Abstract
We explored potential associations of the PD-1/PD-L1/PD-L2 pathway with clinical characteristics, outcome, and expression of EGFR, HER2, HER3 in oropharyngeal squamous cell carcinoma (OPSCC) using an institutional database. Protein expression was assessed by immunohistochemistry on tissue microarray sections (EGFR, HER2, HER3) or whole tissue sections (PD-1/PD-L1/PD-L2). Expression of EGFR, HER2, HER3, PD-L1, and PD-L2 was quantified on tumor cells. Maximum density of PD-1 positive lymphocytes was measured on a scale of 0–4 within the tumor mass and peritumoral stroma. Associations between biomarkers and patient outcomes were tested using descriptive and inferential statistics, logistic regression, and Cox proportional hazards models. We analyzed tissue samples from 97 OPSCC cases: median age 59 years, p16+ (71%), male (83.5%), never smokers (18%), stage 3–4 disease (77%). 25% of cases were PD-L1 positive. The proportion of PD-L1+ tumors was higher in p16+ (29%) than p16- OPSCC (11%, p=0.047). There was no correlation between PD-L1, PD-L2, PD-1, EGFR, HER2, or HER3 expression. Positive PD-L1 status correlated with advanced nodal disease on multivariate analysis (OR 5.53 (CI 1.06–28.77), p=0.042). Negative PD-L2 expression was associated with worse survival (HR 3.99 (1.37–11.58), p=0.011) in p16- OPSCC. Lower density of PD-1+ lymphocytes in peritumoral stroma was associated with significantly increased risk of death on multivariate analysis (HR=3.17 (CI 1.03–9.78), p=0.045) after controlling for prognostic factors such as stage and p16 status. PD-L1 expression on tumor cells correlates with p16 status and advanced nodal status in OPSCC. PD-1+ lymphocytes in peritumoral stroma serve as an independent prognostic factor for overall survival.
INTRODUCTION
Until recently, the standard of care for the treatment of metastatic or recurrent oropharyngeal squamous cell carcinoma (OPSCC) was chemotherapy, with cetuximab as the only targeted treatment option.(1) However, the care of OPSCC patients with recurrent or metastatic disease has been revolutionized with the development of immunotherapy. Immune checkpoint inhibitors have proven to be an effective treatment strategy for a wide range of cancers. Blockade of the programmed cell death-1 (PD-1) pathway has shown promising results (15–40% response rate) in several cancers and PD-1 targeted therapeutics have received FDA approval for the treatment of melanoma, bladder cancer, renal cell carcinoma, non-small cell lung cancer, and squamous cell carcinoma of the head and neck (SCCHN).(2–4) The PD-1 inhibitors pembrolizumab and nivolumab have been approved by the FDA for patients that progress after platinum-based chemotherapy in SCCHN.(5)
Biomarkers that can predict benefit of immunotherapy treatment are still under investigation. The most commonly employed is PD-L1. Despite a seemingly higher likelihood of response to immunotherapy in a number of PD-L1 positive tumors, the adequate cut-off for PD-L1 positivity remains under debate.(6) The correlation between the PD-1/PD-L1 pathway with clinical characteristics and outcomes in OPSCC has not been fully elucidated, with conflicting reports in the literature.(7,8) Additionally, PD-L2 is emerging as a potential biomarker for immunotherapy as well.(9) Furthermore, the relationship between PD-1/PD-L1/PD-L2 and other biologically relevant alterations, including those in the EGFR pathway (EGFR, HER2, HER3), is an understudied yet important area due to existing evidence of interactions between these pathways, especially in the tumor immune microenvironment.(10,11) Our group has previously described HER3 and its ligand as poor prognostic markers in OPSCC.(12) We sought to examine the relationships of PD-1/PD-L1/PD-L2 biomarkers with clinical characteristics, outcome, and biologically relevant alterations of the EGFR family of receptors (EGFR, HER2, and HER3) using our annotated OPSCC database.
MATERIALS AND METHODS
A total of 97 formalin-fixed paraffin-embedded (FFPE) tissue specimens were obtained from patients diagnosed with OPSCC at the Winship Cancer Institute of Emory University from 1994 through 2008. The study was conducted under an Institutional Review Board-approved protocol at Emory University and clinical characteristics were decoded in compliance with the Health Insurance Portability and Accountability Act (HIPAA). No treatments for OPSCC were administered to patients before tissue biopsy or surgery. Information regarding clinical characteristics was retrieved as documented by the treating physicians.
