Abstract
Background
Congestive heart failure (CHF) affects 5.7 million Americans, costing $32 billion annually in treatment expenditures and lost productivity. CHF also contributes to health disparities between black and white Americans: blacks develop CHF at a younger age, and are more likely to be hospitalized and die from it. Improved CHF treatment could generate significant health benefits and reduce health disparities.
Objectives
To illustrate the potential benefit of effective CHF treatment in terms of improved health, greater social value, and reduced health disparities between black and white subpopulations.
Methods
We adapted an established economic-demographic microsimulation to estimate scenarios in which a hypothetical innovation eliminates the incidence of CHF and, separately, six other diseases in patients aged 51-52 in 2016. We followed this cohort until death and estimated total life years, quality-adjusted life years (QALYs) and disability-free life years (DFLYs) with and without the innovation, for the population overall and for race- and gender-defined subpopulations.
Results
CHF prevalence among 65- to 70-year-olds increases from 4.3% in 2012 to 8.5% in 2030. Diagnosis with CHF coincides with significant increase in disability and medical expenditures, particularly among blacks. Preventing CHF among those aged 51-52 in 2016 would generate nearly 2.9 million additional life years, 1.1 million DFLYs, and 2.1 million QALYs worth $210 to $420 billion. These gains are greater among blacks than whites.
Conclusions
CHF prevalence will increase substantially over the next two decades, and will affect black Americans more than whites. Improved CHF treatment could generate significant social value, and reduce existing health disparities.
Keywords: Heart failure, health disparities, disability, medical spending
There is much concern about the increasing share of national income devoted to health care,(1) but spending increases have been accompanied by significant health improvements. Most significantly, age-standardized death rates from all causes have fallen 43% since 1969 (from 1,279 deaths per 100,000 in 1969 to 730 in 2013).(2) Better cardiovascular outcomes have driven much of this improvement, with age-adjusted deaths from heart disease falling from 520 per 100,000 in 1969 to 168.5 in 2015.(2,3) Evidence-based treatment of associated risk factors has been credited with contributing to these declines.(4)
However, progress may be slowing, and — in some disease areas like congestive heart failure (CHF) — may even be reversing. An estimated 5.7 million American adults suffer from CHF, and CHF is a contributing factor in 1 in 9 U.S. deaths.(4) The Centers for Disease Control reports that between 2011 and 2014, age-adjusted death rates from heart failure rose from 16.9 to 18.6 per 100,000.(5)
This trend may also exacerbate existing racial health disparities. African Americans develop heart failure earlier than whites, and are more likely to be admitted to the hospital for it.(6,7) In addition, the 5-year risk-adjusted all-cause mortality rate for CHF patients is 34% higher for African Americans than whites.(8,9) Given these existing racial disparities, the fact that the age-adjusted death rates from CHF are increasing is particularly alarming.
Adding to the personal toll of CHF—premature death, disability and loss of quality of life—its economic costs are substantial: almost $32 billion annually for U.S. treatment costs and lost productivity.(7) Fortunately, recent treatment innovations suggest that the future impact of CHF on patient outcomes, economic productivity, and overall social value could be reduced, perhaps even in a way that mitigates health disparities.(10-12)
This paper models the potential benefits to population health from continued innovation in CHF treatment. Using US population-wide simulations, we estimate trends in CHF prevalence, and how much improved CHF treatments could improve overall social value, and reduce racial and gender differences in health outcomes.
Methods
To illustrate the potential benefits of improved CHF treatment, we adapted the Future Elderly Model (FEM), an established economic-demographic microsimulation that has been used to study a wide variety of health policy questions. The FEM has been developed over time with support from the National Institute on Aging, the Department of Labor, the MacArthur Foundation, and the Centers for Medicare & Medicaid Services to study health care innovation in a wide variety of contexts, including heart disease.(13-17)
Overview
The FEM simulates health and medical spending for Americans aged 51 years and older. The model uses initial demographic characteristics and health conditions for each individual to project their medical spending, health conditions and behaviors, disability status, and quality of life. A key advantage of the FEM is that it tracks individual-level health trajectories and patient outcomes, which allows us to consider the impact of innovation by characteristics such as gender and race.
The FEM’s core module uses individuals’ current characteristics to calculate transition probabilities among health states, including mortality, functional status, body mass index (BMI) and six disease conditions: diabetes, high blood pressure, heart disease (including CHF), cancer (excluding skin cancer), stroke, and lung disease. The model uses inputs from three nationally representative datasets: The Health and Retirement Survey (HRS), a biennial survey of the American population aged 51+ which has been conducted since 1992; the Medical Expenditure Panel Survey (MEPS), a set of large-scale surveys of the non-institutionalized US population, and the Medicare Current Beneficiary Survey (MCBS), a survey of Medicare beneficiaries about their health status, healthcare use and insurance coverage. More detail on the model and data sources is provided in the technical appendix.(18)
Prevalence and incidence of CHF
To predict which individuals have or will get CHF during the simulation, we use HRS historical data to build a two-year CHF incidence model based on predictors including age, sex, education, race, age-race interactions, BMI, smoking behavior, marital status, and the six disease conditions modeled. This is a first-order Markov model in which time-varying components enter via their status two years prior. For example, diabetes status in the prior wave of the survey is a predictor of incident CHF in the current wave. All transition models in the simulation have this structure.
