Abstract
Background
Continuous adherence to antipsychotic treatment is critical for individuals with schizophrenia to benefit optimally, yet studies have shown rates of antipsychotic discontinuation to be high with few differences across medications. We investigated discontinuation of selected first- and second-generation antipsychotics among individuals with schizophrenia receiving usual care in a VA healthcare network in the U.S. midatlantic region.
Methods
We identified 2138 VA patients with schizophrenia who initiated antipsychotic treatment with one of five non-clozapine second-generation antipsychotics or either of the two most commonly prescribed first-generation agents between 1/2004 and 9/2006. The dependent variable was duration of continuous antipsychotic possession from the index prescription until the first gap of more than 45 days between prescriptions. We used the Cox proportional hazards model to compare the hazard of discontinuation among the seven antipsychotics controlling for patient demographic and clinical characteristics. The reference group was olanzapine.
Results
The majority of patients (84%) discontinued their index antipsychotic during the follow-up period (up to 33 months). In multivariable analysis, only risperidone had a significantly greater hazard of discontinuation compared to olanzapine (Adjusted Hazard Ratio=1.15, 95% CI: 1.02–1.30, p=.025). Younger age, non-white race, homelessness, substance use disorder, recent inpatient mental health hospitalization, and prescription of another antipsychotic were also associated with earlier discontinuation.
Conclusions
Examination of a usual care sample of individuals with schizophrenia revealed short durations of antipsychotic use, with only risperidone having a shorter time to discontinuation than olanzapine. These findings demonstrate that current antipsychotic agents have limited overall acceptability by patients in usual care.
Keywords: antipsychotic medication, discontinuation, schizophrenia
1. Introduction
Antipsychotic medications are a cornerstone of treatment for schizophrenia. Although first- and second-generation antipsychotic medications have important differences in side effect profiles, there is increasing consensus that both classes exhibit similar efficacy for reducing positive psychotic symptoms (Jones et al., 2006; Kreyenbuhl et al., 2010; Lieberman et al., 2005). Continuous adherence to therapy is critical for individuals with schizophrenia to benefit optimally from antipsychotic treatment. However, many individuals with schizophrenia do not take antipsychotic medications as prescribed, and an estimated 50% of patients can be characterized as nonadherent to treatment (Byerly et al., 2007; Lacro et al., 2002; Velligan et al., 2006). The clinical and fiscal consequences of antipsychotic nonadherence and subsequent treatment discontinuation are substantial. Reduced adherence is associated with symptom relapse, worse prognosis, increased hospital use, and high health care costs (Byerly et al., 2007; Law et al., 2008; Olfson et al., 2000; Velligan et al., 2006; Weiden and Olfson, 1995).
Discontinuation of antipsychotic therapy has been characterized as an important outcome that integrates both patients’ and clinicians’ judgment of tolerability, safety, and effectiveness of antipsychotic treatment (Lieberman et al., 2005). A surprisingly high rate of discontinuation was reported in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Schizophrenia study (Lieberman et al., 2005), a large, double-blind, randomized trial aimed at evaluating the relative effectiveness of several second-generation antipsychotic (SGA) medications (olanzapine, quetiapine, risperidone, ziprasidone) and the first-generation antipsychotic (FGA) perphenazine for the treatment of chronic schizophrenia. Seventy-four percent of individuals participating in CATIE discontinued their assigned medication before 18 months. CATIE investigators also found that medications differed with regard to median time to discontinuation. Individuals randomly assigned to olanzapine had a significantly longer median time to discontinuation (9.2 months) for any cause than those who received quetiapine (4.6 months) or risperidone (4.8 months), but not perphenazine (5.6 months) or ziprasidone (3.5 months), after analyses were corrected for multiple comparisons (Lieberman et al., 2005). Some clinicians have questioned whether these findings may have been a byproduct of the study protocol, in which switching medications was an invited alternative (Tamminga, 2006). This concern indicates a need for more information on typical rates of discontinuation of antipsychotics among individuals with schizophrenia receiving antipsychotic treatment in usual care settings.