All immunostains were examined and scored by a head and neck pathologist (CCG). Formalin-fixed, paraffin-embedded tissue sections were used for immunohistochemistry (IHC), as described previously.(13) The slides were incubated with primary antibodies for PD-L1 (Cell Signaling Technology, dilution 1:20), PD-L2 (Cell Signaling Technology, dilution 1:50), PD-1 (Abcam, dilution 1:100), EGFR (Biogenex, dilution 1:200), HER2 (Cell Signaling Technology, dilution 1:200), or HER3 (Cell Signaling Technology, dilution 1:100). Staining of the antibodies was observed by 3,3′diaminobenzidine tetrahydrochloride peroxidase substrate solution (Vector Laboratories, Burlingame, CA). Cell surfaces were counterstained using Hematoxylin QS (Vector Laboratories). Mouse immunoglobulin G (IgG) served as a negative control, and normal epithelial tissues with known positive immunoreactions to selected antibodies were used as positive controls. For EGFR, HER2, HER3, PD-L1, and PD-L2 the intensity of staining on tumor cells was measured using a numerical scale (0, no expression; 1+, weak expression; 2+, moderate expression; and 3+, strong expression). The overall score for these stains was taken as the product of the intensity score and the percentage of tumor cells with any staining.
The maximum density of PD-1 positive lymphocytes was quantified within the tumor mass and in the stroma immediately adjacent to the infiltrating tumor (i.e. peritumoral stroma within a single 400x microscopic field of the tumor front). In both the tumor mass and the peritumoral stroma, the slides were examined for the area of maximum density of PD-1 positive lymphocytes and this area of maximum density was given a score of 0–4 (0, no PD-1 positive lymphocytes; 1, rare PD-1 positive lymphocytes [1–4/400x high power field (HPF)]; 2, moderate PD-1 positive lymphocytes [5–19/400x HPF]; 3, high PD-1 positive lymphocytes [20–49/400x HPF]; 4, very high PD-1 positive lymphocytes [≥50/400x HPF]). When scoring the peritumoral stroma, germinal centers were avoided.
Statistical analysis was conducted using SAS Version 9.3, and SAS macros or software developed at the Biostatistics and Bioinformatics Shared Resource at Winship Cancer Institute.(14) The significance level was set at 0.05. Overall survival (OS) was defined as months from date of diagnosis to death or last follow up, and disease free survival (DFS) was defined as months from date of diagnosis to progression or last follow up scan. Descriptive statistics for each variable were reported. The univariate association of each covariate with each biomarker was assessed using the chi-square test for categorical covariates and ANOVA for numerical covariates. The association with OS or DFS was assessed using Cox proportional hazards models and the association with advanced nodal status, defined as N2 disease or above, was carried out by logistic regression model. The multivariable model was fit by a backward variable selection method applying an alpha = 0.20 removal criteria. KM plots were produced to compare survival curves along with the log-rank p-value.
RESULTS
Demographic and clinical information for the 97 OPSCC patients included is shown in Table 1. The median age of the patients was 59 years, 83.5% of patients were male, 81.4% were Caucasian, and 17.7% were never-smokers. Among these oropharyngeal tumors, 71.1% were p16 positive. The majority of tumors were early T stage (T1/2 = 86.2%) compared to late T stage (T3/4 = 13.8%) (p<0.05). The majority of patients had advanced nodal disease (N2–3, 66.7%) and had locally advanced disease (stage 3–4, 77.4%).
Table 1.