CHF status is included as a predictor of other outcomes of interest, including mortality, functional limitations (ADL), and instrumental activities of daily living (IADL) limitations. Mortality is estimated as a two-year probit model, controlling for age, race, sex, education, widowhood, smoking status, the six chronic diseases, ADLs, and IADLs. The number of functional limitations is estimated as an ordered probit with four categories: none, one, two, and three or more. This ADL model controls for the same set of variables as the mortality model, plus BMI. IADL limitations are also modeled with the same predictors, as an ordered probit with three categories: none, one, and two or more.
Valuing health benefits
To value health benefits, we predict quality-adjusted life years (QALYs) using the EQ-5D, a widely-used health-related quality of life index. The EQ-5D instrument includes five questions regarding the extent of problems in mobility, self-care, daily activities, pain, and anxiety/depression, and has been widely used in both Europe and the United States.(19,20) Using the 2001 MEPS, we estimate a linear model fitting EQ-5D scores as a function of six chronic conditions and functional status (details in technical appendix). We predict a QALY measure for every person in the simulation in every year based on their simulated health and functional status.
We simulate outcomes for a representative cohort of 51- and 52-year-olds beginning in 2016 (n=13,040). This cohort, described in the technical appendix, is based on respondents from the HRS. In each year, the spending module predicts medical expenditures over the next two years (the HRS is biennial) based on each individual’s current ‘state’. The health module is then used to predict who will survive to year 2018, and their obesity status, disease, and functional state, and a predicted QALY for that year. The spending module is then used to predict that period’s health care resource use. The simulation iterates in this manner until everyone in the 2016 cohort has died. We repeat the simulation 500 times for each scenario and report the average outcomes and resulting confidence intervals. Primary outcomes are life expectancy, quality-adjusted life expectancy, and lifetime medical spending. All costs and QALYs are discounted using a 3% annual discount rate as suggested by Gold et al.(21)
Scenarios
To predict the prevalence of CHF from 2016 to 2030, we simulate the population aged 50+ in 2016 and beyond, accounting for projected demographic trends over time. To examine the burden of CHF, we model the life trajectories of the cohort of individuals aged 51-52 in 2016 to construct a baseline scenario, and compare this to a “No CHF” scenario in which no individuals in the cohort develop CHF throughout the simulation, maintaining all other transition dynamics. While completely preventing CHF might seem unrealistic, it provides an upper bound for the potential social gain from a medical innovation. For comparison, we perform similar analyses eliminating, in turn, diabetes, high blood pressure, lung disease, cancer, obesity and stroke. To calculate total QALYs added in each scenario, we calculate the number of individuals aged 51-52 in 2016 and multiply this by the average QALYs added.
Implementation
Transition models are estimated using the RAND HRS version P, using nationally representative waves from 1998-2012 (n=114,489 person-waves). Medical costs are estimated using MEPS 2007-2010 for the non-Medicare population and MCBS 2007-2012 for the Medicare population. All estimations performed with Stata 14.0. See the technical appendix for estimates.(18)
Results
Figure 1 shows the prevalence of CHF through 2030—years 1996 through 2010 represent data from the HRS, while years 2012 and beyond reflect simulation estimates. Among 65- to-70-year-olds, the prevalence of CHF is expected to increase from 4.29% in 2010 to 8.45% (95% CI: 8.03-8.87%) in 2030.
Figure 1.
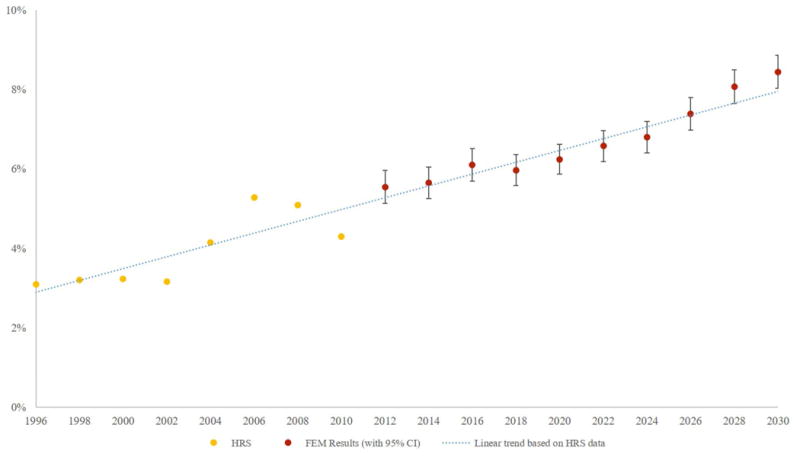
Prevalence of Congestive Heart Failure Among U.S. 65-70 Year Olds
Sources: Health & Retirement Survey, and the Future Elderly Model Simulation
Analysis of the 2010-2012 HRS data among patients with cardiovascular disease shows that the age-adjusted incidence of CHF is higher among blacks than whites, and highest for black females (4.8%) (black males: 4.1%, white females: 4.0%, white males: 3.5%).
