 | Deoxyribonuclease II | 1 | S | No | Hydrolases |
| GA19137 isoform A | 1 | USP8 dimerisation domain; Rhodanese-like domain; Ubiquitin carboxyl-terminal hydrolase | 0 | / | No | Hydrolases |
| N-acetylglucosaminyl-phosphatidylinositol de-N-acetylase | 1 | GlcNAc-PI de-N-acetylase | 1 | S | Yes | Hydrolases |
| Ubiquitin carboxyl-terminal hydrolase 7 | 1 | MATH domain; Ubiquitin carboxyl-terminal hydrolase; ICP0-binding domain of Ubiquitin-specific protease 7; Ubiquitin-specific protease C-terminal | 0 | / | No | Hydrolases |
| Uncharacterized family 31 glucosidase KIAA1161 isoform X1 | 1 | Glycosyl hydrolases family 31 | 0 | / | No | Hydrolases |
| Constitutive coactivator of peroxisome proliferator-activated receptor | 1 | / | 0 | / | No | Hydrolases |
| Endonuclease-reverse transcriptase | 1 | / | 0 | / | No | Hydrolases |
| Neprilysin-11-like isoform X4 | 1 | Peptidase family M13 | 1 | S | No | Peptidases |
| Xaa-Pro dipeptidase | 1 | FAST kinase-like protein, subdomain 1 | 0 | / | No | Peptidases |
| Lon protease-like protein | 1 | ATP-dependent protease La (LON) substrate-binding domain; ATPase family associated with various cellular activities (AAA); Lon protease (S16) C-terminal proteolytic domain | 0 | M | No | Peptidases |
| Serine protease 6 | 1 | Trypsin | 1 | S | Yes | Proteases |
| Stubble-2 | 1 | Trypsin | 0 | / | No | Proteases |
| Activated CDC42 kinase 1 | 1 | GTPase binding; Protein tyrosine kinase | 0 | / | No | Transferases |
| ATP citrate lyase isoform X1 | 1 | ATP-grasp domain; ATP citrate lyase citrate-binding; CoA binding domain; CoA-ligase; Citrate synthase, C-terminal domain | 0 | / | No | Transferases |
| Reverse transcriptase | 3 | Reverse transcriptase (RNA-dependent DNA polymerase) | 0 | / | No | Transferases |
| Hypothetical protein D910_10443 | 1 | Reverse transcriptase (RNA-dependent DNA polymerase) | 0 | / | No | Transferases |
| Uncharacterized protein LOC105556507 | 1 | / | 0 | / | No | Transferases |
| UDP-glucuronosyltransferase 2C1 | 1 | UDP-glucoronosyl and UDP-glucosyl transferase | 1 | / | No | Transferases |
| Histone-lysine N-methyltransferase SETMAR | 1 | / | 0 | / | No | Transferases |
| Protein purity of essence isoform X4 | 1 | E3 ubiquitin-protein ligase UBR4 | 0 | / | No | Transferases |
| RNA-directed DNA polymerase from mobile element jockey-like | 1 | / | 0 | / | No | Transferases |
| Hypothetical protein L798_09082 | 1 | Ring finger domain;CUE domain | 0 | / | No | Transferases |
| FAST kinase domain-containing protein 1 | 1 | FAST kinase-like protein, subdomain 2 | 0 | / | No | Transferases |
| Carbonic anhydrase 2 | 1 | Eukaryotic-type carbonic anhydrase | 1 | S | Yes | Lyases |
| Cytoplasmic aconitate hydratase-like | 1 | Aconitase family (aconitate hydratase); Aconitase C-terminal domain | 0 | / | No | Lyases |
| Ornithine decarboxylase | 1 | Pyridoxal-dependent decarboxylase, pyridoxal binding domain; Pyridoxal-dependent decarboxylase, C-terminal sheet domain | 0 | / | No | Lyases |
| Sphingosine-1-phosphate lyase | 1 | Pyridoxal-dependent decarboxylase conserved domain | 0 | / | No | Lyases |
| Protein disulfide-isomerase | 1 | Thioredoxin | 0 | S | Yes | Isomerases |
| ATP-binding domain-containing protein 4 | 1 | Diphthamide synthase; Endoribonuclease L-PSP | 0 | / | No | Ligases |
| CTP synthase | 1 | CTP synthase N-terminus; Glutamine amidotransferase class-I | 1 | / | No | Ligases |
| ATP synthase F0 subunit 8 | 3 | / | 1 | S | Yes | ATP synthases |
| ATP synthase subunit a | 1 | ATP synthase A chain | 5 | S | No | ATP synthases |
| ATP synthase protein 8 | 1 | ATP synthase protein 8 | 1 | S | Yes | ATP synthases |
| Vacuolar ATP synthase subunit E | 1 | ATP synthase (E/31 kDa) subunit | 0 | / | Yes | ATP synthases |
| ATP synthase-coupling factor 6 | 1 | Mitochondrial ATP synthase coupling factor 6 | 0 | M | No | ATP synthases |
| Uncharacterized protein LOC100632426 | 1 | Iron-sulphur cluster biosynthesis | 0 | / | No | ATP synthases |
| ATP-binding cassette sub-family G member 4-like protein | 3 | ABC-2 type transporter; ABC transporter | 7 | / | No | ABC transporter |
| ATP-binding cassette sub-family D member 2-like protein | 1 | ABC transporter transmembrane region 2; ABC transporter | 4 | / | No | ABC transporter |
| ATP-binding cassette sub-family B member 7 | 1 | ABC transporter transmembrane region | 0 | M | No | ABC transporter |
| ABC protein subfamily ABCH | 1 | ABC transporter; ABC-2 family transporter protein | 5 | / | No | ABC transporter |
| Multidrug resistance-associated protein 1 isoform X1 | 1 | ABC transporter transmembrane region; ABC transporter | 16 | S | Yes | ABC transporter |
| Sly1-like protein | 1 | Sec1 family | 0 | / | No | Endoplasmic reticulum (ER)–Glogi transporter |
| Uncharacterized protein LOC100165575 | 1 | / | 1 | S | No | Ion transporter |
| Piezo-type mechanosensitive ion channel component 2 isoform X7 | 1 | Piezo non-specific cation channel, R-Ras-binding domain; Piezo | 31 | S | Yes | Ion transporter |
| F-box/LRR-repeat protein 7 | 1 | F-box-like; Methyl-CpG binding domain | 0 | / | No | Ion transporter |
| Organic cation transporter protein isoform X2 | 1 | Sugar (and other) transporter | 10 | S | Yes | Ion transporter |
| Uncharacterized protein LOC105568157 | 1 | / | 0 | / | No | Ion transporter |