Patient Characteristics and Tumor Staining
| Variable | Level | N (%) = 97 |
|---|---|---|
|
| ||
| Age | Median | 59.00 |
|
| ||
| p16 | negative | 28 (28.9) |
| positive | 69 (71.1) | |
|
| ||
| Gender | male | 81 (83.5) |
| female | 16 (16.5) | |
|
| ||
| Race | AA | 12 (12.4) |
| White | 79 (81.4) | |
| Other/Unknown | 6 (6.2) | |
|
| ||
| Smoking | never | 17 (17.7) |
| former | 41 (42.7) | |
| current | 38 (39.6) | |
|
| ||
| Differentiation | WD | 5 (5.2) |
| MD | 40 (41.2) | |
| NK | 52 (53.6) | |
|
| ||
| Tumor Stage | 1 | 39 (41.5) |
| 2 | 42 (44.7) | |
| 3–4 | 13 (13.8) | |
|
| ||
| Node Metastasis | N0–1 | 32 (33.3) |
| N2–3 | 64 (66.7) | |
|
| ||
| Overall Stage | 1–2 | 19 (22.6) |
| 3–4 | 65 (77.4) | |
|
| ||
| EGFR average overall | <= median (0.8) | 50 (52.6) |
| > median (0.8) | 45 (47.4) | |
|
| ||
| HER2 average overall | 0 | 71 (74.0) |
| 0.025–0.3 | 25 (26.0) | |
|
| ||
| HER3 average overall | <=median (0.1625) | 47 (49.5) |
| >median (0.1625) | 48 (50.5) | |
|
| ||
| PD-L1 | 0 | 71 (74.7) |
| 1–130 | 24 (25.3) | |
|
| ||
| PD-L2 | 0 | 33 (38.8) |
| 1–130 | 52 (61.2) | |
|
| ||
| PD-1+ lymphocytes in tumor | 0–1 | 33 (35.5) |
| 2–4 | 60 (64.5) | |
|
| ||
| PD-1+ lymphocytes in peritumoral stroma | 0–1 | 11 (11.8) |
| 2–4 | 82 (88.2) | |
AA African-American; WD well-differentiated; MD moderately differentiated; NK non-keratinized
The results of the IHC stains are also shown in Table 1. Based on the methods described above, 25% of tumors were positive (score of 1–130) for PD-L1, 61% PD-L2 positive, and 64.5% had at least a high maximum lymphocyte density within the tumor (scaled value of 2–4). Within the peritumoral stroma, 88.2% had at least a high density of PD-1 positive lymphocytes. Finally, 47.4%, 26%, and 50.5% of carcinomas were positive for EGFR, HER2 and HER3, respectively. Representative samples of tumor PD-L1, PD-1, and peritumoral stroma PD-1 staining are shown in Supplemental Figure 1.
We first sought to establish if the expression of the biomarkers studied correlated with p16 status (Table 2). There was a higher proportion of PD-L1 positivity in p16 positive than in p16 negative OPSCC tumors (29% versus 11%, p=0.047). PD-L2 and PD-1 lymphocyte density, on the other hand, whether examined in the tumor or peritumoral stroma, did not correlate with p16 status. OPSCC tumors that were EGFR positive were associated with p16 tumor negativity (p=0.028). There was no correlation between PD-1, PD-L1, PD-L2, HER2, HER3, and EGFR expression (Table 3).
Table 2.
Univariate Association with p16
| p16 | |||||
|---|---|---|---|---|---|
|
| |||||
| Covariate | Statistics | Level | negative N=28 | positive N=69 | P-value* |
|
| |||||
| PD-L1 | N (Row %) | 0 | 24 (34) | 45 (65)) | 0.047 |
| N (Row %) | 1–130 | 3 (13) | 20 (87 | ||
|
| |||||
| PD-L2 | N (Row %) | 0 | 11 (35) | 20 (65) | 0.243 |
| N (Row %) | 1–130 | 12 (24) | 39 (76) | ||
|
| |||||
| PD-1+ lymphocytes in tumor | N (Row %) | 0–1 | 12 (36) | 21 (64) | 0.316 |
| N (Row %) | 2–4 | 15 (26) | 42 (74) | ||
|
| |||||
| PD-1+ lymphocytes in peritumoral stroma | N (Row %) | 0–1 | 2 (18) | 9 (82) | 0.494 |
| N (Row %) | 2–4 | 25 (32) | 54 (68) | ||
|
| |||||
| EGFR average overall | N (Row %) | <= median (0.8) | 9 (19) | 38 (81) | 0.028 |
| N (Row %) | > median (0.8) | 18 (40) | 27 (60) | ||
|
| |||||
| HER2 average overall | N (Row %) | 0 | 18 (26) | 51 (74) | 0.496 |
| N (Row %) | 0.025–0.3 | 8 (33) | 16 (67) | ||
|
| |||||
| HER3 average overall | N (Row %) | <=median (0.1625) | 15 (33) | 31 (67) | 0.354 |
| N (Row %) | >median (0.1625) | 11 (24) | 35 (76) | ||
Table 3.
