Abstract
There is a higher rate of HPV infection and cervical cancer incidence and mortality in underserved US population who reside in Appalachian mountain region compared to Northern Plains. Social and behavioral factors such as smoking and alcohol consumption are for such a high incidence. However, by and large, the reasons for these discrepancies lie in the reluctance of the underserved population to adopt preventive measures such as prophylactic Human papilloma virus (HPV) vaccines and Pap smear screening that have significantly reduced the incidence and mortality rate of cervical cancer in Caucasian women. Thus, it is clear that drastic change in social behavior and implementation of preventive measures is required to effectively reduce the incidence and mortality from cervical cancer in this underserved population.
Keywords: HPV; HPV genotype; Cervical Cancer; Health Disparity; Vaccination; Appalachian women; American Indian; African American, Review
2. INTRODUCTION
Cervical cancer is the third most common uterine cancer and is the fourth leading cause of cancer deaths in the world (1, 2). Based on Surveillance, Epidemiology, and End Results Program (SEER) data, around 248,920 women suffered from cervical cancer in the United States during 2013 (3) Chronic infection with high-risk HPVs (HR HPV) and inefficient clearance are the major risk factors for cervical cancer and are demonstrated to play a major role in the progression of Cervical Intraepithelial Neoplasia (CIN) (4). Evidently, HR HPV leads to modification of genome sequences, which ultimately affects human physiology resulting in distinct clinical manifestations (5).
An increased emphasis on cervical cancer screening programs in the United States has led to an overall reduction in incidence and mortality rates of cervical cancer. In addition, development of cervical cancer vaccines against HPV has proven to be a major preventive tool in reducing the incidence of this cancer (5). Cost effective and highly sensitive HPV Pappanicolaou (Pap) screening test may be effective than cytology-based cervical cancer primary screening (5–7). However, difficulty in implementing cytology-based screening programs in rural areas and among underserved populations, thus burden of cervical cancer still remains substantially high among these subgroups (8–10). The purpose of this paper is to review disparities in cervical cancer incidence and mortality rate among minorities in underserved areas in the United States and assess the role of socioeconomic conditions on Human Papillomavirus (HPV) infectivity and cervical cancer. The paper also discusses the challenges of implementing successful cervical cancer screening programs among these vulnerable populations.
3. HPV GENOME AND ITS CLASSIFICATION
The HPV is a small (50 to 55 nm in diameter and ~8 kb in length) double stranded DNA virus with an icosahedral capsid (1). A virus genome exists inside the capsid, and harbors eight partially overlapping open reading frames. This genome is divided into three regions: an early region (E), late region (L), and a full form LCR (1). Again, the late region of the genome encodes structural proteins L1 and L2. These proteins comprise the capsid protein and protect the viral genome. Since L1 protein can express its immunogenicity similar to that of other infectious genomes, current vaccines used to prevent HPV infection include L1 protein as a main constituent. In addition, the L2 protein is necessary for viral entry into cells and the transport of viral materials into the nucleus, followed by binding with deoxyribonucleic acid (DNA). Most importantly, the L2 protein evokes production of a broad spectrum of neutralizing antibodies against different types of HPV. These antibodies are cross-reactive between HPV genotypes when compared to the antibodies provoked by L1. Thereby, L2 protein is considered crucial for future vaccines develpment.
The LCR is a non-coding upstream regulatory region located between E6 and L1. It contains a core promoter sequence, as well as enhancer and silencer sequences, and is necessary for viral replication and transcription ((11). The size and nucleotide composition of the LCR display considerable variation between different HPV genotypes. Specific HPV genus is associated with cervical cancer. The pathogenicity of HPV corresponds to specific genotype involved. HPV is divided into five genera (alpha, beta, gamma, mu, and nu), and the genus Alphapapillomavirus includes HPV genotypes that infect both genital and oral mucosa. In general, HPV are classified based on L1 genome sequence, type, intratypic lineage, and sub lineage. The types, intratypic lineages, and sub lineages of HPV are defined by L1 sequences that differ by at least 10%, more than 1%, and less than 1%, respectively. HPVs are also grouped into categories of high-risk (HR) and low-risk (LR) based on their oncogenic capabilities in the cervix. Among 14 high-risk HPV genotypes (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68), the two most common (HPV 16 and 18) account for 70% of cervical cancers. Two LR HPV genotypes (HPV 6 and 11) contribute to the formation of genital warts, most of which require treatment (1).
3.1. Molecular interventions of HPV inside host genome
The transformation of HPV infected cells to cancer cells is a complex process. Essentially, HPV infects basal cells located between the squamous epithelium of the ectocervix and the columnar epithelium of the endocervix. Viral replication is tightly linked to the epithelial cell differentiation cycle (11, 12). Here, early genes coding for pro-mitotic proteins and viral replication factors are expressed. Again, terminal cell differentiation is required for activation of late gene expression and production of viral particles on the top of the epithelium. The initiation of HPV DNA replication occurs as the basal cell DNA is replicated. Two genes, E1 and E2, are primarily required for the maintenance of viral genomes in the host cells as they serve as the initial sites for replication of viral DNA, and use cellular DNA polymerase for replication. Oncoproteins E6 and E7 are responsible for cellular proliferation resulting in increased numbers of infected cells and infectious virions. As overexpression of viral genes is responsible to transform a normal cervical cell into a cervical cancer cell, sequentially cervical epithelial tissue progresses through phases from normal epithelium to CIN (CIN 1, CIN 2, and CIN 3), developing into invasive cervical cancer.
Normally, HPV infections are cleared from the body within 18–24 months. Cis-acting HPV RNA elements, cellular RNA-binding proteins control HPV mRNA splicing, and polyadenylation. These interactions are believed to play an important role in switching from early to late gene expression, thereby contributing to the pathogenesis of HPV. Indeed, it has been shown that the levels of various RNA binding proteins change in response to differentiation and in response to HPV induced cervical lesions and cancer. It is shown that HPV16 and 18 genotypes are the major factors for development of approximately 70% of cervical cancer, while HPV6 and 11 are responsible for genital warts (5). An FDA approved vaccine, Gardasil® is highly for use in females between ages 9 and 26 years. Another HPV vaccine targeting nine HPV genotypes (HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58) has recently been introduced to the market.
3.2. Screening of cervical cancer
Traditional Papanicolaou (Pap) screening was first implemented about five decades ago (6). American Cancer Society guidelines for the early detection of cervical cancer recommended using HPV DNA testing alongside cytology or part of a triage of tests that may be investigated further for abnormal cytology. Implementation of HPV DNA testing has been added to current clinical practice. It exerts improved sensitivity for detecting CIN 2 lesions. The current recommendation is to perform either a follow-up test 12 months later or genotyping of HPV 16 and 18. A negative genotyping of HPV 16 and 18 following 12 months is also recommended; and in different conditions, further examination with colposcopy is suggested (7).
In 2014, the FDA approved an HPV test as the primary screening for cervical cancer. The guidelines recommended managing patients infected with different HPV genotypes identified by HPV genotyping. Patients positive for HPV 16 or 18 must undergo colposcopy, while patients infected with any of 12 other high-risk HPV types can be tested by reflex cytology (7).
Recent finding suggests, when using HPV genotype as a primary screening tool, cervical cancer prevention was improved. In order to increase participations from these underserved socioeconomic groups, self-collected samples were also encouraged. When HPV test was conducted from self and clinical collected samples prevalence and HPV genotype distributions demonstrated similar patterns and less time consuming.
3.3. Oncogenic HPV types
Based on nucleotide similarity, HPVs are hierarchically classified into genera, species and types. All HPV genotypes that cause cervical cancer belong to the Alpha genus. They are HPV 51 (Alpha 5); HPV 56, and HPV 66 (Alpha 6); HPV 18, HPV 39, HPV 45, and HPV 59 (Alpha 7); and HPV 16, HPV 31, HPV 33, HPV 35, HPV 52, and HPV 58 (Alpha 9). Essentially, the Alpha 7 and Alpha 9 types are frequently implicated in cervical cancer cases worldwide.
