1. INTRODUCTION
New treatments and biomarkers for acetaminophen (APAP) hepatotoxicity are needed. APAP overdose is responsible for approximately 50% of all acute liver failure (ALF) cases in the United States, making it the leading cause of ALF and ALF-related deaths (Lee, 2008). It has been reported that 50–80,000 emergency department visits, 20–30,000 hospitalizations, and approximately 500 deaths in the US can be attributed to APAP each year (Nourjah et al., 2006; Budnitz et al., 2011). Currently, the only effective treatment for APAP overdose is N-acetylcysteine (NAC). Although NAC is protective if administered during the early metabolism phase, the protection is significantly reduced ≥16 h after APAP overdose and completely lost by 24 h (Rumack et al., 1981). Unfortunately, many patients do not present until later time points. Craig et al. (2012) found that nearly half of all APAP overdose patients seen at the Royal Infirmary of Edinburgh between 1992 and 2008 presented >24 h after overdose, and late presentation was associated with poor outcome. The major treatment option at that point is a liver transplant, but current biomarkers for liver injury and function do not predict patient outcome or, therefore, the need for a transplant (Antoine et al., 2012; McGill et al., 2014a; McGill, 2016).
A logical approach to treatment of APAP overdose in late-presenting patients is to enhance liver regeneration. The liver has the capacity to regenerate approximately 80% of its functional mass after injury, and liver regeneration is thought to be a critical determinant of outcome (Mehendale, 2005). However, most research to date has focused on the early metabolism and toxicity after APAP overdose rather than the later recovery. Furthermore, the focus of liver regeneration research has been on partial hepatectomy (PHx) and resection rather than acute liver injury caused by drugs and other xenobiotics (Michalopoulos, 2010). More research is needed to understand liver regeneration after toxic injury so that new drug targets and biomarkers can be identified.
Phosphatidic acid (PA) is an intermediate in the synthesis of both triglycerides and membrane phospholipids. Numerous studies have demonstrated that it can also function as a lipid second messenger through activation of mechanistic target of rapamycin (mTOR) signaling (Fang et al., 2001; Foster, 2013). mTOR signaling is well known to inhibit catabolic processes and to promote cell growth and proliferation. Thus, PA may be an important mediator of liver regeneration signaling after acute injury. We hypothesized that PA levels increase after APAP overdose and enhance liver regeneration through a pathway involving mTOR. To test our hypothesis, we treated mice with a hepatotoxic dose of APAP and measured liver injury, regeneration, and PA levels over time. We also measured PA in plasma from APAP overdose patients with and without liver injury as indicated by ALT. Next, we determined the effect of overexpression of the PA phosphohydrolase (PAP) enzymes lipin 1 and lipin 2 on hepatocyte proliferation in vitro. Finally, we tested the effect of inhibition of PA synthesis on liver regeneration in vivo. Overall, our data suggest that PA is elevated during APAP hepatotoxicity due to lipin suppression, and that it promotes liver regeneration.
2. METHODS
2.1. Human subjects
Patients given a diagnosis of APAP overdose at either the University of Kansas Medical Center or the University of Arkansas for Medical Sciences were included in this study. The inclusion criteria were a physician diagnosis based on patient history, elevated serum APAP levels based on the Rumack-Matthew nomogram, abnormal liver function test results, or some combination thereof. Exclusion criteria were reasonable evidence of another major cause of liver injury. Informed consent was obtained from each patient or next of kin, and at least one blood sample was collected in an EDTA plasma or serum tube. The study protocols were approved by the Institutional Review Boards of the University of Kansas Medical Center and the University of Arkansas for Medical Sciences, and conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
2.2. Animals
Male C57Bl/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME). All mice were used at 8–12 weeks age. The animals were fasted overnight to reduce variation in APAP toxicity due to differences in liver glycogen and glutathione content, then injected i.p. with either a single 300 mg/kg dose of APAP (Sigma, St. Louis, MO) dissolved in warm phosphate-buffer saline (PBS) or an equal volume of warm PBS alone. Food was returned 6 h after treatment. In two experiments, mice were also treated with 20 mg/kg FSG67 in DMSO or an equal volume of DMSO vehicle (2 mL/kg). The drug or vehicle was administered either at 2 h post-APAP to assess liver injury at 6 and 24 h, or at 24 and 48 h post-APAP to assess liver regeneration at 52 h. For all other experiments, blood and liver tissue were harvested at the time points indicated in the figures. For each mouse, liver sections were flash frozen in liquid nitrogen for later immunoblotting, PCR, or other biochemical analysis. A single liver section from each mouse was fixed in 10% formalin for histology. Blood was collected in syringes containing EDTA, and plasma was obtained by centrifugation. All animal protocols were reviewed and approved by the Institutional Care and Use Committees of the University of Arkansas for Medical Sciences and the Washington University School of Medicine.
2.3. Cell culture experiments
HepG2 cells were grown in DMEM with 10% fetal bovine serum and pencillin/streptomycin. Lipin sequences were subcloned into the Ad-track CMV vector and recombined into Ad-EASY to generate adenovirus, as described (Mitra et al., 2013). 3H-thymidine incorporation was used to assess DNA synthesis. Briefly, when cells reached approximately 70% confluence, 1 µCis/mL of 3H-thymidine was added to the culture medium and the cells were incubated for 4 h. Cells were then washed with PBS, and proteins were precipitated by addition of cold 10% trichloroacetic acid (TCA). After washing with 10% TCA, the protein pellet was re-solubilized by 30 min incubation in 0.3 N NaOH at room temperature and radioactivity was measured using a scintillation counter.
2.4. Clinical chemistry
Alanine aminotransferase activity was measured in plasma according to the manufacturer’s instructions using a kit purchased from Teco Diagnostics (Anaheim, CA).
2.5. Histology
Formalin-fixed tissues were washed in ethanol and xylene and embedded in paraffin wax. 5–10 µm sections were mounted on glass slides and stained with hematoxylin and eosin (H&E) for microscopy.
2.6. Western blotting
Tissues were homogenized in 25 mM HEPES buffer with 5 mM EDTA (pH 7.4), 0.1% CHAPS, and protease inhibitors, using a bead homogenizer (Qiagen, Valencia, CA). Protein concentration in the homogenates was measured using the bicinchoninic acid (BCA) assay and equal amounts of protein were loaded in each lane of a 4–20% Tris-glycine gel. After electrophoresis, proteins were transferred to PVDF membranes with 0.45 µm pores and blocked with 5% milk in Tris-buffered saline with 0.1% Tween 80. After incubation with the appropriate antibodies, protein bands were visualized using either the Odyssey Imaging System (LiCor Biosciences, Lincoln, NE) or x-ray film. Polyclonal Lipin 1 antibody was purchased from Santa Cruz Biotechnology (Dallas, TX). Lipin 2 antibody was produced as previously described (Gropler et al., 2009). α-Tubulin clone B-5-1-2 antibody was purchased from Sigma (St. Louis, MO). Goat anti-rabbit 800 and donkey anti-mouse 800 secondary antibodies were obtained from LiCor Biosciences. All other antibodies were purchased from Cell Signaling (Boston, MA). All primary antibodies were diluted 1:1,000 in 5% milk, and all secondary antibodies were diluted 1:10,000 in 5% milk.
2.7. Phosphatidic acid measurement
For the time course studies, PA was measured in liver and plasma using LC-ESI-MS/MS. Briefly, mouse liver samples were homogenized in 400 µL of water with the Omni Bead Ruptor 24 (Omni International, Inc., Kennesaw, GA). PA(14:0/14:0) was used as an internal standard (IS) and was added to the samples before extraction. Extraction of PA was performed by protein precipitation from 50 µL of plasma or liver homogenate. PA analysis was performed with a Shimadzu 10A HPLC system and a Shimadzu SIL-20AC HT auto-sampler coupled to a Thermo Scientific TSQ Quantum Ultra triple-quadrupole mass spectrometer operated in SRM mode under ESI(+). Data processing was performed using Xcalibur (Thermo, Waltham, MA). For each experiment, the data were reported as peak AUC ratios relative to the IS, and then normalized to the control group mean. All PA data for each experiment were collected at the same time, so that comparison of the ratios to the within-experiment controls allowed accurate relative quantification. For the FSG67 experiments, total PA (PA+lyso-PA) was measured using a kit from Cell Biolabs (San Diego, CA). All liver PA levels were normalized to protein measured using the BCA assay before expressing as fold over control.