| Golgin subfamily A member 4 | 1 | GRIP domain | 0 | / | No | Golgi transporter |
| Apolipophorin | 1 | von Willebrand factor type D domain | 0 | / | No | Lipid transporter |
| Lipophorin precursor | 1 | Lipoprotein amino terminal region; Domain of unknown function (DUF1943); Domain of Unknown Function (DUF1081); von Willebrand factor type D domain | 0 | S | Yes | Lipid transporter |
| Vitellogenin | 1 | Lipoprotein amino terminal region; Domain of unknown function (DUF1943); von Willebrand factor type D domain | 0 | S | Yes | Lipid transporter |
| Uncharacterized protein LOC100119722 | 1 | Saposin A-type domain; Saposin-like type B, region 1; Saposin-like type B, region 2 | 2 | S | Yes | Lysosome transporter |
| Vam6/Vps39-like protein | 1 | CNH domain; Vacuolar sorting protein 39 domain 1; Vacuolar sorting protein 39 domain 2 | 0 | / | No | Lysosome transporter |
| Vacuolar protein sorting-associated protein 28-like protein | 1 | VPS28 protein | 0 | / | No | Lysosome transporter |
| Run and tbc1 domain-containing protein | 1 | Rab-GTPase-TBC domain | 0 | / | No | Lysosome transporter |
| Hypothetical protein L798_05106 | 1 | Thioredoxin | 2 | S | Yes | Protein transporter |
| Charged multivesicular body protein 3 | 1 | Snf7 | 0 | / | No | Vacuolar transporter |
| Calexcitin-1 isoform X1 | 1 | EF hand; Deoxyribonuclease II | 0 | S | No; | Calcium ion binding protein |
| Hypothetical protein TcasGA2_TC004855 | 1 | LETM1-like protein | 1 | M | No | Calcium ion binding protein |
| EF-hand domain containing protein | 1 | EF hand | 0 | / | No | Calcium ion binding protein |
| Mucin-like protein | 11 | / | 1 | S | Yes | Sheath proteins |
| Actin | 2 | Actin | 0 | / | No | Cytoskeleton protein |
| Myosin-IIIa | 1 | IQ calmodulin-binding motif; Myosin head (motor domain) | 0 | / | No | Cytoskeleton protein |
| Kinesin light chain | 1 | Tetratricopeptide repeat | 0 | / | No | Cytoskeleton protein |
| Microtubule-actin cross-linking factor 1 isoform X4 | 1 | Spectrin repeat; EF-hand domain pair; Growth-Arrest-Specific Protein 2 Domain | 0 | / | No | Cytoskeleton proteins |
| Kinesin-like protein KIF21A isoform X2 | 1 | Kinesin motor domain; WD domain, G-beta repeat | 0 | / | No | Cytoskeleton proteins |
| Cordon-bleu protein-like 1 | 1 | / | 0 | / | No | Cytoskeleton protein |
| Zinc finger protein 239-like | 1 | Zinc finger, C2H2 type; C2H2-type zinc finger | 0 | / | No | Zinc finger protein |
| Zinc finger matrin-type protein | 1 | / | 0 | / | No | Zinc finger protein |
| Apterous a | 1 | LIM domain; Homeobox domain | 0 | / | No | Transcription factors |
| Ecdysteroid receptor | 1 | Ligand-binding domain of nuclear hormone receptor; Zinc finger, C4 type (two domains) | 0 | / | No | Transcription factors |
| Transcriptional regulator ATRX-like | 1 | SNF2 family N-terminal domain; Helicase conserved C-terminal domain | 0 | / | No | Transcription factors |
| RNA polymerase-associated protein CTR9-like protein | 1 | Tetratricopeptide repeat | 0 | / | No | Transcription factors |
| Conserved hypothetical protein | 1 | Leo1-like protein | 0 | / | No | Transcription factors |
| Transcription initiation factor IIA subunit 1 | 1 | Transcription factor IIA, alpha/beta subunit | 0 | M | No | Transcription factors |
| Hypothetical protein L798_07165 | 1 | MH1 domain | 0 | / | No | Transcription factors |
| Hypothetical protein L798_07469 | 1 | / | 0 | M | No | Transcription factors |
| Elongation factor Tu | 1 | Elongation factor Tu GTP binding domain; Elongation factor Tu domain 2; Elongation factor Tu C-terminal domain | 0 | / | No | Translation factors |
| Elongation factor G2 | 1 | Elongation factor Tu; GTP binding domain | 0 | M | No | Translation factors |
| Cryptochrome 2 | 1 | DNA photolyase; FAD binding domain of DNA photolyase | 0 | / | No | DNA repair and recombination protein |
| Thyroid receptor-interacting protein 11 | 1 | GRIP domain | 0 | / | No | DNA repair |
| Uncharacterized protein LOC103516581 | 1 | / | 0 | M | No | DNA binding |
| Cold shock domain-containing protein E1 | 1 | 'Cold-shock' DNA-binding domain | 0 | / | No | DNA binding |
| HMG box-containing protein 1 | 1 | / | 0 | / | No | DNA binding |
| AT-rich interactive domain-containing protein 4B | 1 | RBB1NT (NUC162) domain; ARID/BRIGHT DNA binding domain | 0 | / | No | DNA binding |
| Nucleoprotein TPR | 1 | / | 0 | / | No | DNA binding |
| La-related protein 1B | 1 | / | 0 | / | No | mRNA biosynthesis |
| Mitochondrial fission protein | 1 | Fis1 C-terminal tetratricopeptide repeat; Fis1 N-terminal tetratricopeptide repeat | 1 | S | No | Mitochondrial biogenesis |
| ESF1 homolog | 1 | NUC153 domain | 0 | / | No | Nucleotide binding |
| Bromodomain-containing protein 7 | 1 | Bromodomain; Domain of unknown function (DUF3512) | 0 | / | No | Protein binding |
| Bromodomain and WD repeat-containing protein 2 | 1 | / | 0 | / | No | Protein binding |
| Chaoptin | 1 | / | 6 | M | No | Protein binding |
| Kazrin-A | 1 | SAM domain (Sterile alpha motif) | 0 | / | No | Protein binding |
| Sorting nexin-17 | 1 | / | 0 | / | No | Protein binding |
| Ring finger protein 160 | 1 | Ring finger domain | 0 | / | No | Ring finger protein |
| Cisplatin resistance-associated overexpressed protein | 1 | LUC7 N_terminus | 0 | M | No | RNA binding |
| Teneurin-3-like | 1 | / | 0 | M | No | RNA binding |
