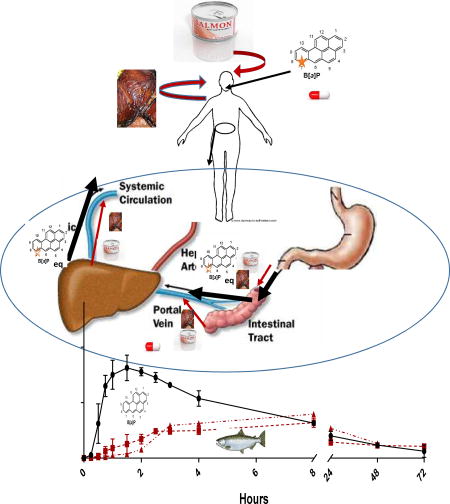
Abstract
Benzo[a]pyrene (BaP), a polycyclic aromatic hydrocarbon (PAH), is a known human carcinogen. In non-smoking adults greater than 95% of BaP exposure is through diet. The carcinogenicity of BaP is utilized by the U.S. EPA to assess relative potency of complex PAH mixtures. PAH relative potency factors (RPFs, BaP=1) are determined from high dose animal data. We employed accelerator mass spectrometry (AMS) to determine pharmacokinetics of [14C]-BaP in humans following dosing with 46 ng (an order of magnitude lower than human dietary daily exposure and million-fold lower than animal cancer models). To assess the impact of co-administration of food with a complex PAH mixture, humans were dosed with 46 ng of [14C]-BaP with or without smoked salmon. Subjects were asked to avoid high BaP-containing diets and a 3-day dietary questionnaire given to assess dietary exposure prior to dosing and three days post-dosing with [14C]-BaP. Co-administration of smoked salmon, containing a complex mixture of PAHs with an RPF of 460 ng BaPeq, reduced and delayed absorption. Administration of canned commercial salmon, containing very low amounts of PAHs, showed the impacts on pharmacokinetics were not due to high amounts of PAHs but rather a food matrix effect.
Keywords: Pharmacokinetics, Benzo[a]pyrene, Accelerator Mass Spectrometry, Dietary Polycyclic Aromatic Hydrocarbons
Graphical abstract
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs), a major human health concern, are formed by the incomplete combustion or volatilization of carbon (e.g., coal, petroleum, wood, tobacco, coal tar-based sealcoat products, automobile tires) and human exposures are associated with multiple diseases including atherosclerosis, asthma, and cancers in a number of target organs including lung (IARC, 2010; Sadiktsis et al., 2012; Titaley et al., 2016). The Agency for Toxic Substances and Disease Registry (ATSDR) lists PAHs as 3 of the top 10 chemicals of concern at priority pollutant sites (ATSDR, 2013). The higher molecular weight PAHs (4 rings and higher) tend to be more carcinogenic and the majority of daily human exposure is through diet (Bansal and Kim, 2015; Dieziel et al., 2011; Domingo and Nadal, 2015; Kazerouni et al., 2001). Benzo[a]pyrene (BaP) is the prototypical carcinogenic PAH, 8th on the ATSDR list (ATSDR, 2013), classified by the International Agency for Research on Cancer (IARC) as a known human carcinogen (IARC, 2010), and used as the standard by agencies such as U.S. Environmental Protection Agency (EPA) in determining relative carcinogenic potency for complex PAH mixtures (the Relative Potency Factor or RPF) (U.S. EPA, 2010) to which we are daily exposed.
Physiologically Based Pharmacokinetic (PBPK) models and cancer studies used for human risk assessment are done primarily in rodent models at doses 5–6 orders of magnitude higher than the average daily BaP exposure, estimated at 270–700 ng (non-smoking adult in the U.S.) (U.S. EPA, 2017). Accelerator mass spectrometry (AMS), with its high sensitivity (low attomole) (Forsgard et al., 2010) allows for study of the pharmacokinetics of human carcinogens at doses that represent a de minimus risk to subjects (Cupid et al., 2004; Garner et al., 1999; Jubert et al., 2009; Lightfoot et al., 2000; Madeen et al., 2015; 2016; Malfatti et al., 2016; Turteltaub et al., 1997). Previously, our laboratory determined the pharmacokinetics of aflatoxin B1 (AFB1) (Jubert et al., 2009), and dibenzo[def,p]chrysene (DBC) (Madeen et al., 2015; 2016) in humans at doses below the LDAL and [14C] amounts (5 nCi) that are orders of magnitude lower than previously used in diagnostic procedures (Atherton and Spiller, 1994) or clinical trials (Ottaviani et al., 2016).
The pharmacokinetic studies of AFB1 (Jubert et al., 2009) and DBC (Madeen et al., 2015; 2016) were performed with individuals that had fasted overnight and with cellulose capsules containing only the chemical under test. A more realistic scenario would be co-administration with food. The use of smoked salmon from the Confederated Tribes of the Umatilla Indian Reservation (CTUIR) provided a food matrix with a complex PAH mixture formed during the wood-smoking of Chinook salmon caught by the tribe from the Columbia River (Forsberg et al., 2011; 2012; Motorykin et al., 2015). Co-administration of this complex PAH mixture allows for a test of assumptions critical to the use of the RPF approach to risk assessment for PAH mixtures, e.g., that a PAH congener does not interfere with the ADME of another PAH (in this case [14C]-BaP) and risk assessment can be done by summing the RPF for each congener multiplied by the percent composition in the mixture of that congener. Thus, this additivity depends upon no inhibition or induction of enzymes involved in ADME or alteration of transport (Jarvis et al., 2014).
BaP and total PAHs are found in almost all foods and are especially high in smoked meats and cheeses and charcoal-broiled foods (Bansal and Kim, 2015; Dieziel et al., 2011; Domingo and Nadal, 2015; Kazerouni et al., 2001; Zelinkova and Wenzl, 2015). In an attempt to reduce the impact on study subjects from even the small BaP dose used in this study, subjects were asked to follow a diet restricted in levels of BaP prior to and during the study.
2. Materials and Methods
2.1. Enrollment Criteria and Demographics of Subjects
Note- This study was conducted under an FDA IND (#117175), Oregon State University IRB approval (#5644) and LLNL-approved IRB Protocol 2017-008.
Volunteers had to meet the following entry criteria: age 21–65; healthy; nonsmoking household; not using medications that can affect gut motility; no history of gastrointestinal surgeries; kidney or liver disease; GI diseases such as Crohn’s, ulcerative colitis, or gastritis. Women volunteers had to be post-menopausal or have had surgical sterilization to eliminate any possibility for fetal exposure as the fetus is expected to have a greater sensitivity to PAH toxicity. Volunteers with potentially high occupational PAH exposures (roofers, asphalt pavers, fire-fighters, etc.) were excluded. A recent routine medical examination was performed (within 4 weeks) by a licensed physician to ensure good general health. The screening assessment included obtaining a menopausal history and performance of a urine pregnancy test for all women. We did not require exclusion based on PAH exposure in air or diet, however, during the screening we provided volunteers with a list of foods containing high levels of PAHs and asked they avoid these foods during the trial. The weight (mean 86.7, range 55.6–131.8 Kg), height (mean 176, range 157–190 cm), BMI (mean 27.9, range 18.5–36.5) gender (3 males and 2 females), age (mean 46, range 30–63 years), and race (all Caucasian, not Hispanic) were recorded under an assigned code number for enrolled subjects. As the research involves dosing human participants with radiolabeled compounds, the Radiation Safety Committee at OSU reviewed and approved the protocol.
2.2. Food Diaries
Each volunteer was requested to avoid intake, for 1 week prior to dosing and during the study, of foods high in PAHs such as smoked meats and cheeses or food broiled over charcoal. Volunteers were required to keep a 3-day food diary prior to dosing and for the 3-day study period (see Table 1 for example). The results were used to estimate dietary intake of BaP over the study period with the latest data from the Joint FAO/WHO Expert Committee on Food Additives (JECFA, 2012).
Table 1.
Example of Food Diary and Calculation of BaP Intake
| Food Item and Method of Preparation |
Portion Size |
Portion Size (g) |
BaP ng/g | BaP ng/portion size | |
|---|---|---|---|---|---|
| Low | High | ||||
| Coffee, black | 32 oz | 960 | 0.011 | 10.56 | |
| Breakfast Hash, pan fried | 1 serving | ||||
| Pork Sausage | 1/4 cup | 32.5 | 0.02 | 0.65 | |
| Egg | 1 each | 50 | 0.03 | 1.50 | |
| Potato | 1 medium | 213 | 0.001–0.17 | 0.21 | 36.21 |
| Grated Cheddar | 1/4 cup | 28.25 | 0.50 | 14.13 | |
| Onion | 2 Tbsp | 20 | 0.46 | 9.20 | |
BaP Meal Intake Range 36.25 – 72.25 ng
2.3. Radiochemical Purity of [7-14C]-BaP and Preparation of [14C]-BaP Capsule
The FDA IND (#117175) and OSU Radiation Safety and IRB committees required quarterly reports of the radiochemical purity of the [7-14C]-BaP (26.7 µCi/µmol) stock used for dosing. If not ≥ 98%, the material had to be purified. We utilized a slight modification of Ramsauer et al. (2011), with reverse-phase LC and UV detection. Fractions were collected and counted via liquid scintillation. The stock solution (toluene) was stored in amber vials under argon at −80°C. For preparation of dosing capsules, a portion of the stock was blown to dryness under argon and re-dissolved in food grade ethanol (25 µL/5 nCi) prior to adding to food-grade cellulose capsules (ethanol evaporated prior to sealing capsule). At least 3 capsules were counted by liquid scintillation to ensure the proper dose/capsule. If the total counts varied (5 nCi = 11,500 dpm) by more than ± 10%, a new batch was made. Each batch was stored in the dark at 4°C and discarded if not used within 1 week.