2.8. Statistics
Normality was tested using the Shapiro-Wilk test. For normally distributed data, Student’s t-test or one-way analysis of variance (ANOVA) with Student-Newman-Keul’s post-hoc test was used to compare two or more than two groups, respectively. For non-normally distributed data, the Mann-Whitney U-test or the Kruskal-Wallis test with Dunn’s multiple comparisons was used. All statistical tests were performed using SigmaPlot 12.5 software (Systat, San Jose, CA). In all cases, p <0.05 was considered significant.
3. RESULTS
3.1. Time course of acetaminophen-induced liver injury and recovery
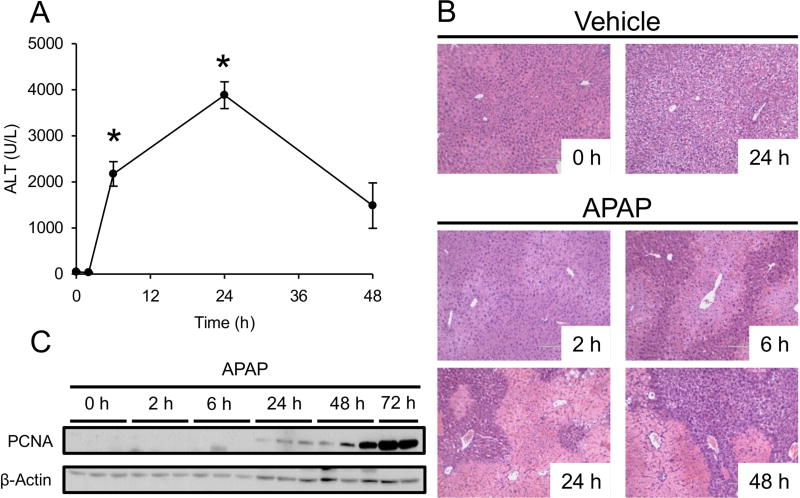
To characterize the time course of acetaminophen-induced acute liver injury and recovery, mice were treated with 300 mg/kg APAP or PBS vehicle for 2, 6, 24 and 48 h and plasma ALT activity was measured. The 2 h PBS-treated mice were used as the 0 h APAP time point. Other mice were treated with PBS vehicle for 6 or 24 h for histology. Plasma ALT was significantly increased by 6 h post-APAP, and peak activity (1,048±79 U/L) was observed at 24 h (Fig. 1A). Activity decreased at 48 h (401±133 U/L). Consistent with that, oncotic necrosis was evident in H&E-stained liver sections by 6 h post-APAP, peaked around 24 h, and was reduced by 48 h (Fig. 1B). Also, more clear space was visible in hepatocytes at 48 h, which is suggestive of glycogen repletion due to improved hepatic function. Finally, proliferating cell nuclear antigen (PCNA) expression was detectable by 24 h and continued to increase until at least 72 h (Fig. 1C). These data demonstrate that APAP-induced liver injury in mice peaks by 24 h, and suggest that liver regeneration begins by 24 h.
Figure 1. Time course of liver injury and regeneration after acetaminophen overdose.
Male C57Bl/6J mice were treated with 300 mg/kg APAP or PBS vehicle and blood and liver tissue were collected at the indicated time points. (A) Plasma ALT activity. (B) H&E-stained liver sections. (C) Proliferating cell nuclear antigen (PCNA) immunoblot. Data are expressed as mean±SEM for n = 3–4. *p<0.05 vs. 0 h and Veh.
3.2. Phosphatidic acid increases in liver and plasma of mice after acetaminophen overdose
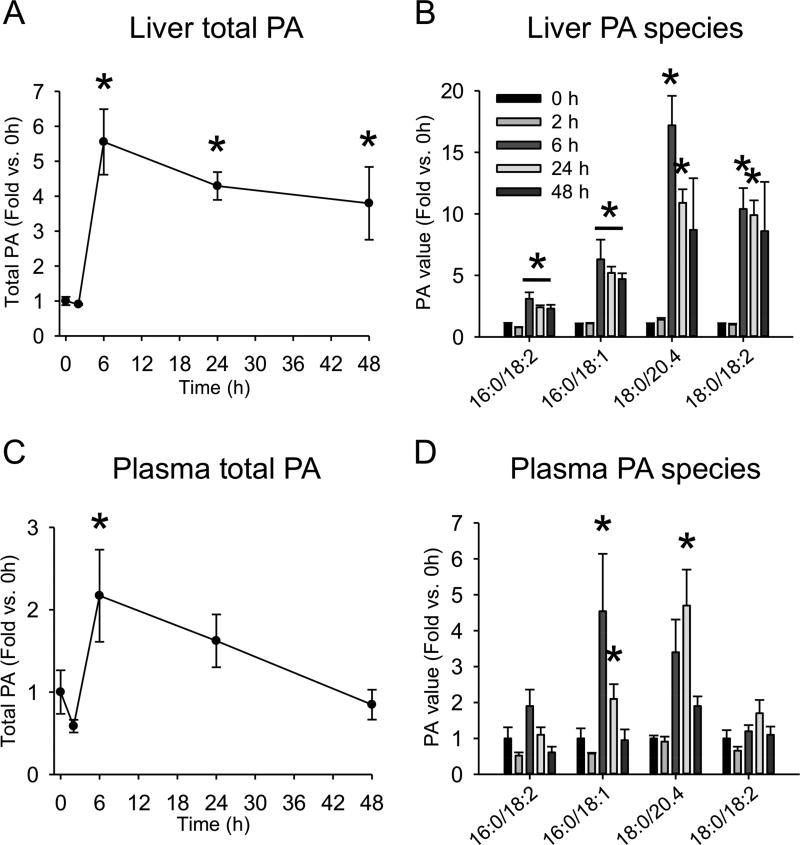
We next measured PA in liver and plasma by LC-MS/MS. We observed a significant increase in total PA level by 6 h (Fig. 2A,C), which was maintained until at least 48 h in the liver (Fig. 2A). Analysis of individual PA species revealed that PA(16:0/18:2), PA(18:0/20:4) and either PA(18:0/18:2) or PA(18:1/18:1) (indistinguishable by LC-MS/MS) were significantly increased in liver tissue, and PA(16:0/18:1) showed a strong trend toward increased levels at 6, 24 and 48 h post-APAP (Fig. 2B). PA(16:0/18:1) and PA(18:0/20:4) accounted for the increase in plasma, and remained elevated until 24 h (Fig. 2D). Altogether, these data demonstrate that PA levels are increased in the liver during APAP hepatotoxicity and recovery, and may be useful as a circulating biomarker.
Figure 2. Time course of phosphatidic acid after acetaminophen overdose.
Male C57Bl/6J mice were treated with 300 mg/kg APAP or PBS vehicle and blood and liver tissue were collected at the indicated time points. (A) Total liver phosphatidic acid (PA) content. (B) Individual PA species in the liver. (C) Total plasma PA content. (D) Individual PA species in the plasma. Data are expressed as mean±SEM for n = 3. *p<0.05 vs. 0 h and Veh.