The extent of antipsychotic discontinuation has been investigated in several studies of patients in naturalistic treatment settings (Cooper et al., 2005; Kilzieh et al., 2008; Moisan and Grégoire, 2010; Mullins et al., 2008). In these studies, the extent of discontinuation has been high, ranging from 60–95% over treatment periods ranging from 6 months to several years. In a study comparing time to discontinuation of several SGAs to FGAs, Ascher-Svanum et al. (2006) found individuals initiated on either olanzapine or risperidone to have a significantly longer time to all-cause discontinuation than those initiated on FGAs (analyzed as a class) or haloperidol combined with prophylactic anticholinergic treatment; only olanzapine demonstrated a longer time to discontinuation compared to the FGA perphenazine. The time to discontinuation for quetiapine and ziprasidone did not differ significantly from FGAs (Ascher-Svanum et al., 2006). In comparisons of time to discontinuation among SGAs other than clozapine, a number of studies in individuals with schizophrenia initiated on either olanzapine or risperidone found that discontinuation rates were significantly lower for olanzapine than risperidone users (Cooper et al., 2005; Kilzieh et al., 2008; Ren et al., 2006). Mullins et al. (2008) found that individuals with schizophrenia receiving quetiapine had a significantly shorter time to discontinuation than individuals prescribed olanzapine; however, time to discontinuation for aripiprazole, risperidone, and ziprasidone was not significantly different from that of olanzapine. Similarly, Moisan and Grégoire (2010) found that individuals prescribed quetiapine had a significantly shorter time to discontinuation than individuals prescribed olanzapine. In contrast to other studies, however, they also found that individuals prescribed risperidone or polytherapy (defined as >1 SGA or a FGA combined with a SGA) to have a significantly greater time to discontinuation than those prescribed olanzapine. Their study sample differed from those of other studies, however, as only 22% had schizophrenia.
Given the inconsistencies in the findings of previous research comparing treatment persistence across antipsychotic medications, we sought to compare rates of discontinuation among first- and second-generation antipsychotic agents other than clozapine in a large sample of veterans diagnosed with schizophrenia. The VA’s health information system provides a unique resource for assessment of antipsychotic persistence in this population. In addition to evaluating time to discontinuation for olanzapine, risperidone, and quetiapine, the time period for the current study (January 1, 2004 to September 30, 2006) permitted the assessment of utilization patterns for aripiprazole and ziprasidone, which were only addressed in two previous studies (Ascher-Svanum et al., 2006; Mullins et al., 2008). Consistent with CATIE and the results of several observational studies, we hypothesized that individuals prescribed olanzapine would experience a longer time to discontinuation than individuals prescribed other antipsychotic agents.
2. Methods
2.1 Data and study population
Data for the study were obtained from U.S. Department of Veterans Affairs’ (VA) pharmacy and health care utilization databases for patients in the mid-Atlantic VA service region that encompasses Maryland and Washington DC as well as northern Virginia and northeastern West Virginia. These areas are served by four VA hospitals and by a network of freestanding VA hospital-affiliated outpatient medical clinics. A total of 2613 individuals with a diagnosis of schizophrenia (ICD-9 code: 295) received at least one prescription for any antipsychotic medication during the study period (January 1, 2004 – September 30, 2006). From this sample, we selected the 2479 individuals prescribed any one of five second-generation antipsychotic (SGA) medications (aripiprazole, olanzapine, quetiapine, risperidone, or ziprasidone) or either of the two most commonly prescribed first-generation antipsychotic (FGA) medications in our sample (chlorpromazine or haloperidol). Because of limitations in sample size, the 141 individuals prescribed the other less commonly used antipsychotic medications, including only 7 prescribed clozapine, were excluded from the study. To be included, a patient must have had a new start of one of the 7 antipsychotic medications of interest during the study period. A new medication start was defined as the presence of a prescription record for the antipsychotic preceded by a period of time equaling the days supply of the prescription plus 45 days during which another prescription for the same antipsychotic was not filled. We excluded 334 individuals who did not have a new antipsychotic medication started after January 1, 2004, of which 161 received the same antipsychotic medication for the entire study period. We included the remaining 2138 patients who had 5629 antipsychotic starts. We selected for analysis one medication start per patient, with the first medication started after January 1, 2004 considered the ‘index’ antipsychotic medication. The study period (i.e., the number of months of prescription data available from the index antipsychotic start date until September 30, 2006) varied across individual patients, depending on the medication start date.