2.4. Acquisition of Smoked Salmon from the CTUIR, Analysis of PAH Composition and Estimated BaPeq Based on Known RPFs
Salmon were smoked in tipi or shed structures over apple or alder wood fire (Forsberg, 2012). The smoking process preserves the salmon as well as cooks it. The salmon was obtained from Tribal fishing grounds on the Columbia River. There were no advisories in these areas with respect to safety. The smoked salmon was placed in sealed food-grade containers in a second container with dry ice and transported to OSU. The quantity of a number of PAHs was determined using the method of Forsberg et al. (2011; 2012), and the results for two batches are shown in Table 2 along with the RPF values used to calculate ng BaPeq/g smoked salmon. That RPF was driven entirely by fluoranthene in the 2015 batch; the 2014 batch also had a small (3.4%) contribution from benzo[g,h,i]perylene (Table 2). Once the salmon was analyzed and the PAH levels determined, it was portioned out, weighed, vacuum-sealed and stored at −20°C in a locked container devoted soled to samples intended for human use. The facility was registered with FDA (#11833682472) and licensed by the Oregon Department of Agriculture (AG-L0077970FP). Subjects were given a portion of smoked salmon containing either 46 or 460 BaPeq corresponding to 22.55 g (2014 batch RPF=2.04) or 125.02 g (2015 batch RPF=3.68), respectively. These two levels corresponded to a 1:1 and 10:1 ratio, respectively of BaPeq:[14C]-BaP. Canned salmon (Ocean Beauty Seafoods, LLC, Icy Point lot #035DR720C) was purchased from Bi Mart (Corvallis, OR). The canned salmon contained a significant amount of water; therefore dry weights of canned and smoked salmon were compared in order to administer equivalent dry weights of each. Liquid was drained from canned salmon and any bones carefully removed from portions of both smoked and canned salmon. Six portions of each were placed in aluminum trays, weighed and placed in a drying oven preheated to 90°C. Salmon was dried for 24 hours, cooled in a desiccator and reweighed. Salmon was then heated in the same oven for an additional 2 hours to ensure all moisture had been removed. Cooling and weighing was repeated for validation. The average percent dry weights of canned and smoked salmon were 35.63 and 51.25%, respectively. Dry weight estimates of 1:1 BaPeq smoked salmon portions were 11.56 g and 64.07 g for both 10:1 BaPeq smoked and canned salmon portions. Canned salmon was not compared at the 1:1 BaPeq ratio. Volunteers consumed 179.74 g wet weight of canned salmon, the equivalent of 125.02 g smoked salmon. Canned salmon was portioned, weighed, and chilled for volunteers 24 hours before serving in order to make it more palatable.
Table 2.
CTUIR Smoked Salmon: PAHa Composition from Two Separate Years and BaPeq Calculated from Published RPFs
| 2014 | 2015 | ||||
|---|---|---|---|---|---|
| Chemical Name | RPF | ng/g | BaPeq | ng/g | BaPeq |
| Naphthalene | 75.4 | 170.00 | |||
| 2-Methylnaphthalene | 42.8 | 63.00 | |||
| 1-Methylnaphthalene | 35.7 | 46.00 | |||
| 2-Ethylenaphthalene | 11.71 | BLOD | |||
| 2,6-Dimethylnaphthalene | 45.6 | 90.00 | |||
| 1,6-Dimethylnaphthalene | 19.04 | BLOQ | |||
| 1,4-Dimethylnaphthalene | BLOD | BLOD | |||
| 1,5-Dimethylnaphthalene | 3.29 | BLOD | |||
| 1,2-Dimethylnaphthalene | BLOD | BLOQ | |||
| 1,8-Dimethylnaphthalene | BLOD | BLOD | |||
| 2,6-Diethylnaphthalene | BLOD | BLOD | |||
| Acenaphthylene | 69.7 | BLOQ | |||
| Acenaphthene | 11.35 | BLOD | |||
| Fluorene | 43 | 74.00 | |||
| Dibenzothiophene | BLOD | BLOD | |||
| Phenanthrene | 102.3 | 150.00 | |||
| Anthracene | 21.6 | BLOQ | |||
| 2-Methylphenanthrene | 13.32 | 20.00 | |||
| 2-Methylanthracene | 10.75 | BLOQ | |||
| 1-Methylphenanthrene | 6.49 | BLOQ | |||
| 9-Methylanthracene | BLOD | BLOD | |||
| 3,6-Dimethylphenanthrene | BLOD | BLOD | |||
| 2,3-Dimethylphenanthrene | BLOD | BLOD | |||
| Fluoranthene | 0.08 | 24.6 | 1.97 | 46.00 | 3.68 |
| 9,10-Dimethylphenanthrene | BLOD | BLOD | |||
| Pyrene | 29.3 | 36.00 | |||
| Retene | BLOD | BLOD | |||
| Benzo(a)fluorene | BLOD | BLOD | |||
| Benzo(b)fluorene | BLOD | BLOD | |||
| Benzo(c)fluorene | 20.00 | BLOD | BLOD | ||
| 1-Methylpyrene | BLOD | BLOD | |||
| Benz(a)anthracene | 0.20 | BLOD | BLOD | ||
| Cyclopenta(cd)pyrene | 0.40 | BLOD | BLOD | ||
| Triphenylene | BLOD | BLOD | |||
| Chrysene | 0.10 | BLOD | BLOD | ||
| 6-Methylchrysene | BLOD | BLOD | |||
| 5-Methylchrysene | BLOD | BLOD | |||
| Benzo(b)fluoranthene | 0.80 | BLOD | BLOD | ||
| 7,12-Dimethylbenz(a)anthracene | BLOD | BLOD | |||
| Benzo(k)fluoranthene | 0.03 | BLOD | BLOD | ||
| Benzo(j)fluoranthene | 0.30 | BLOD | BLOD | ||
| Benz(j) and (e)aceanthrylene | 60.00 | BLOD | BLOD | ||
| Benzo(e)pyrene | BLOD | BLOD | |||
| Benzo(a)pyrene | 1.00 | BLOD | BLOD | ||
| Indeno(1,2,3-c,d)pyrene | 0.07 | BLOD | BLOD | ||
| Dibenzo(a,h)anthracene | 10.00 | BLOD | BLOD | ||
| Benzo(a)chrysene | BLOD | BLOD | |||
| Benzo(g,h,i)perylene | 0.01 | 8.05 | 0.07 | BLOD | |
| Anthanthrene | 0.40 | BLOD | BLOD | ||
| Naphtho(1,2-b)fluoranthene | BLOD | BLOD | |||
| Naphtho(2,3-j)fluoranthene | BLOD | BLOD | |||
| Dibenzo(a,e)fluoranthene | 0.90 | BLOD | BLOD | ||
| Dibenzo(a,l)pyrene | 30.00 | BLOD | BLOD | ||
| Naphtho(2,3-k)fluoranthene | BLOD | BLOD | |||
| Naphto(2,3-e)pyrene | 0.30 | BLOD | BLOD | ||
| Dibenzo(a,e)pyrene | 0.40 | BLOD | BLOD | ||
| Coronene | BLOD | BLOD | |||
| Dibenzo(e,l)pyrene | BLOD | BLOD | |||
| Naphtho(2,3-a)pyrene | BLOD | BLOD | |||
| Benzo(b)perylene | BLOD | BLOD | |||
| Dibenzo(a,i)pyrene | 0.60 | BLOD | BLOD | ||
| Dibenzo(a,h)pyrene | 0.90 | BLOD | BLOD | ||
PAHs with published RPFs are highlighted in gray
BLOD, below the limit of detection (2–10 ng/g)
BLOQ, below the limited of quantification (2–10 ng/g)
2.5. Dosing of Subjects with [14C]-BaP and Salmon and Collection of Blood, DNA Isolation from PBMCs and Determination of Covalent Adduction with [14C]-BaP
Fasted (overnight) subjects were administered a cellulose capsule containing [14C]-BaP (46 ng, 5 nCi) swallowed with 100 mL water. Canned commercial salmon, or smoked salmon from the CTUIR, consumption was prior to dosing with [14C]-BaP. Normal eating and drinking resumed 2 hours after dosing with [14C]-BaP. Blood (up to 10 mL) was drawn by a registered nurse at 0, 0.25, 0.5, 0.75, 1.0, 1.5, 2.0, 2.5, 3, 4, 8, 24, 48 and 72 hours (the 0.25, 2.5 and 72 hour blood draws were not done with the canned salmon cycle) following [14C]-BaP dosing. An indwelling IV catheter was used for blood collection over the first 4 hours and the remainder with straight needle sticks. A minimum 2 week washout between dosing with [14C]-BaP was adopted. Peripheral blood mononuclear cells (PBMCs) were isolated from the buffy coat and used to assess covalent binding of [14C]-BaP to DNA. Briefly, DNA was isolated from PBMCs using a commercial kit for mammalian blood (Roche Diagnostics, Indianapolis, IN). The DNA was precipitated with 100% ethanol and stored at −20°C. Subsequently, the DNA was washed with 100%, precipitated by centrifugation and washed twice with 70% ethanol. The final DNA pellet was solubilized in nuclease-free water at 65°C 30–60 minutes and then stored at 4°C for 49–72 hours. The DNA concentration and purity was determined by using a Nanodrop ND-1000 (Thermo Scientific, Wilmington, DE). The 260/280 nm ratio was 1.7–1.9. Samples were dissolved in water to a concentration of 200 ng/µL and 50 µg placed in amber glass vials and stored at −80°C for subsequent AMS analysis. Twenty µg (100 µL) was used for the AMS analysis.
2.6. Extraction of [14C]-BaPeq from Plasma and Analysis of [14C]-BaPeq by Accelerator Mass Spectrometry
Aliquots of plasma (0.75 mL) from each time point were extracted within two hours after collection according to the method of Crowell et al. (2011) as modified by Madeen et al. (2015; 2016). The samples were acidified with H2SO4, vortexed and extracted with ethyl acetate. Combined extracts were evaporated under nitrogen to dryness in glass vials with PTFE cap liner and stored at −80°C until shipped on dry ice to the Center for Accelerator Mass Spectrometry at LLNL where they were stored at −80°C until processing. As PAHs can adsorb to plastic, care was taken to use glass containers and vials flushed with argon or nitrogen prior to capping to prevent oxidation of samples. Plasma extracts were reconstituted with 50 µL ethyl acetate and converted to graphite by the method of Ognibene et al. (2004) as described in our previous study with [14C]-DBC (Madeen et al., 2015). Briefly, samples were evaporated and flame-sealed in a quartz tube containing Cu(II) and combusted to 900°C, producing CO2. The CO2 was then transferred to a septa sealed glass tube containing Zn and Co and heated to 525°C, producing graphite on the Co catalyst. The graphite was then loaded into an aluminum sample holder for AMS analysis.