4. Statistical Analyses of Incidence and Interpretation
4.1. Geographical population distribution in Appalachia
Of the African American subpopulation in Appalachian, about 18.4% live in Alabama, Mississippi, and South Carolina, whereas, in central Appalachia, the African-American population is much smaller, e.g., in Appalachian Kentucky, it is less than 2% of the total population (8).
The Appalachian region has significant variations in socioeconomic status with a decreasing North-South trend. In 2007, Per capita income in Appalachia was 20% below the national average. Per capita income was only 48% and 57% of the national average in Appalachian Kentucky and Mississippi, respectively. Appalachian Maryland, New York, North Carolina, Ohio, Tennessee, Virginia, and West Virginia also have less than 75% of the national average per capita income. Two-thirds of the adults have college degree in Appalachia. Net population growth in Appalachia between 2000 and 2008 was 3 percent less than the nation as a whole. Appalachian industries grew at a slower rate than the nation as a whole. Appalachian industries are projected to continue adding jobs through 2015, but at a slower pace. Appalachia women experience disparities in healthcare access and utilization (9). In addition, patients cancel physician visits at a higher rate due to lack of monetary support or transportation.
The Appalachian Regional Commission (ARC) has been conducting an analytical classification of each county with national averages on three economic indicators: three-year average unemployment rates, per capita market income, and poverty rates. Each Appalachian county is designated an economic status as, distressed, at-risk, transitional, competitive, or attainment. Distressed counties rank in the worst 10 percent of the nation’s counties. At present, 93 Appalachian counties have been designated as distressed. These counties are located mainly in the rural areas of the highlands that include West Virginia, Ohio, Kentucky, Tennessee, Virginia, and North Carolina. Some of the counties in Appalachian Kentucky and most in Appalachian Mississippi are distressed.
4.2. Socioeconomic conditions and other risk factors for high incidence and mortality rates of cervical cancer among Appalachian and American Indians
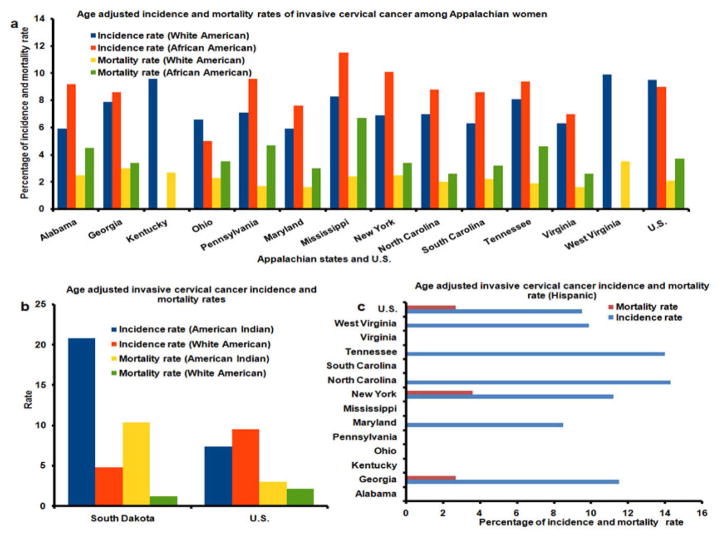
Rural Appalachia has significantly higher overall cervical cancer incidence and mortality rates compared to national rates. There are differences in the incidence and mortality rates for cervical cancer among various counties in Appalachia, with distressed counties experiencing the worst outcomes. Rates of cervical cancer incidence are about 40% higher among African American women than White women in distressed areas of Mississippi. Mortality rates of cervical cancer for African American women are nearly three times higher than those of their White counterparts in Mississippi as shown in Figure 1 (10). Whites are more likely to have diagnosed at early stages of cervical cancer than African Americans. Cervical cancer incidence rates also differ significantly in Tennessee between White and African American women. Mortality rates from cervical cancer among African American in Tennessee is more than twice than White American. Figure 2a and Figure 2c illustrate differences in cervical cancer mortality rates between the northern part and the southeastern part of the Appalachia. Substantial disparity is evident with the two-fold difference between counties with the lowest and highest cervical cancer rates. Many factors have led to increased risk of HPV induced cancers including socioeconomic status and limited access to healthcare services (13, 14).
Figure 1.
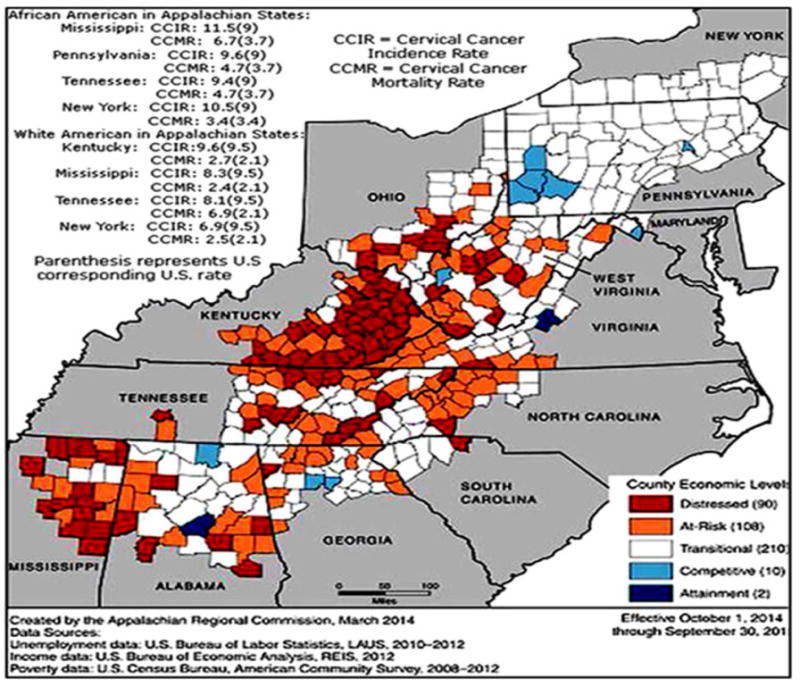
Pictorial representation of high incidence and mortality rate of cervical cancer among Appalachian women and an index-based county economic classification system to identify and monitor the economic status of Appalachian counties. County Economic levels: Each county is categorized into five economic statuses, depending on its position in the national ranking. Distressed: These counties are the most economically depressed counties, ranking worst 10 percent of the nation’s counties. At-Risk: At-Risk counties are those of tending to economically distressed, ranking between the worst 10 percent and 25 percent of the nation’s counties. Transitional: Transitional counties are those transitioning between strong and weak economics, ranking between the worst 25 percent and the best 25 percent of the nation’s counties. Competitive: Competitive counties are those that are able to compete in the national economy but are not in the highest 10 percent of the nation’s counties, ranking between the best 10 percent and 25 percent of the nation’s counties. Attainment: Attainment counties are the economically strongest counties, ranking in the best 10 percent of the nation’s counties. Adopted from: http://www.arc.gov/research/MapsofAppalachia.asp?MAP_ID=116.
Figure 2.
(a) Age Adjusted Cervical Cancer Incidence Rates and Death Rates by U.S. Census Appalachian States, Women and White American, African American, United States. Rates are per 100,000 persons and are age-adjusted to the 2000 U.S. Standard population (19 age groups – Census P25-1130) U.S. Cancer Statistics Working Group. United States Cancer Statics: 1999–2012 Incidence and Mortality Web-based report. Atlanta: U.S. Department of Health and Human Services, Centers for disease Control and Prevention and National Cancer Institute; 2015. Available at: http://www.cdc.gov/uscs. (b) Age Adjusted Cervical Cancer Incidence Rates and Death Rates by U.S. Census Appalachian States, Women and Hispanic American, United States. Rates are per 100,000 persons and are age-adjusted to the 2000 U.S. Standard population (19 age groups – Census P25-1130) U.S. Cancer Statistics Working Group. United States Cancer Statics: 1999–2012 Incidence and Mortality Web-based report. Atlanta: U.S. Department of Health and Human Services, Centers for disease Control and Prevention and National Cancer Institute; 2015. Available at: http://www.cdc.gov/uscs. (c) Age adjusted Cervical Cancer Incidence and Mortality Rates of American Indian and White America in South Dakota. Rates are per 100,000 persons and are age-adjusted to the 2000 U.S. Standard population (19 age groups – Census P25-1130) U.S. Cancer Statistics Working Group. United States Cancer Statics: 1999–2012 Incidence and Mortality Web-based report. Atlanta: U.S. Department of Health and Human Services, Centers for disease Control and Prevention and National Cancer Institute; 2015. Available at: http://www.cdc.gov/uscs.