2.2 Measures
2.2.1 Dependent variable
The dependent variable of the analyses was duration (in days) of the antipsychotic medication episode, a continuous period of medication possession from the date of the index prescription record until the first gap of more than 45 days between prescriptions. Prescribing gaps were calculated by first adding the days’ supply to the dispensing date of each prescription to identify the date the prescription should have been exhausted. Days that the patient was in an inpatient mental health setting were not included in the calculation of episode duration (Cooper et al., 2007; Moisan and Grégoire, 2010). The first gap in prescribing of 46 days or more was considered a discontinuation of the medication.
2.2.2 Demographic and clinical covariates
Covariates were used in multivariable analyses to adjust for demographic and clinical factors that may be related to both the particular antipsychotic prescribed and the likelihood of discontinuation. Demographic covariates included age, gender, and race/ethnicity. Clinical covariates corresponding to the 3-month period immediately preceding the index antipsychotic medication start date included: recent homelessness, presence of any diagnoses for an alcohol or illicit substance abuse or dependence disorder, whether the patient had had any inpatient mental health admissions, and whether the patient used any VA outpatient specialty mental health services. Recent homelessness was defined as having one or more visits to a VA specialty mental health program for homeless veterans, and consequently may be a specific but not a sensitive measure of actual homelessness. In addition to demographic and clinical measures, we included two indicators of access to medications. The first is an indicator of whether the patient had a service connected disability rating ≥50%. VA patients with service connected disability ratings ≥50% are exempt from copayments for prescription medications, and consequently may be less likely to discontinue a medication. The second is whether patients possessed a different antipsychotic medication between the date of discontinuation of the index antipsychotic and the end of the 45-day grace period. Patients who possess a second antipsychotic medication may be more likely to discontinue their first medication.
2.3 Statistical analyses
We first examined the demographic and clinical characteristics of the sample and compared the seven antipsychotic cohorts with respect to these variables using chi-square tests. Second, we estimated the distributions of time until discontinuation and the median time until discontinuation for each antipsychotic cohort using Kaplan-Meier survival curves. In unadjusted analyses, we compared the survival curve for each antipsychotic cohort to the olanzapine cohort with logrank tests. Observations from individuals who continued on their index antipsychotic to the end of the study period were included in the analysis as right censored observations, i.e., these individuals were counted as continuing for the total time they were observed. Third, in adjusted analyses, we used the Cox proportional hazards model (Cox, 1972) to compare times until and adjusted hazard ratios for discontinuation after controlling for patient demographic and clinical characteristics. With olanzapine as the reference group, we estimated the hazard ratio for discontinuation for each of the other antipsychotic cohorts with a 95% confidence interval.
3. Results
3.1 Sample description
The mean (SD) duration of follow up for the 2138 VA patients included in the study was 22.9 (8.8) months. Demographic and clinical characteristics of the total sample and of each antipsychotic cohort are presented in Table 1. Ninety-three percent of individuals were male, 47% were non-white, and the mean (SD) age was 51.7 (11.2) years. Risperidone was the most commonly prescribed antipsychotic (32%), followed by olanzapine (25%), quetiapine (18%), aripiprazole (10%), ziprasidone (7%), haloperidol (5%), and chlorpromazine (2%). When the index antipsychotic agent was discontinued, only 16% of individuals were prescribed another antipsychotic medication.
Table 1.
