Abstract
OBJECTIVE
To summarize and discuss logistic and administrative challenges we encountered during the Benefits of Enhanced Terminal Room (BETR) Disinfection Study and lessons learned that are pertinent to future utilization of ultraviolet (UV) disinfection devices in other hospitals.
DESIGN
Multicenter cluster randomized trial.
SETTING AND PARTICIPANTS
Nine hospitals in the southeastern United States.
METHODS
All participating hospitals developed systems to implement 4 different strategies for terminal room disinfection. We measured compliance with disinfection strategy, barriers to implementation, and perceptions from nurse managers and environmental services (EVS) supervisors throughout the 28-month trial.
RESULTS
Implementation of enhanced terminal disinfection with UV disinfection devices provides unique challenges, including time pressures from bed control personnel, efficient room identification, negative perceptions from nurse managers, and discharge volume. In the course of the BETR Disinfection Study, we utilized several strategies to overcome these barriers: (1) establishing safety as the priority; (2) improving communication between EVS, bed control, and hospital administration; (3) ensuring availability of necessary resources; and (4) tracking and providing feedback on compliance. Using these strategies, we deployed ultraviolet (UV) disinfection devices in 16,220 (88%) of 18,411 eligible rooms during our trial (median per hospital, 89%; IQR, 86%–92%).
CONCLUSIONS
Implementation of enhanced terminal room disinfection strategies using UV devices requires recognition and mitigation of 2 key barriers: (1) timely and accurate identification of rooms that would benefit from enhanced terminal disinfection and (2) overcoming time constraints to allow EVS cleaning staff sufficient time to properly employ enhanced terminal disinfection methods.
TRIAL REGISTRATION
Clinical trials identifier: NCT01579370
Multidrug-resistant organisms (MDROs) are common causes of healthcare-associated infections (HAIs) and lead to adverse patient outcomes, including increased length of hospitalization, morbidity, and mortality.1–3 The hospital environment is increasingly recognized as a source of transmission for these MDROs.4–6 Enhanced terminal room disinfection using ultraviolet (UV) light in addition to standard cleaning is an evidence-based strategy that decreases the risk of MDRO acquisition from the environment.7,8 However, more data are needed to guide hospitals in identifying and overcoming challenges to successful implementation of enhanced disinfection strategies.
We recently completed a large, cluster-randomized multi-center trial to evaluate the impact of enhanced terminal room disinfection strategies on patient outcomes.7 Herein, we summarize data collected during this trial related to the implementation and logistics of using UV disinfection devices. Our objectives in this report are to summarize and discuss (1) logistic and administrative challenges we encountered during the trial and (2) lessons learned that are pertinent to future utilization of UV disinfection devices in other hospitals.
METHODS
The Benefits of Enhanced Terminal Room (BETR) Disinfection Study has been described in detail elsewhere.7 In brief, the BETR Disinfection Study was a pragmatic, prospective, multicenter, cluster-randomized, crossover trial designed to evaluate 4 different strategies for terminal room disinfection in 9 hospitals in the southeastern United States from April 2012 through July 2014. For ease of discussion, the 4 terminal disinfection strategies used in the BETR Disinfection Study will be labeled hereafter as reference, UV, bleach, and bleach and UV. Enhanced strategies were used in targeted rooms, defined as single-patient rooms from which a patient on contact precautions was discharged or transferred.
Our study team engaged local environmental services (EVS), infection control (IC), and physician champions from study hospitals regularly throughout the trial. We met with champions and key hospital personnel in person at the beginning of each study phase. In addition, we held regular “collaborative calls” with the local champions throughout each study phase. We held these calls weekly at the beginning of each phase and less frequently as the phase progressed.