Figure 1 illustrates differences in the economic level within the Appalachian states and their counties. A heightened economic burden is observed in the central and Southern regions, with the most distressed counties in West Virginia, Kentucky, Tennessee, and Mississippi, compared to the Northern Appalachian region. Although data are limited, evidence suggests that African American and White Appalachians exhibit higher cervical cancer incidence and mortality rates compared to the national average. For example, African Americans residing in Mississippi have approximately 40% and 180% greater cervical cancer incidence and mortality rates, compared to their White Appalachian counterparts. In general, Appalachian states tended to exhibit higher age-adjusted incidence and mortality rates of cervical cancer among African American compared to their white counterparts (Figure 2a).
Higher rates of smoking in the most distressed areas and the earlier onset of sexual activity also contribute to the higher rates of cervical cancer. Since the male sex partner is the vector of the infection, the high-risk HPV types are different from that of the low-risk HPV types in women. The most significant risk factors of any HPV are lifetime number of sex partners, youth, uncircumcised partners, duration of sexual relationships, behaviors of own and male sexual partners, alcohol and drug abuse, smoking, and ethnicity. Opioid analgesic injection was common in Appalachian women who consume controlled substances that include heroin, cocaine, crack cocaine, methamphetamine, marijuana, and hallucinogens, psychiatric disorders, HIV risk behaviors (14–19). HPV types and carcinogens substantially increase the risk of high-grade CIN in presence of HR HPV types.
In addition to Appalachian women, cervical cancer is a major health issue in Northern Plains American Indian women. Uterine cervical cancer due to HPV infection is also a huge burden for American Indian Women. They have younger age distributions with advanced stage disease. There is regional diversity in cervical incidence and mortality among them in northern plain region. Figure 2b illustrates age-adjusted cervical cancer incidence and mortality rates for American Indian women in the American Northern Plains. The incidence rate of cervical cancer was 6.0 and 7.4 in South Dakota, respectively, per 100,000 in population (20). Age-adjusted cancer incidence and mortality rates among American Indians residing in South Dakota are approximately 5 times higher compared to their White counterparts (20.8 vs. 4.8; 10 vs. 2). American Indians of South Dakota have about 3 and 2 times higher incidence and mortality rate, respectively, compared to their counterparts in the entire US. A similarly dramatic increase occurred for cervical cancer, which accounted for 10.4% and 1.2% of American Indian and White deaths, respectively. Invasive cervical cancer is specified for 0.7% of all cases reported and 1.2% of all females diagnosed with cancer in South Dakota in 2012. SEER data reported 0.2% of cases were younger than 20 years of age. Importantly, the stage of diagnosis impacts the mortality rate. Cervical cancer at a localized stage showed a 92% survival rate according to the American Cancer Society. Nationally, when diagnosed at late stage, the percentage of five-year survival rate falls to 15%.
Studies have revealed that long-term dependence on cigarette smoking among women is related to cervical cancer. American Indians have the highest cigarette smoking rates when compared to other racial groups in the United States. The binge drinking and other substance use are prevalent in the Northern Plains among American Indian populations, especially those under age group 30. This may be one of the reasons why American Indians suffer from a disproportionate burden of cervical cancer and experience a higher rate of mortality due to persistent HPV infections compared to the general population (21–23). The highest prevalence of drinking and the heaviest drinking occur among those who are below the age of 30. Stretched budgets, inadequate accessible healthcare, and poor healthcare funding, especially in rural and remote areas, have created a wide gap in preventative health care for many Americans Indians on the reservations. Pharmacies and healthcare facilities are almost absent in these communities. Insufficient knowledge about HPV, low levels of HPV examination and vaccination, and low consensus about safety of vaccination also contribute to these higher rates. Other risk factors include nutritional deficiencies, low socioeconomic status; sexual debut at a young age, high-risk multiple male partners, tobacco use, and the use of oral contraceptives (23).
Figure 2c depicts age-adjusted invasive cervical cancer incidence and death rates among Hispanic women in six Appalachian states including West Virginia, Tennessee, North Carolina, New York, Maryland, and Georgia. Incidence rates of cervical cancer among Hispanics were greater than the national average, with the exception of the state of Maryland, which exhibited a 13% lower incidence rate. Hispanics residing in New York had 40% greater mortality rate compared to the national average, whereas mortality rates of those living in Georgia were similar to those of the national average. It is also noteworthy that incidence rates were higher in Hispanic women than in White women, and the high death rate for cervical cancer was the leading cause of cancer death among Hispanic women. Cervical cancer can be prevented in Hispanic women if they receive the same opportunities for screening as women of other ethnicities. There is a notion among Hispanics population that they have the higher rates of cervical cancer and are more likely to die from cervical cancer compared to non-Hispanic Whites. Hispanic women find it apocalyptic when they receive an abnormal result from their Pap tests, which further contribute to their high mortality rate. Lack of screening is an important factor behind this disparity. In fact, most cervical cancers occur in women who have never received a Pap test (24).
4.3. High prevalence of HPV among Appalachian and American Indian women
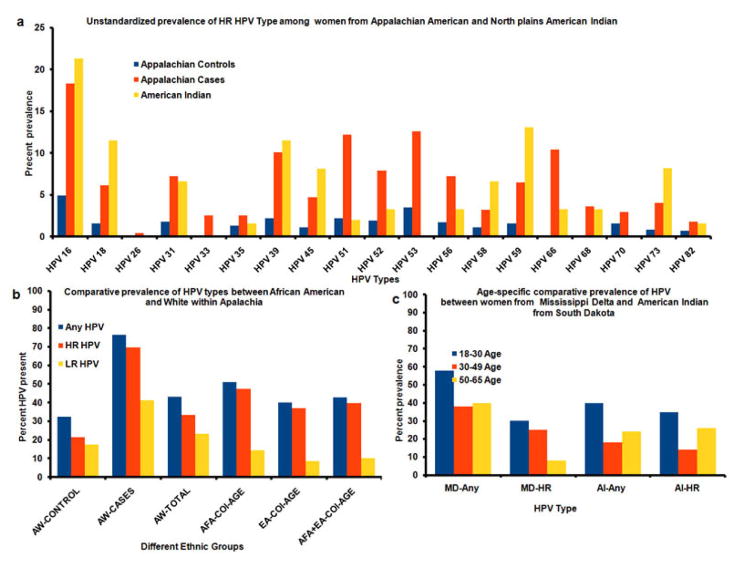
In Appalachian Ohio, HPV incidence and mortality rates from abnormal cytology increased to 12% and 21%, respectively, in 32 specified counties over non-Appalachian counties (25). Figure 3a compares the hypothetical high-risk HPV genotypes prevalence among Appalachian and American Indian females from Northern Plains. Among 37 detected HPV types (6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, and IS39), the prevalence of any HPV type was 43.1%; and a high-risk HPV type was detected in 33.5% of women, while a low-risk HPV type was detected in 23.4% of women. HPV16, a high-risk HPV type, was the most commonly identified HPV type (8.2%) among Appalachian Americans and Northern Plains American Indians. However, it had a 30% higher prevalence among the Northern Plains American Indians. Other more prevalent high-risk HPV strains among American Indians of Northern Plains were 59, 39, 18, and 73, which were found at a higher rate than Appalachian Americans. All other HPV types had a prevalence rate below 4.0%, including types 18, 6, and 11. For Appalachian women, cases were defined as having atypical squamous cells of undetermined significance (ASC-US), atypical squamous cells that cannot exclude HSIL (ASCH), atypical glandular cells (AGC), low-grade squamous intra epithelial lesions (LSIL), High grade squamous intraepithelial lesions (HSIL), adenocarcinoma in situ (AIS), or carcinoma.
Figure 3.