AMS analysis was conducted on the 250 kV Single Stage AMS at the Center for Accelerator Mass Spectrometry at LLNL. AMS operating conditions were optimized to determine the ratio of 14C:C with a precision of 3% and sensitivity of 0.7 attomole 14C per mg of total carbon (Ognibene et al., 2018). Solid sample standards containing a 14C/C content of 1.5 × modern are measured intermittently throughout the analysis to assess the ionization and counting efficiency of the AMS. The biochemical samples are measured 4–10 times with the collection of at least 10,000 14C counts or for 30 seconds for each replicate (Ognibene et al., 2018).
2.7. Pharmacokinetic and Statistical Analysis
The pharmacokinetics of [14C]-BaPeq ([14C]-BaP and metabolites) were evaluated using non-compartmental analysis and a two-compartment model after oral administration of 46 ng [14C]-BaP capsule in one of four treatments: (1) neat or co-administration with (2) 22.55 g smoked salmon (wet weight, 46 ng BaPeq of PAHs), (3) 125.02 g smoked salmon (wet weight, 460 ng BaPeq of PAHs), or (4) 179.74 g commercial canned salmon (wet weight, 460 ng BaPeq of PAHs) as previously detailed in Madeen et al., (2018). AUCs were calculated using the trapezoidal rule. A two-compartment model was used to evaluate the amount (fg) of [14C]-BaPeq in the absorption, central, and peripheral compartments (first-order rate constants ka”, ke”, k12/k21, respectively). A maximum log likelihood objective and Nelder-Mead algorithm was used to optimize model parameters and initial values set adjusting parameters visually. Statistical analysis of PK parameters was performed with “R: A language and environment for statistical computing” Version 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria). Statistical analysis between dosing regiments was typically performed using a two-tailed students t-test.
3. Results
3.1. Estimated Dietary Intake of BaP Prior to and During the 72 Hour Pharmacokinetic Analysis Following Dosing with [14C]-BaP
The BaP dietary ingestion for all 5 subjects was calculated as a range (low and high estimate). The average low estimate was 216 ± 89 ng/day (range 50–613) and the high estimate was 307 ± 113 ng/day (range 112–833) (Table 3). We found the greatest contributors to daily dietary intake of BaP in our subjects were black olives (11.3 ng/g), cooked quinoa (0.5 ng/g), coconut crème (3.4 ng/g) and pan-fried beef (0.4–0.6 ng/g).
Table 3.
Dietary Estimates of Daily BaP Exposure
| Estimated Average Low and High Daily Consumption of Dietary BaP (ng/day) | |||||
|---|---|---|---|---|---|
| Volunteer | Pre-Studya | Cycle 1b | Cycle 4c | Average ± SD Low | Average ± SD High |
| A | 109–157 | 116–255 | 50–112 | 92 ± 36 | 175 ± 73 |
| B | 496–606 | 152–261 | 168–241 | 272 ± 194 | 369 ± 205 |
| C | 292–402 | 268–325 | 187–278 | 249 ± 55 | 335 ± 61 |
| D | 97–162 | 254–311 | 124–151 | 158 ± 84 | 208 ± 89 |
| E | 111–205 | 201–304 | 613–833 | 308 ± 268 | 447 ± 338 |
| Average | 221–306 | 198–291 | 232–319 | 216 | 307 |
| ± SD | 174–195 | 65-31 | 168–195 | 89 | 113 |
Estimate from 3-day diary prior to first dosing with [14C]-BaP
Estimate from 3-day diary following first dosing with [14C]-BaP
Estimate from 3-day diary following first ingestion of smoked salmon with [14C]-BaP (smoked salmon not included in calculation)
3.2. Pharmacokinetics of [14C]-BaP in the Presence or Absence of Smoked Salmon Containing a Complex PAH Mixture at 46 or 460 ng BaPeq
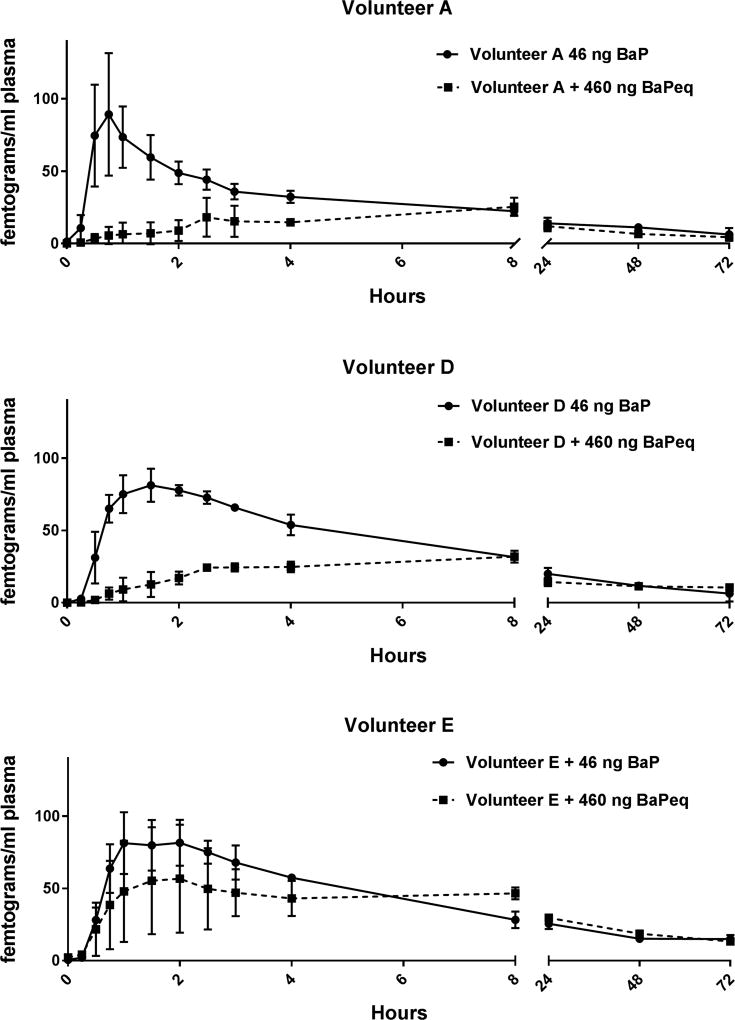
The RPF approach to risk assessment of environmental complex PAH-containing mixtures relies on the assumption that individual PAHs behave independently, i.e., that one PAH does not impact the transport or ADME of another PAH. Typical biomarkers used to predict PAH-dependent carcinogenesis include CYP1 induction (CYP1A1 and CYP1B1, responsible for bioactivation of BaP to 7,8-dihydroxy-9,10-epoxy-7,8,9,10-tetrahydro-BaP, BaPDE) or BaPDE covalent binding to DNA (Kang et al., 2007; Kriek et al., 1993; Lee et al., 2002; Ross et al, 1995). There have been numerous studies done in vitro (Genies et al., 2016; Shimada and Guengerich, 2006; Shimada et al., 2008; Staal et al., 2006; 2007; Tarantini et al., 2011) and in vivo animal models (Nesnow et al., 1998; Schneider et al., 2002) that call into question the reliability of that assumption and RPF-dependent cancer risk assessment including our own work with human cancer cells (Mahadevan et al., 2005; 2007) and the mouse skin tumor model (Courter et al., 2007; Siddens et al., 2012; Tilton et al., 2015). In this study, we examined the pharmacokinetics of the prototypical PAH, BaP, which is the calibrator for cancer RPF determinations of PAHs (BaP has an RPF set at 1) in the presence of a food containing a complex PAH mixture of known composition and RPF. Two of the three volunteers (A & D) administered 125.02 g (460 ng BaPeq) CTUIR smoked salmon prior to the capsule containing 46 ng [14C]-BaP, exhibited markedly different pharmacokinetics compared to when dosed with [14C]-BaP alone (Figure 1 and Tables 4 and 5). A more muted response was seen with volunteer E, primarily due to the high variability between the three cycles of smoked salmon (Figure 1, bottom panel). The fraction absorbed was decreased by 23–30% in volunteers A and D abut actually slightly increased with volunteer E. The Cmax was markedly reduced in all three volunteers and the Tmax increased with volunteers A and D but remained the same with volunteer E. During that same time frame, consuming 22.5 g of salmon (46 BaPeq) actually slightly increased (3–12%) the fraction of [14C]-BaPeq absorbed. Rates of oral absorption of [14C]-BaPeq were reduced with increasing amounts of salmon vehicle (0.921 for neat, 0.590 for 22.5 g salmon vehicle, and 0.140 (volunteers A and D; 0.500 for A, D and E) for 125.02 g salmon vehicle). There was no statistical difference, using the non-compartment model, in T1/2 of [14C]-BaPeq between volunteers that consumed smoked salmon with the [14C]-BaP capsule and those who consumed the capsule neat (p=0.82).
Figure 1. Pharmacokinetics in Plasma of [14C]-BaP Administered Alone or with 10-X BaPeq in Smoked Salmon.
Plasma levels (fg/mL) of [14C]-BaPeq in volunteers A (Top Panel), D (Middle Panel) and E (Bottom Panel) over 72 hours after dosing with 46 ng (5 nCi) of [14C]-BaP with (squares, broken line) or without (circles, solid line) co-administration of 125.02 g of CTUIR-smoked salmon containing a complex PAH mixture with 460 ng BaPeq using published RPF values. The symbols represent the mean of three separate volunteer dosing trials and the bars the S.E. of the mean.
Table 4.
