Abstract
The present study explores the possibilities of using locally available inexpensive waste prawn shell derived chitin reinforced and bioabsorbable polylactic acid (PLA) laminated composites to develop new materials with excellent mechanical and thermal properties for implantable application such as in bone or dental implant. Chitin at different concentration (1–20% of PLA) reinforced PLA films (CTP) were fabricated by solvent casting process and laminated chitin-PLA composites (LCTP) were prepared by laminating PLA film (obtained by hot press method) with CTP also by hot press method at 160 °C. The effect of variation of chitin concentration on the resulting laminated composite's behavior was investigated. The detailed physico-mechanical, surface morphology and thermal were assessed with different characterization technique such as FT-IR, XRD, SEM and TGA. The FTIR spectra showed the characteristic peaks for chitin and PLA in the composites. SEM images showed an excellent dispersion of chitin in the films and composites. Thermogravimetric analysis (TGA) showed that the complete degradation of chitin, PLA film, 5% chitin reinforced PLA film (CTP2) and LCTP are 98%, 95%, 87% and 98% respectively at temperature of 500 °C. The tensile strength of the LCTP was found 25.09 MPa which is significantly higher than pure PLA film (18.55 MPa) and CTP2 film (8.83 MPa). After lamination of pure PLA and CTP2 film, the composite (LCTP) yielded 0.265–1.061% water absorption from 30 min to 24 h immerse in water that is much lower than PLA and CTP. The increased mechanical properties of the laminated films with the increase of chitin content indicated good dispersion of chitin into PLA and strong interfacial actions between the polymer and chitin. The improvement of mechanical properties and the results of antimicrobial and cytotoxicity of the composites also evaluated and revealed the composite would be a suitable candidate for implant application in biomedical sector.
Keywords: Chitin, PLA, LCTP composite, Biomedical application, Bone implants
Graphical abstract
Highlights
-
•
A novel laminated composite has been prepared from chitin and PLA.
-
•
The detailed study revealed that 5% chitin in PLA showed enhanced physico-mechanical properties.
-
•
The composite showed significantly lowered water absorptivity and enhanced antimicrobial sensitivity.
-
•
5% chitin-PLA laminated composite can be a suitable candidate for biomedical application in bone regeneration.
1. Introduction
The development of biomaterials (biodegradable and bioabsorbable) with the desired characteristics to help in the recovery of tissues damaged by accident or human disease is one of the greatest research challenges [1], [2]. Biomaterials have the capability to establish an intimate contact with living tissues [3], [4]. Being a complex and dynamic living tissue bone provides mechanical support for the body and exhibits elastic modulus between 10 and 30 GPa. Over the years, different metallic alloys have been widely used for fracture fixations owing to the good mechanical properties and biocompatibility. But the problem arose firstly, due to the mismatch in elastic moduli between the bone and metallic implants for bone healing And secondly the risk of forming corrosion products by the metal alloy which in turn causes inflammation and infection in the body tissue [5]. By using biodegradable polymer based materials, complications like mismatch of elastic moduli, corrosion, release of metal ions and stress shielding associated with metal implants can be eliminated [6], [7], [8]. Moreover, these biodegradable materials make it highly attractive for biological and medical applications as well as it can be broken down into smaller fractions in physiological environments by aerobic or anaerobic microorganisms, or biologically active processes (e.g. enzyme reactions) or passive hydrolytic cleavage [9], [10], [11], [12]. The most commonly investigated and widely used synthetic biodegradable polymers are polyglycolide/glycolic acid (PGA), polylactide/lactic acid (PLA), poly-ε-caprolactone (PCL), chitin, chitosan, gelatin etc [6], [7], [8].
Polylactic acid (PLA) is a thermoplastic, biocompatible, biodegradable polymer and has an extensive mechanical property profile which has been demonstrated to be a suitable bioabsorbable polymer for fixation devices such as resorbable plates and screw, surgical implant materials, drug delivery systems, and also as a porous scaffold for the growth of neo-tissue. Three-dimensional porous scaffolds of PLA have been created for culturing different cell types, using in cell-based gene therapy for cardiovascular diseases; muscle tissues, bone and cartilage regeneration and other treatments of cardiovascular, neurological, and orthopedic condition [13], [14], [15]. Chitin (β-(1→4)—N-acetyl-D-glucosamine) is the second most important natural polymer in the world and its main sources exploited are two marine crustaceans, shrimp and crabs. It is less toxic, biodegradable in nature, capable of wound healing and also inert in the gastrointestinal tract of mammals [16]. It is widely used to immobilize enzymes and whole cells; enzyme immobilization has applications in the food industry, for example, clarification of fruit juices and processing of milk when α- and β-amylases or invertase are grafted on chitin [17]. However, the main development of chitin film and fiber is in medical and pharmaceutical applications as wound-dressing material [18], [19], [20], [21] and controlled drug release [22], [23]. To regenerate bone tissue,α and β-Chitin membranes, have been developed [24]. In one study, Li et al. reported the development of nHAp/collagen/PLLA scaffolds reinforced with chitin nanofibers for the enhancement of mechanical strength [25]. In a recent study the effects of chitosan on the physicochemical and antimicrobial properties of PLA films were studied, but there was no report in the literature that describes the influence of chitin on the properties of PLA [26].
