SUMMARY
Nucleoside-containing metabolites such as NAD+ can be incorporated as “5′ caps” on RNA by serving as non-canonical initiating nucleotides (NCINs) for transcription initiation by RNA polymerase (RNAP). Here, we report “CapZyme-Seq,” a high-throughput-sequencing method that employs NCIN-decapping enzymes NudC and Rai1 to detect and quantify NCIN-capped RNA. By combining CapZyme-Seq with multiplexed transcriptomics, we determine efficiencies of NAD+ capping by Escherichia coli RNAP for ~16,000 promoter sequences. The results define preferred transcription start-site (TSS) positions for NAD+ capping and define a consensus promoter sequence for NAD+ capping: HRRASWW (TSS underlined). By applying CapZyme-Seq to E. coli total cellular RNA, we establish that sequence determinants for NCIN capping in vivo match the NAD+-capping consensus defined in vitro, and we identify and quantify NCIN-capped small RNAs. Our findings define the promoter-sequence determinants for NCIN capping with NAD+ and provide a general method for analysis of NCIN capping in vitro and in vivo.
eTOC Blurb
Vvedenskaya et al., report a high-throughput-sequencing-based technology that employs NAD+-decapping enzymes to detect and quantify NAD+-capped RNA. Analysis of NAD+ capping for ~16,000 promoter sequences defines a consensus promoter sequence for NAD+ capping.
INTRODUCTION
RNA 5′-end capping provides a layer of “epitranscriptomic” regulation (Jaschke et al., 2016). RNA 5′-end capping influences RNA fate, including stability, processing, localization and translatability; and RNA capping enables cells to distinguish between host and pathogen RNA (Jaschke et al., 2016; Ramanathan et al., 2016; Topisirovic et al., 2011). Cellular processes that add, remove, or modify RNA caps modulate RNA fate and the distinction between host and pathogen RNA (Arribas-Layton et al., 2013; Grudzien-Nogalska and Kiledjian, 2017; Jaschke et al., 2016; Li and Kiledjian, 2010; Ramanathan et al., 2016).
One form of RNA 5′-capping, which has been observed solely in eukaryotes and certain eukaryotic viruses (Furuichi and Shatkin, 2000; Shatkin, 1976; Wei et al., 1975), entails addition of a 7-methylguanylate (m7G) to the RNA 5′ end. The m7G cap is added to the nascent RNA after transcription initiation, when the nascent RNA reaches a length of ~20 nt, and is added by a capping complex that interacts with the nascent RNA transcript as it emerges from RNA polymerase (RNAP) (Ghosh and Lima, 2010; Martinez-Rucobo et al., 2015; Shuman, 2001, 2015). The m7G cap protects RNA from exonuclease digestion, allows cells to distinguish ‘self’ and ‘foreign’ RNA, and enables recognition and binding of proteins that facilitate splicing, polyadenylation, nuclear export, and translation efficiency (Devarkar et al., 2016; Ramanathan et al., 2016; Topisirovic et al., 2011).
Recently, a second form of RNA 5′-capping has been identified, first in bacteria (Bird et al., 2016; Cahova et al., 2015; Chen et al., 2009; Kowtoniuk et al., 2009), and then in eukaryotes (Jiao et al., 2017; Walters et al., 2017). In this second form of RNA capping, a nucleoside-containing metabolite such as nicotinamide adenine dinucleotide (NAD+) is added at the RNA 5′ end. The nucleoside-containing metabolite cap is introduced during the first nucleotide addition step in transcription initiation, and is added by RNAP itself (Barvik et al., 2017; Bird et al., 2016; Hofer and Jaschke, 2016). The nucleoside-containing metabolite serves as a “non-canonical initiating nucleotide” (NCIN) for transcription initiation by RNAP, providing an “ab initio” mechanism of RNA capping (Bird et al., 2016). As with m7G caps, NCIN caps modulate RNA fate by influencing RNA stability and translation efficiency (Bird et al., 2016; Cahova et al., 2015; Jiao et al., 2017). NCIN capping has been observed in vitro with both bacterial RNAP (Bird et al., 2016; Julius and Yuzenkova, 2017; Malygin and Shemyakin, 1979) and eukaryotic RNAP II (Bird et al., 2016). NCIN-capped RNAs have been observed in vivo for bacteria (Bird et al., 2016; Cahova et al., 2015; Chen et al., 2009; Kowtoniuk et al., 2009; Nubel et al., 2017), yeast (Walters et al., 2017), and human cells in culture (Jiao et al., 2017), and the ab initio mechanism of RNA-capping with NCINs has been demonstrated in vivo in bacteria (Bird et al., 2016).
In addition to NAD+, other nucleoside-containing metabolites, including reduced NAD+ (NADH), 5′-desphospho coenzyme A (dpCoA), flavin adenine dinucleotide (FAD), uridine diphosphate glucose (UDP-glucose), and uridine diphosphate N-acetylglucosamine (UDP-GlcNAc), can serve as substrates for NCIN-mediated capping in vitro (Bird et al., 2016; Julius and Yuzenkova, 2017; Malygin and Shemyakin, 1979), suggesting that these nucleoside-containing metabolites also potentially could function in NCIN capping in vivo.
Adenosine-containing NCINs (NAD+, NADH, dpCoA, and FAD; Figure 1A) compete with adenosine triphosphate (ATP) for use by RNAP as initiating nucleotides. Uridine-containing NCINs (UDP-glucose and UDP-glcNAc) compete with uridine triphosphate (UTP) for use by RNAP as initiating nucleotides. We have presented evidence that promoter sequence at and immediately upstream of the transcription start site determines the outcome of the competition between initiation with NCINs and initiation with NTPs (Bird et al., 2016). Julius and Yuzenkova have challenged this evidence, not detecting an effect of promoter sequence upstream of the TSS (Julius and Yuzenkova, 2017). Here, we report “CapZyme-Seq,” a next-generation-sequencing-based method for detection and quantitation of NCIN-capped RNAs. We apply this method to define, comprehensively, the promoter-sequence determinants and promoter-consensus sequence for NCIN capping with NAD+ in vitro, to define the promoter-sequence determinants and promoter-consensus sequence for NCIN capping in vivo, and to identify and quantify NCIN-capped small RNAs in vivo.
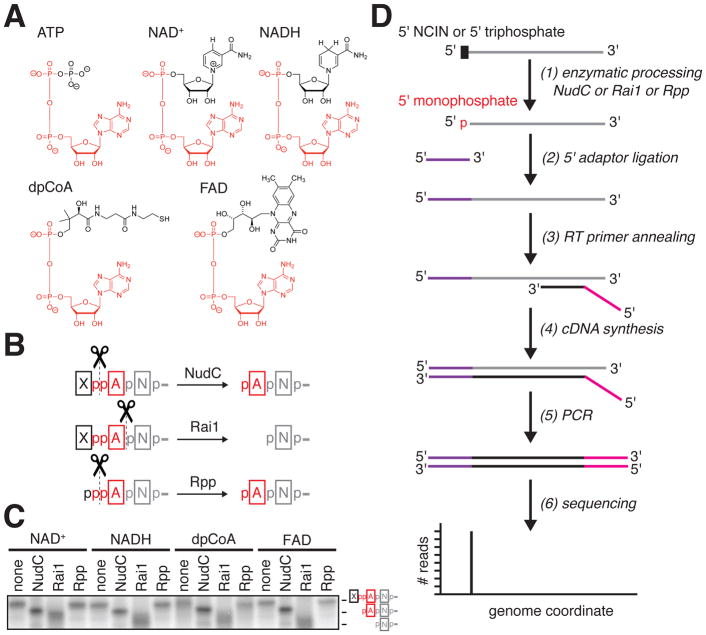
Figure 1. CapZyme-Seq, a high-throughput-sequencing method to detect NCIN-capped RNA.
A. Structures of ATP and adenosine-containing NCINs NAD+, NADH, dpCoA, and FAD. Red, identical atoms.
B. Processing of RNA 5′-ends by NudC, Rai1, and Rpp. Red, common moiety of each 5′-end; black, distinct moiety of each 5′-end; grey, remainder of RNA.
C. Products of processing of NCIN-capped RNA 5′-ends by NudC, Rai1, and Rpp.
D. CapZyme-Seq procedure. Grey, RNA; purple, 5′ adaptor; red, 3′ adaptor; black cDNA.
DESIGN
Methods for high-throughput sequencing of RNA 5′-ends often rely on the ligation of single-stranded oligonucleotide adaptors to RNA 5′-ends. Adaptor ligation requires that the RNA have a 5′-monophosphate. Accordingly, analysis of RNAs that do not have a 5′-monophosphate requires enzymatic processing of the RNAs to yield RNAs having a 5′-monophosphate. Here, we exploit the requirement for enzymatic processing to yield a 5′-monophosphate, together with the use of processing enzymes specific for NCIN-capped RNA and a processing enzyme specific for uncapped, 5′-triphosphate RNA, to enable differential detection and quantitation of NCIN-capped RNA and uncapped, 5′-triphosphate RNA. We term this method “CapZyme-Seq” (Figure 1).
For selective processing of NCIN-capped RNA to 5′-monophosphate RNA, we used the bacterial RNA-decapping enzyme NudC or the fungal RNA-decapping enzyme Rai1. NudC processes NCIN-capped RNA to 5′-monophosphate RNA by cleaving the diphosphate group of the NCIN cap (Cahova et al., 2015; Hofer et al., 2016), yielding products comprising 5′-pNp- (where N is the 3′ nucleoside moiety of the NCIN, for example the adenosine moiety of NAD+) followed by the remainder of the RNA (Figure 1B). Rai1 processes NCIN-capped RNAs to 5′-monophosphate RNA by cleaving the phosphodiester bond connecting the NCIN cap to the remainder of the RNA (Jiao et al., 2017), yielding products comprising 5′-p-followed by the remainder of the RNA (Figure 1B). NudC and Rai1 process RNA capped with at least four of the nucleoside-containing metabolites that can serve as NCINs: NAD+, NADH, dpCoA, and FAD [Figure 1C; (Bird et al., 2016; Cahova et al., 2015; Jiao et al., 2017)]. Accordingly, we propose that CapZyme-Seq using NudC or Rai1 (Figure 1D) can be used to detect NAD+-, NADH-, dpCoA-, and FAD-capped RNA.
For selective processing of uncapped, 5′-triphosphate RNA to 5′-monophosphate RNA we used the RNA processing enzyme RNA 5′ polyphosphatase (Rpp). Rpp processes 5′-triphosphate RNA to 5′-monophosphate RNA by cleaving the phosphodiester bond between the triphosphate β and α phosphates, yielding products comprising 5′-p-followed by the remainder of the RNA (Figure 1B). In initial work, we have found that Rpp does not detectably process RNAs having any of the above NCIN caps: NAD+, NADH, dpCoA, and FAD (Figure 1C).
RESULTS AND DISCUSSION
CapZyme-Seq analysis of NCIN capping with NAD+ in vitro
To define promoter-sequence determinants for NCIN capping, we combined CapZyme-Seq with a multiplexed-transcriptomics method termed “massively systematic transcript end readout” (MASTER; Figure 2A). MASTER enables measurement of RNA 5′-end sequences and RNA yields for RNAs generated during transcription of a template library of up to at least 410 barcoded template sequences (Vvedenskaya et al., 2015; Winkelman et al., 2016). Accordingly, combining CapZyme-Seq with MASTER enables measurement of RNA 5′-end sequences and RNA yields for both NCIN-capped RNA and uncapped, 5′-triphosphate RNA for each of up to at least 410 promoter sequences (Figure 2A).
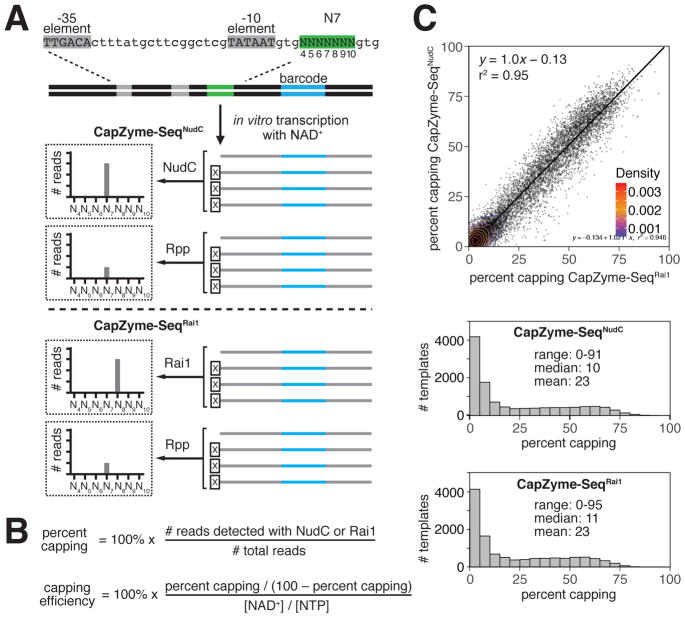
Figure 2. CapZyme-Seq analysis of NCIN capping with NAD+ in vitro.
A. Use of CapZyme-Seq in combination with massively systematic transcript end readout (MASTER). Top, lacCONS-N7 promoter library (47, ~16,000 promoter sequences). Grey, promoter −35 and −10 elements; green, randomized sequences 4–10 bp downstream of promoter −10 element; blue, transcribed-region barcode. The linear DNA template contains ~100 bp of transcribed-region sequence downstream of the green randomized sequence. Thus, RNA products generated from the lacCONS-N7 promoter library are ~100-nt in length. Middle, CapZyme-Seq using NudC for processing of NCIN-capped RNA and Rpp for processing of uncapped 5′-triphosphate RNA (CapZyme-SeqNudC). Bottom, CapZyme-Seq using Rai1 for processing of NCIN-capped RNA and Rpp for processing of uncapped 5′-triphosphate RNA (CapZyme-SeqRai1).
