Abstract
Poly lactic acid is a biodegradable, biocompatible, and non-toxic polymer, widely used in many pharmaceutical preparations such as controlled release formulations, parenteral preparations, surgical treatment applications, and tissue engineering. In this study, we prepared lipid-polymer hybrid nanoparticles for topical and site targeting delivery of Norfloxacin by emulsification solvent evaporation method (ESE). The design of experiment (DOE) was done by using software to optimize the result, and then a surface plot was generated to compare with the practical results. The surface morphology, particle size, zeta potential and composition of the lipid-polymer hybrid nanoparticles were characterized by SEM, TEM, AFM, and FTIR. The thermal behavior of the lipid-polymer hybrid nanoparticles was characterized by DSC and TGA. The prepared lipid-polymer hybrid nanoparticles of Norfloxacin exhibited an average particle size from 178.6 ± 3.7 nm to 220.8 ± 2.3 nm, and showed very narrow distribution with polydispersity index ranging from 0.206 ± 0.36 to 0.383 ± 0.66. The surface charge on the lipid-polymer hybrid nanoparticles were confirmed by zeta potential, showed the value from +23.4 ± 1.5 mV to +41.5 ± 3.4 mV. An Antimicrobial study was done against Staphylococcus aureus and Pseudomonas aeruginosa, and the lipid-polymer hybrid nanoparticles showed potential activity against these two. Lipid-polymer hybrid nanoparticles of Norfloxacin showed the %cumulative drug release of 89.72% in 24 h. A stability study of the optimized formulation showed the suitable condition for the storage of lipid-polymer hybrid nanoparticles was at 4 ± 2 °C/60 ± 5% RH. These results illustrated high potential of lipid-polymer hybrid nanoparticles Norfloxacin for usage as a topical antibiotic drug carriers.
Keywords: Lipid-polymer hybrid nanoparticles, Norfloxacin, Antimicrobial activity, Carbopol gel
Graphical abstract
Highlights
-
•
Efficient topical drug delivery systems of norfloxacin have been synthesized.
-
•
Norfloxacin loaded to the core of lipid- polymer hybrid nanoparticles were prepared.
-
•
The formulations were optimized by factorial design and characterization techniques.
-
•
A unique formulation of norfloxacin that offer prolonged and control delivery.
1. Introduction
Norfloxacin is a fluoroquinolone derivative, differ from quinolones by having fluorine atom at six position and a piperazine moiety at seventh position. It is a broad spectrum antibiotic, has activity against Gram-negative and some Gram-positive aerobic bacteria. Norfloxacin acts by inhibiting synthesis of bacterial deoxyribonucleic acid (DNA) and have bactericidal property. They can be administered through oral route but the oral bioavailability is only 30–40% on a single dose of 200–400 mg [1].
Lipid-polymer hybrid nanoparticles (LPNPs) are emerging nanoparticles drug delivery systems that have advantage of both state i.e. liquid and solid state. LPNPs remains in solid state at body temperature [2], hence incorporation into the carbopol gel make them easy and consistent delivery of the drug to the targeted site. Due to their existent in both state, they showed a control release of drug. LPNPs are polymeric nanoparticle basically composed of three subsequent layers as 1) an inner hydrophobic core layer where the encapsulation of large amount of hydrophobic drug is possible; 2) an interfacial lipid layer that act as a flexible and biocompatible shell; and 3+) an outer hydrophilic polymer stealth layer to enhance the circulation time and stability of the LPNPs [4].
The coexistence of lipid and polymer possessing different physicochemical properties, such as their lipophilic and hydrophilic behavior lead to design a large variety of delivery system, and also have versatile capability of loading varying types of drugs, (Y.W. Xiao suggested that LPNPs are the best drug delivery system for the highly hydrophilic drug, because these drug possess the problem of rapid clearance from the body and low therapeutic recovery) [2] as well as they can be easily conjugated with the targeting moiety to deliver a drug to its target site.
Topical infections cover, localized surface infection due to accidental injury, surgery, abrasion and major complication of burns and topical disease covers bacterial infection, plant warts attack, and fungal growth etc. [3]. Topical antibiotics of lipid-polymer hybrid nanoparticles play an important role to deliver drug in such kind of topical infection and disease, due to its controlled and prolonged drug delivery to the surface of the infection, and avoid frequent application of the medicament to the infected and painful area, hence, lead to an increase in patient compliances.
On the other hand making use of biodegradable, biocompatible and non-toxic polymer such as Poly lactic acid have shown great therapeutic potential as a drug delivery system. PLA is a hydrophobic polymer and exhibits a good mechanical strength widely used in the manufacturing of containers, surgical equipment, and other delivery appliances. In this study the PLA is used as a polymer in an optimized concentration to control the release of drug.
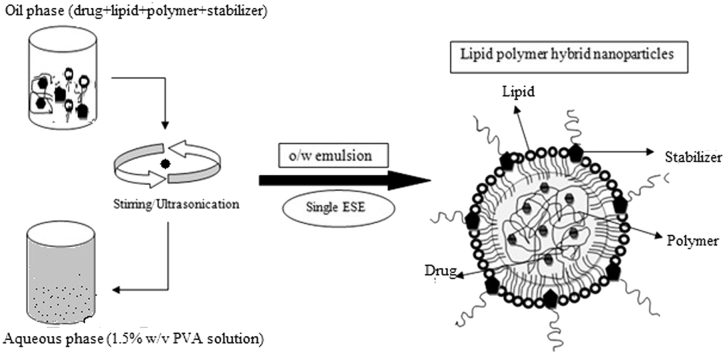
The emulsification solvent evaporation (ESE) is a technique in which a primary emulsion oil/water was prepared, where the water act as a non-solvent system for the polymer (PLA) and finally the organic solvent in which the polymer was dissolved were evaporated by using Rota-evaporator. This method also known as ‘water in drying’ system for the hydrophobic polymer system because the water serves as a non-solvent that enable a hydrophobic polymer to form particle size in a Nano range. In this study the ESE was used to prepare the lipid-polymer hybrid nanoparticles of Norfloxacin to enhance the drug loading to the polymer and lipid core material, so that a controlled release formulation can be obtained.
However, it is challenging to design an optimal formulation with all desirable characteristics for site specific drug delivery of the norfloxacin, hence, an experiment design was used to overcome this problem. This research article provides an optimized formulation development, antimicrobial study, factors that influence the optimized formulation and there related characterization, and future in development of the effective delivery of LPNPs as an emerging tool for the topical drug delivery system.
2. Experimental
2.1. Materials
Norfloxacin was purchased from Sisco Research Laboratory, Mumbai, India. Poly lactic acid was obtained as a gift sample from Sun pharma advanced research company Ltd. Vadodara, Gujarat, India. Soya lecithin (Lipid), stearylamine (charge-inducer), poly vinyl alcohol (PVA), was purchased from Sigma Aldrich, India, Dichloromethane (DCM), Acetic acid (HPLC grade) was purchased from Merck specialties Pvt, Ltd., Mumbai, India. All other analytical grade chemicals were used during experiment. Millipore water (Millipore, Bedford, MA, USA) was used throughout the study.
2.2. Formulation of norfloxacin loaded lipid polymer hybrid nanoparticles
The lipid-polymer hybrid nanoparticles were prepared by emulsification solvent evaporation method firstly described by Gurny 1981 [5]. Briefly, soya lecithin with different PLA ratios (as showed in Table 1) were dissolved in 3 ml of DCM in a beaker and 200 mg of norfloxacin was separately dissolve in 0.3 ml acetic acid, which was further added to the above lipid-polymer (oil) phase. Then 20 ml of PVA (1.5% w/v) solution was used as an aqueous phase and that also act as a stabilizing agent for the formulation. Lipid phase was added dropwise into the aqueous phase under the high speed stirring for 24 h. Leaving continuous stirring of the formulation till total evaporation of organic solvent (DCM) at room temperature (RT). Nanoparticle dispersion was then subjected to sonication at 4 °C using probe sonicatior (PCI Analytics) for each 5 min of cycle leaving rest of 3min in between each cycles of sonication to avoid excessive heat generation that may lead to product degradation [6]. The lipid-polymer hybrid nanoparticles were centrifuged at 14000 rpm for 15 min at 4 °C. The supernatant were discarded and the solid mass were washed thrice with distilled water and then suspended in Millipore water. The LPNPs were lyophilized and stored in a tightly closed container.