In the present we wish to fabricate laminated chitin-PLA bio absorbable composite by hot press method to use in artificial bone implant. The complete characterization of the raw materials and the composite have done and described in the following section to clinch the application of the proposed materials as a simulated form of human bones.
2. Materials and methods
2.1. Materials
The prawn shells (Litopenaeus setiferus) were purchased from a local market. They were washed and crushed in a sample mill (SM-450TR, mrc scientific instruments). Poly (lactic acid) (PLA), Nature WorksTM 3251 D, was supplied by NatureWorks LLC, Minneapolis, USA. The material has a density of 1.25 g/cm3, glass transition temperature (Tg) of 58 °C and melting point of 160 °C. The molecular weight (Mw) of the PLA is between 195,000–205,000 g/mol. Sodium hydroxide pellet (E. Merck, Germany), 37% hydrochloric acid (E. Merck, Germany), sodium chlorite flakes (Loba Chemie Pvt. Ltd. India), extra pure 1,4-Dioxane (E. Merck, Germany) and glacial acetic acid (E. Merck, Germany) were purchased and used in this work as obtained.
2.2. Extraction of chitin from prawn shell
The prawn shells were washed, dried and milled. The pulverized shell was stirred by magnetic stirrer with 1M NaOH for 3 h and treated with 1% NaClO2 and glacial acetic acid at 80 °C for completing deproteinization and bleaching step respectively. Bleached prawn shell was then demineralized by using 3NHCl. Finally, the demineralized shell was washed and dried at 60 °C in an oven to obtain dried chitin.
2.3. Preparation of hot pressed PLA film
PLA pellets were dried in a vacuum oven at 60 °C for 4 h. Accurate amount of PLA pellets were weighed and hot pressed in two plates of a hot press (Weber Pressen Hydraulic Press, WEBER-HYDRAULI K, Germany) for 2–3 min after reaching process temperature of 160 °C. Thin film of approximately 0.15 mm thick was prepared by compression molding of PLA pellets between Teflon® lined mild steel (MS) platens. The sample in hot press were pressed at 50-kN for 2–3 min at a pressure of 3 MPa and quenched at cold water. Thin films of 6 inch × 6 inch were obtained in this processes.
2.4. Preparation of chitin reinforced PLA film (CTP)
Chitin powder was dispersed well in 20 mL 1,4- dioxane solution by ultrasonic sonication. In a typical procedure 4.0 g PLA pellets and 0.20 g chitin whiskers were stirred at 60 °C for 30 min in 20 mL 1,4-dioxane. The mixture was then poured in silicone paper and dried in drier at 40 °C for 3 h at constant air flow and CTP2 was obtained. Depending on the various amount of chitin and PLA beads, the films were named as CTP1, CTP2, CTP3, CTP4 and CTP5 (as shown in Table 1).
Table 1.
Chitin-PLA film (CTP) of different compositions.
| PLA Films containing different wt% of chitin | Amount of Chitin (gm) | Amount of PLA (gm) | Nomenclature |
|---|---|---|---|
| 1% Chitin-PLA Film | 0.04 | 4.00 | CTP1 |
| 5% Chitin-PLA Film | 0.20 | 4.00 | CTP2 |
| 10% Chitin-PLA Film | 0.40 | 4.00 | CTP3 |
| 15% Chitin-PLA Film | 0.60 | 4.00 | CTP4 |
| 20% Chitin-PLA Film | 0.80 | 4.00 | CTP5 |
2.5. Preparation of laminated CTP2-PLA composite (LCTP)
The laminated chitin PLA-PLA composite (LCTP) has been prepared by hot-press method. In a typical procedure 0.15 mm PLA and chitin-PLA (CTP2) containing 5% chitin was heat pressed with 5 kN load at 160 °C under 3 MPa for 1 min, 5 MPa for 2 min, and 10 MPa for 5 min to liberate the trapped air bubbles followed by quenched with cold water. After cooling the final laminated film was obtained having a thickness of 0. 8 mm and 6 inch × 6 inch dimension film was obtained which then kept in desiccator for further characterization.
2.6. Characterization
2.6.1. Fourier transform infrared spectroscopy (FTIR) analysis
Fourier transform infrared (FTIR) spectra (transmission) of chitin, hot pressed PLA film, CTP2 film and LCTP were measured by a FTIR spectrophotometer (Model 01831 Shimadzu Corporation, Japan).