(a) Unstandardized prevalence in each HPV genotype among American Indian women from South Dakota and from Appalachian Indian women. Appalachian Cases serve the genotype prevalence from Appalachian white women with atypical squamous cells of undetermined significance (ASC-US) while Appalachian controls are from women with normal cervical cytology. (b) Age-specific prevalence in HPV type among African American and European American within Appalachia. AW-CONTROL Appalachian White with normal cytology; AW-CASES, Appalachian White with abnormal cytology AFA-COL-AGE, African American College Age; EA-COL-AGE, European American College Age (c) Age-specific prevalence in HPV type among American Indian women from Northern Plains and African American from Mississippi Delta. AF, African American; HR, high-risk; AI, American Indian; MD, Mississippi Delta.
In the Northern Plains, 67% of the women were infected with high-risk HPV types, and 33% of the women had low-risk HPV types. In American Indian women, HPV 16 was present in most of the cases; and HPV 59 was the second most prevalent HPV infection. While the prevalence of HR HPV types is similar to the pattern identified in other populations, where HPV 16 was reported as the most common HR HPV types, women had a higher prevalence of certain HR types, mainly HPV 59, 39, and 73. The occurrence of the Pap test was significantly higher in women who were infected with high-risk HPV types compared to HPV negative or low-risk HPV types infected women.
There was considerable variation in HPV prevalence between Appalachian white and American Indian women. For example, HPV16 was identified more often in American Indian than in Appalachian Whites. HPV16 is genetically related to types 31, 33, 52 and 58, but not 35. Also, HPV18 was more prevalent in American Indians than in Appalachian Whites. The HPV18-related type 45 was also more prevalent in American Indians. Comparison of HPV-specific prevalence by region is shown in Figure 3b. In cases from Appalachia, the prevalence of HPV45 (4%) was less than 31 (7.5%) but more than 33 (2.5%). In cases from Northern Plains, HPV58 (5.8%) and 52 (4.4%) were found more commonly than HPV45, 31 and 33. Other HPV types varied considerably in their prevalence from region to region, but accounted for no more than 2% of cases from any region.
In the Mississippi delta, age specific variation was involved in HPV, HR HPV and alpha 5, 6, 7, and 8 genotypes, leaving 3, 4, and 15 genotypes. The distribution of the five HPV genotypes commonly available from clinician collected samples in the delta are HPV 54 (4.9%), HPV 62 (4.7%), HPV 83 (4.4%), HPV 52 (3.9%), and HPV 71 (3.4%). The most dramatic difference in HPV prevalence is that HPV 16 and HPV 18, most targeted by current vaccine, were found only in 2.5% and 1.5% of the cases. The presence of HPV genotypes from samples in Mississippi Delta (about 91% African American, 8% White, and 0.7% other) demerits hypothetical HPV genotype prevalence study.
4.4. Comparison of the age-specific HPV prevalence
Figure 3c presents the age-adjusted total and high-risk HPV genotype prevalence between African Americans from Mississippi Delta and American Indian women from Northern Plains. The prevalence of high risk HPV among women from Mississippi Delta is negatively associated with increasing age. The highest prevalence was detected among women 18 to 30 years of age (about 25%), whereas the lowest prevalence was observed among women older than 50 years of age (approximately 8%). Although the pattern is different in at Northern Plains American Indian women. Where HR HPV distribution was highest among women 30 years of age and younger (approximately 32%), it plummeted after the age of 30 (10%) and again increased to around 25% after age 50. Comparing high-risk prevalence of HPV between women residing in the two regions, a greater percentage of HR HPV was detected among American Indian women of Northern Plains younger than 30 and older than 50 years of age, whereas the 30–49 years of age women of Mississippi Delta exhibited a higher prevalence of HR-HPV compared to their age counterparts in South Dakota. Therefore, HR and LR HPV types were common at age 18–30 years of age. Figure 3c shows the prevalence of HR HPV types between ages 18–30 years, the prevalence of HPV 16 and 18 among American Indian women in Northern Plain.
The Genotypes detected were given commercially available vaccines according to their coverage by the bivalent HPV (16 and 18) and quadrivalent (16, 18, 6, and 11), as well as by the seven valent (16, 18, 31, 33, 45, 52, and 58). An HPV infection was preventable by a vaccine if more than one of the types identified to infect that woman was among a vaccine’s component antigens. Therefore, a vaccine’s antigens may only correspond to some HR HPV but not all of the HR HPV infections in a woman co-infected with multiple types.
The effectiveness was calculated among females 20–65 years of age and separately for the maximum age-specific prevalence. The overall prevalence of HPV was 76% with multiple and single genotypes prevalent in 56% and 20% of women, respectively. In Mississippi Delta, alpha HPV species groups 3, 5, 6, 7, 9, 10, 11, and single and multiple HPV infections predominantly existed. Alpha 7 and 9 species were detected in 14% and 83% of CIN3, respectively, but in 57% and 47% of adenocarcinoma in situ (AIS). Classification of HPV prevalence by age for the types targeted by the bivalent, quadrivalent, and nonavalent vaccines. The prevalence of alpha 9 papillomavirus species was 74%, 44% and 17% in women aged 30 years, 30–39 years, and 40 years, respectively. In contrast, the alpha 7 papillomavirus species increased across these same age groups, 49%, 64%, and 64%, respectively. The relative percent of HPV positive by diagnosis within age groups followed similar patterns to the percent HPV positive for all age groups combined. HPV types included in the bivalent, quadrivalent, and nonavalent HPV vaccines tended to decrease with increasing age. One HR genotype, HPV 58, was more frequently detected with respect to HPV and therefore, nonavalent vaccine should be effective to prevent a larger number of infections caused by HR genotypes.
4.5. Higher prevalence on HPV infection in younger women
In young women from the Northern Plains, HPV infection was 41% higher compared to all other age groups. In contrast, fewer women from the 65 to 74 age group exhibited HPV infection. There was a significant difference between African American and White women within Appalachian States of South Carolina. White women were HPV positive on 40% of the visits, while African American women were HPV positive on 51% of the visits. Figure 2b shows that prevalence of HR-HPV types in White women was 37.1%, while for African American women, it was 47.4%. LR HPV types in White American women was among 8.7% and for African American women, was 14.4%. African American and White participants exhibited very similar frequencies of simultaneous infections by multiple HPV types. The prevalence of multiple HPV types for Appalachian African is 10–20% greater than the White women.
4.6. Clearance of HPV infection among Appalachian women
Relevant HR HPV persistence in the cervix increases the risk for cervical cancer. The process of cervical cancer transformation needs an environment of less HR HPV clearance, persistence, and the multiple HR HPV infections. Fifty percent of HPV clearance was nearly twice for African American women (601 days), compared with White women at college admission age. Uterine cervices have the abilities to clear HPV infections by using the efficient immune system. Contributing factors for high-grade cervical lesions are HR HPV infection with specific HPV type, number of HPV types, and increased viral load, plasma levels of micronutrients, and various life-style variables.
5. Highlights
Appalachian women living in the most distressed counties are detected with higher rates of cervical cancer compared with less distressed counties (8). This significant association between socioeconomic indicators and premature cancer morbidity and mortality are prominently found among counties in the Southeastern states, Mississippi Delta region, Central Appalachian, and Western regions of the country. Furthermore, healthcare disparities persist within these areas and are believed to be triggered by lower uptake of cervical screening. Enormous work needs to be done to combat cancer in these low-income counties. However, it is difficult to get exact cancer incidence rates in Appalachia within regions of distressed and better-off counties due to uneven availability of the healthcare data across the region. Currently, population-based cancer incidence data within distressed counties for all of Appalachia are not available.
In the Appalachian region, higher incidence and mortality rates of uterine cervical cancer among women is also dependent on the age. Over the past years, a low interest has been observed in women taking up on their screening invitation, especially younger women and those in distressed rural areas. The reason is their closely held cultural beliefs (26). Unless they understand and follow the importance of prevention through removal of their fatalistic belief, Appalachian women will continue to experience premature death due to cervical cancer. The level of education must help them to overcome this belief system and to show willingness to face the unpleasant truths in life by altering their perceptions. Cervical cancer screening can save many lives, and it is important for women to consider this opportunity to accept invitation for screening. A new screening program, put offered by the FDA, gives hope for possible type-specific replacement upon introduction of nonavalent HPV vaccination.