Table 1.
Formulation of Norfloxacin loaded LPNPs.
| Batch No. | LPNPs-1 | LPNPs-2 | LPNPs-3 | LPNPs-4 | LPNPs-5 | LPNPs-6 | LPNPs-7 | LPNPs-8 | LPNPs-9 |
|---|---|---|---|---|---|---|---|---|---|
| Drug: lipid: polymer (mg: mg: mg) | 200:10:10 | 200:10:20 | 200:10:30 | 200:20:10 | 200:20:20 | 200:20:30 | 200:30:10 | 200:30:20 | 200:30:30 |
| Stearylamine (mg) | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 |
| Acetic acid (ml) | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| DCM (ml) | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| PVA solution (ml) 1.5% w/v | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
Drug: Norfloxacin; Lipid: Soya lecithin; Polymer: PLA(poly lactic acid); DCM: Dichloromethane; PVA: Polyvinylalcohol.
2.3. Incorporation of lipid-polymer nanoparticles to the carbopol K-940 gel
Prepared LPNPs were further incorporated into 1% w/v carbopol K-940 gel. Briefly, 0.5% of the carbopol K-940 was dispersed in slight amount of distilled water and stirred continuously to form gel like mass, subsequently volume was made up to 50 ml with distilled water. Then the prepared LPNPs of norfloxacin (20 ml) was incorporated to it using high speed stirring at 1000 rpm for 5 min. The gel was further made alkaline by using 1–2 drop of Triethanolamine (TEA). Then 0.5 ml of glycerin was added to it that act as a humectant. Then the lipid-polymer nanoparticle gel of norfloxacin was left equilibrating for 24 h at room temperature (25 ± 1 °C) [7].
2.4. Experimental design
Design of experiment (DoE) and Response surface methodology (RSM) have been proved to be useful statistical and mathematical tool in experiment modeling and process optimization of the variables which may influence the responses of the designed experiments [8]. In the present study, Box-Behnken statistical (32 Full-factorial) design was employed by using Statistica V.10_ software (StatSoft, Inc. USA) to provide an appropriate set of experiment runs [9]. RSM explores the relationship between several independent variables (factors) and one or more dependent variable (responses). In this study, nine LPNPs were prepared to determine the effect of independent variable as concentration of soya lecithin (X1) and concentration of PLA (X2) on responses (dependent factor). The responses that taken in measure were: % entrapment efficiency (Y1), particle size (Y2), and % cumulative drug release (Y3) [20]. Table 2, Table 3 summarizes the experimental run, their coded form and corresponding actual values, and their responses.
Table 2.
Coded levels for the experimental design.
| Independent variable | Coded Levels |
||
|---|---|---|---|
| +1 | 0 | −1 | |
| Concentration of soya lecithin (lipid), mg | 30 | 10 | 20 |
| Concentration of PLA (Polymer), mg | 30 | 10 | 20 |
Table 3.
Experimental runs, independent variables, & measures responses of 32 full factorial experimental designs of the formulated LPNPs.
| Runs | X1 | X2 | %EE | PS (nm) | %CDR |
|---|---|---|---|---|---|
| LPNPs-1 | 0 | 0 | 56.42 ± 0.43 | 220.8 ± 2.3 | 68.02 ± 0.42 |
| LPNPs-2 | 0 | −1 | 54.32 ± 0.09 | 218.9 ± 4.0 | 66.62 ± 1.32 |
| LPNPs-3 | 0 | +1 | 53.29 ± 0.30 | 216.5 ± 1.9 | 67.4 ± 0.46 |
| LPNPs-4 | −1 | 0 | 67.17 ± 0.36 | 206.7 ± 4.2 | 71.02 ± 0.6 |
| LPNPs-5 | −1 | −1 | 65.39 ± 0.33 | 201.1 ± 2.9 | 78.02 ± 0.62 |
| LPNPs-6 | −1 | +1 | 62.75 ± 0.61 | 197.5 ± 3.5 | 79.89 ± 0.86 |
| LPNPs-7 | +1 | 0 | 70.7 ± 0.56 | 189.8 ± 2.0 | 86.1 ± 0.72 |
| LPNPs-8 | +1 | −1 | 72.34 ± 0.23 | 178.6 ± 3.7 | 89.72 ± 0.21 |
| LPNPs-9 | +1 | +1 | 69.25 ± 0.27 | 200.3 ± 3.6 | 77.25 ± 0.61 |
Data represent mean ± SD of 3 determination.
Independent variable; X1 = Concentration of soya lecithin, X2 = Concentration of polymer, the level of independent variable were set at 0, -1, +1 i.e. lower, middle, and higher.
Dependent variable; %EE = entrapment efficiency, PS = particle size, %CDR = cumulative drug release.
2.5. Particle size, polydispersity and zeta potential
The prepared LPNPs were characterized for their particle size (nm), polydispersity index (PDI) and zeta potential (mV) by dynamic light scattering (DLS) method using Malvern Zetasizer Nano ZS (Malvern Instruments, UK) at standard temperature and experimental condition [10]. The average particle size defined as the relative size of the particle in the LPNPs, and a narrow PDI reveals about particle homogeneity in the prepared formulation. The zeta potential value having larger than +30 mV or −30 mV reveals about its maximum repulsion force and long term stability of the prepared LPNPs. All the measures of particle size, PDI, and zeta potential was run trice for 15 cycle by using software.
2.6. Entrapment efficiency (%EE)
The entrapment efficiency (%EE) of the prepared lipid-polymer hybrid nanoparticles was determined by measuring the concentration of unentrapped drug in the supernatant when it exposed to a high speed centrifugation. Briefly, the LPNPs were subjected to ultracentrifugation (REMI equipped with TLA-45 rotor) at 14000 rpm at 4 °C for 15 min, the procedure was repeated- till a clear supernatant was obtained. Then the amount of unentrapped drug present in the supernatant was determined by UV/Vis-spectrophotometry at 272 nm [10], [11]. The each supernatant were analyzed thrice and the %EE was calculated by following formula:
| %EE = [(Drug added − Free “unentrapped drug”)/Drug added]*100 | (1) |
2.7. Particle morphology of LPNPs by scanning electron microscopy (SEM), transmission electron microscopy (TEM) and atomic force microscopy (AFM)
In the present study, the surface morphology of the prepared lipid-polymer hybrid nanoparticles was studied by using SEM, TEM, and AFM. The Scanning electron microscope (SEM) was performed by using lyophilized sample of LPNPs. Briefly, the LPNPs were lyophilized by Freeze-dryer (Labconco lyophilizer, ¼ hp refrigeration system) using trehalose (50 mg/ml) as a cryoprotectant in deionized water by maintaining the freezing temperature for LPNPs at −60 °C and vacuum pressure of 0.018 mbar, till complete drying of the LPNPs and stored in closed tight container. Then lyophilized LPNPs sample were mounted on double sided carbon tape and uniformly coated with gold by the help of ion-sputter for 10 min and examined under SEM (CSIR-CEERI, Pilani, Rajasthan) operated at 20 kV at different magnification of 2,700×, and 19000×.
TEM (AIIMS, Delhi) operated at 200 kV at a magnification of 9900× was used to assess the size and morphology of the LPNPs. TEM visualizes transparent LPNPs as bright spherical areas against dark background. The TEM study was performed accordingly reported elsewhere. Briefly, 1 ml of the LPNPs was taken in an Eppendorf tube and then diluted 10 times with deionized water, then 200 μl of the sample was pipetted and stained with 2% w/v phosphotungustic acid for 30 s on a coated copper grid and the excess of the material was removed by filter paper, and then allowed to completely dried. Two grids were prepared for each sample, the microscope magnification was calibrated and then viewed randomly.