2.6.2. Thermal analysis
To analyze the thermal behavior, thermogravimetric analysis (TGA)/Differential Thermal Analysis (DTA) of chitin, hot pressed PLA film, CTP5 film and LCTP were performed in aluminum cell under nitrogen flow from room temperature to 600 °C at a rate of 20 °C/min with a TG/DTA EXTAR 6000 STATION (Seiko Instrument Inc. Japan TGA-50) instrument. A differential scanning calorimeter (DSC-60, Shimadzu Corp., Japan) was used to determine the glass transition and/or decomposition temperature of the materials. Thermo-mechanical analyses (TMA) of all the aforesaid samples were carried out by using a Shimadzu TMA-50 analyzer.
2.6.3. Scanning electron microscopy (SEM)
A high-performance scanning electron microscope (SEM) (JEOL JSM-6490) with a high resolution of 3.0 nm at 20 kV was used to determine the surface morphological characteristics of chitin, CTP2 film, and LCTP. A small portion of the samples was fixed on conductive carbon tape and mounted on the support and then sputtered with an approximately 5 nm layer of graphite.
2.6.4. X-ray diffraction (XRD) analysis
For the analysis of structure and crystallinity of chitin, CTP2 and LCTP, XRD analysis was carried out by an X-ray diffractometer (model D8 Philips Advance X-ray diffractometer). The monochromatic Cu α1 line of wavelength ?? = 1.54 and a graphite monochromator with a current of 30 mA, and voltage of 30 mV was used for the analysis of chitin and composites. The diffraction intensity was in the range of 5°–30° of 2?? (Bragg angle) and the scanning speed was 0.02° sec−1.
2.6.5. Mechanical properties
Tensile strength (TS) and percent elongation at break (Eb) and E-modulus of hot pressed PLA film, CTP films and LCTP composite were analyzed by Universal Testing Machine (Hounsfield, Model H50 Ks 0404, UK).
2.6.6. Water uptake test
Water uptake of the PLA film, CTP film and LCTP composite were calculated to observe the water absorptivity and sustainability in water. The water absorption was determined by using the ASTM test method D570-81.
2.6.7. Antimicrobial sensitivity analysis
The measurement of the antimicrobial activity of CTP2 film was done by both Mueller-Hinton Agar disk diffusion susceptibility testing method and Kirby-Bauer method. Two bacterial strains i.e., selected gram positive (Staphylococcus aureous) and gram negative (Escherichia coli) were used to asses susceptibility pattern. The media used for antimicrobial activity was poured into a sterile petri dish and allowed to cool. Then the test culture was inoculated properly onto the media. The samples were autoclaved for 2 h and 10 m to remove any bacterial contamination. The plates were incubated overnight at 37 °C and the inhibition zone was measured to evaluate the antimicrobial activity of the samples.
3. Results and discussion
3.1. Characterization of chitin-PLA films (CTP)
3.1.1. FTIR analysis
FTIR data revealed very important information about the interactions between the chitin–
PLA matrix. The FTIR spectra of chitin, PLA, 5% chitin - PLA (CTP2) and laminated composite LCTP are shown in Fig. 1. . In the case of chitin, the main absorption bands were assigned at 3445 cm−1 (O—H stretching vibrations overlapping with the N—H stretching vibrations), 1643 cm−1 (C O stretching vibrations of amide bonds), 1321 cm−1 (bending vibration of —CH3) and 1562 cm−1 (N—H bending vibrations of amide bonds, which overlapped with C O stretching vibrations of amide bonds which overlapped with C O stretching vibrations of amide bonds).
Fig. 1.
FTIR spectra of (a) Chitin, (b) pure PLA, (c) 5% Chitin Reinforced PLA (CTP2) film and (d) laminated chitin-PLA composite (LCTP).
The FTIR spectrum of pure PLA film displayed characteristic bands at 1755 cm−1 and 1078 cm−1, which are attributed to the backbone ester group of PLA [27], [28]. The —C—O— stretching vibration from the ester units was observed at 1078 cm−1. The peak at 3505 cm−1 indicates the terminal —OH of L-lactic acid. The bands at 3001 cm−1 and 2885 cm−1 were assigned to the —C—H asymmetric and symmetric vibration of —CH3 groups in the side chains. The band at 2945 cm−1 was attributed to the —CH— groups in the main chain of PLA. From the FTIR spectra of the chitin and PLA (CTP2), the bands around 1654, 1599 and 1550 cm1 were assigned to amide I (C O), amino (—NH2) and amide II (—NH) of chitin in the organic matrix, respectively. However, there was a significant difference between the two spectra: the characteristic peak of carbonyl group (C O) of PLA at 1745 cm1 nearly disappeared in the FTIR spectrum of CTP2. The peak at 3444 cm−1 corresponding to —OH group of chitin and that was significantly shifted to higher wave number at 3502 cm−1 in CTP2. Actually, three peaks in the range of 3650–3400 cm−1 were observed due to —OH group of PLA and —OH group of chitin. No significant changes were observed in the FTIR-spectra before and after blending [28]. It was also observed in Fig. 4 that an absorption band at 1036 cm−1 is due to the stretching vibration for —C—O—C— of the glucosamine ring of chitin. Chitin gave peak at 2916 cm−1 for the −C—H stretching vibration on the other hand in case of LCTP, it has shifted to 2997 cm−1. Another characteristic band at 3001 cm−1 of PLA film has also been shifted to lower wave number of 2997 cm−1 For LCTP.