B. Equations used to calculate percent capping and capping efficiencies.
C. Results of CapZyme-SeqNudC and CapZyme-SeqRai1. Top, mean percent capping from CapZyme-SeqNudC (n=3) vs. mean percent capping from CapZyme-SeqRai1 (n=3) for ~16,000 promoter sequences (Density from Gaussian kernel density estimation method). Middle and bottom, percent capping histograms.
In this work, we used the MASTER promoter library “lacCONS-N7” (Vvedenskaya et al., 2015), which contains 47 (~16,000) derivatives of a consensus E. coli σ70-dependent promoter comprising all possible sequence variants at the positions 4 to 10 base pairs (bp) downstream of the promoter −10 element (positions 4, 5, 6, 7, 8, 9 and 10; Figure 2A, top). We performed in vitro transcription experiments using the lacCONS-N7 promoter library and E. coli RNAP σ70 holoenzyme, in parallel, in the presence or absence of NAD+. RNA products from each reaction were analyzed with CapZyme-Seq using NudC (CapZyme-SeqNudC; Figure 2A, middle) or Rai1 (CapZyme-SeqRai1; Figure 2A, bottom). We determined “percent capping” (capped RNA yields relative to total RNA yields) and “capping efficiency” [NAD+-mediated initiation relative to NTP-mediated initiation; (Kcat/Km,NAD+)/(Kcat/Km,NTP)] from the resulting RNA yields using the equations in Figure 2B. Comparison of results obtained using CapZyme-SeqNudC with results obtained using CapZyme-SeqRai1 indicates the results are well correlated (r2 ~ 0.95; slope ~ 1.0; Figure 2C, top). The mean percent capping observed is ~23%, the median percent capping is ~10%, and the range of percent capping is 0–95% for the 47 promoter sequences (Figure 2C, bottom). The majority of percent capping values fall within the range of 0–15%. The distribution of percent capping values is highly skewed with a high peak of 0–5% and a long tail extending to greater than 90% (Figure 2C, bottom). The skewed, long-tailed distribution of percent capping confirms that different promoter sequences differ markedly in efficiency of NCIN capping with NAD+.
Determinants for transcription start site selection in NCIN capping with NAD+ in vitro
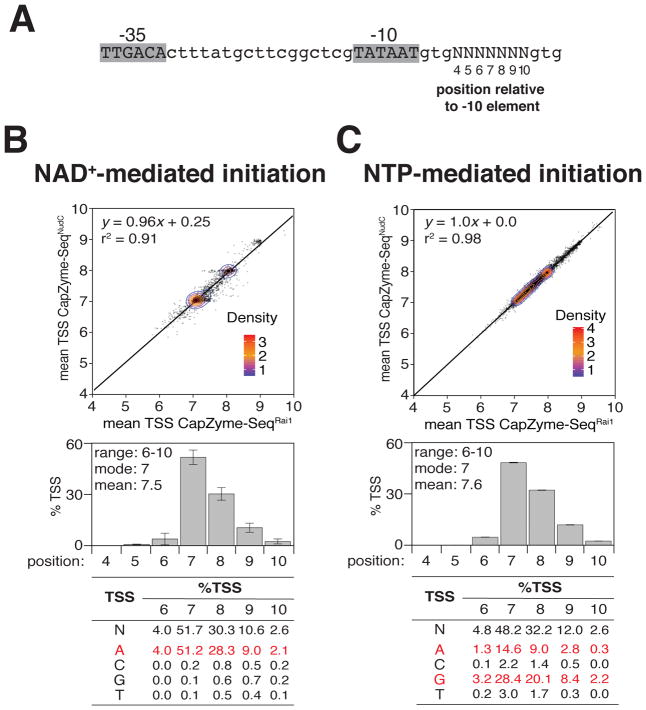
In bacterial transcription initiation, RNAP selects a transcription start site (TSS) at a variable distance downstream of the promoter −10 element. In prior work, we used MASTER to analyze TSS selection in NTP-mediated initiation with the lacCONS-N7 promoter library (Vvedenskaya et al., 2015). Results indicated that TSS selection occurs over a range of five positions located 6–10 bp downstream of the −10 element (positions 6, 7, 8, 9 and 10), that the preferred, modal position for TSS selection is position 7, and that the order of preference for TSS selection is 7 > 8 > 9 > 6 > 10. Results further indicated that there is a strong sequence preference for G or A (R) at each TSS position.
To define, comprehensively, the determinants for TSS selection in NAD+-mediated initiation, we used the combination of CapZyme-Seq and MASTER to determine 5′-end sequence and yields of NAD+-capped RNA in NAD+-mediated initiation with the lacCONS-N7 promoter library (Figure 3A–B). To compare these determinants with determinants for TSS selection in NTP-mediated initiation under identical reaction conditions, we used the combination of CapZyme-Seq and MASTER to determine 5′-end sequence and yields of uncapped, 5′-triphosphate RNA in NTP-mediated initiation with the lacCONS-N7 promoter library in the presence or absence of NAD+ (Figures 3C and S1). As with the results above for percent capping (Figure 2C), the results here for TSS selection obtained using CapZyme-SeqNudC and CapZyme-SeqRai1 are well correlated (r2 ~ 0.95; slope ~ 1.0; Figure 3B,C and Figure S1B, top). The positional preferences for TSS selection in NAD+-mediated initiation (range = positions 6–10; mode = 7 bp downstream of −10 element; mean 7.5 bp downstream of −10 element; order of preference = 7 > 8 > 9 > 6 > 10; Figure 3B, middle) are indistinguishable to the positional preferences for TSS selection in NTP-mediated initiation (range = positions 6–10; mode = 7 bp downstream of −10 element; mean 7.6 bp downstream of −10 element; order of preference = 7 > 8 > 9 > 6 > 10 in reactions performed both in the presence or absence of NAD+; Figure 3C, middle, and Figure S1B, middle). However, the sequence preferences for TSS selection in NAD+-mediated initiation differ from the sequence preferences for TSS selection in NTP-mediated initiation, exhibiting an essentially absolute preference for TSS positions where the base pair is A:T (Figure 3B, bottom), instead of preference for TSS positions where the base pair is either A:T or G:C (Figure 3C, bottom), consistent with expectation based on the base pairing preferences of the adenosine moiety of NAD+ (Figure 1A). Furthermore, in the presence of NAD+, there is a decrease in ATP-mediated initiation, but not GTP-mediated initiation (Figure S1B, bottom), consistent with competition between initiation with NAD+ and initiation with ATP.
Figure 3. Determinants for transcription start site selection in NCIN capping with NAD+ in vitro.
A. lacCONS-N7 promoter library (47, ~16,000 promoter sequences).
B–C. Data for NAD+-mediated initiation (B) and NTP-mediated initiation (C). Top panels, mean TSS from CapZyme-SeqNudC (n=3) vs. mean TSS from CapZyme-SeqRai1 (n=3; mean TSS = [(4 × %TSS at position 4) + (5 × %TSS at position 5) +(6 × %TSS at position 6) + (7 × %TSS at position 7) + (8 × %TSS at position 8) + (9 × %TSS at position 9) + (10 × %TSS at position 10)] / 100). Middle panels, histograms of TSS positions (positions numbered relative to promoter −10 element; mean±SD of percentage of TSS at each position; n=6). Bottom panels, nucleotide frequencies for TSS selection at positions 6, 7, 8, 9, and 10 bp downstream of the −10 element (data for consensus nucleotides in red).
See also Figure S1.
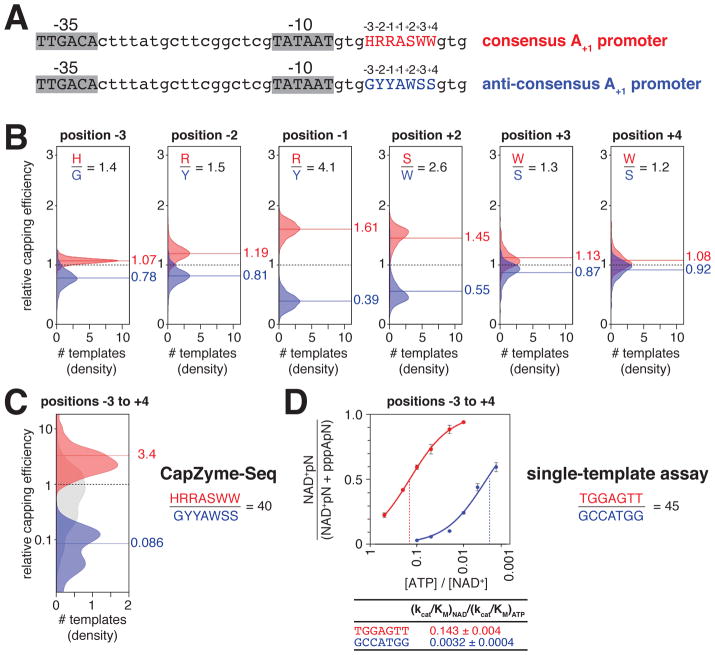
Promoter sequence determinants for NCIN capping with NAD+ in vitro
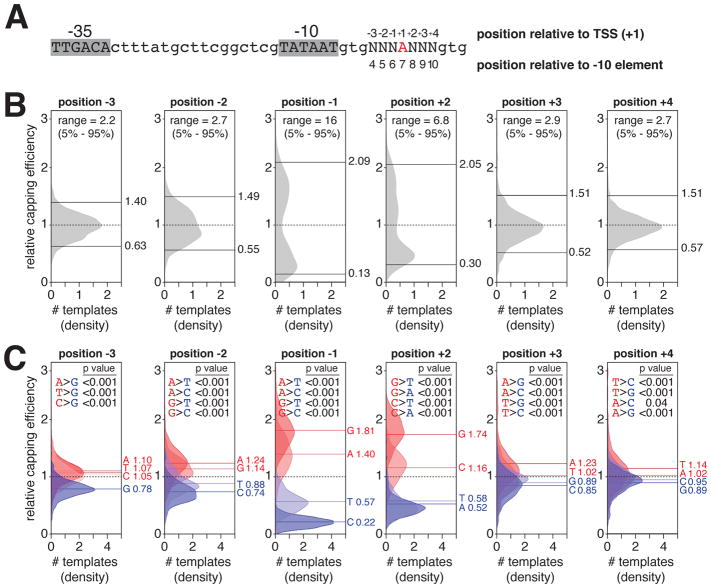
The results in the previous section show that the modal, consensus TSS position for NAD+-mediated initiation is 7 bp downstream of the promoter −10 element and that the consensus TSS base pair for NAD+-mediated initiation is A:T. Considering the subset of ~4,000 promoter sequences in the lacCONS-N7 promoter library that have A:T at the modal, consensus TSS position for NAD+-mediated initiation, 7 bp downstream of the promoter −10 element (A+1 promoters), we next assessed promoter sequence determinants for NAD+-mediated initiation at each of the three positions upstream of the TSS (positions 4, 5, and 6 bp downstream of the −10 element; positions −3, −2, and −1 relative to the TSS, position +1; Figure 4) and at each of the three positions downstream of the TSS (positions 8, 9, and 10 bp downstream of the −10 element; positions +2, +3, and +4 relative to the TSS; Figure 4). We find that capping efficiency depends on the identity of the nucleotide at each of these positions (Figures 4B,C and S2). At position −3, capping efficiency is higher for A, T, and C than for G, yielding the consensus H and anti-consensus G; at position −2, capping efficiency is higher for G and A than for T and C, yielding the consensus R and anti-consensus Y; at position −1, capping efficiency is higher for G and A than for T and C, yielding the consensus R; at position +2, capping efficiency is higher for G and C than for A and T, yielding the consensus S; at position +3, capping efficiency is higher for A and T than for G and C, yielding the consensus W and anti-consensus S; and at position +4, capping efficiency is higher for A and T than for G and C, yielding the consensus W and anti-consensus S (Figure 4C). The strongest dependence of capping efficiency on nucleotide identity, at positions other than the TSS, is observed at position −1. At this position, the mean relative capping efficiencies for promoters having A and G are ~2 to ~3 times higher than for promoters having T and ~7 to ~8 times higher than for promoters having C (Figure 4C). The second-strongest dependence of capping efficiency on nucleotide identity is observed at position +2. At this position, capping efficiencies for promoters having G and C are ~2 to ~3 times higher than for promoters having A and T (Figure 4C). The dependences of capping efficiencies on nucleotide identity at each of the other positions (−3, −2, +3 and +4) are smaller, but significant (Figure 4C).
Figure 4. Promoter sequence determinants for NCIN capping with NAD+ in vitro.
A. Subset of lacCONS-N7 promoter library having A (red) at the position 7 bp downstream of −10 element (~4,000 sequences).
B. Distributions of relative capping efficiency (calculated using the equation in Figure S2B; n=6) for ~4,000 A+1 promoter sequences at the positions immediately upstream of the TSS (positions −1, −2, and −3) and immediately downstream of the TSS (positions +2, +3, and +4). The dashed line is the mean relative capping efficiency, the upper and lower solid lines are the 95th percentile and 5th percentile, respectively, and the “range” is defined as the 95th percentile relative capping efficiency divided by the 5th percentile relative capping efficiency.