| R3H domain-containing protein 1 isoform X1 | 1 | R3H domain; SUZ domain | 0 | / | No | RNA binding |
| Coiled-coil domain-containing protein 12 | 1 | cwf18 pre-mRNA splicing factor | 0 | / | No | Spliceosome |
| Transcription elongation regulator 1 | 1 | WW domain | 0 | / | No | Spliceosome |
| Integrator complex subunit 1 isoform X1 | 1 | / | 0 | / | No | Spliceosome |
| Anaphase-promoting complex subunit | 1 | / | 0 | / | No | Ubiquitin |
| CD109 antigen-like | 1 | MG2 domain; Alpha-2-macroglobulin family N-terminal region; Alpha-2-macroglobulin family; Alpha-macro-globulin thiol-ester bond-forming region; A-macroglobulin complement component; A-macroglobulin receptor | 0 | S | Yes | Antigen |
| Tubulin-specific chaperone C | 1 | Tubulin-specific chaperone C N-terminal domain; Tubulin binding cofactor C | 0 | / | No | Chaperone protein |
| Interaptin isoform X2 | 1 | / | 1 | S | Yes | Ribosome-binding protein |
| Notchless protein homolog 1-like | 1 | NLE (NUC135) domain; WD domain, G-beta repeat | 0 | / | No | Ribosome biogenesis |
| mRNA turnover protein 4 homolog | 1 | Ribosomal protein L10 | 0 | / | No | Ribosome biogenesis |
| Hypothetical protein KGM_12879 | 1 | RNA recognition motif. (a.k.a. RRM, RBD, or RNP domain) | 0 | / | No | Ribosome biogenesis |
| Putative RNA-binding protein 19 | 1 | RNA recognition motif. (a.k.a. RRM, RBD, or RNP domain) | 0 | / | No | Ribosome biogenesis |
| Nucleolar complex protein 3-like protein | 1 | Nucleolar complex-associated protein; CBF/Mak21 family | 0 | / | No | Ribosome biogenesis |
| 30S ribosomal protein S2 | 1 | Ribosomal protein S2 | 0 | / | No | Ribosomal protein |
| 39S ribosomal protein L38 | 1 | Phosphatidylethanolamine-binding protein | 0 | / | No | Ribosomal protein |
| Uncharacterized protein LOC105558474 | 1 | Ribosomal protein S12/S23 | 0 | / | No | Ribosomal protein |
| Heat shock cognate 70 protein | 1 | Hsp70 protein | 0 | / | No | Heat shock protein |
| DEP domain-containing protein 5 isoform X4 | 1 | / | 0 | / | No | Signal transduction protein |
| Mothers against decapentaplegic homolog 3 | 1 | MH2 domain | 0 | M | No | Signal transduction protein |
| Protein numb | 1 | Phosphotyrosine interaction domain (PTB/PID) | 0 | / | No | Signal transduction protein |
| Protein numb-like | 1 | NUMB domain | 0 | M | No | Signal transduction protein |
| Methoprene-tolerant protein | 1 | PAS domain | 0 | / | No | Unknown |
| Salivap-5 | 1 | / | 0 | / | No | Unknown |
| Cchamide 1 | 1 | / | 1 | S | Yes | Unknown |
| Uncharacterized protein LOC105672384 | 1 | Putative peptidase (DUF1758) | 0 | / | No | Unknown |
| Uncharacterized protein LOC105692561 | 1 | / | 3 | / | No | Unknown |
| Protein Daple | 1 | / | 0 | / | No | Unknown |
| Rhogtpase | 1 | BTB/POZ domain | 0 | / | No | Unknown |
| Uncharacterized protein LOC105149357 isoform X1 | 1 | / | 0 | / | No | Unknown |
| Collagen alpha-1(III) chain | 1 | / | 0 | / | No | Unknown |
| Glutamate receptor ionotropic | 1 | / | 0 | / | No | Unknown |
| Protein retinal degeneration B isoform X3 | 1 | / | 0 | / | No | Unknown |
| Hypothetical protein L798_11130 | 1 | / | 0 | M | No | Unknown |
| Triple functional domain | 1 | / | 0 | / | No | Unknown |
| Uncharacterized protein LOC100680511 | 1 | / | 0 | / | No | Unknown |
| Hypothetical protein TcasGA2_TC010304 | 1 | / | 0 | / | No | Unknown |
| Hypothetical protein | 1 | / | 0 | S | Yes | Unknown |
| Uncharacterized protein LOC103508838 isoform X1 | 1 | / | 0 | / | No | Unknown |
| Uncharacterized protein KIAA1143 homolog | 1 | Domain of unknown function (DUF4604) | 0 | M | No | Unknown |
| Hypothetical protein L798_03213 | 1 | / | 0 | / | No | Unknown |
| Glucocorticoid-induced transcript 1 protein-like | 1 | Protein Family FAM117 | 0 | M | No | Unknown |
a The unique matched peptides are shown in S3 Table.
b Protein domains were determined by Pfam version 31.0 (http://pfam.xfam.org/)).
c THMHH Server v. 2.0 (http://www.cbs.dtu.dk/services/TMHMM/) was used to predict transmembrane helices in proteins.
d Subcelluar localization was predicted by TargetP 1.1 Server (http://www.cbs.dtu.dk/services/TargetP/). M stands for mitochondrial targeting peptide. S stands for secretory pathway signal peptide. “/” stands for other.
eSignal peptide was determined by SignalP 4.1 Server (http://www.cbs.dtu.dk/services/SignalP/).
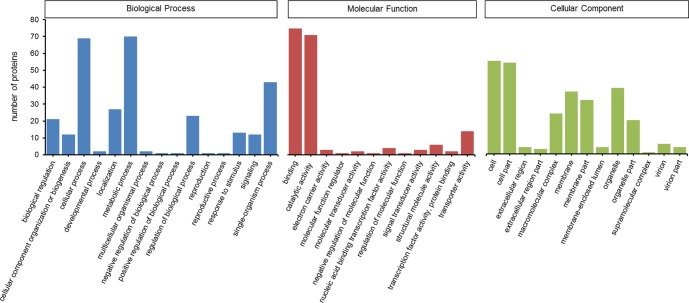
Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were used to identify the potential functions of S. furcifera water salivary proteins. For GO annotation, saliva components were classified at the second level under three root GO domains: biological process, molecular function and cellular component (Fig 1). The most two categories were metabolic process (including 70 proteins) and cellular process (69) in biological process, binding (75) and catalytic activity (71) in molecular function, cell (55) and cell part (54) in cellular component. KEGG pathways involved in the salivary proteins were divided into 5 branches: metabolism, genetic information processing, environmental information processing, cellular processes and organismal systems (Fig 2). Most proteins were distributed in metabolism (34) and genetic information processing (33) at the first level, while the majority proteins were related to energy metabolism (14), transcription (14) and transport and catabolism (15) at the second level.