Atomic Force Microscope (AFM) was carried out to determine the surface properties as height and diameter of the LPNPs. An AIST FP tip no. 01 and AC mod was used for imaging the LPNPs. Controlled software, and an Advance Integrated Scanning tool for Nano Technology (AIST-NT) Model: Smart SPM 1000, (NIIFP, Russia) in tapping mode was used to analyze the images. In this imaging technique, around 10 μl of LPNPs was safely secured on a freshly cleaved Mica slips and incubated for 5 min. The surplus matter was removed from the surface of slips gently by deionized water. Then sample was dried on spin coater to produce a thin film. Each sample was scanned by placing under the lens and analyzed at different magnification and three dimensional structure was observed by displaying amplitude, height and phase signal of the cantilever in the traced direction simultaneously [7].
2.8. Attenuated total reflection- Fourier transform infrared spectroscopy (ATR-FTIR)
The Infrared spectra of LPNPs loaded norfloxacin, composed of soya lecithin (lipid), PLA (polymer), stearylamine (charge inducer), and PVA (poly vinyl alcohol, stabilizer) were analyzed by using a ATR-FTIR spectra (Bruker EQUINOX 55 FTIR spectrophotometer equipped with a liquid nitrogen cooled mercury cadmium telluride (MCT)) at room temperature (25 ± 1 °C) and a set detector of nominal resolution of 2 cm−1 for each spectra. A diamond was used as an internal reflection element, placed at an incidence angle of 45°, scans 32 times and give one reflection that is equivalent to 21 resolutions. An advanced ATR correction was applied to all spectra, and the region from 4000 to 400 cm-1 was selected to run the spectra and peak fitting was done by using Opus software.
2.9. Differential scanning calorimetry (DSC)
In this study, DSC was performed by using DSC (Model: DSC 204 F1 PHOENIX). Equipped with calorimeter (DSC 60), flow meter (flow controller FCL 60), thermal analyzer (TA 60) and an operating software (NETZSCH Proteus Thermal software). In this study, the optimized sample LPNPs-8 was placed in aluminium pans, crimped with hydraulic press and then heated under a nitrogen flow (30 mL/min) at a scanned temperature rate of 5 °C/min from 25 °C to 300 °C [12], set on isothermal programming. The same procedure was repeated for the physical mixture of the drug and excipient and a concordance result was predicted.
2.10. Thermogravimetric analysis (TGA)
TGA studies of norfloxacin, soya lecithin, PLA, polyvinyl alcohol (PVA) and optimized formulation LPNPs-8 was done in order to study physical and chemical properties with the help of PROTEUS Thermal analysis (TGA 400). TGA is also used to determine weight loss, vaporization, sublimation, absorption, adsorption, etc. TGA is generally used to conclude selected characteristics of samples that show either weight loss or gain due to decomposition. The small amount of samples was taken in a crucible and after tarring the weight of crucible was kept in assembly and software was made to run. The amount of weight loss graphs were obtained and reported [13].
2.11. In-vitro drug release
In-vitro drug release of the optimized formulation was studied on cellulose acetate membrane which was soaked for 24 h prior work so that it can easily tie to diffusion tube. Diffusion tube was clamped and dipped in phosphate buffer pH 6.8 in beaker maintained at temperature 37 °C. About 1 gm of LPNPs loaded norfloxacin gel was added in diffusion tube which is a donor compartment and covered with parafilm to avoid evaporation of formulation. The phosphate buffer 6.8 was kept in receiver compartment and stirred continuously at 500 rpm. From receptor compartment 3 ml solution was withdrawn at 0,1,2 … …8, ….and 24 h respectively at particular time interval and replaced by buffer solution so that volume of receptor solution kept constant during drug release. The drug concentrations in the aliquot were determined at 272 nm by UV/VIS spectroscopy against appropriate blank. The experiments were carried out in triplicate and expressed as the Mean ± SD. Drug release data was normalized by converting the drug concentrations in solution to a percentage of cumulative drug release (% CDR) and was shown graphically [14], [15], [19].
2.12. Kinetic modeling
To study the release kinetics from LPNPs loaded norfloxacin, the release data of the two optimized formulation LPNPs-7 and LPNPs-8 were fitted to Zero order (Eq. (2)), First order (Eq. (3)), Higuchi (Eq. (4)) and Korsemeyer-Peppas (Eq. (5)). These kinetic modeling were analyzed by using Microsoft Office Excel (2013) to obtain the best fit model for the in-vitro release [8].
| Qt = Q0 + K0t | (2) |
| Log C = log C0 − Kt/2.303 | (3) |
| ʄ = t1/4 KH t1/2 | (4) |
| ʄt = atn | (5) |
2.13. Antimicrobial activity studies
Bacterial strains were procured from MTCC (Microbial Type Culture Collection and Gene Bank), Chandigarh, India. The microbiological assay of standard drug (norfloxacin) solution and optimized formulation (LPNPs-8) was performed in nutrient agar plate employing cup plate method. The nutrient agar media (2.8% w/v) was prepared and sterilized at 121 °C for 21 min at 15 lbs pressure and poured into sterile petri plates. Media was allowed to solidify and then loop full bacteria was inoculated (Staphylococcus aureus MTCC 3160 and Pseudomonas aeruginosa MTCC 1688 from grown bacterial suspension) with the help of swapping method followed by boring in the plate (9 cm in diameter and 5 cm in thickness) with the help of cork-borer to obtain definite size of hole. Drug solution and optimized LPNPs formulation equivalent to 2, 4, 6, and 8 μg/ml of drug was poured in hole with crystal violet dye and incubated for 24 h at 37 °C. Further the zone of inhibition was measured by using Vernier caliper in mm and antimicrobial effect was compared with the standard drug. The entire operation except the incubation was carried out in a laminar airflow unit [16].
2.14. Skin irritation studies
The skin irritancy study was done on Wistar rats procured from Chaudhary Charan Singh Haryana Agricultural University after approval by IAEC of Banasthali University with protocol number BV/IAEC/2016/19. Plain norfloxacin solution and optimized formulation (LPNPs-8) was assessed by Draize patch tested on albino Wistar rat. The animals were housed at 25 °C and hair of the back was trimmed short, 24 h prior to beginning of the assay. The animals were divided into three groups of six each. Two milliliters of norfloxacin solution and LPNPs-08 were applied on the hair-free skin of rats by spreading uniformly. The animals were protected by using nylon mesh, supported by the plastic squares having small pores. Any visible changes such as erythema at 24, 48 and 72 h after the application of formulations was observed. The mean erythema scores were recorded (ranging from 0 to 4), depending on the degree of erythema, as follows: no erythema = 0; slight erythema (barely perceptible-light pink) = 1; moderate erythema (dark pink) = 2; moderate to severe erythema (light red) = 3; and severe erythema (extreme redness) = 4 grade [18].
2.15. Stability studies
Stability studies were performed for both lipid-polymer hybrid nanoparticles suspension and lyophilized nanoparticles to investigate the loss of drug from nanoparticle and change in nanoparticle structure during storage condition. Optimized formulation LPNPs-08 nanoparticles were subjected to accelerated stability studies as per ICH guidelines (4 °C/60 ± 5% RH and25 °C/60 ± 5% RH) [17], for a period of 9 month. Further the sample evaluation were made by microscopic observation, particle size, PDI, zeta potential, and entrapment efficiency.