Univariate Association of EGFR, HER2, HER3 and PD-1, PD-L1, PD-L2
| PD-1+ lymphocytes in tumor | |||||
|---|---|---|---|---|---|
| Covariate | Statistics | Level | 0–1 N=33 | 2–4 N=57 | P-value* |
| EGFR average overall | N (Row %) | <= median (0.8) | 17 (39.53) | 26 (60.47) | 0.5 |
| N (Row %) | > median (0.8) | 14 (32.56) | 29 (67.44) | ||
| HER2 average overall | N (Row %) | 0 | 23 (33.82) | 45 (66.18) | 0.505 |
| N (Row %) | 0.025–0.3 | 8 (42.11) | 11 (57.89) | ||
| HER3 average overall | N (Row %) | <=median (0.1625) | 19 (44.19) | 24 (55.81) | 0.116 |
| N (Row %) | >median (0.1625) | 12 (27.91) | 31 (72.09) | ||
| PD-1+ lymphocytes in peritumoral stroma | |||||
| Covariate | Statistics | Level | 0–1 N=11 | 2–4 N=79 | P-value* |
| EGFR average overall | N (Row %) | <= median (0.8) | 6 (13.95) | 37 (86.05) | 0.747 |
| N (Row %) | > median (0.8) | 5 (11.63) | 38 (88.37) | ||
| HER2 average overall | N (Row %) | 0 | 8 (11.76) | 60 (88.24) | 0.699 |
| N (Row %) | 0.025–0.3 | 3 (15.79) | 16 (84.21) | ||
| HER3 average overall | N (Row %) | <=median (0.1625) | 6 (13.95) | 37 (86.05) | 0.747 |
| N (Row %) | >median (0.1625) | 5 (11.63) | 38 (88.37) | ||
| PD-L1 | |||||
| Covariate | Statistics | Level | 0 N=69 | 1–130 N=23 | P-value* |
| EGFR average overall | N (Row %) | <= median (0.8) | 38 (84.44) | 7 (15.56) | 0.061 |
| N (Row %) | > median (0.8) | 29 (67.44) | 14 (32.56) | ||
| HER2 average overall | N (Row %) | 0 | 51 (73.91) | 18 (26.09) | 0.770 |
| N (Row %) | 0.025–0.3 | 16 (80) | 4 (20) | ||
| HER3 average overall | N (Row %) | <=median (0.1625) | 34 (77.27) | 10 (22.73) | 0.622 |
| N (Row %) | >median (0.1625) | 32 (72.73) | 12 (27.27) | ||
| PD-L2 | |||||
| Covariate | Statistics | Level | 0 N=31 | 1–130 N=51 | P-value* |
| EGFR average overall | N (Row %) | <= median (0.8) | 15 (41.67) | 21 (58.33) | 0.686 |
| N (Row %) | > median (0.8) | 16 (37.21) | 27 (62.79) | ||
| HER2 average overall | N (Row %) | 0 | 22 (36.07) | 39 (63.93) | 0.520 |
| N (Row %) | 0.025–0.3 | 8 (44.44) | 10 (55.56) | ||
| HER3 average overall | N (Row %) | <=median (0.1625) | 13 (36.11) | 23 (63.89) | 0.755 |
| N (Row %) | >median (0.1625) | 17 (39.53) | 26 (60.47) | ||
The p-value is calculated by chi-square test or Fisher’s exact, where appropriate.
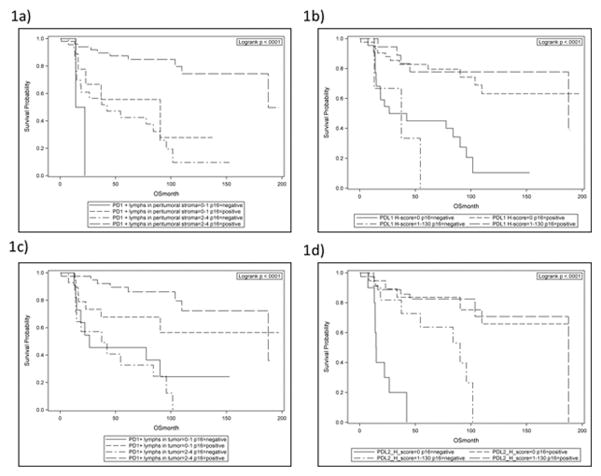
Subsequently, we examined whether PD-L1, PD-L2 tumor expression and PD-1 positive lymphocyte density in our OPSCC population correlated with advanced nodal status, defined as N2 disease or above. On multivariate analysis, tumors that were PD-L1 positive were significantly associated with advanced nodal status (OR 5.53, CI 1.06–28.77, p=0.042). Conversely, PD-1 positive lymphocyte density did not correlate with nodal status. PD-L2 positive tumors trended towards more advanced nodal disease (OR 3.12, CI 0.71–10.99, p=.054), but this did not reach statistical significance (Table 4). Interestingly, patients with a high density of PD-1 positive lymphocytes in the peritumoral stroma had improved survival compared to patients that had lower PD-1 positive lymphocyte density in the peritumoral stroma in a multivariable analysis controlling for other prognostic factors such as age, p16 status, and stage (HR = 3.17, CI 1.03–9.78, p = 0.045). The improved survival seen in this population was observed regardless of p16 status (Figure 1a). Tumor expression of PD-L1, EGFR, HER2, and HER3, and PD-1 positive lymphocyte density within the tumor were not associated with overall survival (Table 5, Figures 1b and 1c). Additionally, PD-L2 overall was not associated with survival, however, for p16- OPSCC tumors, those that did not express PD-L2 had worse survival outcomes (HR 5.29, CI 1.64–17.03, p=0.005) (Figure 1d).