C. Distributions of relative capping efficiency for ~4,000 A+1 promoter sequences parsed by position and nucleotide (A, T, C, or G). The dashed line is the mean relative capping efficiency for all sequences, the solid lines are the means for sequences having the indicated nucleotide. Distributions and lines are colored by consensus nucleotide (mean relative capping efficiency greater than 1; red) or anti-consensus nucleotide (mean relative capping efficiency less than 1; blue). Shown are p values for pairwise comparisons of consensus and anti-consensus nucleotides (Kolmogorov–Smirnov test).
See also Figure S2.
To validate the CapZyme-Seq results, we analyzed capping efficiencies for individual promoters using a single-template gel assay [Figure S3; procedures as in (Bird et al., 2017)]. The results of the ~1,000-template CapZyme-Seq assays and the single-template gel assays were in full agreement (Figure S3).
The finding that position −1 is a crucial sequence determinant, with G or A as the preferred nucleotides, confirms our previous results (Bird et al., 2016) and contradicts results of (Julius and Yuzenkova, 2017). Julius and Yuzenkova did not observe specificity at position −1, most likely because they measured only 1/Km, and not kcat/Km, and thus were unable to detect specificity manifest at the level of kcat. The findings for sequence specificity at positions −3, −2, +2, +3, and +4 are new to this work.
Promoter consensus sequence for NCIN capping with NAD+ in vitro
The results in the previous section provide a promoter consensus and anti-consensus sequence for NCIN capping with NAD+: H−3R−2R−1A+1S+2W+3W+4 and G−3Y−2Y−1A+1W+2S+3S+4, respectively (Figure 5A). The results in Figure 5B indicate that the mean relative capping efficiencies for promoters having a consensus nucleotide at positions −3, −2, −1, +2, +3 and +4 are ~1.4-fold, ~1.5-fold, ~4.1-fold, ~2.6-fold, ~1.3-fold, and ~1.2-fold, respectively, greater than the mean relative capping efficiencies for promoters having an anti-consensus nucleotide at these positions. Mean capping efficiency values for consensus A+1 promoter sequences vs. anti-consensus A+1 promoter sequences differ by ~40-fold (Figure 5C). Single-template gel assays comparing a consensus A+1 promoter and anti-consensus A+1 promoter confirm the ~40-fold difference in capping efficiency observed by CapZyme-Seq (Figure 5D).
Figure 5. Promoter consensus sequence for NCIN capping with NAD+ in vitro.
A. lacCONS promoter derivatives with consensus A+1 sequence (red) and anti-consensus A+1 sequence (blue) for NAD+ capping.
B. Distributions of relative capping efficiency, for ~4,000 A+1 promoter sequences parsed by position and nucleotide (H, G, R, Y, S, or W) and colored by consensus nucleotide (red) or anti-consensus nucleotide (blue). The dashed line is the mean relative capping efficiency for all sequences, the solid lines are the means for sequences having a consensus nucleotide (red) or an anti-consensus nucleotide (blue).
C. Distributions of relative capping efficiency for consensus A+1 sequences (red), anti-consensus A+1 sequences (blue), or all A+1 sequences (grey).
D. Dependence of NAD+ capping on [ATP] / [NAD+] ratio for representative consensus A+1 promoter sequence (red) and anti-consensus A+1 promoter sequence (blue): data from single-template gel assays (mean±SD; n=3). The dashed line indicates the value of [ATP] / [NAD+] when the value of NAD+pN / (NAD+pN + pppApN) = 0.5.
See also Figure S3.
Strand specificity of NCIN capping with NAD+ in vitro
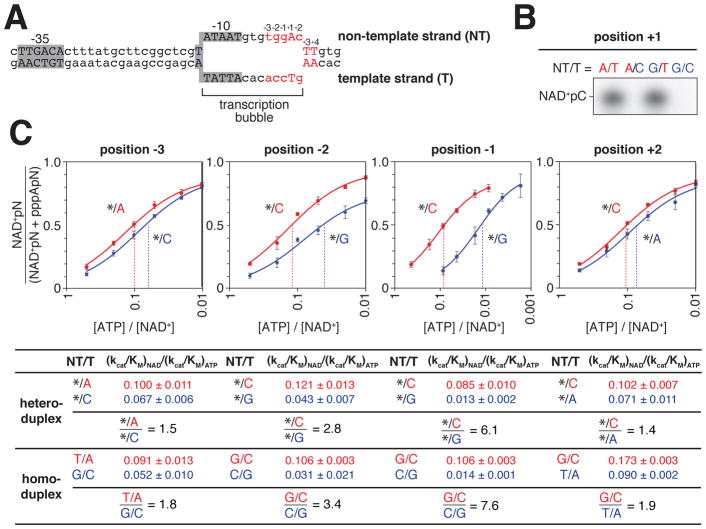
In a catalytically competent transcription initiation complex (RNAP-promoter open complex), positions −3, −2, −1, +1, and +2 are part of an unwound, non-base-paired “transcription bubble” (Zhang et al., 2012). This raises the question whether specificity for positions −3, −2, −1, +1, and +2 is carried by the single-stranded “template strand” of the transcription bubble (the strand that templates incoming nucleotide substrates), the single stranded “non-template strand” of the transcription bubble, or both (Figure 6A). To address this question for the TSS, position +1, we analyzed NAD+ capping for promoter derivatives having consensus nucleotides at this position on both the template and non-template strands, on the template strand only, on the non-template strand only, or on neither (Figure 6B). NAD+ capping was observed when consensus nucleotides were present on both strands or on the template strand only, but not when consensus nucleotides were present on the non-template strand only or on neither strand (Figure 6B). We conclude that, at position +1, the sequence information for NAD+ capping resides exclusively in the template strand. To address this question for positions −3, −2, −1, and +2, we compared NAD+ capping efficiencies for heteroduplex promoter derivatives having the consensus or anti-consensus nucleotides on the template strand and an abasic site (*) on the non-template strand (Figure 6C). For each position, the capping efficiency ratio for constructs having the consensus vs. anti-consensus only on the template strand matched the capping efficiency ratio for homoduplex promoter derivatives having consensus vs. anti-consensus on both strands (Figure 6C). We conclude that, as at position +1, at positions −3, −2, −1, and +2, the sequence information for NAD+ capping resides exclusively in the template strand.
Figure 6. Strand specificity of NCIN capping with NAD+ in vitro.
A. lacCONS promoter derivative containing consensus A+1 promoter sequence in context of RNAP-promoter open complex. DNA non-template strand (NT) on top; DNA template strand (T) on bottom; Unwound, non-base-paired DNA region, “transcription bubble,” indicated by raised and lowered nucleotides
B. Products of transcription reactions with NAD+ as initiating nucleotide and [α32P]-CTP as extending nucleotide for templates having the consensus nucleotides at the TSS, position +1, on both DNA strands, non-template strand only, template strand only, or neither.
C. Dependence of NAD+ capping on [ATP] / [NAD+] ratio for templates having an abasic site (*) on the DNA non-template strand and either consensus base (red) or anti-consensus base (blue) on the DNA template strand at each of positions −3, −2, −1, and +2, relative to TSS. Below, capping efficiencies and consensus/anti-consensus capping efficiency ratios for heteroduplex templates with an abasic site on the DNA non-template strand or, for comparison, for homoduplex templates having a complementary nucleotide on the DNA non-template strand (mean±SD; n=3). The dashed line indicates the value of [ATP] / [NAD+] when the value of NAD+pN / (NAD+pN + pppApN) = 0.5.
See also Figure S5.
CapZyme-Seq analysis of NCIN capping in vivo
To demonstrate the utility of CapZyme-Seq for analysis of RNA isolated from living cells we applied CapZyme-Seq to (i) define promoter sequence determinants for NCIN capping in vivo in E. coli, and (ii) to identify and quantify small RNAs (sRNAs) in vivo. Both of these objectives were pursued, in parallel, using RNA isolated from a single culture.
Promoter-sequence determinants for NCIN capping in vivo
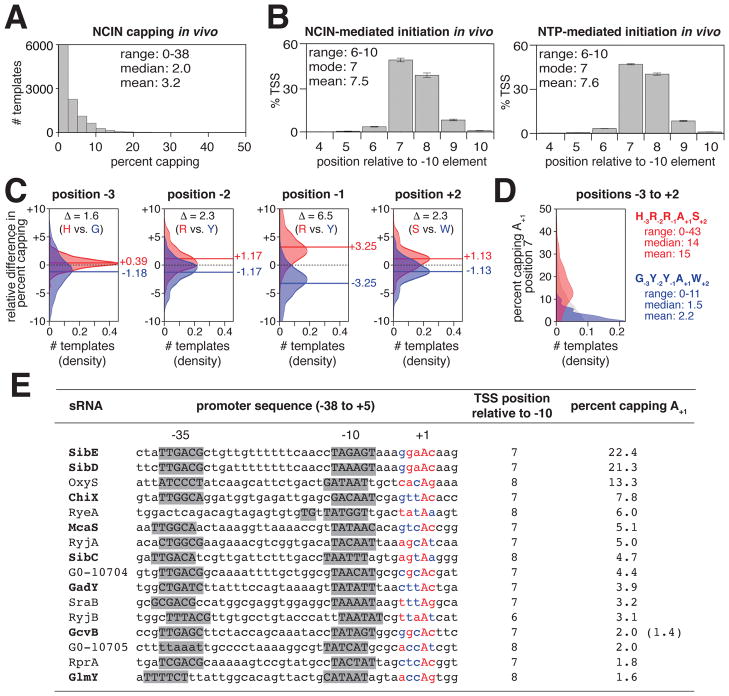
To determine total levels of NCIN capping and to define promoter-sequence determinants for NCIN capping we isolated RNA products from E. coli cells containing the MASTER lacCONS-N7 template library used in the experiments in Figures 2–5 and analyzed RNA products from the 47 MASTER lacCONS-N7 promoter sequences using CapZyme-SeqNudC (Figure 7A). We observed NCIN capping with many promoter sequences in vivo, extending and generalizing our conclusion from previous work with a single promoter sequence (Bird et al. 2016). The level of NCIN capping differs for RNA products from the 47 different promoter sequences, ranging from 0 to 38%, with a mean of 3%, and a median of 2% (Figure 7A). We see a broad distribution of percent capping values in vivo (Figure 7A) reminiscent of the broad distribution of percent capping values observed in vitro (Figure 2C).
Figure 7. CapZyme-Seq analysis of NCIN capping in vivo.
A–D. Promoter sequence determinants for NCIN capping in vivo in E. coli.
Percent capping histogram (A); TSS position histograms (B; mean±SD of percentage of TSS at each position; n=3); relative percent capping difference distributions (C; calculated using the equation in Figure S4B; the dashed line is 0, the solid lines are the means, consensus nucleotides are colored red, anti-consensus nucleotides are colored blue); percent capping histograms for −3 through +2 consensus (red) and anti-consensus (blue) sequences for NCIN-capping in vivo (D).
E. Identification and quantitation of NCIN-capped sRNAs in vivo in E. coli. Bold indicates sRNA sequences previously identified as NAD+ capped (Cahova et al., 2015). In the promoter sequences, grey shading represents the promoter −10, extended −10, and −35 promoter elements, and colors indicate matches to the −3 through +2 consensus (red) and anti-consensus (blue) sequences for NCIN capping in vivo. Percent capping values represent the mean of three independent measurements. In the column for percent capping the number reported previously in (Nubel et al., 2017) for GcvB is in parentheses.
See also Figure S4.
The preferred TSS positions for NCIN capping in vivo matches the preferred TSS positions for NCIN capping with NAD+ in vitro (mode = 7 bp downstream of −10 element; mean = 7.5 bp downstream of −10 element; Figures 3B and 7B). The preferred TSS base pair for NCIN capping in vivo (A:T) also matches the preferred TSS base pair for NCIN capping with NAD+ in vitro (Figures 3B and S4A).
Considering the subset of ~4,000 promoter sequences in the lacCONS-N7 promoter library that have A:T at the modal, consensus TSS position for NCIN capping, 7 bp downstream of the promoter −10 element (A+1 promoters), we next assessed promoter sequence determinants for NCIN capping in vivo at each of the three positions upstream and downstream of the TSS (Figures 7C and S4C–D). We find that, for positions −3 to +2, the sequence determinants for NCIN capping in vivo (Figures 7C and S4C) match those for NCIN capping with NAD+ in vitro (Figures 4, 5, and S3). At position +3 we observe no sequence preferences in vivo (Figure S4D) and at position +4 we observe sequence preferences similar to, but weaker than those observed in vitro (Figures 4 and S4D). The results provide promoter consensus and anti-consensus sequences for NCIN capping in vivo—H−3R−2R−1A+1S+2 and G−3Y−2Y−1A+1W+2 (Figure 7C,D)—that match the corresponding promoter consensus and anti-consensus sequences for positions −3 to +2 for NCIN capping with NAD+ in vitro (Figure 5). The strongest promoter sequence dependence of NCIN capping in vivo, apart from that at the TSS, is observed at position −1 (Figure 7C), matching the pattern observed for NCIN capping with NAD+ in vitro (Figure 4B,C). At this position, the difference, Δ, in mean percent capping for consensus vs. anti-consensus is ~6.5% (Figure 7C). At positions −3, −2, and +2, the difference, Δ, in mean percent capping for consensus vs. anti-consensus is ~2%. Considering positions −3 through +2, the difference, Δ, in mean percent capping for consensus vs. anti-consensus is ~13% (Figure 7D).