Fig. 4.
Comparative XRD analysis of (a) Chitin, (b) 5% Chitin-reinforced PLA film (CTP2) and (c) laminated chitin-PLA composite (LCTP).
3.1.2. Morphological study
Fig. 2 shows the SEM photographs of prawn shell derived chitin which exhibited rough and thick surface morphology under electron microscopic examination at 50 × magnification (in Fig. 2 A). At higher magnification (3000 × ), chitin from shrimp (Fig. 2B) was found to be distinctly arranged in a microfibrillar crystalline structure. Similar observation was also observed in the literature [29], [30]. The PLA film is transparent [31] and heat treated laminated PLA film surface was observed uniform. Fig. 3A and B represented SEM images of CTP1 and CTP2. CTP2 exhibited a relatively smooth surface, indicating a high compatibility between PLA and chitin or a good uniform dispersion of chitin in the reinforced film. The film also showed better entanglement of chitin and PLA in the surface. As a result CTP2 film have chosen to make laminated composite sheet by heat pressed of PLA film with CTP2.
Fig. 2.
SEM images of chitin at low and high magnification.
Fig. 3.
SEM images of chitin-PLA film (A) 1% chitin and (B) 5% chitin.
3.1.3. XRD analysis
Fig. 4 demonstrated the XRD spectrum of chitin, CTP2 and LCTP. It is observed that, chitin showed peaks at 2θ = 10° and 19.3° indicating the existence of α-chitin as reported by Cho et al. [32]. The peaks were prominent and sharp that indicates the crystalline nature of chitin structure (Fig. 4). In a previous study, the XRD pattern of pure PLA was described by Mihai et al. [33] and the XRD data of pure PLA was given in Fig. S1 in the supporting information. It was observed that CTP2 produced three peaks in its XRD spectrum at 16.50°, 19° and 22.3°. The peaks CTP2 at 2θ = 16.5° and 22.3° for CTP2 indicated the presence of α-chitin structure [32]. All these results revealed that the chitin structure was retained after the reinforcing process. The highest crystallinity of CTP2 film was compared to pure PLA film and showed that chitin present in CTP2 has given rise to the crystallinity to CTP2 film. This may be due to the fact that chitin acts as a nucleating agent that accelerates the crystallization of PLA. Moreover, the percentage of crystallinity in PLA (64.07%) has been observed to decrease in CTP2 (48.54%) (as given in the supporting documents Fig. S1 and S2).
3.1.4. Mechanical properties
3.1.4.1. Tensile properties
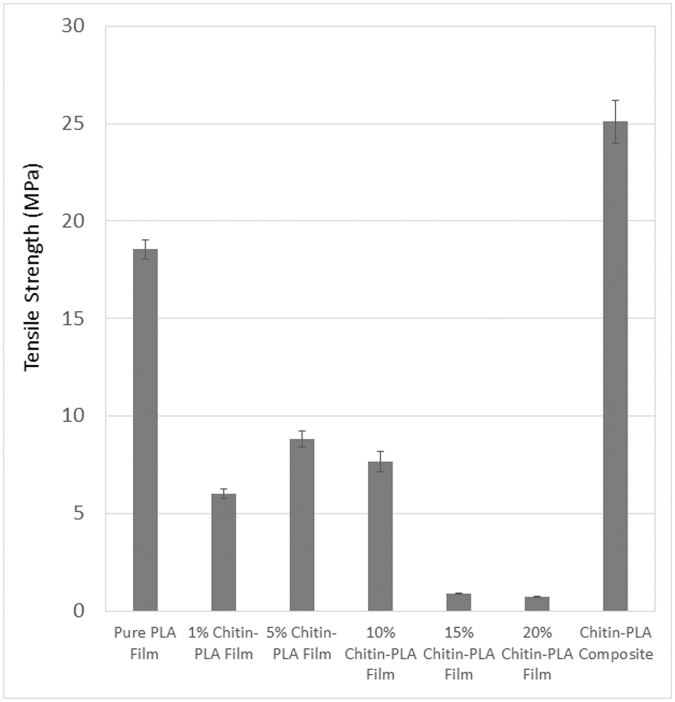
The tensile properties of the CTP films (CTP1, CTP2, CTP3, CTP4 and CTP5) are given in Fig. 5. The results demonstrated that solution casted PLA films with various chitin content showed less tensile strength than of pure PLA film. It is also observed that tensile strength increases with increasing chitin concentration from 1% (CTP1) to 5% (CTP2). The increased tensile strength of the films with the increasing chitin content indicated good dispersion of chitin into PLA and strong interfacial actions between the polymer and chitin.