3.3. Phosphatidic acid increases in plasma of acetaminophen overdose patients with liver injury
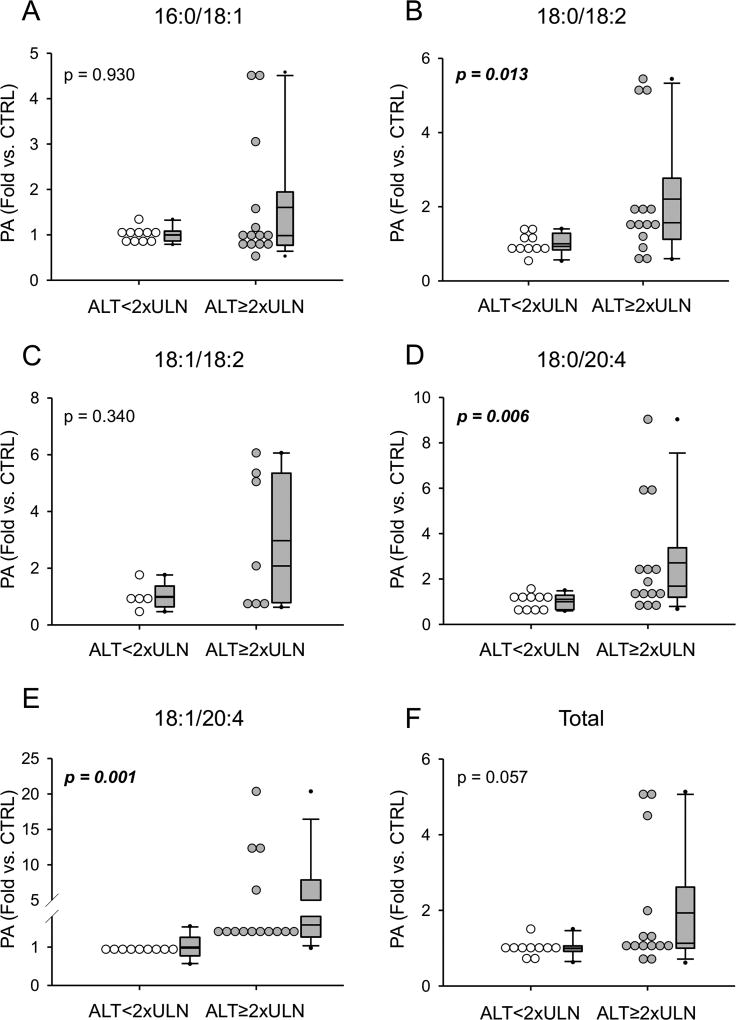
To determine if PA also increases in humans with liver injury due to APAP overdose, blood was collected from patients diagnosed with APAP overdose and PA was measured in the plasma. Patient demographics and clinical laboratory test results are listed in Table 1. Similar to mice, we observed a significant increase in three PA species in overdose patients that developed evidence of acute liver injury (peak ALT≥2xULN) compared to those who did not (peak ALT≤2xULN) (Fig. 3). We also observed a strong trend toward increased total PA in the patients with injury (Fig. 3F). Although there were no significant correlations between absolute values for PA and ALT based on Pearson correlation coefficients, receiver operating characteristic curve analysis revealed AUC values in the range of 0.80 to 0.90 for PA and peak ALT≥2xULN (Table 2).
Table 1.
Patient demographics and clinical laboratory test results.
| ALT <2xULN | ALT ≥2xULN | |
|---|---|---|
| N | 10 | 13 |
| Age (yr) (median, range) | 41, 18–80 | 42, 19–63 |
| Sex (% F) | 78 | 69 |
| Peak ALT (median, range) | 43, 29–69 | 3895, 800–5365 |
| Peak PT (s) (median, range)* | 16, 11–23 | 43, 14–165 |
| Peak MELD (median, range)* | 7, 6–16 | 22, 8–46 |
| Outcome (% survival) | 100 | 92 |
When available.
Figure 3. Phosphatidic acid values in acetaminophen overdose patients.
Phosphatidic acid species were measured in plasma or serum from acetaminophen overdose patients and compared between patients that did not develop liver injury (ALT less than 2x the upper limit of normal [ULN]) and those that did (ALT≥2xULN). (A) 16:0/18:1 PA. (B) 18:0/18:2 PA. (C) 18:1/18:2 PA. (D) 18:0/20:4 PA. (E) 18:1/20:4 PA. (F) Total PA. Data are expressed as fold over ALT<2xULN patients. Whiskers show the 5th to 95th percentile, box plots show the 25th to 75th percentile, and lines show the median and mean.
Table 2.
Receiver operating characteristic curve results for PA and acute liver injury.
| PA species | AUC | Cutoff (Fold) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| 18:0/18:2 | 0.81 | 1.43 | 71 | 100 |
| 18:0/20:4 | 0.84 | 1.29 | 71 | 90 |
| 18:1/20:4 | 0.89 | 1.41 | 71 | 90 |
| Total | 0.74 | 1.10 | 57 | 90 |
3.4. Lipin 1 and 2 are suppressed during acetaminophen-induced liver injury and recovery
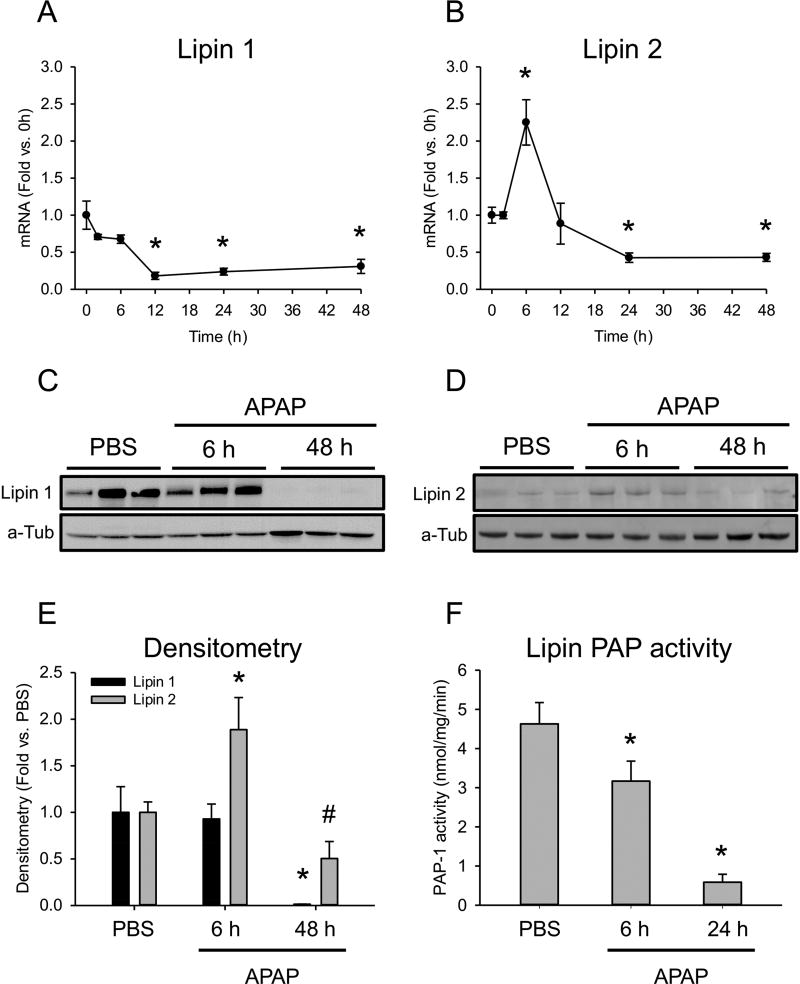
Next, we wanted to investigate the mechanism of increased PA in liver and plasma in mice. The lipin enzymes are PA phosphohydrolases that convert PA to diacylglycerol (DAG). The major lipin isoforms in the liver are lipin 1 and lipin 2. To determine if reduced lipin 1 and/or 2 expression could explain the increased PA, we measured mRNA of those enzymes. Lipin 1 mRNA significantly decreased by 12 h (0.24±0.05-fold vs. 0 h) and remained suppressed until at least 48 h (Fig. 4A). On the other hand, lipin 2 mRNA increased at 6 h post-APAP, but then showed a strong trend toward reduced expression by 24 h (Fig. 4B) like lipin 1. Western blotting for lipin 1 and lipin 2 in liver from PBS control and 6 h and 48 h post-APAP mice confirmed these results (Fig. 4C,D). These data demonstrate that lipin 1 expression is decreased by 12 h after APAP overdose, and that both lipin 1 and 2 are decreased at later time points, during recovery.
Figure 4. Time course of lipin 1 and 2 expression and activity after APAP overdose.
Male C57Bl/6J mice were treated with 300 mg/kg APAP or PBS vehicle and liver tissue was collected at the indicated time points. (A) Lipin 1 mRNA in the liver. (B) Lipin 2 mRNA in the liver. (C) Liver lipin 1 immunoblot. (D) Liver lipin 2 immunoblot. (E) Densitometry for lipin 1 and lipin 2 immunoblots. (F) Lipin-specific Mg2+-dependent PAP activity in the liver. Data are expressed as mean±SEM for n=3–6. *p<0.05 vs. PBS or 0 h. #p = 0.1 vs. PBS.
Because PA levels increased in the liver at 6 h post-APAP, but lipin expression did not decrease until 12 h or later, we wondered if the early rise in PA could be due to suppression of lipin activity. To test that possibility, we measured lipin-specific Mg2+-dependent PAP activity in the liver at 0, 6 and 24 h post-APAP. Importantly, we observed a decrease in lipin activity by 6 h (Fig. 4E,F). These data suggest that the elevated PA levels early after APAP overdose are due to suppression of lipin activity, rather than expression. Thus, the early reduction in lipin activity was likely due to post-translational modifications, as there was no change in lipin 1 protein at that time point and lipin 2 protein even increased.