Non-Compartmental Pharmacokineticsa of [14C]-BaP Following Micro-Dosing with or without 46 or 460 ng [14C]-BaPeq Smoked Salmon or Canned Salmon
| Subject | Fa (fraction absorbed) |
Tmax (hr) |
Cmax (fg/mL) |
AUC0–72hr (fgxhrxmL−1) |
AUC0-∞ (fgxhrxmL−1) |
T1/2 (hr) |
|---|---|---|---|---|---|---|
| Impact of Smoked Salmon Containing 460 ng BaPeq on [14C]-BaP Pharmacokinetics | ||||||
| A | - | 0.75 | 79.2 | 1135 | 2118 | 50 |
| A + 460 ng BaPeq | 0.700 | 8 | 25.3 | 794 | 992 | 30 |
| D | - | 1.5 | 78.1 | 1450 | 1832 | 31 |
| D + 460 ng BaPeq | 0.773 | 8 | 31.9 | 1121 | 2779 | 103 |
| E | - | 2 | 81.6 | 1709 | 3052 | 60 |
| E + 460 ng BaPeq | 1.13 | 2 | 56.7 | 1929 | 2738 | 40 |
| Impact of Smoked Salmon Containing 46 ng BaPeq on [14C]-BaP Pharmacokinetics | ||||||
| B | - | 1.0 | 29.4 | 649 | 1769 | 114 |
| B + 46 ng BaPeq | 1.026 | 3 | 22.8 | 666 | 1880 | 125 |
| C | - | 1.0 | 82.4 | 2060 | 4306 | 79 |
| C + 46 ng BaPeq | 1.121 | 2.5 | 71.1 | 2315 | 4074 | 60 |
| Impact of Canned Salmon on [14C]-BaP Pharmacokinetics | ||||||
| A + Canned Salmon | 0.736 | 8 | 32.8 | 815 | 1108 | 23 |
| D + Canned Salmon | 0.837 | 8 | 39.7 | 1213 | 1699 | 27 |
Table 5.
Two-Compartment Pharmacokineticsa of [14C]-BaP Following Micro-Dosing with or without 46 or 460 ng [14C]-BaPeq Smoked Salmon or Canned Salmon
| Subject | Ka (hr−1) |
K12 (hr−1) |
K21 (hr−1) |
K1e (hr−1) |
Kelα (hr−1) |
T1/2α (hr) |
V (L) |
Kelβ (hr−1) |
T1/2β (hr) |
|---|---|---|---|---|---|---|---|---|---|
| Impact of Smoked Salmon Containing 460 ng BaPeq on [14C]-BaP Pharmacokinetics | |||||||||
| A | 1.394 | 0.773 | 0.164 | 0.083 | 1.006 | 0.689 | 304 | 0.014 | 51.1 |
| A + 460 ng BaPeq | 0.126 | 0.086 | 0.011 | 0.012 | 0.108 | 6.416 | 822 | 0.001 | 570 |
| D | 0.672 | 0.422 | 0.128 | 0.089 | 0.620 | 1.118 | 280 | 0.018 | 38.0 |
| D + 460 ng BaPeq | 0.154 | 0.134 | 0.021 | 0.001 | 0.156 | 4.445 | 603 | 0.0001 | 5725 |
| E | 0.591 | 0.466 | 0.077 | 0.050 | 0.586 | 1.183 | 235 | 0.007 | 106 |
| E + 460 ng BaPeq | 1.221 | 0.006 | 0.174 | 0.025 | 0.248 | 2.798 | 727 | 0.017 | 40 |
| Impact of Smoked Salmon Containing 46 ng BaPeq on [14C]-BaP Pharmacokinetics | |||||||||
| B | 1.280 | 0.307 | 0.102 | 0.019 | 0.423 | 1.639 | 1146 | 0.004 | 155.7 |
| B + 46 ng BaPeq | 0.730 | 0.165 | 0.063 | 0.015 | 0.239 | 2.900 | 1333 | 0.004 | 172 |
| C | 0.666 | 0.486 | 0.145 | 0.034 | 0.658 | 1.054 | 294 | 0.007 | 93.1 |
| C + 46 ng BaPeq | 0.450 | 0.161 | 0.091 | 0.034 | 0.274 | 2.529 | 354 | 0.01 | 62 |
| Impact of Canned Salmon on [14C]-BaP Pharmacokinetics | |||||||||
| A + Canned Salmon | 0.301 | 0.050 | 0.031 | 0.037 | 0.107 | 6.49 | 847 | 0.011 | 65 |
| D + Canned Salmon | 0.093 | 0.055 | 0.011 | 0.028 | 0.090 | 7.690 | 460 | 0.003 | 209 |
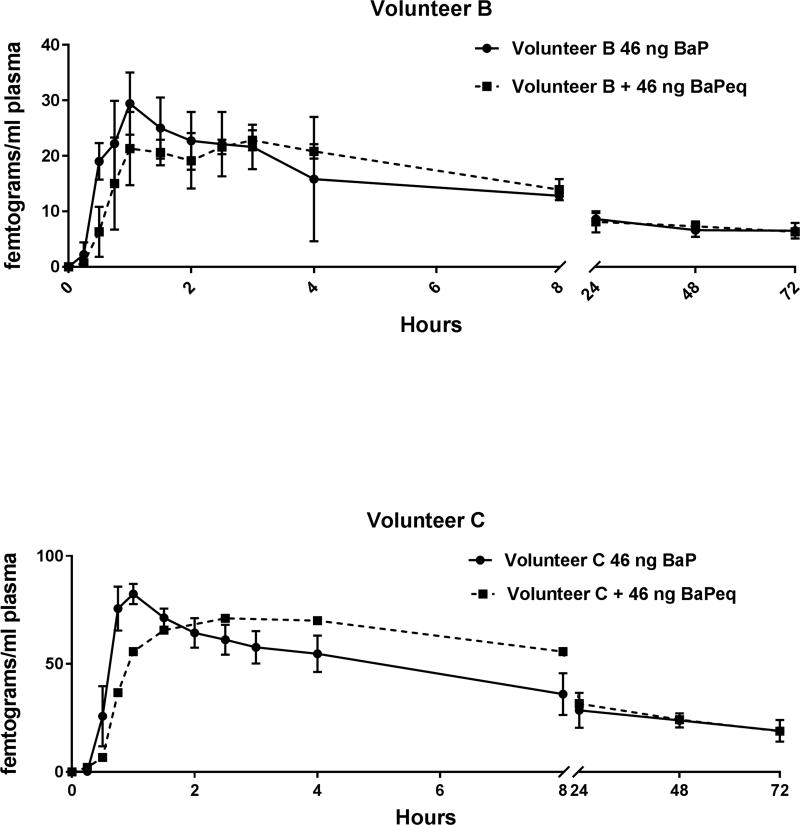
With ingestion of smoked salmon the Kelα in the two-compartment model was markedly reduced (again, with the exception of volunteer E) whereas T1/2α and T1/2β were increased (except Volunteer E) in a dose-response fashion. Together, these changes in pharmacokinetics could have been interpreted as not supporting the RPF approach to cancer risk assessment as it appears that, in the presence of a much greater amount and number of PAHs, the pharmacokinetics of orally administered [14C]-BaP is markedly altered. This effect was dampened when the level of BaPeq in the smoked salmon was reduced to 46 ng (Figure 2 and Tables 4 and 5). There was a slight, non-significant reduction in Cmax. A smaller portion of smoked salmon with a 1:1 ratio of BaPeq to [14C]-BaP also resulted in a delay in absorption. The impact of metabolism on the pharmacokinetics of [14C]-BaP with fasted individuals or those given the smoked salmon cannot be assessed from this study.
Figure 2. Pharmacokinetics in Plasma of [14C]-BaP Administered Alone or with 1-X BaPeq in Smoked Salmon.
Top Panel- Plasma levels (fg/mL) of [14C]-BaPeq in volunteer B over 72 hours after dosing with 46 ng (5 nCi) of [14C]-BaP with (squares, broken line) or without (circles, solid line) co-administration of 22.55 g of CTUIR-smoked salmon containing a complex PAH mixture with 46 ng BaPeq using published RPF values. The symbols represent the mean of three separate volunteer dosing trials and the bars the S.E. of the mean. Bottom Panel- Plasma levels (fg/mL) of [14C]-BaPeq in volunteer C over 72 hours after dosing with 46 ng (5 nCi) of [14C]-BaP with (squares) or without (circles) co-administration of 22.7 g of CTUIR-smoked salmon containing a complex PAH mixture with 46 ng BaPeq using published RPF values. Volunteer C only underwent 1 dosing trial (squares, broken line) with co-administration of 22.55 g smoked salmon. For clarity, for volunteer E with salmon, only 1 directional error bars were plotted.
3.3. Pharmacokinetics of [14C]-BaP in the Presence or Absence of Canned Salmon
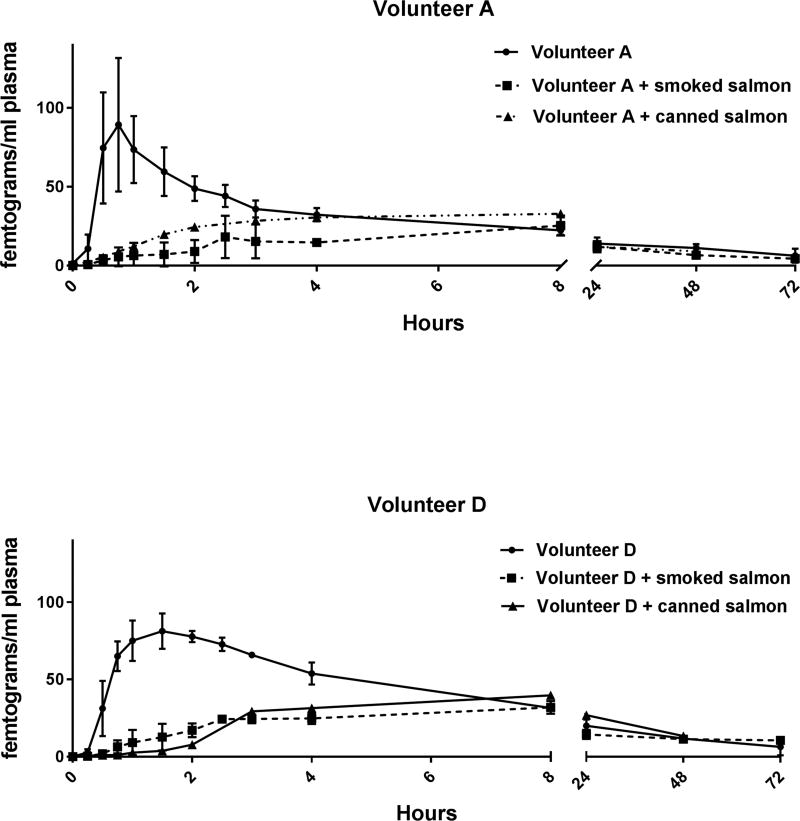
To ensure that the alteration in [14C]-BaP pharmacokinetics was due to PAHs in the smoked salmon and not any food matrix effect we dosed two individuals with 179.4 g of canned commercially obtained non-smoked salmon that had about 500-fold lower total PAHs (and no detectable carcinogenic PAHs). The impact on [14C]-BaP pharmacokinetics was similar to the high smoked salmon intake (Figure 3 and Tables 4 and 5) indicating that the alteration in absorption and elimination from plasma was due to the food matrix itself and not the high levels of PAHs in the CTUIR smoked salmon.