Fig. 5.
Comparison of tensile strength of pure PLA film, Chitin reinforced PLA films (CTP) and laminated chitin-PLA composite (LCTP).
The orientation of chitin effectively occurred in PLA matrix and, therefore, the TS rises. According to Dufresne et al. [34], the overall mechanical performance of composites, depends on adhesion and compatibility between the polymer matrix and the additives or reinforcement and crystallinity of the polymer matrix and reinforcement. Hence the increase of TS upon the addition of chitin is probably due to good dispersion and crystalline nature of the chitin.
Further increase of chitin content in the PLA made the films brittle and the films did not show enough strength since CTP5 film showed the lowest tensile strength of 0.719 MPa.
3.1.4.2. Elongation at break
Elongation at break (Eb) of PLA film was found 4.65%. For 1% chitin-PLA (CTP1), 5% chitin-PLA (CTP2), 10% chitin-PLA (CTP3), 15% chitin-PLA (CTP4) and 20% chitin-PLA (CTP5) film Eb were observed as 5.57%, 7.36%, 7.36%, 3.5% and 2.5% respectively (Fig. 6).
Fig. 6.
Comparison of elongation break of pure PLA film, Chitin reinforced PLA films and LCTP.
3.1.4.3. E-modulus
The E-modulus of the pure PLA and CTP films (CTP1, CTP2, CTP3, CTP4 and CTP5) are depicted in Fig. 7. Among the CTP films, the highest E-modulus was observed for CTP2, on the other hand pure PLA film showed E-modulus of approximately 350 MPa, which is about 2.5 times higher than CTP2. From the above data 5% chitin-PLA (CTP2) film was selected for lamination as it showed the best tensile properties among the 5 different compositions of chitin reinforced PLA films.
Fig. 7.
Comparison of E-modulus of pure PLA film, Chitin reinforced PLA films (CTP) and LCTP.
3.1.5. Thermogravimetric analysis
The TGA curves as depicted in Fig. 8 showed that chitin had initial weight loss due to the removal of moisture, however PLA (curve 'a' in Fig. 8) and CTP2 film (curve 'b' in Fig. 8) had no initial loss due to moisture because PLA is hydrophobic in nature. After the addition of chitin (curve 'c' in Fig. 8) no significant loss was observed because chitin content is very low compared to PLA. Fig. 8 showed that the onset temperature for chitin, PLA and CTP2 are at around 343 °C, 352 °C and 342 °C respectively. The DTA curve of chitin showed an endothermic peak at around 366 °C due to thermal degradation. The curves of pure PLA film and CTP2 film revealed that both of the melting point temperature (Tm) is in the range of 168 °C. From the derivative curves (DTG) (as shown in Fig. S3 and S4 in the supporting information), it is observed that the maximum weight loss (Tmax) of chitin, pure PLA film and CTP2 occurred at temperature 379 °C, 367 °C and 364 °C respectively.
Fig. 8.
TGA thermogram (a) PLA, (b) CTP2, (c) Chitin and (d) laminated chitin-PLA composite (LCTP).
3.1.6. Thermomechanical analysis (TMA)
As the temperature increased the PLA film softens as it goes through its glass transition temperature (Tg) (Fig. 9 A). The softening of PLA composite started at 58.9 °C and contraction continued up to 100 °C. Then expansion started and continued up to 137.9 °C and after that it melted down. So, from this phenomena 58.9 °C is considered as its Tg. This expansion occurred because the film sample still contained some crystalline PLA in it. TMA of CTP2 are described in Fig. 9B. Softening of CTP2 film started at 51.9 °C and contraction continues up to 118.1 °C (Fig. 9B). Then expansion started and continues up to 137.3 °C and then molten down.
Fig. 9.
TMA Analysis of (a) pure PLA, (b) 5% chitin reinforced PLA and (c) laminated chitin-PLA composite.