3.5. Expression of other phosphatidic acid metabolism genes after acetaminophen treatment
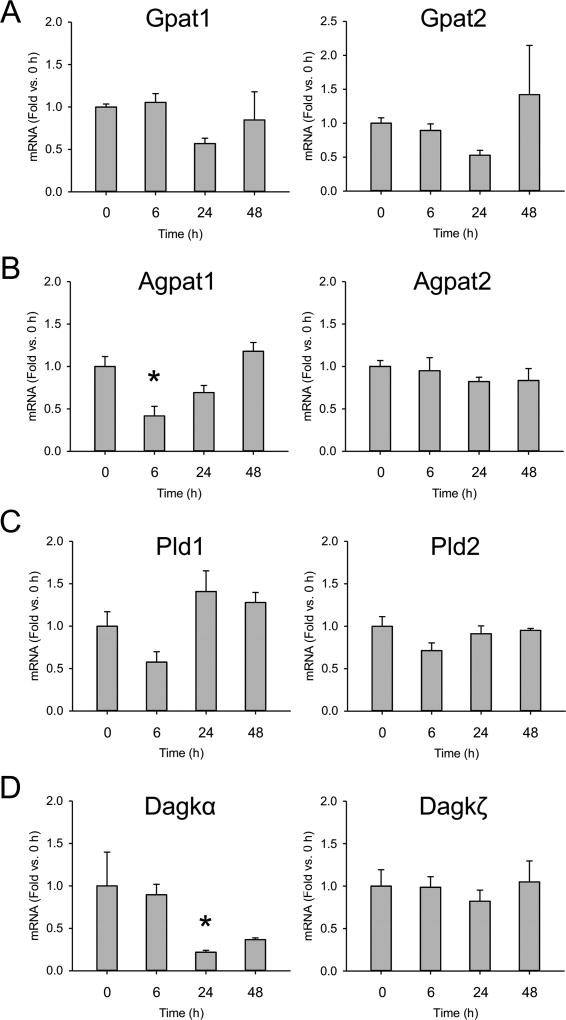
To determine if changes in expression of other PA-metabolizing genes could also contribute to the observed changes in liver PA levels, we measured mRNA for the major liver isoforms of glycerol-3-phosphate acyltransferase (Gpat), 1-acylglycerol-3-phosphateacyl-transferase (Agpat), phospholipase D (Pld), and diacylglycerol kinase (Dagk) (Fig. 5). While lipin 1 and lipin 2 are important for metabolism of PA, these other enzymes are important for synthesis of PA. Although we did find a transient decrease in mRNA for Agpat1 at 6 h (Fig. 5B) and for Dagkα at 24 h (Fig. 5D), there were no long-term changes in expression of any of these genes and none increased (Fig. 5). Thus, suppression of lipin enzymes appears to be the primary mechanism of increased PA levels in the liver during APAP-induced liver injury and recovery.
Figure 5. Expression of genes for phosphatidic acid synthesis enzymes.
Male C57Bl/6J mice were treated with 300 mg/kg APAP or PBS vehicle control and liver tissue was collected at the indicated time points. (A) mRNA for the major isoforms of glycerol-3-phosphate acyltransferase (Gpat) in the liver. (B) mRNA for the major isoforms of acylglycerol phosphate acyltransferase (Agpat) in the liver. (C) mRNA for the major isoforms of phospholipase D (Pld) in the liver. (D) mRNA for the major isoforms of diacylglycerol kinase (Dagk) in the liver. Data are expressed as mean±SEM for n = 3–6. *p<0.05 vs. vehicle control (CTRL).
3.6. Lipin overexpression reduces hepatocyte proliferation in vitro
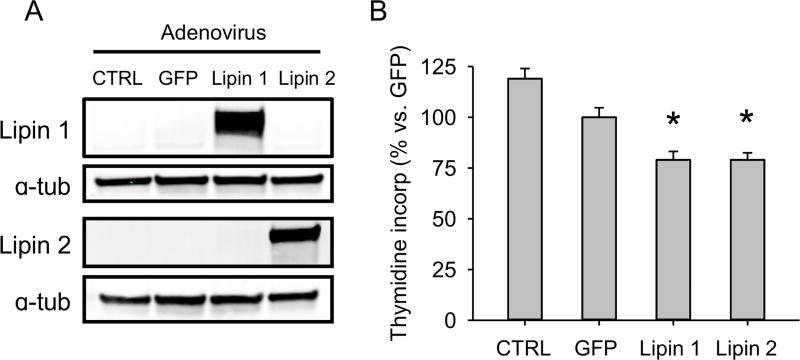
We next wanted to test the effect of PA on hepatocyte proliferation. It is thought that direct treatment of cultured cells with PA causes off-target effects due to 1) conversion of PA to lysophosphatidic acid (lyso-PA) by phospholipase A2 and other PA-metabolizing enzymes often present in cell culture medium, and 2) interactions with cell surface receptors. Thus, to test the effect of PA on cell proliferation, indirect methods of PA modulation must be used. We overexpressed lipin 1 and lipin 2 individually in the human hepatocyte cell line HepG2 to reduce PA content and measured DNA synthesis by 3H-thymidine incorporation. Importantly, overexpression of either lipin enzyme (Fig. 6A) resulted in reduced DNA synthesis (Fig. 6B). These data indicate that PA promotes hepatocyte proliferation.
Figure 6. Effect of lipin enzymes on DNA synthesis in vitro.
HepG2 cells were untreated or transduced to express GFP, lipin 1, or lipin 2, and DNA synthesis was measured by 3H-thymidine incorporation. (A) Immunoblots for lipin 1 and 2 after adenovirus transduction. (B) Thymidine incorporation. Values are compared to the GFP transduction control. Data are expressed as mean±SEM for n = 4 independent experiments, each performed in triplicate. *p<0.05 vs. GFP control.
3.7. Inhibition of PA synthesis reduces liver regeneration in vivo
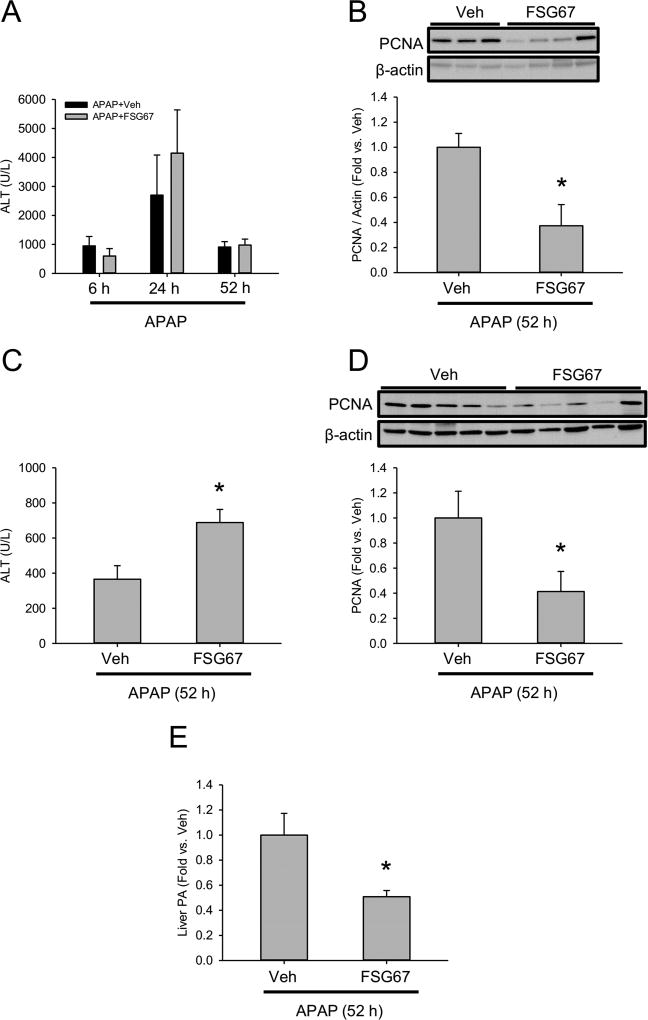
To determine if reducing PA content in the liver can affect liver injury or blunt hepatocyte proliferation in vivo, we treated mice with APAP followed by the Gpat inhibitor FSG67 or DMSO vehicle control at 2, 24, and 48 h post-APAP. APAP metabolism is rapid, and complete by 2 h post-APAP (McGill et al., 2013), so this post-treatment regimen is not expected to affect APAP bioactivation. We measured ALT at 24, 48 h, and 52 h post-APAP, and PCNA protein at 52 h. There was no difference in ALT between the vehicle and FSG67 treated mice at any time point (Fig. 7A), which confirmed that the post-treatment regimen did not affect APAP bioactivation. However, PCNA was significantly decreased at 52 h in liver tissue from the FSG67-treated animals (Fig. 7B). To avoid the potential for any unexpected effects of vehicle or FSG67 on the early liver injury, we also repeated the experiment with only the 24 and 48 h post-APAP treatments (Fig. 7C,D). Again, FSG67 reduced PCNA levels at 52 h post-APAP (Fig. 7D). We also observed a significant increase in ALT in the FSG67-treated mice. The reason for the difference in ALT results at 52 h between the two experiments is not clear, but an increase in ALT is consistent with reduced recovery. Finally, we measured PA in all of the FSG67 and vehicle-treated mice at 52 h post-APAP to ensure that the drug did in fact reduce PA content in the liver. As expected, PA values were significantly lower in the FSG67-treated mice (Fig. 7E). Together, these data demonstrate that Gpat inhibition reduces hepatocyte proliferation in vivo. These results are consistent with a role for PA in liver regeneration after acute injury.