3. Results and discussion
3.1. Optimization
For optimization of the LPNPs, the following quadratic mathematical model equation was generated by using software Statistica V.10_ software (StatSoft, Inc. USA):
| Y (responses) = b0 + b1X + b2Y + b3X2 + b4XY + b5Y2 |
The model equation relating Entrapment efficiency (%EE), Particle size (PS), and % Cumulative drug release (%CDR) as response is shown in equations:
| %EE = 41.6522 + 1.6737X + 0.0673Y − 0.0238X2 + 0.0042XY − 0.0075Y2 |
| PS (nm) = 274.9111 – 3.1217X – 3.05334 + 0.0233X2 + 0.0362XY + 0.0568Y2 |
| %CDR = 38.95 + 1.3093X + 1.7592Y – 0.0013X2 – 0.0203XY – 0.0336Y2 |
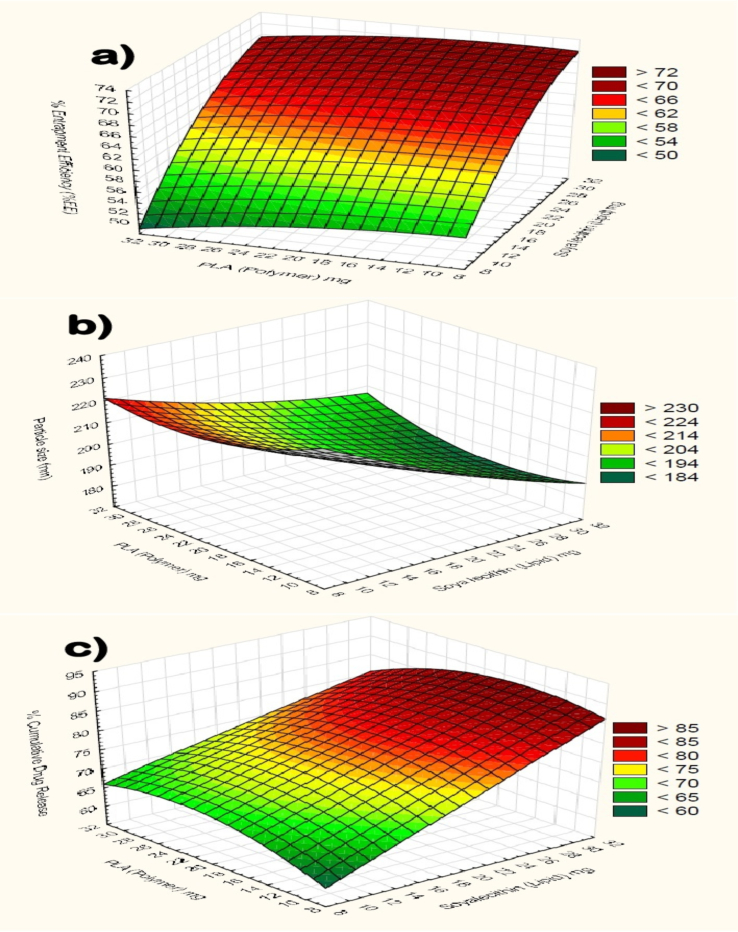
In the above equation the positive value favors the model while the negative value opposes the model fit. The quadric equation showed that the optimized formulation could be get at a specific ratio of lipid and polymer. Because the lipid and polymer both have their significant effect on the entrapment efficiency, particle size and the %cumulative drug release, responses can be observed clearly in their surface plot Fig. 1. Later on in this study these quadric model were further studied for various parameter and there results were explained accordingly.
Fig. 1.
Response surface plot showing effect of independent variables (Drug: lipid: polymer concentration) on a) %EE, b) PS, c) %CDR.
3.2. Particle size, polydispersity and zeta potential
The mean vesicle size, size distribution and zeta potential are the essential parameters that describes the quality and stability of LPNPs. The particle size and polydispersity index (PDI) were determined by using dynamic light scattering technique and observed values are shown in Table 4. All the formulations, LPNPs-1 to LPNPs-9 showed mean particle diameter sizes ranged between 178.6 ± 3.7 nm to 220 ± 2.3 nm with PDI ranged between 0.206 ± 0.36 to 0.383 ± 0.66. In the present study, it was observed that the particle size was affected by the polymer and lipid concentration. It was seen that upon increasing the concentration of polymer (PLA), there was drastic change in particle size and PDI, at high concentration of PLA the particle size was found to be less (i.e.178.6 ± 3.7 nm) and showed narrow distribution (i.e. PDI value less than 0.3), as observed in the optimized formulation LPNPs-8. But, it was also observed that at high concentration of the PLA, when soya lecithin (lipid) concentration were too high (beyond 20 mg), the particle size as well as PDI value were drastically increases. Hence, it was concluded that the LPNPs loaded nanoparticle with the lipid: polymer ratios of 3:2 was suitable for the optimized formulations. Either increase or decrease of these ratios, widely affect the particle size of the formulations.
Table 4.
Particle size, PDI and Zeta potential.
| Parameters | LPNPs-1 | LPNPs-2 | LPNPs-3 | LPNPs-4 | LPNPs-5 | LPNPs-6 | LPNPs-7 | LPNPs-8 | LPNPs-9 |
|---|---|---|---|---|---|---|---|---|---|
| Particle size (nm) | 220.8 ± 2.3 | 218.9 ± 4.0 | 216.5 ± 1.9 | 206.7 ± 4.2 | 201.1 ± 2.9 | 197.5 ± 3.5 | 189.8 ± 2.0 | 178.6 ± 3.7 | 200.3 ± 3.6 |
| Polydispersity | 0.383 ± 0.66 | 0.357 ± 2.02 | 0.325 ± 1.03 | 0.308 ± 0.80 | 0.291 ± 0.78 | 0.268 ± 1.20 | 0.240 ± 0.59 | 0.206 ± 0.36 | 0.219 ± 0.25 |
| Zeta potential | +23.4 ± 1.5 | +24.7 ± 4.1 | +27.3 ± 2.1 | +29.9 ± 2.3 | +33.4 ± 3.4 | +35.2 ± 3.3 | +36.34 ± 4.3 | +41.5 ± 3.4 | +37.8 ± 2.9 |
Data represent mean ± SD; n = 3.
The stability of LPNPs was calculated by measuring zeta potential of the particles by Malvern Zeta siezer Nano ZS (Malvern Instruments, UK). If all the particles in suspension have a highest positive zeta potential (above or equal to +30 mV) and lowest negative zeta potential (above or equal to −30 mV), then they will tend to repel each other and there will be no tendency for the particles to form agglomerate. Zeta potential measurements showed that the variation in vesicle sizes might exert an influence on the charges carried by the vesicles. The results of zeta potential for different formulations are show in Table 4. All the formulations from LPNPs-1 to LPNPs-9 showed zeta potential values from +23.4 ± 1.5 mV to +41.5 ± 3.4 mV. In all the formulations the zeta potential value were noted that it has cationic charge, this is due to the effect of the charge inducer stearyl amine. But it was also observed that the each formulation possessed a different zeta potential value and this may arise due to the interaction of charge inducer with the lipid surface. Because it was observed that when the concentration of lipid increased from 20 mg to 30 mg, the zeta potential values also increases, and this can be clearly observed in the optimized formulation LPNPs-8 that has a zeta potential value of +41.5 ± 3.4 mV which showed a highly stable formulation. All these findings clearly reveal that an accurate combination of lipid, polymer and charge inducer will give highly stable, narrow dispersed and acceptable LPNPs with controlled particle size.
3.3. Entrapment efficiency (%EE)
The entrapment efficiency is the most important parameter that suggest loading capacity of drug by any polymer and other excipients. The entrapment efficiency determined from all nine batches were 56.42 ± 0.43, 54.32 ± 0.09, 53.29 ± 0.30, 67.17 ± 0.36, 65.17 ± 0.33, 62.75 ± 0.61, 70.7 ± 0.56, 72.34 ± 0.23, and 69.25 ± 0.27, all are also summarized in Table 3. It was observed that the formulation LPNPs-8 showed the maximum percentage entrapment efficiency i.e. 72.34 ± 0.23% followed by formulation LPNPs-7 having entrapment efficiency 70.7 ± 0.56%, this may be possible due to higher concentration of the lipid (30 mg). But it was also observed that the formulation LPNPs-9 containing lipid concentration of 30 mg, but it showed a slight decrease in the percentage entrapment efficiency i.e. 69.25 ± 0.27%, this may be possible due to an increased in the concentration of polymer (PLA) that bound to the surface of soya lecithin (lipid), leaved less free surfaces for drug entrapment. The formulations LPNPs-1, 2 and 3 have very less amount of entrapment efficiency due to less amount of lipid content in these formulation. Hence, we can conclude that an appropriate amount of Lipid/polymer ratio is necessary for the formulation of lipid-polymer hybrid nanoparticles [19], [21]. The order for entrapment was found to be LPNPs-8> LPNPs7> LPNPs-9> LPNPs-4> LPNPs-5> LPNPs-6> LPNPs-1> LPNPs-2> LPNPs-3. The optimized formulation with highest encapsulation efficiency having high lipid/polymer to drug ratio, was used for further studies.