3.1.7. Water uptake test
Fig. 10 showed the water absorption behavior of PLA film, 5% chitin-PLA film (CTP) and laminated composite (LCTP) from 30 min to 24 h duration. Interestingly pure PLA film showed 1–3% water absorption up to 24 h although it has hydrophobic nature. After the incorporation of chitin (CTP2 film) the graph displayed less water absorption than pure PLA film. It absorbed 0.2–1.5% water in a 24 h period. The absorption of water decreases after 2 h. The films containing higher percentage of chitin were very much brittle and non-uniform in character and thus absorbed more water than pure PLA film.
Fig. 10.
Water uptake of PLA film, 5% Chitin reinforced PLA film (CTP2) and laminated chitin-PLA composite (LCTP).
3.1.8. Antimicrobial sensitivity analysis
After incubation it was found that the CTP2 film had antimicrobial effect as clear zone (10 mm diameter) of inhibition was found underneath and in the vicinity of these films placed onto the Escherichia coli bacterial cultured by Kirby-Bauer method (Fig. 11). Mueller-Hinton Agar disk diffusion susceptibility testing method did not show any zone of inhibition. . The result obtained was expected as chitin had good antimicrobial activity.
Fig. 11.
Antimicrobial test for CTP2 for two bacterial strain.
3.2. Characterization of laminated composites (LCTP)
3.2.1. FTIR analysis
The FTIR spectra LCTP is shown in Fig. 1 along with PLA and CTO. The spectra of LCTP demonstrated that chitin showed a peak at 2916 cm−1 for the —C—H stretching vibration however in LCTP, it has shifted to 2997 cm−1. Another characteristic band at 3001 cm−1 of PLA film has been shifted to lower wave number of 2997 cm−1. The characteristics peak from both PLA and chitin also appeared in LCTP but in a slightly shifted position.
3.2.2. Morphological study
SEM of image of the surface of LCTP at high and low magnification are shown in Fig. 12 which revealed very compact surface with few crevices on it.
Fig. 12.
SEM images of LCTP at low and high magnification.
3.2.3. XRD analysis
The XRD of LCTP, PLA and CTP2 films are shown in Fig. 4 as mentioned in the previous section. The figure showed that LCTP exhibited a small and broad peak at 2θ = 16.3° as well as a less prominent peak at 32.2°; which is very close to the peak of pure PLA. After lamination the amount of chitin obtained is significantly lower than PLA which demonstrated that chitin is not the dominate phase and thus did not show any intense characteristic peak.
3.2.4. Mechanical properties
3.2.4.1. Tensile properties
The tensile properties LCTP have also shown in in Fig. 5. As expected the laminated composite LCTP exhibited extremely enhanced tensile strength than CTP and PLA as described in Section 3.1.4.1. The highest TS 25.09 MPa was observed for LCTP which is significantly higher than PLA and CTP2 18.55 and 8.83 MPa respectively. This enhanced TS of LCTP can be explained by the presence of crystalline nature and uniform dispersion of chitin as mentioned in the previous section. This high tensile strength of the laminated composites results a good indication that the material can be efficiently used as bone implants.
3.2.4.2. Elongation at break
The LCTP yielded 8.83% of elongation at break (as shown in Fig. 6) indicated its high strength and low elongation and be a suitable candidate for bone implant application.
3.2.4.3. E-modulus
The E-modulus of the LCTP is given in Fig. 7 as described in the previous section. The results showed that the E-modulus of the LCTP are 560 MPa which is significantly higher than CTPs and PLA films.
This enhanced physicomechanical properties of LCTP than CTP and PLA are due to (a) the reinforcing effect from the finely dispersed high performance chitin particles throughout the PLA matrix and (b) strong interaction between chitin and PLA which enhances interfacial adhesion.
3.2.5. Thermal properties
The TGA curves showed that chitin (Fig. 7c) had initial loss due to moisture, however PLA (Fig. 7a) and laminated chitin-PLA (Fig. 7d) had no initial loss due to moisture because PLA is hydrophobic in nature. This indicated that the materials could be a promising bone implant while placing in the natural fluid of the body with minimal losses due to moisture. The maximum slope (50% degradation) for chitin, PLA and LCTP were at 380.6 °C, 372.5 °C and 371.5 °C respectively. After addition of chitin this property did not been show any significant change because chitin content is very less compared to PLA.
The DTA curves of pure PLA film and laminated chitin-PLA composite showed that their Tm were at around 168 °C and 166 °C respectively (as shown in Fig.S3 and S4 in the supporting information). From the derivative curves (DTG), the temperature of maximum weight loss (Tmax) of chitin, pure PLA film and laminated chitin-PLA occurred at around 379 °C, 367 °C and 365 °C respectively. This is because; chitin and PLA did not bonded chemically (as shown in Fig. S3 and S4 in the supporting information).
3.2.6. Thermomechanical analysis (TMA)
TMA of laminated chitin-PLA composites are depicted in Fig. 9 as discussed in the previous section. Softening of laminated chitin-PLA composite started at 56.2 °C and contraction continued up to 92.4 °C. The expansion of the composite started and continued up to 139.4 °C and finally melted down.