We simplified the room selection process by using enhanced disinfection in all contact precaution rooms, regardless of indication for the use of contact precautions. We used a “Swiss cheese” model of multiple redundant strategies to increase our ability to identify contact precaution rooms for enhanced terminal disinfection: (1) bed control and EVS staff had daily (morning) discussions about patients expected to be discharged; (2) EVS housekeepers were instructed to use contact precaution signs to determine the need for enhanced or standard disinfection at the time of terminal room cleaning; and (3) local infection preventionists made regular rounds to ensure contact precaution signage was accurate. At the beginning of each study phase, housekeepers were provided laminated pocket cards that provided information about the correct chemical to use and whether a UV device was required. Each study hospital developed its own strategy for UV device deployment. Ultimately, EVS supervisors were responsible for deployment of the UV devices at all 9 study hospitals.
We collected data on compliance with study protocols, including the use of the UV disinfection device in targeted rooms during the study. We also administered electronic surveys to EVS supervisors and nursing unit managers from each study hospital after every study phase to obtain qualitative data on perceptions about each terminal room disinfection strategy. We compared responses (1) during the use of quaternary ammonium versus bleach and (2) with and without UV device deployment.
Data were summarized using standard statistical measures. Proportions were compared using a 2-tailed χ2 test or Fisher exact test, as appropriate. All analyses were completed using SAS software version 9.2 (SAS Institute, Cary, NC). The Duke University Health System Institutional Review Board (IRB) served as the central institutional review board for the trial.
RESULTS
Process Considerations
In total, UV disinfection devices were deployed in 16,220 of 18,411 eligible contact precaution rooms (88%). The median hospital compliance was 89% (IQR, 86%–92%), but variation was observed among study hospitals (Supplementary Figure 1).
A UV disinfection device was used a total of 21,844 times during the 28-month study period. A vegetative cycle (targeting MRSA, VRE, and Acinetobacter in our study) was used 16,313 times (75%), and a spore cycle (targeting C. difficile) was used 3,651 times (17%). The type of cycle was not documented 1,880 times (9%). Of 21,431 cycles with documented times, the cycle completed 21,189 times (97%; range per hospital, 90.8%–98.8%). Among 1,272 aborted or blocked opportunities, the 2 most common causes were “room needed immediately for patient” (n = 906, 71%) and “device malfunction” (n = 72, 5.7%). Device and personnel availability and perception of difficulty moving the machine were infrequent causes of missed or aborted opportunities (n = 30, 2.3%).
Overall, the median cycle time was 33 minutes (IQR, 25–46), with vegetative cycles taking a median of 30 minutes (IQR, 24–41) and spore cycles taking a median of 55 minutes (IQR, 41–71). Considerable variation was noted in the amount of time needed to deploy the UV devices at the study hospitals (Figure 1). Variation appeared to be primarily related to differences in room size, room configurations, and the number of items in the room at the time the UV device was employed. For example, we heard numerous anecdotes about local nursing staff placing additional materials in rooms (eg, IV poles) so they would “be cleaned.” We observed that dust accumulated easily on the UV bulbs in the early phases of the study. This dust accumulation was correlated with increased run times, so we initiated a policy to clean the bulbs weekly approximately 2 months into the study.
Figure 1.
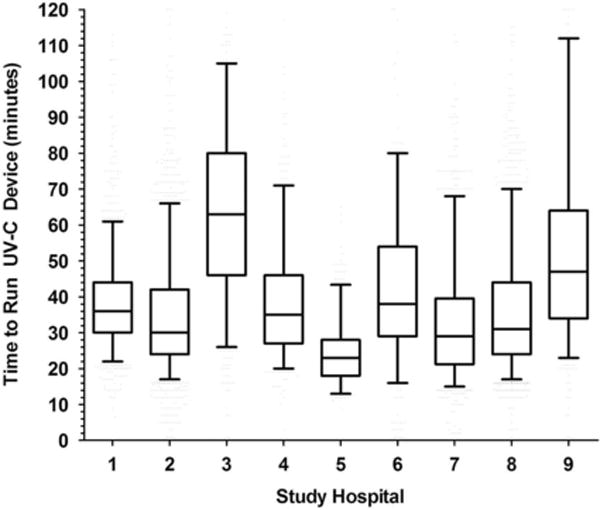
Median time* required to complete ultraviolet (UV) disinfection device cycles in 9 study hospitals during the Benefits of Enhanced Terminal Room (BETR) Disinfection Study. NOTE: *Median, IQR, and 95% confidence interval provided for each of the 9 study hospitals using the standard box and whiskers approach.