Identification and quantitation of NCIN-capped small RNAs in vivo
Identities of several NAD+-capped small RNA (sRNA) and sRNA-like 5′-RNA fragments in E. coli total cellular RNA have been reported (Cahova et al., 2015). Here, we applied CapZyme-Seq to identify and quantify NCIN capping of all annotated E. coli sRNAs. We isolated E. coli total cellular RNA and performed CapZyme-SeqNudC using primers for the cDNA synthesis step designed to target 77 annotated sRNAs of E. coli (Keseler et al., 2017). Analysis of uncapped, 5′-triphosphate RNA using Rpp identified 16 sRNAs arising from promoters having A:T at the TSS position (Figure 7E). Analysis of NCIN-capped RNA using NudC shows that all 16 sRNAs exhibit NCIN capping (Figure 7E), including eight previously reported as NAD+-capped (Cahova et al., 2015) (Figure 7E text in bold). NCIN capping levels for the 16 sRNAs ranged from 1.6% to 22.4%. Three RNAs shows particularly high NCIN capping levels: SibE, SibD and OxyS (22.4%, 21.3%, and 13.3%). SibE and SibD are anti-toxin sRNAs and OxyS is an sRNA regulator of oxidative stress. We note that the two most highly capped sRNAs are transcribed from promoters that match the four most important positions, −2 to +2, of our consensus sequence for NCIN capping in vivo.
Proposed basis for promoter sequence specificity for NCIN capping with NAD+
The results in Figures 3–6 show that the efficiency of NCIN capping with NAD+ is determined by sequence at the TSS (+1), sequence at the three positions immediately upstream of the TSS (−3 to −1), and sequence at the three positions immediately downstream of the TSS (+2 to +4). There is an essentially absolute preference for A at the TSS (Figure 3B). At the first position upstream of the TSS, position −1, sequence has very strong effects on efficiency of NAD+ capping (up to at least 16-fold; Figure 4B). At the second and third positions upstream of the TSS, positions −2 and −3, sequence has small, but significant, effects (up to at least 2.7-fold and 2.2-fold, respectively; Figure 4B). At the first position downstream of the TSS, position +2, sequence has large effects (up to at least 6.8-fold; Figure 4B). At the next two positions downstream of the TSS, positions +3 and +4, promoter sequence has small but significant effects (up to at least 2.9-fold and 2.7-fold, respectively; Figure 4B). The results in Figure 6 show that the specificity determinants at positions −3 to +2 reside exclusively in the template strand of the promoter DNA within the unwound transcription bubble of the RNAP-promoter open complex.
The essentially absolute preference for an A:T base pair at the at the TSS, position +1, results from the Watson-Crick base-paring preference of the adenosine moiety of NAD+ with a T on the template-strand (Figure 1A, and Figure S5A).
Structural modeling suggests that the very strong preference for R (Y on template strand) at the position immediately upstream of the TSS, position −1, can be understood in terms of “pseudo-base pairing” of the NAD+ nicotinamide moiety with the DNA template strand base at position −1 (Figure S5). The NAD+ nicotinamide can be positioned to form a nicotinamide:Y pseudo-base pair with template-strand C at position −1 or, with a 180° rotation about the pyridine-amide bond of the NAD+ nicotinamide, with template-strand T at position −1, in each case, forming two H-bonds with Watson-Crick H-bonding atoms of the template-strand and stacking on the NAD+ adenine base (Figure S5A,B). In contrast, the NAD+ nicotinamide moiety would experience severe steric clash with template strand A or G at position −1 (Figure S5C).
Structural modeling suggests that the specificity for R (Y on template strand) at position −2 also can be understood in terms of stacking preferences for pseudo-base pairing by the NAD+ nicotinamide moiety to the template-strand base at position −1. A template-strand Y at position −2 can stack favorably on the NAD+ nicotinamide moiety of a nicotinamide:Y pseudo-base pair (Figure S5A), but a template-strand R at position −2 would clash with the NAD+ nicotinamide moiety.
The strong specificity for S (S on template strand) at the first position downstream of the TSS, position +2, can be understood in terms of differences of 1/Km for the incoming extending NTP, which base pairs with the template-strand base at position +2 (Jensen et al., 1986; Rhodes and Chamberlin, 1974), together with different sensitivities to this parameter of NAD+-mediated initiation to ATP-mediated initiation.
The specificity for W:W base pairs at positions +3 and +4 observed in vitro, potentially can be understood in terms of differences in DNA duplex stabilities, and corresponding DNA unwinding energies for W:W base pairs vs. S:S base pairs, together with different sensitivities to these parameters of NAD+-mediated initiation to ATP-mediated initiation.
Prospect
Jaschke and co-workers have reported a method that combines click-chemistry covalent capture with high-throughput sequencing to isolate and identify NAD+-capped RNAs, “NAD+-capture-seq” (Cahova et al., 2015; Winz et al., 2017). NAD+-capture-seq has enabled detection of NAD+ capped RNAs in bacteria, yeast, and human cells in culture (Cahova et al., 2015; Jiao et al., 2017; Walters et al., 2017). However, NAD+-capture-seq does not enable single-nucleotide resolution identification of RNA 5′-ends, does not enable quantitation of relative yields of NAD+-capped and uncapped RNA, and does not enable detection of RNAs carrying NCIN caps other than NAD+. In contrast, the method we report in this work, “CapZyme-Seq” (Figure 1), which combines selective enzymatic processing of NCIN-capped 5′ ends and uncapped 5′ ends with high-throughput sequencing, enables single-nucleotide resolution identification of RNA 5′-ends (Figures 3, S1, 7B and S4A), enables quantitation of relative yields of NAD+-capped and uncapped RNA (Figures 2, 4, 5, and 7), and enables detection of RNAs carrying NCIN-caps other than NAD+ (Figure 1).
By combining CapZyme-Seq with multiplexed transcriptomics, we determined the efficiencies of NAD+ capping by E. coli RNAP σ70 holoenzyme for ~16,000 promoter sequences (Figure 2). A priority for future studies will be to adapt the methods employed in this work to define the promoter-sequence determinants for NAD+ capping by bacterial RNAP holoenzymes carrying alternative σ factors, archaeal RNAP, eukaryotic RNAP I, II, and III, mitochondrial RNAP, and chloroplast RNAP.
The prevalence of nucleoside-containing metabolites that can function as NCINs underscores the need to determine, for each NCIN, the promoter-sequence determinants for NCIN capping. The method reported here, either using the same decapping enzymes or using alternative decapping enzymes with alternative decapping specificities, should enable analysis of determination of NCIN-capping efficiencies and promoter sequence determinants thereof for each nucleoside-containing metabolite that can serve as an NCIN (e.g., NADH, dpCoA, FAD, UDP-glucose, and UDP-GlcNAc).
NCIN caps provide a layer of epitranscriptomic regulation by modulating RNA stability and translation efficiency. Understanding the full impact of NCIN capping as a mechanism for altering RNA fate requires understanding the mechanism(s) by which the distributions of NCIN caps for different RNA products are determined—i.e. the mechanism(s) of “NCIN targeting.” The method reported here was developed, validated, and applied to RNA generated in vitro and in vivo in E. coli. This same method should be applicable, essentially without modification, to RNA isolated from any source, including eukaryotic cells, tissues, organs, and organisms.
Limitations
One issue to note when using CapZyme-Seq is that the efficiency of RNA 5′-end processing by the enzymes used potentially can be influenced by RNA 5′-end sequence or secondary structure. For example, NudC shows low RNA 5′-end processing efficicency for RNA substrates having stable secondary structures at any of the first three nucleotides (Hofer et al., 2016). In the application of CapZyme-Seq in this work, we analyzed DNA-sequence determinants for NCIN capping with NAD+ at the three promoter positions immediately upstream of the TSS and the three promoter positions immediately downstream of the TSS. For the promoter positions upstream of the TSS, outside the transcribed region, changing DNA sequence does not change RNA 5′-end sequence and RNA 5′-end processing efficiency, and thus CapZyme-Seq analysis of DNA-sequence determinants at these positions, by itself, provides a definitive measure of effects on capping efficiencies. For the positions downstream of the TSS, inside the transcribed region, changing DNA sequence changes RNA 5′-end sequence and possibly changes RNA 5′-end processing efficiency, and thus CapZyme-Seq analysis of DNA-sequence determinants at these positions, by itself, provides a provisional measure of effects on capping efficiencies. In this work, this provisional measure of effects of positions downstream of the TSS on the efficiency of NCIN capping with NAD+ was corroborated by two additional findings. First, CapZyme-Seq results for the subset of RNAs predicted to have stable secondary structures at any of the first three nucleotides were shown to match CapZyme-Seq results obtained for the subset of RNAs predicted not to have secondary structures at any of the first three nucleotides (Figure S6). Second, CapZyme-Seq results for effecs of substitution of promoter position +2 on capping efficiency were confirmed in single-template gel assays (Figure S3).
Another issue to note when using CapZyme-Seq is that the method, in its current form, using NudC and RaiI as decapping enzymes, identifies NCIN-capped RNA but does not identify the NCIN cap (i.e., does not distinguish between NAD+ caps and other NCIN caps). This is not an issue for experiments in vitro, where the experimenter controls the identity of the NCIN. However, this can be an issue for experiments in vivo, where NCIN capping may occur not only with NAD+, but also with other NCINs. Accordingly, for experiments in vivo, identification of the NCIN cap may require additional analysis [e.g., metabolic labelling, electrophoretic mobility, boronic-acid reactivity, immunoassay, or mass spectrometry (Bird et al., 2016; Cahova et al., 2015; Chen et al., 2009; Kowtoniuk et al., 2009; Nubel et al., 2017)]. A goal of future work will be to develop embodiments of CapZyme-Seq that employ additional RNA 5′-end processing enzymes that selectively report on specific 5′-end modifications.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Requests for further information or reagents should be directed to Bryce E. Nickels (bnickels@waksman.rutgers.edu).
METHOD DETAILS
Proteins
E. coli RNAP core enzyme used in transcription experiments was prepared from E. coli strain NiCo21(DE3) transformed with plasmid pIA900 (Svetlov and Artsimovitch, 2015) using culture and induction procedures, immobilized-metal-ion affinity chromatography on Ni-NTA agarose, and affinity chromatography on Heparin HP as in (Artsimovitch et al., 2003). E. coli σ70 used in transcription experiments was prepared from E. coli strain BL21(DE3) transformed with plasmid pσ70–His using culture and induction procedures, immobilized-metal-ion affinity chromatography on Ni-NTA agarose, and anion-exchange chromatography on Mono Q as in (Marr and Roberts, 1997). RNAP holoenzyme was formed by mixing 2 μM RNAP core and 10 μM σ70 in 10 mM Tris-Cl, pH 8.0, 100 mM KCl, 10 mM MgCl2, 0.1 mM EDTA, 1 mM DTT, and 50% glycerol and incubating for 30 min at 25°C.
E. coli NudC was prepared from E. coli strain NiCo21(DE3) transformed with plasmid pET NudC-His (Bird et al., 2016) using culture and induction prodcedures, immobilized-metal-ion affinity chromatography on Ni-NTA agarose, and size exclusion chromatography on Superdex S200 16/600 as in (Cahova et al., 2015). S. pombe Rai1 was prepared from E. coli strain BL21(DE3) Rosetta using culture and induction procedures, immobilized-metal-ion affinity chromatography on Ni-NTA agarose, and size exclusion chromatography using Sephacryl S-300 as in (Xiang et al., 2009). 5′ RNA polyphosphatase (Rpp) was purchased from Epicentre.
Oligonucleotides
A complete list of oligodeoxyribonucleotides and oligoribonucleotides are provided in Table S1. Adapters and primers used in MASTER library construction were HPLC purified. All other oligodeoxyribonucleotides and oligoribonucleotides were purified with standard desalting purification unless otherwise specified.
Linear in vitro transcription templates for single-template gel assays were generated by PCR using Phusion HF Polymerase master mix, 5 nM of template oligo, 0.5 mM of forward primer and 0.5 mM of reverse primer. PCR products were purified using a QIAquick PCR purification kit prior to use in transcription reactions.
Templates with abasic sites on the DNA non-template strand (Figure 6) were generated by mixing 1.1 μM non-template strand oligo with 1 μM template strand oligo in 10 mM Tris pH 8.0. Mixtures were heated to 95°C for 10 minutes, cooled to 25°C, and incubated for 20 minutes using a Dyad PCR machine (Bio-Rad).
NudC, Rai1, and Rpp processing assays
For assays shown in Figure 1C, radiolabeled NCIN-capped RNA products were generated by in vitro transcription. 10 nM linear lacCONS A-less cassette template (made by PCR using oligos JB277, JB285 and JB455) was mixed with 50 nM RNAP holoenzyme in 10 mM Tris HCl pH 8.0, 50 mM NaCl, 10 mM MgCl2, 0.1 mM EDTA, 1 mM DTT, 0.1 mg/ml BSA, 2% glycerol. Reactions were incubated at 37°C for 15 minutes to form open complexes, mixed with 1 mM of NCIN (NAD+, NADH, dpCoA, or FAD), 6 mCi [α-32P]-UTP (3000 Ci/mmol), 200 μM CTP, 200 μM UTP, and 200 μM GTP and incubated at 37°C for 10 minutes to allow for product formation. Reactions were stopped by addition of 1.5× stop solution (0.6 M Tris HCl pH 8.0, 18 mM EDTA, 0.1 mg/ml glycogen). Samples were extracted with acid phenol:chloroform and NCIN-capped products were recovered by ethanol precipitation. The NCIN-capped RNA products generated in these reactions have the sequence: 5′-XppACUUGUUUUGGUGUCUGCGCUCCUCCUUGCCUGUUUCCUCGGUUCUUUGUGUUGGUUGCUCUGUGUUCCUUCGUUUUUCCGCCCUGCUUGGCGGUUUUUUCGUUUUCUGUGC-3′, where XppA = NAD+, NADH, dpCoA, or FAD. The minimum free energy structure for this RNA product determined by RNAfold (Lorenz et al., 2011) is not predicted to have secondary structures at any of the first ten nucleotides.