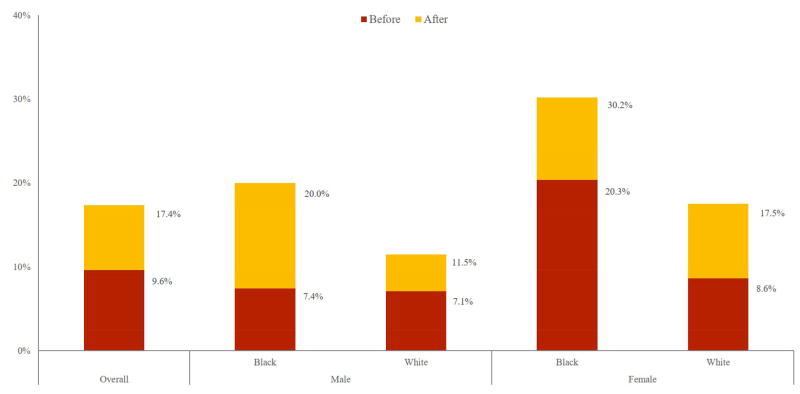
We also considered the impact of CHF on disability status, and how this varies with race and gender. Using 2000-2012 HRS data, we identified all patients without CHF in one period who went on to develop CHF in the subsequent period, and their reported ability to perform five ADLs: eating, bathing, dressing, walking across a room, and getting in or out of bed. Figure 2 reports the age-adjusted proportion of these patients who report limitations in three or more ADLs before and after CHF diagnosis.
Figure 2.
Age-Adjusted Percent of CHF Population Reporting Limitations in 3 or More Activities of Daily Living, Before and After CHF Diagnosis, by Race and Gender
Source: Health & Retirement Survey 2000 to 2012 Data
Immediately before CHF diagnosis, 9.6% of patients report three or more limitations, rising to 17.4% after CHF diagnosis. The onset of significant disability with CHF diagnosis is particularly severe among black men: Before diagnosis, 7.4% of black males who will develop CHF report three or more limitations, increasing to 20% immediately after diagnosis. Among black females who develop CHF, the proportion reporting three or more limitations is 20.3% before diagnosis and 30.2% afterwards. The proportion of the population that did not develop CHF across two consecutive waves saw no significant changes in age-adjusted disability.
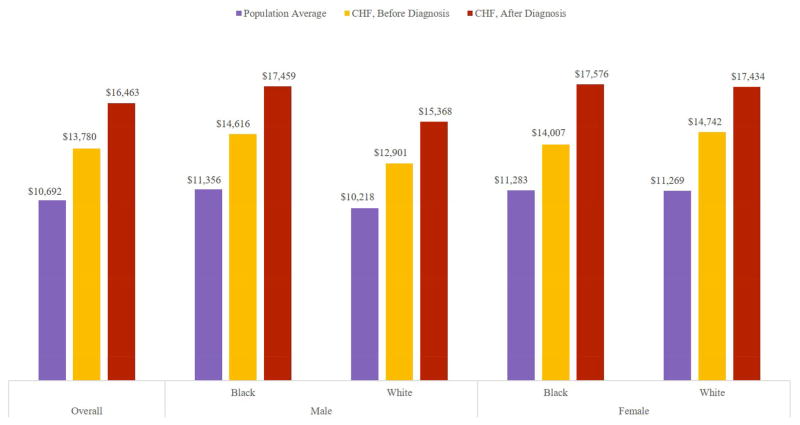
Medical expenditures follow a similar pattern (Figure 3). In the 2000-2012 HRS data we found that, prior to diagnosis, patients who will develop CHF are somewhat sicker than the average person of the same age, with medical expenditures 25-30% higher than those of people without CHF. After diagnosis, CHF patients have medical expenditures 50-56% higher. The increment is especially large among black females.
Figure 3.