The identification of HPV in the uterine cervix is relevant for better clinical outcomes through vaccination. The use of the HPV test for monitoring of cervical lesions is supplementary to cervical smear cytology. It has been shown by many investigators that HPV 16 and HPV 18 are prevalent in cervical carcinomas and high-grade precancerous lesions. Schmidt-Grimminger et al., (2011) found that lower percentage of American Indian women tested negative for HPV infection compared to Whites (58% vs. 77%), and a higher percentage of American Indian females were infected by oncogenic types (30% vs. 16%) (21). Also, American Indian women exhibited a differential pattern of HPV types, including a higher prevalence of mixed HPV infections (19% vs. 7%), and showed a higher percentage of HPV infections that were not preventable by HPV vaccination (32% vs. 15%) (21). Bell et al., (2007) reported that American Indian women exhibited more risk factors than White women. These risk factors were sexual debut at younger age, low level of education, less vegetable consumption, multiple sexual partners, and more pregnancies. American Indian women indulged more frequently with recreational drugs, had history of sexually transmitted diseases, and had histories of smoking and alcohol consumption. However, White women were also reported more alcohol consumption (22).
Among 37 HR HPV genotypes that have been associated with cervical carcinoma and precancerous lesions, Bell et al., (2007) also reported that the women infected with oncogenic HPV types (48.7. %) having HPV16 and 18 and the remaining (51.3%) were infected with other oncogenic types HPV59, HPV 39, and HPV 73 (22). American Indians are highly susceptible to HR HPV infections and associated with multiple sexual partners to aggravate the cervical microenvironment. Therefore, the detection of HPV genotypes provides a suitable way of examining the relations between the HR HPV genotypes and the stages of cervical precancerous lesions and carcinomas.
The HPV 16 is the prevalent HPV type in patients with carcinoma and the prevalence of HPV16 progressively increase with the severity of the cervical lesion. The frequency of HPV 16 was 18.3. % in cases while the control group showed only 4.9% in Appalachia (27). Prevalence of HPV in women with cervical carcinoma HPV DNA was detected in 93% of tumors throughout the world, and HPV 16 was detected in 50% of these specimens in most countries. Reiter et al., examined prevalence of HPV among Appalachian women and detected 43.1% for any HPV type, 33.5% for HR HPV types, 23.4% for LR HPV types, and 12.5% for vaccine-preventable HPV type. Detection of any HPV type was more common among women ages 18–26, smokers, had at least five male lifetime sexual partners, or had multiple male sexual partners during the examination year. Similar correlates were identified for detection of a high-risk HPV type. HPV 53(12.6%), HPV 51(12.2%), and HPV 62 (11.5%) were the second common types which were detected in patients with carcinoma. However, they were the most common HPV types after HPV 16 in patients with dysplasia. Therefore, it is likely that the differences between studies with respect to the second most common types depended on ethnic or geographic variations.
The relationship between HPV infection from multiple sexual partners and the initiation of cervical cancer is unknown. People in impoverished communities tend to have multiple HPV infections from multiple sexual partners. In addition, early age of sexual debut contributes to the development of cervical neoplasia. It was observed that HPV infection rates are nearly three times higher in American Indian women than in White women (19% vs. 7%). This data supports a possible role for multiple HPV types in the development or progression of cervical dysplasia. Moreover, different results are available with respect to the multiplicity of HPV infection in patients with cervical neoplasia. American Indian women are also susceptible to increased prevalence estimates for infection by Alpha 7, Alpha 9, and other oncogenic types, as well as nearly double the prevalence of infection by any oncogenic HPV type, compared to White women (30% vs. 16%).
The incidence and prevalence rates are determined by the coexistence of HPV types in a specific infection for a prolonged period of time. The retention of HR HPV types suggests risk of high-grade cervical cancer. Results indicate HR HPV was longer lasting among African American college-age women than their White counterpart in South Carolina. It took almost twice as long for 50% of the HR HPV infections to clear (601 days) than for the low risk HPV (LR HPV) types (316 days). Risk factors for infection are difficult to ascertain because of the high frequency of infection. It is known that major risk factors for acquiring HR HPV infection involve sexual behavior, particularly multiple sex partners and impairment of cell-mediated immunity, because distressed immunity is directly related to the physiologic and immunologic conditions of the cervical microenvironment.
Usually HR HPVs only transit in a uterine cervix for about six months, but when they stay, they remain in cervix for a much longer period, as in cases of African American women (28). This makes the transit quite important, strong, and influential by virtue of anchoring and staying for a long time. Anchoring occurs through multiple infections and is not an easy pairing, especially because it intensifies its transformable ability and is disruptive to receptive cervical tissue (29). Because of this persistent cycle, their action is going to linger for around twenty-four months or more. This prolonged action can result at times in aggressive or at least quite adversarial response because they tend to work at odds with each other when acting through their favorable expressions in diminishing or deleting oncogene suppressors (30). This is because HPV tends to push forward assertively with time, but suppressors which tend to resist fail. HPV deals with transformation and, in order to change, the cervix has to let go of acquired immunity, tumor suppressors, and allow an environment for growth.
Innate immunity is related to HPV clearance through accelerating humoral or cellular immune response. Molecules activate innate immune response, and may be the opportunities to unravel the mechanism of innate immune response by HPV, and also by participating in the microenvironment surrounding HPV infection. Such research may find a new target for HPV eradication through tumor suppressor mechanisms. Cytokines induced by HPV may mediate its adverse effects by causing hypersensitivity and promoting tumor formation (30, 31). Changes in local level of TNFα, IL-6, or IFNγ influence the sensitivity of HPV variants. An infection with different HPV genotypes is associated with distinct pro-inflammatory cytokine expression profiles (32–35). It is still unknown if this explains some of the racial differences observed in cervical cancer.
Most importantly, considering the high prevalence of cervical cancer in Appalachia, limited studies have been conducted in this region to establish which HPV types are closely related to the development of high-grade CIN and cervical cancer. The objective of this review was to analyze the spectrum of HPV genotypes and prevalence of cervical abnormalities in Appalachian women living in underserved Appalachian regions ranging from Ohio to Southern Mississippi and to compare them to American Indians from the Northern Plains. HPV genotypes were detected of which 11 types (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, and 58) are epidemiologically classified as carcinogenic to humans, accounting for two thirds of all infections. HPV16 was the most common viral type in all groups with frequency rates ranging from 2.5% in Mississippi Delta to 18% in Ohio and among Indian American from Northern Plain.
We also discuss here in particular on HPV vaccines that are made from empty protein coats using recombinant DNA technology, having no DNA material, and are not infectious. Previously, HPV vaccines were fabricated to protect against HPV 16 and 18; the quadrivalent vaccine also protects against low-risk genotypes 6 and 11. The nonavalent vaccine may provide high and consistent protection against cervical and other HPV related cancers and pre-cancers in low-income Appalachian women that lack screening programs and may alter screening attitude. The bivalent, quadrivalent, and nonavalent HPV vaccine capabilities decrease with increasing age.
Age-related reduction of HR and LR HPV genotype distributions has received less attention with respect to vaccination outcome. The highest distribution of any HPV is observed in ages between 18–30 years in both women from Mississippi delta and in American Indians from Northern Plain. HR and LR HPV followed by a rapid reduction of HR HPV than LR HPV. HR and LR HPV were equally common in younger ages, by age 30 LR HPV; especially LR types of the phylogenetic group were detected more frequently than carcinogenic HPV. Because of the age related reduction in HPV prevalence prevented by the bivalent and quadrivalent vaccine was greater in some instances than that obtained for the nonavalent vaccine. Interestingly, LR HPVs are prevalent in the Mississippi delta. Therefore, a future analysis of these variables by comparing pathological evaluation and comparing with the HPV genotyping data will be helpful in selecting ideal vaccination. Again, health economic analysis of HPV vaccine implications must account for age-specific differences in HPV type distribution. A vaccine preventable HPV type (6, 11, 16, or 18) was found in 12% of women, including 21% of ages 18–26 in Ohio Appalachian region. This study shows that the distribution of HPV types in women selected for cervical cancer screening in Appalachian and American women is in accordance with observations found worldwide. This observation confirms that the current HPV vaccine against specific HPV genotypes may have the proper effectiveness in controlling cervical cancer among Appalachian and American Indian women. More research on the underlying risk factors would provide outlines for the prevention of cervical cancer in the population of Northern Plains.