NudC processing reactions were performed by adding 400 nM NudC to products resuspended in 10μl of NudC reaction buffer (10 mM Tris HCl pH 8.0, 50 mM NaCl, 10 mM MgCl2, 1 mM DTT). Rai1 processing reactions were performed by adding 400 nM Rai1 to RNA products resuspended in 10 μl Rai1 reaction buffer [10 mM Tris-HCl (pH 7.5), 100 mM KOAc, 2 mM MgOAc, 1 mM MnCl2, 2 mM DTT]. Rpp processing reactions were performed by adding 10U Rpp to RNA products resuspended in 10 μl of Rpp reaction buffer [50 mM HEPES-KOH (pH 7.5), 100 mM NaCl, 1 mM EDTA, 0.1% β-mercaptoethanol, and 0.01% Triton X-100]. Reactions were incubated at 37°C for 30 minutes, stopped by addition 10 μl 2× RNA loading dye (95% deionized formamide, 18 mM EDTA, 0.25% SDS, xylene cyanol, bromophenol blue, amaranth), and separated by electrophoresis on 8% TBE-Urea polyacrylamide gels (UreaGel system, National Diagnostics). Radioactive products were detected by autoradiography using storage phosphor screens and a Typhoon 9400 variable mode imager (GE Life Science).
CapZyme-Seq
Isolation of RNA products generated in vitro
The pMASTER-lacCONS-N7 plasmid library (Vvedenskaya et al., 2015; Winkelman et al., 2016) contains the promoter cassette shown in Figure 1A fused to the tR2 terminator. A linear DNA fragment containing the placCONS-N7 promoter library was used as a template for in vitro transcription assays. To generate this template, the plasmid pMASTER-lacCONS-N7 library variant described in (Winkelman et al., 2016) was diluted to ~109 molecules/μl. 1μl of diluted DNA was amplified by emulsion PCR (ePCR) using a Micellula DNA Emulsion and Purification Kit in detergent-free Phusion HF reaction buffer containing 5 μg/ml BSA, 0.4 mM dNTPs, 0.5 μM Illumina RP1 primer, 0.5 μM Illumina RPI1 primer and 0.04 U/μl Phusion HF polymerase. ePCR reactions were performed with an initial denaturation step of 10 seconds at 95°C, amplification for 30 cycles (denaturation for 5 seconds at 95°C, annealing for 5 seconds at 60°C and extension for 15 seconds at 72°C), and a final extension for 5 minutes at 72°C. DNA was purified from emulsions according to the manufacturer’s recommendations, recovered by ethanol precipitation, and resuspended in nuclease-free water to a final concentration of ~1 μM.
The sequence of the linear DNA template used in the transcription assays is: 5′-gttcagagttctacagtccgacgatcaggcTTGACActttatgcttcggctcgTATAATgtgNNNNNNNgtgagcggataacaatNNNNNNNNNNNNNNNcctgcaggtggaattctcgggtgccaaggaactccagtcacatcacgatctcgtatgccgtcttctgcttg-3′, where the −35 and −10 elements are in bold, and the 7-bp randomized transcription start site region and 15-bp transcribed-region barcode are underlined.
In vitro transcription was performed by mixing 10 nM of template DNA with 50 nM RNAP holoenzyme in 50 mM Tris HCl (pH 8.0), 10 mM MgCl2, 0.01 mg/ml BSA, 100 mM KCl, 5% glycerol, 10 mM DTT, and 0.4U/μl RNase OUT. Reactions were incubated at 37°C for 10 minutes to form open complexes. A single round of transcription was initiated by addition of 100 μM ATP, 100 μM CTP, 100 μM UTP, 100 μM GTP, and 0.1 mg/ml heparin, or by addition of 100 μM ATP, 100 μM CTP, 100 μM UTP, 100 μM GTP, 2 mM NAD+, and 0.1 mg/ml heparin. Reactions were incubated at 37°C for 15 minutes and stopped by addition of 0.5M EDTA (pH 8) to a final concentration of 50 mM. Nucleic acids were recovered by ethanol precipitation and resuspended in 30 μl of nuclease-free water. Reactions performed in the absence or presence of NAD+ were performed in triplicate, and each replicate was analyzed by both CapZyme-SeqNudC and CapZyme-SeqRai1.
The resuspended nucleic acids were mixed with 30 μl of 2× RNA loading dye and separated by electrophoresis on 10% 7M urea slab gels (equilibrated and run in 1× TBE). The gel was stained with SYBR Gold nucleic acid gel stain, bands were visualized on a UV transilluminator, and RNA products ~100 nt in size were excised from the gel. The excised gel slice was crushed, 300 μl of 0.3 M NaCl in 1× TE buffer was added, and the mixture was incubated at 70°C for 10 minutes. Eluted RNAs were collected using a Spin-X column. After the first elution, the crushed gel fragments were collected and the elution procedure was repeated, nucleic acids were collected, pooled with the first elution, isolated by ethanol precipitation, and resuspended in 10 μl of RNase-free water.
Isolation of RNAs generated in vivo in E. coli
To analyze RNA products generated from the pMASTER-lacCONS-N7 library in vivo, three independent 50 ml cell cultures of DH10B-T1R cells containing the pMASTER-lacCONS-N7 plasmid library variant described in (Vvedenskaya et al., 2015) were grown in LB media containing chloramphenicol (25 μg/ml) in a 250 ml DeLong flask shaken at 210 RPM at 37°C to mid-exponential phase (OD600 ~0.5). 2 ml aliquots of the cell suspensions were placed in 2 ml tubes and cells were collected by centrifugation (1 min, 21,000 × g at room temperature). Supernatants were removed and cell pellets were rapidly frozen on dry ice and stored at −80°C.
RNA was isolated from each cell pellet as in (Vvedenskaya et al., 2015). Cell pellets were resuspended in 600 μl of TRI Reagent solution. The suspensions were incubated at 70°C for 10 min and centrifuged (10 min, 21,000 × g) to remove insoluble material. The supernatant was transferred to a fresh tube, ethanol was added to a final concentration of 60.5%, and the mixture was applied to a Direct-zol spin column. DNase I treatment was performed on-column according to the manufacturer’s recommendations. RNA was eluted from the column using nuclease-free water that had been heated to 70°C (3 × 30 μl elutions; total volume of eluate = 90 μl). RNA was treated with 2U TURBO DNase at 37°C for 1 h to remove residual DNA. Samples were extracted with acid phenol:chloroform, RNA was recovered by ethanol precipitation and resuspended in RNase-free water. A MICROBExpress Kit was used to deplete rRNAs from 9 μg of the recovered RNA. The rRNA-depleted RNA was isolated by ethanol precipitation and resuspended in 30 μl of RNase-free water.
pMASTER-lacCONS-N7 plasmid DNA was isolated from each of the three cultures using a Plasmid Mini-prep kit. Plasmid DNA isolated from these cultures was used as template in ePCR reactions to generate linear DNA products that were analyzed by high-throughput sequencing (DNA template libraries Vv828, Vv830, and Vv832, see below).
The sequence from the −35 element of the lacCONS-N7 promoter to the end of the tR2 terminator on the plasmid used for the in vivo experiments is: 5′-TTGACACTTTATGCTTCGGCTCGTATAATGTGNNNNNNNGTGAGCGGATAACAATNNNNNNNNNNNNNNNTGGAATTCTCGGGTGCCAAGGGCCCCAGCGGCCGTCTTCAAGAGCTCATGGATCCGAATAGCCATCCCAATCGAACAGGCCTGCTGGTAATCGCAGGCCTTTTTATTTGGAT-3′, where the −35 and −10 elements are in bold, and the 7-bp randomized transcription start site region and 15-bp transcribed-region barcode are underlined. Note that the pMASTER-lacCONS-N7 library variant described in (Winkelman et al., 2016) used to generate the linear DNA template for in vitro assays contains an 8-bp sequence insertion (CCTGCAGG) downstream of the transcribed-region barcode that is not present in the pMASTER-lacCONS-N7 plasmid library variant described in (Vvedenskaya et al., 2015) used for the in vivo assays.
Enzymatic treatments of RNA products generated in vitro with Rpp, NudC, or Rai1
To convert 5′ triphosphate RNA to 5′ monophosphate RNA, products were mixed with 20U Rpp and 40U RNaseOUT in 1× Rpp reaction buffer in a 20 μl reaction volume.
To convert NAD+-capped RNA to 5′ monophosphate RNA, products were mixed with 1× NudC reaction buffer, 3.6 μM NudC, and 40U of RNaseOUT in a 20 μl reaction volume or with 1× Rai1 reaction buffer, 0.3 μM Rai1, and 40U RNaseOUT in a 20 μl reaction volume. In parallel, we added RNA products to each of the reaction buffers without addition of enzyme. Reactions were incubated at 37°C for 30 minutes, 20 μl of 2× RNA loading dye was added, and products were separated by electrophoresis on 10% 7M urea slab gels (equilibrated and run in 1× TBE). The gel was stained with SYBR Gold nucleic acid gel stain, bands were visualized on a UV transilluminator, and ~100-nt products were excised from the gel. The excised gel slice was crushed, 300 μl of 0.3 M NaCl in 1× TE buffer was added, and the mixture was incubated at 70°C for 10 minutes. The eluate was collected using a Spin-X column. After the first elution step, the elution procedure was repeated, eluates were pooled, and RNA was isolated by ethanol precipitation and resuspended in 10 μl of RNase-free water.
Enzymatic treatments of RNA products generated in vivo with Rpp or NudC
To convert 5′-triphosphate RNA to 5′-monophosphate RNA, 2 μg rRNA-depleted RNA were mixed with 20U Rpp and 40U RNaseOUT in 1× Rpp reaction buffer in a 30 μl reaction volume. In parallel, we added 2 μg rRNA-depleted RNA to 1× Rpp reaction buffer without addition of enzyme. Reactions were incubated at 37°C for 30 minutes. Samples were extracted with acid phenol:chloroform, RNA was recovered by ethanol precipitation, and resuspended in 10 μl RNase-free water.
Prior to treating total cellular RNA with NudC to convert NCIN-capped RNA to 5′-monophosphate RNA we first treated 2 μg of rRNA-depleted RNA with 2U CIP in the presence of 40U RNaseOUT in a 30 μl reaction volume at 37°C for 1h to remove 5′-terminal phosphates. RNA was extracted with acid phenol:chloroform, recovered by ethanol precipitation, and resuspended in 20 μl of RNase-free water. CIP-treated RNA was mixed with 3.75 μM NudC and 40U RNaseOUT in 1× NudC reaction buffer in a 30 μl reaction volume. In parallel, CIP-treated RNA was incubated in 1× NudC reaction buffer without NudC. Reactions were incubated at 37°C for 30 minutes, 30 μl of 2× RNA loading dye was added, and products were separated by electrophoresis on 10% 7M urea slab gels (equilibrated and run in 1× TBE). The gel was stained with SYBR Gold nucleic acid gel stain, RNA was visualized on a UV transilluminator, and excised from the gel. The excised gel slice was crushed, 400 μl of 0.3 M NaCl in 1× TE buffer was added, and the mixture was incubated at 70°C for 10 minutes. The eluate was collected using a Spin-X column. After the first elution step, the elution procedure was repeated, eluates were pooled, and recovered RNA isolated by ethanol precipitation and resuspended in 10 μl of RNase-free water.
5′ adaptor ligation
RNA products (in 10 μl of nuclease-free water) were combined with PEG 8000 (10% final concentration), 1 mM ATP, 40U RNaseOUT, 1× T4 RNA ligase buffer, and 10 U T4 RNA ligase 1, in 30 μl reaction volume. 0.3 μM barcoded 5′ adaptor oligo was added to in vitro generated RNAs, and 1 μM barcoded 5′ adaptor oligo was added to in vivo generated RNAs, respectively. Reactions were incubated at 16°C for 16 h.
To enable quantitative comparisons between samples treated with Rpp, samples treated with NCIN-processing enzymes, samples incubated in Rpp reaction buffer (“mock Rpp treatment”), and samples incubated in NCIN-processing enzyme reaction buffer (“mock NudC treatment” or “mock Rai1 treatment”), we performed the 5′ adaptor ligation step using barcoded 5′-adaptor oligonucleotides. For libraries prepared from RNA generated in vitro or in vivo, oligo i105 was used in ligation reactions performed with products isolated after Rpp treatment, oligo i106 was used in ligation reactions performed with products isolated after mock Rpp treatment, oligo i107 was used in ligation reactions performed with products isolated after NudC treatment (for CapZyme-SeqNudC) or Rai1 treatment (for CapZyme-SeqRai1), and oligo i108 was used in ligation reactions performed with products isolated after mock NudC treatment or mock Rai1 treatment.