Annual Per-Capita Medical Expenditures, by Race and Gender
Source: Health & Retirement Survey 2000 to 2012 Data
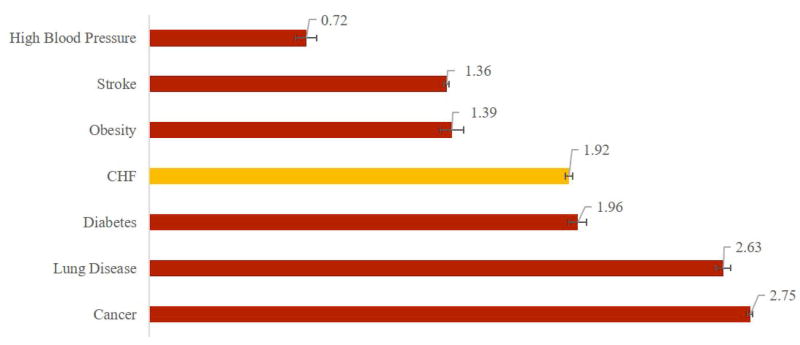
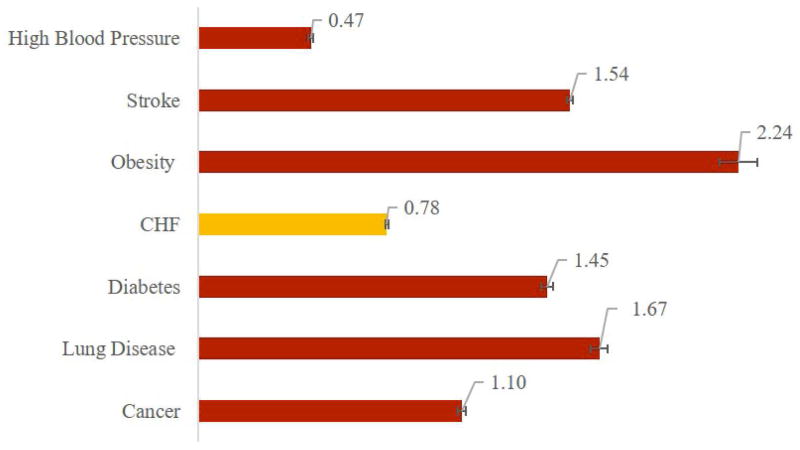
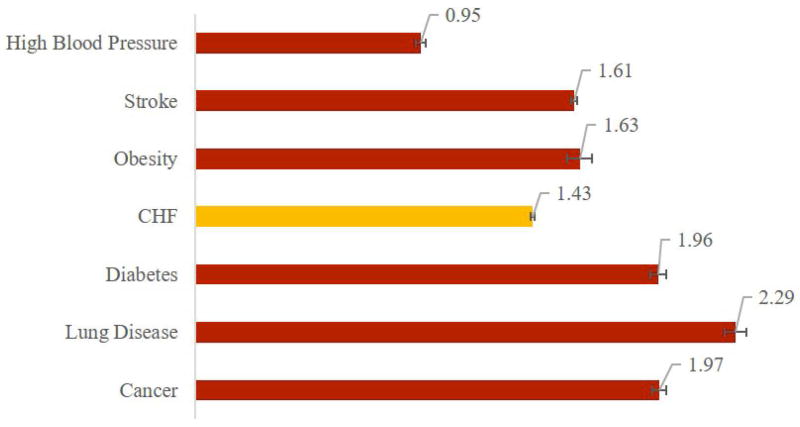
Increasing prevalence of a disease such as CHF, with significant mortality, disability and expenditure implications for the overall population and differential implications by race and gender underscores the potential benefits from improved treatment. We explored these benefits by simulating scenarios in which we eliminate seven diseases—CHF, cancer, diabetes, high blood pressure, lung disease, obesity, and stroke—and compare the resulting gains in life expectancy, QALYs and disability-free life years (DFLYs). Affected patients retain all the other characteristics and comorbidities of a patient with the disease in question; they do not return to “average” health. Figure 4 presents these results: Among patients who otherwise would have developed CHF, eliminating the disease increases average life expectancy by 1.92 (95% CI: 1.91-1.93) years, increases the average time lived without a disability by 0.78 (0.78-0.79) years and increases quality-adjusted life expectancy by 1.43 (1.42-1.44) years. Only eliminating cancer, lung disease and diabetes generate greater life expectancy increases for their affected populations. Table 1 presents simulation results including the lifetime risk of each condition, and the impact of eliminating each on the life expectancy for the entire population, which combines the impact on the affected population with prevalence.
Figure 4.
Average Gain in Selected Outcomes from Eliminating Seven Conditions (Among Those Affected)
Panel A –Life Expectancy in Years
Panel B – Disability-Free Life Years
Panel C – Quality-Adjusted Life Years
Source: The Future Elderly Model Analysis
Table 1.