Figure 3. Pharmacokinetics in Plasma of [14C]-BaP Administered Alone, with Smoked Salmon or Canned Salmon.
Plasma levels (fg/mL) of [14C]-BaPeq in volunteers A (Top Panel) and D (Bottom Panel) over 72 hours after dosing with 46 ng (5 nCi) with [14C]-BaP alone (circles, solid line) or immediately following consumption of 125 g of smoked salmon (460 BaPeq, squares, dashed line) or 179.4 g of commercially obtained canned salmon (triangles, broken line). The symbols represent the mean of three separate volunteer dosing trials with [14C]-BaP alone or with smoked salmon and the bars the S.E. of the mean. Co-administration of canned salmon was analyzed following a single dosing.
3.4 Lack of Covalent Adduction of [14C]-BaPeq to DNA from PBMCs
Covalent adduction to DNA has been used as a biomarker of cancer risk for numerous carcinogens including BaP (Boysen and Hecht, 2003). We found no [14C]-BaPeq covalently bound to total DNA isolated from PBMCs out to 72 hours post-dosing (LOD 10 fg/mg DNA or 0.5 adducts/1011 nucleotides).
4. Discussion
BaP, a class 1 known human carcinogen (IARC, 2010), is found in almost all food and is especially high in charcoal-broiled meats or smoked meats and cheeses. The concentration of BaP in a particular food stuff varies markedly world-wide, e.g., eggs in the U.S. have been reported to contain an average of 0.03 ng/g (Kazerouni et al., 2001) whereas in Kuwait (post Iraq War) the number is over two orders of magnitude higher (7.49 ng/g) (Husain et al., 1997). White bread in the U.S. contains 0.10 ng BaP/g (Kazerouni et al., 2001) compared to Italy with 0.017 ng/g (Lodovici et al., 1995). Therefore, it is not surprising that reports of the estimated daily dietary intake of BaP range widely from 5–3,440 ng (Domingo and Nadal, 2015). In this study, based on our dietary questionnaire, the average intake for the five volunteers prior to study initiation was 221–306 ng/day. Using the recently revised cancer risk slope factor of 1 (mg/kg-day)−1 this would equate to a lifetime excess cancer risk of 3.1 to 4.4 × 10−6.
The RPF approach to cancer risk assessment with complex environmental PAH mixtures assumes that there is a common mode of action and additivity applies across a wide range of structures (U.S. EPA, 2010). Thus, there should not be evidence of synergism or inhibition when comparing binary or complex mixtures. There are a number of studies using both in vitro markers of carcinogenic potency with human cell lines and in vivo tumor studies with animal models that raise serious doubts about this approach. The exquisite sensitivity of AMS allowed us, for the first time, to examine the impact (alteration in pharmacokinetics) of co-administration of a food containing a complex PAH mixture with BaP in humans at a defined dose. To that end, 46 ng of [14C]-BaP was administered with or without smoked salmon containing either a 1:1 or a 10:1 ratio of BaPeq as determined by the quantity and profile of PAH congeners by GC-MS in smoked salmon supplied by the CTUIR. In the study by Forsberg et al. (2012), 40 samples of Chinook salmon from the Columbia River, smoked for 22–33 hours with 2 types of wood in two different structures (tipi or shed), were analyzed for 62 PAHs. All PAHs detected were due to the smoking process as the unsmoked fillets were all below the limit of detection (2–10 ng/g for the PAHs analyzed). Approximately 98% of the total PAHs in the smoked salmon were 2–4 ring PAHs. The larger carcinogenic PAHs (chrysene, benz[a]anthracene, benzo[b]fluoranthene, benzo[a]pyrene, benzo[k]fluoranthene and benzo[b]fluoranthene) were present at levels of 26–100 ng/g wet weight. As reported in this previous study, the major carcinogenic (assigned RPF) PAH in the CTUIR salmon used in this study contributing to the BaPeq was fluoranthene (46 ng/g). BaP was below the limit of detection (2 ng/g) which Forsberg et al. (2012) found in 10% of the samples they analyzed.
A caveat in comparing pharmacokinetic data from rodents and humans in this current study is the orders of magnitude difference in dosage. Even so, in fasted individuals dosed with 46 ng [14C]-BaP the Tmax (1.25 hours) was similar to that (0.92–1.20 hours) found in non-fasted C57BL/6 mice (Fang and Zhang, 2010) after an oral dose (gavage in DMSO:corn oil, 1:9 v/v) of 15–30 mg/kg (approximately 7 orders of magnitude higher, on a mg/kg b.w. basis, than the human dose used here); the T1/2 values were also similar (0.8 and 1.1 hours for mouse and human, respectively). Not unexpectedly, the Cmax ratio was also about 7 orders of magnitude different (233 ng/mL versus 70 fg/mL). Pharmacokinetic studies in the rat indicate a much longer time for absorption with a Tmax of 5–8 hours (Olesen et al., 2016; Ramesh et al., 2001). The Olesen et al. (2016) study dosed male Sprague-Dawley rats (fasted overnight and for 8 hours post-dosing) with 1.05 µg/kg b.w [3H]-BaP by oral gavage in ethanol whereas the Ramesh et al. (2001) study dosed male Fisher-344 rats (fasted for 16 hours prior to dosing) with 100 mg/kg b.w. BaP by gavage in peanut oil. Administration of smoked salmon containing 460 ng BaPeq markedly reduced the Cmax, increased Tmax and essentially eliminated Kelα (two-compartment model) of [14C]-BaPeq in volunteers A and D (actually increased with volunteer E) all of which support rejection of the tenants of the RPF approach for PAH mixtures. A less marked delay in absorption and reduction in elimination was observed when the quantity of smoked salmon was reduced 10-fold (22.55 g of CTUIR smoked salmon, 46 ng BaPeq). However, in order to establish that these alterations in [14C]-BaP were due to the high levels of co-administered PAHs and not a food matrix effect, we repeated the analysis with commercial canned salmon (containing at least 500-fold less total PAHs and no carcinogenic PAHs) at an amount (179.74 g) equivalent to the smoked salmon portion containing 460 ng BaPeq. The impact was similar to alteration in pharmacokinetics seen with the 125.02 g co-administration of CTUIR smoked salmon. Thus, it appears that the food matrix is an important factor in oral BaP pharmacokinetics.
Rate and extent of oral absorption is dependent on chemical solubility, chemical concentration, and GI permeability (Mudie 2010). Here, we observed reduction of BaP bioavailability and rate of absorption with increasing dose vehicle. Salmon is a food source rich in lipid and there have been studies indicating enhanced GI absorption of PAHs with high-lipid containing foods or lipid vehicles (Laher et al., 1983) although not all studies have observed this effect (Laher et al., 1984). Since PAHs are highly lipophilic, salmon lipids could increase BaP solubility in the gut. However, the volume of salmon can dramatically reduce the BaP concentration, which we hypothesize is reducing the rate and extent of BaP absorption. Additionally, BaP could bind to insoluble salmon further reducing abosorption.
A reduction in Kelα with increasing salmon vehicle was observed. This reduction of Kelα could be a result of reduced absorption or perhaps a change in overall BaP metabolism. A more definitive answer requires evaluation of BaP metabolites, which is not possible with this application of AMS as only total [14C] is measured. A number of metabolic pathways exist for BaP, some leading to detoxication and others to bioactivation (reviewed in ATSDR, 2013; U.S. EPA, 2017). A portion of BaP carcinogenicity/toxicity is also likely due to canonical AhR signally as BaP and some metabolites are ligands (ATSDR, 2013; U.S. EPA, 2017). A great deal of research has documented the metabolism of BaP in hepatic and pulmonary tissues (reviewed in ATSDR, 2013). Extensive GI and hepatic metabolism could lead to a “first pass” effect wherein the [14C]-BaPeq measured in plasma in this study would be expected to be comprised of predominantly BaP metabolites. The mouse and human GI have a complement of phase 1 and phase 2 BaP metabolizing enzymes although not as extensive as in liver (Buesen et al., 2002; Uno et al., 2008). This observation, plus estimates that greater than 95% of BaP (and other high MW carcinogenic PAHs) exposure in non-smoking humans is dietary (ATSDR, 2013), suggests that further work on carcinogenic PAH risk assessment needs to take into account the role of the GI in addition to the liver. In a previous study we micro-dosed (29 ng) human volunteers with [14C]-DBC and assessed the pharmacokinetics of [14C]-DBCeq (Madeen et al., 2015). Almost all of the pharmacokinetic parameters were similar (caveat that only 1/5 volunteers were in both studies) to [14C]-BaPeq except there was a non-significant trend with Ka (slower with [14C]-DBCeq, p=0.057) and Tmax (longer with [14C]-DBC, p=0.081). As the 6-membered ring PAH, DBC, has a log Kow (7.2) higher than BaP (6.0), it would appear than absorption of an oral dose in the absence of food is not determined primarily by hydrophobicity.