The ligation reactions were stopped by adding 30 μl of 2× RNA loading dye and heated at 95°C for 3 minutes. Each set of four adaptor ligation reactions were combined, mixed with an equal volume of 2× RNA loading dye, and separated by electrophoresis on 10% 7M urea slab gels (equilibrated and run in 1× TBE). Gels were incubated with SYBR Gold nucleic acid gel stain, and bands were visualized with UV transillumination. For RNA generated in vitro, ≃150 nt products were recovered from the gel (procedure as above) and resuspended in 10 μl of nuclease-free water. For RNA generated in vivo, products migrating above the 5′-adapter oligo were recovered from the gel (procedure as above) and resuspended in 50 μl of nuclease-free water.
First strand cDNA synthesis: analysis of RNAs generated from the lacCONS-N7 promoter library in vitro
5′-adaptor-ligated products (in 10 μl of nuclease-free water) were mixed with 1.5 μM s128A oligonucleotide, incubated at 65°C for 5 minutes, then cooled to 4°C. To this mixture was added 9.7 μl of a solution containing 4 μl of 5× First-Strand buffer, 1 μl of 10 mM dNTP mix, 1 μl of 100 mM DTT, 1 μl (40U) RNaseOUT, 1 μl (200U) of SuperScript III Reverse Transcriptase and 1.7 μl of nuclease-free water. Reactions were incubated at 25°C for 5 minutes, 55°C for 60 minutes, 70°C for 15 minutes, then cooled to 25°C. 10U of RNase H was added, the reactions were incubated at 37°C for 20 minutes and 20 μl of 2× RNA loading dye was added. Nucleic acids were separated by electrophoresis on 10% 7M urea slab gels (equilibrated and run in 1× TBE). Gels were incubated with SYBR Gold nucleic acid gel stain, bands were visualized with UV transillumination, and species ~80 to ~150 nt in length were recovered from the gel (procedure as above) and resuspended in 20 μl of nuclease-free water.
First strand cDNA synthesis: analysis of RNAs generated in vivo
5′-adaptor-ligated products were divided into two equal portions (each in 25 μl of nuclease-free water). One portion was mixed with 0.5 μl of 100 μM s128A oligonucleotide to enable analysis of RNA products generated from the lacCONS-N7 promoter library, while the other portion was mixed with 0.5 μl of 100 μM sRNA oligo pool (a mixture of 77 oligonucleotides each having a 3′-end sequence complementary to positions +50 to +30 of one of 77 annotated sRNAs in E. coli; see Key Resources Table). The mixtures were incubated at 65°C for 5 min, then cooled to 4°C. To these mixtures was added 24.5 μl of a solution containing 10 μl 5× First-Strand buffer, 2.5 μl 10 mM dNTP mix, 2.5 μl 100 mM DTT, 2.5 μl (100U) RNaseOUT, 2.5 μl (500U) SuperScript III Reverse Transcriptase and 4.5 μl of nuclease-free water. Reactions were incubated at 25°C for 5 min, 55°C for 60 min, 70°C for 15 min, then cooled to 25°C. 20U RNase H was added, the reactions were incubated at 37°C for 20 min and 50 μl of 2× RNA loading dye was added. Nucleic acids were separated by electrophoresis on 10% 7M urea slab gels. To isolate cDNA derived from the lacCONS-N7 promoter library, ~80- to ~150-nt products were recovered from the gel (procedure as above) and resuspended in 20 μl of nuclease-free water. To isolate cDNA derived from sRNA, ~80- to ~225-nt products were recovered from the gel and resuspended 20 μl of nuclease-free water.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| MG1655 | ATCC | 47076 |
| ElectroMax DH10B-T1R electrocompetent cells | ThermoFisher | 1521050 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Nuclease-free water (not DEPC-treated) | ThermoFisher | AM9932 |
| Bacto agar | VWR | 90000-760 |
| Bacto tryptone | VWR | 90000-286 |
| Bacto yeast extract | VWR | 90000-726 |
| Chloramphenicol | Gold Biotech | C-105-25 |
| Spectinomycin | Gold Biotech | S0188-25 |
| SOC Outgrowth Medium | NEB | B9020S |
| dNTP solution mix, 10 mM of each NTP | NEB | N0447S |
| NTP set (ultra-pure), 100 mM solutions | GE Healthcare | 27-2025-01 |
| NAD+ | Roche | 10127965001 |
| NADH | Roche | 10107735001 |
| desphospho-CoA | Sigma-Aldrich | D3385 |
| FAD | Sigma-Aldrich | F6625 |
| Tris base (Amresco) | VWR | 97061-800 |
| Boric Acid (ACS grade) | VWR | 97061-980 |
| EDTA disodium salt dyhydrate | VWR | 97061-018 |
| 0.5 M EDTA pH 8 | ThermoFisher | AM9260G |
| Sodium Chloride | EMD Milipore | SX0420-3 |
| Potassium Chloride | EMD Milipore | 7300-500GM |
| Sodium Acetate, trihydrate | VWR | MK736406 |
| Formamide, deionized | VWR | EM-4610 |
| Sodium dodecylsulfate (SDS) | VWR | 97064-470 |
| Magnesium chloride hexahydrate | VWR | EM-5980 |
| Glycerol (ACS grade) | VWR | EMGX0185-5 |
| Bovine Serum Albumin (BSA) fraction V | VWR | 101174-932 |
| Bromophenol Blue | VWR | EM-BX1410-7 |
| Xylene Cyanol | Sigma-Aldrich | X4126-10G |
| Amaranth Dye | VWR | 200030-400 |
| Temed (JT Baker) | VWR | JT4098-1 |
| Ammonium Persulfate | VWR | 97064-594 |
| Dithiothreitol (DTT) | Gold Bio | DTT50 |
| Glycogen from Oyster (type II) | Sigma-Aldrich | G8751 |
| Hydrochloric Acid (ACS plus) | Fisher Scientific | A144-212 |
| Ethyl Alcohol | Pharmco-AAPER | 111000200 |
| Isopropyl Alcohol | BDH | BDH1133-1LP |
| Heparin sulfate | Sigma-Aldrich | H-3393 |
| GeneMate LE Quick Dissolve agarose | BioExpress | E-3119-500 |
| SequaGel sequencing system | National Diagnostics | EC833 |
| 10% TBE-Urea gels, 1mm x 10 wells | ThermoFisher | EC6875Box |
| 10% TBE gels, 1mm x 10 wells | ThermoFisher | EC6275Box |
| α-32P CTP EasyTide 250 uCi | Perkin Elmer | BLU508H250UC |
| α-32P GTP EasyTide 250 uCi | Perkin Elmer | BLU506H250UC |
| α-32P UTP EasyTide 250 uCi | Perkin Elmer | BLU507H250UC |
| Low Range ssRNA Ladder | NEB | N0364S |
| O’Gene Ruler Ultra Low Range DNA Ladder | ThermoFisher | SM1223 |
| 6X Orange DNA Loading Dye | ThermoFisher | R0631 |
| SYBR Gold nucleic acid gel stain | ThermoFisher | S11494 |
| TRI Reagent | Molecular Research Center | TR118 |
| Acid phenol:chloroform (CHCl3) pH 4.5 | ThermoFisher | AM9720 |
| TURBO DNAse | ThermoFisher | AM2238 |
| DNase I | Zymo Research | E1009A |
| RNaseOUT, Recombinant Ribonuclease Inhibitor | ThermoFisher | 10777-019 |
| Calf Intestine Alkaline Phosphatase | NEB | M0290S |
| RNA 5′ Polyphosphatase | Lucigen (Epicentre) | RP8092H |
| NudC | (Cahova et al., 2015) | |
| Rai1 | (Xiang et al., 2009) | |
| T4 RNA Ligase 1 (ssRNA Ligase) | NEB | M0204L |
| Superscript III Reverse Transcriptase | ThermoFisher | 18080-044 |
| RNAse H | ThermoFisher | AM2293 |
| Phusion HF DNA Polymerase | ThermoFisher | F-530L |
| 5X Detergent-free Phusion HF Buffer Pack | NEB | B0520S |
| Phusion Flash HF master mix | ThermoFisher | F-548L |
| E. coli RNA polymerase core (β′-6xHis) | (Artsimovitch et al., 2003) | |
| E. coli σ70-6xHis | (Marr and Roberts, 1997) | |
| Critical Commercial Assays | ||
| Direct-zol RNA MiniPrep | Zymo Research | R2051 |
| MICROBExpress Bacterial mRNA purification Kit | ThermoFisher | AM1905 |
| QIAprep Spin Miniprep Kit | Qiagen | 27106 |
| Qubit dsDNA HS assay kit | ThermoFisher | Q32851 |
| Qubit ssDNA Assay kit | ThermoFisher | Q10212 |
| Micellula DNA Emulsion PCR Kit | Chimerx | 3600-02 |
| QIAquick PCR purification kit | Qiagen | 28104 |
| Deposited Data | ||
| Sequencing data | NIH Sequence Read | accession number |
| Unprocessed gel images | Archive | PRJNA411835 |
| Mendeley Data | http://dx.doi.org/10.17632/kjc9rypnzr.2 | |
| Oligonucleotides (sequences provided 5′ to 3′) | ||
| Sequences of the DNA and RNA primers used in this work are provided in Table S1 | Integrated DNA Technologies (IDT) | |
| Recombinant DNA | ||
| pIA900 | Gift of I. Artsimovitch | |
| pσ70–His | Gift of J. Roberts | |
| pET NudC-His | (Bird et al., 2016) | |
| pMASTER-lacCONS-N7 | (Vvedenskaya et al., 2015) | |
| Software and Algorithms | ||
| Excel | Microsoft | 2011 |
| ImageQuant | GE Healthcare | TL 5.1 |
| SigmaPlot | Systat Software Inc. | Ver. 10 |
| MASTER-EX-CLT | Nickels Lab, Rutgers University | https://github.com/NickelsLabRutgers/MASTER-EX-CLT |
| R programming language | R Core Team | http://www.R-project.org/ |
| ggplot2 | (Wickham, 2016) | http://www.springer.com/us/book/9780387981413 |
| Dplyr | (Wickham and Francois, 2016) | https://CRAN.R-project.org/package=dplyr |
| ViennaRNA package (RNAfold) | (Lorenz et al., 2011) | Version 2.4.3 https://www.tbi.univie.ac.at/RNA/index.html |
| Pymol | Schrodinger, LLC | http://www.pymol.org |
| Illustrator | Adobe | Ver. CS6 |
| Other | ||
| Bench protocol | Methods S1 | |
| Illumina high-throughput sequencing | RUCDR, Rutgers University | www.rucdr.org |
| Illumina high-throughput sequencing | Waksman Genomics Core Facility, Rutgers University | ngs.lab@rutgers.edu |
| Molecular Dynamics PhosphorImager | GE Healthcare | Typhoon 9400 |
cDNA amplification
cDNA derived from RNA products generated in vitro or in vivo were diluted with nuclease-free water to a concentration of ~109 molecules/μl. 2μl of the diluted cDNA solution was used as a template for ePCR reactions containing Illumina index primers using a Micellula DNA Emulsion and Purification Kit (20 cycles; conditions as above). The emulsion was broken and DNA was purified according to the manufacturer’s recommendations. DNA was recovered by ethanol precipitation and resuspended in 20 μl of nuclease-free water.
High-throughput sequencing
Barcoded libraries were pooled and sequenced on an Illumina NextSeq platform in high-output mode using custom primer s1115.
Sample serial numbers for CapZyme-SeqRai1 in vitro
Samples Vv1225, Vv1229, Vv1230, and Vv1231 are cDNA derived from RNA products generated in vitro in the absence of NAD+. Vv1225 and Vv1229 are cDNA generated from the same RNA products, and thus were considered as a single replicate. Samples Vv1227, Vv1232, Vv1233, and Vv1234 are cDNA derived from RNA products generated in vitro in the presence of NAD+. Samples Vv1227 and Vv1232 are cDNA generated from the same RNA products, and thus were considered as a single replicate.
Sample serial numbers for CapZyme-SeqNudC in vitro
Samples Vv1226, Vv1235, Vv1236, and Vv1237 are cDNA derived from RNA products generated in vitro in the absence of NAD+. Samples Vv1226 and Vv1235 are cDNA generated from the same RNA products and thus were considered as a single replicate. Samples Vv1228, Vv1238, Vv1239, and Vv1240 are cDNA derived from RNA products generated in vitro in the presence of NAD+. Samples Vv1228 and Vv1238 are cDNA libraries generated from the same RNA products, and thus were considered as a single replicate Sample Vv1168 is the DNA template used in the MASTER-lacCONS-N7 in vitro reactions.
Sample serial numbers for CapZyme-SeqNudC in vivo
Samples Vv1273, Vv1275, and Vv1277 are cDNA derived from RNA products generated in vivo from the lacCONS-N7 promoter library. Samples Vv1274, Vv1276, and Vv1278 are cDNA derived from sRNAs products present in total cellular RNA isolated from E. coli. Samples Vv828, Vv830, and Vv832 are DNA products generated in ePCR reactions using pMASTER-lacCONS-N7 plasmid DNA isolated from cells.
CapZyme-Seq data analysis
Transcribed-region barcode identification for MASTER-lacCONS-N7 experiments
The DNA template used for in vitro transcription reactions was analyzed by high-throughput sequencing (sample serial number Vv1168) to identify transcribed-region barcodes, and to assign these barcodes to individual placCONS-N7 template sequences (Vvedenskaya et al., 2015).
For in vivo MASTER-lacCONS-N7 experiments, DNA products generated in ePCR reactions using pMASTER-lacCONS-N7 plasmid DNA isolated from cells were analyzed by high-throughput sequencing (sample serial numbers Vv828, Vv830, and Vv832) to identify transcribed-region barcodes, and to assign these barcodes to individual placCONS template sequences (Vvedenskaya et al., 2015). In particular, analysis of sample Vv828 was used to assign transcribed-region barcodes for analysis of sample Vv1273, analysis of sample Vv830 was used to assign transcribed-region barcodes for analysis of sample Vv1275, and analysis of sample Vv832 was used to assign transcribed-region barcodes for analysis of sample Vv1277.