Fig 1. Gene Ontology classification of S. furcifera water salivary proteins.
Saliva components were classified at the second level under three root GO domains: biological process, molecular function and cellular component.
Fig 2. KEGG pathway classification of S. furcifera salivary proteins.
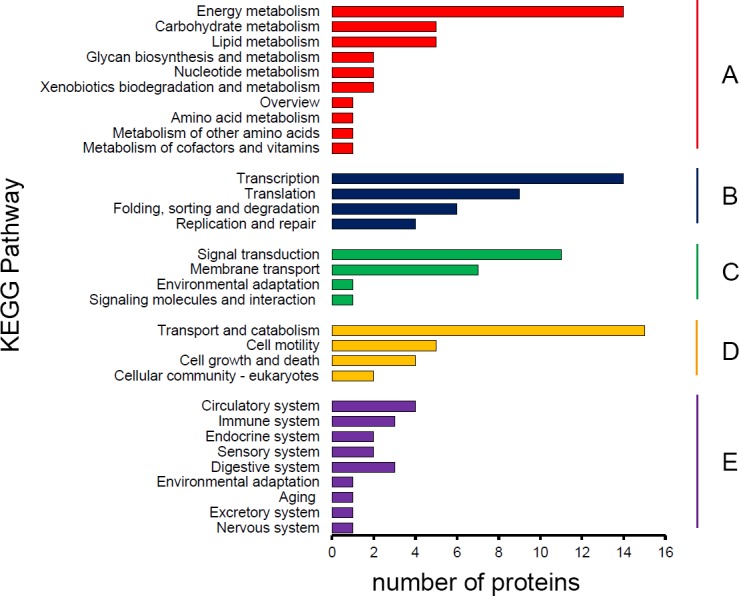
The proteins according to KEGG pathway involved was divided into five branches: A. Metabolism; B. Genetic information processing; C. Environmental information processing; D. Cellular processes; E. Organismal systems.
Predicting, cloning and sequencing of the secretory proteins
Among the 161 proteins, 21 proteins have a putative secretory peptide and no transmembrane domain or the only transmembrane domain was in range of the signal peptide, suggesting secretory proteins. Other proteins without signal peptide indicate unknown secretory mechanism. The putative secretory proteins were searched in the transcriptome of S. furcifera salivary glands, 11 proteins were found to have the complete ORF, suggesting reliable for sequencing and cloning. Other proteins are not found in the transcriptome or only have a short fragment, so we excluded these proteins. All of the 11 salivary secretory proteins were manually searched by blastx program and then named according to the highest protein similarities with the high amio acid identities range from 54% to 100% in National Center for Biotechnology Information (NCBI) (Table 2). Complete ORFs of 9 proteins were verified by cloning and sequencing, while other two proteins were confirmed with partial ORF. The data were deposited on NCBI (accession number from MF189025 to MF189034) except mucin-like protein, which was the same as the Accession number KX670544.
Table 2. Genes of secretory protein identified in S. furcifera watery saliva.
| Gene name | Accession number | Query length (bp) | ORF (aa) | Completeness | Blastx annotation | Score | E-value | Identity |
|---|---|---|---|---|---|---|---|---|
| Peptidylglycine α-hydroxylating monooxygenase | MF189025 | 1044 | 347 | Complete | Peptidylglycine alpha-hydroxylating monooxygenase [Bactrocera dorsalis] | 459 | 6e-159 | 69% |
| Carboxylesterase | MF189026 | 1650 | 549 | Complete | Carboxylesterase [Nilaparvata lugens] | 554 | 0 | 54% |
| Neprilysin-11-like isoform X4 | MF189027 | 1452 | 483 | Complete | Neprilysin-11-like isoform X4 [Nasonia vitripennis] | 584 | 0 | 58% |
| Serine protease 6 | MF189028 | 819 | 272 | Complete | Serine protease 6 [Nilaparvata lugens] | 406 | 2e-141 | 76% |
| Carbonic anhydrase 2 | MF189029 | 924 | 307 | Complete | Carbonic anhydrase 2 [Lygus hesperus] | 303 | 3e-99 | 54% |
| Protein disulfide-isomerase | MF189030 | 1509 | 502 | Complete | Protein disulfide-isomerase [Nilaparvata lugens] | 894 | 0 | 95% |
| Vacuolar ATP synthase subunit E | MF189031 | 681 | 226 | Complete | Vacuolar ATP synthase subunit E [Nilaparvata lugens] | 378 | 2e-131 | 90% |
| Lipophorin precursor | MF189032 | 1555 | 518 | Partial | Lipophorin precursor [Nilaparvata lugens] | 5894 | 0 | 86% |
| Vitellogenin | MF189033 | 1324 | 441 | Partial | Vitellogenin [Laodelphax striatella] | 2590 | 0 | 89% |
| Calexcitin-1 isoform X1 | MF189034 | 558 | 185 | Complete | Calexcitin-1 isoform X1 [Athalia rosae] | 265 | 6e-88 | 63% |
| Mucin-like protein | KX670544 | 2160 | 719 | Complete | Mucin-like protein [Sogatella furcifera] | 768 | 0 | 100% |
Tissue, development and sex-specific expression analysis
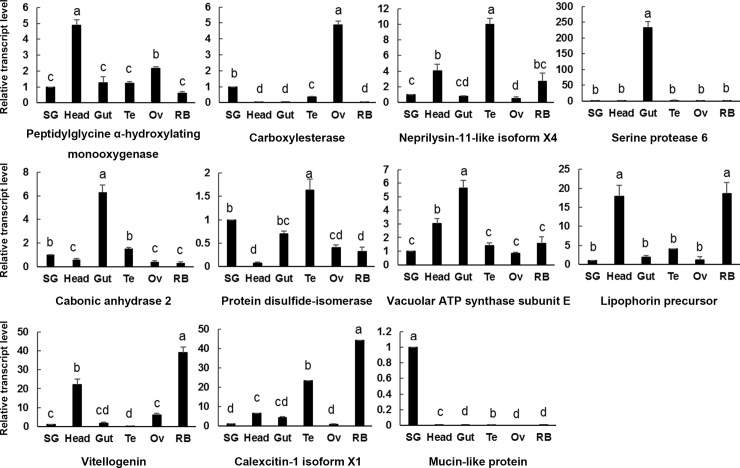
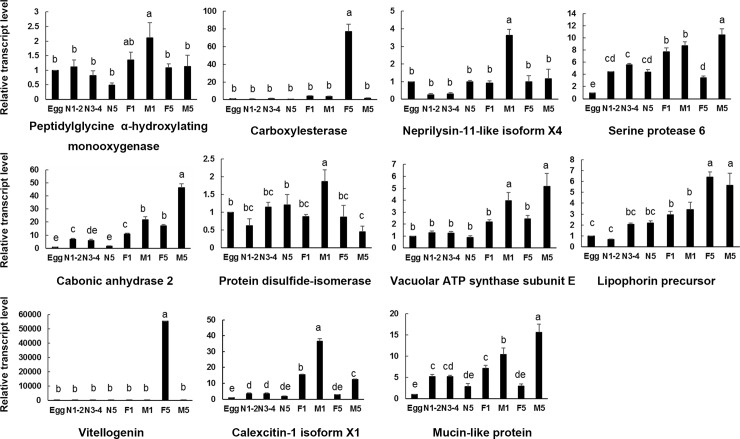
The expression of 11 secretory salivary protein genes in different tissues (salivary gland, head, gut, testis, ovary and remaining body) and different developmental stages and sexes (egg, 1st-2nd instar nymphs, 3rd-4th instar nymphs, 5th instar nymphs, newly emerged female and male adults, and female and male adults after molting for 5 days) were determined by using qPCR. Figs 3 and 4 showed the results.