Demographic and clinical characteristics of the study sample by antipsychotic medication
| All drugs n (%) (N=2138) |
Aripiprazole n (%) (N=214) |
Olanzapine n (%) (N=539) |
Quetiapine n (%) (N=394) |
Risperidone n (%) (N=690) |
Ziprasidone n (%) (N=139) |
Chlorpromazine n (%) (N=47) |
Haloperidol n (%) (N=115) |
|
|---|---|---|---|---|---|---|---|---|
| Age (years) | ||||||||
| 19–34 | 118 ( 5.6) | 19 ( 9.0) | 27 ( 5.0) | 16 ( 4.1) | 42 ( 6.1) | 13 ( 9.4) | 0 (0.0) | 1 ( 0.9) |
| 35–49 | 773 (36.4) | 89 (42.0) | 195 (36.3) | 161 (41.2) | 220 (32.1) | 61 (44.2) | 12 (25.5) | 35 (30.4) |
| 50–64 | 975 (45.9) | 91 (42.9) | 243 (45.3) | 172 (44.0) | 336 (49.0) | 50 (36.2) | 25 (53.2) | 58 (50.4) |
| 65+ | 260 (12.2) | 13 ( 6.1) | 72 (13.4) | 42 (10.7) | 88 (12.8) | 14 (10.1) | 10 (21.3) | 21 (18.3) |
| Race | ||||||||
| White | 606 (28.3) | 72 (33.6) | 152 (28.2) | 124 (31.5) | 163 (23.6) | 50 (36.0) | 12 (25.5) | 33 (28.7) |
| Non-White | 1005 (47.0) | 75 (35.0) | 268 (49.7) | 178 (45.2) | 361 (52.3) | 46 (33.1) | 27 (57.4) | 50 (43.5) |
| Missing | 527 (24.6) | 67 (31.3) | 119 (22.1) | 92 (23.4) | 166 (24.1) | 43 (30.9) | 8 (17.0) | 32 (27.8) |
| Male | 1979 (92.6) | 193 (90.2) | 512 (95.0) | 358 (90.9) | 643 (93.2) | 118 (84.9) | 45 (95.7) | 110 (95.7) |
| Homeless | 251 (11.7) | 19 ( 8.9) | 57 (10.6) | 56 (14.2) | 91 (13.2) | 12 ( 8.6) | 4 ( 8.5) | 12 (10.4) |
| Substance use disorder | 539 (25.2) | 43 (20.1) | 138 (25.6) | 126 (32.0) | 176 (25.5) | 27 (19.4) | 5 (10.6) | 24 (20.9) |
| Service-connected disability rating >50% | 953 (44.8) | 109 (51.2) | 218 (40.5) | 162 (41.3) | 308 (44.8) | 65 (47.8) | 30 (63.8) | 61 (53.0) |
| Any mental health/substance abuse hospitalization in prior 3 months | 373 (17.4) | 27 (12.6) | 104 (19.3) | 63 (16.0) | 114 (16.5) | 27 (19.4) | 6 (12.8) | 32 (27.8) |
| Any mental health/substance abuse outpatient visit in prior 3 months | 1616 (75.6) | 176 (82.2) | 405 (75.1) | 314 (79.7) | 500 (72.5) | 108 (77.7) | 33 (70.2) | 80 (69.6) |
| Prescribed a different antipsychotic at the time of discontinuation of index antipsychotic | 338 (15.8) | 54 (25.2) | 76 (14.1) | 72 (18.3) | 69 (10.0) | 30 (21.6) | 9 (19.1) | 28 (24.3) |
3.2 Treatment discontinuation
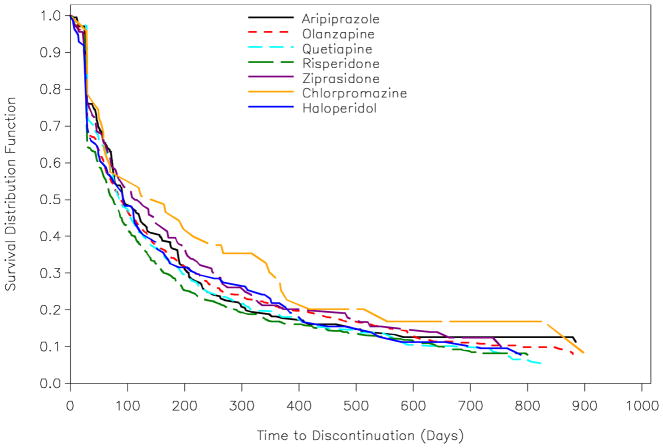
A total of 1799 individuals (84%) discontinued their index antipsychotic agent during the study period, with the median time to discontinuation for each antipsychotic displayed in Table 2. The Kaplan-Meier survival curves for time to discontinuation by antipsychotic are shown in Figure 1. In the unadjusted comparison of the survival curve for each antipsychotic to olanzapine, only risperidone was significantly different (Logrank χ2 = 4.12, df = 1, p = .042). Close inspection of Figure 1 shows that at any point along the follow-up time (beyond the first 30 days or so), a lower cumulative proportion of individuals persisted on risperidone compared to olanzapine. After adjustment for covariates using Cox proportional hazards regression, individuals prescribed risperidone had a significantly greater risk (i.e., hazard) of discontinuation than those prescribed olanzapine (HR = 1.15, p = .025), consistent with the unadjusted analysis. All other antipsychotics were not significantly different from olanzapine with regard to discontinuation