Analysis of sequencing reads derived from cDNA
All cDNA libraries were generated from the same input RNA that had been split into four portions and subjected to two distinct 5′ processing reactions (Rpp and NudC or Rai1) and two distinct control reactions (mock Rpp treatment and mock NudC or Rai1 treatment). To distinguish cDNA derived from each of the four reactions, RNA from each reaction was ligated to a 5′-adaptor oligonucleotide containing a unique 4-nt barcode sequence (i105, i106, i107, or i108; see above). Each 5′-adaptor oligonucleotide also contains 11 nt of random sequence at the 3′ end that improves ligation efficiency (by reducing sequence-dependent effects), and that marks individual RNA products with an 11-nt sequence tag that is used to reduce effects of PCR amplification bias. Thus, sequencing reads having identical 11-nt sequence tags and identical cDNA insert sequences are counted as a single read count during the data analysis.
Due to the presence of the 4-nt barcode sequence and 11-nt sequence tag at the 3′ end of the 5′-adaptor oligonucleotide, the first four bases of each read provide the sequence of the 4-nt barcode, the next 11 bases provide the sequence of the 11-nt sequence tag, and the 16th base provides the sequence of the RNA 5′ end from which the cDNA was generated.
Analysis of sequencing reads derived from cDNA: MASTER-lacCONS-N7 analysis in vitro
For analysis of cDNA libraries generated from RNA products produced from the MASTER-lacCONS-N7 library in vitro the 15-base transcribed-region barcode was identified and used to associate reads derived from RNA transcripts with their template of origin (Vvedenskaya et al., 2015). We considered only RNA 5′-end-sequences that could be aligned to the sequence of their template of origin with no mismatches. These reads were associated with one of the four reaction conditions based on the identity of the 4-nt barcode sequence.
For each of the ~16,000 sequence variants, we determined the number of reads emanating from each position of the N7 region for samples treated with Rpp (#Rpp), samples treated with NCIN-processing enzymes (#NudC or #Rai1), samples subjected to mock Rpp treatment (#Rppmock), and samples subjected to mock NCIN-processing enzyme treatment (NudCmock or #Rai1mock). From these values, we calculated #ppp and #NCIN, where #ppp = (#Rpp - #Rppmock), and #NCIN = (#NudC - #NudCmock) for CapZyme-SeqNudC and #NCIN = (#Rai1 - #Rai1mock) for CapZyme-SeqRai1. Negative values for #ppp or #NCIN were replaced with a value of 0.
Analysis of reactions performed in the absence of NAD+ revealed activity of NudC and Rai1 on 5′-triphosphate RNA. By comparison with analysis of reactions performed in the absence of NAD+ with Rpp we estimate, on average, NudC converted ~19% of 5′-triphosphate RNA to 5′-monophosphate RNA, and Rai1 converted ~3.5% of 5′-triphosphate RNA to 5′-monophosphate RNA. Therefore, to account for the conversion of 5′-triphosphate RNA to 5′-monophosphate RNA by NudC or Rai1 we used values of #ppp and #NCIN obtained in reactions performed in the absence of NAD+ to calculate a correction factor (cf), where cf = (#NCIN / #ppp), to apply to the analysis of reactions performed in the presence of NAD+.
To analyze results of reactions performed in the presence of NAD+ we used the value of cf, the values for #ppp, and the value for #NCIN to calculate a “background corrected” value of #NCIN (#NCINBkd_Cor), where #NCINBkd_Cor = [#NCIN – (cf × #ppp)]. Next, using the value of #NCINBkd_Cor and #ppp we calculated a value of “percent capping,” where percent capping = 100% × [#NCINBkd_Cor / (#NCINBkd_Cor + #ppp)]. (Note that values of (#NCINBkd_Cor + #ppp) of 0 were replaced by “1” prior to calculating percent capping.)
For results of experiments performed in vitro we calculated a value for “capping efficiency,” where capping efficiency = [percent capping / (100 - percent capping)] / 20. (Note that the value of 20 corresponds to the [NAD+]/[ATP] for the in vitro transcription experiments performed in this work.
Results of Figure 2C represent a plot of the mean percent capping value (n=3; replicates of CapZymeNudC or CapZymeRai1) for template sequences with (#NCINBkd_Cor + #ppp) ≥ 50.
Results of Figure 3B represent the mean TSS value (n=3; replicates of CapZymeNudC or CapZymeRai1) and mean %TSS values (n=6; replicates of CapZymeNudC and CapZymeRai1) for template sequences with #NCINBkd_Cor ≥ 50. Results of Figure 3C and Figure S1B represent the mean TSS value (n=3; replicates of CapZymeNudC or CapZymeRai1) and mean %TSS values (n=6; replicates of CapZymeNudC and CapZymeRai1) for template sequences with #ppp ≥ 50. Mean TSS and %TSS were calculated using the formulas below.
Results of Figures 4 and 5B represent relative capping efficiency values calculated as shown in Figure S2 for the position 7 bp downstream of the promoter −10 element for template sequences with (#NCINBkd_Cor at position 7 + #ppp at position 7) ≥ 25 in each of the three CapZyme-SeqNudC replicates and in each of the three CapZyme-SeqRai1 replicates. Relative capping efficiency at each position was determined for groups of four promoter sequences, “quartets,” having A, G, C, or T, along with sequences identical at each other position. The relative capping efficiency of each A+1 promoter sequence, Y, at each position, X, is calculated by dividing the capping efficiency of Y by the mean capping efficiency of Y’s quartet at position X. For each promoter sequence, the mean capping efficiency (n=6; replicates of CapZymeNudC and CapZymeRai1) was used to calculate relative capping efficiency at each position. For results of Figure 5C, the relative capping efficiency of the consensus and anti-consensus promoter sequences were calculated by dividing the capping efficiency of each consensus or anti-consensus promoter sequence by the mean capping efficiency of all A+1 promoter sequences.
Uncapped RNA products generated in vitro from A+1 promoters have the sequence: 5′-ANNNGUGAGCGGAUAACAAUNNNNNNNNNNNNNNNCCUGCAGGUGGAAUUCUCGGGUGCCAAGGAACUCCAGUCACAUCACGAUCUCGUAUGCCGUCUUCUGCUUG-3′.
Analysis of effects of RNA secondary structure
Each of the ~4,000 A+1 promoter sequences in the lacCONS-N7 MASTER library is associated with multiple different transcribed-region barcode sequences (Figure S6A, top and middle). Accordingly, for each A+1 promoter sequence, multiple different RNA products with different 5′-end secondary structures are generated (Figure S6A, bottom). We took advantage of this feature of the lacCONS-N7 MASTER library to assess effects of predicted RNA 5′-end secondary structure on CapZyme-Seq results. Specifically, for each A+1 promoter sequence, we used RNAfold (Lorenz et al., 2011) to predict the minimum free energy structure for each RNA product, we compared the observed percent-capping values for the subset of RNA products predicted to have secondary structures at any of the first three nucleotides, to the observed percent-capping values for the subset of RNA products predicted not to have secondary structures at any of the first three nucleotides (Figure S6B). We find that the observed percent capping for the subset of RNA products predicted to have RNA 5′-end secondary structures and the observed percent capping for the subset of RNA products predicted to not have RNA 5′-end secondary structures agree well (r2 ~ 0.9 for CapZyme-SeqNudC and CapZyme-SeqRai1; slope ~ 1 for CapZyme-SeqNudC and CapZyme-SeqRai1; Figure S6B), indicating that CapZyme-SeqNudC results and CapZyme-SeqRai1 results reported in this work are not strongly affected by RNA 5′-end secondary structure.
Figure S6B reports observed mean percent capping values determined in vitro for A+1 promoters for which, in each of three replicates, (#NCINBkd_Cor at position 7 + #ppp at position 7) ≥ 25 for the subset of RNA products predicted to have secondary structure and (#NCINBkd_Cor at position 7 + #ppp at position 7) ≥ 25 for the subset of RNA products predicted not to have secondary structure (422 A+1 promoters for CapZyme-SeqNudC; 354 A+1 promoters for CapZyme-SeqRai1).
Analysis of sequencing reads derived from cDNA: MASTER-lacCONS-N7 analysis in vivo
For analysis of cDNA libraries generated from RNA products produced from the MASTER-lacCONS-N7 library in vivo the 15-base transcribed-region barcode was used to associate reads derived from RNA transcripts with their template of origin (Vvedenskaya et al., 2015). As with RNAs generated in vitro, we also considered only RNA 5′-end-sequences that could be aligned to the sequence of their template of origin with no mismatches (Vvedenskaya et al., 2015). These reads were associated with one of the four reaction conditions based on the identity of the 4-nt barcode sequence.
For each of the ~16,000 sequence variants, we determined the number of reads emanating from each position of the N7 region for samples treated with Rpp (#Rpp), samples subjected to mock Rpp treatment (#Rppmock), samples subjected to NudC treatment (#NudC), and samples subjected to mock NudC treatment (NudCmock). Using the values for #NudC and #NudCmock we calculated the value of #NCIN, where #NCIN = (#NudC - #NudCmock). Negative values for #NCIN were replaced with a value of 0.
To avoid complications due to the background activity of NudC on 5′-triphosphate RNA we treated RNA generated in vivo with CIP to remove 5′-terminal phosphates prior to treatment with NudC. In contrast to the analysis of RNA produced in vitro, removal of 5′-terminal phosphates by CIP prior to NudC treatment enabled us to directly use the value of #NCIN to calculate percent capping for RNA produced in vivo.
To calculate percent capping in vivo we used the value for #Rpp instead of that for #ppp in order to include 5′-monophosphate RNA in our analysis. Thus, we calculated a value of percent capping in vivo using the formula: percent capping = 100% × [#NCIN / (#NCIN + #Rpp)].
For results shown in Figures 7A–D, analysis was done using read count sums of the three independent CapZyme-SeqNudC replicates. Results of Figure 7A represent a plot of the mean percent capping values for template sequences with (#NCIN + #Rpp) ≥ 50. Results of Figure 7B (left) and Figure S4A (left) represent the mean TSS value and mean %TSS values calculated using the sum of #NCIN of all template sequences. Results of Figure 7B (right) and Figure S4A (right) represent the mean TSS value and mean %TSS values calculated using the sum of #ppp of all template sequences. %TSS and the mean TSS were calculated using the formulas below.
Results of Figures 7C and S4C,D represent relative difference in percent capping values calculated using the formula in Figure S4B for the position 7 bp downstream of the promoter −10 element for template sequences with (#NCIN at position 7 + #ppp at position 7) ≥ 25. Relative difference in percent capping at each position was determined for groups of four promoter sequences, “quartets,” having A, G, C, or T, along with sequences identical at each other position. The relative difference in percent capping of each A+1 promoter sequence, Y, at each position, X, is calculated by subtracting the percent capping of Y by the mean percent capping of Y’s quartet at position X. For results of Figure 7D, percent capping values of the consensus and anti-consensus promoter sequences are shown.
Uncapped RNA products generated in vivo from A+1 promoters have the sequence: 5′-ANNNGUGAGCGGAUAACAAUNNNNNNNNNNNNNNNUGGAAUUCUCGGGUGCCAAGGGCCCCAGCGGCCGUCUUCAAGAGCUCAUGGAUCCGAAUAGCCAUCCCAAUCGAACAGGCCUGCUGGUAAUCGCAGGCCUUUUUAUUUGGAU-3′
Analysis of sequencing reads derived from cDNA: sRNA analysis
Reads were associated with one of the four reaction conditions based on the identity of the 4-nt barcode sequence, the next 11 bases provide the sequence of the 11-nt sequence tag, and the 16th base provides the sequence of the RNA 5′ end from which the cDNA was generated. The first 20 bases of the RNA 5′-end-sequence of each sequencing read was mapped exactly to the sequences from 50 bp upstream to 50 bp downstream of the annotated 5′-end position of each sRNA. Read counts derived from samples treated with Rpp (#Rpp), samples subjected to mock Rpp treatment (#Rppmock), samples subjected to NudC treatment (#NudC), and the number of reads for samples subjected to mock NudC treatment (NudCmock). Using the values for #NudC and #NudCmock we calculated the value of #NCIN, where #NCIN = (#NudC - #NudCmock). Negative values for #NCIN were replaced with a value of 0. As mentioned above, removal of 5′-terminal phosphates by CIP prior to NudC treatment enabled us to directly use the value of #NCIN to calculate percent capping for sRNA produced in vivo.
We first used values for #Rpp and #Rppmock to identify positions with the highest value of #Rpp for each sRNA. Next, for these positions we identified those representing primary TSS where the value of #Rpp was at least two times greater than #Rppmock. In this manner, we identified 16 primary TSS positions where the base pair is A:T and the sum of #Rpp and #NCIN is greater than 100 in each of the three replicates (Figure 7E). For these primary TSS, we calculated a value of percent capping using the formula: percent capping = 100% × [#NCIN / (#NCIN + #Rpp)]. Values reported in Figure 7E are the mean of three replicates.