The impact of current HPV vaccination initiatives is undercut by poor immunization rates, which are well below target levels. Immunization is not practiced evenly across age, demographic, and socioeconomic divisions within the distressed regions of vaccine recipients (27). To get highest impact on health improvement initiation, we should focus on immunization coverage in the poorest regions. Exploration of significant determinants of HPV vaccine initiation and completion, such as the attitudes of parents and healthcare providers and factors that stimulate HPV-related health outcomes, including smoking and human immunodeficiency virus-mediated immune suppression, is necessary. Vaccination campaigns will require a health disparities approach that both identifies and remedies the underlying causes of population differences in HPV vaccination uptake (36).
In conclusion, the prevalence of HPV genotypes across the Appalachian region of cervical cancer in this unique national resource using tissue-based HPV genotype. By striving for universal use of effective bivalent, quadrivalent and nonavalent vaccines on the rates of initiation, progression, and ultimately invasive cervical cancers, we can help to reduce burden on public health. Here, caregiver organizations must reconcile the disparity in the Appalachians socioeconomic conditions and the vaccination through cervical screening in the distressed areas. This is especially important since 42% of the people in Appalachia are still living in rural areas, compared with 20 percent nationally. Greater awareness on the additional cervical screening is key to reducing the risk of cervical cancer in these economically distressed areas. Other predominant cervical cancer risk factors, like obesity, unhealthy diet, lack of physical activity, smoking, and alcohol consumption, are prevalent among African American women in Tennessee and Mississippi. Ethnic, racial, and regional differences in the incidence and mortality of cervical cancers among American Indian, Appalachian White, African American, and Hispanic women in the United States are remarkable. Further studies on evidence-based interventions in implementing healthcare policies and systems are needed to reduce the cancer burden on distressed Appalachian and American Indian women. We propose reducing inequalities in cancer incidence and uptake of cancer HPV screening as a strategy in the cervical cancer control.
6. INACCESSIBILITY OF DISTRESSED APPALACHIAN AREAS FOR CERVICAL CANCER SCREENING AND DIRECTIONS FOR FUTURE RESEARCH
In the US, despite the introduction of national guidelines, screening coverage is still very low among distressed socioeconomic Appalachia and Northern Plains. Self-esteem, disparity, trust, safety, disclosure, pain, sexual discrimination, method of examination, fatalistic belief, fear and anxiety, communication barriers, and impact of physical examination are responsible for the failure to implement effective screening programs in Appalachia and Northern Plains (8). Younger age at vaccination, lower stage, and HPV positive status could improve woman’s health and strengthen families’ economic self-sufficiency.
6.1. Utilization of Pap test
Prophylactic vaccinations have increased survival from anti-HPV treatments throughout United States. HR HPV type prevalence has been identified among demographic population persistence in Appalachia (19). Screening in underserved special categories is important for the target population; it must guarantee screening, management, and adequate follow-up of patients. The screening must be given on-site and at low-cost, with minimum infrastructure requirement that can lead to immediate treatment, if found abnormal. Cervical cancer screening should be planned in line with other program for cancer control (7). This technique has been able to reduce the incidence and mortality from cervical cancer in the US, but failed to attain the required results in Appalachian areas and in Northern plains. Previous experience has shown no decline in the incidence and/or mortality of cervical cancer and this is probably because of low-quality cytology smears. Consequently, implementation and execution of the whole process is too complex and expensive. Moreover, even if implementing a high-quality cytology program in these countries is possible, it would only be moderately effective. This is because the currently used Pap test misses approximately 50% of high-grade precursor lesions and cancers with a single screening. Additionally, in low-resource settings, women would probably only be screened once or twice in their lifetime.
6.2. New trend in molecular test
Cervical cancer can be described as the integration of HPV DNA into the host cell genome causing proliferation of basal and parabasal cells (1). Sustained expression of viral oncoproteins lead to the development of cervical cancer. Therefore, the qualitative detection of HPV DNA is used by many assays and is the only molecular marker fully developed and approved for primary cervical cancer screening. These tests can be based on the detection of specific types of oncogenic HPV that identify women at a higher cancer risk (e.g., HPV genotypes 16 and 18) (37). However, many other molecular mechanisms associated with HPV infection are necessary for cervical cancer development, such as chromosomal abnormalities, expression of oncogenes, epigenetic regulation (hyper methylation), and apoptotic markers, which cover a large number of potential biomarkers (38–40). Molecular tests have been lately under intensive study as a potential alternative and triage tests for cervical cancer screening.
6.3. Selective oncogene expressions
Elevated expressions of oncoproteins are generally considered as biomarkers in the HPV-positive women. Only the E6 protein from HR HPV type potentially leads to carcinogenesis. This allows the E6 protein to bind to cellular molecules and deregulate cellular proliferation and differentiation, which may lead to the development of cancer. The oncogenic activity of E7 protein is also indirectly related to host cyclin-dependent kinase inhibitor. This kinase inhibitor decreases the cell cycle by inactivating the cyclin-dependent kinases (CDK4/CDK6) involved in retinoblastoma protein phosphorylation (41).
6.4. miRNAs and HPV/cervical cancer variation
MicroRNAs (miRNAs) are a class of small non-coding RNAs eliciting functions at post-transcriptional level i.e. epigenetic (42). These miRNAs have expressions at many levels in cellular organisms, including cell cycle regulation, apoptosis, and differentiation. The consequence of miRNA regulation is thought to reduce the expression variation of target genes. However, it is possible that changes or mutations in miRNAs and target sites cause modulation of the miRNA regulatory networks resulting in increased alterations in gene expression. After critical evaluation on gene expression patterns in human populations, genes regulated by miRNAs signature might have higher expression variation at the population level. They exhibit greater variation in expression among human ethnic groups (43–46).
The expression variation in miRNAs, genetic variants in miRNA loci, and mutations in miRNA target sites are important sources of elevated expression variation of miRNA target genes. Regulation of miRNAs is responsible for initiation and propagation of tumor formation and can serve as potential biomarkers. HR-HPVs can regulate gene expression of host microRNAs (miRNAs) by deletion, amplification, or genomic rearrangement. Several miRNAs are disturbed in cervical cancer, such as miR 21, miR 127, miR 143, miR 145, miR 155, miR 203, miR 218 and miR 214. The miRNA 203 is down-regulated in HPV-positive cells and its repression leads to maintenance of increased levels of p63 in infected cells, maintaining cells in an active state of the cell cycle. MiRNA 21 up-regulation has been responsible for poor prognosis in cervical cancer and emphasize role of miRNAs involved to determine their potential as prognostic biomarkers and as therapeutic targets.
The chronic infections of HPV oncoproteins induce specific cellular pathway modifications that lead to carcinogenesis. Innate and adaptive immune failure leads to severe tissue and systemic damage, impaired tumor surveillance, and concomitant carcinogenesis promotion by selecting for metastatic and therapeutically resistant tumor phenotypes. The hypothesis is that the impacts of genes expression targeted by miRNAs are intrinsically highly changeable, and the miRNA regulation has evolved to reduce the inter-individual stochastic variability of target gene expression. The miRNAs result in qualitative canalization on the expression of target genes. At present, the level of intrinsic stochastic variation in gene expression that would be seen in the absence of miRNA targeting is unknown, and is not adjudged the intensity of canalization of miRNAs in ethnic populations. Therefore, interactions of the post-transcriptional factors such as RNA binding proteins with miRNAs to regulate gene expression patterns will provide deeper understanding in terms of the broad genetic mechanisms underlying human abnormal genetic variation in cervical cancer among American Indian, African Americans, Caucasians, and Hispanics and local adaptation for cervical cancer tumorigenesis. These approaches may be tailored to various ethnic groups according to age, specific drug abuse, alcoholism, smoking, and other comorbid conditions.