Figure 7. Effect of phosphatidic acid synthesis inhibition on liver injury and regeneration after APAP overdose.
Male C57Bl/6J mice were treated with 300 mg/kg APAP or PBS vehicle, followed by 20 mg/kg FSG67 at either 2, 24, and 48 h post-APAP (A,B) or at 24 and 48 h post-APAP (C,D). Blood and liver tissue were collected at the indicated time points. (A) Plasma ALT at 6, 24 and 52 h post-APAP. (B) Immunoblot for liver PCNA and associated densitometry at 52 h post-APAP. (C) Plasma ALT at 52 h post-APAP. (D) Immunoblot for liver PCNA and associated densitometry at 52 h post-APAP. (E) Liver PA content at 52 h post-APAP. Data are expressed as mean±SEM for n = 3–5. *p<0.05 vs. PBS treatment. #p<0.05 vs. Veh.
3.8. Time course of mTOR phosphorylation after acetaminophen overdose
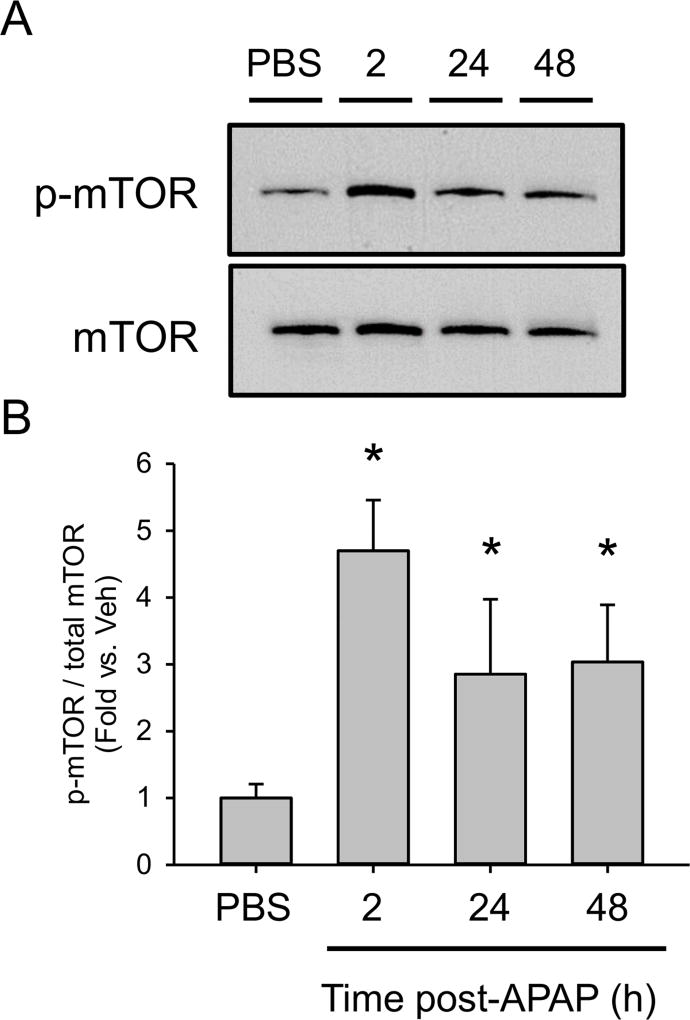
Finally, to begin to test if the increased PA activates mTOR signaling after APAP overdose, we measured mTOR phosphorylation by immunoblot. Surprisingly, p-mTOR was increased by 2 h post-APAP, before the increase in PA (Fig. 8). p-mTOR levels were then maintained until at least 48 h (Fig. 8). It is possible that some initial stimulus activates mTOR signaling at early time points after APAP overdose and PA then maintains those levels. Additional studies are needed to address the role of PA in late mTOR activation after APAP overdose.
Figure 8. Phosphorylation of mTOR after acetaminophen treatment.
Male C57Bl/6J mice were treated with 300 mg/kg APAP or saline vehicle and liver tissue was collected at the indicated time points. (A) Immunoblots for p-mTOR and total mTOR from pooled samples. (B) Densitometry for phosphorylated mTOR (p-mTOR) and total mTOR from non-pooled samples. Data are expressed as mean±SEM for n = 3. *p<0.05 vs. Veh.
4. DISCUSSION
Little is known about the mechanisms of liver regeneration after APAP overdose. Most studies of liver regeneration have relied on the partial hepatectomy model (PHx) in which 2/3 of the liver is removed and the remaining lobes grow to restore functional mass. It is clear from those studies that growth factors such as hepatocyte growth factor (HGF) and epidermal growth factor receptor (EGFR) ligands are necessary for liver regeneration (Paranjpe et al., 2016; Michalopoulos, 2017), that various cytokines help to prime hepatocytes for growth (Fausto et al., 2006; Michalopoulos, 2017), and that large changes in nutrient homeostasis resulting from the PHx can also affect regeneration (Huang and Rudnick, 2014; Huang et al., 2016). The details of those signaling pathways have been studied for decades, but few groups have attempted to investigate the mechanisms of liver regeneration after drug-induced liver injury. It is known that interleukin-6 (IL-6) deficiency delays liver regeneration after APAP overdose in mice (James et al., 2003). More recent data have also demonstrated that EGFR and the HGF receptor c-Met are activated in APAP hepatotoxicity, and that inhibition of EGFR reduces hepatocyte proliferation (Bhushan et al., 2014; 2017). Similar effects have been described in mice treated with other acute hepatotoxicants (Phaneuf et al., 2004; Scheving et al., 2015). However, no effort has been made to study the effects of metabolic changes during APAP hepatotoxicity on liver regeneration, even though defects in mitochondrial respiration and lipid metabolism after APAP overdose have been described in both mice and humans in numerous reports (Meyers et al., 1988; Coen et al., 2003; Chen et al., 2009; McGill et al., 2014b; Bhattacharyya et al., 2014).
We observed an increase in the lipid PA in both plasma and liver tissue from mice during APAP hepatotoxicity. The increase in PA coincided with a decrease in expression of the enzymes lipin 1 and lipin 2, and with reduced lipin-specific PAP activity, suggesting that the mechanism of PA elevation was suppression of lipin enzymes. The fact that lipin activity decreased before the decrease in expression also suggests that the activity is regulated by post-translational modification early after APAP overdose. It is already known that mTOR-mediated phosphorylation deactivates lipin 1 (Harris et al., 2007; Eaton et al., 2013). Future experiments will determine whether this or other post-translational modifications occur after APAP overdose. To determine if PA levels affect hepatocyte proliferation and liver regeneration, we overexpressed lipin 1 or lipin 2 in HepG2 cells. Importantly, transduction of either enzyme reduced DNA synthesis. Finally, we found that the PA synthesis inhibitor FSG67 reduced an important marker of liver regeneration in vivo. Together, the data are consistent with our hypothesis that PA enhances liver regeneration after APAP hepatotoxicity.