We reviewed the timing of room discharges for 8 study hospitals and the timing of the use of the UV devices for all 9 study hospitals. Of 598,291 documented room cleanings during the 28-month study period, most occurred during first shift (07:00–15:00) (Figure 2a). Only 35,380 (6%) occurred during third shift (23:00–07:00). However, of the 19,163 uses of the UV device with complete timing data, the largest number of room cleanings with the UV device occurred during second shift (15:00–23:00). The highest volume of room cleaning per hour occurred from 12:00 to 16:00, while the highest volume of UV device utilization per hour occurred from 13:00 to 18:00 (Figure 2b).
Figure 2A.
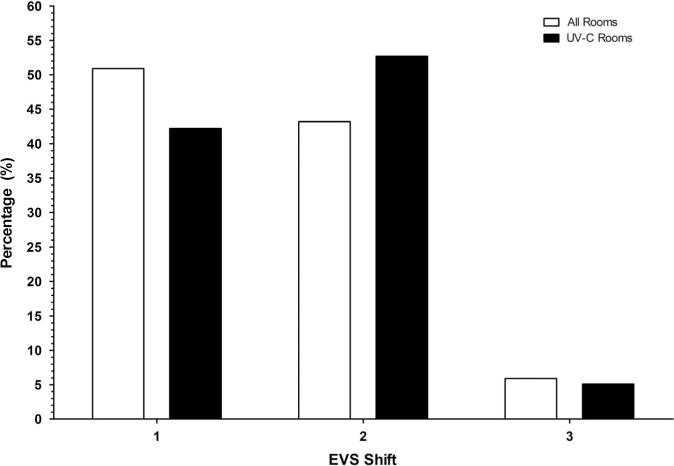
Percentage of 598,291 patient discharges per shift during the Benefits of Enhanced Terminal Room (BETR) Disinfection Study. Data are provided for any type of patient discharge (all rooms) and for patient discharges after which a UV device was deployed (UV rooms).
Figure 2B.
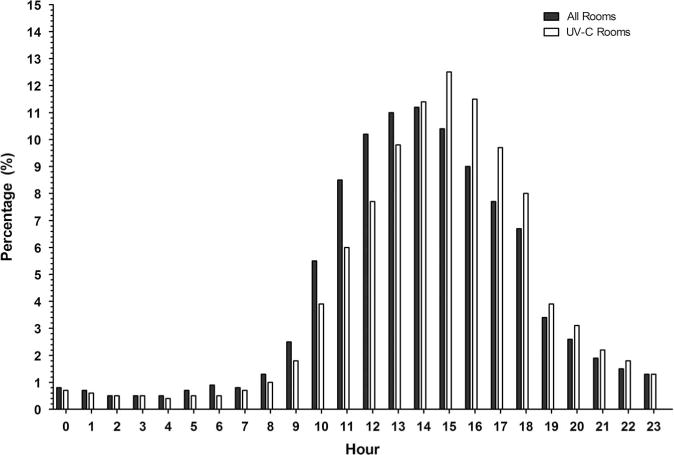
Percentage of 598,291 patient discharges per hour during the Benefits of Enhanced Terminal Room (BETR) Disinfection Study. Data are provided for any type of patient discharge (all rooms) and for patient discharges after which a UV device was deployed (UV rooms).
Qualitative Data
We sent surveys to 517 nurse managers and 270 EVS supervisors during the study; 309 nurse managers (60%) and 184 EVS supervisors (68%) responded (Table 1). Nurse managers and EVS supervisors both perceived more room cleaning delays during study phases when UV devices were used compared to study phases without UV devices (nurse managers: 45% vs 16%, P < .001; EVS supervisors: 47% vs 21%; P < .001). Nurse managers perceived more complaints from staff concerning odor when UV devices were being used (53% vs 22%; P < .001). The EVS supervisors perceived more complaints about delays from nurse managers (69 vs 38%; P = .0002) and bed control personnel (54 vs 34%; P = .01) with the use of UV devices compared to periods when UV devices were not being used. Regardless of the cleaning strategy, EVS supervisors believed that the delay in the emergency department was the primary cause of the delay in hospital room turnover, whereas nurse managers perceived that room disinfection was the principal source of delay.