Daniel W. Nebert and colleagues performed a series of elegant experiments on BaP toxicity and carcinogenesis in mice employing single, double and triple knockouts (Cyp1a1−/−, Cyp1a2−/−, Cyp1b1−/−, Cyp1a1−/−/1a2−/−, Cyp1a1−/−/1b1−/−, Cyp1a2−/−/1b1−/− and Cyp1a1−/−/1a2−/−/1b1−/−) of the mouse Cyp1 genes (Dragin et al., 2008; Nebert et al., 2013; Shi et al., 2010a; Uno et al., 2001; 2004; 2006; 2008). Surprisingly, loss of expression of CYP1a1 enhanced toxicity and promoted carcinogenesis. Liver- and intestinal-specific Cyp1a1 knockout mice further established the importance of GI Cyp1a1 metabolism of BaP in reducing uptake and distribution of BaP to target tissues (Shi et al., 2010b). A humanized Cyp1a1 mouse has been employed to establish that the human gene is similar to the mouse in expression in the GI tract and in the metabolism of BaP (Dragin et al., 2007). The overall conclusion from these studies was that in the mouse GI metabolism of BaP is the predominant factor in toxicity and carcinogenesis. One caveat when comparing these studies to the present study is that the mouse studies were done with chronic high dose oral BaP exposure with presumed induction of CYP1a1 whereas it is unlikely that the single micro-dose of BaP employed here would significantly impact expression of BaP-metabolizing enzymes although the PAHs in the smoked salmon could induce GI (and perhaps liver) CYP1A1. Consistent with the tissue-specific mouse Cyp1a1 knockout studies, intestinal-specific NADPH cytochrome P450 reductase null mice (which would eliminate all CYP-dependent metabolism) have shown intestinal BaP metabolism is important in pharmacokinetics of orally (but not intraperitoneally) administered BaP (Fang and Zhang, 2010). In the Fang and Zhang (2010) study, there was evidence that induction of Cyp1a1 metabolism of BaP could be induced as early as 2 hours following a 30 mg/kg oral dose. How might the salmon matrix impact intestinal CYP1A1-dependent BaP metabolism in humans?
In order to address these questions, we have initiated studies with “moving wire” technology developed at LLNL, in which UPLC can be interfaced with AMS allowing for quantification and identification (and thus pharmacokinetics) of [14C]-BaP metabolites (Thomas et al., 2011). It will be important to determine if alteration in [14C]-BaP metabolism occurs in the presence of smoked salmon containing high levels of multiple PAHs and how that impacts pharmacokinetics. Ramesh et al. (2001), performed a comprehensive examination of BaP and BaP metabolite levels in multiple tissues over time in the rat to determine pharmacokinetic values after oral dosing of rats with 100 mg/kg. Furthermore, they utilized HPLC to resolve and quantify tissue- and time-dependent levels of BaP and phase 1 and phase 2 metabolites. It will be of great interest to compare their results with respect to BaP metabolite profiles over time to plasma and urine of micro-dosed humans (a limitation of our study is the lack of availability of tissues).
5. Conclusion
Accelerator mass spectrometry was utilized to quantify [14C]-BaPeq in plasma of humans over a 72 hour period following oral dosing with 46 ng in the presence or absence of quantities of smoked salmon containing a complex PAH mixture at BaPeq of 1:1 or 1:10 ([14C]-BaP:BaPeq smoked salmon). Co-administration of the large portion (125.02 g) of smoked salmon containing 460 ng BaPeq altered the pharmacokinetics of [14C]-BaPeq. The rate of absorption was decreased, the Tmax increased and Cmax and K1e decreased. Reducing the amount of co-administered smoked salmon 10-fold reduced the extent of alteration in pharmacokinetic parameters. An initial interpretation of this study was that the presence of large amounts of complex PAHs altered processes involved in the ADME and/or transport of orally administered [14C]-BaPeq. However, co-administration of commercially obtained canned salmon (with total PAH levels 500-fold lower than smoked salmon and with no carcinogenic PAHs), at amounts equivalent to the large portion of smoked salmon, had the equivalent impact on [14C]-BaPeq pharmacokinetics suggesting that this was a food matrix effect (perhaps the lipid-rich salmon somehow sequestered [14C]-BaPeq or changed the route of absorption to primarily lymphatic).
To assess the utility of high dose animal data for pharmacokinetics, toxicokinetic and tumor studies for carcinogenic PAHs, it is critical that we have data from humans following administration of an environmentally relevant dose. A comprehensive comparison requires analysis of [14C]-BaP and its metabolites which is now possible with a UPLC-AMS “moving wire” approach and that will be the focus of future research. Finally, analysis of DNA isolated from PBMCs had no detectable (LOD = 0.5 adduct per 1011 nucleotides) [14C]-BaPeq covalent binding negating its usefulness as a biomarker of cancer risk.
Supplementary Material
Highlights.
Oral micro-dosing of humans with [14C]-BaP, at a level in the low range of lifetime dietary BaP exposure, was performed in the presence or absence of food (smoked salmon) containing a complex mixture of polycyclic aromatic hydrocarbons (PAHs) (1-or 10-fold the BaPeq). This test of the relative potency factor approach (RPF) for oral cancer risk assessment for PAH mixtures appeared to call into question the validity of this method as smoked salmon impacted a number of pharmacokinetic parameters resulting in delayed absorption (higher Tmax), reduced maximum blood levels (Cmax) and reduction in the rate of elimination (longer T1/2 and slower Ke). However, administration of canned salmon containing no detectable PAHs, at identical amounts had similar impacts on pharmacokinetics of [14C]-BaP supporting a food matrix effect rather than interference by high levels of multiple PAHs. Physiologically-based pharmacokinetic models, toxicokinetics and risk assessments are currently performed with rodent models at doses orders of magnitude higher than actual human exposures. This unique dataset should be useful for further analysis of cancer risk in humans following exposure to environmentally relevant levels.
Acknowledgments
The authors would like to thank Alexandria VanScoyk, Hannah You, Chelsea Meedom, and Youngjoo Lee for their contribution of extracting samples for AMS analysis.
Competing interests and funding statements:
This study was funded by PHS grants P42ES016465, K.C. Donnelly Supplement to P42ES016465, R01ES028600 and T32ES07060. Work performed at the Research Resource for Biomedical AMS, which is operated at LLNL under the auspices of the U.S. Department of Energy under contract DE-AC52-07NA27344, is supported by the National Institutes of Health (NIH), National Institute of General Medical Sciences (NIGMS) and the Biomedical Technology Research Resources (BTRR) under grant number P41GM103483. Pacific Northwest National Laboratory (PNNL) is a multi-program national laboratory operated by Battelle for the DOE under Contract DE-AC06-76RL01830.
Abbreviations
- ADME
absorption distribution metabolism and excretion
- AFB1
aflatoxin B1
- AMS
accelerator mass spectrometry
- ATDSR
Agency for Toxic Substances and Disease Registry
- BaP
benzo[a]pyrene
- BaPeq
benzo[a]pyrene equivalents
- BLOD
below the limit of detection
- BLOQ
below the limit of quantitation
- BMI
body mass index
- CTUIR
Confederated Tribes of the Umatilla Indian Reservation
- CYP
cytochrome P450
- DBC
dibenzo[def,p]chrysene
- EPA
Environmental Protection Agency
- FDA
Food and Drug Administration
- IARC
International Agency for Research on Cancer
- IND
investigative new drug
- IRB
institutional review board
- JECFA
Joint FAO/WHO expert committee on food additives
- LDAL
lowest daily allowable level
- LLNL
Lawrence Livermore National Laboratory
- LOD
limit of detection
- OSU
Oregon State University
- PAH
polycyclic aromatic hydrocarbon
- PBMCs
peripheral blood mononuclear cells
- PBPK
physiologically based pharmacokinetics
- PK
pharmacokinetics
- RPF
relative potency factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions
JMH, EPM, KAA, KWT, JNS, SCT, WMB and DEW all contributed to the conceptual design of this study. LKS, SLU, SKK and DEW were responsible for all certifications and approvals associated with human use. JMH, EPM, LKS, TJM, KAA, TJO, SH, and JNS all contributed to sample collections, preparations and analytical assessments. Data analysis was primarily conducted by JMH, EPM, KAA, GB, JNS and SCT. The initial drafts of this manuscript were done by JMH, EPM and DEW. All coauthors contributed to revisions of drafts and approval of the final submission.
References
- Atherton JC, Spiller RC. The urea breath test for Helicobacter pylori. Gut. 1994;35:723–5. doi: 10.1136/gut.35.6.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR. [accessed 12-21-2017];2013 http://www.atsdr.cdc.gov/spl/resources/ATSDR_2013_SPL_Detailed_Data_Table.pdf.