hazard (Table 3). These results were unchanged in a sensitivity analysis in which we excluded the 115 individuals who discontinued their antipsychotic treatment after less than 30 days, indicating they had short episodes of antipsychotic treatment (data not shown). Further, because risperidone had the lowest observed median time to discontinuation and was the most frequently prescribed antipsychotic, in another sensitivity analysis we re-fit the Cox regression model specifying risperidone as the reference group. In addition to olanzapine, individuals prescribed aripiprazole (HR = .84, p = .042) and ziprasidone (HR=.76, p = .008) also had significantly reduced risk of discontinuation versus risperidone.
Table 2.
Unadjusted median time to discontinuation by antipsychotic medication
| Index Drug | Number of index episodes | Median time to discontinuation (days) | 95% CI |
|---|---|---|---|
| Aripiprazole | 214 | 93 | (76 – 131) |
| Olanzapine | 539 | 90 | (75 – 105) |
| Quetiapine | 394 | 87 | (77 – 105) |
| Risperidone | 690 | 76 | (65 – 87) |
| Ziprasidone | 139 | 114 | (79 – 170) |
| Chlorpromazine | 47 | 164 | (59 – 268) |
| Haloperidol | 114 | 95 | (58 – 127) |
Figure 1.
Kaplan-Meier estimated survival curves by antipsychotic medication
Table 3.
Adjusted hazard ratios for risk of discontinuation of antipsychotic medications
| Variable | Hazard Ratio | 95% CI | p-value |
|---|---|---|---|
| Age (years) | |||
| 35–49 | .97 | (.78–1.20) | .770 |
| 50–64 | .81 | (.65–1.01) | .061 |
| 65+ | .77 | (.60–.99) | .044 |
| 19–34 | reference | ||
| Male | 1.13 | (.94–1.36) | .207 |
| Race | |||
| Non-White | 1.26 | (1.12–1.41) | <.001 |
| Missing | 1.40 | (1.22–1.60) | <.001 |
| White | reference | ||
| Homeless | 1.20 | (1.03–1.39) | .018 |
| Substance use disorder | 1.35 | (1.20–1.52) | <.001 |
| Service connected disability rating >50% | .92 | (.83–1.01) | .076 |
| Any mental health/substance abuse hospitalization in prior 3 months | 1.18 | (1.04–1.33) | .013 |
| Any mental health/substance abuse outpatient visit in prior 3 months | .91 | (.80–1.04) | .175 |
| Prescribed a different antipsychotic at the time of discontinuation of index antipsychotic | 1.25 | (1.11–1.42) | <.001 |
| Index antipsychotic medication | |||
| Aripiprazole | .94 | (.79–1.12) | .501 |
| Quetiapine | 1.02 | (.89–1.18) | .746 |
| Risperidone | 1.15 | (1.02–1.30) | .025 |
| Ziprasidone | .88 | (.71–1.09) | .255 |
| Chlorpromazine | .89 | (.64–1.24) | .489 |
| Haloperidol | 1.01 | (.80–1.26) | .947 |
| Olanzapine | reference |
Several patient demographic and clinical characteristics were also significantly related to discontinuation, independent of the antipsychotic prescribed. Individuals 65 years of age and older had a 33% lower risk of discontinuation relative to those ages 19–34. Individuals with a history of homelessness had a 20% higher risk of discontinuation, non-white patients had a 26% higher risk, those with a substance abuse disorder had a 35% higher risk, and those with an inpatient hospitalization in the 3 months prior to the start of the index antipsychotic had a 18% higher risk of treatment discontinuation. The risk of discontinuation was increased by 25% if the patient was prescribed another antipsychotic at the time of discontinuation of the index antipsychotic.