3.4. Particle morphology of LPNPs by scanning electron microscopy (SEM), transmission electron microscopy (TEM) and atomic force microscopy (AFM)
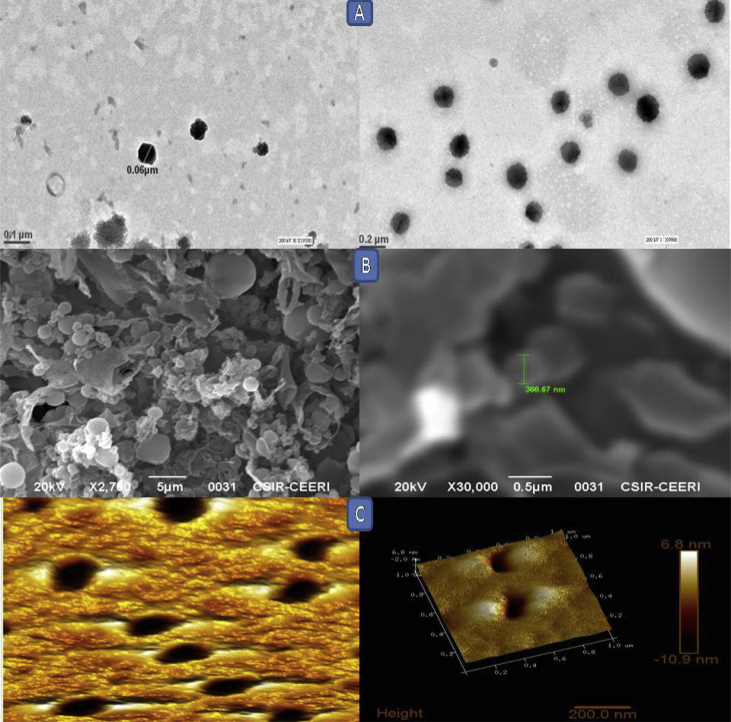
The SEM photomicrograph of the optimized formulation LPNPs-8 reveals a spherical shape having roughed and rugged surface, showed in Fig. 2 the dense and cross-linked particle in the SEM image suggested that the polymer was of high density and act as controlled release for the formulation.
Fig. 2.
TEM, SEM and AFM images of Norfloxacin loaded LPNPs (LPNPs-8): (A) TEM (B) SEM and (C) AFM.
The surface morphology of the optimized formulation lPNPs-8 was further confirmed by TEM, showed in Fig. 2 TEM showed dark spherical nanoparticles against light background. In the Fig. 2, it can be clearly observed that prepared nanoparticles were well identified with essentially spherical morphology. The images confirmed that nanoparticle were round, smooth, and free from drug crystals.
Atomic force microscopy images can reveal a lot of information about the behavior of the carriers that cannot be obtained so easily by the SEM and TEM. AFM images of nanoparticles formulation are shown in Fig. 2 that provides information about the morphological characteristics of optimized nanoparticles formulation. Further, the surface morphology demonstrated the height of the nanoparticles is 3.2 nm, 1635.481 (nm2), diameter 42.658 (nm) which clearly indicates that the interaction with the substrate resulted in a smooth spherical shape since the measured diameter of the average sized structure is ≃150 nm, same is reported by Müllera et al. Nanoparticles displayed no visible cracks or pinholes.
3.5. Attenuated total reflection- Fourier transform infrared spectroscopy (ATR-FTIR)
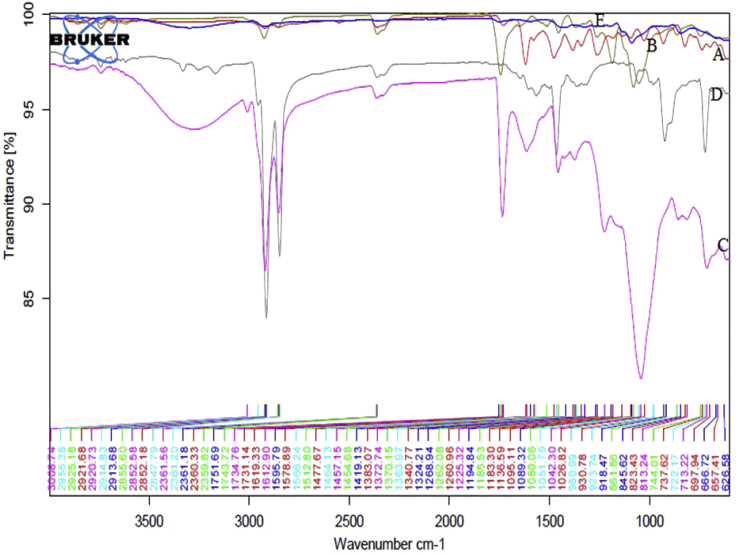
The ATR-FTIR results of pure drug, polymer and optimized formulation (LPNPs-8) are depicted in Fig. 3 ATR-FTIR spectra of the drug showed major characteristic peaks at 3855.12 cm−1 due to 0-H stretching, 2913.68 cm−1 due to = CH and aromatic —H stretching, and 2852.18 cm−1 due to CH₂ stretch, 2360.33 cm−1 due to acidic O—H stretch, 1751.69 cm−1 due to —C O stretching, 1477.67 cm−1 due to stretching of O—C—O group of acid, 1619.33 and 1578.89 cm−1 due to quinolones N—H bending, 1383.07, 1324.21 and 1260.96 cm−1 due to O—H bending, 1089.32 and 1026.82 cm−1 due to C—F bending, 930.78 cm−1 due to N—H bending and 823.48 cm−1 due to distribution of aromatic protons. The result revealed that there is no considerable change in the IR peaks of Norfloxacin and optimized formulation indicating the absence of any interaction between the drug and polymer.
Fig. 3.
Overlay of FTIR spectra of [A] Norfloxacin, [B] PLA, [C] Soya lecithin, [D] stearylamine and [E] optimized formulation LPNPs-8.
3.6. Differential scanning calorimetry (DSC)
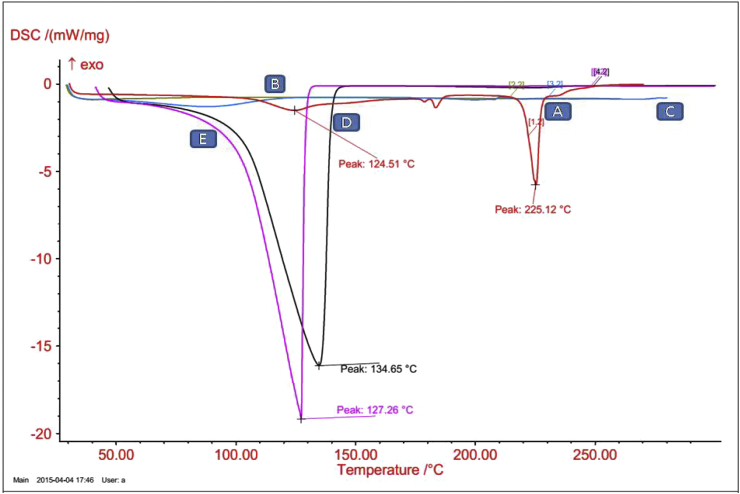
In this study, DSC was used to investigate the interactions and thermal stability between the drug, excipients, lipid and polymer. In the present study, DSC experiments involved the study of thermal behavior for pure drug (norfloxacin), polymer (PLA), soya lecithin (lipid), stearylamine and drug loaded optimized formulation (LPNPs-8). The peaks of the drug loaded lyophilized nanoparticles was similar with melting peaks and heat of enthalpy of pure drug, and an overlapped graphs were showed in Fig. 4. The DSC thermogram of Norfloxacin exhibited a sharp endothermic peak at 225.12 °C. Melting point of polymer PLA, soya lecithin, and stearylamine was seen at 40.2 °C, 87.84 °C, and 52.48 °C. The melting peaks obtained for optimized formulation LPNPs-8 was 216.14 °C, with low intensity and broad endothermic peaks compared to the pure drug. This indicates the reduced structure of drug in the lipid bilayer structures forming a new phase. Thus from the study it was concluded that the results of DSC analysis for optimized formulation LPNPs-8 indicate all the lipid and polymer components interact with each other to a great extent. Further, the results of this study were also supported by TGA.