Fig 3. Tissue-specific expression of S. furcifera genes encoding salivary proteins.
SG, salivary gland; Head, head without salivary gland; Gut, gut; Te, testis; Ov, ovary; RB, remaining body.
Fig 4. Developmental stage- and sex-specific expression of S. furcifera genes encoding salivary proteins.
Egg, egg period; N1-2, 1st-2nd instar nymphs; N3-4, 3rd-4th instar nymphs; N5, 5th instar nymphs; F1, newly emerged female adults; M1, newly molted male adults; F5, female adults after molting for 5 days; M5, male adults after molting for 5 days.
Oxidoreductases are common enzymes in insect saliva. These enzymes can detoxify phenolic compounds in plant-defense reactions by changing the redox balance [3]. Oxidoreductase peptidylglycine α-hydroxylating monooxygenase was highly expressed in head, followed by ovary. This enzyme was extensively expressed in all developmental stages and no significant difference between female and male.
Hydrolases are related to plant-cell degradation by facilitating stylets penetration and movement in plant cell [3]. Carboxylesterase had the highest transcript level in ovary, followed by salivary gland, and then testis. Other tissues were almost not detected gene expression. This gene was highly expressed in female adult after molting for 5 days, suggesting it may play an important role in reproduction.
Proteases (peptidases) generally function as digestive enzymes and are important in detoxification of plant defense compounds [7]. We investigated two proteases in this study. Serine protease 6 had a unique expression in gut, indicating its digestive function. However, neprilysin-11-like isoform X4 was highly expressed in testis, followed by head and remaining body. The developmental stages and sex specific expression pattern between these two enzymes were also different. The expression level of serine protease 6 was increasing along with the development of insect in general except a decline in female adult. Neprilysin-11-like isoform X4 had a significantly high transcript level in newly emerged male adult.
Lyases degrade substance without hydrolysis and oxidation and lyases in insect saliva were not widely known [3]. Carbonic anhydrase was expressed in all tissues but highly expressed in gut. Adult expression level of this gene was higher than nymph and male expression was higher than female adult. Adult molting for 5 days was expressed higher than newly emerged adult.
We tested an isomerase, protein disulfide-isomerase, which is involved in protein folding and synthesis. This gene was highest expressed in testis and then salivary gland and gut. Protein disulfide-isomerase was expressed in all developmental stages and had the highest expression level in newly emerged male adult. However, the expression decreased significantly in male adult after molting for 5 days, indicating it may function in the male mating with female adult.
ATP synthases are related to energy metabolism. Vacuolar ATP synthase subunit E had an extensive expression in all tissues and developmental phases, but it was expressed higher in gut and head than other tissues. Expression level of this gene in male adult was higher than female.
Lipophorin precursor and vitellogenin are lipid transport proteins that function in modulating immune responses in insects [18, 19]. They share a similar expression pattern in tissues. They were both expressed highly in remaining body and head. As to developmental stages and sexes, they preform differently. Vitellogenin expressed significantly high in female adult molting for 5 days, indicating an important role in female reproduction. Lipophorin precursor expressed in all developmental stages and the expression level was increasing together with the insect development.
Calcium ion binding proteins are recorded in saliva of many aphids. This kind of protein facilitates insects to feed on phloem sap of plants by preventing sieve element occlusion [6]. Calexcitin-1 isoform X1 was mainly expressed in remaining body and testis but hardly expressed in salivary gland. It will be interesting to find how calexcitin enters saliva of S. furcifera. Calexcitin-1 isoform X1 had a higher expression level in newly emerged male and female adult and male expression was higher than female.
Mucin-like protein was reported to play an integral role in the formation of salivary sheath [20]. The transcript of this protein was detected at significantly high level in salivary gland but was not detectable in head, gut, testis, ovary and remaining body. It was expressed in all stages and sexes while egg period had the lowest expression level and male adult had the highest one.