- Bansal V, Kim KH. Review of PAH contamination in food products and their health hazards. Environm. Internl. 2015;84:26–38. doi: 10.1016/j.envint.2015.06.016. [DOI] [PubMed] [Google Scholar]
- Boysen G, Hecht SS. Analysis of DNA and protein adducts of benzo[a]pyrene in human tissues using structure-specific methods. Mutat. Res. 2003;543:17–30. doi: 10.1016/s1383-5742(02)00068-6. [DOI] [PubMed] [Google Scholar]
- Buesen R, Mock M, Seidel A, Jacob J, Lampen A. Interaction between metabolism and transport of benzo[a]pyrene and its metabolites in enterocytes. Toxicol. Appl. Pharmacol. 2002;183:168–78. doi: 10.1006/taap.2002.9484. [DOI] [PubMed] [Google Scholar]
- Courter LA, Musafia-Jeknic T, Fischer K, Bildfell R, Giovanini J, Pereira C, Baird WM. Urban dust particulate matter alters PAH-induced carcinogenesis by inhibition of CYP1A1 and CYP1B1. Toxicol. Sci. 2007;95:63–73. doi: 10.1093/toxsci/kfl137. [DOI] [PubMed] [Google Scholar]
- Crowell SR, Amin SG, Anderson KA, Krishnegowda G, Sharma AK, Soelberg JJ, Williams DE, Corley RA. Preliminary physiologically based pharmacokinetic models for benzo[a]pyrene and dibenzo[def,p]chrysene in rodents. Toxicol. Appl. Pharmacol. 2011;257:365–76. doi: 10.1016/j.taap.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupid BC, Lightfoot TJ, Russell D, Gant SJ, Turner PC, Dingley KH, Curtis KD, Leveson SH, Turteltaub KW, Garner RC. The formation of AFB1-macromolecular adducts in rats and humans at dietary levels of exposure. Fd. Chem. Toxicol. 2004;42:559–69. doi: 10.1016/j.fct.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Dieziel NC, Strickland PT, Platz EA, Abubaker S, Buckley TJ. Comparison of standard methods for assessing dietary intake of benzo[a]pyrene. Cancer Epidemiol. Biomarkers Prev. 2011;20:962–70. doi: 10.1158/1055-9965.EPI-10-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo J, Nadal M. Human dietary exposure to polycyclic aromatic hydrocarbons: A review of the scientific literature. Fd. Chem. Toxicol. 2015;86:144–53. doi: 10.1016/j.fct.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Dragin N, Uno B, Dalton TP, Nebert DW. Generation of “humanized” hCYP1A1_1A2_Cyp1a1/1a2(−/−) mouse line. Biochem. Biophys. Res. Commun. 2007;359:635–42. doi: 10.1016/j.bbrc.2007.05.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragin N, Shi Z, Madan R, Karp CL, Sartor MA, Chen C, Gonzalez FJ, Nebert DW. Phenotype of the Cyp1a1/1a2/1b1(−/−) triple-knockout mouse. Molec. Pharmacol. 2008;73:1844–56. doi: 10.1124/mol.108.045658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang C, Zhang Q-Y. The role of small-intestinal P450 enzymes in protection against systemic exposure of orally administered benzo[a]pyrene. J. Pharmacol. Exptl. Therap. 2010;334:156–63. doi: 10.1124/jpet.110.167742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg ND, Wilson GR, Anderson KA. Determination of parent and substituted polycyclic aromatic hydrocarbons in high-fat salmon using a modified QuEChERs extraction, dispersive SPE and GC-MS. J. Ag. Fd. Chem. 2011;59:8109–16. doi: 10.1021/jf201745a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg ND, Stone D, Harding A, Harper B, Harris S, Matzke MM, Cardenas A, Waters KM, Anderson KA. Effect of Native American fish smoking methods on dietary exposure to polycyclic aromatic hydrocarbons and possible risks to human health. J. Ag. Fd. Chem. 2012;60:6899–6906. doi: 10.1021/jf300978m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgard N, Salehpour M, Possnert G. Accelerator mass spectrometry in the attomolar concentration range for 14C–labeled biologically active compounds in complex mixtures. J. Analy. Atomic Spectom. 2010;25:74–8. [Google Scholar]
- Garner RC, Lightfoot TJ, Cupid BC, Russell D, Coxhead JM, Kutschere W, Priller A, Rom W, Steier P, Alexander DJ, Leveson SH, Dingley KH, Mauthe RJ, Turteltaub KW. Comparative biotransformation studies of MeIQx and PhIP in animal models and humans. Cancer Lett. 1999;143:161–5. doi: 10.1016/s0304-3835(99)00118-4. [DOI] [PubMed] [Google Scholar]
- Genies C, Jullien A, Lefebvre E, Revol M, Maitre A, Douki T. Inhibition of the formation of benzo[a]pyrene adducts to DNA in A549 lung cells exposed to mixtures of polycyclic aromatic hydrocarbons. Toxicol. In Vitro. 2016;35:1–10. doi: 10.1016/j.tiv.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Husain A, Naeemi E, Dashti B, al-Omirah H, al-Zenki S. Polycyclic aromatic hydrocarbons in food products originating from locally reared animals in Kuwait. Food Addit. Contam. 1997;14:295–9. doi: 10.1080/02652039709374527. [DOI] [PubMed] [Google Scholar]
- IARC. Monographs on the evaluation of carcinogenic risks to humans. Lyon: France; 2010. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. [PMC free article] [PubMed] [Google Scholar]
- Jarvis IWH, Dreij K, Mattsson A, Jernström B, Stenius U. Interactions between polycyclic aromatic hydrocarbons in complex mixtures and implications for cancer risk assessment. Toxicol. 2014;321:27–39. doi: 10.1016/j.tox.2014.03.012. [DOI] [PubMed] [Google Scholar]
- JECFA. [accessed 12-22-2017];2012 http://www.food.gov.uk/multimedia/pdfs/poly-aromatic-hydrocarbons.pdf.
- Jubert C, Mata J, Bench G, Dashwood R, Pereira C, Tracewell W, Turteltaub K, Williams D, Bailey G. Effects of chlorophyll and chlorophyllin on low-dose aflatoxin B1 pharmacokinetics in human volunteers. Cancer Prev. Res. 2009;2:1015–22. doi: 10.1158/1940-6207.CAPR-09-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HG, Jeong SH, Cho MH, Cho JH. Changes of biomarkers with oral exposure to benzo(a)pyrene, phenanthrene and pyrene in rats. J. Vet. Sci. 2007;8:361–8. doi: 10.4142/jvs.2007.8.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazerouni N, Sinha R, Hsu C-H, Greenberg A, Rothman N. Analysis of 200 food items for benzo[a]pyrne and estimation of its intake in an epidemiologic study. Fd. Chem. Toxicol. 2001;39:423–36. doi: 10.1016/s0278-6915(00)00158-7. [DOI] [PubMed] [Google Scholar]
- Kriek E, Van Schooten FJ, Hillebrand MJX, Van Leeuwen FE, Den Engelse L, De Looff AJA, Dijkmans APG. DNA adducts as a measure of lung cancer risk in humans exposed to polycyclic aromatic hydrocarbons. Environ. Hlth. Perspect. 1993;99:71–5. doi: 10.1289/ehp.939971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laher JM, Chernenko GA, Barrowman JA. Studies on the absorption and enterohepatic circulation of 7,12-dimethylbenz[a]anthracene in the rat. Can. J. Physiol. Pharmacol. 1983;61:1368–73. doi: 10.1139/y83-196. [DOI] [PubMed] [Google Scholar]
- Laher JM, Rigler MW, Vetter RD, Barrowman JA, Patton JS. Similar bioavailability and lymphatic transport of benzo(a)pyrene when administered to rats in different amounts of dietary fat. J. Lipid Res. 1984;25:1337–42. [PubMed] [Google Scholar]
- Lee BM, Yoo SD, Kim S. A proposed methodology of cancer risk assessment modeling using biomarkers. J. Toxicol. Environ. Hlth. A. 2002;65:341–54. doi: 10.1080/15287390252808019. [DOI] [PubMed] [Google Scholar]
- Lightfoot TJ, Coxhead JB, Cupid BC, Nicholson S, Garner RC. Analysis of DNA adducts by accelerator mass spectrometry in human breast tissue after administration of 2-amino-1-methyl-6-phenylimidzao[4,5-b]pyridine and benzo[a]pyrene. Mutat. Res. 2000;472:119–27. doi: 10.1016/s1383-5718(00)00134-0. [DOI] [PubMed] [Google Scholar]
- Lodovici M, Dolara P, Casalini C, Ciappellano S, Testolin G. Polycyclic aromatic hydrocarbon contamination in the Italian diet. Fd. Addit. Contam. 1995;12:703–13. doi: 10.1080/02652039509374360. [DOI] [PubMed] [Google Scholar]
- Madeen E, Corley RA, Crowell S, Turteltaub K, Ognibene T, Malafatti M, McQuistan TJ, Garrard M, Sudakin D, Williams DE. Human in vivo pharmacokinetics of [14C]dibenzo[def,p]chrysene by accelerator mass spectrometry following oral micro-dosing. Chem. Res. Toxicol. 2015;28:126–34. doi: 10.1021/tx5003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeen EP, Ognibene TJ, Corley RA, McQuistan TJ, Baird WM, Bench G, Turteltaub KW, Williams DE. Human micro-dosing with carcinogenic polycyclic aromatic hydrocarbons: In vivo pharmacokinetics of dibenzo[def,p]chrysene and metabolites by UPLC accelerator mass spectrometry. Chem. Res. Toxicol. 2016;29:1641–50. doi: 10.1021/acs.chemrestox.6b00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeen E, Siddens LK, Uesugi S, McQuistan T, Corley RC, Smith J, Waters KM, Tilton SC, Anderson KA, Ognibene T, Turteltaub K, Baird WM, Williams DE. Pharmacokinetics in humans of benzo[a]pyrene, an IARC class 1 carcinogen, following a truly environmentally relevant oral dose. Cancer Res. 2018 in review. [Google Scholar]
- Mahadevan B, Marston CP, Dashwood WM, Li Y, Pereira C, Baird WM. Effect of standardized complex mixture derived from coal tar on the metabolic activation of carcinogenic polycyclic aromatic hydrocarbons in human cells in culture. Chem. Res. Toxicol. 2005;18:224–31. doi: 10.1021/tx0497604. [DOI] [PubMed] [Google Scholar]
- Mahadevan B, Marston CP, Luch A, Dashwood WM, Brooks E, Pereira C, Doehmer J, Baird WM. Competitive inhibition of carcinogen-activating CYP1A1 and CYP1B1 enzymes by a standardized complex mixture of PAH extracted from coal tar. Int. J. Cancer. 2007;120:1161–8. doi: 10.1002/ijc.22466. [DOI] [PubMed] [Google Scholar]