Single-template gel analysis of NCIN capping with NAD+ (Bird et al., 2017)
10 nM of linear template was mixed with 50 nM RNAP holoenzyme in transcription buffer and incubated at 37°C for 15 minutes to form open complexes. 1 mM NAD+, and increasing concentrations of ATP (0, 10, 20, 50, 100, 200, 500 μM) were added along with 200 nM of non-radiolabeled extending nucleotide (CTP, UTP or GTP) plus 6 mCi of radiolabeled extending nucleotide ([α32P]-CTP, [α32P]-UTP, or [α32P]-GTP; 3000 Ci/mmol). Upon addition of nucleotides, reactions were incubated at 37°C for 10 minutes to allow for product formation. Reactions were stopped by addition of an equal volume of gel loading buffer [90% formamide, 100 mM Tris-HCl pH 8.0, 18 mM EDTA, 0.025% xylene cyanol, 0.025% bromophenol blue].
Samples were run on 20% TBE-Urea polyacrylamide gels. Bands were quantified using ImageQuant software. Observed values of NAD+pC / (pppApC + NAD+pC) were plotted vs. [NAD+]/[ATP] on semi-log plot (Sigmaplot). Non-linear regression was used to fit the data to the equation: y = (ax) / (b+x); where y is NAD+pC / (pppApC + NAD+pC), x is [NAD+] / [ATP], and a and b are regression parameters. The resulting fit yields the value of x for which y = 0.5. The relative efficiency (kcat/KM, NAD+) / (kcat/KM, ATP) is equal to 1/x.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analyses and representation of results was done using the R programming language (Version 3.4.2). The number of replicates, statistical test procedures, and definitions of center and dispersions, are in the figure legends and CapZyme-Seq data analysis section above. Statistical significance is defined using the p-value derived from the indicated statistical test.
DATA AND SOFTWARE AVAILABILITY
Source code and documentation for CapZyme-Seq analysis are provided at: https://github.com/NickelsLabRutgers.
Sequencing reads have been deposited in the NIH/NCBI Sequence Read Archive under the study accession number PRJNA411835.
Original imaging data for gels shown in Figure 1 and Figure 6 have been uploaded to Mendeley Data: http://dx.doi.org/10.17632/kjc9rypnzr.2
Supplementary Material
Methods S1 (Related to STAR Methods). Detailed protocol.
Table S1 (Related to STAR Methods). List of oligonucleotides.
Highlights.
high-throughput detection and quantitation of NAD+-capped RNA
efficiencies of NAD+ capping by E. coli RNA polymerase for ~16,000 promoter sequences
consensus promoter sequence for NAD+ capping by Escherichia coli RNA polymerase
sequence determinants for capping in vivo match consensus defined in vitro
Acknowledgments
We thank Premal Shah for advice. Work was supported by Czech Science Foundation grant 15-05228S (L.K.), and by NIH grants GM067005 (M.K), GM041376 (R.H.E), and GM118059 (B.E.N).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
Conceptualization, M.K., R.H.E, and B.E.N.; Methodology, Yu Zhang, I.B., L.K., and B.E.N.; Formal Analysis, J.B., Yuanchao Zhang, M.K., D.M.T., R.H.E. and B.E.N.; Investigation, J.G.B., I.O.V., and X.J.; Data Curation, Yuanchao Zhang; Writing – Original Draft, R.H.E. and B.E.N.; Writing – Review & Editing, J.G.B., I.O.V., L.K., and M.K.; Visualization, J.G.B., Yuanchao Zhang, and B.E.N.; Supervision, L.K., M.K., D.M.T., R.H.E., and B.E.N.; Project Administration, R.H.E. and B.E.N.; Funding Acquisition, L.K., M.K., R.H.E., and B.E.N.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arribas-Layton M, Wu D, Lykke-Andersen J, Song H. Structural and functional control of the eukaryotic mRNA decapping machinery. Biochim Biophys Acta. 2013;1829:580–589. doi: 10.1016/j.bbagrm.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artsimovitch I, Svetlov V, Murakami KS, Landick R. Co-overexpression of Escherichia coli RNA polymerase subunits allows isolation and analysis of mutant enzymes lacking lineage-specific sequence insertions. JBC. 2003;278:12344–12355. doi: 10.1074/jbc.M211214200. [DOI] [PubMed] [Google Scholar]
- Barvik I, Rejman D, Panova N, Sanderova H, Krasny L. Non-canonical transcription initiation: the expanding universe of transcription initiating substrates. FEMS Microbiol Rev. 2017;41:131–138. doi: 10.1093/femsre/fuw041. [DOI] [PubMed] [Google Scholar]
- Bird JG, Nickels BE, Ebright RH. RNA Capping by Transcription Initiation with Non-canonical Initiating Nucleotides (NCINs): Determination of Relative Efficiencies of Transcription Initiation with NCINs and NTPs. Bio-protocol. 2017:7. doi: 10.21769/BioProtoc.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird JG, Zhang Y, Tian Y, Panova N, Barvik I, Greene L, Liu M, Buckley B, Krasny L, Lee JK, et al. The mechanism of RNA 5′ capping with NAD+, NADH and desphospho-CoA. Nature. 2016;535:444–447. doi: 10.1038/nature18622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahova H, Winz ML, Hofer K, Nubel G, Jaschke A. NAD captureSeq indicates NAD as a bacterial cap for a subset of regulatory RNAs. Nature. 2015;519:374–377. doi: 10.1038/nature14020. [DOI] [PubMed] [Google Scholar]
- Chen YG, Kowtoniuk WE, Agarwal I, Shen Y, Liu DR. LC/MS analysis of cellular RNA reveals NAD-linked RNA. Nature Chem Biol. 2009;5:879–881. doi: 10.1038/nchembio.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarkar SC, Wang C, Miller MT, Ramanathan A, Jiang F, Khan AG, Patel SS, Marcotrigiano J. Structural basis for m7G recognition and 2′-O-methyl discrimination in capped RNAs by the innate immune receptor RIG-I. PNAS. 2016;113:596–601. doi: 10.1073/pnas.1515152113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y, Shatkin AJ. Viral and cellular mRNA capping: past and prospects. Adv Virus Res. 2000;55:135–184. doi: 10.1016/S0065-3527(00)55003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Lima CD. Enzymology of RNA cap synthesis. Wiley interdisciplinary reviews. RNA. 2010;1:152–172. doi: 10.1002/wrna.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grudzien-Nogalska E, Kiledjian M. New insights into decapping enzymes and selective mRNA decay. Wiley interdisciplinary reviews RNA. 2017:8. doi: 10.1002/wrna.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer K, Jaschke A. Molecular biology: A surprise beginning for RNA. Nature. 2016;535:359–360. doi: 10.1038/nature18908. [DOI] [PubMed] [Google Scholar]
- Hofer K, Li S, Abele F, Frindert J, Schlotthauer J, Grawenhoff J, Du J, Patel DJ, Jaschke A. Structure and function of the bacterial decapping enzyme NudC. Nature Chem Biol. 2016;12:730–734. doi: 10.1038/nchembio.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaschke A, Hofer K, Nubel G, Frindert J. Cap-like structures in bacterial RNA and epitranscriptomic modification. Cur Opin Microbiol. 2016;30:44–49. doi: 10.1016/j.mib.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Jensen KF, Fast R, Karlstrom O, Larsen JN. Association of RNA polymerase having increased Km for ATP and UTP with hyperexpression of the pyrB and pyrE genes of Salmonella typhimurium. J Bacteriol. 1986;166:857–865. doi: 10.1128/jb.166.3.857-865.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao X, Doamekpor SK, Bird JG, Nickels BE, Tong L, Hart RP, Kiledjian M. 5′ End Nicotinamide Adenine Dinucleotide Cap in Human Cells Promotes RNA Decay through DXO-Mediated deNADding. Cell. 2017;168:1015–1027. e1010. doi: 10.1016/j.cell.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius C, Yuzenkova Y. Bacterial RNA polymerase caps RNA with various cofactors and cell wall precursors. NAR. 2017;45:8282–8290. doi: 10.1093/nar/gkx452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keseler IM, Mackie A, Santos-Zavaleta A, Billington R, Bonavides-Martinez C, Caspi R, Fulcher C, Gama-Castro S, Kothari A, Krummenacker M, et al. The EcoCyc database: reflecting new knowledge about Escherichia coli K-12. NAR. 2017;45:D543–D550. doi: 10.1093/nar/gkw1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowtoniuk WE, Shen Y, Heemstra JM, Agarwal I, Liu DR. A chemical screen for biological small molecule-RNA conjugates reveals CoA-linked RNA. PNAS. 2009;106:7768–7773. doi: 10.1073/pnas.0900528106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kiledjian M. Regulation of mRNA decapping. Wiley interdisciplinary reviews. RNA. 2010;1:253–265. doi: 10.1002/wrna.15. [DOI] [PubMed] [Google Scholar]
- Lorenz R, Bernhart SH, Honer Zu Siederdissen C, Tafer H, Flamm C, Stadler PF, Hofacker IL. ViennaRNA Package 2.0. Algorithms Mol Biol. 2011;6:26. doi: 10.1186/1748-7188-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malygin AG, Shemyakin MF. Adenosine, NAD and FAD can initiate template-dependent RNA synthesis catalyzed by Escherichia coli RNA polymerase. FEBS letters. 1979;102:51–54. doi: 10.1016/0014-5793(79)80926-6. [DOI] [PubMed] [Google Scholar]
- Marr MT, Roberts JW. Promoter recognition as measured by binding of polymerase to nontemplate strand oligonucleotide. Science. 1997;276:1258–1260. doi: 10.1126/science.276.5316.1258. [DOI] [PubMed] [Google Scholar]
- Martinez-Rucobo FW, Kohler R, van de Waterbeemd M, Heck AJ, Hemann M, Herzog F, Stark H, Cramer P. Molecular Basis of Transcription-Coupled Pre-mRNA Capping. Mol Cell. 2015;58:1079–1089. doi: 10.1016/j.molcel.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Nubel G, Sorgenfrei FA, Jaschke A. Boronate affinity electrophoresis for the purification and analysis of cofactor-modified RNAs. Methods. 2017;117:14–20. doi: 10.1016/j.ymeth.2016.09.008. [DOI] [PubMed] [Google Scholar]
- Ramanathan A, Robb GB, Chan SH. mRNA capping: biological functions and applications. NAR. 2016;44:7511–7526. doi: 10.1093/nar/gkw551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes G, Chamberlin MJ. Ribonucleic acid chain elongation by Escherichia coli ribonucleic acid polymerase. I. Isolation of ternary complexes and the kinetics of elongation. JBC. 1974;249:6675–6683. [PubMed] [Google Scholar]
- Shatkin AJ. Capping of eucaryotic mRNAs. Cell. 1976;9:645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Shuman S. Structure, mechanism, and evolution of the mRNA capping apparatus. Prog Nucleic Acid Res Mol Biol. 2001;66:1–40. doi: 10.1016/s0079-6603(00)66025-7. [DOI] [PubMed] [Google Scholar]
- Shuman S. RNA capping: progress and prospects. RNA. 2015;21:735–737. doi: 10.1261/rna.049973.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetlov V, Artsimovitch I. Purification of bacterial RNA polymerase: tools and protocols. Methods Mol Biol. 2015;1276:13–29. doi: 10.1007/978-1-4939-2392-2_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topisirovic I, Svitkin YV, Sonenberg N, Shatkin AJ. Cap and cap-binding proteins in the control of gene expression. Wiley interdisciplinary reviews. RNA. 2011;2:277–298. doi: 10.1002/wrna.52. [DOI] [PubMed] [Google Scholar]
- Vvedenskaya IO, Zhang Y, Goldman SR, Valenti A, Visone V, Taylor DM, Ebright RH, Nickels BE. Massively Systematic Transcript End Readout, “MASTER”: Transcription Start Site Selection, Transcriptional Slippage, and Transcript Yields. Mol Cell. 2015;60:953–965. doi: 10.1016/j.molcel.2015.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters RW, Matheny T, Mizoue LS, Rao BS, Muhlrad D, Parker R. Identification of NAD+ capped mRNAs in Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:480–485. doi: 10.1073/pnas.1619369114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CM, Gershowitz A, Moss B. Methylated nucleotides block 5′ terminus of HeLa cell messenger RNA. Cell. 1975;4:379–386. doi: 10.1016/0092-8674(75)90158-0. [DOI] [PubMed] [Google Scholar]
- Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer; 2009. [Google Scholar]
- Wickham H, Francois R. dplyr: a grammar of data manipulation R package version 0.5.0 2016 [Google Scholar]
- Winkelman JT, Vvedenskaya IO, Zhang Y, Zhang Y, Bird JG, Taylor DM, Gourse RL, Ebright RH, Nickels BE. Multiplexed protein-DNA cross-linking: Scrunching in transcription start site selection. Science. 2016;351:1090–1093. doi: 10.1126/science.aad6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winz ML, Cahova H, Nubel G, Frindert J, Hofer K, Jaschke A. Capture and sequencing of NAD-capped RNA sequences with NAD captureSeq. Nature Prot. 2017;12:122–149. doi: 10.1038/nprot.2016.163. [DOI] [PubMed] [Google Scholar]
- Xiang S, Cooper-Morgan A, Jiao X, Kiledjian M, Manley JL, Tong L. Structure and function of the 5′-->3′ exoribonuclease Rat1 and its activating partner Rai1. Nature. 2009;458:784–788. doi: 10.1038/nature07731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Feng Y, Chatterjee S, Tuske S, Ho MX, Arnold E, Ebright RH. Structural basis of transcription initiation. Science. 2012;338:1076–1080. doi: 10.1126/science.1227786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods S1 (Related to STAR Methods). Detailed protocol.
Table S1 (Related to STAR Methods). List of oligonucleotides.