Discussion
We investigated the watery saliva component of S. furcifera in an attempt to understand the interaction between the insects and their host plants. This study has identified 161 proteins which have been classified into 8 categories according to the function. Recently, Huang et al. detected 177 proteins in the secreted saliva of S. furcifera. After comparison, we found that 10 salivary proteins in our study were the same with Huang’s research, including salivap-5, plancitoxin-1, golgin subfamily A member 4-like, lipophorin precursor, stubble-2, RNA polymerase-associated protein CTR9-like protein, nucleoprotein TPR, AT-rich interactive domain-containing protein 4B, carboxylesterase and vitellogenin. Some similar cytoskeleton proteins like actin, myosin, kinesin-like protein and a transcription factor like elongation factor were also found in our research. However, the majority of identified proteins were different from them, which may be caused by insect geographical population, saliva collection method and LC/MS-MS analysis method. For example, Chaudhary et al. [8] reported saliva of M. euphorbiae obtained in different diet (0.4% resorcinol, 15% sucrose plus amino acids or water) had different compositions. Our investigated salivary proteins will be useful in increasing the existing knowledge of watery saliva of S. furcifera and enriching the saliva composition database of S. furcifera. Some salivary proteins of N. lugens and Laodelphax striatellus that have been found in the study of Huang et al. [13] was also detected in our research, such as carbonic anhydrase, EF-hand domain containing protein, protein disulfide isomerase and mucin-like protein. It is worth mentioning that Huang et al. [20] found the mucin-like protein in N. lugens was necessary in feeding, especially reared on the resistant rice variety. This protein was confirmed to be involved in the formation of salivary sheath and suppression of resistant plant defense [20].
Since secretory proteins are most likely to be candidate effectors that can secrete into plant tissues and modulate plant defense [21, 22, 23], we selected 11 secretory proteins from the proteomic and transcriptomic analysis for intensive study.
Peptidylglycine α-hydroxylating monooxygenase (PHM), a copper binding oxidoreductase, was highly expressed in the head of S. furcifera and intensively expressed in all developmental stages. Zabriskie et al. [24] isolated a PHM from heads of honeybees (Apis mellifera) and found the enzyme catalyze the amidation of C-terminal peptides, which play important roles in insect reproduction, development and defense. The research about PHM was rarely conducted in invertebrates. The expression pattern about this gene was firstly reported in sap-sucking insects. It may play the same role in formatting of neuropeptides in head as in mammals, and it works in all developmental stages in S. furcifera.
Carboxylesterases (CarEs) occupy crucial roles in detoxification of xenobiotics, degradation of pheromone, neurogenesis and developmental regulations [25]. We found the CarE was highly expressed in ovary, followed by the salivary gland, suggesting its function in regulation of reproduction and detoxification of plant allelochemicals. The significantly high expression level in female after molting for 5 days also verified its important role in reproduction. CarE was previously detected in the saliva of N. lugens [4, 12], while the expression pattern was a little different. CarE was expressed in all tissues, with the highest level in salivary gland and the lowest in ovary [12]. This could be because different families of carboxylesterases were detected in saliva of S. furcifera and N. lugens.
Peptidases (proteases) are the protein-hydrolysing enzymes which have been reported in many hemipterans [3]. We detected two secreted peptidases in saliva of S. furcifera. Serine protease 6, acting as digesting and detoxifying enzyme, had a specific expression in gut of S. furcifera and an extensive expression in all developmental stages except egg. Serine protease was detected in saliva of Schizaphis graminum biotype GB-E, GB-G and GB-H other than GB-C (biotype GB-H, GB-G, GB-E, GB-C ranging in virulence from high to low) [6], suggesting its function in detoxification. Serine protease 6 found in saliva of S. furcifera may play a role in preoral digestion. Neprilysins belong to zinc metalloendopeptidase and play an important role in turning off peptide signaling events at the cell surface [26]. Research on neprilysin and neprilysin-like were conducted on Locusta migratoria [27], Drosophila melanogaster [28] and Bactrocera dorsalis [29], but report about this protein in insect saliva was not found. Neprilysin-11-like isoform X4, detected in S. furcifera saliva, was highly expressed in testis and newly emerged male adults, suggesting its role in testis development and spermatogenesis. This result was in accord with expression pattern of neprilysin in B. dorsalis and D. melanogaster. Neprilysin of B. dorsalis was specifically expressed in testis [29] and different types of neprilysin in D. melanogaster were reported to be expressed in malpighian tubules and testis, suggesting roles for the peptidase in excretory function and in spermatogenesis [28]. However, the role of this protein in insect saliva requires further research.
Carbonic anhydrase (CA) and vacuolar ATP synthase were responsible for intracellular pH homeostasis [30]. Slaymaker et al. [31] claimed that tobacco chloroplast carbonic anhydrase binds salicylic acid (SA), indicating function in plant defense response. CA was previously detected in saliva of S. graminum [6], Macrosiphum euphorbiae [8] and N. cincticeps [10]. It was also found in midgut of larval Aedes aegypti [32] and malpighian tubules of D. melanogaster [33]. CA in S. furcifera had a highest expression level in gut and relatively high level in testis and salivary gland, while it was specifically expressed in salivary gland in N. lugens [12]. This may be because CA in different species owns different tissue distribution and function. Vacuolar ATP synthase exists in almost all eukaryotic cells functioning in membrane trafficking, cytosolic alkalinization and extracellular acidification [34]. It was highly expressed in gut and head in S. furcifera. Hu et al. [35] found that vacuolar ATPase plays an important role in male reproductive physiological processes in B. dorsalis. CA and vacuolar ATP synthase expression in male adult both were higher than female, indicating more important function in male. These two proteins in saliva of S. furcifera could involve in the regulation of pH homeostasis.
Protein disulfide isomerase (PDI) is essential for protein folding [36]. PDI was expressed relatively high in testis and salivary gland in S. furcifera, suggesting its role in regulation of seminal fluid and salivary protein folding. It has also been detected in saliva of Acyrthosiphon pisum [9] and N. lugens [12] previously. Carolan [9] indicated that PDIs were related to an increased yield of salivary proteins. PDI in saliva of S. furcifera may function in synthesis of salivary proteins.
Lipophorin precursor and vitellogenin (Vg), function as lipid transporter, were found in saliva of S. furcifera. Lipophorin was reported to act in lipid-based plant defense [6] and previously discovered in water saliva of S. graminum, A. pisum, M. euphorbiae and N. lugens and L. striatellus [1, 4, 6, 8, 13]. Vg is known as a nutritional source for embryonic development [37]. It has been reported in gelling saliva of N. lugens [12]. These two proteins were both expressed highly in remaining body and head. This may be because they were synthesized in fat body, which is abundant in remaining body and head. Lipophorin precursor had a valid expression in all developmental stages while Vg had a significantly high transcript level in female molting for 5 days, indicating its crucial role in reproduction. However, it is still unknown how Vg secretes into the saliva of S. furcifera and what its role in saliva.
Calcium ion binding proteins exist in saliva of many sap-sucking insects. Innate plant defense mechanism enables occlusion of sieve-tube element when suffering damage by insects [3]. Calexcitin (CE), a calcium ion binding protein, appears as a Ca2+-activated signaling molecular [38, 39] and involves in suppressing plant defense. CE had a high transcript level in remaining body and testis, and was highly expressed in newly emerged adults, suggesting a role in regulation of mating and reproduction. CE had the lowest transcript level in salivary gland, leaving us the confusion that how it works in inhibiting of plant defense.