Lifetime risk of seven diseases, and gain in population-wide life expectancy from eliminating each one (95% confidence intervals in parentheses)
| Disease/Condition for Intervention* | Lifetime Risk/Percentage of Population Affected | LE gain for the entire population† | LE gain for the sub-population affected by the intervention |
|---|---|---|---|
| Cancer | 39.2% (38.8 – 39.6) | 1.09 (1.08 – 1.10) | 2.75 (2.71 – 2.79) |
| CHF | 36.2% (35.9 – 36.4) | 0.70 (0.69 – 0.71) | 1.92 (1.91 – 1.93) |
| Diabetes | 57.4% (56.8 – 58.0) | 1.13 (1.11 – 1.15) | 1.96 (1.93 – 2.00) |
| High Blood Pressure | 89.0% (88.8 – 89.3) | 0.64 (0.63 – 0.65) | 0.72 (0.70 – 0.73) |
| Lung Disease | 27.8% (27.4 – 28.3) | 0.74 (0.73 – 0.75) | 2.63 (2.57 – 2.68) |
| Obesity | 42.1% (40.9 – 43.2) | 0.59 (0.56 – 0.61) | 1.39 (1.34 – 1.44) |
| Stroke | 37.5% (37.3 – 37.7) | 0.51 (0.51 – 0.52) | 1.36 (1.35 – 1.37) |
The intervention eliminates the disease or condition
Entire population refers to the cohort of 51-52 year old in the year 2016 Source: The Future Elderly Model Simulation
If an innovation to eliminate heart failure is applied to the 4.1 million individuals aged 51-52 in 2016, it could generate nearly 2.9 million additional life years, 2.1 million QALYs, and 1.2 million DFLYs. Depending on the value of each additional QALY, the population health benefits of such an innovation range from $210 to $420 billion.
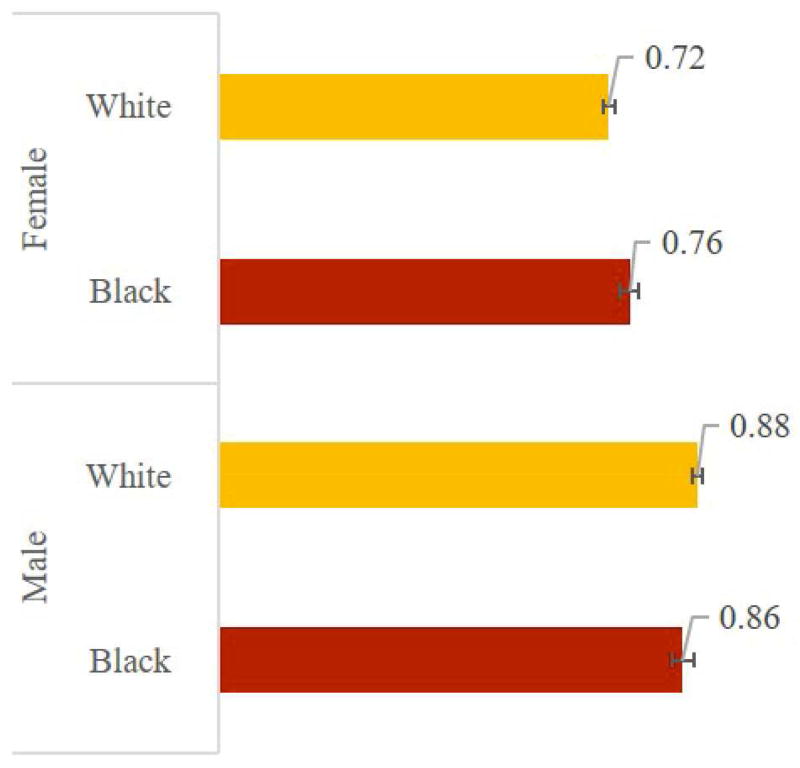
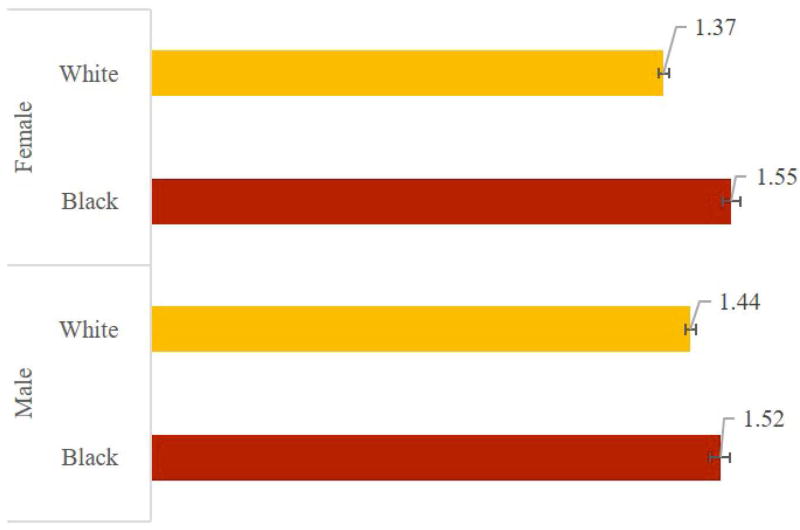
Figure 5 Panel A shows that eliminating heart failure increases average life expectancy among those affected by 2.10 (95% CI: 2.06-2.14) years for black males, 1.90 (1.88-1.92) for white males, 2.18 (2.15-2.22) for black females, and 1.84 (1.82-1.86) for white females. Panel B shows that eliminating CHF adds 0.86 (0.84-0.88) disability-free life years for black males, 0.88 (0.87-0.89) for white males, 0.76 (0.74-0.78) for black females, and 0.72 (0.71-0.73) for white females. Panel C shows QALY gains: 1.52 (1.50-1.55) for black males, vs. 1.44 (1.43-1.46) for white males, and 1.55 (1.53-1.58) for black females compared to 1.37 (1.36-1.39) for white females.