Table 4.
Multivariate Analysis of Advanced Nodal Metastasis
| Node Metastasis = N2–3 | |||
|---|---|---|---|
|
| |||
| Covariate | Level | Odds Ratio (95% CI) | OR P-value |
|
| |||
| PD-L1 | 1–130 | 5.53 (1.06–28.77) | 0.042 |
| 0 | - | - | |
|
| |||
| PD-L2 | 1–130 | 3.12 (0.71–10.99) | 0.054 |
| 0 | - | - | |
|
| |||
| PD-1+ lymphocytes in tumor | 0–1 | 0.87 (0.31–2.48) | 0.795 |
| 2–4 | - | - | |
|
| |||
| PD-1 + lymphocytes in peritumoral stroma | 0–1 | 2.44 (0.43–13.91) | 0.316 |
| 2–4 | - | - | |
Figure 1.
Figure 1a: Overall Survival Based on PD-1 in Peritumoral Stroma
Figure 1b: Overall Survival Based on PD-L1 in Tumor
Figure 1c: Overall Survival Based on PD-1 in Tumor
Figure 1d: Overall Survival Based on PD-L2 in Tumor
Table 5.
Multivariate Survival Analysis
| OS month | |||||
|---|---|---|---|---|---|
|
| |||||
| Covariate | Level | N | Median Survival (m)(95% CI) | Hazard Ratio (95% CI) | HR P-value |
|
| |||||
| EGFR average overall | <= median (0.8)) | 47 | 109.8 (90.3, NA) | 0.77 (0.35–1.69) | 0.516 |
| > median (0.8 | 43 | 90.2 (37.4, 187.6) | - | -- | |
|
| |||||
| HER3 average overall | <=median (0.1625) | 46 | 103.7 (84, 187.6) | 0.89 (0.39–2.03) | 0.778 |
| >median (0.1625) | 44 | 109.8 (22.4, NA) | - | - | |
|
| |||||
| HER2 average overall | 0 | 68 | 103.7 (90.2, 187.6) | 0.70 (0.29–1.65) | 0.414 |
| 0.025–0.3 | 23 | 42.3 (18.6, NA) | - | - | |
|
| |||||
| PD-L1 | 0 | 67 | 101.6 (84, NA) | 1.06 (0.61–3.01) | 0.911 |
| 1–130 | 23 | 187.6 (45.2, 187.6) | - | - | |
|
| |||||
| PD-L2 | 0 | 30 | 109.8 | 1.44 (0.61–3.43) | 0.406 |
| 1–130 | 50 | 103.7 | - | - | |
|
| |||||
| PD-1+ lymphocytes in tumor | 0–1 | 33 | 90.2 (23.2, NA) | 1.11 (0.51–2.42) | 0.797 |
| 2–4 | 55 | 109.8 (95.8, 187.6) | - | - | |
|
| |||||
| PD-1+ lymphocytes in peritumoral stroma | 0–1 | 11 | 37 (14.2, NA) | 3.17 (1.03–9.78) | 0.045 |
| 2–4 | 77 | 187.6 (90.2, NA) | - | - | |
DISCUSSION
As immunotherapy has ushered in a new era for the treatment of SCCHN and PD-1 inhibitors are now standard of care in the treatment of recurrent or metastatic SCCHN, further investigation of immune-related biomarkers and their interaction with other cancer-related pathways is warranted. In our study, we examined potential correlations between EGFR, HER2, HER3, PD-1, PD-L1, and PD-L2 expression and their association with clinical characteristics and outcomes in patients with OPSCC.