Mucin-like protein was related to the formation of salivary sheath [4], existing in saliva of N. lugens [4, 12, 13], L. striatellus [13] and N. cincticeps [10]. Huang et al. [12, 20] investigated mucin-like protein had a unique expression in salivary gland of N. lugens and its role in N. lugens virulence and adaptation to host resistance. Shangguan et al. [40] revealed that mucin-like protein of N. lugens can induce plant cell death, the expression of defense-related genes and callose deposition. Expression pattern of mucin-like protein was generally the same in S. furcifera, indicating its function in feeding and interacting with host plant. Male adult had a higher expression than female, suggesting it plays a more important role in male.
In conclusion, we investigated the water saliva component in S. furcifera and tested the expression pattern of 11 secretory proteins. Many proteins were expressed relatively high in salivary gland of S. fuicifera, suggesting its role in saliva. While some proteins had a low expression level in salivary gland but were found in saliva, leaving us to find their potential function in saliva. Mucin-like protein was specifically expressed in salivary gland, which is most likely to be an effector functioning in plant defense. Intensive studies are needed to understand the function of this protein.
Supporting information
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 31772538 and 31370439).
Data Availability
All data are available in the Figs 1–4, Table 1, and supplementary materials S1–S3 Tables.
Funding Statement
The work was supported by National Natural Science Foundation of China (No. 31772538 and 31370439) to YL, http://www.nsfc.gov.cn/.
References
- 1.Vandermoten S, Harmel N, Mazzucchelli G, De Pauw E, Haubruge E, Francis F. Comparative analyses of salivary proteins from three aphid species. Insect Mol Biol. 2014; 23(1):67–77. doi: 10.1111/imb.12061 [DOI] [PubMed] [Google Scholar]
- 2.Miles PW. Aphid saliva. Biol Rev. 1999; 74(1):41–85. [Google Scholar]
- 3.Sharma A, Khan AN, Subrahmanyam S, Raman A, Taylor GS, Fletcher MJ. Salivary proteins of plant-feeding hemipteroids-implication in phytophagy. B Entomol Res. 2014; 104(2):117–136. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, Zhou H, Zhao J, Hua H, He Y. Identification of the secreted watery saliva proteins of the rice brown planthopper, Nilaparvata lugens (Stal) by transcriptome and Shotgun LC-MS/MS approach. J Insect Physiol. 2016; 89:60–69. doi: 10.1016/j.jinsphys.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Tang N, Gao X, Chang Z, Zhang L, Zhou G, et al. Genome sequence of a rice pest, the white-backed planthopper (Sogatella furcifera). GigaScience. 2017; 6(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholson SJ, Puterka GJ. Variation in the salivary proteomes of differentially virulent greenbug (Schizaphis graminum Rondani) biotypes. J Proteomics. 2014; 105:186–203. doi: 10.1016/j.jprot.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 7.Nicholson SJ, Hartson SD, Puterka GJ. Proteomic analysis of secreted saliva from Russian wheat aphid (Diuraphis noxia Kurd.) biotypes that differ in virulence to wheat. J Proteomics. 2012; 75(7):2252–2268. doi: 10.1016/j.jprot.2012.01.031 [DOI] [PubMed] [Google Scholar]
- 8.Chaudhary R, Atamian HS, Shen Z, Briggs SP, Kaloshian I. Potato aphid salivary proteome: enhanced salivation using resorcinol and identification of aphid phosphoproteins. J Proteome Res. 2015; 14(4):1762–1778. doi: 10.1021/pr501128k [DOI] [PubMed] [Google Scholar]
- 9.Carolan JC, Caragea D, Reardon KT, Mutti NS, Dittmer N, Pappan K, et al. Predicted effector molecules in the salivary secretome of the pea aphid (Acyrthosiphon pisum): a dual transcriptomic/proteomic approach. J Proteome Res. 2011; 10(4):1505–1518. doi: 10.1021/pr100881q [DOI] [PubMed] [Google Scholar]
- 10.Hattori M, Komatsu S, Noda H, Matsumoto Y. Proteome analysis of watery saliva secreted by green rice leafhopper, Nephotettix cincticeps. PloS one. 2015; 10(4):e0123671 doi: 10.1371/journal.pone.0123671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper WR, Nicholson SJ, Puterka GJ. Salivary proteins of Lygus hesperus (Hemiptera: Miridae). Ann Entomol Soc Am. 2013; 106(1):86–92. [Google Scholar]
- 12.Huang HJ, Liu CW, Huang XH, Zhou X, Zhuo JC, Zhang CX, et al. Screening and functional analyses of Nilaparvata lugens salivary proteome. J Proteome Res. 2016; 15(6):1883–1896. doi: 10.1021/acs.jproteome.6b00086 [DOI] [PubMed] [Google Scholar]
- 13.Huang HJ, Lu JB, Li Q, Bao YY, Zhang CX. Combined transcriptomic/proteomic analysis of salivary gland and secreted saliva in three planthopper species. J Proteome Res. 2017; http://dx.doi.org/10.1016/j.jprot.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Li Z, An XK, Liu YD, Hou ML. Transcriptomic and expression analysis of the salivary glands in white-backed planthoppers, Sogatella furcifera. PloS one. 2016; 11(7):e0159393 doi: 10.1371/journal.pone.0159393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wisniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009; 6:359–362. doi: 10.1038/nmeth.1322 [DOI] [PubMed] [Google Scholar]
- 16.An XK, Hou ML, Liu YD. Reference gene selection and evaluation for gene expression studies using qRT-PCR in the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). J Econ Entomol. 2016; 109(2):879–886. doi: 10.1093/jee/tov333 [DOI] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25(4):402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 18.Gupta L, Noh JY, Jo YH, Oh SH, Kumar S, Noh MY, et al. Apolipophorin-III mediates antiplasmodial epithelial responses in Anopheles gambiae (G3) mosquitoes. PloS one. 2010; 5(11):e15410 doi: 10.1371/journal.pone.0015410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amdam GV, Simoes ZL, Hagen A, Norberg K, Schroder K, Mikkelsen O, et al. Hormonal control of the yolk precursor vitellogenin regulates immune function and longevity in honeybees. Exp Gerontol. 2004; 39(5):767–773. doi: 10.1016/j.exger.2004.02.010 [DOI] [PubMed] [Google Scholar]