Fig. 4.
DSC thermogram of [A] Norfloxacin, [B] PLA, [C] Soya lecithin and [D] optimized formulation LPNPs-8 gel [E] optimized nanoparticle suspension.
3.7. Thermogravimetric analysis (TGA)
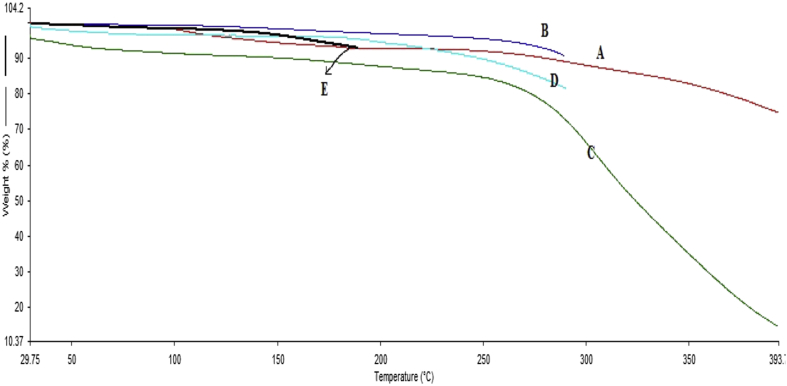
Thermogravimetric analysis (TGA) was performed for PLA, norfloxacin, and optimized formulation LPNPs-8 in order to investigate its thermal property. The TGA study was done to validate the result obtained from the DSC study and to study. The thermal degradation profile of drug, excipient, and their physical mixture and the optimized formulation are depicted in Fig. 5. It was observed in Fig. 5 that the thermal degradation profile of norfloxacin, PLA, and optimized formulation showed three regions of weight loss, which were different from a single mass loss for the norfloxacin. Each turning point marked the combustion of a new component. The TGA weight loss % of norfloxacin, PLA, optimized formulation was started at 85%, 90%, and 82% at 275–450 °C, 225–290 °C and 260–400 °C respectively. Thus, TGA was a convenient means for evaluating the weight loss of the polymers and drug.
Fig. 5.
TGA thermograph of [A] Norfloxacin, [B] PLA, [C] optimized formulation LPNPs-8, [D] Soya lecithin, [E] Stearylamine.
3.8. In-vitro drug release
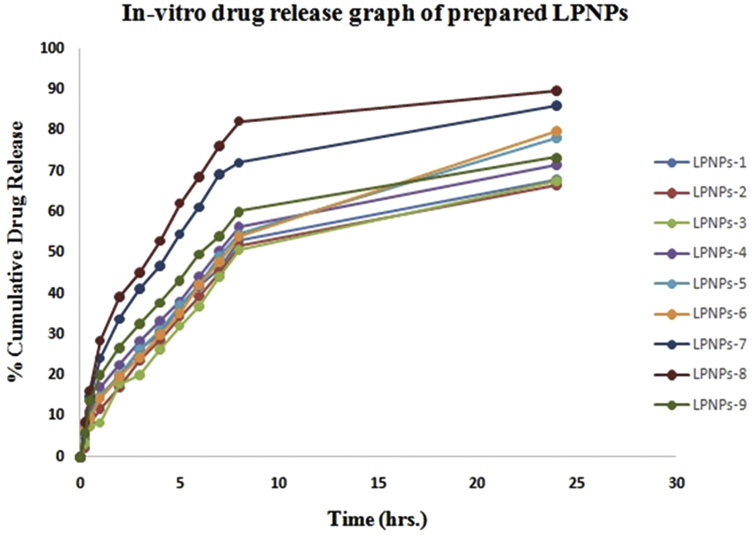
The release of Norfloxacin from the lipid-polymer hybrid core vary upon varying concentration of the lipid: polymer ratio. The in vitro release studies of various formulations are depicted in Fig. 6. The % cumulative drug release of formulations LPNPs-1 to LPNPs-9 were showed as 68.02 ± 0.42, 66.62 ± 1.32, 67.4 ± 0.46, 71.02 ± 0.06, 78.02 ± 0.62, 79.89 ± 0.86, 86.1 ± 0.72, 89.72 ± 0.21 and 77.25 ± 0.61% drug release in 24 h respectively. Among all formulations the formulation LPNPs-8 showed the maximum drug release i.e. 89.72 ± 0.21% in 24 h followed by formulation LPNPs-7 i.e. 86.1 ± 0.72% in 24 h. The cumulative percentage of drug release from nanoparticles showed initially burst release and sustained release thereafter. The sustained release is attributed to the presence of lipid bilayer made up of lipid and stearylamine that acts as a rate limiting membrane for release of the encapsulated drug. A rapid release was obtained for LPNPs-1 and LPNPs-3 formulations which have low lipid concentration. Lipid coat and stearylamine retards the release of norfloxacin in nanoparticles formulations. A moderate drug release pattern were observed in the formulation LPNPs-4 to LPNPs-6 due to moderate concentration of lipid and thus their entrapment efficiency. It can be concluded that variation in lipid and polymer concentration may modified the drug release [22], [24].
Fig. 6.
In-vitro drug release of nanoparticles formulation from LPNPs-1 to LPNPs-9.
3.9. Kinetic modeling
For the kinetic modeling, the two best formulation were opted for the release study. Both formulation LPNPs-7 and LPNPs-8 were attributed the release kinetic with Korsmeyer-Peppas model, and showed overall R2 values ranged in between 0.9316 and 0.9178 and the release exponent ‘n’ value were 0.3935 and 0.3927, showed that formulation mimic the slab like structure and releases drug through diffusion mechanism. The interpretation of kinetic data was based on value of regression coefficient (R2) near to 1, all data of the kinetic modeling is depicted in Table 5.
Table 5.
Kinetic equation parameter of the optimized nanoparticle formulations.
| Release kinetics of Optimized nanoparticle formulation | Zero order |
First order |
Higuchi |
Korsmeyer-Peppas's |
||||
|---|---|---|---|---|---|---|---|---|
| K | R2 | K | R2 | KH | R2 | n | R2 | |
| LPNPs-7 | 2.8789 | 0.6043 | −0.0231 | 0.7603 | 16.609 | 0.8629 | 0.3935 | 0.9316 |
| LPNPs-8 | 2.9059 | 0.5933 | −0.0239 | 0.7373 | 16.675 | 0.8552 | 0.3927 | 0.9178 |
3.10. Antimicrobial activity studies
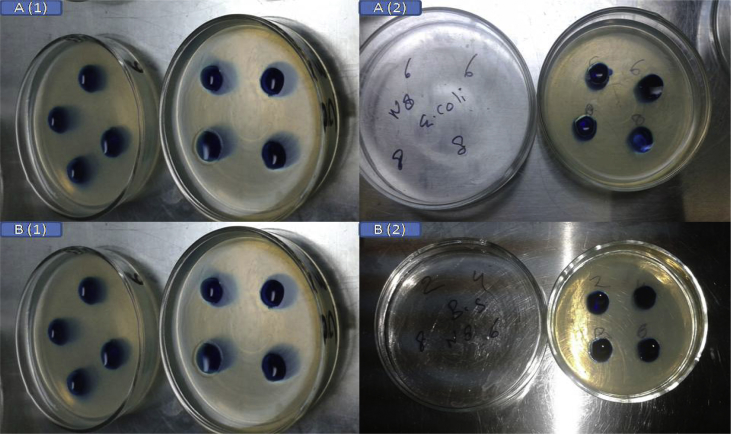
The result of the antimicrobial efficacy tests are shown in Fig. 7 and Table 6. An optimized formulation LPNPs-8 was tested for anti-microbial activity against Staphylococcus aureus and Pseudomonas aeruginosa using norfloxacin as standard, showed good antibacterial activity against gram negative bacteria (Pseudomonas aeruginosa). The formulation showed moderate activity against gram positive bacteria (Staphylococcus aureus) [7]. The study indicate that norfloxacin loaded lipid polymer hybrid nanoparticles are able to retain their antimicrobial efficacy as same as norfloxacin standard [23].