It has been known for decades that PA is mitogenic. As early as 30 years ago, it was shown that treatment of cultured cells with PA can induce DNA synthesis (Siegmann, 1987). Recent data suggest that the pro-proliferative effects of PA occur through mTOR. Fang et al. (2001) were the first to demonstrate that PA activates mTOR signaling in cultured cells. Although it was later demonstrated that exogenous PA is converted to lyso-PA by the action of PLA2 in the culture medium and that lyso-PA is actually responsible for the effect on mTOR in an ERK-dependent manner (Winter et al., 2010), numerous other studies have also revealed a direct interaction of PA with mTOR (Fang et al., 2001; Veverka et al., 2008; You et al., 2012) and it has been demonstrated that PA activates mTOR in cells through both ERK-dependent and -independent mechanisms (You et al., 2012). Finally, treatment with di-8:0 PA activates mTOR in cultured cells (Yoon et al., 2011; 2015), and di-8:0 PA has biological actions in cell culture that 8:0 lyso-PA does not have (Fischer et al., 2001). Thus, the weight of the evidence supports a direct effect of intracellular PA on mTOR, though indirect effects through extracellular lyso-PA likely also occur. Importantly, mTOR signaling enhances liver regeneration. Treatment with rapamycin after PHx inhibits cell proliferation in the liver (Palmes et al., 2008; Espeillac et al., 2011; Fouraschen et al., 2013), and genetic deficiency of the mTOR target S6K1 has a similar effect (Espeillac et al., 2011). Moreover, chronic mTOR activation is carcinogenic in the liver (Menon et al., 2011), also demonstrating that it promotes hepatocyte proliferation. Interestingly, we observed increased phosphorylation of mTOR as early as 2 h after APAP overdose, and that effect was sustained until at least 48 h. Therefore, it is possible that the initial mTOR activation leads to the increase in PA in the liver through inhibition of lipin 1, and the PA then maintains mTOR activation at later time points resulting in a feed-forward loop that enhances regeneration. Additional studies are needed to test that possibility and to investigate the mechanism of the early mTOR activation and lipin suppression.
The fact that the Gpat inhibitor FSG67 reduced PCNA expression after APAP hepatotoxicity is consistent with a role for PA in liver regeneration. However, Gpat inhibition would also be expected to reduce lyso-PA. Thus, we cannot rule out the possibility that lyso-PA is also important. Our in vitro experiments with overexpression of lipin 1 and lipin 2 in HepG2 cells may be more specific for PA, but we did not demonstrate that experimentally. While a more direct approach to test the effect of PA on liver regeneration or hepatocyte proliferation would be to treat with exogenous PA, we did not do that for two reasons. First, the major effect that we observed in our experiments was an increase in intrahepatic PA, and it is well known that the mechanisms of intracellular and extracellular PA signaling differ (Shad et al., 2015). Although both activate mTOR, extracellular PA activates multiple additional signaling pathways that could cause off-target effects (Shad et al., 2015). For example, as discussed above, extracellular PA is likely converted to lyso-PA in plasma (Winter et al., 2010), and lyso-PA activates cell surface receptors such as the lysophosphatidic acid receptor 1 (Lpar1), a G protein-coupled receptor (Winter et al., 2010; Shad et al., 2015). PA itself likely also binds to some extracellular receptors. Second, PA was already increased by APAP overdose. Thus, we believed that the best approach would be to prevent or reduce the accumulation of PA, rather than trying to enhance it.
Our discovery that PA is increased in plasma from both mice and humans suggests that it could serve as a biomarker of liver regeneration. Regeneration is generally thought to be critical for survival in ALF in humans. Several studies support that idea. Indices of proliferative activity in liver sections correlate with liver function and patient outcome (Kayano et al., 1992; Katoonizadeh et al., 2006). It is also known that α-fetoprotein (AFP), a marker of hepatocyte immaturity and proliferation, is a strong predictor of survival after APAP overdose (Schmidt and Dalhoff, 2005), although its utility is limited by the fact that its levels do not increase until after the liver injury has occurred. Finally, a recent meta-analysis of clinical trials also demonstrated that administration of granulocyte-colony stimulating factor (G-CSF), which is known to accelerate liver repair in animal models by promoting migration of stem cells into the damaged liver, reduces mortality in acute-on-chronic liver failure patients (Chavez-Tapia et al., 2015). Due to the apparent importance of regeneration for survival, it seems likely that biomarkers that reflect regenerative signaling could be useful for prediction of patient outcome. Also, the FDA and European Medicines Agency have recently expressed support for development of biomarkers that can differentiate between patients who are likely to develop true, severe liver injury, and those who will adapt and survive (EMA, 2016; Teschke et al., 2017). It is possible that PA and similar lipid metabolites could be used for that purpose.
One potential problem with the use of PA as a circulating biomarker of regeneration and survival revealed by our data is that PA was not elevated compared to the control group for each individual patient with liver injury. Because most of the patients in our study were survivors, that suggests that PA is not elevated in all survivors and therefore has poor sensitivity for survival. However, our data are limited by the fact that we only measured PA at one time point for each patient, and the time points were not standardized to either the time of presentation or the reported time of APAP overdose. Thus, although there was considerable overlap in values for most PA species between the two groups, that overlap could simply be due to variation in sample timing. It is also possible that PA has high intra-individual variation, such that a baseline value would need to be taken at the time of patient presentation in order to be clinically useful, similar to the use of cardiac troponins to diagnose myocardial infarction. Clearly, additional studies are needed to determine the clinical utility of circulating PA.
Importantly, while this is the first study to explore the role of hepatic lipin enzymes and PA in liver regeneration, we are not the first to investigate the role of lipin enzymes in liver injury. The effects of lipin 1 have been thoroughly investigated in mouse models of alcoholic liver disease (ALD). In contrast to our results,Hu et al. (2012) reported that ethanol induces expression of lipin 1 in the liver. Induction of lipin 1 appears to be protective in ALD; hepatic lipin 1 deficiency actually enhances alcoholic steatosis and inflammation in mice (Hu et al., 2013). Interestingly, those effects are probably due to loss of the co-regulatory transcription factor function of lipin 1 within hepatocytes (Hu et al., 2013). It is not yet clear what role that function of lipin 1 has in APAP toxicity, but it is interesting to note that mitochondrial biogenesis is important in liver regeneration after APAP overdose (Du et al., 2017) and lipin 1 directly interacts with the mitochondrial biogenesis mediator PGC-1α to amplify its function (Finck et al., 2006).
The effects of lipin 1 in liver injury are not limited to hepatic lipin 1. Surprisingly, myeloid-specific lipin 1 deficiency has the opposite effect of hepatic deficiency on ALD, causing reduced inflammation and liver injury (Wang et al., 2016). That finding may also have implications for APAP hepatotoxicity, as inflammation has been thought to play a role in both injury and regeneration after APAP overdose. The importance of inflammation in APAP hepatotoxicity is highly controversial (Woolbright and Jaeschke, 2017a, 2017b; Kennon-McGill and McGill, 2018) and experiments with myeloid-specific lipin 1 deficient or transgenic mice could add clarity. Another study revealed that overexpression of lipin 1 in adipose tissue is also protective against ALD (Zhang et al., 2018). Clearly, future studies should examine the role of extrahepatic lipin enzymes in APAP hepatotoxicity.
Taken together, our data demonstrate that PA is increased in liver and plasma during APAP hepatotoxicity, and may enhance liver regeneration and therefore recovery. Our data have broad implications for all forms of liver injury and disease that involve hepatocyte proliferation, including cancer, hepatic resection, hypoxic hepatitis, and cirrhosis. Our future studies will focus on the regulation of lipin enzymes during liver injury, and will explore the roles of mTOR and PA in liver regeneration after APAP hepatotoxicity in much greater detail.
Supplementary Material
HIGHLIGHTS.
-
-
Phosphatidic acid increases in liver and circulation in mice after APAP overdose
-
-
Phosphatidic acid increases in circulation of humans with APAP hepatotoxicity
-
-
Reducing phosphatidic acid content in the liver blunts liver regeneration
-
-
Phosphatidic acid may affect liver regeneration through mTOR signaling
Acknowledgments
This work was supported in part by start-up funds from the University of Arkansas for Medical Sciences (to MRM). Other sources of support included an intramural grant (to BNF and MRM) and core facility services from the Washington University Institute of Clinical and Translational Sciences, which is funded in part by grant UL1TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH), and NIH grants R01 DK078187 and R01 HL119225 (to BNF). Mass spectrometry was performed in the Metabolomics Facility at Washington University (NIH P30 DK056341). Additional support came from NIH grant T32 DK0077653 (AJL). MRM would also like to thank Drs. Mitchell G. Scott, Ann M. Gronowski, Dennis J. Dietzen, and Sarah Riley at Washington University School of Medicine and St. Louis University for additional support, and for excellent training in laboratory medicine. The funding sources had no role in design or execution of experiments, nor in writing this manuscript.