Figure 5.
Average Gain in Selected Outcomes from Eliminating Congestive Heart Failure (Among Those Affected), by Race and Gender
Panel A –Life Expectancy in Years
Panel B –Disability-Free Life Years
Panel C – Quality-Adjusted Life Years
Source: The Future Elderly Model Analysis
Discussion
CHF prevalence and lifetime risk
Our estimates of the future prevalence of CHF are generally higher than in other studies.(22,23) This is in part because our estimates focus on prevalence among the older population, while other estimates report prevalence among the entire US population. However, our simulation also incorporates trends in the risk factors that lead to CHF, which are themselves increasing.
For example, Heidenreich et al. project CHF prevalence increasing from 2.4% in 2012 to 3.0% in 2030.(22) Their estimates are driven by changes in the size of subpopulations—defined by age, gender, and ethnicity—but they do not allow the prevalence within a subpopulation to change over time. Our subpopulation-specific CHF prevalence estimates incorporate projected trends in comorbidities and other health indicators that accompany CHF, including obesity, hypertension, etc. Thus, increasing obesity over time will increase the prevalence of CHF even within demographic subpopulations, leading to higher CHF prevalence estimates than those using the Heidenreich methods.(22,23)
Our model projects a lifetime risk of CHF incidence of 35% for patients aged 51-52, similar to other lifetime risk estimates based on large-scale population studies.(24) Our findings of disparities between blacks and whites in the risk of CHF in the HRS data mirrors results of previous studies. Most notably, the Atherosclerosis Risk in Communities Study found that the lifetime risk of CHF for those aged 45-75 was higher for blacks than for whites, and highest for black females (24% vs. 21% for black males, 19% for white males, 13% for white females).(7)
Disability and disparities
People with CHF often have other serious medical conditions, such as arthritis (62%) or diabetes (38%); are unable to walk two to three blocks or walk up 10 steps (57%); need help with activities of daily living (11%); and take 6.4 prescription medications on average.(25) Such factors may affect CHF patients’ ability to live independently, with needs ranging from help from an informal caregiver to moving to a nursing facility.
We show (Figure 2) that disability outcomes vary by race—black patients with CHF diagnoses are much more likely to report limitations in three or more ADLs than their white counterparts. Among men diagnosed with CHF, roughly the same fractions of black and white patients report three or more ADLs before diagnosis (7.4% vs. 7.1%), but after diagnosis, that fraction increases by more than 170% for black males, and only 62% for white males. Similar trends appear in the expenditure data (Figure 3).
While the correlation between CHF and disability may be the result of CHF occurring in patients who are already very sick and disabled, our findings suggest that CHF may also play a causal role in patients’ decline: For many, the onset of CHF can be relatively sudden, and preceded by relatively good health, but disability may progress rapidly after diagnosis. If so, then perhaps the appearance of significant disability could be forestalled if a diagnosis of CHF could be delayed or eliminated. And if health disparities are driven by differential disability outcomes by race and gender, then more effective treatments for CHF could reduce health disparities.
Value of innovation in CHF
Until recently, there has been relatively little innovation in CHF treatment, with standard care involving medications to treat symptoms, including angiotensin-converting enzyme inhibitors, beta blockers, and diuretics.(11) New drugs for heart failure have recently been approved that reduced the risk of death or hospitalization from heart failure by 20 percent in clinical trials.(26,27) While these new treatments do not eliminate CHF, they point at the potential for significant innovation in this disease area.
Our results demonstrate that eliminating CHF, even without changing patients’ underlying health characteristics, would add 1.92 years to each affected patient’s life—more than affected patients would gain by eliminating stroke, obesity, or high blood pressure. Improvements in CHF treatment can also enhance patients’ quality of life, with elimination adding 1.43 QALYs or 0.78 DFLYs to the average CHF patient’s life. These also compare favorably with innovations to eliminate high blood pressure, a condition that receives far more public health attention than CHF.