Our study demonstrated that the density of PD-1 positive lymphocytes in peritumoral stroma correlates with increased mortality. This highlights the potential role the tumor microenvironment may play in OPSCC. However, given that the PD-L1/PD-1 pathway acts as a “brake” of the immune system, it was expected that increased PD-1 signaling on tumor infiltrating lymphocytes (TILs) and/or the peritumoral stroma would be associated with worse outcomes.(15) Previous studies have demonstrated the activity of the PD-L1/PD-1 pathway in HPV-driven OPSCC, theorizing the potential role immune resistance may have in these tumors.(16) Badoual et al. examined the role of PD-1 positive TILs as a prognostic biomarker in HPV associated OPSCC. Not only did HPV associated OPSCC have higher levels of TILs, but the increased infiltration of TILs around the tumor correlated with better prognosis for this group of patients. A possible explanation of these findings is that PD-1 status could be a marker of immune activation during HPV driven OPSCC tumorigenesis, leading to the observed improved outcomes.(8) Other studies have also demonstrated that the density of TILs in SCCHN may predict outcome.(17,18) However, evidence is mounting that there is heterogeneity within the tumor microenvironment and more complete characterization of TILs is lacking.(19) This problem is noted in other tumor types as well. Increased PD-1 TIL signaling in the tumor microenvironment has been shown to be a positive prognostic marker in a variety of tumor types, including ovarian and pancreatic tumors, while high PD-1 TIL expression was associated with worse outcomes in other histologies such as renal cell carcinoma.(20–22) Our study demonstrates a correlation between PD-1 staining on TILs within the tumor microenvironment, specifically the peritumoral region, and better survival in both p16 positive and negative cohorts. Interestingly, PD-L1 expression was not associated with survival. There was a correlation between higher PD-L1 expression and increased nodal status (p-value = 0.042). Additionally, PD-L2 is currently being examined as potentially playing an important role in the PD-1 pathway. Although less studied than PD-L1, PD-L2 has been shown to play an important role in immunosuppression and T cell function.(23) Therefore, it holds interest as a potential biomarker and target for immunotherapy treatment. In fact, Yearley et al. examined PD-L2 expression across tumor types, and found that HNSCC had the highest level of tumor staining, at greater than 50% of HNSCC tested. This correlates with our data where we found 61% of tumors were PD-L2 positive. Additionally, they found that PD-L2 was an independent predictor of PFS and OS for patients undergoing treatment with pembrolizumab.(9) Our study showed that PD-L2 is a poor prognostic marker for p16- HNSCC patients, and highlights that this group may get superior benefit from checkpoint blockade.
The EGFR, HER2, and HER3 pathways have been proven to play important roles in the tumorigenesis of SCCHN.(24) Prior work from our group demonstrated that high heregulin mRNA and high HER3 protein levels were associated with worse prognosis.(12) The importance of these pathways has led to the development of novel therapeutics for the treatment of this patient population, such as the anti-EGFR antibody cetuximab, now FDA-approved for SCCHN in both the definitive and metastatic setting.(1,25) Of additional interest is that several studies have demonstrated an association of the EGFR pathway with the immune system in SCCHN. This has been less well defined for HER2 and HER3.(26,27) We did not see a correlation between EGFR, HER2, and HER3 expression analyzed by IHC and immune markers or overall outcomes in our study. Dynamic expression of these markers is expected during the course of therapy and therefore analyses such as ours may not reflect an accurate picture of the interaction between these pathways. PD-1 and PD-L1 expression have been shown to be upregulated in relation to treatment with concurrent chemotherapy and radiation in HPV positive SCCHN patients as well as other tumors.(28,29) Further insight into the dynamic interplay of the EGFR pathway and PD-1/PD-L1 is needed and may help guide the design of future clinical trials using these combinations.