Abbreviations
- ALF
acute liver failure
- ALT
alanine aminotransferase
- APAP
acetaminophen
- Gpat
glycerol-3-phosphate acyltransferase
- LC-ESI-MS/MS
liquid chromatography with electrospray ionization and tandem triple quadrupole mass spectrometry
- lyso-PA
lysophosphatidic acid
- mTOR
mechanistic target of rapamycin
- NAC
N-acetylcysteine
- PA
phosphatidic acid
- PAP
phosphatidic acid phosphohydrolase
- PCNA
proliferating cell nuclear antigen
- PHx
partial hepatectomy
- ULN
upper limit of normal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, Craig DG, Simpson KJ, Jaeschke H, Park BK. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol. 2012;56(5):1070–9. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bhattacharyya S, Yan K, Pence L, Simpson PM, Gill P, Letzig LG, Beger RD, Sullivan JE, Kearns GL, Reed MD, Marshall JD, Van Den Anker JN, James LP. Targeted liquid chromatography-mass spectrometry analysis of serum acylcarnitines in acetaminophen toxicity in children. Biomark Med. 2014;8(2):147–59. doi: 10.2217/bmm.13.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan B, Chavan H, Borude P, Xie Y, Du K, McGill MR, Lebofsky M, Jaeschke H, Krishnamurthy P, Apte U. Dual role of epidermal growth factor receptor in liver injury and regeneration after acetaminophen overdose in mice. Toxicol Sci. 2017;155(2):363–78. doi: 10.1093/toxsci/kfw213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan B, Walesky C, Manley M, Gallagher T, Borude P, Edwards G, Monga SP, Apte U. Pro-regeneration signaling after acetaminophen-induced acute liver injury in mice identified using a novel incremental dose model. Am J Pathol. 2014;184(11):3013–25. doi: 10.1016/j.ajpath.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnitz DS, Lovegrove MC, Crosby AE. Emergency department visits for overdoses of acetaminophen-containing products. Am J Prev Med. 2011;40(6):585–92. doi: 10.1016/j.amepre.2011.02.026. [DOI] [PubMed] [Google Scholar]
- Chavez-Tapia NC, Mendiola-Pastrana I, Ornelas-Arroyo VJ, Norena-Herrera C, Vidana-Perez D, Delgado-Sanchez G, Uribe M, Barrientos-Gutierrez T. Granulocyte-colony stimulating factor for acute-on-chronic liver failure: systematic review and meta-analysis. Ann Hepatol. 2015;14(5):631–41. [PubMed] [Google Scholar]
- Chen C, Krausz KW, Shah YM, Idle JR, Gonzalez FJ. Serum metabolomics reveals irreversible inhibition of fatty acid beta-oxidation through the suppression of PPARalpha activation as a contributing mechanism of acetaminophen-induced hepatotoxicity. Chem Res Toxicol. 2009;22(4):699–707. doi: 10.1021/tx800464q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen M, Lenz EM, Nicholson JK, Wilson ID, Pognan F, Lindon JC. An integrated metabonomic investigation of acetaminophen toxicity in the mouse using NMR spectroscopy. Chem Res Toxicol. 2003;16(3):295–303. doi: 10.1021/tx0256127. [DOI] [PubMed] [Google Scholar]
- Du K, Ramachandran A, McGill Mr, Mansouri A, Asselah T, Farhood A, Woolbright BL, Ding WX, Jaeschke H. Induction of mitochondrial biogenesis protects against acetaminophen hepatotoxicity. Food Chem Toxicol. 2017;108(Pt A):339–50. doi: 10.1016/j.fct.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton JM, Mullins GR, Brindley DN, Harris TE. Phosphorylation of lipin 1 and charge on the phosphatidic acid head group control its phosphatidic acid phosphatase activity and membrane association. J Biol Chem. 2013;288(14):9933–45. doi: 10.1074/jbc.M112.441493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeillac C, Mitchell C, Celton-Morizur S, Chauvin C, Koka V, Gillet C, Albrecht JH, Desdouets C, Pende M. S6 kinase 1 is required for rapamycin-sensitive liver proliferation after mouse hepatectomy. J Clin Invest. 2011;121(7):2821–32. doi: 10.1172/JCI44203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency. Letter of support for drug-induced liver injury (DILI) biomarker. Rassi G, editor. [accessed on 20 December 2017];EMA/423870/2016. 2016 Last Updated: 30 September 2016. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Other/2016/09/WC500213479.pdf.
- Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;29:1942–5. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43(2 Suppl 1):S45–53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- Finck BN, Gropler MC, Chen Z, Leone TC, Croce MA, Harris TE, Lawrence JC, Jr, Kelly DP. Lipin 1 is an inducible amplifier of the hepatic PGC-1alpha/PPARalpha regulatory pathway. Cell Metab. 2006;4(3):199–210. doi: 10.1016/j.cmet.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Fischer DJ, Nusser N, Virag T, Yokoyama K, Wang Da, Baker DL, Bautista D, Parrill AL, Tigyi G. Short-chain phosphatidates are subtype-selective antagonists of lysophosphatidic acid receptors. Mol Pharmacol. 2001;60(4):776–84. [PubMed] [Google Scholar]
- Foster DA. Phosphatidic acid and lipid-sensing by mTOR. Trends Endocrinol Metab. 2013;24(6):272–8. doi: 10.1016/j.tem.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouraschen SM, de Ruiter PE, Kwekkeboom J, de Bruin RW, Kazemier G, Metselaar HJ, Tilanus HW, van der Laan LJ, de Jonge J. mTOR signaling in liver regeneration: rapamycin combined with growth factor treatment. World J Transplant. 2013;3(3):36–47. doi: 10.5500/wjt.v3.i3.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TE, Huffman TA, Chi A, Shabanowitz J, Hunt DF, Kumar A, Lawrence JC., Jr Insulin controls subcellular localization and multisite phosphorylation of the phosphatidic acid phosphatase, lipin 1. J Biol Chem. 2007;282(1):277–86. doi: 10.1074/jbc.M609537200. [DOI] [PubMed] [Google Scholar]
- Hu M, Wang F, Li X, Rogers CQ, Liang X, Finck BN, Mitra MS, Zhang R, Mitchell DA, You M. Regulation of hepatic lipin-1 by ethanol: role of AMP-activated protein kinase/sterol regulatory element-binding protein 1 signaling in mice. Hepatology. 2012;55(2):437–46. doi: 10.1002/hep.24708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Yin H, Mitra MS, Liang X, Ajmo JM, Nadra K, Chast R, Finck BN, You M. Hepatic-specific lipin-1 deficiency exacerbates experimental alcohol-induced steatohepatitis in mice. Hepatology. 2013;58(6):1953–63. doi: 10.1002/hep.26589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Rudnick DA. Elucidating the metabolic regulation of liver regeneration. Am J Pathol. 2014;184(2):309–21. doi: 10.1016/j.ajpath.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Schriefer AE, Clifften PF, Dietzen D, Kulkarni S, Sing S, Monga SP, Rudnick DA. Postponing the hypoglycemic response to partial hepatectomy delays mouse liver regeneration. Am J Pathol. 2016;186(3):587–99. doi: 10.1016/j.ajpath.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James LP, Lamps LW, McCullough S, Hinson JA. Interleukin 6 and hepatocyte regeneration in acetaminophen toxicity in the mouse. Biochem Biophys Res Commun. 2003;309(4):857–63. doi: 10.1016/j.bbrc.2003.08.085. [DOI] [PubMed] [Google Scholar]
- Katoonizadeh A, Nevens F, Verslype C, Pirenne J, Roskams T. Liver regeneration in acute severe liver impairment: a clinicopathological correlation study. Liver Int. 2006;26(10):1225–33. doi: 10.1111/j.1478-3231.2006.01377.x. [DOI] [PubMed] [Google Scholar]
- Kayano K, Yasunaga M, Kubota M, Takenaka K, Mori K, Yamashita A, Kubo Y, Sakaida I, Okita K, Sanuki K. Detection of proliferating hepatocytes by immunohistochemical staining for proliferating cell nuclear antigen (PCNA) in patients with acute hepatic failure. Liver. 1992;12(3):132–6. doi: 10.1111/j.1600-0676.1992.tb00571.x. [DOI] [PubMed] [Google Scholar]