Fig. 7.
Zone of inhibition of [A] Staphylococcus aureus (1) standard (pure Norfloxacin) (2) Norfloxacin loaded nanoparticle and, [B] Pseudomonas aeruginosa (1) standard (pure Norfloxacin) (2) Norfloxacin loaded nanoparticle.
Table 6.
Antibacterial activity of optimized formulation LPNPs-8 (Zone of inhibition in mm).
| Concentration (μg/ml) | Standard ZOI (mm) | Test formulation (LPNPs-08) |
|
|---|---|---|---|
| ZOI (mm) | Percentage efficacy | ||
| Staphylococcus aureus | |||
| 2 | 8 | 7 | 87.5 |
| 4 | 14 | 13 | 92.8 |
| 6 | 16 | 15 | 93.7 |
| 8 | 17 | 15 | 88.2 |
| Pseudomonas aeruginosa | |||
| 2 | 12 | 11 | 91.6 |
| 4 | 13 | 11 | 84.6 |
| 6 | 19 | 18 | 94.7 |
| 8 | 22 | 21 | 95.4 |
3.11. Skin irritation studies
The skin irritancy were scored on Albino Wistar rat by applying blank formulation (free drug) and drug loaded LPNPs and observed each for 72 h. The moderate to severe erythema was observed in case of free norfloxacin solution, showing primary irritation index (PII) of 1.6 which strongly restricts its applicability and acceptability by the patients. The LPNPs-8 formulation caused relatively less irritation showing PII of 1.0. Norfloxacin is associated with noticeable skin irritation, which strongly restricts its applicability and acceptability by the patients. Ideally, the norfloxacin containing nanoparticles should be able to reduce irritation. The reduction in the drug irritation may be due to the drug behaving itself as antimicrobial properties. Thus LPNPs-8 could be considered safe for topical formulation.
3.12. Stability studies
The optimizations of physical and chemical stability are essential parameter for the preparation of LPNPs. The stability of vesicles is one of the major problems in the formulation of LPNPs because of accumulation and leaching of drug from the polymer core and lipid layers. The results of physical stability of optimized formulation LPNPs-8 in various intervals of time are shown in Table 7. LPNPs-8 was selected for the stability study and its suspension was divided into two batches and kept in sealed vials (10 ml) at refrigerator temperature 4 ± 2 °C/60 ± 5% RH and room temperature 25 ± 2 °C/60 ± 5% RH. As the function of time, after 7, 15, 30, 60 and 90 days of storage, lyophilized nanoparticles were redispersed in Millipore water, sonicated for 10–15 s and characterized for morphology, particle size, zeta potential, and PDI and entrapment efficiency. All the analysis was carried out in triplicate and the results are reported as mean ± SD. The nanoparticles which were kept at RT showed considerable increase in particle size (from 178.6 ± 3.7 nm to 187.04 ± 1.7 nm after 7 days) and decrease in % encapsulation efficiency from 89.72 ± 0.23 to 84.54 ± 3.54. This was allied with significant increase in PDI from 0.206 ± 0.36 to 0.225 ± 0.74. Thus, these results confirm the particle aggregation and leakage of the encapsulated drug. After 7 days the RT samples had too much aggregation with increasing in particle size with 187.04 ± 1.7, 205.36 ± 4.6, 235.4 ± 2.54, 275.4 ± 4.05 and 370.7 ± 3.43 with decrease in % entrapment efficiency 84.54 ± 3.54, 80.65 ± 5.09, 78.88 ± 2.79, 76.54 ± 3.33 and 72.42 ± 2.54 after day 15, 30, 60 and 90 respectively. Nanoparticles stored at 4 °C were quite stable. The results were in accordance with the earlier reported studies [25], [26], [27]. Thus, the optimized storage condition for formulation LPNPs-8 was found to be stable at 4 ± 2 °C/60 ± 5% RH.
Table 7.
Stability study of optimized formulation LPNPs-8.
| Formulation LPNPs-8 | |||||
|---|---|---|---|---|---|
| Time (days) | Microscopic observation | % Encapsulation efficiency | Particle size (nm) | PDI | Zeta potential (mV) |
| At 4 °C/60 ± 5% RH (n = 3) | |||||
| Initial | Smooth spherical vesicles | 89.72 ± 0.23 | 178.6 ± 3.7 | 0.206 ± 0.36 | +41.5 ± 3.4 |
| 7 | Smooth spherical vesicles | 87.11 ± 2.51 | 189.7 ± 1.87 | 0.227 ± 0.78 | +39.43 ± 2.2 |
| 15 | Smooth spherical vesicles | 84.62 ± 1.98 | 197.45 ± 5.2 | 0.279 ± 0.32 | +36.16 ± 2.5 |
| 30 | Smooth spherical vesicles | 81.82 ± 3.41 | 205.8 ± 3.36 | 0.305 ± 0.63 | +34.08 ± 4.5 |
| 60 | Rough spherical vesicles | 80.03 ± 6.2 | 219 ± 4.32 | 0.328 ± 0.78 | +33.62 ± 3.6 |
| 90 | Rough spherical vesicles | 79.29 ± 1.94 | 274 ± 3.66 | 0.353 ± 0.71 | +30.3 ± 3.33 |
| At 25 °C/60 ± 5% RH (n = 3) | |||||
| Initial | Smooth spherical vesicles | 89.72 ± 0.23 | 178.6 ± 3.7 | 0.206 ± 0.36 | +41.5 ± 3.4 |
| 7 | Smooth spherical vesicles | 84.54 ± 3.54 | 187.04 ± 1.7 | 0.225 ± 0.74 | +39.66 ± 3.2 |
| 15 | Rough spherical vesicles | 80.65 ± 5.09 | 205.36 ± 4.6 | 0.271 ± 0.54 | +35.43 ± 1.98 |
| 30 | Rough spherical vesicles | 78.88 ± 2.76 | 235.4 ± 2.54 | 0.334 ± 0.19 | +32.76 ± 1.55 |
| 60 | Agglomerate vesicles | 76.54 ± 3.33 | 275.4 ± 4.05 | 0.402 ± 0.72 | +29.12 ± 4.5 |
| 90 | Agglomerate vesicles | 72.42 ± 2.54 | 370.7 ± 3.43 | 0.471 ± 0.29 | +23.09 ± 3.4 |
Data represent mean ± SD; n = 3.
4. Conclusion
Lipid polymer hybrid nanoparticles prepared by single emulsification solvent evaporation technique showed high drug encapsulation efficiency with desired particle size, polydispersity index and better drug release profile with higher stability at 4 ± 2 °C. These nanoparticles have been found to exhibit desired antibacterial activity against gram positive and negative bacteria.
The results justify the formulation of LPNPs containing Norfloxacin is a good alternative and suitable carrier for the treatment of burn bacterial infection. The data obtained from article expect the safe, effective and very efficient as a drug carrier for topical drug delivery system. Based on this future research works needed to bring the LPNPs more closely to its clinical realization.