6.5. Detection of molecular biomarkers
Molecular markers for triage of HPV-positive women are molecular markers expressing aberrant S-phase induction (47). Topoisomerase IIA and minichromosome maintenance proteins are overexpressed in HPV-infected cells as a result of the differential gene transcription and are associated with cervical lesions. Again, a carcinoma embryonic antigen has shown to be biomarker for cervical cancer prognosis and disease management (48). Integral membrane protein CD44, enzyme cyclooxygenase-2, cytokine vascular endothelial growth factor, and membrane protein caveolin-1 are the promising areas in cervical cancer screening, depending on severity, disease progression, and prognosis.
Acknowledgments
This work was primarily supported by National Institutes of Health (UO1CA62106A, RO1CA204552, RO1CA206069, RO1CA210192 and K22CA174841). Authors Ashok Rajan Karuri and Vivek Kumar Kashyap contributed equally to this manuscript.
Abbreviations
- SEER
Surveillance, Epidemiology, and End Results Program
- CIN
cervical intraepithelial neoplasia
- ARC
Appalachian Regional Commission
- HPV
Human papilloma virus
- HR HPV
high-risk human papilloma virus
- FDA
Food and Drug Administration
- LCR
long control region
- DNA
deoxyribo nucleic acid
- Pap
papanicolaou
- CDC
Centers for Disease Control and Prevention
- ASCH
atypical squamous cells cannot exclude HSIL
- AGC
atypical glandular cells
- LSIL
low-grade squamous intra epithelial lesions
- HSIL
high grade squamous intraepithelial lesions
- AIS
adenocarcinoma in situ
- LR HPV
low-risk human papilloma virus
References
- 1.Chauhan SC, Jaggi M, Bell MC, Verma M, Kumar D. Epidemiology of Human Papilloma Virus (HPV) in Cervical Mucosa. Methods Mol Biol. 2009;471:439–56. doi: 10.1007/978-1-59745-416-2_22. [DOI] [PubMed] [Google Scholar]
- 2.Peng G, Dan W, Jun W, Junjun Y, Tong R, Baoli Z, Yang X. Transcriptome profiling of the cancer and adjacent nontumor tissues from cervical squamous cell carcinoma patients by RNA sequencing. Tumour Biol. 2015;36(5):3309–17. doi: 10.1007/s13277-014-2963-0. [DOI] [PubMed] [Google Scholar]
- 3.Howlader NAN, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER data submission. Nov, 2015. Cancer Statistics Review, 1975–2013. [Google Scholar]
- 4.Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16(1):1–17. doi: 10.1128/CMR.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hariri S, Bennett NM, Niccolai LM, Schafer S, Park IU, Bloch KC, Unger ER, Whitney E, Julian P, Scahill MW, Abdullah N, Levine D, Johnson ML, Steinau M, Markowitz LE Group H-IW. Reduction in HPV 16/18-associated high grade cervical lesions following HPV vaccine introduction in the United States - 2008–2012. Vaccine. 2015;33(13):1608–13. doi: 10.1016/j.vaccine.2015.01.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338(7):423–8. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 7.Hariri S, Unger ER, Schafer S, Niccolai LM, Park IU, Bloch KC, Bennett NM, Steinau M, Johnson ML, Markowitz LE Group H-IW. HPV type attribution in high-grade cervical lesions: assessing the potential benefits of vaccines in a population-based evaluation in the United States. Cancer Epidemiol Biomarkers Prev. 2015;24(2):393–9. doi: 10.1158/1055-9965.EPI-14-0649. [DOI] [PubMed] [Google Scholar]
- 8.Jackson HM. Letter: The Appalachian Regional Commission. JAMA. 1975;234(12) doi: 10.1001/jama.1975.03260250013007. [DOI] [PubMed] [Google Scholar]
- 9.Wilson SL, Kratzke C, Hoxmeier J. Predictors of access to healthcare: what matters to rural Appalachians? Glob J Health Sci. 2012;4(6):23–35. doi: 10.5539/gjhs.v4n6p23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castle PE, Rausa A, Walls T, Gravitt PE, Partridge EE, Olivo V, Niwa S, Morrissey KG, Tucker L, Katki H, Scarinci I. Comparative community outreach to increase cervical cancer screening in the Mississippi Delta. Prev Med. 2011;52(6):452–5. doi: 10.1016/j.ypmed.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johannsen E, Lambert PF. Epigenetics of human papillomaviruses. Virology. 2013;445(1–2):205–12. doi: 10.1016/j.virol.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delury CP, Marsh EK, James CD, Boon SS, Banks L, Knight GL, Roberts S. The role of protein kinase A regulation of the E6 PDZ-binding domain during the differentiation-dependent life cycle of human papillomavirus type 18. J Virol. 2013;87(17):9463–72. doi: 10.1128/JVI.01234-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez M, Findley SE, Mejia M, Martinez J. The impact of community health worker training and programs in NYC. J Health Care Poor Underserved. 2006;17(1 Suppl):26–43. doi: 10.1353/hpu.2006.0011. [DOI] [PubMed] [Google Scholar]
- 14.Kelly KM, Ferketich AK, Ruffin MT, IV, Tatum C, Paskett ED. Perceived risk of cervical cancer in Appalachian women. Am J Health Behav. 2012;36(6):849–59. doi: 10.5993/AJHB.36.6.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Downs LS, Smith JS, Scarinci I, Flowers L, Parham G. The disparity of cervical cancer in diverse populations. Gynecol Oncol. 2008;109(2 Suppl):S22–30. doi: 10.1016/j.ygyno.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Blake KD, Ottenbacher AJ, Finney Rutten LJ, Grady MA, Kobrin SC, Jacobson RM, Hesse BW. Predictors of human papillomavirus awareness and knowledge in 2013: gaps and opportunities for targeted communication strategies. Am J Prev Med. 2015;48(4):402–10. doi: 10.1016/j.amepre.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shell R, Tudiver F. Barriers to cancer screening by rural Appalachian primary care providers. J Rural Health. 2004;20(4):368–73. doi: 10.1111/j.1748-0361.2004.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 18.Reiter PL, Katz ML, Ferketich AK, Ruffin MTt, Paskett ED. Measuring cervical cancer risk: development and validation of the CARE Risky Sexual Behavior Index. Cancer Causes Control. 2009;20(10):1865–71. doi: 10.1007/s10552-009-9380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paskett ED, McLaughlin JM, Lehman AM, Katz ML, Tatum CM, Oliveri JM. Evaluating the efficacy of lay health advisors for increasing risk-appropriate Pap test screening: a randomized controlled trial among Ohio Appalachian women. Cancer Epidemiol Biomarkers Prev. 2011;20(5):835–43. doi: 10.1158/1055-9965.EPI-10-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh SD, Henley SJ, Ryerson AB. Summary of Notifiable Noninfectious Conditions and Disease Outbreaks: Surveillance for Cancer Incidence and Mortality - United States, 2011. MMWR Morb Mortal Wkly Rep. 2015;62(54):11–51. doi: 10.15585/mmwr.mm6254a3. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt-Grimminger DC, Bell MC, Muller CJ, Maher DM, Chauhan SC, Buchwald DS. HPV infection among rural American Indian women and urban white women in South Dakota: an HPV prevalence study. BMC Infect Dis. 2011;11:252. doi: 10.1186/1471-2334-11-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bell MC, Schmidt-Grimminger D, Jacobsen C, Chauhan SC, Maher DM, Buchwald DS. Risk factors for HPV infection among American Indian and white women in the Northern Plains. Gynecol Oncol. 2011;121(3):532–6. doi: 10.1016/j.ygyno.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duvall J, Buchwald D. Human papillomavirus vaccine policies among american Indian tribes in Washington State. J Pediatr Adolesc Gynecol. 2012;25(2):131–5. doi: 10.1016/j.jpag.