Health disparities
We estimate that eliminating CHF could narrow the disparity between black and white average life expectancy. In our baseline scenario, for the subgroup that developed CHF, white males live 5.1 years longer than black males, and white females 3.5 years longer than black females, on average. Curing CHF reduces this gap by 0.1 and 0.3 years, respectively.
Limitations
We note several limitations. First, our results are derived from simulations estimated by the FEM, which uses simplifications of the dynamic relationships driving outcomes in the real world, and parameterizes those relationships using estimates from the literature. If these simplifications are incomplete, or the parameter estimates are imperfect, model results may not correspond with actual outcomes.
Second, our “No-CHF” scenario assumes an innovation that eliminates CHF, although medical innovation is unlikely to completely eliminate CHF in the near term. Instead, actual innovations are more likely to reduce CHF incidence or lessen its severity, but not eliminate it completely. This scenario does however also help answer the question: what is the social cost of CHF in older populations?
Third, we do not explicitly model the social determinants of health disparities, such as greater poverty, poorer access to care, and lower health literacy among black patients with CHF.(28) Our “No-CHF” scenario implicitly assumes that a cure for CHF is applied equally to all CHF patients, regardless of social determinants, without modeling how that penetration would happen. Nevertheless, understanding the social value that such a cure, uniformly applied, would unlock helps inform what kinds of policies would be most beneficial.
Finally, our model of the relationship between CHF and outcomes (disability, quality-of-life, and mortality) is based on associations observed in cohorts of thousands of older Americans followed over decades. We have not demonstrated a direct causal link.
Policy Implications
Policy programs to improve public health have often focused on interventions for high-prevalence diseases and conditions such as diabetes, obesity, and hypertension. Cancer has also received much policy attention, including the recent Cancer Moonshot, with the goal of hastening cures, despite limited progress. Our work suggests that similar emphasis, focus and investment in finding ways to eliminate CHF could have as much or more impact in terms of adding life years, QALYs and DFLYs, and potentially reducing racial disparities among the population.
Conclusions
Heart failure is one example of the growing disease burden older Americans bear as they live longer but face growing risks of disability.(29) From a societal standpoint, policymakers and other decision makers must balance competing aims to benefit all people generally and disadvantaged groups specifically to achieve goals of both efficiency and equity. Innovations that improve disease outcomes—not just eliminate them—can improve efficiency by increasing benefits to society through longer, healthier, more productive lives. Some treatment innovations also can improve equity by narrowing longstanding health disparities among minorities and women.
Innovation in CHF deserves scientific and policy attention not simply because it can extend lives and reduce disability and decline in older Americans but also because it could ameliorate some racial and gender disparities in health outcomes associated with cardiovascular disease.
Clinical Perspectives.
Competency in Medical Knowledge
CHF prevalence will increase substantially over the next two decades, affecting black Americans more than whites. A CHF diagnosis coincides with significant increase in disability and medical expenditures, particularly among blacks compared with whites. Improving CHF treatment could generate significant social value, and reduce existing racial/ethnic health disparities.
Translational Outlook
Future research to identify more effective treatment and prevention of CHF could both improve the quality and length of life for patients with CHF, and reduce disparities among patients affected by CHF.
Acknowledgments
None
Funding:
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health (Bethesda, MD) under Awards P30AG024968 and P30AG043073, and by the Schaeffer Center for Health Policy and Economics at the University of Southern California (Los Angeles, CA). Additional support provided by Novartis, Inc. (NY, NY). The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsors.