Declaration of interest
The authors report no conflict of interest.
Acknowledgement
This research was carried out at Banasthali University Rajasthan. The authors would like to thank Department of Chemistry & Department of Physics of Banasthali University, for their kind support during the work.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Bratu I., Borodi G., Kacso I., Moldovana Z., Filip C., et al. New solid form of Norfloxacin: structural studies. Spectroscopy. 2011;25:53–62. [Google Scholar]
- 2.Xiao Y.W. Strategies for optimizing polymer-lipid hybrid nanoparticle-mediated drug delivery. Expert Opin. Drug Del. 2016;13:609–612. doi: 10.1517/17425247.2016.1165662. [DOI] [PubMed] [Google Scholar]
- 3.Dua K., Kavita P., Ramana M.V. Preparation, characterization and in vitro evaluation of accecloffenac solid dispersions. Ars Pharm. 2010;51:57–76. [Google Scholar]
- 4.Liua Y., Pana J., Feng S.S. Nanoparticles of lipid monolayer shell and biodegradable polymer core for controlled release of paclitaxel: effects of surfactants on particles size, characteristics and in vitro performance. Int. J. Pharmaceu. 2010;395:243–250. doi: 10.1016/j.ijpharm.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Gurny R., Peppas N., Harrington D., Banker G. Development of biodegradable and injectable latices for controlled release of potent drugs. Drug Dev. Ind. Pharm. 1981;7:1–25. [Google Scholar]
- 6.Cheow W.S., Hadinoto K. Factors affecting drug encapsulation and stability of lipid–polymer hybrid nanoparticles. Colloids Surf. B. 2011;85:214–220. doi: 10.1016/j.colsurfb.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 7.Agrawal U., Gupta M., Vyas S.P. Capsaicin delivery into the skin with lipidic nanoparticles for the treatment of psoriasis. Artif. Cells Nanomed. Biotechnol. 2013 doi: 10.3109/21691401.2013.832683. [DOI] [PubMed] [Google Scholar]
- 8.Sahu A.K., Verma A. Development and statistical optimization of chitosan and eudragit based gastroretentive controlled release multiparticulate system for bioavailability enhancement of metformin HCl. J. Pharmaceu. Investig. 2016;46:239–252. doi: 10.1007/s40005-016-0229-0. [DOI] [Google Scholar]
- 9.Box George E.P., Hunter J.S., Hunter W.G. second ed. Wiley; NewYork: 1978. Statistics for Experimenters: Design, Innovation, and Discovery; p. 510. [Google Scholar]
- 10.Li Y., Wong H.L., Shuhendler A.J., Rauth A.M., et al. Molecular interactions, internal structure and drug release kinetics of rationally developed polymer-lipid hybrid nanoparticles. J. Contr. Rel. 2008;1:60–70. doi: 10.1016/j.jconrel.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Shah P., Desai P., Singh M. Effect of oleic acid modified polymeric bilayered nanoparticles on percutaneous delivery of spantide II and ketoprofen. J. Contr. Rel. 2012;158:336–345. doi: 10.1016/j.jconrel.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ranjan O.P., Shavi G.V., Nayak U.Y., Arunuqam K., Averineni R.K., et al. Controlled release chitosan microspheres of mirtazapine: in vitro and in vivo evaluation. Arch. Pharm. Res. 2011;11:1919–1929. doi: 10.1007/s12272-011-1112-1. [DOI] [PubMed] [Google Scholar]
- 13.Babu R.J., Kanikkannan N., Kikwai L., Ortega C., Andega S., et al. The influence of various methods of cold storage of skin on the permeation of melatonin and nimesulide. J. Control Rel. 2003;86:49–57. doi: 10.1016/s0168-3659(02)00368-1. [DOI] [PubMed] [Google Scholar]
- 14.Zhao P., Wang H., Yu M., Liao Z., Wang X., et al. Paclitaxel loaded folic acid targeted nanoparticles of mixed lipid-shell and polymer-core: In vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2012;81:248–256. doi: 10.1016/j.ejpb.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Narvekar M., Xue H.Y., Wong H.L. A novel hybrid delivery system: polymer-oil nanostructured carrier for controlled delivery of highly lipophilic drug all-trans-retinoic acid (ATRA) Pharm. Nano. Tech. Int. J. Pharmaceu. 2012;436:721–731. doi: 10.1016/j.ijpharm.2012.07.042. [DOI] [PubMed] [Google Scholar]
- 16.Jubie S., Rajeshkumar R., Yellareddy B., Siddhartha G., Sandeep M., et al. Microwave assisted synthesis of some novel benzimidazole substituted Fluoroquinolones and their antimicrobial evaluation. J. Pharmaceu. Sci. Res. 2010;(2):69–76. [Google Scholar]
- 17.ICH Q1A (R2), Stability Testing of New Drug Substances and Products, International Conference on Harmonization. U.S. Department of Health and Human Service Food and Drug Administration; 2003. pp. 4–20. CPMP/ICH/2736/99. [Google Scholar]
- 18.Liua Y., Pana J., Feng S.S. Nanoparticles of lipid monolayer shell and biodegradable polymer core for controlled release of paclitaxel: effects of surfactants on particles size, characteristics and in vitro performance. Int. J. Pharm. 2010;395:243–250. doi: 10.1016/j.ijpharm.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Ling G., Zhang P., Zhang W., Sun J., Meng X., et al. Development of novel self-assembled DS-PLGA hybrid nanoparticles for improving oral bioavailability of vincristine sulfate by P-gp inhibition. J. Contr. Rel. 2010;148:241–248. doi: 10.1016/j.jconrel.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Trapani A., Laquintana V., Denora N., Lopedota A., Cutrignelli A., et al. Eudragit RS 100 microparticles containing 2-hydroxypropyl-beta-cyclodextrin and glutathione: physicochemical characterization, drug release and transport studies. Eur. J. Pharm. Sci. 2007;30:64–74. doi: 10.1016/j.ejps.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Song X., Zhao Y., Wu W., Bi Y., Cai Z., Chen Q. PLGA nanoparticles simultaneously loaded with vincristine sulfate and verapamil hydrochloride: systematic study of particle size and drug entrapment efficiency. Int. J. Pharm. 2008;35:320–329. doi: 10.1016/j.ijpharm.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 22.Hasan W., Chu K., Gullapalli A., Dunn S.S., et al. Delivery of multiple siRNAs using lipid-coated PLGA nanoparticles for treatment of prostate cancer. Nano Lett. 2011;12:287–292. doi: 10.1021/nl2035354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harer S.L., Rajurkar V.G., Patil P., Harer P.S., et al. Synthesis, characterization and anti-microbial evaluation of some 2-Iodo-N'-[(1e)-substituted phenylmethylidene] benzohydrazide analogues. Int. J. Pharmaceu. Sci. Drug Res. 2010;2:134–136. [Google Scholar]
- 24.Shah R., Eldridge D., Palombo E., Harding I. Optimisation and stability assessment of solid lipid nanoparticles using particle size and zeta potential. J. Phys. Sci. 2014;1:59–75. [Google Scholar]
- 25.Dixit A.R., Rajput S.J., Patel S.G. Preparation and bioavailability assessment of SMEDDS containing valsartan. AAPS Pharm. Sci. Tech. 2010;11:314–321. doi: 10.1208/s12249-010-9385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narvekar M., Xue H.Y., Wong H.L. A novel hybrid delivery system: polymer-oil nanostructured carrier for controlled delivery of highly lipophilic drug all-trans-retinoic acid (ATRA) Pharm. Nano Tech. Int. J. Pharmaceu. 2012;436:721–731. doi: 10.1016/j.ijpharm.2012.07.042. [DOI] [PubMed] [Google Scholar]
- 27.Müllera R.H., Radtkeb M., Wissingb S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Del. Rev. 2002;1:S131–S155. doi: 10.1016/s0169-409x(02)00118-7. [DOI] [PubMed] [Google Scholar]