3.2.7. Water uptake test
Fig. 10 showed the effect of water absorption of LCTP from 30 min to 24 h duration. After lamination of pure PLA and CTP2 film, the chitin-PLA laminated composite LCTP showed much less water absorption than CTP2 as expected in this research work. LCTPshowed 0.265–1.061% water absorption from 30 min to 24 h. This results clearly demonstrated that the composite is suitable for bone implant material because it absorbs water very slowly and thus degradation will be minimum.
3.2.8. Antimicrobial analysis
The details antimicrobial results of CTP2 for two bacterial strain has been discussed in Section 3.1.8. Similar experiments has also done for LCTP and same results are obtained. There were no inhibition zone for both CTP and LCTP indicated its suitability as bone replacement.
4. Conclusion
The laminated composite material (LCTP) was found to have combined advantages of their components, namely higher tensile strength, elongation break and E-modulus. The composite also showed suitable thermal stability as well as lower water absorption capacity and antimicrobial activity that made it suitable for using as a bone implant. Considering the various physico-mechanical and thermal properties it is revealed that the materials can be an excellent candidate for the replacement of traditional metallic, ceramic based implants.
Acknowledgements
The author highly acknowledge to the Alexander von Humboldt (AvH) Foundation (3.4-8151/RAHMAN-15 025) for the Grant in the form of equipment to carry out this research.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.bioactmat.2017.09.003.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Razak SA, Sharif N, Rahman W. Biodegradable polymers and their bone applications: a review. Int. J. Basic Appl. Sci. 12:31–49.
- 2.Marolt D, Knezevic M, Vunjak-Novakovic G. Bone tissue engineering with human stem cells. Stem Cell Res. Ther. 1:10. [DOI] [PMC free article] [PubMed]
- 3.RodrÃ-guez B., Romero A., Soto O., de Varorna O. Biomaterials for orthopedics. Applications of engineering mechanics in medicine. GED. 2004:1–26. [Google Scholar]
- 4.Seal C.K., Vince K., Hodgson M.A. IOP Publishing; 2009. Biodegradable Surgical Implants Based on Magnesium Alloys. A Review of Current Research. IOP Conference Series: Materials Science and Engineering; p. 012011. [Google Scholar]
- 5.Middleton J.C., Tipton A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000;21:2335–2346. doi: 10.1016/s0142-9612(00)00101-0. [DOI] [PubMed] [Google Scholar]
- 6.Gunatillake P.A., Adhikari R. Biodegradable synthetic polymers for tissue engineering. Eur. Cell Mater. 2003;5:1–16. doi: 10.22203/ecm.v005a01. [DOI] [PubMed] [Google Scholar]
- 7.Adeosun S.O., Lawal G.I., Gbenebor O.P. Characteristics of biodegradable implants. J. Min. Mater. Charact. Eng. 2014;2:88–106. [Google Scholar]
- 8.Sravanthi R. National Institute of Technology; Rourkela: 2009. Preparation and Characterization of Poly (μ-caprolactone) PCL Scaffolds for Tissue Engineering Applications. [Google Scholar]
- 9.Gupta B., Revagade N., Hilborn Jn. Poly (lactic acid) fiber: an overview. Prog. Polym. Sci. 2007;32:455–482. [Google Scholar]
- 10.Mooney D.J., Sano K., Kaufmann P.M., Majahod K., Schloo B., Vacanti J.P., et al. Long term engraftment of hepatocytes transplanted on biodegradable polymer sponges. J. Biomed. Mater. Res. Part A. 1997;37:413–420. doi: 10.1002/(sici)1097-4636(19971205)37:3<413::aid-jbm12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 11.Satyanarayana D., Chatterji P.R. Biodegradable polymers: challenges and strategies. J. Macromol. Sci. Part C Polym. Rev. 1993;33:349–368. [Google Scholar]
- 12.Hutmacher D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–2543. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 13.Coutu D.L., Yousefi A.M., Galipeau J. Three†dimensional porous scaffolds at the crossroads of tissue engineering and cell†based gene therapy. J. Cell. Biochem. 2009;108:537–546. doi: 10.1002/jcb.22296. [DOI] [PubMed] [Google Scholar]