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramirez AS, Rutten LJ, Oh A, Vengoechea BL, Moser RP, Vanderpool RC, Hesse BW. Perceptions of cancer controllability and cancer risk knowledge: the moderating role of race, ethnicity, and acculturation. J Cancer Educ. 2013;28(2):254–61. doi: 10.1007/s13187-013-0450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reiter PL, Katz ML, Ruffin MT, Hade EM, DeGraffenreid CR, Patel DA, Paskett ED, Unger ER. HPV prevalence among women from Appalachia: results from the CARE project. PLoS One. 2013;8(8):e74276. doi: 10.1371/journal.pone.0074276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanderpool RC, Dressler EV, Stradtman LR, Crosby RA. Fatalistic beliefs and completion of the HPV vaccination series among a sample of young Appalachian Kentucky women. J Rural Health. 2015;31(2):199–205. doi: 10.1111/jrh.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reiter PL, Fisher JL, Hudson AG, Tucker TC, Plascak JJ, Paskett ED. Assessing the burden of HPV-related cancers in Appalachia. Hum Vaccin Immunother. 2013;9(1):90–6. doi: 10.4161/hv.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banister CE, Messersmith AR, Cai B, Spiryda LB, Glover SH, Pirisi L, Creek KE. Disparity in the persistence of high-risk human papillomavirus genotypes between African American and European American women of college age. J Infect Dis. 2015;211(1):100–8. doi: 10.1093/infdis/jiu394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prata TT, Bonin CM, Ferreira AM, Padovani CT, Fernandes CE, Machado AP, Tozetti IA. Local immunosuppression induced by high viral load of human papillomavirus: characterization of cellular phenotypes producing interleukin-10 in cervical neoplastic lesions. Immunology. 2015;146(1):113–21. doi: 10.1111/imm.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang LY, Scott ME, Ma Y, Moscicki AB. Higher levels of cervicovaginal inflammatory and regulatory cytokines and chemokines in healthy young women with immature cervical epithelium. J Reprod Immunol. 2011;88(1):66–71. doi: 10.1016/j.jri.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kemp TJ, Hildesheim A, Garcia-Pineres A, Williams MC, Shearer GM, Rodriguez AC, Schiffman M, Burk R, Freer E, Bonilla J, Herrero R, Pinto LA. Elevated systemic levels of inflammatory cytokines in older women with persistent cervical human papillomavirus infection. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1954–9. doi: 10.1158/1055-9965.EPI-10-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Govan VA, Constant D, Hoffman M, Williamson AL. The allelic distribution of −308 Tumor Necrosis Factor-alpha gene polymorphism in South African women with cervical cancer and control women. BMC Cancer. 2006;6:24. doi: 10.1186/1471-2407-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deshpande A, Nolan JP, White PS, Valdez YE, Hunt WC, Peyton CL, Wheeler CM. TNF-alpha promoter polymorphisms and susceptibility to human papillomavirus 16-associated cervical cancer. J Infect Dis. 2005;191(6):969–76. doi: 10.1086/427826. [DOI] [PubMed] [Google Scholar]
- 34.Rosa MI, Moraes MV, Vuolo F, Petronilho F, Bozzetti MC, Medeiros LR, Igansi CN, Silva FR, Dal-Pizzol F, Rosa DD. Association of interleukin-6 in women with persistence of DNA-HPV: a nested case-control study. Arch Gynecol Obstet. 2012;285(1):143–8. doi: 10.1007/s00404-011-1925-7. [DOI] [PubMed] [Google Scholar]
- 35.Hibma MH. The immune response to papillomavirus during infection persistence and regression. Open Virol J. 2012;6:241–8. doi: 10.2174/1874357901206010241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reiter PL, Katz ML, Paskett ED. HPV vaccination among adolescent females from Appalachia: implications for cervical cancer disparities. Cancer Epidemiol Biomarkers Prev. 2012;21(12):2220–30. doi: 10.1158/1055-9965.EPI-12-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres-Poveda K, Burguete-Garcia AI, Cruz M, Martinez-Nava GA, Bahena-Roman M, Ortiz-Flores E, Ramirez-Gonzalez A, Lopez-Estrada G, Delgado-Romero K, Madrid-Marina V. The SNP at −592 of human IL-10 gene is associated with serum IL-10 levels and increased risk for human papillomavirus cervical lesion development. Infect Agent Cancer. 2012;7(1):32. doi: 10.1186/1750-9378-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel DA, Rozek LS, Colacino JA, Van Zomeren-Dohm A, Ruffin MT, Unger ER, Dolinoy DC, Swan DC, Onyekwuluje J, DeGraffinreid CR, Paskett ED. Patterns of cellular and HPV 16 methylation as biomarkers for cervical neoplasia. J Virol Methods. 2012;184(1–2):84–92. doi: 10.1016/j.jviromet.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gustinucci D, Giorgi Rossi P, Cesarini E, Broccolini M, Bulletti S, Carlani A, D’Angelo V, D’Amico RM, Di Dato E, Galeazzi P, Malaspina M, Martinelli N, Spita N, Tintori B, Giaimo MD, Passamonti B. Use of Cytology, E6/E7 mRNA, and p16INK4a-Ki-67 to Define the Management of Human Papillomavirus (HPV)-Positive Women in Cervical Cancer Screening. Am J Clin Pathol. 2016;145(1):35–45. doi: 10.1093/ajcp/aqv019. [DOI] [PubMed] [Google Scholar]
- 40.Filippova M, Filippov VA, Kagoda M, Garnett T, Fodor N, Duerksen-Hughes PJ. Complexes of human papillomavirus type 16 E6 proteins form pseudo-death-inducing signaling complex structures during tumor necrosis factor-mediated apoptosis. J Virol. 2009;83(1):210–27. doi: 10.1128/JVI.01365-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Narasimha AM, Kaulich M, Shapiro GS, Choi YJ, Sicinski P, Dowdy SF. Cyclin D activates the Rb tumor suppressor by mono-phosphorylation. Elife. 2014;3 doi: 10.7554/eLife.02872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Wang Q, Liu H, Shao N, Tan B, Zhang G, Wang K, Jia Y, Ma W, Wang N, Cheng Y. The association of miR-146a rs2910164 and miR-196a2 rs11614913 polymorphisms with cancer risk: a meta-analysis of 32 studies. Mutagenesis. 2012;27(6):779–88. doi: 10.1093/mutage/ges052. [DOI] [PubMed] [Google Scholar]
- 43.Gocze K, Gombos K, Kovacs K, Juhasz K, Gocze P, Kiss I. MicroRNA expressions in HPV-induced cervical dysplasia and cancer. Anticancer Res. 2015;35(1):523–30. [PubMed] [Google Scholar]
- 44.Bermudez-Morales VH, Gutierrez LX, Alcocer-Gonzalez JM, Burguete A, Madrid-Marina V. Correlation between IL-10 gene expression and HPV infection in cervical cancer: a mechanism for immune response escape. Cancer Invest. 2008;26(10):1037–43. doi: 10.1080/07357900802112693. [DOI] [PubMed] [Google Scholar]
- 45.Wang L, Wang Q, Li HL, Han LY. Expression of MiR200a, miR93, metastasis-related gene RECK and MMP2/MMP9 in human cervical carcinoma--relationship with prognosis. Asian Pac J Cancer Prev. 2013;14(3):2113–8. doi: 10.7314/APJCP.2013.14.3.2113. [DOI] [PubMed] [Google Scholar]
- 46.Reshmi G, Surya R, Jissa VT, Babu PS, Preethi NR, Santhi WS, Jayaprakash PG, Pillai MR. C-T variant in a miRNA target site of BCL2 is associated with increased risk of human papilloma virus related cervical cancer--an in silico approach. Genomics. 2011;98(3):189–93. doi: 10.1016/j.ygeno.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 47.Malinowski DP. Molecular diagnostic assays for cervical neoplasia: emerging markers for the detection of high-grade cervical disease. Biotechniques. 2005;(Suppl):17–23. doi: 10.2144/05384su03. [DOI] [PubMed] [Google Scholar]
- 48.Lim SJ, Kim HJ, Kim JY, Park K, Lee CM. Expression of HuR is associated with increased cyclooxygenase-2 expression in uterine cervical carcinoma. Int J Gynecol Pathol. 2007;26(3):229–34. doi: 10.1097/01.pgp.0000236946.82334.07. [DOI] [PubMed] [Google Scholar]