- Malfatti MA, Kuhn EA, Turteltaub KW, Vickers SM, Jensen EH, Strayer L, Anderson KE. Disposition of the dietary mutagen 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in health and pancreatic cancer compromised humans. Chem. Res. Toxicol. 2016;29:352–8. doi: 10.1021/acs.chemrestox.5b00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motorykin O, Santiago-Delgado L, Rohlman D, Schrlau JE, Harper B, Harris S, Harding A, Kile ML, Simonich SLM. Metabolism and excretion rates of parent and hydroxyl-PAHs in urine collected after consumption of traditionally smoked salmon for Native American volunteers. Sci. Total Environ. 2015;514:170–7. doi: 10.1016/j.scitotenv.2015.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudie DM, Amidon GL, Amidon GE. Physiological parameters for oral delivery and in Vitro testing. Molec. Pharmaceut. 2010;7:1388–1405. doi: 10.1021/mp100149j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert DW, Shi Z, Gálvez-Peralta M, Uno S, Dragin N. Oral benzo[a]pyrene: understanding pharmacokinetics, detoxication, and consequences-Cyp1 knockout mouse lines as a paradigm. Molec. Pharmacol. 2013;84:304–13. doi: 10.1124/mol.113.086637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesnow S, Mass MJ, Ross JA, Galati AJ, Lambert GR, Gennings C, Carter WH, Jr, Stoner GD. Lung tumorigenic interactions in strain A/J mice of five environmental polycyclic aromatic hydrocarbons. Environ. Hlth. Perspect. 1998;106(Suppl. 6):1337–46. doi: 10.1289/ehp.98106s61337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ognibene TJ, Bench G, Brown TA, Vogel JS. The LLNL accelerator mass spectrometry system for biochemical 14C-measurements. Nucl. Instr. Meth. Phys. Res. 2004;23:12–5. [Google Scholar]
- Ognibene TJ, Haack KW, Bench G, Turteltaub KW. Trials and tribulations in the first three years in operation of the SSAMS for biomedical 14C–AMS at LLNL.. Nucl. Instr. Meth. Phys. Res. Section B-Beam Interact. Materials Atoms. 2018 doi: 10.1016/j.nimb.2018.05.008. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen NE, Vana V, Holm R. Does the digestibility of cyclodextrins influence the in vivo absorption of benzo[a]pyrene in rats? J. Pharmaceut. Sci. 2016;105:2698–702. doi: 10.1016/j.xphs.2015.10.027. [DOI] [PubMed] [Google Scholar]
- Ottaviani JI, Borges G, Momma TY, Spencer JPE, Keen CL, Crozier A, Schroeter H. The metabolome of [2-14C] (−)-epicatechin in humans: implications for the assessment of efficacy, safety, and mechanisms of action of polyphenolic bioactives. Nature Sci. Reports. 2016;6:29034. doi: 10.1038/srep29034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh A, Inyang F, Hood DB, Archibong AE, Knuckles M, Nyanda AM. Metabolism, bioavailability, and toxicokinetics of benzo[α]pyrene in F-344 rats following oral administration. Exptl. Toxicol. Pathol. 2001;53:275–90. doi: 10.1078/0940-2993-00192. [DOI] [PubMed] [Google Scholar]
- Ramsauer B, Sterz K, Hagedorn H-W, Engl J, Scherer G, McEwan M, Errington G, Shepperd J, Cheung F. A liquid chromatography/tandem mass spectrometry (LC-MS/MS) method for the determination of phenolic polycyclic aromatic hydrocarbons (OH-PAH) in urine of non-smokers and smokers. Analy. Bioanaly. Chem. 2011;399:877–89. doi: 10.1007/s00216-010-4355-7. [DOI] [PubMed] [Google Scholar]
- Ross JA, Nelson GB, Wilson KH, Rabinowitz JR, Galati A, Stoner GD, Newnow S, Mass MJ. Adenomas induced by polycyclic aromatic hydrocarbons in strain A/J/ mouse lung correlate with time-integrated DNA adduct levels. Cancer Res. 1995;55:1039–44. [PubMed] [Google Scholar]
- Sadiktsis I, Bergvall C, Johansson C, Westerholm R. Automobile tires- a potential source of highly carcinogenic dibenzopyrenes to the environment. Environ. Sci. Technol. 2012;46:3326–34. doi: 10.1021/es204257d. [DOI] [PubMed] [Google Scholar]
- Schneider K, Roller M, Kalberlah F, Schuhmacher-Wolz U. Cancer risk assessment for oral exposure to PAH mixtures. J. Appl. Toxicol. 2002;22:73–83. doi: 10.1002/jat.828. [DOI] [PubMed] [Google Scholar]
- Shi Z, Dragin N, Miller ML, Stringer KF, Johansson E, Chen J, Uno S, Gonzalez FJ, Rubio CA, Nebert DW. Oral benzo[a]pyrene-induced cancer: two distinct types in different target organs depend on the mouse Cyp1 genotype. Int. J. Cancer. 2010a;127:2334–50. doi: 10.1002/ijc.25222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Dragin N, Gálvez-Peralta M, Norge-Nebert LF, Miller ML, Wang B, Nebert DW. Organ-specific roles of CYP1A1 during detoxication of dietary benzo[a]pyrene. Molec. Pharmacol. 2010b;78:46–57. doi: 10.1124/mol.110.063438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Guengerich FP. Inhibition of human cytochrome P450 1A1-, 1A2, and 1B1-mediated activation of procarcinogens to genotoxic metabolites by polycyclic aromatic hydrocarbons. Chem. Res. Toxicol. 2006;19:288–94. doi: 10.1021/tx050291v. [DOI] [PubMed] [Google Scholar]
- Shimada T, Murayama N, Tanaka K, Takenaka S, Imai Y, Hopkins NE, Foroozesh MF, Alworth WL, Yamazaki H, Guengerich FP, Komori M. Interaction of polycyclic aromatic hydrocarbons with human cytochrome P450 1B1 in inhibiting catalytic activity. Chem. Res. Toxicol. 2008;21:2313–23. doi: 10.1021/tx8002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddens LK, Larkin A, Krueger SK, Bradfield CA, Waters KM, Tilton SC, Pereira CB, Löhr CV, Arlt VM, Phillips DH, Williams DE, Baird WM. Polycyclic aromatic hydrocarbons as skin carcinogens: comparison of benzo[a]pyrene, dibenzo[def,p]chrysene and three environmental mixtures in the FVB/N mouse. Toxicol. Appl. Pharmacol. 2012;264:377–86. doi: 10.1016/j.taap.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal YCM, van Herwijnen MHM, van Schooten FJ, van Delft JHM. Modulation of gene expression and DNA adduct formation in HepG2 cells by polycyclic aromatic hydrocarbons with different carcinogenic potencies. Carcinogenesis. 2006;27:646–55. doi: 10.1093/carcin/bgi255. [DOI] [PubMed] [Google Scholar]
- Staal YCM, Hebels DGAJ, van Herwijnen MHM, Gottschalk RWH, van Schooten FJ, van Delft JHM. Binary PAH mixtures cause additive or antagonistic effects on gene expression but synergistic effects on DNA adduct formation. Carcinogenesis. 2007;28:2632–40. doi: 10.1093/carcin/bgm182. [DOI] [PubMed] [Google Scholar]
- Tarantini A, Maître A, Lefèbvre E, Marques M, Rajhi A, Douki T. Polycyclic aromatic hydrocarbons in binary mixtures modulate the efficiency of benzo[a]pyrene to form DNA adducts in human cells. Toxicology. 2011;279:36–44. doi: 10.1016/j.tox.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Thomas AT, Ognibene T, Daley P, Turteltaub K, Radousky H, Bench G. Ultrahigh efficiency moving wire combustion interface for online coupling of high-performance liquid chromatography (HPLC) Analy. Chem. 2011;83:9413–7. doi: 10.1021/ac202013s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilton SC, Siddens LK, Krueger SK, Larkin AJ, Löhr CV, Williams DE, Baird WM, Waters KM. Mechanism-based classification of PAH mixtures to predict carcinogenic potential. Toxicol. Sci. 2015;146:135–45. doi: 10.1093/toxsci/kfv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titaley IA, Chlebowski A, Truong L, Tanguay RL, Simonich SLM. Identification and toxicological evaluation of unsubstituted PAHs and novel PAH derivatives in pavement sealcoat products. Environ. Sci. Technol. Lett. 2016;3:234–42. [PMC free article] [PubMed] [Google Scholar]
- Turteltaub KW, Mauthe RJ, Dingley KH, Vogel JS, Frantz C, Garner RC, Shen N. MeIQx-DNA adduct formation in rodent and human tissues at low doses. Mutat. Res. 1997;376:243–52. doi: 10.1016/s0027-5107(97)00049-3. [DOI] [PubMed] [Google Scholar]
- Uno S, Dalton TP, Shertzer HG, Genter MB, Warshawsky D, Talaska G, Nebert DW. Benzo[a]pyrene-induced toxicity: paradoxical protection in Cyp1a1(−/−) knockout mice having increased hepatic BaP-DNA adduct levels. Biochem. Biophys. Res. Commun. 2001;289:1049–56. doi: 10.1006/bbrc.2001.6110. [DOI] [PubMed] [Google Scholar]
- Uno S, Dalton TP, Derkenne S, Curan CP, Miller ML, Shertzer HG, Nebert DW. Oral exposure to benzo[a]pyrene in the mouse: detoxication by inducible cytochrome P450 is more important that metabolic activation. Molec. Pharmacol. 2004;65:1225–37. doi: 10.1124/mol.65.5.1225. [DOI] [PubMed] [Google Scholar]
- Uno S, Dalton TP, Dragin N, Curran CP, Derkenne S, Miller ML, Shertzer HG, Gonzalez FJ, Nebert DW. Oral benzo[a]pyrene in Cyp1 knockout mouse lines: CYP1A1 important in detoxication, CYP1B1 metabolism required for immune damage independent of total-body burden and clearance rate. Molec. Pharmacol. 2006;69:1103–14. doi: 10.1124/mol.105.021501. [DOI] [PubMed] [Google Scholar]
- Uno S, Dragin N, Miller ML, Dalton TP, Gonzalez FJ, Nebert DW. Basal and inducible CYP1 mRNA quantitation and protein localization throughout the mouse gastrointestinal tract. Free Radic. Biol. Med. 2008;44:570–83. doi: 10.1016/j.freeradbiomed.2007.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA, U.S. Development of a relative potency factor (RPF) approach for polycyclic aromatic hydrocarbon (PAH) mixtures. United States Environmental Protection Agency 2010 [Google Scholar]
- U.S. EPA, U.S. [accessed 01/06/2018];Toxicological review of benzo[a]pyrene (CASRN 50-32-8) 2017 www.epa.gov/iris.
- Zelinkova Z, Wenzl T. The occurrence of 16 EPA PAHs in food- a review. Polycyc. Arom. Hydrocarb. 2015;35:248–84. doi: 10.1080/10406638.2014.918550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.