Table 1.
Perceptions of Nurse (RN) Managers and Environmental Services (EVS) Supervisors During the 28-Month BETR Disinfection Study
| Study Arm
|
||||
|---|---|---|---|---|
| Quat, No. (%) | Quat/UV, No. (%) | Bleach, No. (%) | Bleach and UV, No. (%) | |
| Did you perceive increase in delay with room cleaning? | ||||
| EVS | N = 39 | N = 41 | N = 32 | N = 33 |
| No | 27 (69.2) | 11 (26.8) | 17 (53.1) | 19 (57.6) |
| Yes – slight | 8 (20.5) | 17 (41.5) | 5 (15.6) | 12 (36.4) |
| Yes – substantial | 2 (5.1) | 5 (12.2) | 0 (0.0) | 1 (3.0) |
| RN | N = 83 | N = 68 | N = 44 | N = 93 |
| No | 43 (51.8) | 30 (44.1) | 26 (59.1) | 41 (44.1) |
| Yes – slight | 7 (8.4) | 25 (36.8) | 9 (20.5) | 36 (38.7) |
| Yes – substantial | 1 (1.2) | 4 (5.9) | 3 (6.8) | 8 (8.6) |
| Did EVS supervisors receive complaints about delays? | N = 39 | N = 41 | N = 32 | N = 33 |
| From housekeepers | ||||
| No | 29 (74.4) | 22 (53.7) | 21 (65.6) | 19 (57.6) |
| Yes – few | 9 (23.1) | 16 (39.0) | 8 (25.0) | 11 (33.3) |
| Yes – a lot | 0 (0.0) | 2 (4.9) | 2 (6.3) | 2 (6.1) |
| From nurse managers | ||||
| No | 23 (59.0) | 10 (24.4) | 16 (39.0) | 12 (36.4) |
| Yes – few | 11 (28.2) | 19 (46.3) | 12 (29.3) | 18 (54.5) |
| Yes – a lot | 2 (5.1) | 11 (26.8) | 2 (4.9) | 3 (9.1) |
| From bed control managers | ||||
| No | 27 (69.2) | 17 (41.5) | 19 (46.3) | 14 (42.4) |
| Yes – few | 11 (28.2) | 17 (41.5) | 11 (26.8) | 16 (48.5) |
| Yes – a lot | 1 (2.6) | 6 (14.6) | 1 (2.4) | 1 (3.0) |
| How did RN managers feel that the rooms were cleaned? | N = 83 | N = 68 | N = 44 | N = 93 |
| Consistently every day | 16 (19.3) | 15 (22.1) | 11 (25.0) | 24 (25.8) |
| Consistently most every day | 35 (42.2) | 23 (33.8) | 12 (27.3) | 34 (36.6) |
| Consistently but occasionally ask housekeepers to return | 24 (28.9) | 23 (33.8) | 16 (36.4) | 28 (30.1) |
| Not cleaned consistently | 8 (9.6) | 7 (10.3) | 0 (0.0) | 8 (8.6) |
Note. Quat, quaternary ammonium; UV, ultraviolet light.
Only 1 UV exposure event occurred during the study when a charge nurse who was not trained to operate the UV machine attempted to abort a cycle and entered the room while the machine was operating. The nurse was exposed to the UV-C for approximately 1 minute. The nurse subsequently reported to occupational health with headaches and seeing “sun spots.” The nurse received symptomatic therapy and subsequently had no permanent complaints. This event, however, led to the cessation of the use of the UV device in this hospital for approximately 6 weeks while safety concerns were evaluated, and additional precautionary steps were developed and implemented. The event occurred because of several system failures, including a malfunctioning door sensor designed to shut the machine off if the door was breached.