4. Discussion
Among an observational sample of VA patients with schizophrenia beginning a new episode of antipsychotic treatment, the overwhelming majority (84%) discontinued their medication during the study period. This finding is consistent with the CATIE Schizophrenia study (Lieberman et al., 2005), in which 74% of patients discontinued antipsychotic treatment within 18 months, and supports the results of studies in similar usual care samples (Cooper et al., 2005; Moisan and Grégoire, 2010; Mullins et al., 2008). Although several studies document similarly high rates of antipsychotic discontinuation, it is striking that only 16% of patients who discontinued their index antipsychotic in this study had a prescription filled for a different antipsychotic agent within 45 days. This suggests that a majority of patients had an interruption in antipsychotic treatment which increased their risk for serious adverse outcomes. Taken together, these findings suggest that despite the introduction of several new antipsychotic medications over the past 15 years, available treatments may not address the needs and preferences of most individuals with schizophrenia.
Although evidence-based treatment guidelines for schizophrenia recommend ongoing antipsychotic treatment for all patients (Kreyenbuhl et al., 2010) and the risks of antipsychotic discontinuation can be considerable, it should not be assumed that every decision to interrupt treatment is necessarily irrational. For example, the decision to discontinue a treatment that is not providing adequate relief of target symptoms or whose side effect profile is intolerable and thus impeding rather than facilitating recovery may be a logical response, signaling the need for the patient and clinician to jointly consider an alternate treatment strategy. More research is needed to understand why episodes of antipsychotic treatment continue to be so brief and whether strategies that facilitate shared decision-making between patients and their clinicians around antipsychotic treatment enhance continuity of treatment (Kreyenbuhl et al., 2009).
Contrary to our hypothesis, we found the median length of treatment for each of the SGAs and FGAs evaluated in this study to be generally similar to that of olanzapine. The only exception was risperidone, which demonstrated a statistically significantly greater risk of discontinuation compared to olanzapine, a finding that was observed in CATIE and some (Cooper et al., 2005; Ren et al., 2006; Kilzieh et al., 2008), but not all (Moisan and Grégoire, 2010; Mullins et al., 2008) previous observational studies. Our results add to the accumulating evidence that the effectiveness of olanzapine, and possibly aripiprazole and ziprasidone, may be superior in some ways to that of risperidone. Unfortunately, the administrative data we used for this study provide no insight into the reasons certain antipsychotics are discontinued more quickly than others. For example, we don’t know if risperidone was prescribed in dosages above recommended ranges, possibly leading to side effects that promoted early discontinuation of treatment.
Our results did not confirm earlier findings (Moisan and Grégoire, 2010; Mullins et al., 2008) of a greater risk of treatment discontinuation for quetiapine, nor did we observe any specific advantages for olanzapine over the two most recently introduced SGAs during the study period, ziprasidone and aripiprazole. In contrast to earlier research (Ascher-Svanum et al., 2006), we also did not find evidence of a greater rate of discontinuation for the two most frequently prescribed FGAs, haloperidol and chlorpromazine, relative to olanzapine. However, more investigation of this is warranted as our statistical power to detect these differences may have been reduced due to the relatively small sample sizes of those prescribed chlorpromazine (n=47) and haloperidol (n=115) in this study.
Consistent with previous research, however, we did find evidence that greater illness severity and complexity is associated with a higher risk of antipsychotic discontinuation. Recent homelessness, substance use disorder (Cooper et al., 2007) and prior inpatient psychiatric hospitalization (Mullins et al., 2008; Ren et al., 2006) were all significantly associated with an increased risk of treatment discontinuation. On the other hand, we found that older individuals had a lower risk of discontinuing antipsychotic treatment. Older individuals may have more stable symptom presentations than younger patients or over time, have found an acceptable antipsychotic regime after several trials. These findings remind us that, regardless of the antipsychotic prescribed, in involving patients in shared decision-making around antipsychotic choice, close attention should be paid to whether patients possess other known risk factors for treatment discontinuation.