- 14.Kellomäki M., Niiranen H., Puumanen K., Ashammakhi N., Waris T. Bioabsorbable scaffolds for guided bone regeneration and generation. Biomaterials. 2000;21:2495–2505. doi: 10.1016/s0142-9612(00)00117-4. [DOI] [PubMed] [Google Scholar]
- 15.Papenburg B.J., Liu J., Higuera G.A., Barradas A.M.C., de Boer J., van Blitterswijk C.A., et al. Development and analysis of multi-layer scaffolds for tissue engineering. Biomaterials. 2009;30:6228–6239. doi: 10.1016/j.biomaterials.2009.07.057. [DOI] [PubMed] [Google Scholar]
- 16.Hudson S.M., Jenkins D.W. Chitin and chitosan. Encycl. Polym. Sci. Technol. 2001 [Google Scholar]
- 17.Krajewska B. Application of chitin-and chitosan-based materials for enzyme immobilizations: a review. Enzyme Microb. Technol. 2004;35:126–139. [Google Scholar]
- 18.Yusof N.L.B.M., Wee A., Lim L.Y., Khor E. Flexible chitin films as potential wound dressing materials: wound model studies. J. Biomed. Mater. Res. Part A. 2003;66:224–232. doi: 10.1002/jbm.a.10545. [DOI] [PubMed] [Google Scholar]
- 19.Hudson S.M. Applications of chitin and chitosan as fiber and textile chemicals. Adv. Chitin Sci. 1997;2:590–599. [Google Scholar]
- 20.Rathke T.D., Hudson S.M. Review of chitin and chitosan as fiber and film formers. J. Macromol. Sci. Part C Polym. Rev. 1994;34:375–437. [Google Scholar]
- 21.Hudson S.M. The applications of chitin and chitosan to fiber and textile products. Adv. chitin Sci. 1998;3:80–87. [Google Scholar]
- 22.Kanke M., KatayAma H., Tsuzuki S., KuRamoTo H. Application of chitin and chitosan to pharmaceutical preparations. I.: film preparation and in vitro evaluation. Chem. Pharm. Bull. 1989;37:523–525. [Google Scholar]
- 23.Kato Y., Onishi H., Machida Y. Application of chitin and chitosan derivatives in the pharmaceutical field. Curr. Pharm. Biotechnol. 2003;4:303–309. doi: 10.2174/1389201033489748. [DOI] [PubMed] [Google Scholar]
- 24.Jayakumar R., Rani V.V.D., Shalumon K.T., Kumar P.T.S., Nair S.V., Furuike T., et al. Bioactive and osteoblast cell attachment studies of novel α-and Î2-chitin membranes for tissue-engineering applications. Int. J. Biol. Macromol. 2009;45:260–264. doi: 10.1016/j.ijbiomac.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Li X., Feng Q., Cui F. In vitro degradation of porous nano-hydroxyapatite/collagen/PLLA scaffold reinforced by chitin fibres. Mater. Sci. Eng. C. 2006;26:716–720. [Google Scholar]
- 26.Bonilla J., Fortunati E., Vargas M., Chiralt A., Kenny J. Effects of chitosan on the physicochemical and antimicrobial properties of PLA films. J. food Eng. 2013;119:236–243. [Google Scholar]
- 27.Xu J., Zhang J., Gao W., Liang H., Wang H., Li J. Preparation of chitosan/PLA blend micro/nanofibers by electrospinning. Mater. Lett. 2009;63:658–660. [Google Scholar]
- 28.Zhou Z.F., Huang G.Q., Xu W.B., Ren F.M. Chain extension and branching of poly (L-lactic acid) produced by reaction with a DGEBA-based epoxy resin. Express Polym. Lett. 2007;1:734–739. [Google Scholar]
- 29.Gopalan Nair K., Dufresne A. Crab shell chitin whisker reinforced natural rubber nanocomposites. 1. Processing and swelling behavior. Biomacromolecules. 2003;4:657–665. doi: 10.1021/bm020127b. [DOI] [PubMed] [Google Scholar]
- 30.Saravanan D, Gomathi T, Sudha PN. Comparative study of thermal stability using natural polymer blend by cross linking. Archives Appl. Sci. Res. 3:342–350.
- 31.Jin T., Zhang H. Biodegradable polylactic acid polymer with nisin for use in antimicrobial food packaging. J. Food Sci. 2008;73:M127–M134. doi: 10.1111/j.1750-3841.2008.00681.x. [DOI] [PubMed] [Google Scholar]
- 32.San Román MaS, Holgado MaJ, Salinas B, Rives V. Drug release from layered double hydroxides and from their polylactic acid (PLA) nanocomposites. Appl. Clay Sci. 71:1–7.
- 33.Mihai M., Huneault M.A., Favis B.D., Li H. Extrusion foaming of semi-crystalline PLA and PLA/thermoplastic starch blends. Macromol. Biosci. 2007;7:907–920. doi: 10.1002/mabi.200700080. [DOI] [PubMed] [Google Scholar]
- 34.Dufresne A., Dupeyre D., Paillet M. Lignocellulosic flour-reinforced poly (hydroxybutyrate-co-valerate) composites. J. Appl. Polym. Sci. 2003;87:1302–1315. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.