DISCUSSION
The BETR Disinfection Study was the first large, multicenter randomized controlled trial to investigate the efficacy of enhanced disinfection strategies. We discovered that implementation of enhanced terminal room disinfection strategies using UV devices requires recognition and mitigation of 2 key barriers: (1) timely and accurate identification of rooms that would benefit from enhanced terminal disinfection and (2) overcoming time constraints to allow EVS cleaning staff sufficient time to properly employ enhanced terminal disinfection methods. We learned several lessons during our study to help overcome these barriers.
Establish Priorities
The use of enhanced terminal disinfection with UV disinfection devices may be perceived by administrative leaders to be in conflict with the need to promptly admit patients waiting in the emergency department or admission area.7 We believe that this conflict needs to be viewed as a safety issue because enhanced disinfection using UV devices is an evidence-based strategy to improve patient safety.7,8 Administrators in charge of bed control must be educated about the need for enhanced disinfection and the additional safety provided by the strategy. Similarly, EVS directors must understand and work with infection prevention personnel to ensure that enhanced strategies are prioritized in appropriate rooms, even if EVS services are obtained through contracted groups. Establishing safety as the highest priority increases the likelihood of success for all other strategies discussed below.
Establish Regular Communication
The conflict between personnel working in bed control and EVS was the primary initial barrier to the use of UV disinfection devices in our study hospitals. Improved communication between these groups is essential to the successful implementation of these devices. We recommend daily direct communication with personnel working in these 2 areas. An effective way to increase both communication and cooperation between these 2 groups is to include EVS personnel in the daily meetings between bed control personnel and nurse managers to facilitate bed assignments and to identify problems that can be negotiated or mitigated. For example, rooms that do not need enhanced terminal room disinfection may be identified and prioritized for patient assignment by bed control staff to provide additional time needed in rooms that require enhanced terminal room disinfection. In addition to facilitating the use of enhanced disinfection by EVS, communication can help overcome the perception held by nurse managers that EVS is the primary cause of room delays observed through our questionnaires. We recommend that infection prevention personnel participate in these daily scheduled discussions whenever possible.
Communication with patients and staff was also important. In response to complaints of a noticeable odor following use of the UV devices, we created and placed tent cards in rooms following the use of the UV device. These cards contained a simple message that explained why there was an unusual odor in the room. This message stated, “This room has been cleaned with ultraviolet light. You may notice a faint odor, but it will dissipate quickly. This smell is the new smell of clean!” Complaints from patients and staff greatly decreased after this strategy was initiated.
Get the Device in the Room
No perfect systems exist to timely identify all rooms eligible for enhanced disinfection. Our “Swiss cheese” model was successful; we were able to deploy the UV disinfection device in almost 90% of eligible rooms. We often received concerned feedback that the arrival of an admitted patient would require termination of the UV cycle before completion. In our experience, however, there was almost always adequate time to complete the cycle. This point is particularly important, as one of the biggest barriers encountered during the study was the concept of a “room under pressure”—a room that was needed urgently or immediately for a patient. In our experience, this pressure was related more to perception than an actual barrier to use of the UV device. During our pretrial investigations, we tracked the amount of time required to bring a patient to a room labeled as “under pressure” and observed that there was always sufficient time to run a standard UV device cycle. Nevertheless, this scenario led to 70% of missed opportunities in our study. Thus, we regularly emphasized the importance of being allowed to start the cycle and run it until the patient arrived. When the patient arrived, the cycle could be aborted and the device could be quickly removed from the room. We believe this approach has some biological plausibility, as even small amounts of UV irradiation can decrease the bioburden of bacteria.9 Ultimately, this issue is related to the prioritization of safety and underscores the importance of communication.
Tracking Compliance
We tracked EVS compliance data related to appropriate use of disinfectant chemicals, room cleaning (through liquid UV markers), device utilization, and room turnover times. These data were regularly fed back to EVS directors and supervisors as well as infection preventionists during the study. As is familiar to infection prevention personnel, these data were used in multiple ways in our study, including feedback, problem solving, and incentivizing target compliance. These data also helped us overcome the “power of the anecdote” during discussions with bed control and nurse managers. Inevitably, the handful of processes that broke down were more memorable than the numerous times that things went smoothly. Feedback of data on compliance and room turnover time helped remind people of how well functioning the processes were. We recommend that hospitals (1) set goals for compliance with room cleaning and device utilization, (2) regularly and objectively monitor compliance, and (3) feed these data back to EVS and infection prevention personnel at routine intervals.