- 20.Huang HJ, Liu CW, Xu HJ, Bao YY, Zhang CX. Mucin-like protein, a saliva component involved in brown planthopper virulence and host adaptation. J Insect Physiol. 2017; 98:223–230. doi: 10.1016/j.jinsphys.2017.01.012 [DOI] [PubMed] [Google Scholar]
- 21.Bos JI, Prince D, Pitino M, Maffei ME, Win J, Hogenhout SA. A functional genomics approach identifies candidate effectors from the aphid species Myzus persicae (green peach aphid). PLoS Genet. 2010; 6(11):e1001216 doi: 10.1371/journal.pgen.1001216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villarroel CA, Jonckheere W, Alba JM, Glas JJ, Dermauw W, Haring MA, et al. Salivary proteins of spider mites suppress defenses in Nicotiana benthamiana and promote mite reproduction. Plant J. 2016; 86(2):119–131. doi: 10.1111/tpj.13152 [DOI] [PubMed] [Google Scholar]
- 23.Jaouannet M, Rodriguez PA, Thorpe P, Lenoir CJ, MacLeod R, Escudero-Martinez C, et al. Plant immunity in plant-aphid interactions. Front Plant Sci. 2014; 5:663 doi: 10.3389/fpls.2014.00663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zabriskie TM, Klinge M, Szymanski CM, Cheng HM, Vederas JC. Peptide amidation in an Invertebrate: purification, characterization, and inhibition of peptidylglycine a-hydroxylating monooxygenase from the heads of honeybees (Apis mellifera). Arch Insect Biochem Physiol. 1994; 26:27–48. doi: 10.1002/arch.940260104 [DOI] [PubMed] [Google Scholar]
- 25.Yu QY, Lu C, Li WL, Xiang ZH, Zhang Z. Annotation and expression of carboxylesterases in the silkworm, Bombyx mori. BMC genomics. 2009; 10:553 doi: 10.1186/1471-2164-10-553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner AJ, Isaac RE, Coates D. The neprilysin (NEP) family of zinc metalloendopeptidases: genomics and function. BioEssays. 2001; 23(3):261–269. doi: 10.1002/1521-1878(200103)23:3<261::AID-BIES1036>3.0.CO;2-K [DOI] [PubMed] [Google Scholar]
- 27.Macours N, Poels J, Hens K, Luciani N, De LA, Huybrechts R. An endothelin-converting enzyme homologue in the locust, Locusta migratoria: functional activity, molecular cloning and tissue distribution. Insect Mol Biol. 2003; 12(3):233–240. [DOI] [PubMed] [Google Scholar]
- 28.Thomas JE, Rylett CM, Carhan A, Bland ND, Bingham RJ, Shirras AD, et al. Drosophila melanogaster nep2 is a new soluble member of the neprilysin family of endopeptidases with implications for reproduction and renal function. Biochem J. 2005; 386(2):357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei D, Li HM, Yang WJ, Wei DD, Dou W, Huang Y, et al. Transcriptome profiling of the testis reveals genes involved in spermatogenesis and marker discovery in the oriental fruit fly, Bactrocera dorsalis. Insect Mol Biol. 2015; 24:41–57. doi: 10.1111/imb.12134 [DOI] [PubMed] [Google Scholar]
- 30.Schewe B, Schmalzlin E, Walz B. Intracellular pH homeostasis and serotonin-induced pH changes in Calliphora salivary glands: the contribution of V-ATPase and carbonic anhydrase. J Exp Biol. 2008; 211:805–815. doi: 10.1242/jeb.002667 [DOI] [PubMed] [Google Scholar]
- 31.Slaymaker DH, Navarre DA, Clark D, del Pozo O, Martin GB, Klessig DF. The tobacco salicylic acid-binding protein 3 (SABP3) is the chloroplast carbonic anhydrase, which exhibits antioxidant activity and plays a role in the hypersensitive defense response. Proc Natl Acad Sci USA. 2002; 99(18):11640–11645. doi: 10.1073/pnas.182427699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corena M, Seron TJ, Lehman HK, Ochrietor JD, Kohn A, Tu C, et al. Carbonic anhydrase in the midgut of larval Aedes aegypti: cloning, localization and inhibition. J Exp Biol. 2002; 205(5):591–602. [DOI] [PubMed] [Google Scholar]
- 33.Bertram G, Zierold K, Wessing A. Carbonic anhydrase supports electrolyte transport in Drosophila malpighian tubules. evidence by X-ray microanalysis of cryosections. J Insect Physiol. 1997; 43(1):17–28. [DOI] [PubMed] [Google Scholar]
- 34.Forgac M. Structure, function and regulation of the vacuolar (h+)-atpases. Annu Rev Cell Dev Bi. 1997; 13(13):779–808. [DOI] [PubMed] [Google Scholar]
- 35.Hu LM, Shen JM, Y. BS, Lin JT. Cloning and tissue-specific expression analysis of V-ATPase G subunit gene in Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Acta Entomol Sin. 2011; 54(12):1452–1458. [Google Scholar]
- 36.Bekendam RH, Bendapudi PK, Lin L, Nag PP, Pu J, Kennedy DR, et al. A substrate-driven allosteric switch that enhances PDI catalytic activity. Nat Commun. 2016; 7:12579 doi: 10.1038/ncomms12579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tufail M, Takeda M. Molecular characteristics of insect vitellogenins. J Insect Physiol. 2008; 54(12):1447–1458. doi: 10.1016/j.jinsphys.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 38.Nelson TJ, Quattrone A, Kim J, Pacini A, Cesati V, Alkon DL. Calcium-regulated GTPase activity in the calcium-binding protein calexcitin. Comp Biochem Physiol B: Biochem Mol Biol. 2003; 135(4):627–638. [DOI] [PubMed] [Google Scholar]
- 39.Nelson TJ, Cavallaro S, Yi CL, Mcphie D, Schreurs BG, Gusev PA, et al. Calexcitin: a signaling protein that binds calcium and gtp, inhibits potassium channels, and enhances membrane excitability. P Natl Acid Sci USA. 1996; 93(24):13808–13813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shangguan XX, Zhang J, Liu BF, Zhao Y, Wang HY, Wang ZZ, et al. A mucin-like protein of planthopper is required for feeding and induces immunity response in plants. Plant Physiology. 2017; doi: 10.1104/pp.17.00755 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All data are available in the Figs 1–4, Table 1, and supplementary materials S1–S3 Tables.