This study has several limitations, including several inherent in the use of electronic databases for research purposes. We could not tell from the administrative data the extent to which individuals who we classified as discontinuing antipsychotic treatment were receiving such treatment or other mental health services from non-VA settings because of moving or other reasons. However, prior research has shown that less than 20% of veterans with schizophrenia receive mental health services outside of the VA (Hoff and Rosenheck, 2000; Desai et al., 2001). Another study found that while around 20% of veterans with schizophrenia move residences in a given year, many actually move closer to VA providers (McCarthy et al., 2007). Finally, there are incentives for veterans with schizophrenia to obtain their medications from VA pharmacies where they are required to pay only minimal or no pharmacy copayments because of their low income or service-connected health conditions. Taken together, this suggests the extent of misclassifying true antipsychotic discontinuation in this study due to veterans using non-VA mental health or pharmacy services was likely to be modest. Our source of data also did not permit us to elucidate the circumstances under which antipsychotic treatment was discontinued, including whether it was the decision of the patient or the clinician or the specific reason for discontinuation. In addition, some of the measures we derived from administrative data, including that of recent homelessness and substance use, may not be particularly sensitive indicators of these problems.
Other limitations of the study include that although we attempted to minimize selection bias by adjusting our analyses for potential confounders (e.g., substance use), unmeasured differences across the antipsychotic cohorts may have been related to rates of discontinuation. Also, the results of this study may only generalize to VA patients with schizophrenia, most of who are male and older and receive care in an integrated healthcare system. Further, despite the known superiority of clozapine over other antipsychotics for the treatment of schizophrenia, it is relatively underused in VA settings (Kreyenbuhl et al., 2006), and consequently we were unable to evaluate it due to a small sample size (n=7). These limitations are mitigated to some extent by our ability to evaluate discontinuation of treatment across a variety of widely prescribed antipsychotic agents over an extended period of time in a large sample of individuals with schizophrenia.
In summary, we found high rates of antipsychotic discontinuation in individuals with schizophrenia being treated in a VA healthcare setting and few differences across several first- and second-generation medications. Reasons for such short durations of antipsychotic treatment remain elusive. Such information is needed in order to develop strategies that foster longer periods of treatment continuation.
References
- Ascher-Svanum H, Zhu B, Faries D, Landbloom R, Swartz M, Swanson J. Time to discontinuation of atypical versus typical antipsychotics in the naturalistic treatment of schizophrenia. BMC Psychiatry. 2006;6:8. doi: 10.1186/1471-244X-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly MJ, Nakonezny PA, Lescouflair E. Antipsychotic medication adherence in schizophrenia. Psychiatr Clin North Am. 2007;30:437–452. doi: 10.1016/j.psc.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Cooper D, Moisan J, Grégoire JP. Adherence to atypical antipsychotic treatment among newly treated patients: a population-based study in schizophrenia. J Clin Psychiatry. 2007;68(6):818–25. doi: 10.4088/jcp.v68n0601. [DOI] [PubMed] [Google Scholar]
- Cooper D, Moisan J, Gaudet M, Abdous B, Grégoire JP. Ambulatory use of olanzapine and risperidone: a population-based study on persistence and the use of concomitant therapy in the treatment of schizophrenia. Can J Psychiatry. 2005;50(14):901–908. doi: 10.1177/070674370505001404. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression models and life tables (with discussion) J Roy Statistical Society. 1972;34:187–220. [Google Scholar]