Gather Necessary Resources
The volume of patient discharges per hour varied widely in our study hospitals, but most discharges occurred between 11:00 and 18:00. Thus, hospitals planning to use enhanced terminal disinfection strategies must ensure that adequate staffing is available at the time the strategies are most often used. In our study, the peak use of the UV emitting devices occurred at the transition from first shift (07:00 to 15:00) to second shift (15:00 to 23:00). Thus, EVS supervisors need to develop systems for “hand offs” and/or develop unique positions that work the end of first shift and beginning of second shift. Similarly, a sufficient number of devices must also be available during times of peak use and/or devices should be located and kept adjacent to high volume locations. In our study, hospitals had approximately 1 machine per 150–200 beds. In fact, device unavailability was rarely cited as a reason for a missed opportunity in our study.
Our study has limitations. Our study took place in 2 tertiary-care hospitals, 6 community hospitals, and a Veterans Affairs medical center in the southeastern United States. Our observations occurred during a randomized controlled trial during which significant resources and time were invested in the use of enhanced disinfection strategies. For example, infection preventionists at several study hospitals increased routine ward rounding during the study to track contact precaution door signs. Thus, some of our findings and process strategies may not be generalizable to other locations or settings. We utilized a single type of UV-C device in our study; other enhanced disinfection devices may require different deployment strategies. In addition, this device and others now have electronic monitoring and notification systems that improve efficiency. However, we suspect that the strategies and lessons discussed above can reasonably be generalized to any type of enhanced terminal disinfection strategy.
In conclusion, implementation of enhanced disinfection strategies utilizing UV devices is difficult. In our experience, key barriers to success included perceptions about EVS, interplay between personnel working in EVS and bed control, and conflict between increased safety and room turnover time. However, implementation of these important strategies can be successful when these barriers are identified and directly addressed. We recommend that hospital infection control and EVS programs review the checklist provided in Supplementary Table 1 prior to implementing enhanced terminal room disinfection strategies to better assess potential barriers and to identify strategies to overcome these barriers.
Supplementary Material
Acknowledgments
The authors would like to specifically thank Joyce Frederick for her significant contributions to this study. We would also like to thank the following for their contributions to the successful completion of this study: Alamance Regional Medical Center: Mike Gover, Ron Halleen, Jean Keck, Melba Phillips, Clea Pulliam, Cameron Satterfield, Ashlei Williamson, and Sara Wall; Chesapeake Regional Medical Center: Cynthia Boydston, Melanie Buski, Rollyn Cartwright, Joleen Connor, Monica Fuller, Larry Hires, Allison Johnson, Amie Prado, Arnell Pugh, Billy Richmond, MD, Barbara Shields, Monita Short, Tiffany Silmon, Scavellas Slater, Mary Summerlin, Tammy Von Moll, and Elizabeth Wade; Duke Raleigh Hospital: Linda Alford, Donald Brown, Kim Catron, Connie Clark, Glenda Debord, Tammy Green, Neal Seigler, Kathryn Whitfield, and Brittain Wood; Duke Regional Hospital: Kint Greenhouse, Mary Justesen, Cameron Satterfield, James Sessoms, Laura Smith, Vicki Tutor, Joy Vollers, and James Walker; Duke University Hospital: Orlando Ackerson, Demario Harris, Pam Isaacs, Judith Megelich, Christopher O’Connor, Ornella Stewart, Christopher Vogelheim, and Jessica Vondy; Durham Veterans Affairs Medical Center: Ben Blount, Joel Boggan, MD, Orlando Brown, James Bunn, Dwayne Hicks, Sara Hoffman, William Hubbell, Steve McLeod, Mike Mejia, Larry Park, PhD, Jeffrey Robinson, Susan Wilkins, Roger Walsh, and Christopher Woods, MD, MPH; High Point Regional Hospital: Angelina Drews, Rocky Jameson, Judy Pemberton, Jennifer Pridgen, Cherrie Speagle, and Barbara Tillman; Rex Healthcare: Sean Agard, Jeffrey Agricola, Dr Paul Becherer, Joe Brown, Vicki Clark, Zena Farkas, Aku Fudzie, Zoe Gjertson, Debra Harris, and Christopher Riley; University of North Carolina Health Care: Katie Crandall, Lula Daniels, Lauren DiBase, Sherie Goldbach, Kimberly Green, Kandi Herndon, Lori Osborne, Emily Pfaff, Orlando Reyes, Emily Sickbert-Bennett, PhD, Jeff Strickler, and Jason Smith.