Despite the unique results of our study, there are important limitations that should be noted. Given the retrospective nature of our analysis and that patients were treated with curative modalities, we were unable to assess the predictive value of the biomarkers studied in regards to immunotherapy. Furthermore, biopsies may not provide enough information regarding the topography of the entire tumor to relay a more accurate status for PD-L2/PD-1/PD-L1 and TILs. In the biomarker analysis of the KEYNOTE-012 study, PD-L1 expression correlated with response and PFS in patients treated with pembrolizumab in the metastatic setting, although the sample size was too small to make any firm conclusions.(30,31) Likewise, in the Checkmate-141 phase 3 trial examining nivolumab in comparison to investigator’s choice chemotherapy in recurrent or metastatic SCCHN post-platinum exposure, a trend for improved survival in patients receiving nivolumab was noted in the PD-L1 positive (PD-L1 ≥1%) group.(32) These results cannot, however, be considered conclusive given the nature of the analysis.(2) Further exploration of relevant biomarkers is underway in these landmark studies. More work is needed to better define the population that will benefit from these new drugs.
As the future of SCCHN treatment will rely on different combinations of immunotherapeutic agents with or without cytotoxic or targeted systemic therapy, understanding the interactions between anti-cancer agents, tumor biology, and the microenvironment is of utmost importance. Our results add to existing evidence suggesting that the tumor immune microenviroment plays a role in OPSCC and ought to be a major focus of future research.
Supplementary Material
Acknowledgments
Research reported in this publication was supported in part by the biostatistics and bioinformatics of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors report there are no relevant disclosures or conflicts of interest.
References
- 1.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. The New England journal of medicine. 2008;359(11):1116–27. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 2.Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. The New England journal of medicine. 2016;375(19):1856–67. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizvi NA, Mazieres J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. The Lancet Oncology. 2015;16(3):257–65. doi: 10.1016/s1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32(10):1020–30. doi: 10.1200/jco.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauml J, Seiwert TY, Pfister DG, Worden F, Liu SV, Gilbert J, et al. Pembrolizumab for Platinum- and Cetuximab-Refractory Head and Neck Cancer: Results From a Single-Arm, Phase II Study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2017 doi: 10.1200/jco.2016.70.1524. Jco2016701524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ilie M, Hofman V, Dietel M, Soria JC, Hofman P. Assessment of the PD-L1 status by immunohistochemistry: challenges and perspectives for therapeutic strategies in lung cancer patients. Virchows Archiv: an international journal of pathology. 2016;468(5):511–25. doi: 10.1007/s00428-016-1910-4. [DOI] [PubMed] [Google Scholar]
- 7.Cho YA, Yoon HJ, Lee JI, Hong SP, Hong SD. Relationship between the expressions of PD-L1 and tumor-infiltrating lymphocytes in oral squamous cell carcinoma. Oral oncology. 2011;47(12):1148–53. doi: 10.1016/j.oraloncology.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, et al. PD-1–Expressing Tumor-Infiltrating T Cells Are a Favorable Prognostic Biomarker in HPV-Associated Head and Neck Cancer. Cancer Research. 2013;73(1):128–38. doi: 10.1158/0008-5472.can-12-2606. [DOI] [PubMed] [Google Scholar]
- 9.Yearley JH, Gibson C, Yu N, Moon C, Murphy E, Juco J, et al. PD-L2 Expression in Human Tumors: Relevance to Anti-PD-1 Therapy in Cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2017;23(12):3158–67. doi: 10.1158/1078-0432.ccr-16-1761. [DOI] [PubMed] [Google Scholar]
- 10.Concha-Benavente F, Srivastava RM, Trivedi S, Lei Y, Chandran U, Seethala RR, et al. Identification of the Cell-Intrinsic and -Extrinsic Pathways Downstream of EGFR and IFNgamma That Induce PD-L1 Expression in Head and Neck Cancer. Cancer Res. 2016;76(5):1031–43. doi: 10.1158/0008-5472.can-15-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Concha-Benavente F, Ferris RL. Oncogenic growth factor signaling mediating tumor escape from cellular immunity. Current opinion in immunology. 2017;45:52–9. doi: 10.1016/j.coi.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian G, Jiang N, Wang D, Newman S, Kim S, Chen Z, et al. Heregulin and HER3 are prognostic biomarkers in oropharyngeal squamous cell carcinoma. Cancer. 2015;121(20):3600–11. doi: 10.1002/cncr.29549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muller S, Su L, Tighiouart M, Saba N, Zhang H, Shin DM, et al. Distinctive E-cadherin and epidermal growth factor receptor expression in metastatic and nonmetastatic head and neck squamous cell carcinoma: predictive and prognostic correlation. Cancer. 2008;113(1):97–107. doi: 10.1002/cncr.23557. [DOI] [PubMed] [Google Scholar]
- 14.Nickleach D, Liu Y, Shrewsberry A, Ogan K, Kim S, Wang Z. Common Biostatistical Analyses and Generate Reports. SESUG 2013: The Proceeding of the SouthEast SAS User SESUG 2013. The Proceeding of the SouthEast SAS User Group. http://analytics.ncsu.edu/sesug/2013/PO-05.pdf2013.