- Desai RA, Rosenheck RA, Rothbard A. Cross-system service use among VA mental health patients living in Philadelphia. Adm Policy Ment Health. 2001;28(4):299–309. doi: 10.1023/a:1011137630558. [DOI] [PubMed] [Google Scholar]
- Hoff RA, Rosenheck RA. Cross-system service use among psychiatric patients: Data from the Department of Veterans Affairs. J Behav Health Serv Res. 2000;27(1):98–106. doi: 10.1007/BF02287807. [DOI] [PubMed] [Google Scholar]
- Jones PB, Barnes TR, Davies L, Dunn G, Lloyd H, Hayhurst KP, Murray RM, Markwick A, Lewis SW. Randomized controlled trial of the effect on Quality of Life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1) Arch Gen Psychiatry. 2006;63(10):1079–1087. doi: 10.1001/archpsyc.63.10.1079. [DOI] [PubMed] [Google Scholar]
- Kilzieh N, Todd-Stenberg JA, Kennedy A, Wood AE, Tapp AM. Time to discontinuation and self-discontinuation of olanzapine and risperidone in patients with schizophrenia in a naturalistic outpatient setting. J Clin Psychopharmacol. 2008;28(1):74–7. doi: 10.1097/jcp.0b013e3181602cf3. [DOI] [PubMed] [Google Scholar]
- Kreyenbuhl J, Valenstein M, McCarthy JF, Ganoczy D, Blow FC. Long-term combination antipsychotic treatment in VA patients with schizophrenia. Schizophr Res. 2006;84(1):90–99. doi: 10.1016/j.schres.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Kreyenbuhl J, Nossel IR, Dixon LB. Disengagement from mental health treatment among individuals with schizophrenia and strategies for facilitating connections to care: A review of the literature. Schizophr Bull. 2009;35(4):696–703. doi: 10.1093/schbul/sbp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreyenbuhl J, Buchanan RW, Dickerson FB, Dixon LB. The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull. 2010;36(1):94–103. doi: 10.1093/schbul/sbp130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacro JP, Dunn LB, Dolder CR, Leckband SG, Jeste DV. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63(10):892–909. doi: 10.4088/jcp.v63n1007. [DOI] [PubMed] [Google Scholar]
- Law MR, Soumerai SB, Ross-Degnan D, Adams AS. A longitudinal study of medication nonadherence and hospitalization risk in schizophrenia. J Clin Psychiatry. 2008;69(1):47–53. doi: 10.4088/jcp.v69n0107. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- McCarthy JF, Valenstein M, Blow FC. Residential mobility among patients in the VA health system: associations with psychiatric morbidity, geographic accessibility, and continuity of care. Adm Policy Ment Health. 2007;34(5):448–55. doi: 10.1007/s10488-007-0130-2. [DOI] [PubMed] [Google Scholar]
- Moisan J, Grégoire JP. Patterns of discontinuation of atypical antipsychotics in the province of Québec: A retrospective prescription claims database analysis. Clin Ther. 2010;32(Suppl 1):S21–S31. doi: 10.1016/j.clinthera.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Mullins CD, Obeidat NA, Cuffel BJ, Naradzay J, Loebel AD. Risk of discontinuation of atypical antipsychotic agents in the treatment of schizophrenia. Schizophr Res. 2008;98(1–3):8–15. doi: 10.1016/j.schres.2007.04.035. [DOI] [PubMed] [Google Scholar]
- Olfson M, Mechanicn D, Hansell S, Boyer CA, Walkup J, Weiden PJ. Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatr Serv. 2000;51(2):216–222. doi: 10.1176/appi.ps.51.2.216. [DOI] [PubMed] [Google Scholar]
- Ren XS, Qian S, Lee AF, Herz L, Miller DR, Kazis LE. Treatment persistence: a comparison among patients with schizophrenia who were initiated on atypical antipsychotic agents. J Clin Pharm Ther. 2006;31(1):57–65. doi: 10.1111/j.1365-2710.2006.00711.x. [DOI] [PubMed] [Google Scholar]
- Tamminga CA. Practical treatment information for schizophrenia. Am J Psychiatry. 2006;163(4):563–565. doi: 10.1176/ajp.2006.163.4.563. [DOI] [PubMed] [Google Scholar]
- Velligan DI, Lam YW, Glahn DC, Barrett JA, Maples NJ, Ereshefsky L, Miller AL. Defining and assessing adherence to oral antipsychotics: a review of the literature. Schizophr Bull. 2006;32(4):724–742. doi: 10.1093/schbul/sbj075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiden PJ, Olfson M. Cost of relapse in schizophrenia. Schizophr Bull. 1995;21(3):419–429. doi: 10.1093/schbul/21.3.419. [DOI] [PubMed] [Google Scholar]