The authors would also like to acknowledge TruD SmartUVC, Ecolab, and Clorox for their significant material contributions to the study. In addition, the authors would like to thank Angelica Corporation and Shared Linen Services, each for donating microfiber cloths to a study hospital. All of these companies provided materials for the completion of the study but played no role in the funding, design, analysis, or manuscript preparation.
Financial support: This study was supported by grants from the Centers for Disease Control and Prevention Epicenters Program (grant no. U54CK000164 to Dr Sexton), the National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) (grant no. K23AI095357 to Dr Anderson) and the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (grant no. UL1TR001111). The views expressed in this article are those of the authors and do not necessarily represent the views of the CDC or the NIH.
Footnotes
Potential conflicts of interest: W.A.R. and D.J.W. report receiving consulting fees from Clorox. All other authors report no conflicts of interest relevant to this article.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2017.268
References
- 1.Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis. 2003;36:53–59. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 2.DiazGranados CA, Jernigan JA. Impact of vancomycin resistance on mortality among patients with neutropenia and enterococcal bloodstream infection. J Infect Dis. 2005;191:588–595. doi: 10.1086/427512. [DOI] [PubMed] [Google Scholar]
- 3.Dubberke ER, Butler AM, Reske KA, et al. Attributable outcomes of endemic Clostridium difficile-associated disease in nonsurgical patients. Emerg Infect Dis. 2008;14:1031–1038. doi: 10.3201/eid1407.070867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang SS, Datta R, Platt R. Risk of acquiring antibiotic-resistant bacteria from prior room occupants. Arch Intern Med. 2006;166:1945–1951. doi: 10.1001/archinte.166.18.1945. [DOI] [PubMed] [Google Scholar]
- 5.Shaughnessy MK, Micielli RL, DePestel DD, et al. Evaluation of hospital room assignment and acquisition of Clostridium difficile infection. Infect Control Hosp Epidemiol. 2011;32:201–206. doi: 10.1086/658669. [DOI] [PubMed] [Google Scholar]
- 6.Drees M, Snydman DR, Schmid CH, et al. Prior environmental contamination increases the risk of acquisition of vancomycin-resistant enterococci. Clin Infect Dis. 2008;46:678–685. doi: 10.1086/527394. [DOI] [PubMed] [Google Scholar]
- 7.Anderson DJ, Chen LF, Weber DJ, et al. Enhanced terminal room disinfection and acquisition and infection caused by multidrug-resistant organisms and Clostridium difficile (the Benefits of Enhanced Terminal Room Disinfection Study): a cluster-randomised, multicentre, crossover study. Lancet. 2017;389:805–814. doi: 10.1016/S0140-6736(16)31588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber DJ, Rutala WA, Anderson DJ, Chen LF, Sickbert-Bennett EE, Boyce JM. Effectiveness of ultraviolet devices and hydrogen peroxide systems for terminal room decontamination: focus on clinical trials. Amer J Infect Control. 2016;44(Suppl 5):e77–e84. doi: 10.1016/j.ajic.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nerandzic MM, Cadnum JL, Pultz MJ, Donskey CJ. Evaluation of an automated ultraviolet radiation device for decontamination of Clostridium difficile and other healthcare-associated pathogens in hospital rooms. BMC Infect Dis. 2010;10:197. doi: 10.1186/1471-2334-10-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