- 15.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer. 2012;12(4):252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer research. 2013;73(6):1733–41. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balermpas P, Michel Y, Wagenblast J, Seitz O, Weiss C, Rodel F, et al. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. British journal of cancer. 2014;110(2):501–9. doi: 10.1038/bjc.2013.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen N, Bellile E, Thomas D, McHugh J, Rozek L, Virani S, et al. Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head & neck. 2016;38(7):1074–84. doi: 10.1002/hed.24406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lei Y, Xie Y, Tan YS, Prince ME, Moyer JS, Nor J, et al. Telltale tumor infiltrating lymphocytes (TIL) in oral, head & neck cancer. Oral oncology. 2016;61:159–65. doi: 10.1016/j.oraloncology.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darb-Esfahani S, Kunze CA, Kulbe H, Sehouli J, Wienert S, Lindner J, et al. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget. 2016;7(2):1486–99. doi: 10.18632/oncotarget.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diana A, Wang LM, D’Costa Z, Allen P, Azad A, Silva MA, et al. Prognostic value, localization and correlation of PD-1/PD-L1, CD8 and FOXP3 with the desmoplastic stroma in pancreatic ductal adenocarcinoma. Oncotarget. 2016;7(27):40992–1004. doi: 10.18632/oncotarget.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, et al. PD-1 Is Expressed by Tumor-Infiltrating Immune Cells and Is Associated with Poor Outcome for Patients with Renal Cell Carcinoma. Clinical Cancer Research. 2007;13(6):1757–61. doi: 10.1158/1078-0432.ccr-06-2599. [DOI] [PubMed] [Google Scholar]
- 23.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nature immunology. 2001;2(3):261–8. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 24.Rysman B, Mouawad F, Gros A, Lansiaux A, Chevalier D, Meignan S. Human epidermal growth factor receptor 3 in head and neck squamous cell carcinomas. Head & neck. 2016;38(Suppl 1):E2412–8. doi: 10.1002/hed.24367. [DOI] [PubMed] [Google Scholar]
- 25.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. The New England journal of medicine. 2006;354(6):567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 26.Srivastava RM, Trivedi S, Concha-Benavente F, Gibson SP, Reeder C, Ferrone S, et al. CD137 Stimulation Enhances Cetuximab-Induced Natural Killer: Dendritic Cell Priming of Antitumor T-Cell Immunity in Patients with Head and Neck Cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016 doi: 10.1158/1078-0432.ccr-16-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trivedi S, Srivastava RM, Concha-Benavente F, Ferrone S, Garcia-Bates TM, Li J, et al. Anti-EGFR Targeted Monoclonal Antibody Isotype Influences Antitumor Cellular Immunity in Head and Neck Cancer Patients. Clinical Cancer Research. 2016;22(21):5229. doi: 10.1158/1078-0432.CCR-15-2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parikh F, Duluc D, Imai N, Clark A, Misiukiewicz K, Bonomi M, et al. Chemoradiotherapy-induced upregulation of PD-1 antagonizes immunity to HPV-related oropharyngeal cancer. Cancer research. 2014;74(24):7205–16. doi: 10.1158/0008-5472.CAN-14-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheng J, Fang W, Yu J, Chen N, Zhan J, Ma Y, et al. Expression of programmed death ligand-1 on tumor cells varies pre and post chemotherapy in non-small cell lung cancer. Scientific reports. 2016;6:20090. doi: 10.1038/srep20090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. The Lancet Oncology. 2016;17(7):956–65. doi: 10.1016/s1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 31.Chow LQ, Mehra R, Haddad R, Mahipal A, Weiss J, Berger R, et al. Biomarkers and response to pembrolizumab (pembro) in recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC) Chicago: USA: 2016. [Google Scholar]
- 32.Ferris R, Concha-Benavente F, Blumenschein G, Jr, Harrington K, Fayette J, Colevas AD, et al. Characterization of potential predictive biomarkers of response to nivolumab in CheckMate-141 in patients with squamous cell carcinoma of the head and neck (SCCHN) Orlando, USA: 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.