Abbreviations List
- ADL
Activities of Daily Living
- BMI
Body Mass Index
- CHF
Congestive Heart Failure
- EQ-5D
EuroQol five dimensions questionnaire
- FEM
Future Elderly Model
- HRS
Health and Retirement Survey
- IADL
Instrumental Activities of Daily Living
- MEPS
Medical Expenditure Panel Survey
- MCBS
Medicare Current Beneficiary Survey
- QALY
Quality-Adjusted Life Year
- DFLY
Disability-Free Life Year
Footnotes
Disclosures:
Dr. Goldman is a co-founder of Precision Health Economics and holds equity in its parent company. Dr. Van Nuys has served as a consultant to Precision Health Economics. Dr. Hlatky has received consulting fees from Acumen and Sanofi-Aventis, and research grants from HeartFlow, Milestone Pharmaceuticals, and St. Jude Medical. The remaining authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Health Expenditure Data Highlights. Center for Medicare & Medicaid Services Report. 2015 [Google Scholar]
- 2.Ma J, Ward EM, Siegel RL, Jemal A. TEmporal trends in mortality in the united states, 1969-2013. JAMA. 2015;314:1731–1739. doi: 10.1001/jama.2015.12319. [DOI] [PubMed] [Google Scholar]
- 3.Statistics C-NCfH. Underlying Causes of Death 1999-2015. 2016 [Google Scholar]
- 4.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics—2016 Update. American Heart Association. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 5.Ni H, Xu J. Recent Trends in Heart Failure-related Mortality: United States, 2000–2014. NCHS Data Brief. 2015 Dec;(No. 231) [PubMed] [Google Scholar]
- 6.Bibbins-Domingo K, Pletcher MJ, Lin F, et al. Racial Differences in Incident Heart Failure among Young Adults. New England Journal of Medicine. 2009;360:1179–1190. doi: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huffman MD, Berry JD, Ning H, et al. Lifetime Risk for Heart Failure Among White and Black AmericansCardiovascular Lifetime Risk Pooling Project. Journal of the American College of Cardiology. 2013;61:1510–1517. doi: 10.1016/j.jacc.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.East MA, Peterson ED, Shaw LK, Gattis WA, O’Connor CM. Racial differences in the outcomes of patients with diastolic heart failure. American Heart Journal. 2004;148:151–156. doi: 10.1016/j.ahj.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Durstenfeld MS, Ogedegbe O, Katz SD, Park H, Blecker S. Racial and Ethnic Differences in Heart Failure Readmissions and Mortality in a Large Municipal Healthcare System. JACC: Heart Failure. 2016;4:885–893. doi: 10.1016/j.jchf.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fonarow GC, Hernandez AF, Solomon SD, Yancy CW. Potential mortality reduction with optimal implementation of angiotensin receptor neprilysin inhibitor therapy in heart failure. JAMA Cardiology. 2016;1:714–717. doi: 10.1001/jamacardio.2016.1724. [DOI] [PubMed] [Google Scholar]
- 11.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 12.Sacubitril/valsartan (entresto) for heart failure. JAMA. 2015;314:722–723. doi: 10.1001/jama.2015.9398. [DOI] [PubMed] [Google Scholar]
- 13.Goldman DP, Shang B, Bhattacharya J, et al. Consequences of health trends and medical innovation for the future elderly. Health Affairs. 2005;24(Suppl 2):W5R5-17. doi: 10.1377/hlthaff.w5.r5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldman DP, Cutler D, Rowe JW, et al. Substantial health and economic returns from delayed aging may warrant a new focus for medical research. Health Affairs. 2013;32:1698–705. doi: 10.1377/hlthaff.2013.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldman DP, Orszag PR. The Growing Gap in Life Expectancy: Using the Future Elderly Model to Estimate Implications for Social Security and Medicare. American Economic Review: AEA Papers and Proceedings. 2014;104:230–233. doi: 10.1257/aer.104.5.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaudette E, Goldman DP, Messali A, Sood N. Do Statins Reduce the Health and Health Care Costs of Obesity? PharmacoEconomics. 2015 doi: 10.1007/s40273-014-0234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldman DP, Zheng Y, Girosi F, et al. The benefits of risk factor prevention in Americans aged 51 years and older. American Journal of Public Health. 2009;99:2096–101. doi: 10.2105/AJPH.2009.172627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldman DP, Leaf DE, Sullivan J, Tysinger B, Xie Z. Innovation in Heart Failure Treatment: Life Expectancy, Disability, and Health Disparities - Technical Appendix. USC Schaeffer Center Working Paper. 2017 [Google Scholar]
- 19.Dolan P. Modeling valuations for EuroQol health states. Medical Care. 1997;35:1095–108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Medical Care. 2005;43:203–20. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Gold M, Siegel J, Russell L, Weinstein M. Cost-effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 22.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the Impact of Heart Failure in the United States. A Policy Statement From the American Heart Association. 2013 doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the Future of Cardiovascular Disease in the United States. A Policy Statement From the American Heart Association. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 24.Huffman MD, Berry JD, Ning H, et al. Lifetime Risk for Heart Failure Among White and Black Americans: Cardiovascular Lifetime Risk Pooling Project. Journal of the American College of Cardiology. 2013;61:1510–1517. doi: 10.1016/j.jacc.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong CY, Chaudhry SI, Desai MM, Krumholz HM. Trends in Comorbidity, Disability, and Polypharmacy in Heart Failure. The American Journal of Medicine. 124:136–143. doi: 10.1016/j.amjmed.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMurray JJV, Packer M, Desai AS, et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. New England Journal of Medicine. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 27.Fala L. Entresto (Sacubitril/Valsartan): First-in-Class Angiotensin Receptor Neprilysin Inhibitor FDA Approved for Heart Failure. Am Health Drug Benefits. 2016;9:78–82. [PMC free article] [PubMed] [Google Scholar]
- 28.Quality AfHRa. National Healthcare Disparities Report 2007. 2008 Feb; [Google Scholar]
- 29.Étienne Gaudette BT. Alwyn Cassil and Dana Goldman. Health and Health Care of Medicare Beneficiaries in 2030. USC Schaeffer Center Working Paper. 2015 doi: 10.1515/fhep-2015-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]