- Kennon-McGill S, McGill MR. Extrahepatic toxicity of acetaminophen: critical evaluation of the evidence and proposed mechanisms. J Clin Transl Res. 2017;3(3):5. doi: 10.18053/jctres.03.201703.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WM. Etiologies of acute liver failure. Semin Liver Dis. 2008;28(2):142–52. doi: 10.1055/s-2008-1073114. [DOI] [PubMed] [Google Scholar]
- McGill MR. The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J. 2016;15:817–28. doi: 10.17179/excli2016-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Staggs VS, Sharpe MR, Lee WM, Jaeschke H Acute Liver Failure Study Group. Serum mitochondrial biomarkers and damage-associated molecular patterns are higher in acetaminophen overdose patients with poor outcome. Hepatology. 2014;60(4):1336–45. doi: 10.1002/hep.27265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Lebofsky M, Norris HR, Bajt ML, Xie Y, Williams CD, Wilkins DG, Rollins DE, Jaeschke H. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: dose-response, mechanisms, and clinical implications. Toxicol Appl Pharmacol. 2013;269(3):240–9. doi: 10.1016/j.taap.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Li F, Sharpe MR, Williams CD, Curry SC, Ma X, Jaeschke H. Circulating acylcarnitines as biomarkers of mitochondrial dysfunction after acetaminophen overdose in mice and humans. Arch Toxicol. 2014;88(2):391–401. doi: 10.1007/s00204-013-1118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehendale HM. Tissue repair: an important determinant of final outcome of toxicant-induced injury. Toxicol Pathol. 2005;33(1):41–51. doi: 10.1080/01926230590881808. [DOI] [PubMed] [Google Scholar]
- Meyers LL, Beierschmitt WP, Khairallah EA, Cohen SD. Acetaminophen-induced inhibition of hepatic mitochondrial respiration in mice. Toxicol Appl Pharmacol. 1988;93(3):387–87. doi: 10.1016/0041-008x(88)90040-3. [DOI] [PubMed] [Google Scholar]
- Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176(1):2–13. doi: 10.2353/ajpath.2010.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos GK. Hepatostat: liver regeneration and normal liver tissue maintenance. Hepatology. 2017;65(4):1384–92. doi: 10.1002/hep.28988. [DOI] [PubMed] [Google Scholar]
- Mitra MS, Chen Z, Ren H, Harris TE, Chambers KT, Hall AM, Nadra K, Klein S, Chrast R, Su X, Morris AJ, Finck BN. Mice with an adipocyte-specific lipin 1 separation-of-function allele reveal unexpected roles for phosphatidic acid in metabolic regulation. Proc Natl Acad Sci USA. 2013;110(2):642–7. doi: 10.1073/pnas.1213493110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourjah P, Ahmad SR, Karwoski C, Willy M. Estimates of acetaminophen (paracetamol)-associated overdoses in the United States. Pharmacoepidemiol Drug Saf. 2006;15(6):398–405. doi: 10.1002/pds.1191. [DOI] [PubMed] [Google Scholar]
- Palmes D, Zibert A, Budny T, Bahde R, Minin E, Kebschull L, Hӧlzen J, Schmidt H, Spiegel HU. Impact of rapamycin on liver regeneration. Virchows Arch. 2008;452(5):545–57. doi: 10.1007/s00428-008-0604-y. [DOI] [PubMed] [Google Scholar]
- Paranjpe S, Bowen WC, Mars WM, Orr A, Haynes MM, DeFrances MC, Liu S, Tseng GC, Tsagianni A, Michalopoulos GK. Combined systemic elimination of MET and epidermal growth factor receptor signaling completely abolishes liver regeneration and leads to liver decompensation. Hepatology. 2016;64(5):1711–24. doi: 10.1002/hep.28721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phaneuf D, Moscioni AD, LeClair C, Raper SE, Wilson JM. Generation of a mouse expressing a conditional knockout of the hepatocyte growth factor gene: demonstration of impaired liver regeneration. DNA Cell Biol. 2004;23(9):592–603. doi: 10.1089/dna.2004.23.592. [DOI] [PubMed] [Google Scholar]
- Rumack BH, Peterson RC, Koch GG, Amara IA. Acetaminophen overdose. 662 cases with evaluation of oral acetylcysteine. Arch Intern Med. 1981;141(3):380–5. doi: 10.1001/archinte.141.3.380. [DOI] [PubMed] [Google Scholar]
- Scheving LA, Zhang X, Stevenson MC, Threadgill DW, Russell WE. Loss of hepatocyte EGFR has no effect alone but exacerbates carbon tetrachloride-induced liver injury and impairs regeneration in hepatocyte Met-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2015;308(5):G364–77. doi: 10.1152/ajpgi.00364.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt LE, Dalhoff K. Alpha-fetoprotein is a predictor of outcome in acetaminophen-induced liver injury. Hepatology. 2005;41(1):26–31. doi: 10.1002/hep.20511. [DOI] [PubMed] [Google Scholar]
- Siegmann DW. Stimulation of quiescent 3T3 cells by phosphatidic acid-containing liposomes. Biochem Biophys Res Commun. 1987;145(1):228–33. doi: 10.1016/0006-291x(87)91310-6. [DOI] [PubMed] [Google Scholar]
- Teschke R, Shulze J, Eickhoff A, Danan G. Drug induced liver injury: can biomarkers assist RUCAM in causality assessment? Int J Mol Sci. 2017;18(4):803. doi: 10.3390/ijms18040803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veverka V, Crabbe T, Bird I, Lennie G, Muskett FW, Taylor RJ, Carr MD. Structural characterization of the interaction of mTOR with phosphatidic acid and a novel class of inhibitor: compelling evidence for a central role of the FRB domain in small molecule-mediated regulation of mTOR. Oncogene. 2008;27(5):585–95. doi: 10.1038/sj.onc.1210693. [DOI] [PubMed] [Google Scholar]
- Wang J, Kim C, Jogasuria A, Han Y, Hu X, Wu J, Shen H, Chrast R, Finck BN, You M. Myeloid cell-specific lipin-1 deficiency stimulates endocrine adiponectin-FGF15 axis and ameliorates ethanol-induced liver injury in mice. Sci Rep. 2016;6:34117. doi: 10.1038/srep34117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter JN, Fox TE, Kester M, Jefferson LS, Kimball SR. Phosphatidic acid mediates activation of mTORC1 through the ERK signaling pathway. Am J Physiol Cell Physiol. 2010;299(2):C335–44. doi: 10.1152/ajpcell.00039.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolbright BL, Jaeschke H. The impact of sterile inflammation in acute liver injury. J Clin Transl Res. 2017a;3(Suppl 1):170–88. doi: 10.18053/jctres.03.2017S1.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolbright BL, Jaeschke H. Role of the inflammasome in acetaminophen-induced liver injury and acute liver failure. J Hepatol. 2017b;66(4):836–848. doi: 10.1016/j.jhep.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon MS, Rosenberger CL, Wu C, Truong N, Sweedler JV, Chen J. Rapid mitogenic regulation of the mTORC1 inhibitor, DEPTOR, by phosphatidic acid. Mol Cell. 2015;58(3):549–56. doi: 10.1016/j.molcel.2015.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon MS, Sun Y, Arauz E, Jiang Y, Chen J. Phosphatidic acid activates mammalian target of rapamycin complex 1 (mTORC1) kinase by displacing FK506 binding protein 38 (FKBP38) and exerting an allosteric effect. J Biol Chem. 2011;286(34):29568–74. doi: 10.1074/jbc.M111.262816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You JS, Fey JW, Hornberger TA. Mechanical stimulation induces mTOR signaling via an ERK-independent mechanism: implications for a direct activation of mTOR by phosphatidic acid. PLoS One. 2012;7(10):e47258. doi: 10.1371/journal.pone.0047258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zhong W, Sun Q, Sun X, Zhou Z. Adipose-specific lipin1 overexpression in mice protects against alcohol-induced liver injury. Sci Rep. 2018;8(1):408. doi: 10.1038/s41598-017-18837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.